Temperature-driven evolution of ceria–zirconia-supported AuPd and AuRu bimetallic catalysts under different atmospheres: insights from IL-STEM studies†
Lidia
Chinchilla
 *,
Ramón
Manzorro
*,
Ramón
Manzorro
 ,
Carol
Olmos
,
Xiaowei
Chen
,
Carol
Olmos
,
Xiaowei
Chen
 ,
José J.
Calvino
,
José J.
Calvino
 and
Ana B.
Hungría
and
Ana B.
Hungría

Departamento de Ciencia de los Materiales, Ingeniería Metalúrgica y Química Inorgánica, Facultad de Ciencias, Universidad de Cádiz, Campus Río San Pedro, Puerto Real (Cádiz), E-11510, Spain. E-mail: lidia.chinchilla@uca.es
First published on 7th December 2023
Abstract
The evolution of the structure and composition of the system of particles in two Ce0.62Zr0.38O2-supported bimetallic catalysts based on Au and a 4d metal (Ru or Pd) under high temperature conditions and different reducing and oxidizing environments has been followed by means of Identical Location Scanning Transmission Electron Microscopy (IL-STEM). As an alternative to in situ microscopy, this technique offers valuable insights into the structural modifications occurring in chemical environments with the characteristics of a macro-scale reactor. By tracking exactly the same areas on a large number of metallic entities, it has been possible to reveal the influence of particle size and the nature of the redox environment on the temperature-driven mobilization of the different metals involved. Thus, oxidizing environments evidenced a much higher capacity to mobilize the three metals, preferentially Au. Moreover, the typical storage conditions (under air) of catalysts during the prolonged exposure time has been proved to induce significant modifications in these bimetallic systems, even at room temperature. Regardless of the type of redox environment, bimetallic systems showed better thermal resistance, which demonstrates a beneficial effect of the second metal. In summary, IL-STEM is an invaluable and complementary methodology for characterizing heterogeneous catalysts under realistic reaction conditions and is within the reach of most laboratories.
Introduction
The introduction of a second active metal in gold-based heterogeneous catalysts offers a myriad of opportunities to tailor their surface structure and composition. This can significantly impact catalytic performance, promoting stability and selectivity for specific products.1–11 Electron microscopy has played a pivotal role in unraveling the complex relationships between synthesis, structure and catalytic functionality.12,13 Recent advances in aberration correction for transmission electron microscopy (TEM), high brightness electron sources as well as the design of new X-ray detection systems have enabled images and elemental maps to be obtained with atomic resolution.14–19 Therefore, TEM has played a crucial role in investigating the distribution of supported metal nanoparticles, their morphology and composition, their structural or epitaxy relationships, and metal–support interaction phenomena in catalyst materials.4,20–23However, a crucial consideration is the dynamic nature of the catalyst under reaction conditions, in which the morphology, the crystallographic nature of the exposed surfaces of the nanoparticles, and their structure and composition may change in response to several factors, such as temperature, redox atmospheres, etc. These dynamic processes are vital to understanding the catalytic mechanism and designing new formulations for enhanced catalytic performance.24
In order to observe the dynamic process at the nanoscale, breaking the requirement of ultra-high vacuum of TEM, in situ microscopy has been implemented.25,26 Thus, the development of a specially modified TEM allows gaseous reactants into the sample chamber at low pressures (<5 × 10−3 bar). Alternatively, specialized TEM holders based on windowed cells have been also designed, which can be operated with flowing gases at pressures reaching up to 2 bar.27,28 In both approaches, annealing treatments can be performed and their use in aberration-corrected microscopes would allow the recording of atomic resolution images, as well as performing X-ray energy dispersive spectroscopy (XEDS) and electron energy loss spectroscopy (EELS) analyses.29–31
However, in situ microscopy still faces challenges related to the interaction of the electron beam with the gas surrounding the sample. Therefore, it is essential to develop specific methodologies for each catalyst reaction system that guarantee that the results correspond to the interaction of the sample with the real chemical process.25 The combination of spectroscopic techniques has also its own challenges, for example the geometry of an in situ holder hinders reliable XEDS spectroscopy, among others.32,33 These inherent drawbacks of the system architecture have prevented so far quantitative analyses. Despite the continuous progress, in situ microscopy is still beyond reach for many laboratories due to its intrinsic complexity and cost.
In this context, Identical Location Transmission Electron Microscopy (IL-TEM) has emerged as a valuable alternative and complementary technique, primarily used in electrocatalysis.34–37 In this methodology, analysis of the identical areas of the sample was performed prior and post-reaction by exposing the TEM grid to the same reaction systems as for the ex situ experiments, thus ensuring that the pressure and temperature are as close as possible to real operating conditions and without the need for such sophisticated technology as the in situ infrastructure. However, IL-TEM only provides a picture of the catalyst once the reaction is completed, missing intermediate structural insights concerning the dynamic processes related to reactions, only accessible with full in situ techniques. While information on the intermediate stages of the reaction is lost, IL-TEM is useful not only to reveal how aging under reaction affects the initial structure of the catalyst by looking at the modifications suffered by individual particles, but also to establish the optimum working conditions in the in situ experiments.
Here, we employ IL-TEM to understand the structural and compositional modification behaviour of AuPd and AuRu bimetallic catalysts supported on a ceria–zirconia mixed oxide under high temperature conditions and different reducing and oxidizing redox environments. Considering the synergistic effects previously demonstrated on these bimetallic catalysts for the selective oxidation of alcohols (glycerol, octanol and benzyl alcohol),3,4,8,38 we extend the study of a potential synergistic effect of thermal stabilization of Au by intermixing with 4d metals. This approach in scanning transmission electron microscopy mode (IL-STEM) combined with elemental analysis with a good signal-to-noise ratio acquired by a high efficiency detection XEDS system has allowed us to achieve reliable quantifications, which are difficult to obtain by using an in situ holder. Our results provide information which is out of reach for conventional STEM studies, in which spatial correlation between the final and initial states of the sample is lost. The subtle details of the structural transformations taking place during the activation of bimetallic catalysts are revealed. The whole set of results evidence large differences in the mobilization of the different metals as well as a large influence of the chemical environment and particle size on this particular question, which is key to understand the effect of activation pre-treatments on the catalyst nanostructure.
Experimental
All catalysts presented in this study have been supported on a ceria–zirconia mixed oxide (CZ) provided by Grace Davison. The monometallic AuCZ reference catalyst was synthetized using the deposition–precipitation technique as previously detailed.4 To this end, a HAuCl4 solution was dropped onto an aqueous suspension of cerium–zirconia oxide. This suspension was kept at constant pH ≈ 8 during the whole Au precursor addition process using an automatic titrator which dosed the necessary amounts of a Na2CO3 solution. The resultant precipitate was filtered, washed until no chloride ions were detected, and dried overnight at 110 °C. For the AuRu bimetallic system, the previously obtained fresh AuCZ solid sample was impregnated with a Ru(NO)(NO3)3 solution to achieve a 1Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Ru molar ratio as is explained elsewhere.4 The catalysts, AuCZ and 1
1Ru molar ratio as is explained elsewhere.4 The catalysts, AuCZ and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1AuRuCZ, were activated under reduction treatment at 350 °C (1 h) using a H2(5%)/Ar flow, further evacuated (1 h) under a He flow at the same temperature, cooled in helium to room temperature (RT), and finally subjected to a passivation treatment to minimize the effects of uncontrolled exothermic oxidation on the catalysts.
1AuRuCZ, were activated under reduction treatment at 350 °C (1 h) using a H2(5%)/Ar flow, further evacuated (1 h) under a He flow at the same temperature, cooled in helium to room temperature (RT), and finally subjected to a passivation treatment to minimize the effects of uncontrolled exothermic oxidation on the catalysts.
In the case of the Au–Pd bimetallic system, it was prepared by a simultaneous deposition–precipitation method using a combination of HAuCl4 and PdCl2 precursors in a single solution as was described elsewhere.8 Finally, the dried AuPdCZ was activated by an oxidized treatment in O2(5%)/He flow at 250 °C.
Catalyst characterization
TEM imaging was performed using a JEOL 2010F and a double-corrected FEI Titan3 60–300 Themis microscope operated at 200 kV in High Angle Annular Dark Field Scanning Transmission Electron Microscopy (HAADF-STEM) mode. The latter is considered to be the best technique to characterize these kinds of materials as the image intensity is proportional to the thickness of the sample and to roughly the square of the atomic number of the scattering element;39 therefore, the morphology and location of the particles containing heavier elements, such as Au (Z = 79), can be distinguished from the ceria–zirconia mixed oxide (Z ≈ 50). Since the catalysts also contain lighter elements, such as Pd (Z = 46) or Ru (Z = 44), which may result in lower contrast images compared to Au supported on CZ, the samples were additionally characterized by STEM X-ray energy dispersive spectroscopy to map the spatial distribution of all elements. The FEI Titan3 microscope is equipped with a high sensitivity ChemiSTEM™ system, which implements 4 windowless silicon drift detectors, resulting in a large sensor area of about 120 mm2 and a solid angle of 0.7 sr in combination with a high readout speed electronics (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 spectra per second).
000 spectra per second).
In general, HAADF-STEM images were recorded using a convergence semi-angle of α ≈ 19 mrad, a spot size of 9 and a beam current of 30 pA. For XEDS spectroscopy experiments, the beam current was increased to 100–120 pA using a dwell time ranging from 50 to 75 μs per pixel and an area of analysis of 400 × 400 pixels. Each spectrum-image dataset was collected as a series of 100 frames using a drift compensation tool.
All HAADF-STEM and XEDS-STEM datasets were acquired and processed using Velox software. This program allows to employ the well-known Cliff–Lorimer method to perform XEDS quantifications using Au-Lα, Pd-Lα, Ru-Lα and Ce-Lα series.40
Identical location STEM study
To obtain a representative view of the structural and compositional modifications taking place under reducing or oxidizing treatments at high temperature, several areas were tracked before and after thermal treatments. As previously described, all experiments were carried out in STEM mode employing XEDS. TEM samples were prepared by depositing 2 drops of 0.1% (w/v) catalyst solution in ethanol on PELCO® silicon nitride support films for TEM grids with a membrane thickness of 15 nm. These grids were previously proved to be mechanically and thermally stable under conditions characteristic of the thermochemical treatments used in this study.Firstly, a detailed HAADF-STEM and XEDS analysis of the initial state of the catalysts was performed on different areas of the grid. Then the TEM grid was cautiously transferred into a quartz reactor and subjected to heating treatments for 2 h either under reducing or oxidizing atmospheres. The thermal treatment was performed at 700 °C to favour the formation of bimetallic entities and to compare the stability of these systems with that of their Au monometallic counterparts. At the Tamman temperature (TTamman), atomic mobility and diffusion are observed in bulk materials. The TTamman values for Au and Pd are 396 and 640 °C respectively, but in the case of Ru, it is much higher, about 990 °C.41 Although the TTamman is not reached in the latter, actually exceeds the Hutting barrier, the temperature at which the surface atoms start to exhibit mobility.42 We will refer to treatments as R700 and O700. In R700, the catalysts were heated in a temperature ramp of up to 700 °C and further maintained at this temperature for 1 h under a flow of H2 (5%)/Ar and in O700, the catalysts were heated under a flow of O2 (5%)/He. In both cases, the gas was switched at high temperature to a flow of pure argon or helium and kept under an inert atmosphere for an additional hour. After completing the heating step, the TEM grid was cooled in an inert flow down to room temperature (RT). In all cases a flow rate of 1 ml min−1 and a heating ramp of 5 °C min−1 were used. Finally, the TEM grid was carefully collected from the reactor and transferred to the electron microscope in order to characterize the same locations initially studied.
Results
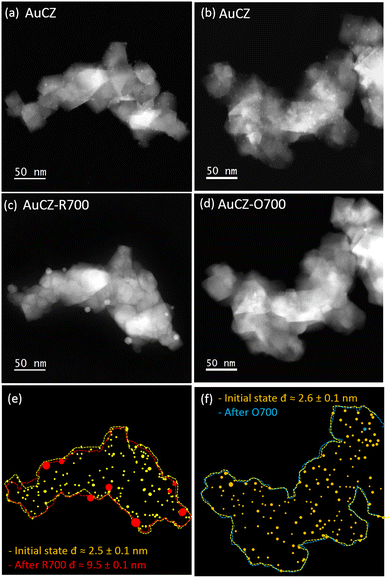
Here, we study the effects of thermochemical treatments on the AuPd and AuRu nanoparticles by tracking identical nanometric areas by HAADF-STEM and XEDS-STEM mapping. To this end, a reference gold catalyst and two bimetallic catalysts with a similar Au/M molar ratio (M = Pd, Ru), as shown in Table 1, were compared before (initial state) and after the thermal treatments at 700 °C.Au monometallic reference catalysts
Fig. 1a and c illustrate the impact of R700 on the AuCZ reference catalysts through a series of identical location HAADF-STEM images. Note how the number of particles detected decreased from 208 to 8 and the average particle size increased from 2.5 (±0.1) to 9.5 (±0.1) nm. Such a decrease in the number of particles is in the order of magnitude expected for the increase in the diameter of the particles. In contrast, after O700 in Fig. 1b and d, an intensive particle disappearance is observed. Note also that the comparison of the support configuration before and after the thermal treatments in Fig. 1e and f reveals no obvious morphological changes, demonstrating its high thermal stability as catalytic support.A careful analysis of the whole set of IL-HAADF-STEM experiments (Fig. S1a and b in the ESI†) confirms that under a reducing atmosphere, R700, the variation in particle size is the main degradation detected, whereas under an oxidizing atmosphere, O700, Au particles are missing in the majority of locations and only a couple of areas retains large Au particles (Fig. S1c).† This phenomenon of disappearance of the metal particles has already been described for in situ microscopy experiments performed on PtPd particles supported on alumina under oxidizing conditions at 700 °C. Such behaviour was attributed to the occurrence of evaporation and diffusion processes.43
To investigate if this particle disappearance effect occurs in the whole system, a general inspection of several areas different from those already studied by IL-STEM was carried out in the TEM grid. Thus, the examination of the AuCZ-R700 sample showed similar results to those discussed in Fig. 1e and S1a.† In comparison, crystallites of the CZ support with no gold entities are frequently observed in AuCZ-R700, while several large particles of gold (in the size range of 10 nm) are found distributed as isolated structures onto the grid membrane (Fig. S2).† It is, therefore, clear from the IL-HAADF-STEM images that the mobility of Au species on the surfaces of the CZ oxide is higher under oxidizing conditions. Note in this sense that most of them migrate out of the support agglomerates and end up scattered onto the grid membrane.
In any case, it is evident that the tendency of gold particles to increase in size is primarily affected by temperature rather than the type of atmosphere in which heating takes place. However, this result is not particularly surprising as it is well known that highly dispersed gold nanoparticles have a high surface to volume ratio and consequently a lower melting temperature than the bulk material.44
Regarding the high mobility observed for agglomerated Au structures during O700, Chen et al. established that the desorption of oxygen from the nanoparticles of a gold film above 200 °C accelerates coarsening, this indicates an oxygen-induced mobilization of Au atoms. In contrast, materials annealed in reductive CO gas showed better stability.45 Related to this, experimental observations of Au clusters supported on an amorphous carbon film subjected to O2 treatment at 250 °C have reported that molecular oxygen at elevated temperature can bind to surface defects to further attenuate interactions between particles and the support, allowing greater mobility by releasing the Au clusters from their original binding sites at the surface.46 Similarly, comparing annealing treatments on Au/TiO2 under ultrahigh-vacuum (UHV) conditions and an oxidation under an O2 atmosphere revealed that oxygen has a pronounced effect on the growth of Au nanoparticles at elevated temperatures (≈400 °C).47 In contrast, it has been widely reported that the generation of a strong metal–support interaction (SMSI) in metal particles supported on reducible oxides, triggered by high-temperature reduction treatments, is an efficient way to prevent metal sintering. It is proposed that such an effect is mediated by physical barriers that either separate metal nanoparticles from each other or anchor them against moving.48 However, Au nanoparticles have long been considered unable to manifest SMSI behaviour due to their lower work function and surface energy compared with other noble metals, and it has only recently been demonstrated that such an interaction is possible under severe pre-treatment conditions (500–800 °C).49 Therefore, it cannot be ruled out that the AuCZ catalyst has evidenced a less pronounced metal loss during R700 due to possible SMSI interaction.
AuPd bimetallic catalysts
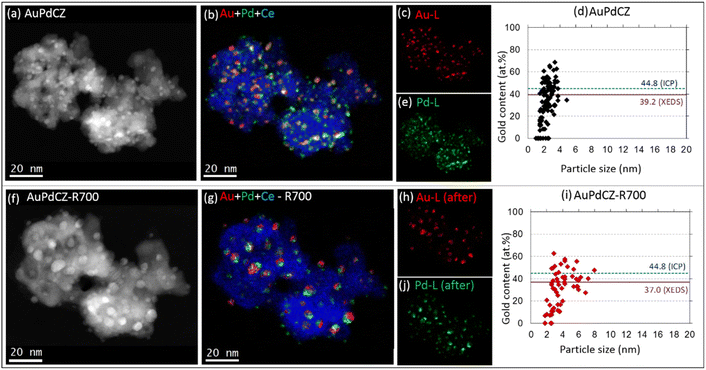
A representative set of IL-HAADF-STEM-XEDS results of the AuPdCZ catalyst before and after being subjected to R700 treatment is shown in Fig. 2. The spatial distribution of Au and Pd is represented in coloured maps extracted using the Au-Lα and Pd-Lα XEDS signals, respectively. The corresponding RGB image overlaying the Ce-Lα signal in blue is also shown. Complementary information is gathered in Fig. 2d and i, in which diagrams depicting the percentage of gold versus the particle size for each particle analyzed from the XEDS maps in Fig. 2b and g are represented.As can be seen in Fig. 2a, b and d, the AuPdCZ catalyst initially presents a fairly uniform distribution of particles ranging in size from 1 to 5 nm. The analysis of individual particles reveals that the vast majority contained both Au and Pd metals spanning the composition range from 4.6 to 68.3 at% Au, and only a relatively small fraction of monometallic Pd particles in the size range from 1 to 3 nm. The corresponding average gold content of the whole set of particles is 39.2% (red solid line), which is only 5.6% lower than that measured at the macroscopic level, 44.8% as estimated by ICP-AES, and is represented in Fig. 2d by the blue dashed line. This discrepancy will be discussed further in the light of additional data.
After R700, a modest change in the nanoparticle size is observed, as revealed by the presence of larger entities in Fig. 2f. Likewise, the elemental maps in Fig. 2g demonstrate that the larger nanoparticles contain both Au and Pd. It is also worth noting that most of those bimetallic particles are made up of two domains: an Au-rich domain and a Pd-rich domain, resembling the typical asymmetric structures of Janus-like particles, whose chemical species are not uniformly distributed through the entire volume of the particle.50
A comparison of the corresponding composition versus particle size plots before and after R700 (Fig. 2dvs.2i) shows that the number of particles decreased from 107 to 63. Regarding the reduction of the number of particles, it is observed that the group of particles between 1 and 2 nm in size, being mostly Pd rich (Au content < 20 at%), and the small fraction of Au-rich particles (Au content > 60 at%) of 2–4 nm in size have been importantly affected. In contrast, a new group of particles appears in the range of 4–8 nm in size and between 30 and 55 at% Au in composition. From these results, it was determined that the mean particle size increases from 2.3 (±0.1) to 4.2 (±0.2) nm and the average gold content from all particles in this location remains approximately constant, around 37.0 at%, close to that estimated for the original state (39.0 at%), demonstrating that the relative amounts of Au and Pd remain fairly constant within the same CZ aggregates during the treatment, although mobilization of both phases evidently drives them to merge into larger particles. Note, in any case, that the Au composition range spanned by the particles after the reducing treatments is practically the same observed in the untreated catalyst.
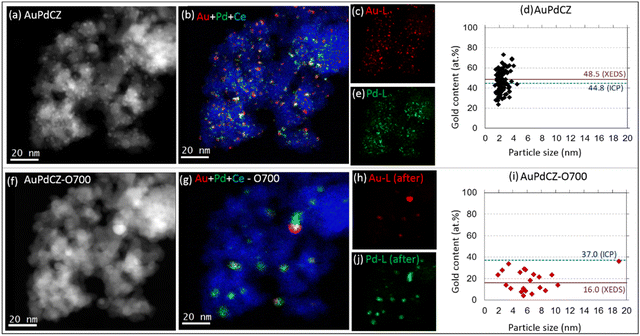
The impact of O700 treatment on AuPdCZ catalysts is presented in Fig. 3, from which a severe modification is evident. The XEDS elemental maps and the analysis of the metal content–particle size diagrams (Fig. 3d–i) indicate that the initial population of 125 small Au–Pd entities, below 5 nm in size and with composition in a wide range of Au contents, decays to only 21 sintered Pd-rich structures of 3 to 19 nm in size and Au contents between 4.0 and 36.0 at%. In line with these changes, the average particle size evolves from 2.5 (±0.1) to 6.5 (±1.0) nm. According to the quantitative analysis of gold content from all particles, the initial value of 48.5 at% dropped drastically to 16.0 at%, demonstrating a considerable preferential loss of gold from the aggregates under oxidizing conditions. It is also worth noting in this case that the Au composition range shrinks significantly with respect to that observed in the untreated sample. This compositional homogenization effect was already observed in conventional STEM studies and evidences a higher mixing efficiency of the two metals under oxidizing conditions.38
Comparison of both sets of results indicates that increasing the temperature up to 700 °C stimulates the Au–Pd particles to sinter, regardless of the type of atmosphere present. However, in the reductive environment, R700, particle growth is less severe than under oxidizing conditions, O700.
To obtain more statistically-meaningful information, we performed quantitative analysis over large regions of the IL-STEM-XEDS experiments. Furthermore, to understand the influence of the thermochemical treatments on the content of both metals in absolute terms, Fig. 4 gathers quantitative data about Au and Pd atomic percentages with respect to the Ce content, which is the major metallic component of the support content (details in Table S1 in the ESI†). Likewise, the corresponding Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio values are also included to refine the discussion presented above. Measurements were obtained from 5 different large areas on each sample, which are shown in Fig. 2, 3, and S3.† Previous studies have shown that both the Ce/Zr and O/(Ce + Zr) ratios are homogeneous all over the support crystallites in the R700 and O700 samples, which guarantees a proper comparison of metallic contents not only on the same areas, before and after the thermochemical treatment, but also between different areas of the same sample.
Pd ratio values are also included to refine the discussion presented above. Measurements were obtained from 5 different large areas on each sample, which are shown in Fig. 2, 3, and S3.† Previous studies have shown that both the Ce/Zr and O/(Ce + Zr) ratios are homogeneous all over the support crystallites in the R700 and O700 samples, which guarantees a proper comparison of metallic contents not only on the same areas, before and after the thermochemical treatment, but also between different areas of the same sample.
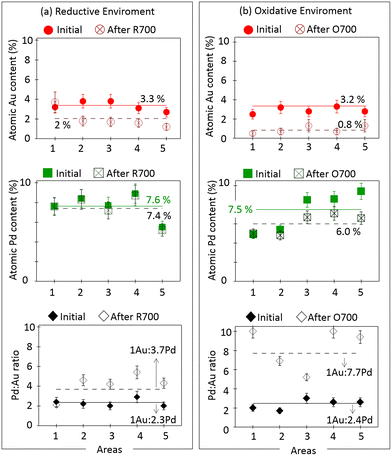 | ||
Fig. 4 Quantitative XEDS analysis of the atomic percentages of Au (red) and Pd (green) of the AuPdCZ catalyst of different areas followed by IL-STEM methodology contrasted at the initial state versus after thermal treatments: (a) R700 and (b) O700. Evolution of the atomic Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio values (black) is included. In all graphs the solid lines represent the initial average determined from the whole set of areas included in the plot and the dashed lines represent the final average determined from the whole set of areas after thermal treatments. The sets of IL-HAADF-STEM-XEDS maps are shown in Fig. 2, 3 and S3 of the ESI.† Pd ratio values (black) is included. In all graphs the solid lines represent the initial average determined from the whole set of areas included in the plot and the dashed lines represent the final average determined from the whole set of areas after thermal treatments. The sets of IL-HAADF-STEM-XEDS maps are shown in Fig. 2, 3 and S3 of the ESI.† | ||
Before going into further details, it is necessary to discuss on the difference observed between the quantitative analysis performed at the nanoscale (by XEDS) and that at the macroscopic level (by ICP-AES). As can be seen in Fig. 4, before treatments, the metal content ranged from 2.5 to 3.8 at% for Au and from 4.9 to 9.4 at% for Pd, which gives average values of 3.2 Au at% and 7.5 Pd at%. Note how the Au/Pd molar ratio in the two sets of areas monitored during IL-STEM studies before treatment is quite close (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 for the areas followed during the R700 study and 1
2.3 for the areas followed during the R700 study and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 in the case of the areas used for the O700 IL-STEM experiment). The 1Au
2.4 in the case of the areas used for the O700 IL-STEM experiment). The 1Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3Pd ratio is about twice richer in Pd than that determined by ICP-AES (1Au
2.3Pd ratio is about twice richer in Pd than that determined by ICP-AES (1Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2Pd). Therefore, this implies that we have analysed areas richer in Pd than those expected for the sample as a whole. This must be related to the fact that a very small fraction (<1%) of very large, 10–30 nm, nearly pure Au nanoparticles, such as those in Fig. S4,† is rarely found. This type of particles, which embed a huge number of atoms, contribute a lot to the metal loading but only minorly in terms of the percentage of particles. Therefore, we decided to concentrate our analysis on the behaviour of the fraction of metallic entities in the small size range (<6 nm), which accounts for nearly 99% of the whole system of particles. The differential behaviour of large size nanoparticles with compositions in the Au-rich limit will be commented in another section of this work, dealing with Au–Ru bimetallic catalysts.
1.2Pd). Therefore, this implies that we have analysed areas richer in Pd than those expected for the sample as a whole. This must be related to the fact that a very small fraction (<1%) of very large, 10–30 nm, nearly pure Au nanoparticles, such as those in Fig. S4,† is rarely found. This type of particles, which embed a huge number of atoms, contribute a lot to the metal loading but only minorly in terms of the percentage of particles. Therefore, we decided to concentrate our analysis on the behaviour of the fraction of metallic entities in the small size range (<6 nm), which accounts for nearly 99% of the whole system of particles. The differential behaviour of large size nanoparticles with compositions in the Au-rich limit will be commented in another section of this work, dealing with Au–Ru bimetallic catalysts.
Continuing with the analysis of data in Fig. 4, let us mention that the average relative content of gold, calculated from all areas, is roughly 30 at% Au-balanced Pd, which is lower than that obtained from the average of analysis performed on individual particles (39.2 and 48.5 Au at% in Fig. 2d and 3d, respectively). However, we have to keep in mind that in the particle size–composition plots the Pd content has been underestimated, due to the difficulties to take into account the large number of very small, in the sub-nanometre range, Pd clusters present in this catalyst. This underestimation partly compensates the effect of excluding the largest size Au nanoparticles present in the sample (Fig. S4†).
In essence, the actual Pd content is better captured in the quantitative measurements over large areas monitored by IL-STEM-XEDS (Fig. 4). Consequently, the present study fully captures the relative amounts of Au–Pd in the catalyst and it is valid to monitor the behaviour of the largest fraction of metallic entities under oxidizing and reducing atmospheres at high temperatures.
Concerning the effect of the thermochemical treatments on the gold content, it can be observed that the concentrations decrease after R700 to average values of 2.0 at%. In this respect, note that the Pd content remains roughly constant, 7.4 at%, but the gold content decreases by a factor of about 40% in almost all locations. As we will prove later, this decrease is related to migration of the metal out of the support crystallites. These results clearly prove that Au is the metal that preferentially mobilizes in the reducing environment. Therefore, there is a tendency to increase the local Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd molar ratio, except for area 1 (Fig. 2), in which the relative amounts of metals remain similar, wherein no long distance migration of the active phases takes place. The average Au
Pd molar ratio, except for area 1 (Fig. 2), in which the relative amounts of metals remain similar, wherein no long distance migration of the active phases takes place. The average Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd molar ratio from all IL-STEM data changes from 1
Pd molar ratio from all IL-STEM data changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 to 1
2.3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.7, which corresponds to a decrease of gold content from 30 to 21 at%.
3.7, which corresponds to a decrease of gold content from 30 to 21 at%.
When the treatment is performed under oxidizing conditions (Fig. 4b), the changes in metal content in all areas are more dramatic and affect both Au and Pd. In the case of gold, the average initial content of 3.2 at% drops down to 0.8 at% and for palladium it decreases more moderately, from 7.5 to 6.0 at%. This corresponds to changes in the metal content of about 75% for Au and just 20% for Pd with respect to the initial values. Hence, the local Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd molar ratio transforms from 1
Pd molar ratio transforms from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 to 1
2.3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.7, which corresponds to a Pd-rich system of particles. Alternatively, the average gold content in the areas drops from 30 at% down to 12 at% under O700. The measurement of the gold content at the macroscopic level by ICP-AES before and after O700 provided values of 2.4 to 2.0 wt%, respectively (Table S3†). This result supports those obtained at the nanoscopic level even though the loss is relatively smaller on a macroscopic scale (Fig. 3d and i), which is justified by the phase mobility in the system as a whole.
7.7, which corresponds to a Pd-rich system of particles. Alternatively, the average gold content in the areas drops from 30 at% down to 12 at% under O700. The measurement of the gold content at the macroscopic level by ICP-AES before and after O700 provided values of 2.4 to 2.0 wt%, respectively (Table S3†). This result supports those obtained at the nanoscopic level even though the loss is relatively smaller on a macroscopic scale (Fig. 3d and i), which is justified by the phase mobility in the system as a whole.
In summary, the identical location STEM-HAADF-XEDS experiments highlight that the AuPd system is very susceptible to high temperatures in both reducing and oxidizing environments. Upon R700 treatment, the catalyst preserves better the local amounts of active phases than O700, modification in gold being more pronounced than in palladium, which practically remains stable, particularly in the reducing environment. In this respect, many studies have concluded that a strong metal support interaction occurs at the interface between ceria in CZ and Pd under reducing conditions,51–53 which may lead to stabilizing the active metals in this case, as it has been described for AuCZ. While the same pattern has been detected in an oxidizing scenario, the loss of both metals is much more pronounced and affects preferentially to Au.
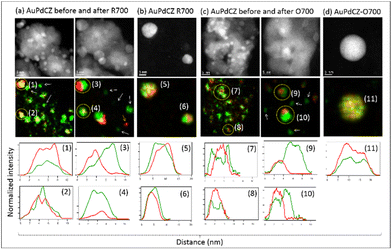
An additional question which merits a more in-depth comment refers to the details of the spatial distribution of the two elements in the bimetallic particles. In this respect, Fig. 5 shows high-magnification IL-HAADF-STEM-XEDS analysis of the AuPdCZ catalyst both at the initial stage and after the R700 and O700 treatments. First of all, we observed that a large fraction of the bimetallic nanoparticles in the starting AuPdCZ catalyst shows a heterogeneous distribution of the two elements within the nanoparticles. This is illustrated in the intensity line profile analysis of the Au and Pd XEDS signals (net counts) recorded across particles numbered 1, 2, 7 and 8 in Fig. 5a and c. These results clearly reveal Au-rich areas surrounded by Pd-rich domains. After the thermochemical treatments, larger particles are in general observed in which Au and Pd have spatially redistributed, preferentially adopting a Janus-type structure (e.g. particles numbered 3, 9 and 10 in Fig. 5). Apart from these bimetallic particles, a fraction of particles which remain as unalloyed small Pd monometallic entities are observed (marked with arrows over XEDS maps in Fig. 5a and c) regardless of the redox nature of the treatment applied. The formation of bimetallic particles depicting a heterogeneous distribution of gold and palladium have already been described after high temperature oxidation processes for alumina-supported systems.54
However, when we compare these results with those obtained in our previous conventional analytical STEM study of the same AuPdCZ catalyst oxidized at increasing temperatures,38 some discrepancies are observed with respect to how gold and palladium are distributed. In that study it was proved that, in the O700 case, the system evolved from an initial situation in which Au and Pd were only partially mixed into particles, depicting a narrow size range and a wide compositional range, to a final one in which Au and Pd became mixed at the atomic level into larger but compositionally homogeneous particles. These, alloy type, particles ranged in composition from 50 to 60 at% Au and between 3 and 20 nm in diameter. Surprisingly, alloy-type nanoparticles were also found in the IL-STEM study of both kind of treatments, but mainly as isolated particles on the surface of the silicon nitride membrane, as shown in Fig. 5b and d (e.g. particles 5, 6 and 11). Also, in clear contrast to the conventional analytical STEM studies, in the IL-TEM results the particles which remain attached to the CZ support crystallites are Janus type.
These differences can be attributed to the distinct boundary conditions characteristic of each type of experiments, associated with the scaling effect related to moving from the macro- to the nano-scale. Thus, for example, differences have been observed between the ex situ oxidation of bimetallic PtPd nanoparticles carried out in a muffle furnace compared to an in situ oxidation in a TEM gas cell specimen holder.42 However, in our case, despite employing the same heating protocol, heating device, reactor, and gas supply system for both types of experiments, the large difference in the following details should be considered: (i) the amount of catalysts subjected to the treatment is different, being about 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times lower in the IL-STEM experiments. This causes that the agglomerates of crystals containing hundreds of metal particles remain isolated, at large distance from each other, on the grid membrane. This situation clearly contrasts with the tight contact between neighbouring aggregates prevailing on a large scale macroscopic experiment; (ii) in the nano-scale experiments, the catalyst particles are supported onto the silicon nitride membrane of the TEM grid which, in turn, is placed on the reactor bed. In the macroscopic experiment, as already mentioned, the catalyst aggregates are in direct contact with each other. It can then be easily understood that mass transfer phenomena between aggregates will be neatly different. In fact, interparticle migration of metallic species will necessarily proceed in the case of IL-STEM experiments by diffusion out of one particle via the Si3N4 membrane and into neighbouring aggregates. In conventional experiments such a process will very likely take place at interparticle contacts or through the small volumes involved in the microporosity of the reactor bed; (iii) although the flow rate was set to a minimum during the IL-STEM experiments (60 times lower than in macro-experiments), the spatial velocity of the gas flow (i.e. flow rate per unit mass of the catalyst) is about 500 times higher in IL-STEM experiments, which may also influence heat or mass transport processes. All these factors may have some implications in the differences observed between IL-STEM and conventional experiments but will not influence neither the intrinsic behaviour of any of the two metals in the catalyst or the more general trends observed in the particle and compositional evolution of the catalysts in the two chemical environments.
000 times lower in the IL-STEM experiments. This causes that the agglomerates of crystals containing hundreds of metal particles remain isolated, at large distance from each other, on the grid membrane. This situation clearly contrasts with the tight contact between neighbouring aggregates prevailing on a large scale macroscopic experiment; (ii) in the nano-scale experiments, the catalyst particles are supported onto the silicon nitride membrane of the TEM grid which, in turn, is placed on the reactor bed. In the macroscopic experiment, as already mentioned, the catalyst aggregates are in direct contact with each other. It can then be easily understood that mass transfer phenomena between aggregates will be neatly different. In fact, interparticle migration of metallic species will necessarily proceed in the case of IL-STEM experiments by diffusion out of one particle via the Si3N4 membrane and into neighbouring aggregates. In conventional experiments such a process will very likely take place at interparticle contacts or through the small volumes involved in the microporosity of the reactor bed; (iii) although the flow rate was set to a minimum during the IL-STEM experiments (60 times lower than in macro-experiments), the spatial velocity of the gas flow (i.e. flow rate per unit mass of the catalyst) is about 500 times higher in IL-STEM experiments, which may also influence heat or mass transport processes. All these factors may have some implications in the differences observed between IL-STEM and conventional experiments but will not influence neither the intrinsic behaviour of any of the two metals in the catalyst or the more general trends observed in the particle and compositional evolution of the catalysts in the two chemical environments.
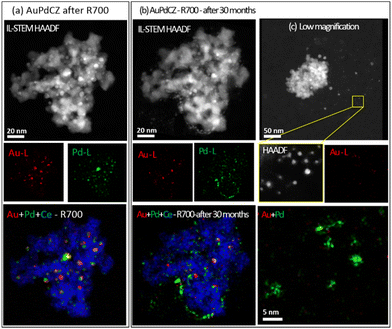
Evolution at room temperature of AuPdCZ bimetallic systems
Due to the strong structural rearrangements observed under the oxidizing treatment at high temperature, we studied the influence of prolonged exposure to an oxidising atmosphere at room temperature. For this purpose, IL-STEM areas of the AuPdCZ-R700 catalysts were monitored after storing for 30 months under ambient conditions in the absence of light. The results are shown in Fig. 6 and S5,† which gather sets of elemental maps obtained from: (a) after R700 treatment and (b) after 30 months of storage at RT.As can be observed in Fig. 6b, the nanoparticles suffer a severe redistribution at RT under air. The elemental maps of Au and Pd in Fig. 6b clearly show the impact of the particle volume and fine details on the spatial distribution of both elements. In particular, a decrease in particle size is observed, which leads to a significantly high number of small Pd-rich entities. In comparison, the Au-rich entities exhibit the same tendency but to a lesser extent, maintaining a similar location but a smaller particle size. The average particle diameter decreased from 4 (±0.2) to just 2.7 (±0.4) nm, ranging the size of the new set of small Pd-rich particles between 1 and 3 nm, whereas that of the gold-rich ones is between 3 and 6 nm. This particle shrinkage is particularly noticeable since, in contrast, gold metal particle growth has been reported during storage at RT (in the absence of light).55,56 Some studies have demonstrated that the corrosion of gold nanoplates is responsible for a significant fragmentation in solutions containing cyanide or halide ions when using O2 or H2O2 as oxidizing agents.57 However, to the best of our knowledge, Pd redispersion at RT has not been reported before. Typically, such redispersion has been evidenced at high temperatures in oxidizing environments, where PdO mobility and eventual fracture into smaller particles are promoted.58,59 An example of anodic Pd corrosion under hard oxidative conditions has reported the formation of small colloidal palladium particles in the working solution.60 Consequently, the decrease in the particle size of AuPdCZR700 catalysts might be associated with oxidation phenomena during long exposure times.
The results of the quantitative analysis of all IL-STEM-XEDS data show (Table S4†) that the average of Au and Pd contents decreases from 1.9 to 1.5 at% Au and from 7.8 to 4.6 at%. The apparent mass changes in each identical location are mainly due to the mobility of the particles, since many of them were found outside the support, supported on the silicon nitride membrane instead as shown in the low-magnification image in Fig. 6c. In the O700 experiments, a higher mobility of the Au phase was observed, in contrast, at room temperature a higher mobility is manifested in the palladium phase. In this respect, the formation of Pd(OH)x species in the presence of H2O, which are known to be highly mobile, has been reported.61 Therefore, we cannot discard that environmental factors, such humidity and oxygen from the environment, may favour the formation of Pd mobile species that are able to migrate in the AuPdCZ system.
The dynamic evolution of catalysts after long storage periods is rarely considered. Our results, however, highlight the importance of considering not only the optimal storage conditions for catalysts, but also to take into account the restructuring phenomena they may undergo, which strongly affect the reactivity, selectivity, and deactivation or may eventually favour the regeneration of a catalytic system.62 Although the results evidence that the stability of the AuPdCZ catalysts is affected by the mobilization of the metallic phase, this does not show signs of agglomeration, preserving in such a case some catalytic potential in the reaction where a small particle size is an essential feature.
AuRu bimetallic catalysts
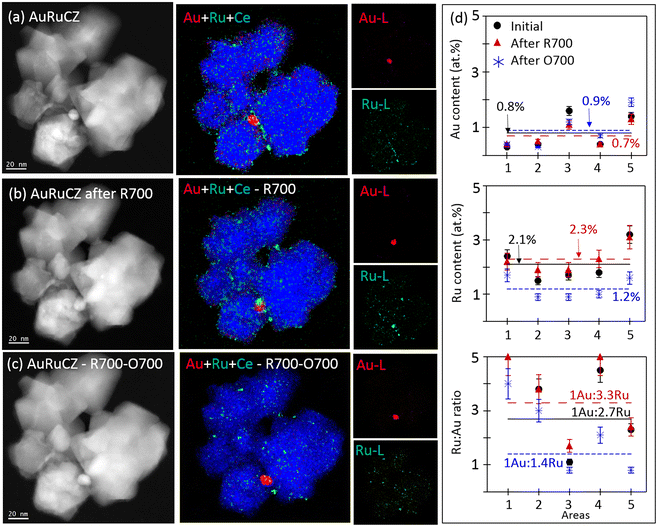
In the case of the AuRuCZ system, the influence of both the molar ratio and the reducing atmosphere at pre-treatment temperature on the selective oxidation of alcohols in the liquid phase has been previously described.3,4 These studies have provided clues about the synergy effect of a small amount of ruthenium during the reaction and reducing environments at temperatures up to 700 °C. In this work, we have extended the analysis of the Au–Ru system by monitoring the behaviour of a AuRuCZ catalyst in 3 different consecutive steps: (1) as-prepared, (2) after R700 and finally (3) upon O700. In this case, for the reasons explained below, the O700 treatment was applied after R700.Fig. 7a–c show some representative HAADF-STEM images and XEDS maps of the IL-STEM study of the AuRuCZ catalyst cycled consecutively in R700 and then O700. Note that in the as-prepared state, the elemental Au map evidences a poor dispersion of the gold phase, as only one particle of about 10 nm was found in the area. After R700, the gold particle retains its size and slightly moves on the CZ surface. In the same way, after O700, no significant change is observed, revealing thermal stability during both treatments.
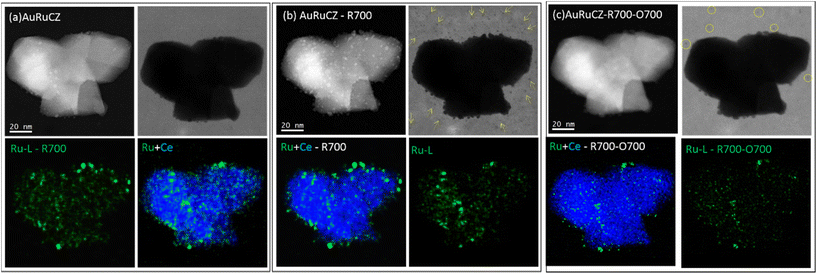
Regarding Ru, in the as-prepared state the elemental maps evidence a high degree of dispersion, with a large population of nanoparticles smaller than 4 nm. After R700, most of the Ru particles retain their size, except for a few ones that grow up to 5–7 nm. Besides, according to the AuRuCe overlays, a few Ru particles have migrated to decorate the surface of the Au particles. Similarly, Ru particles retain their small size also after O700, although this treatment clearly induces a severe loss of ruthenium, including those entities in contact with the Au particles. Finally, the set of HAADF-STEM images does not evidence noticeable modifications in the morphology of the mixed oxide support throughout the consecutive thermochemical treatments, in line with the observations previously commented for AuCZ and AuPdCZ.
Fig. 8 shows some data that allow us to understand the loss of Ru detected after O700. In this case, a different catalyst aggregate, populated mostly by pretty small Ru particles, was imaged as prepared (Fig. 8a), after R700 (Fig. 8b), and after O700 (Fig. 8c). Note, once more, that ruthenium particles remain almost unchanged after R700 and depicts a more pronounced degradation only after O700, particularly in terms of the number of particles detected in the area. However, the group of bright-field (BF) STEM images in Fig. 8 reveals that after the R700 step an important population of sub-nanometer Ru species becomes dispersed on the grid membrane (marked with arrows). This indicates that during the high-temperature reduction treatment, Ru migration takes place. In contrast, the identical location results of the same area after O700 demonstrates the loss of these sub-nanometer Ru species, as well as part of the particles which remained attached to the CZ support after R700. In fact, only a small fraction of the ruthenium phase remains attached to the CZ crystallite after O700.
To provide a quantitative assessment of the overall compositional changes detected, the relative atomic contents of Au and Ru, considering Ce as the third element, of 5 areas tracked by IL-STEM experiments (shown in Fig. 7 and S6†) were compared and are shown in Fig. 7d in 3 different states: (1) as prepared (black), (2) after R700 (red) and finally (3) upon O700 (blue), and the details are included in Table S2 in the ESI.† As expected, after R700 only slight deviations of the initial Au and Ru contents can be observed, which fall under the limits of quantification errors. After O700, the gold contents do not change significantly, and the ruthenium content drops by 50% of the original stage, pointing out the high sensibility of this phase to the O700 treatment. Therefore, the AuRuCZ catalyst, which preserves a load about 3 times richer in Ru than Au after R700, becomes a more equitable ratio (1Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4Ru) after O700.
1.4Ru) after O700.
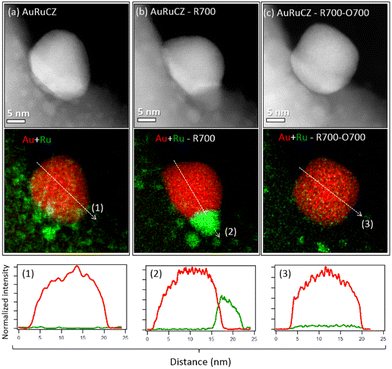
XEDS analyses from individual particles at high magnification were also performed to monitor, together with the evolution of the particle morphology, the fine details of the spatial distribution of the two metals in the nanoparticles. Thus, in the as-prepared catalyst, the configuration observed is that of large Au-rich particles, in the range of 10 nm, surrounded by a high number of highly dispersed Ru-rich particles, as shown in Fig. 9a, in which Ru particles eventually decorate the surface of the large Au particles. After R700 (Fig. 9b), gold particles do not grow, but ruthenium migrates and attaches to Au particles, leading to bimetallic entities with the so-called Janus-type structure.50 Finally, after O700, small Ru particles are mostly lost from the area (Fig. 9c), while the gold particle retains both position and size.
The Janus-type configuration is also lost, though a small amount of Ru becomes mixed within the Au particles, as revealed by the weak Ru signal observed in the corresponding XEDS intensity line profile at the bottom row of Fig. 9c. Quantification of the XEDS spectra indicates a Ru content in the order of 2%, which corresponds quite close to the maximum solubility reported for Ru within Au.63
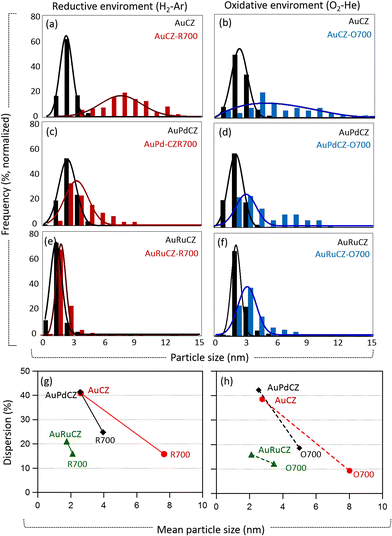
Comparative analysis of particle size distribution (PSD) and dispersion (D) for AuCZ and bimetallic catalysts
In order to evaluate the thermal stability of the system of particles in both catalysts following R700 and O700 treatments, in comparative terms with respect to their monometallic gold counterpart, a detailed analysis of the PSDs, mean particle size and their dispersion was performed. To this end, the diameters of over 500 particles detected in the different areas of all identical location STEM experiments were measured (Fig. S1, S3 and S6).† In the particular case of AuRuCZ catalysts, a study of its thermal stability after O700 was performed on the catalysts already subjected to R700, taking into account that the results discussed in the previous section reveal a significant similarity between the initial state and after R700 in all areas of these catalysts in terms of particle size and metal loadings.The PSDs obtained from all catalysts both before and after thermal treatments were compared and are presented in Fig. 10a–f. The highest degree of sintering is unambiguously observed in the monometallic AuCZ, which suffers a clear loss of small particles (<3 nm in size), while the distribution tail of larger particles is more pronounced. The growth in the number of large Au particles is more pronounced after O700 (Fig. 10b), even though the percentage of these larger entities is still low. The effect of high temperature treatments, R700 and O700, is much more moderate in the bimetallic systems, particularly in the AuRuCZ catalyst. Thus, the PSD curves of AuPdCZ show a profile which skews toward larger particles, in the size range of 4–9 nm, upon R700 (Fig. 10c). After O700, the PSD spans a much wider size range, which reaches up to 12 nm, and its average clearly shifts to larger values (Fig. 10d). However, the change is more moderate than in AuCZ. Note also that the AuRuCZ system presents the lowest susceptibility to the thermochemical treatments (Fig. 10e and f), especially after R700, in which the PSD faintly shifts to the right, spanning in fact a size range similar to that observed in the as-prepared state. In all cases, annealing in O700 provokes larger modifications than R700.
The relationship between the average particle size and dispersion of all catalysts is summarized in Fig. 10g and h. The initial AuCZ and AuPdCZ catalysts present nearly identical average particle size ≈ 2.6 (±0.1) nm and a total dispersion of about 41%. In contrast, the dispersion of the original AuRuCZ catalyst with a smaller average particle size (đ = 1.8 ± 0.1 nm) exhibits a lower dispersion value, D ≈ 21%. These results arise from the contribution of gold-rich particles (đ > 10 nm), despite the fact that very few were found (frequency less than 1%, Fig. 7a and S6†). Since the metallic dispersion is a ratio of the surface atoms to the total number of atoms in the particle, D = NS/NT, the total number of atoms increases very rapidly in the presence of larger particles and therefore the ratio decreases proportionally.
After the thermal treatments, the average particle diameter in all catalysts becomes larger than that of the fresh state, while the metallic dispersion decreases. As can be seen in Fig. 10g and h, although AuRuCZ is the catalyst exhibiting the lowest initial dispersion, it is also the one showing the highest stability against sintering, basically preserving the high frequency of small nanoparticles, associated with the ruthenium phase. In fact, the dispersion decreases only by roughly 5%. In the AuPdCZ catalyst in reducing and oxidizing environments, the decrease is still moderate compared to AuCZ, being of about 17% and 24%, respectively.
Clearly, the combination of Au with Pd or Ru improves the stability of the gold particles supported on CZ and limits the severe loss of Au surface sites by sintering observed in the AuCZ. As a final point, in all cases, thermal annealing is more deleterious, both in terms of PSD and metallic dispersion, in oxidizing than reducing environments.
The behaviour of gold agrees with that expected for this metal, characterized by a very low resistance to sintering. Regarding Ru, previous works have reported the occurrence of redispersion of ceria-supported nanoparticles of this metal, with size in the 2–6 nm range, under mild oxidizing conditions; in particular, at 230 °C under O2.64 The formation of isolated single atom Ru species is proposed in this work. Such a phenomenon has been attributed to decomposition of the corresponding RuOx surface species and further interaction with the ceria support. Our results evidence a significant mobilization of Ru under an oxidizing atmosphere, though the temperatures used in our study are much higher. The formation of such isolated Ru species on the basis of HAADF images is out of the limits of the technique, due to the very low atomic number of Ru (Z = 44) in comparison with that of Ce (Z = 58). In any case, our XEDS data indicate that apart from the formation of such species, their migration must also take place, at least at 700 °C, since the amounts of detected Ru were in all the areas lower than those measured in the as-prepared catalyst. Concerning the stability of such oxides, while RuO2 has been found to be stable up to 450–650 °C, volatile RuO4 species form above 650 °C.65,66 Therefore, it is possible that the Ru volatilization phenomenon may have participated in the loss of this metal under O700 treatment.
In the case of palladium, sintering and redispersion phenomena in oxidizing environments at elevated temperatures (500–800 °C) have been reported.67,68 Redispersion has been found to be governed by the presence of PdO crystallites that spread over the surface of the substrate accompanied by rupturing into smaller particles.58 Our findings demonstrate that this mechanism could be valid even at room temperature during prolonged aging under air.
Previous works have reported a beneficial effect of H2 pre-treatment atmospheres on the Pd-supported catalyst, pointing out that metal dispersion after such treatments remains higher than that observed after treatments under oxidizing atmospheres.69 In our case, apart from the influence on particle size, it is shown that the stability of the metal loading is also favoured. As explained above, metal–support interaction effects are likely to be involved.
Conclusions
We investigated the thermal behaviour of the system of particles present in ceria–zirconia supported-bimetallic catalysts based on gold using the identical location-STEM technique, allowing us to track details of their thermally-driven structural and compositional evolutions under oxidizing and reducing atmospheres, which fall out of reach for conventional STEM analysis. We concluded that thermal treatments at high temperatures highlight the preferential sintering of the monometallic gold particles regardless of the redox environment. In contrast, the bimetallic catalysts retain a large fraction of highly dispersed particles (2 to 6 nm), indicating that the combination with a 4d metal partially mitigates Au agglomeration. The thermal stability of these particles depends critically on the redox environment, with the oxidizing atmosphere favoring the mobility of the three metals to a greater extent, preferentially Au. However, bimetallic systems moderately diminish this mobilization, as part of the gold remains stable under oxidation at elevated temperatures, especially in the AuRu catalysts. In contrast, part of Ru and Pd is partially sacrificed, showing a moderate degree of mobilization. These differential mobilization capacities allow us to understand the strong sintering and alloying effects observed in conventional STEM characterization studies, which, as the IL-STEM studies performed here, evidence the formation of bimetallic particles in which Au and the 4d metal become mixed at the atomic level in AuPd or AuRu alloy-type particles. In clear contrast, reducing environments lead to a much more moderate mobilization of Ru and Pd. In this case, Au and the 4d element evolve into Janus-type entities, in which the two metals, though in contact along a sharp interface, remain spatially segregated.Clues about catalyst modifications during prolonged exposure to air at room temperature have been provided, pointing out the importance of catalyst storage conditions to preserve their pristine nanostructure. Likewise, a much higher nanostructure transformation potential of oxidizing treatments has been proved.
In addition, characterization by identical location TEM has shown how scaling from macroscopic to nanoscopic conditions involves intrinsic changes in heat and mass transfer phenomena, which must be taken into account in the comparative analysis. Likewise, IL-TEM has proven to be an invaluable tool for studying “live” systems such as catalysts, whose structure, composition and morphology are prone to modify under reaction conditions, as demonstrated in the set of findings presented in this work.
Author contributions
L.Ch. and A.B.H conceived, planned the study and performed TEM characterization. L.Ch, X. Ch. and C.O. fabricated the catalysts. R.M. performed the thermal annealing treatments. J.J.C. supervised the research. L.Ch., R.M. and A.B.H. prepared the manuscript. All authors contributed to the discussion and revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Junta de Andalucía project P20_00968 and the Ministry of Science, Innovation and Universities of Spain projects PID2019-110018GA-I00, PID2020-113006-RB-I00, PID2020-113809RB-C33 and PID2022-142312NB-I00 funded by MCIN/AEI/10.13039/501100011033.References
- A. Abad, C. Almela, A. Corma and H. García, Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts, Tetrahedron, 2006, 62(28), 6666–6672 CrossRef CAS.
- G. C. Bond and D. T. Thompson, Catalysis by Gold, Catal. Rev., 1999, 41(3–4), 319–388 CrossRef CAS.
- L. E. Chinchilla, C. Olmos, M. Kurttepeli, S. Bals, G. Van Tendeloo, A. Villa, L. Prati, G. Blanco, J. J. Calvino, X. Chen and A. B. Hungría, Combined Macroscopic, Nanoscopic, and Atomic-Scale Characterization of Gold–Ruthenium Bimetallic Catalysts for Octanol Oxidation, Part. Part. Syst. Charact., 2016, 33(7), 419–437 CrossRef CAS.
- L. E. Chinchilla, C. M. Olmos, A. Villa, A. Carlsson, L. Prati, X. Chen, G. Blanco, J. J. Calvino and A. B. Hungría, Ru-modified Au catalysts supported on ceria–zirconia for the selective oxidation of glycerol, Catal. Today, 2015, 253, 178–189 CrossRef CAS.
- N. Dimitratos, J. A. Lopez-Sanchez, D. Lennon, F. Porta, L. Prati and A. Villa, Effect of Particle Size on Monometallic and Bimetallic (Au,Pd)/C on the Liquid Phase Oxidation of Glycerol, Catal. Lett., 2006, 108(3), 147–153 CrossRef CAS.
- G. J. Hutchings and C. J. Kiely, Strategies for the Synthesis of Supported Gold Palladium Nanoparticles with Controlled Morphology and Composition, Acc. Chem. Res., 2013, 46(8), 1759–1772 CrossRef CAS PubMed.
- C. M. Olmos, L. E. Chinchilla, A. M. Cappella, A. Villa, J. J. Delgado, A. B. Hungría, G. Blanco, J. J. Calvino, L. Prati and X. Chen, Selective Oxidation of Veratryl Alcohol over Au-Pd/Ce0.62Zr0.38O2 Catalysts Synthesized by Sol-Immobilization: Effect of Au:Pd Molar Ratio, Nanomaterials, 2018, 8(9), 669 CrossRef.
- C. M. Olmos, L. E. Chinchilla, E. G. Rodrigues, J. J. Delgado, A. B. Hungría, G. Blanco, M. F. R. Pereira, J. J. M. Órfão, J. J. Calvino and X. Chen, Synergistic effect of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for selective oxidation of glycerol, Appl. Catal., B, 2016, 197, 222–235 CrossRef CAS.
- C. M. Olmos, L. E. Chinchilla, A. Villa, J. J. Delgado, H. Pan, A. B. Hungría, G. Blanco, J. J. Calvino, L. Prati and X. Chen, Influence of pretreatment atmospheres on the performance of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for benzyl alcohol oxidation, Appl. Catal., A, 2016, 525, 145–157 CrossRef CAS.
- H. Sun, S. Chen, W. Yang, L. Wang, R. Tang, X. Zhang, R. Zheng, S. Gu, Y. Jiang, W. Liang and J. Huang, Plasmon-enhanced alcohol oxidations over porous carbon nanosphere-supported palladium and gold bimetallic nanocatalyst, Appl. Catal., B, 2021, 292, 120151 CrossRef CAS.
- J. Zhang, W. Xu, Y. Liu, S.-F. Hung, W. Liu, Z. Lam, H. B. Tao, H. Yang, W. Cai, H. Xiao, H. Chen and B. Liu, In Situ Precise Tuning of Bimetallic Electronic Effect for Boosting Oxygen Reduction Catalysis, Nano Lett., 2021, 21(18), 7753–7760 CrossRef CAS PubMed.
- D. Su, Advanced electron microscopy characterization of nanomaterials for catalysis, Green Energy Environ., 2017, 2(2), 70–83 CrossRef.
- J. M. Thomas, Reflections on the value of electron microscopy in the study of heterogeneous catalysts, Proc. R. Soc. A, 2017, 473(2197), 20160714 CrossRef PubMed.
- V. Beermann, M. Gocyla, E. Willinger, S. Rudi, M. Heggen, R. E. Dunin-Borkowski, M.-G. Willinger and P. Strasser, Rh-Doped Pt–Ni Octahedral Nanoparticles: Understanding the Correlation between Elemental Distribution, Oxygen Reduction Reaction, and Shape Stability, Nano Lett., 2016, 16(3), 1719–1725 CrossRef CAS.
- P.-C. Chen, M. Gao, S. Yu, J. Jin, C. Song, M. Salmeron, M. C. Scott and P. Yang, Revealing the Phase Separation Behavior of Thermodynamically Immiscible Elements in a Nanoparticle, Nano Lett., 2021, 21(15), 6684–6689 CrossRef CAS.
- C. Cui, L. Gan, M. Heggen, S. Rudi and P. Strasser, Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis, Nat. Mater., 2013, 12(8), 765–771 CrossRef CAS.
- M. López-Haro, J. M. Cíes, S. Trasobares, J. A. Pérez-Omil, J. J. Delgado, S. Bernal, P. Bayle-Guillemaud, O. Stéphan, K. Yoshida, E. D. Boyes, P. L. Gai and J. J. Calvino, Imaging Nanostructural Modifications Induced by Electronic Metal−Support Interaction Effects at Au||Cerium-Based Oxide Nanointerfaces, ACS Nano, 2012, 6(8), 6812–6820 CrossRef PubMed.
- D. Wang, Y. Yu, H. L. Xin, R. Hovden, P. Ercius, J. A. Mundy, H. Chen, J. H. Richard, D. A. Muller, F. J. DiSalvo and H. D. Abruña, Tuning Oxygen Reduction Reaction Activity via Controllable Dealloying: A Model Study of Ordered Cu3Pt/C Intermetallic Nanocatalysts, Nano Lett., 2012, 12(10), 5230–5238 CrossRef CAS PubMed.
- Y. Zhang, S. Bals and G. Van Tendeloo, Understanding CeO2-Based Nanostructures through Advanced Electron Microscopy in 2D and 3D, Part. Part. Syst. Charact., 2019, 36(1), 1800287 CrossRef.
- L. Bednarova, C. E. Lyman, E. Rytter and A. Holmen, Effect of Support on the Size and Composition of Highly Dispersed Pt–Sn Particles, J. Catal., 2002, 211(2), 335–346 CrossRef CAS.
- R. E. Lakis, C. E. Lyman and H. G. Stenger, Alumina-Supported Pt-Rh Catalysts: I. Microstructural Characterization, J. Catal., 1995, 154(2), 261–275 CrossRef CAS.
- J. Liu, Advanced Electron Microscopy of Metal–Support Interactions in Supported Metal Catalysts, ChemCatChem, 2011, 3(6), 934–948 CrossRef CAS.
- M. G. Willinger, W. Zhang, O. Bondarchuk, S. Shaikhutdinov, H.-J. Freund and R. Schlögl, A Case of Strong Metal–Support Interactions: Combining Advanced Microscopy and Model Systems to Elucidate the Atomic Structure of Interfaces, Angew. Chem., Int. Ed., 2014, 53(23), 5998–6001 CrossRef CAS.
- K. F. Kalz, R. Kraehnert, M. Dvoyashkin, R. Dittmeyer, R. Gläser, U. Krewer, K. Reuter and J.-D. Grunwaldt, Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions, ChemCatChem, 2017, 9(1), 17–29 CrossRef CAS.
- T. W. Hansen and J. B. Wagner, Catalysts under Controlled Atmospheres in the Transmission Electron Microscope, ACS Catal., 2014, 4(6), 1673–1685 CrossRef CAS.
- Y. Zhou, C. Jin, Y. Li and W. Shen, Dynamic behavior of metal nanoparticles for catalysis, Nano Today, 2018, 20, 101–120 CrossRef CAS.
- J. R. Jinschek, Advances in the environmental transmission electron microscope (ETEM) for nanoscale in situ studies of gas–solid interactions, Chem. Commun., 2014, 50(21), 2696–2706 RSC.
- N. Kamiuchi, K. Sun, R. Aso, M. Tane, T. Tamaoka, H. Yoshida and S. Takeda, Self-activated surface dynamics in gold catalysts under reaction environments, Nat. Commun., 2018, 9(1), 2060 CrossRef.
- M. J. Coombes, E. J. Olivier, E. Prestat, S. J. Haigh, E. du Plessis and J. H. Neethling, Iron-silica interaction during reduction of precipitated silica-promoted iron oxides using in situ XRD and TEM, Appl. Catal., A, 2021, 613, 118031 CrossRef CAS.
- X. Ye, C. Wei, S. Xue, W. Xing, X. Liang, H. Nie, M. Shen, Y. Du, J. Zhang, X. Wang, W. Lin and Z. Yu, Atomistic Observation of Temperature-Dependent Defect Evolution within Sub-stoichiometric WO3−x Catalysts, ACS Appl. Mater. Interfaces, 2022, 14(1), 2194–2201 CrossRef CAS.
- S. Zhang, M. Cargnello, W. Cai, C. B. Murray, G. W. Graham and X. Pan, Revealing particle growth mechanisms by combining high-surface-area catalysts made with monodisperse particles and electron microscopy conducted at atmospheric pressure, J. Catal., 2016, 337, 240–247 CrossRef CAS.
- N. J. Zaluzec, M. G. Burke, S. J. Haigh and M. A. Kulzick, X-ray Energy-Dispersive Spectrometry During In Situ Liquid Cell Studies Using an Analytical Electron Microscope, Microsc. Microanal., 2014, 20(2), 323–329 CrossRef CAS.
- J. B. Wagner, F. Cavalca, C. D. Damsgaard, L. D. L. Duchstein and T. W. Hansen, Exploring the environmental transmission electron microscope, Micron, 2012, 43(11), 1169–1175 CrossRef CAS PubMed.
- C. Baldizzone, L. Gan, N. Hodnik, G. P. Keeley, A. Kostka, M. Heggen, P. Strasser and K. J. J. Mayrhofer, Stability of Dealloyed Porous Pt/Ni Nanoparticles, ACS Catal., 2015, 5(9), 5000–5007 CrossRef CAS.
- L. Dubau, L. Castanheira, G. Berthomé and F. Maillard, An identical-location transmission electron microscopy study on the degradation of Pt/C nanoparticles under oxidizing, reducing and neutral atmosphere, Electrochim. Acta, 2013, 110, 273–281 CrossRef CAS.
- K. J. J. Mayrhofer, S. J. Ashton, J. C. Meier, G. K. H. Wiberg, M. Hanzlik and M. Arenz, Non-destructive transmission electron microscopy study of catalyst degradation under electrochemical treatment, J. Power Sources, 2008, 185(2), 734–739 CrossRef CAS.
- F. R. Nikkuni, L. Dubau, E. A. Ticianelli and M. Chatenet, Accelerated degradation of Pt3Co/C and Pt/C electrocatalysts studied by identical-location transmission electron microscopy in polymer electrolyte environment, Appl. Catal., B, 2015, 176–177, 486–499 CrossRef CAS.
- C. M. Olmos, L. E. Chinchilla, A. Villa, J. J. Delgado, A. B. Hungría, G. Blanco, L. Prati, J. J. Calvino and X. Chen, Size, nanostructure, and composition dependence of bimetallic Au–Pd supported on ceria–zirconia mixed oxide catalysts for selective oxidation of benzyl alcohol, J. Catal., 2019, 375, 44–55 CrossRef CAS.
- S. J. Pennycook and P. D. Nellist, Z-Contrast Scanning Transmission Electron Microscopy, in Impact of Electron and Scanning Probe Microscopy on Materials Research, ed. D. G. Rickerby, G. Valdrè and U. Valdrè, Springer Netherlands, Dordrecht, 1999, pp. 161–207 Search PubMed.
- G. Cliff and G. W. Lorimer, The quantitative analysis of thin specimens, J. Microsc., 1975, 103(2), 203–207 CrossRef.
- M. D. Argyle and C. H. Bartholomew, Heterogeneous Catalyst Deactivation and Regeneration, A Review, Catalysts, 2015, 5 CAS.
- Y. Dai, P. Lu, Z. Cao, C. T. Campbell and Y. Xia, The physical chemistry and materials science behind sinter-resistant catalysts, Chem. Soc. Rev., 2018, 47(12), 4314–4331 RSC.
- A. C. Meng, K.-B. Low, A. C. Foucher, Y. Li, I. Petrovic and E. A. Stach, Anomalous metal vaporization from Pt/Pd/Al2O3 under redox conditions, Nanoscale, 2021, 13(26), 11427–11438 RSC.
- J.-H. Shim, B.-J. Lee and Y. W. Cho, Thermal stability of unsupported gold nanoparticle: a molecular dynamics study, Surf. Sci., 2002, 512(3), 262–268 CrossRef CAS.
- A. Y. Chen, S. S. Shi, F. Liu, Y. Wang, X. Li, J. F. Gu and X. F. Xie, Effect of annealing atmosphere on the thermal coarsening of nanoporous gold films, Appl. Surf. Sci., 2015, 355, 133–138 CrossRef CAS.
- K.-J. Hu, S. R. Plant, P. R. Ellis, C. M. Brown, P. T. Bishop and R. E. Palmer, Atomic Resolution Observation of a Size-Dependent Change in the Ripening Modes of Mass-Selected Au Nanoclusters Involved in CO Oxidation, J. Am. Chem. Soc., 2015, 137(48), 15161–15168 CrossRef CAS PubMed.
- S. Kielbassa, M. Kinne and R. J. Behm, Thermal Stability of Au Nanoparticles in O2 and Air on Fully Oxidized TiO2(110) Substrates at Elevated Pressures. An AFM/XPS Study of Au/TiO2 Model Systems, J. Phys. Chem. B, 2004, 108(50), 19184–19190 CrossRef CAS.
- J. Yu, X. Sun, X. Tong, J. Zhang, J. Li, S. Li, Y. Liu, N. Tsubaki, T. Abe and J. Sun, Ultra-high thermal stability of sputtering reconstructed Cu-based catalysts, Nat. Commun., 2021, 12(1), 7209 CrossRef CAS.
- H. Tang, Y. Su, B. Zhang, A. F. Lee, M. A. Isaacs, K. Wilson, L. Li, Y. Ren, J. Huang, M. Haruta, B. Qiao, X. Liu, C. Jin, D. Su, J. Wang and T. Zhang, Classical strong metal–support interactions between gold nanoparticles and titanium dioxide, Sci. Adv., 2017, 3(10), e1700231 CrossRef PubMed.
- H. Su, C. A. Hurd Price, L. Jing, Q. Tian, J. Liu and K. Qian, Janus particles: design, preparation, and biomedical applications, Mater. Today Bio, 2019, 4, 100033 CrossRef CAS.
- A. Badri, C. Binet and J.-C. Lavalley, Metal-support interaction in Pd/CeO2 catalysts. Part 2.-Ceria textural effects, J. Chem. Soc., Faraday Trans., 1996, 92(9), 1603–1608 RSC.
- W. T. Figueiredo, G. B. Della Mea, M. Segala, D. L. Baptista, C. Escudero, V. Pérez-Dieste and F. Bernardi, Understanding the Strong Metal–Support Interaction (SMSI) Effect in CuxNi1−x/CeO2 (0 < x < 1) Nanoparticles for Enhanced Catalysis, ACS Appl. Nano Mater., 2019, 2(4), 2559–2573 CrossRef CAS.
- H. P. Sun, X. P. Pan, G. W. Graham, H. W. Jen, R. W. McCabe, S. Thevuthasan and C. H. F. Peden, Partial encapsulation of Pd particles by reduced ceria-zirconia, Appl. Phys. Lett., 2005, 87(20), 201915 CrossRef.
- A. A. Herzing, A. F. Carley, J. K. Edwards, G. J. Hutchings and C. J. Kiely, Microstructural Development and Catalytic Performance of Au−Pd Nanoparticles on Al2O3 Supports: The Effect of Heat Treatment Temperature and Atmosphere, Chem. Mater., 2008, 20(4), 1492–1501 CrossRef CAS.
- R. Zanella and C. Louis, Influence of the conditions of thermal treatments and of storage on the size of the gold particles in Au/TiO2 samples, Catal. Today, 2005, 107–108, 768–777 CrossRef CAS.
- N. F. P. Ribeiro, R. P. F. Bonfim, M. M. V. M. Souza and M. Schmal, Investigation of activity losses of gold nanoparticles in the CO selective oxidation, J. Power Sources, 2010, 195(21), 7386–7390 CrossRef CAS.
- Y. Cheng, C. Qiu, H. Ma, X. Zhang and X. Gu, Unusual corrosion process of gold nanoplates and the mechanism study, Nanoscale, 2010, 2(5), 685–688 RSC.
- J. J. Chen and E. Ruckenstein, Role of interfacial phenomena in the behavior of alumina-supported palladium crystallites in oxygen, J. Phys. Chem., 1981, 85(11), 1606–1612 CrossRef CAS.
- E. D. Goodman, A. C. Johnston-Peck, E. M. Dietze, C. J. Wrasman, A. S. Hoffman, F. Abild-Pedersen, S. R. Bare, P. N. Plessow and M. Cargnello, Catalyst deactivation via decomposition into single atoms and the role of metal loading, Nat. Catal., 2019, 2(9), 748–755 CrossRef CAS.
- M. D. Vedenyapina, V. V. Kuznetsov, S. A. Kulaishin, N. N. Makhova and M. M. Kazakova, The first example of anodic corrosion of Pd in aqueous ethylenediamine with formation of colloidal palladium, Mendeleev Commun., 2021, 31(5), 638–640 CrossRef CAS.
- J. Lee, Y. Ryou, S. Hwang, Y. Kim, S. J. Cho, H. Lee, C. H. Kim and D. H. Kim, Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging, Catal. Sci. Technol., 2019, 9(1), 163–173 RSC.
- Y. N. Wu, D. B. Shieh, L. X. Yang, H. S. Sheu, R. Z. Zheng, P. Thordarson, D. H. Chen and F. Braet, Characterization of Iron Core-Gold Shell Nanoparticles for Anti-Cancer Treatments: Chemical and Structural Transformations During Storage and Use, Materials, 2018, 11(12), 2572 CrossRef CAS.
- H. Okamoto and T. B. Massalski, The Au-Ru (Gold-Ruthenium) system, Bull. Alloy Phase Diagrams, 1984, 5(4), 388–390 CrossRef CAS.
- A. Aitbekova, L. Wu, C. J. Wrasman, A. Boubnov, A. S. Hoffman, E. D. Goodman, S. R. Bare and M. Cargnello, Low-Temperature Restructuring of CeO2-Supported Ru Nanoparticles Determines Selectivity in CO2 Catalytic Reduction, J. Am. Chem. Soc., 2018, 140(42), 13736–13745 CrossRef CAS PubMed.
- T. Li, P. A. J. Bagot, E. A. Marquis, S. C. E. Tsang and G. D. W. Smith, Characterization of Oxidation and Reduction of Pt–Ru and Pt–Rh–Ru Alloys by Atom Probe Tomography and Comparison with Pt–Rh, J. Phys. Chem. C, 2012, 116(33), 17633–17640 CrossRef CAS.
- H. Over, Ruthenium dioxide, a fascinating material for atomic scale surface chemistry, Appl. Phys. A, 2002, 75(1), 37–44 CrossRef CAS.
- C. Y. Seo, X. Chen, K. Sun, L. F. Allard, G. B. Fisher and J. W. Schwank, Palladium redispersion at high temperature within the Pd@SiO2 core@shell structure, Catal. Commun., 2018, 108, 73–76 CrossRef CAS.
- S. Zhang, C. Chen, M. Cargnello, P. Fornasiero, R. J. Gorte, G. W. Graham and X. Pan, Dynamic structural evolution of supported palladium–ceria core–shell catalysts revealed by in situ electron microscopy, Nat. Commun., 2015, 6(1), 7778 CrossRef PubMed.
- C. Wen, Y. Liu, Y. Guo, Y. Wang and G. Lu, Influence of pretreatment on the structural and catalytic properties of supported Pd catalysts for CO oxidation, Surf. Rev. Lett., 2013, 20(02), 1350013 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr02304d |
| This journal is © The Royal Society of Chemistry 2024 |