Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Small animals with unique chemistry – the natural product chemistry of Collembola
Anton
Möllerke
 * and
Stefan
Schulz
* and
Stefan
Schulz
 *
*
Technische Universität Braunschweig, 38106 Braunschweig, Germany. E-mail: stefan.schulz@tu-braunschweig.de; a.moellerke@tu-braunschweig.de
First published on 12th November 2024
Abstract
Covering up to September 2024
Collembola, commonly known as springtails, are abundant and important members of soil ecosystems. Due to their small size and hidden life, not much is known about their secondary metabolites. This chemistry is remarkably different from that of insects, with which they share a common ancestor, although they diverged already around 450 mya. Here we describe what is known so far, mainly compounds for chemical defence and cuticular lipids, as well as chemical signals. The uniqueness of the structures found is striking, many of which are not known from other natural sources. These include polychlorinated benzopyranones, small alkaloids, hetero-substituted aromatic compounds, and a diverse terpene chemistry, including highly branched compounds.
1 Introduction
Collembola are tiny arthropods that play an important role in nearly all soil ecosystems.1,2 Developing from arthropod ancestors, their phylogenetic lineage split from the insects about 450 mya.3 Currently they are regarded as basal hexapods, separating from insects already around 400 mya (Fig. 1). The more than 9.500 recognised species of Collembola4 represent about 32% of all terrestrial arthropods in the world.1 These soil-dwelling arthropods are important members of the food web due to their ability to decompose plant material, as they feed on litter, algae, and fungi. They can reach large numbers of individuals sometimes exceeding densities of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ind. per m2, especially in temperate regions.1 Several Collembola are social arthropods living in colonies, where they show coordinated group behaviour, e.g. in moulting,5 reproductive behaviour, and aggregation,6 suggesting some type of communication between individuals.
000 ind. per m2, especially in temperate regions.1 Several Collembola are social arthropods living in colonies, where they show coordinated group behaviour, e.g. in moulting,5 reproductive behaviour, and aggregation,6 suggesting some type of communication between individuals.
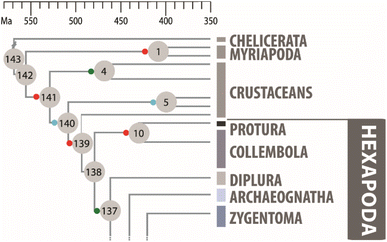 | ||
| Fig. 1 Phylogenetic relationship of Collembola with other arthropods, adopted from Misof et al. Science, 2014, 346, 763.3 Collembola are basal hexapods. Recent insect lineages evolved mostly much later. The arthropods went ashore about 400 mya, with the three lineages Chelicerata, Myriapoda, and Hexapoda, while Crustacea mostly stayed in water. Reprinted with permission from AAAS. | ||
The chemistry of Collembola is of interest especially from an evolutionary perspective because of the early division between insects and Collembola. While the chemistry of insects is relatively well understood, the chemistry of Collembola has only been studied in a few cases, which are described here. Identifying similarities and differences between the chemistry of Collembola and Insecta is therefore of special interest. Diplura and especially Protura are two other basal hexapod lineages, relatively close to Collembola. To our knowledge, there are no reports of secondary metabolites of these tiny arthropods.
Epicuticular lipids and defence compounds (allomones) are two groups of compounds we know best from Collembola. They employ several remarkable structural motifs that are otherwise rare in arthropods, including cyclopropanes, polychlorination, or benzooxathiolanes.
Insects frequently utilise complex mixtures of mainly n- and methyl-branched long-chain alkanes and alkenes,7 but also alcohols, ethers, esters, aldehydes, and dialkyltetrahydrofurans as epicuticular lipids.8,9 In contrast, Collembola tend to rely on higher terpenes, which are rare in insects.10 Furthermore, the data suggest that they generally produce only a few epicuticular compounds instead of the complex hydrocarbon mixtures typical for insects.
The epicuticular lipids of Collembola are of high interest since the collembolan surface is often super- and omniphobic.11 While this effect is partly attributed to the nanostructure of their cuticle, the lipid layer also seems to play a role as the hydrophobicity is affected by seasonal changes or solvent washes.12
The fine-tuning of the cuticular hydrophobicity is key for the water management of Collembola. Unlike e.g. insects or spiders, Collembola respite through their body surface, exposing them to a high risk of desiccation.13 As an adaptation, they developed thinner (permeable) and thicker (impermeable) cuticular areas covered by a wax layer.14 Adjustment of this wax layer allows habitat and seasonal adaptation.
In response to numerous predators, Collembola developed effective defence strategies.2 The main defence mechanism is their ability to catapult themselves out of dangerous situations.15 This is made possible by their furca, a tail-like appendage that has given Collembola their common name ‘springtails’. In soil-dwelling species, the furca can be reduced or absent. These species, but also others, often use chemical deterrents. In some Collembola, this is actively enforced by reflex bleeding of the hemolymph that contains deterrents.16,17
Insects and spiders regularly use contact pheromones for communication.8,18 There is increasing evidence that this is also the case in springtails.19,20 Nonetheless, the structure of such semiochemicals was only determined in a few cases.21,22
The phylogenetic relations of Collembola are still under discussion but three major groups of species are established: Symphypleona, Poduromorpha, and Entomobryomorpha.23 Similarities in chemical profiles of phylogenetic closely related species have been reported e.g. inside Poduromorpha and Entomobryomorpha.10 There have been first attempts to utilise chemical profiles for phylogenetic studies.24
While there is still much room for further investigation, we will summarise here what is known about the secondary metabolites of Collembola.
2 The chemical analysis of Collembola
Collembola are mostly collected in the wild, as only a few species are known to be culturable.25,26 While some Collembola can occur in large numbers, often only small numbers of individuals can be found. Furthermore, species determination is difficult and co-occurrences of different species is common, which demands time consuming inspection of each tiny individual. All in all, often only small quantities of Collembola are available for chemical analysis, with exceptions for species with mass occurrences. Because of their small body size, usually cuticular washes and whole body extracts are prepared, with few exceptions.16 While in some cases enough material for NMR analysis can be obtained, the extracts are commonly analysed with GC/MS. The analysis of electron impact mass spectra and comparison with commercial databases such as NIST,27 or more specific openly available ones such as MACE,28 will lead to the identification of known compounds, while unknown ones often need additional methods such as GC/DD-IR,29 derivative formation in microscale, or computer calculations of spectra. The combined data then lead to a structure proposal that has to be verified by synthesis. These methods have been proven to be effective in the analysis of cuticular (semi-)volatile compounds, while larger and more polar compounds are not detected. HPLC/MS has been used only in few cases,30 often due to limitations in material availability.3 Defence compounds
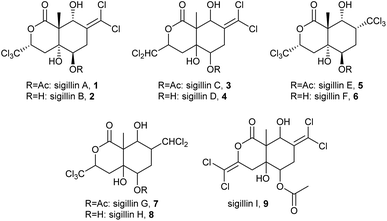
Sigillins (1–9, Fig. 2) are a group of highly chlorinated compounds from the snow flea, Ceratophysella sigillata.31 Mass occurrence of the snow flea allowed for the isolation and crystallisation of sigillin A (1),31 the most abundant compound of this group, and sigillin F (6).30 X-ray crystallography gave the absolute configuration of the compounds.30,31 Yamaoka et al. developed a total synthesis of sigillin A (1) from (R)-4-(trichloromethyl)oxetan-2-one in seven steps.32 Sigillin A (1) is a deterrent against the ant Myrmica rubra in a two-choice feeding assay. Both sigillin A (1) and sigillin F (6) are effective insecticides against aphids, Aphis craccivora, Myzus persicae, and Megoura viciae, the boll weevil, Anthonomus grandis, and the yellow fever mosquito, Aedes aegypti.30,31 Further investigation of the mode of action showed that sigillin A (1) binds to the DmRDL GABA receptor, which is also the target of other insecticides such as fipronil, with similar activity (IC50 24 nM).33 This indicates that nature focused on the same insecticide target as humans did millions of years later! The configuration of sigillin E (5) was established by NMR, while the structures of the other sigillins were deduced from MS data.31 The sigillins contain an unusually large amount of chlorine, only occasionally found in other natural products. The occurrence of di- and trichlormethyl groups suggested a radical formation, maybe by an non-heme iron halogenase requiring ketoglutarate.31 Such enzymes have been found to be active in the biosynthesis of other trichlormethyl-group carrying natural products such as barbamide.34 Whether the springtails produce sigillins de novo or by symbiotic microorganisms, or whether they take them up by food is unclear, although the large amounts found may hint to de novo biosynthesis of these polyketides.31The ‘giant springtail’ Tetrodontophora bielanensis secretes a sticky defence fluid rich in the pyridopyrazines 10–12 (Fig. 3), which are also abundant in minor amounts in other compartments of the springtail.16,35 These compounds were synthesized.16 Pyrazine 10 was also reported from Onychiurus circulans and Deuteraphorura scotaria (as Onychiurus scotaria).36 The predator Nebria brevicollis shows immediate cleansing behavior and disorientation when it comes in contact with these pyridopyrazines.16 A similar structure was found in Hypogastrura viatica which contains the pyridoimidazole 13. The biosynthesis of the pyridopyrazines has not been investigated. While these alkaloids have not been reported from other organisms, genes encoding functional isopenicillin N synthase (IPNS) were found in the genome of Folsomia candida and other springtails.37,38 A likely β-lactam compound was detected using an ELISA assay in F. candida extracts, although it did not show antibiotic activity.38
Several heteroatom-substituted aromatic compounds have been associated with defence functions in Collembola. The compounds 14–17 and 47 were identified in extracts of Neanura muscorum and tested for their deterrent properties against the predatory mite Pergamasus norvegicus.17 In this bioassay, only 16 showed significant deterrence. The biological function of 15 and 14 is unknown, they might be biosynthetic precursor for 16 or be effective deterrents against other predators.
The two phenolic acids 18 and 19 were identified in C. denticulata.39 They showed deterrent effects against the predator Stenus comma (Staphylinidae). Recently, the compounds 20–21 were reported from C. denticulata. They feature some unique structural elements, such as a benzooxathiolane motif otherwise rare in nature.25 Furthermore, 22 is the first compound reported from nature that contains a benzene ring substituted by six heteroatoms. The compounds 20–22 were synthesized and tested for their deterrent properties against the ant Lasius niger in a two-choice feeding assay. While 20 proved to be a highly effective deterrent, 22 showed a significant deterrence only for parts of the test period. Compound 21 did not deter the predator, it may nonetheless be effective against other predators. Benzyl benzoate (17), a known insecticide also used as a plasticizer, was found in Brasilimeria assu sp. nov, among other compounds of unclear origin.40
Additionally, to the small molecules described here, Collembola also utilizes antimicrobial peptides in its immune system, which will not be discussed here.41
4 Cuticular compounds
The epicuticular wax layer forms an important barrier between the body of an arthropod and the environment, thereby fulfilling a variety of functions. The regulation of water diffusion is of tremendous importance to arthropods, which due to their small size have a high surface-to-volume ratio.42 In contrast to insects and arachnids, Collembola respire primarily through their body surface, thereby increasing their vulnerability to water loss.Collembola often contains only a few cuticular compounds, insects on the other hand often use complex mixtures.10 In the following sections, the structural features of the collembolan epicuticular lipids will be discussed.
4.1 Terpenes
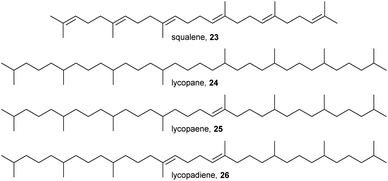
The use of terpenes is widespread within Collembola. Cholesterol and squalene (23) are present in nearly every species, but the most notable difference between Collembola and insects is the widespread use of higher terpenes (7–9 isoprene units) which is rare in insects.10 The majority of tri- and tetraterpenes are formed by tail-to-tail-condensation of two smaller terpene precursors. Squalene (23), a [31+31]-terpene (see ref. 43 for an explanation of the numbering system) is formed by the condensation of two farnesyl pyrophosphate precursors and lycopene, a [41+41]-terpene, by the connection of two geranylgeranyl pyrophosphate precursors.The [41+41]-terpene lycopane (24, Fig. 4) and its mono- and diunsaturated derivatives 25 and 26 have been reported from several species of the order Poduromorpha (C. denticulata, C. sigillata, T. bielanensis).10,44
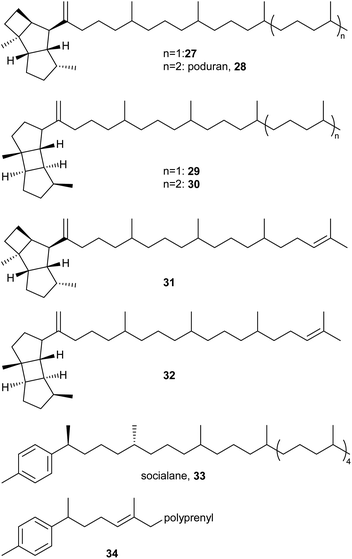
The formation of higher terpenes via only head-to-tail connections is rare in nature but is essential in the ubiquinone biosynthetic pathway. It has been suggested that Collembola exploit this pathway for the production of extracellular lipids of unique structure. There are several examples of fully head-to-tail-connected terpenes bearing a cyclic head group, including poduran (28)45 from P. aquatica and the derivative 27, which is shortened by one isoprene group, reported from X. griesea (Fig. 5).10 In both species, they are accompanied by corresponding prenylprespatanes 29 and 30. Additionally, unsaturated derivatives 31 and 32 occur in X. griesea.10 Both kelsoene- and prespatane-type compounds feature a complex head group similar to sesquiterpenes.46 Socialane (33) is a [9]-terpene with a tolyl-headgroup reported from Hypogastrura socialis, probably formed by a type I terpene synthase.47 The stereochemistry of socialane (33) remains partly unsolved. Four of the 64 possible diastereomers were synthesized,47 showing that the methyl groups next to the aromatic ring are anti-configured and that the others are atactically configured. Partly separation of diastereomers was only possible using a high-temperature polar GC phase with more than 10 h runtime. Similar compounds (34) were reported from F. candida, which contains unsaturated analogues of 33.10
Linear, head-to-tail-connected terpenes have also been reported, like the [8]-terpene 35 from Protaphorura fimata (Fig. 6).10 Interestingly branched prenylated or geranylated terpenes have been identified as well, e.g. viaticenes A (36) and B from H. viatica,43 as well as nitidane (37) from Heteromurus nitidus.26 Such branched terpenes are otherwise rare in nature, an example being the so-called ‘highly branched isoprenoids’ from diatom algae,48 which are smaller. Viaticene A (36), a [614+21]-terpene, is composed of a head-to-tail-connected triterpene core, which is prenylated by a geranyl unit.43 Nitidane (37), however, is based on a diterpene-core, which is prenylated by a geranyl unit, which is also prenylated, resulting in a [46+(22+11)1]-terpene.26 Both terpenes seem to be formed by the addition of the branch to a double bond resulting in the rearrangement of said double bond. The rearranged double bond is formed in a Z-configuration in both nitidane (37) and viaticene A (36), which may indicate a similar enzymatic mechanism.
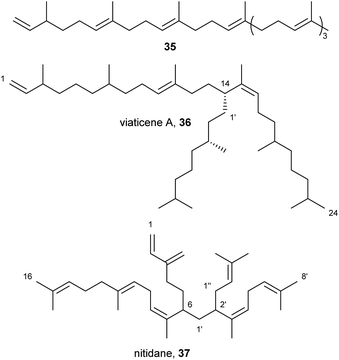 | ||
| Fig. 6 Open-chain, head-to-tail-connected terpenes from Collembola with prenylated (36) and geranylated (36, 37) side chains. | ||
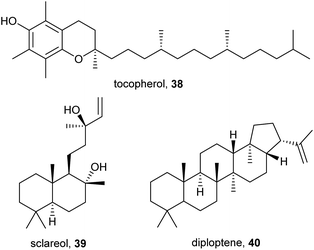
The vitamin tocopherol (38) and the diterpene sclareol (39) have been reported from the cuticle of Megaphorura arctica and F. quadrioculata, respectively (Fig. 7).10 Their biological function is unclear, they might act as parts of the lipid layer or as (semi-)volatile signaling molecules. The hopanoid diploptene (40) occurs in C. denticulata and C. sigillata.10 Hopanoids have been reported from bacteria, lichens, and some plants, where they fulfill similar functions as sterols.49
4.2 Steroids
It was previously assumed that insects and the majority of arthropods cannot synthesize triterpenes, tetraterpenes, and sterols de novo, due to the absence of the requisite genes. However, the majority of the genes from the cholesterol biosynthetic pathway are present in genomes of Collembola, e.g. F. candida, Orchesella cincta, and Allacma fusca, although only a few high-quality genome data are available for Collembola so far.10 In addition, cholesterol and squalene (23) are ubiquitous in Collembola and frequently represent the main components of epicuticular extracts, also in cases where laboratory cultures were fed exclusively with yeast,10 which lacks cholesterol. These data suggest that springtails can synthesize 23 and cholesterol de novo. A recent study has demonstrated, that the cuticular cholesterol layers restrict protein adsorption and bacterial adhesion, thereby contributing to the antiadhesive properties of Collembola.50In addition to cholesterol, desmosterol is also widespread in the order Poduromorpha.10,44 Other sterols reported from Collembola include cholestanol, cholest-3,5-diene, ergosta-5,22-dien-3-ol, cholest-7-en-3-ol, and cholesteryl acetate.10
4.3 Non-terpenoid hydrocarbons and related compounds
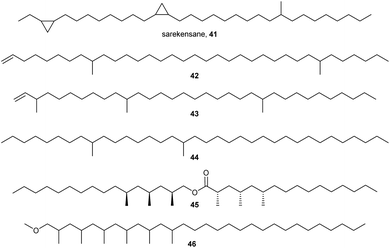
Similarly to insects, some Collembola employ a range of hydrocarbons as epicuticular lipids. However, in the majority of species, only one or two of these lipids are present as major components and the chain length seems to be slightly longer than usually encountered in insects. Furthermore, different structural features are present (Fig. 8). The most striking example of this is sarekensane (41) from Vertagopus sarekensis, a long-chain hydrocarbon containing two cyclopropanes and a methyl branch.51 The identification needed degradation and total synthesis to verify the structures because NMR analysis could not solve the positional arrangement. Cyclopropanes have not been reported from other arthropod hydrocarbons. Two long-chain terminal alkenes with two (42) and three methyl-branches (43) have been reported from O. cincta as well as an alkane (44) with two methyl-branches from Tomocerus vulgaris.10 In these compounds, the methyl branches are placed distantly from each other, while in insects a 1,5- or 1,7-arrangement is common.Anurida maritima uses no hydrocarbons, but a 1,3-polymethyl branched ester (45) as epicuticular lipid.10 While this motif has not been reported from other Collembola, the spider Argiope bruennichi uses similar esters but shorter and in complex mixtures.52 The absolute configuration has been elucidated by total synthesis. Another polymethyl branched compound is the methyl ether 46 found in X. maritima. Several simpler linear alkanes have been reported from Collembola in a few cases.10,44
5 Chemical communication
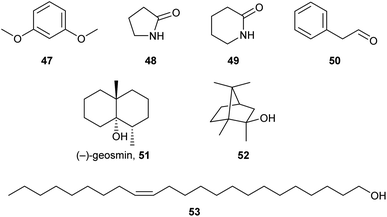
Arthropods often rely on chemical channels for communication, as acoustic and visual signalling can be less effective, due to their small body size. While several studies have demonstrated the involvement of chemical cues in the aggregation behaviour and interspecific recognition of Collembola,19,53 as well as in moulting5 and reproductive behaviour,6,22 only limited information is available regarding the structures of the pheromones involved. While some species-specific compounds have been reported, they have seldom been tested on biological functions.10An example is 1,3-dimethoxybenzene (47, Fig. 9), which has a strong smell and proved to function as an alarm signal for Collembola.21 While it was previously believed to be a chemotaxonomic marker for the Neanurinae subfamily,54 it was recently identified in C. denticulata.25 High concentrations of the amides 48 and 49, as well as phenylacetaldehyde (50) from F. candida repel conspecifics.55
Geosmin (51), a known bacterial sesquiterpenoid with a distinct and intense odour was found to be released by colonies of C. sigillata through headspace analysis.31 The terpene is also produced by soil actinomycetes bacterial colonies, which serve as a food source for F. candida. A recent study demonstrated that geosmin (51) and 2-methylisoborneol (52) elicited an electrophysiological response in F. candida and attracted Collembola in field experiments.56 The dispersal of bacterial spores in the soil is thus enhanced. Other reports show detection using electroantennography or deterrence of certain Collembola by fungal volatiles including 1-octen-3-ol and methyl cinnamate.57
Linolenic acid has been reported from various Collembola36,44,58 and shows repellent effects against the springtail Protaphorura armata.36 This common acid was therefore postulated as an alarm signal of Collembola. The ability to synthesise linolenic acid de novo is widespread in Collembola.59
Palmitic acid, certainly present in all Collembola, has been investigated for pheromonal activity in P. armata and F. candida, attracting and inducing aggregation in P. armata, but not in F. candida.36 This indicates a signalling function of this ubiquitous acid and the species specificity of the signal.36 Virgin females of O. cincta are attracted to spermatophores of the same species. The attraction pheromone was identified as (Z)-14-tricosenol (53), while co-occurring (Z)-13-docosenol was not attracting virgin females.22
6 Other compounds
The fatty acid composition of Collembola is dependent on diet,60 environmental temperature, and life stage.58 The C16/C18 ratio in neutral lipid fatty acids increased with decreasing temperature, while the unsaturation index changed.58 Interestingly, Collembola, like Crustacea, often contain a high proportion of C20 polyunsaturated fatty acids,60,61 while this is rare in insects.62 Such alterations in lipid composition could be employed to enhance cold tolerance,63 as membrane fluidity represents a pivotal factor in cold tolerance.64 Exposure to α-pinene, which is emitted by numerous plants, was shown to increase cold tolerance in Folsomia candida, despite its toxicity at high doses.657 Conclusions
The existing literature on the chemistry of Collembola is comparatively limited, yet it does contain several distinctive structural motifs. The use of higher terpenes is a prevalent phenomenon, these terpenes are often fully head-to-tail linked. Cyclic head groups are frequently used, as are branched terpenes. Additionally, non-terpenoid lipids exhibit rare structural motifs, e.g. the cyclopropane sarekensane (41) or the polymethyl-branched ester 45.Collembola have also developed several effective deterrents. These often contain high amounts of heteroatoms e.g. the polychlorinated sigillins (1–9), benzodioxolanes (20), and benzooxathiolanes (21–22), as well as the pyridopyrazines (10–12). These or related compounds are not reported from other natural sources.
The composition of the epicuticular lipid profiles of Collembola with only a few compounds compared is relatively simple, in comparison to those of insects. However, species specificity occurs and is reflected in more complex molecular structures. Given the high energy costs to synthesise them, they might fulfill important biological tasks, such as species recognition, as has been reported from insects. Although pheromone activity has been reported several times, our knowledge of the molecular structure is almost absent. Further investigation in these areas is certainly necessary, especially because of the importance of Collembola in soil ecosystems, although this may be hindered by the difficulties encountered when working with these tiny creatures.
While there has been accumulated at least a moderate amount of knowledge about the chemistry of Poduromorpha and Entomobryomorpha, reports on Symphyleona remain scarce.10 Further investigation of the Symphyleona and other orders or families, will enhance our understanding of the taxonomic influence of chemical profiles.
8 Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.9 Author contributions
All authors contributed equally to the manuscript.10 Conflicts of interest
The authors declare no conflict of interest.11 Acknowledgements
We thank the Deutsche Forschungsgemeinschaft for a grant Schu984/13-1.12 References
- A. M. Potapov, C. A. Guerra, J. van den Hoogen, A. Babenko, B. C. Bellini, M. P. Berg, S. L. Chown, L. Deharveng, Ľ. Kováč, N. A. Kuznetsova, J.-F. Ponge, M. B. Potapov, D. J. Russell, D. Alexandre, J. M. Alatalo, J. I. Arbea, I. Bandyopadhyaya, V. Bernava, S. Bokhorst, T. Bolger, G. Castaño-Meneses, M. Chauvat, T.-W. Chen, M. Chomel, A. T. Classen, J. Cortet, P. Čuchta, A. L. M. de Pedrosa, S. S. D. Ferreira, C. Fiera, J. Filser, O. Franken, S. Fujii, E. G. Koudji, M. Gao, B. Gendreau-Berthiaume, D. F. Gomez-Pamies, M. Greve, I. Tanya Handa, C. Heiniger, M. Holmstrup, P. Homet, M. Ivask, C. Janion-Scheepers, M. Jochum, S. Joimel, B. C. S. Jorge, E. Jucevica, O. Ferlian, L. C. I. de Oliveira Filho, O. Klauberg-Filho, D. Baretta, E. J. Krab, A. Kuu, E. C. A. de Lima, D. Lin, Z. Lindo, A. Liu, J.-Z. Lu, M. J. Luciañez, M. T. Marx, M. A. McCary, M. A. Minor, T. Nakamori, I. Negri, R. Ochoa-Hueso, J. G. Palacios-Vargas, M. M. Pollierer, P. Querner, N. Raschmanová, M. I. Rashid, L. J. Raymond-Léonard, L. Rousseau, R. A. Saifutdinov, S. Salmon, E. J. Sayer, N. Scheunemann, C. Scholz, J. Seeber, Y. B. Shveenkova, S. K. Stebaeva, M. Sterzynska, X. Sun, W. I. Susanti, A. A. Taskaeva, M. P. Thakur, M. A. Tsiafouli, M. S. Turnbull, M. N. Twala, A. V. Uvarov, L. A. Venier, L. A. Widenfalk, B. R. Winck, D. Winkler, D. Wu, Z. Xie, R. Yin, D. Zeppelini, T. W. Crowther, N. Eisenhauer and S. Scheu, Nat. Commun., 2023, 14, 674 CrossRef CAS PubMed.
- P. S. Hopkin, Biology of the Springtails (Insecta: Collembola), Oxford University Press, Oxford, 1997 Search PubMed.
- B. Misof, S. Liu, K. Meusemann, R. S. Peters, A. Donath, C. Mayer, P. B. Frandsen, J. Ware, T. Flouri, R. G. Beutel, O. Niehuis, M. Petersen, F. Izquierdo-Carrasco, T. Wappler, J. Rust, A. J. Aberer, U. Aspöck, H. Aspöck, D. Bartel, A. Blanke, S. Berger, A. Böhm, T. R. Buckley, B. Calcott, J. Chen, F. Friedrich, M. Fukui, M. Fujita, C. Greve, P. Grobe, S. Gu, Y. Huang, L. S. Jermiin, A. Y. Kawahara, L. Krogmann, M. Kubiak, R. Lanfear, H. Letsch, Y. Li, Z. Li, J. Li, H. Lu, R. Machida, Y. Mashimo, P. Kapli, D. D. McKenna, G. Meng, Y. Nakagaki, J. L. Navarrete-Heredia, M. Ott, Y. Ou, G. Pass, L. Podsiadlowski, H. Pohl, B. M. von Reumont, K. Schütte, K. Sekiya, S. Shimizu, A. Slipinski, A. Stamatakis, W. Song, X. Su, N. U. Szucsich, M. Tan, X. Tan, M. Tang, J. Tang, G. Timelthaler, S. Tomizuka, M. Trautwein, X. Tong, T. Uchifune, M. G. Walzl, B. M. Wiegmann, J. Wilbrandt, B. Wipfler, T. K. F. Wong, Q. Wu, G. Wu, Y. Xie, S. Yang, Q. Yang, D. K. Yeates, K. Yoshizawa, Q. Zhang, R. Zhang, W. Zhang, Y. Zhang, J. Zhao, C. Zhou, L. Zhou, T. Ziesmann, S. Zou, Y. Li, X. Xu, Y. Zhang, H. Yang, J. Wang, J. Wang, K. M. Kjer and X. Zhou, Science, 2014, 346, 763–767 CrossRef CAS.
- C. Leo, A. Carapelli, F. Cicconardi, F. Frati and F. Nardi, Diversity, 2019, 11, 169 CrossRef CAS.
- H. P. Leinaas, Science, 1983, 219, 193–195 CrossRef CAS.
- E. S. Waldorf, Environ. Entomol., 1974, 3, 916–918 CrossRef.
- (a) G. J. Blomquist, in Insect Hydrocarbons. Biology, Biochemistry, and Chemical Ecology, ed. G. J. Blomquist and A.-G. Bagnères, Cambridge University Press, Cambridge, UK, New York, 2010, pp. 53–74 CrossRef; (b) D. A. Carlson, U. R. Bernier and B. D. Sutton, J. Chem. Ecol., 1998, 24, 1845–1866 CrossRef CAS; (c) H. Holze, L. Schrader and J. Buellesbach, Heredity, 2020, 126, 219–234 CrossRef.
- Insect Hydrocarbons. Biology, Biochemistry, and Chemical Ecology, ed. G. J. Blomquist and A. G. Bagnères, Cambridge University Press, Cambridge, UK, New York, 2010 Search PubMed.
- (a) S. Schulz, G. Beccaloni, R. Nishida, Y. Roisin, R. I. Vane-Wright and J. N. McNeil, Z. Naturforsch., 1998, 53c, 107–116 CrossRef; (b) P. A. Sutton, M. J. Wilde, S. J. Martin, J. Cvacka, V. Vrkoslav and S. J. Rowland, J. Chromatogr. A, 2013, 1297, 236–240 CrossRef CAS.
- A. Möllerke, G. Brasse, J. Bello, D. M. Vidal, K. Dettner, J. Zettel, M. P. Berg, S. Scheu, H. P. Leinaas and S. Schulz, iScience, 2024, 27, 110416 CrossRef.
- R. Hensel, C. Neinhuis and C. Werner, Chem. Soc. Rev., 2016, 45, 323–341 RSC.
- (a) H. Gundersen, H. P. Leinaas and C. Thaulow, PLoS One, 2014, 9, e86783 CrossRef PubMed; (b) H. Gundersen, H. P. Leinaas and C. Thaulow, Beilstein J. Nanotechnol., 2017, 8, 1714–1722 CrossRef CAS PubMed; (c) H. Gundersen, C. Thaulow and H. P. Leinaas, Zoomorphology, 2015, 134, 211–218 CrossRef; (d) L. Schmüser, W. Zhang, M. T. Marx, N. Encinas, D. Vollmer, S. Gorb, J. E. Baio, H. J. Räder and T. Weidner, ACS Appl. Mater. Interfaces, 2020, 12, 12294–12304 CrossRef; (e) J. Guo, M. Jiang, X. Li, M. U. Farid, B. J. Deka, B. Zhang, J. Sun, Z. Wang, C. Yi, P. W. Wong, S. Jeong, B. Gu and A. K. An, Nat. Commun., 2024, 15, 7750 CrossRef CAS PubMed.
- C. W. Kærsgaard, M. Holmstrup, H. Malte and M. Bayley, J. Insect Physiol., 2004, 50, 5–15 CrossRef.
- H. Ghiradella and W. Radigan, J. Insect Physiol., 1974, 20, 301–306 CrossRef CAS.
- V. M. Ortega-Jimenez, E. J. Challita, B. Kim, H. Ko, M. Gwon, J.-S. Koh and M. S. Bhamla, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2211283119 CrossRef CAS.
- K. Dettner, A. Scheuerlein, P. Fabian, S. Schulz and W. Francke, J. Chem. Ecol., 1996, 22, 1051–1074 CrossRef CAS.
- C. Messer, J. Walther, K. Dettner and S. Schulz, Pedobiologia, 2000, 44, 210–220 CrossRef CAS.
- S. A. Adams and N. D. Tsutsui, Philos. Trans. R. Soc., B, 2020, 375, 20190476 CrossRef PubMed.
- S. Salmon, S. Rebuffat, S. Prado, M. Sablier, C. D'Haese, J.-S. Sun and J.-F. Ponge, Biol. Fertil. Soils, 2019, 55, 425–438 CrossRef.
- D. Porco, L. Deharveng and C. Gers, Pedobiologia, 2004, 48, 581–583 CrossRef.
- C. Messer, K. Dettner, S. Schulz and W. Francke, Pedobiologia, 1999, 43, 174–182 CrossRef CAS.
- Z. V. Zizzari, T. Engl, S. Lorenz, N. M. van Straalen, J. Ellers and A. T. Groot, Anim. Behav., 2017, 124, 221–227 CrossRef.
- (a) Y. Xiong, Y. Gao, W. Yin and Y. Luan, Mol. Phylogenet. Evol., 2008, 49, 728–735 CrossRef CAS PubMed; (b) D. Yu, S. Du, X. Wei, J. Zhu, Y. Ding, F. Hu, M. Liu and F. Zhang, Mol. Phylogenet. Evol., 2024, 200, 108169 CrossRef CAS.
- (a) D. Porco and L. Deharveng, Sci. Nat., 2009, 96, 943–954 CrossRef CAS; (b) D. Porco, A. Bedos and L. Deharveng, PLoS One, 2010, 5, e14405 CrossRef CAS PubMed.
- A. Möllerke, M. Stell, C. Schlawis, U. Trauer-Kizilelma, J. Herrmann, H. P. Leinaas, S. Scheu and S. Schulz, Chem. Sci., 2024, 35, 15332–15338 RSC.
- A. Möllerke and S. Schulz, J. Nat. Prod., 2024, 87, 1454–1458 CrossRef PubMed.
- National Institute of Standards, NIST mass spectral library, Gaithersburg, 2023 Search PubMed.
- S. Schulz and A. Möllerke, J. Chem. Ecol., 2022, 48, 589–597 CrossRef CAS.
- C. Schlawis and S. Schulz, Nat. Prod. Rep., 2020, 37, 1561–1567 RSC.
- M. Steinbiss, M. Maczka, J. Langewald, B. London, R. Vallinayagam, P. G. Jones, H. Bastiaans and S. Schulz, J. Nat. Prod., 2020, 83, 468–472 CrossRef CAS PubMed.
- W. Schmidt, T. M. Schulze, G. Brasse, E. Nagrodzka, M. Maczka, J. Zettel, P. G. Jones, J. Grunenberg, M. Hilker, U. Trauer-Kizilelma, U. Braun and S. Schulz, Angew. Chem., Int. Ed., 2015, 54, 7698–7702 CrossRef CAS.
- Y. Yamaoka, T. Nakayama, S. Kawai and K. Takasu, Org. Lett., 2020, 22, 7721–7724 CrossRef CAS PubMed.
- M. Gassel, C. Wolf, S. Noack, H. Williams and T. Ilg, Insect Biochem. Mol. Biol., 2014, 45, 111–124 CrossRef CAS PubMed.
- C. S. Neumann, D. G. Fujimori and C. T. Walsh, Chem. Biol., 2008, 15, 99–109 CrossRef CAS PubMed.
- M. Buděšínský, A. Trka, K. Stránský and M. Streibl, Collect. Czech. Chem. Commun., 1986, 51, 956–963 CrossRef.
- E. Nilsson and G. Bengtsson, J. Chem. Ecol., 2004, 30, 1431–1443 CrossRef CAS.
- D. Roelofs, M. J. T. N. Timmermans, P. Hensbergen, H. van Leeuwen, J. Koopman, A. Faddeeva, W. Suring, T. E. de Boer, J. Mariën, R. Boer, R. Bovenberg and N. M. van Straalen, Mol. Biol. Evol., 2013, 30, 541–548 CrossRef CAS PubMed.
- W. Suring, K. Meusemann, A. Blanke, J. Mariën, T. Schol, V. Agamennone, A. Faddeeva-Vakhrusheva, M. P. Berg, A. Brouwer, N. M. van Straalen and D. Roelofs, Mol. Ecol., 2017, 26, 3217–3229 CrossRef CAS.
- C. Bitzer, G. Brasse, K. Dettner and S. Schulz, J. Chem. Ecol., 2004, 30, 1591–1602 CrossRef CAS PubMed.
- D. Zeppelini, G. C. Queiroz, N. P. Lopes and F. J. B. Mendonça-Junior, PLoS One, 2019, 14, e0212451 CrossRef CAS.
- G. Pradhan and P. Engsontia, Insects, 2023, 14, 215 CrossRef.
- J. W. Beament, Biol. Rev., 1961, 36, 281–320 CrossRef CAS.
- J. E. Bello, P. Stamm, H. P. Leinaas and S. Schulz, Eur. J. Org Chem., 2019, 2019, 2158–2162 CrossRef CAS.
- K. Stránský, A. Trka, M. Budesínský and M. Streibl, Collect. Czech. Chem. Commun., 1986, 51, 948–955 CrossRef.
- S. Schulz, C. Messer and K. Dettner, Tetrahedron Lett., 1997, 38, 2077–2080 CrossRef CAS.
- G. M. König and A. D. Wright, J. Org. Chem., 1997, 62, 3837–3840 CrossRef.
- A. Möllerke, D. Montes Vidal, H. P. Leinaas and S. Schulz, Chem.–Eur. J., 2024, 30, e202400272 CrossRef.
- S. T. Belt, T. A. Brown, L. Smik, A. Tatarek, J. Wiktor, G. Stowasser, P. Assmy, C. S. Allen and K. Husum, Org. Geochem., 2017, 110, 65–72 CrossRef CAS.
- B. J. Belin, N. Busset, E. Giraud, A. Molinaro, A. Silipo and D. K. Newman, Nat. Rev. Microbiol., 2018, 16, 304–315 CrossRef CAS.
- J. Friedrichs, R. Helbig, J. Hilsenbeck, P. R. Pandey, J.-U. Sommer, L. D. Renner, T. Pompe and C. Werner, Nature, 2023, 618, 733–739 CrossRef CAS PubMed.
- A. Möllerke, J. Bello, H. P. Leinaas and S. Schulz, J. Nat. Prod., 2023, 87, 85–97 CrossRef.
- M. Gerbaulet, A. Möllerke, K. Weiss, S. Chinta, J. M. Schneider and S. Schulz, J. Chem. Ecol., 2022, 48, 244–262 CrossRef CAS.
- (a) H. A. Verhoef, C. J. Nagelkerke and E. N. G. Joosse, J. Insect Physiol., 1977, 23, 1009–1013 CrossRef; (b) H. A. Verhoef, J. Insect Physiol., 1984, 30, 665–670 CrossRef; (c) A. Manica, F. K. McMeechan and W. A. Foster, Entomol. Exp. Appl., 2001, 99, 393–395 CrossRef.
- D. Porco and L. Deharveng, Biochem. Syst. Ecol., 2007, 35, 160–161 CrossRef CAS.
- J. Liu and D. Wu, J. Insect Behav., 2017, 30, 331–341 CrossRef.
- P. G. Becher, V. Verschut, M. J. Bibb, M. J. Bush, B. P. Molnár, E. Barane, M. M. Al-Bassam, G. Chandra, L. Song, G. L. Challis, M. J. Buttner and K. Flärdh, Nat. Microbiol., 2020, 5, 821–829 CrossRef CAS PubMed.
- K. Hedlund, G. Bengtsson and S. Rundgren, Funct. Ecol., 1995, 9, 869–875 CrossRef.
- D. Haubert, M. M. Häggblom, S. Scheu and L. Ruess, Eur. J. Soil Biol., 2008, 44, 213–219 CrossRef CAS.
- M. Malcicka, J. Ruther and J. Ellers, J. Chem. Ecol., 2017, 43, 911–919 CrossRef CAS PubMed.
- P. M. Chamberlain, I. D. Bull, H. Black, P. Ineson and R. P. Evershed, Soil Biol. Biochem., 2005, 37, 1608–1624 CrossRef CAS.
- (a) M. Graeve, P. Dauby and Y. Scailteur, Polar Biol., 2001, 24, 853–862 CrossRef; (b) P. M. Chamberlain and H. I. J. Black, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2005, 140, 299–307 CrossRef PubMed.
- L. R. Shen, C. Q. Lai, X. Feng, L. D. Parnell, J. B. Wan, J. D. Wang, D. Li, J. M. Ordovas and J. X. Kang, J. Lipid Res., 2010, 51, 2985–2992 CrossRef CAS PubMed.
- M. Holmstrup and S. Slotsbo, J. Comp. Physiol., B, 2018, 188, 225–236 CrossRef CAS PubMed.
- (a) E. Z. Drobnis, L. M. Crowe, T. Berger, T. J. Anchordoguy, J. W. Overstreet and J. H. Crowe, J. Exp. Zool., 1993, 265, 432–437 CrossRef CAS; (b) A. R. Cossins, Biochim. Biophys. Acta, 1977, 470, 395–411 CrossRef CAS.
- T. G. Jensen, M. Holmstrup, R. B. Madsen, M. Glasius, L. N. Trac, P. Mayer, B. Ehlers and S. Slotsbo, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2020, 229, 108681 CAS.
| This journal is © The Royal Society of Chemistry 2025 |