Realizing a high OER activity in new single-atom catalysts formed by introducing TMNx (x = 3 and 4) units into carbon nanotubes using high-throughput calculations†
Xia
Yang
a,
Guangtao
Yu
 *a and
Wei
Chen
*a and
Wei
Chen
 *abc
*abc
aEngineering Research Center of Industrial Biocatalysis, Fujian Provincial Key Laboratory of Advanced Materials Oriented Chemical Engineering, Fujian-Taiwan Science and Technology Cooperation Base of Biomedical Materials and Tissue Engineering, College of Chemistry and Materials Science, Fujian Normal University, Fuzhou, 350007, China. E-mail: yugt@fjnu.edu.cn; chenwei@fjnu.edu.cn
bAcademy of Carbon Neutrality of Fujian Normal University, Fujian Normal University, Fuzhou, 350007, China
cFujian Provincial Key Laboratory of Theoretical and Computational Chemistry, Xiamen University, Xiamen, 361005, China
First published on 27th November 2023
Abstract
Exploring highly efficient electrocatalysts for the oxygen evolution reaction (OER) is of great significance for hydrogen production through water splitting. By means of high-throughput density functional theory (DFT) calculations, we investigated the OER catalytic activity of a series of one-dimensional carbon nanotube (CNT)-based systems containing TMN4 or TMN3 functional units. Through the screening of 3d/4d/5d transition metals (TMs) from Group IVB to Group VIII, eight newly obtained TMNx@CNT (x = 3 and 4) systems were found to exhibit excellent OER activity, with very low overpotentials in the range 0.29–0.51 V, where the Co, Rh, Ir, Ti, Fe, and Ru atoms could be used as active sites. It was found that under the framework of TMN3@CNTs, the pre-adsorption of some species from water dissociation on the relevant TM sites (TM = Ti, Fe, and Ru) could lead to a high OER catalytic activity, which was different from the general situation where OER reactions directly occur on the clean surfaces of the remaining systems with Co/Rh/Ir metal centers. Moreover, the catalytic mechanisms were analyzed in detail. This work can be conducive to obtaining low-cost and high-performance OER single-atom electrocatalysts based on excellent CNT nanomaterials.
Introduction
In order to solve the global energy crisis and the environmental problems caused by fossil fuel consumption, researchers have made many efforts to find sustainable alternative energy sources.1–3 Hydrogen can be considered as a promising energy carrier for sustainable development owing to its high energy density and zero carbon emissions.4–6 Currently, electrochemical water splitting is recognized as one of the most effective methods for hydrogen production.7–9 As one half-reaction for water splitting, the oxygen evolution reaction (OER) at the anode has a very slow kinetics owing to its multi-electron transfer process. Therefore, to ensure the smooth progress of the reaction, it is necessary to employ highly efficient catalysts to reduce its overpotential.10,11 It is well known that RuO2 and IrO2 can be viewed as the best-performing OER catalysts due to their low overpotential and high efficiency. However, their widespread application is largely limited because of the small reserves and high price of the metals.12,13 Therefore, developing cost-effective OER catalysts is critical for eliminating the bottleneck in the practical application of electrochemical water splitting.Generally, there are two ways for developing ideal OER electrocatalysts. One approach is to use fewer precious metals without reducing the catalytic efficiency. Another approach is to design nonprecious metal alternatives.14–16 In the development process of OER electrocatalysts, the emergence of single-atom catalysts (SACs) can provide new opportunities for achieving nonprecious, highly efficient OER catalysts. The concept of SACs was first proposed by Qiao and coworkers in their report on the incredible performance of Pt1/FeOx in CO oxidation.17 It is well known that SAC systems can have the unique advantages of a high utilization rate of active atoms, uniform active center, low coordination number of active center atoms, and high selectivity.18,19 At present, some progress has been made in the research on SACs for the OER.20–23
Among them, the development of low-dimensional carbon-based SACs for the OER has attracted much attention owing to their enormous potential applications.24–26 So far, some typical carbon structures have been used to construct the SAC electrocatalysts for the OER, such as graphene,27,28 graphyne,29–32 macroporous T-carbon,33 and fullerene.34 As a classic type of nanocarbon, carbon nanotubes (CNTs) can be considered as one of the most important members of the low-dimensional carbon-based material family. They can be regarded as a one-dimensional (1D) cylindrical structure with a nanoscale diameter, which has a large area π-conjugated skeleton composed of sp2 hybridized C atoms. Due to the unique structure, CNTs can exhibit many unprecedented properties, such as a high Young's modulus, large tensile strength, and excellent electrical and thermal conductivity.35,36 In particular, CNTs have become attractive support materials because of their high conductivity, structural stability, large surface area, low cost, and corrosion resistance in acid and alkali solutions.37–39 In addition, it can be found that the electronic structure and reactivity of CNT materials is closely related to their inherent structural curvature.40,41 It is worth mentioning that the CNT-based SAC systems have been used as effective electrocatalysts for both the HER42–44 and ORR.45–48 However, relevant research is quite scarce, especially on the OER electrocatalytic activity of SACs based on CNTs.
Unlike the previous investigations, in this study, we propose an effective strategy to construct novel one-dimensional (1D) SAC catalysts for the OER by introducing the structural units TMNx (x = 3 or 4) into CNTs. High-throughput DFT calculations were carried out to screen the series of 3d/4d/5d transition metals from Group IVB to Group VIII. It is worth mentioning that some different structures with TMNx units exhibited good OER catalytic performance, such as M-C3N4 (M = Cr, Mn, Fe, Co, Ni, Cu, and Zn),49 Mn-CoN,50 M-COF (M = Mn, Fe, Co, Ni, and Cu),51,52 and MOF (M = Fe, Co, Ni, and Ru).53–55 It is highly expected that these typical CNT-based SAC systems would possess excellent OER catalytic activity. Indeed, our calculation results indicated that a series of CNT-based SACs involving the TMN3 or TMN4 units (TM = Co, Rh, Ir, Ti, Fe, or Ru) would exhibit excellent OER catalytic performance with very low overpotentials (η) ranging from 0.29 to 0.51 V, which are comparable to or even lower than IrO2 (0.56 V).56,57 Obviously, this work can provide new ideas for achieving low-cost and high-performance OER electrocatalysts.
Computational method
All calculations were carried out using density functional theory (DFT), as implemented in the Vienna Ab initio Simulation Package (VASP).58,59 The exchange correlation energy was described by the Perdew–Burke–Ernzerhof (PBE) functional within the Generalized Gradient Approximation (GGA),60 which includes the semiempirical van der Waals (vdW) method proposed by Grimme (DFT-D2) to account for the dispersion interactions.61–63 The nuclei–electron interactions were described by the projector augmented wave (PAW) pseudo-potentials. The 1 × 1 × 5 Monkhorst–Pack k-points were used to sample the Brillouin zone for the structural optimization, and the 21 Line-mode k-points along the (0, 0, 0) → (0, 0, 0.5) high symmetry line were employed to calculate the density of states (DOSs). The convergence criteria of the total energy and force were set to 10−4 eV and 0.05 eV Å−1, respectively. The cutoff energy for the plane wave basis set was 450 eV. A vacuum space of 20 Å along the x- and y-directions was utilized to prevent spurious interactions between the periodically repeated images.To evaluate the structural stability of the studied TMNx@CNT (x = 3 and 4) systems, the corresponding binding energy (Eb) was calculated by the following formula:
| Eb = ETM + ENx@CNT − ETMNx@CNT | (1) |
In acid media, the overall OER can be expressed as:
| 2H2O → O2 + 4H+ + 4e− | (2) |
The OER processes have the following four electron reaction paths:64–66
| H2O + * → HO* + H+ + e− | (a) |
| HO* → O* + H+ + e− | (b) |
| O* + H2O → HOO* + H+ + e− | (c) |
| HOO* → * + O2 + H+ + e− | (d) |
The adsorption energies of the oxygen-containing intermediates on the TMNx@CNT (x = 3 and 4) structures were defined as:67
| ΔEOH* = EOH* − E* − (EH2O − 1/2EH2) | (3) |
| ΔEO* = EO* − E* − (EH2O − EH2) | (4) |
| ΔEOOH* = EOOH* − E* − (2EH2O − 3/2EH2) | (5) |
Then, the adsorption free energy can be obtained by including the zero-point energy (ZPE) and the entropy (S) corrections in the following equation:65
| ΔG = ΔE + ΔZPE − TΔS + ΔGU + ΔGpH | (6) |
The reaction free energies of the four steps (a)–(d) for the OER were defined as ΔGa, ΔGb, ΔGc, and ΔGd, respectively. In view of ΔGa + ΔGb + ΔGc + ΔGd = 4.92 eV, the best scenario corresponded to the case where they are all the same and equal to 1.23 eV, which is an ideal OER catalyst. Then the overpotential (η) can be calculated to evaluate the performance for the OER by:67
| η = max{ΔGa, ΔGb, ΔGc, ΔGd}/e − 1.23 V | (7) |
In addition, we also conducted a computational test to explore the solvation effect on the OER catalytic activity by sampling the RhN3@CNT system (Fig. S1†). The calculation results showed that the overpotential of the OER in solvation could be as small as 0.41 V, which was close to the corresponding result in vacuum (0.46 V), indicating a negligible solvation influence. Therefore, to make the computational cost less demanding, in this work we calculated the η value under vacuum conditions for estimating the OER activity of the studied systems.
Results and discussion
Geometric structures and stability
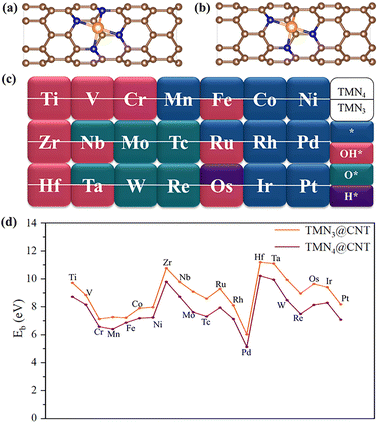
Initially, we optimized the structure of a pristine (6, 0) CNT, and then removed two adjacent C atoms to form a divacancy defect. Further, one 3d/4d/5d TM atom ranging from Group IVB to Group VIII was inserted into the divacancy defect, while the surrounding C atoms were replaced with three or four N atoms (Fig. 1a and b). All the TM atoms considered are listed in Fig. 1c. For convenience, these new systems are labeled as TMNx@CNT (x = 3 and 4), according to the number of N atoms. As shown in Fig. 1a and b, the TM atom was usually located at the center of the divacancy after optimization, and slightly protruded from the plane formed by the four surrounding atoms, in view of the curvature of the nanotube. The involved TM-N/TM-C bond lengths ranged from 1.848 to 2.238 Å. The calculated lattice parameter a of TMN3@CNT and TMN4@CNT systems could be in the range of 12.789 to 12.868 Å, very close to that of a pure CNT (12.793 Å), which indicated that doping a TM atom into the CNT did not cause significant structural deformation. Moreover, the binding energy (Eb) of the TM atom was calculated to estimate the stability of TMNx@CNT (x = 3 and 4). As shown in Fig. 1d, all these doped structures exhibited large binding energies in the range of 6.027–11.198 eV and 5.132–10.218 eV for TMN3@CNT and TMN4@CNT, respectively. This indicates that all these doped systems had high structural stability, with the TMN3@CNT system generally slightly more stable than its corresponding counterpart in the TMN4@CNT series.Furthermore, the calculated electron location function (ELF) results revealed that all the C–C and C–N bonds exhibited strong covalent characteristic, in view of the fact that the ELF isovalues at the center of these chemical bonds were approximately 0.8 (Fig. S4 and S5†). In contrast, strong ionic bonds could be formed between the TM and N/C atom, where the ELF isovalues around TM atom could be close to zero, while the ELF isovalues around its adjacent N/C atoms could be larger than 0.9. The formation of all these strong chemical bonds could jointly promote the high structural stability of these doped systems. This is advantageous for them to become potential SACs for the OER in the process of water splitting, where the TMNx (x = 3 and 4) part is very promising to become high active sites.
OER catalytic activity
In this section, we conducted a systematic study on the OER catalytic activity of these stable TMN3@CNT and TMN4@CNT structures by screening almost all 3d/4d/5d transition metals. According to the approach proposed by Rossmeisl et al.,56 the OER process typically consists of four elementary reaction steps involving some intermediates (i.e., OH*, O*, and OOH*), each of which involves a proton/electron coupled transfer process, as shown in eqn (a)–(d) above. The overpotential (η) was used to evaluate the catalytic activity of the material systems, which could be obtained by assessing the difference between the minimum voltages required for the four reaction steps.Here, the intermediates with an OH group adsorbed at the TM sites were optimized for the TMN3@CNT and TMN4@CNT series, where 21 different TM metal atoms were considered (Fig. 1c).
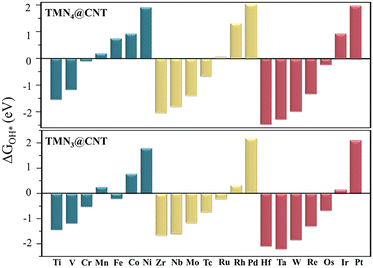
The corresponding Gibbs free energies are shown in Fig. 2. It could be found that the calculated ΔGOH* value generally presented a periodic change trend with the number of valence electrons of the TM atom. In these doped CNT systems, the TM atoms with fewer valence electrons could have negative ΔGOH* values, while those with more valence electrons could have positive ΔGOH* values. Among them, we first focused on the TMN4@CNT systems (TM = Mn, Fe, Co, Ni, Ru, Rh, Pd, Ir, and Pt) with positive ΔGOH* values (0.04–2.03 eV), considering that for a good catalyst, all the steps in the OER reaction should usually move uphill when no bias is applied. As shown in Fig. S6,† the OER free energy diagrams of these systems were constructed, revealing that the CoN4@CNT, RhN4@CNT, and IrN4@CNT systems exhibited very small overpotential (η) values, which were 0.51, 0.29, and 0.33 V, respectively. It is worth mentioning that all of them were even smaller than that of the state-of-the-art IrO2 system (0.56 V),56,57 indicating that these SAC systems doped with TM atoms with 9 valence electrons in the Group VIII could exhibit a considerably high OER catalytic activity. Besides, the neighboring NiN4@CNT system, doped with Ni atom in the Group VIII, could also present good OER activity, in view of the comparable η value (0.64 V) to IrO2. In contrast, the other TMN4@CNT systems (TM = Mn, Fe, Ru, Pd, and Pt) could be considered to have poor OER electrocatalytic performance, as reflected by their large η values (0.92–1.51 V).
In addition, the remaining TMN4@CNT systems (TM = Ti, V, Cr, Zr, Nb, Mo, Tc, Hf, Ta, W, Re, and Os) were also investigated. All of these could have negative ΔGOH* values (−2.42 to −0.08 eV), indicating that these TM sites may have relatively strong interactions with some related species in the solution, which may cause additional reaction processes before the regular OER occurs. As is well known, the dissociation of water can usually form OH, O, and H species. Therefore, before the OER process, their possible occupation of TM sites should be investigated by calculating the ΔGOH*, ΔGO*, and ΔGH* values based on the formulas (1)–(3) in the ESI.† All the calculation results are shown in Fig. 1c and Table S1.†
Specifically, we found that the TMN4@CNT systems doped with Ti, V, Cr, Zr, or Hf atoms exhibited the most negative ΔGOH* value, suggesting that the corresponding TM site had the strongest interaction with OH*. Comparatively, the CNT systems doped by the 4d/5d TM atoms (i.e., Nb, Mo, Tc, Ta, W, and Re) from Group VB to VIIB had the strongest interaction with O*, as reflected by their more negative ΔGO* values (Table S1†). This indicates that during the electrocatalytic OER process, the TM site of the former tends to be occupied by OH species, while that of the latter favors being occupied by O species. Furthermore, given the more negative ΔGH* values, the Os atom in Group VIII elements is more likely to be occupied by H species. Clearly, different from the TM sites with more valence electrons (such as Group VIII elements), these TM sites with less valence electrons will be first occupied by the related different species in the OER process, which could be expected to have an important impact on the OER catalytic performance.
Then, the overpotential (η) values of these TMN4@CNT systems (TM = Ti, V, Cr, Zr, Nb, Mo, Tc, Hf, Ta, W, Re, and Os) were estimated based on the relevant structural models, where the corresponding TM site was first occupied by the OH, O, or H species before the OER reaction occurs. By performing a computational screening (Fig. S6†), it was found that these TMN4@CNT systems could exhibit large η values in the range of 0.70–2.09 V, indicating their relatively poor OER catalytic activity.
Subsequently, we investigated the OER catalytic activity of the TMN3@CNT series, and explored whether more TM-doped CNT structures with high OER performance could be achieved by changing the functional unit from TMN4 to TMN3. Initially, we focused on the TMN3@CNT systems with positive ΔGOH* values (TM = Mn, Co, Ni, Rh, Pd, Ir, and Pt). Our computed results reveal that when the number of N atoms decreased, the obtained CoN3@CNT and RhN3@CNT systems could still exhibit small η values as low as 0.39 and 0.46 V, indicating that they could maintain a high OER catalytic activity. Additionally, other TMN3@CNT systems doped with TM = Ir, Mn, Ni, Pd, and Pt could possess relatively inert OER catalytic activity, in view of their large overpotential values (0.72–1.47 V).
Further, we found that the TM sites with less valence electrons would also be occupied by the OH, O, or H species, when converting the functional unit from TMN4 to TMN3. However, some differences could also be observed. For instance, the doped CNT systems by the Nb, Ta, or Os atom will be occupied by the OH* group instead of the original O* or H*. Especially, different from the clean surface of TMN4@CNT, the Fe and Ru sites will also be occupied by OH* species within the TMN3@CNT framework (Fig. 1c), which could cause the extremely low η value of about 0.35 V, indicating the excellent OER activity. Obviously, the occupation of OH at the TM site can be considered as a self-optimization process for the OER catalytic activity of FeN3@CNT and RuN3@CNT in the process of electrochemical water splitting, which can effectively modulate the adsorption strength of the relevant species involved in the OER process, ultimately leading to a high OER catalytic performance. This is significantly different from the general situation that the OER reaction can occur directly on the surface of a clean material through the four elementary reaction steps. Moreover, the TiN3@CNT system could also exhibit high OER catalytic activity, as reflected by the small η value of about 0.48 V. Obviously, by converting the functional unit from TMN4 to TMN3, more new carbon nanotube-based SAC structures with high OER activity could be obtained.
In addition, by using the overpotential (η) as a function of ΔGOOH* − ΔGO*, activity volcano plots were constructed to display the overall OER catalytic activity in the TMN4@CNT and TMN3@CNT frameworks (Fig. 3). As is well known, constructing a volcano plot has been widely used as a powerful method for screening high-performance electrocatalysts. Usually, the top of the volcano plot indicates the optimal catalytic activity of the catalyst, because a lower overpotential implies a better electrochemical activity. From Fig. 3a, it can be found that the calculated η values of the TMN4@CNT series were in the range of 0.29–2.10 V. The CoN4@CNT, RhN4@CNT, and IrN4@CNT systems were located at the peak of the volcano, with very small η values of 0.51, 0.29, and 0.33 V, respectively. Additionally, the η values of the TMN3@CNT series were in the range of 0.35–1.57 V (Fig. 3b). We can observe that more TMN3@CNT (TM = Ti, Fe, Ru, Co, and Rh) systems could be located at the peak of the volcano plot, exhibiting very small overpotentials of approximately 0.48, 0.35, 0.35, 0.39, and 0.46 V, respectively. All these TMN4@CNT and TMN3@CNT systems could even have lower η values than that of the state-of-the-art IrO2 (0.56 V), indicating their excellent OER catalytic activity.
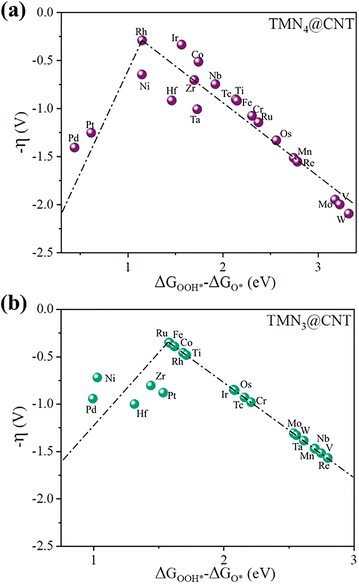 | ||
| Fig. 3 The volcano plots of the calculated overpotential η versus ΔGOOH* − ΔGO* for (a) TMN4@CNT and (b) TMN3@CNT systems. | ||
Furthermore, the stability of these eight one-dimensional TMN4@CNT (TM = Co, Rh, and Ir) and TMN3@CNT (TM = Ti, Fe, Ru, Co, and Rh) systems with high OER activity were further evaluated by AIMD simulations at 500 K, as shown in Fig. S8.† This temperature has been widely accepted for evaluating thermal stability.71–74 It was found that after 5000 fs, they could still maintain structural integrity, and their average potential energy remained almost unchanged, indicating their high thermal stabilities. Moreover, we also investigated the electronic behavior of these eight structures by calculating their density of states (DOSs). Our computed results show that they uniformly exhibited a metallic behavior in view of the relevant states crossing the Fermi level (Fig. S9†), suggesting their high conductivity. All of these can be beneficial for the high OER catalytic activity.
Obviously, introducing the TMN4 or TMN3 units into one-dimensional CNTs with curvature can be considered as a new and effective strategy to achieving SACs with high OER performance. As the number of N atoms decreased from four to three, more new SAC structures with high OER activity could be obtained. In particular, compared to the general case of the OER on a clean material surface, the pre-adsorption of some species from water dissociation on the TM sites could play an important role in realizing high OER catalytic activity in some SAC systems.
OER catalytic mechanisms
From the above discussions, we know that the TMN4@CNT and TMN3@CNT systems doped with Co/Rh/Ir/Fe/Ru atoms in Group VIII and Ti atom in Group IVB would possess excellent OER catalytic performance. In this section, we conducted a detailed analysis of the catalytic mechanism to reveal the underlying reasons behind their high OER catalytic activity.Initially, the influence of different TM metals on the overpotential η value was explored by plotting the relationship between η and the number of valence electrons (Nval) of TM in the TMN4@CNT and TMN3@CNT frameworks. It can be seen from Fig. 4a that for the TMN4@CNT series, the η values showed a fluctuating trend with the increase in the number of valence electrons of TM. To be specific, when employing the TM atoms with four valence electrons, the doped systems exhibited moderate η values. Then, as the number of valence electrons increased from four to six in succession, the overpotential η value increased monotonically, and then decreased when Nval further increased from six to nine, and increased again for the system with Nval = 10. Obviously, the number of valence electrons of the TM atom can play an important role in determining the overpotential η value of the TMN4@CNT system, where the CNT systems doped by Co/Rh/Ir atoms with nine valence electrons exhibited the optimal OER catalytic performance.
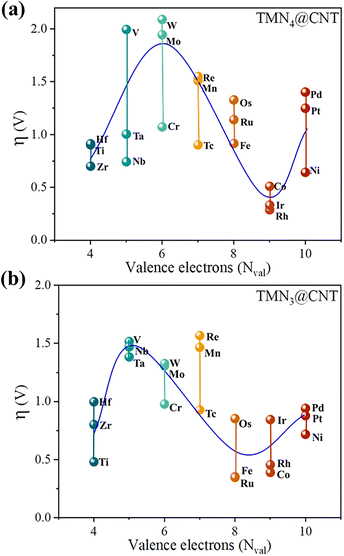 | ||
| Fig. 4 Relationship between the calculated overpotential (η) and the valence electrons (Nval) of TM on (a) TMN4@CNT and (b) TMN3@CNT. | ||
A similar fluctuation could also be observed in the TMN3@CNT series. As shown in Fig. 4b, the relationship curve between η and Nval still showed a trend of first rising, then falling, and then rising with the increase in the number of valence electrons of the TM atom. The difference is that when Nval increased from four to five, the η value monotonously increased, and then monotonically decreased until Nval increased to eight. Eventually, the η value monotonically increased again when increasing Nval from eight to ten. Clearly, the effective influence of the Nval of the TM atoms on the η value was reflected again in the TMN3@CNT series.
Further, the relationships of ΔGOH*vs. ΔGO* and ΔGOOH*vs. ΔGO* were fitted for the two series including TMN4@CNT and TMN3@CNT. It can be found that they exhibited good linear correlations, as shown in Fig. S10.† Specifically, for ΔGOH* and ΔGO*, the linear relationships could be expressed as ΔGOH* = 0.55ΔGO* − 0.49 and ΔGOH* = 0.66ΔGO* − 0.58 for TMN4@CNT and TMN3@CNT, respectively, where the corresponding correlation coefficients (R) were about 0.86 and 0.87. As for ΔGOOH* and ΔGO*, the relational expressions of ΔGOOH* = 0.47ΔGO* + 2.83 (R = 0.90) and ΔGOOH* = 0.50ΔGO* + 2.97 (R = 0.88) could be obtained for TMN4@CNT and TMN3@CNT, respectively. Clearly, regardless of the number of N atoms, both ΔGOH* and ΔGOOH* were linearly proportional to ΔGO*, thus ΔGO* can be regarded as a valid descriptor for determining the overpotential η values for the two series. This situation can also be reflected by the volcano diagram of ΔGO* and η, as shown in Fig. 5a and b.
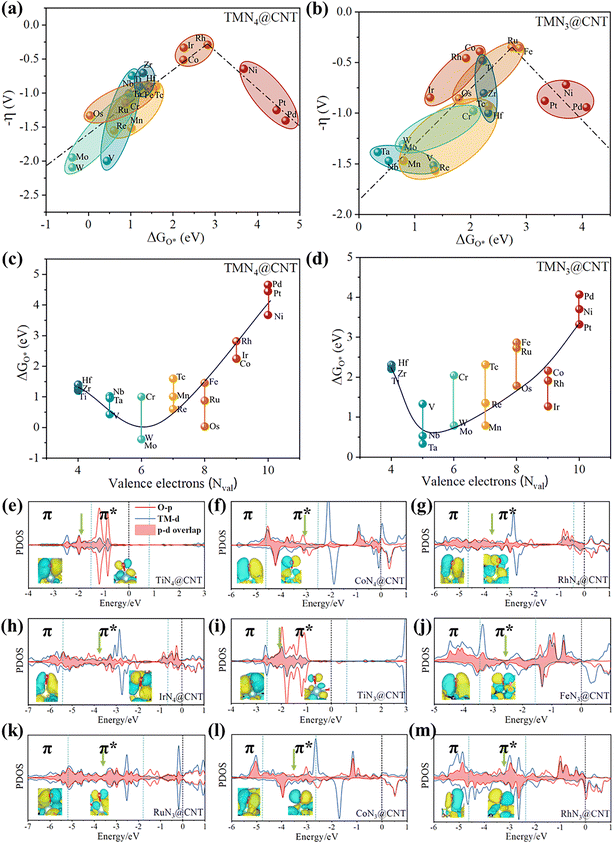
In Fig. 5a, we can observe that the TMN4@CNT systems were located on the left side of the volcano plot when the TM atoms come from Group IVB (Ti, Zr, and Hf), VB (V, Nb, and Ta), VIB (Cr, Mo, and W), and VIIB (Mn, Tc, and Re) elements, as well as some atoms in Group VIII, such as Fe, Ru, and Os. This means that the interaction between O* and these TM atoms is relatively strong. In contrast, the TMN4@CNT systems doped with Ni, Pd, and Pt in Group VIII were located on the right side of this volcano map, indicating weak interactions between O* and these TM sites. Obviously, the adsorption strength of the intermediates O* can play a crucial role in determining the OER activity of such CNT-based SACs. As is well known, too strong an adsorption of reactants can usually block the catalytic sites and hinder the catalytic reaction, while too weak an adsorption cannot provide sufficient driving force to bind with the adsorbent, resulting in a low catalytic efficiency. Therefore, the appropriate adsorption strength of O* is necessary for efficient catalysis. Indeed, the TMN4@CNT systems doped by Co, Rh, and Ir were located at the peak of the volcano plot, and exhibited a suitable adsorption state for O*, leading to their high OER catalytic activity. Similarly, the CoN3@CNT and RhN3@CNT systems were also located at the peak of the volcanic curve (Fig. 5b). Besides, the TiN3@CNT, FeN3@CNT, and RuN3@CNT systems were located at the peak of the volcano plot, different from the corresponding TMN4@CNT systems. Clearly, the appropriate adsorption of O* can also endow these five TMN3@CNT systems with high OER catalytic activity.
These findings raise the question, why can doping these TM atoms induce the appropriate adsorption state of O* within the framework of TMN3@CNT and TMN4@CNT? To solve this issue, we plotted the relationships between ΔGO* and the valence electron number Nval of TM atom, as shown in Fig. 5c and d. It was found that for the TMN4@CNT series, the ΔGO* values showed a trend of first decreasing and then increasing with the increase in Nval, and the systems doped by Cr, Mo, and W with Nval = 6 displayed the smallest ΔGO* values. A similar trend could also be observed in the TMN3@CNT series, where the systems doped by V, Nb, and Ta with Nval = 5 had the lowest ΔGO* values. Obviously, the filling of valence electrons can play a key role in determining the ΔGO* value. To be specific, when there are fewer valence electrons, the bonding ability between O* and TM metals is relatively strong, thus showing relatively small ΔGO* values. In contrast, a continuous increase in valence electrons in the d orbitals will lead to the existence of antibonding characteristic, which can weaken the bonding ability between O* and the TM metal, and lead to an increase in the ΔGO* value. Clearly, during the gradient process, moderate ΔGO* values were obtained in the TMN4@CNT (TM = Co, Rh, and Ir) and TMN3@CNT (TM = Ti, Fe, Ru, Co, and Rh) systems, resulting in high OER catalytic activity.
In order to better understand the catalytic mechanism, we further conducted bonding analysis on all the TMN4@CNT and TMN3@CNT systems with excellent OER catalytic performance. For comparison, the TiN4@CNT system with poor OER catalytic activity was also considered. From Fig. 5e, we can find that for TiN4@CNT, the overlapping center of the O-p and Ti-d orbitals was located in the π bonding area, meaning a relatively strong interaction between the O* and Ti-site, which is the reason for the inert OER catalytic performance. Comparatively, for the three TMN4@CNT (TM = Co, Rh, and Ir) systems, the overlapping centers of the O-p and TM-d orbitals were located in the π* antibonding area, which could effectively weaken the interaction between the O* and TM, and bring about the appropriate the adsorption state of O*, leading to their excellent OER catalytic activity (Fig. 5f–h).
When converting the functional unit from TiN4 to TiN3, the overlapping center of the O-p and Ti-d orbitals also moved into the π* antibonding region (Fig. 5i). The appearance of π* antibonding characteristic endowed the TiN3@CNT system with the moderate ΔGO* values, ultimately leading to a high OER catalytic performance. In addition, a similar situation was further reflected in the TMN3@CNT systems (TM = Fe, Ru, Co, and Rh), where all the overlapping centers of the O-p and TM-d orbitals were also located in the π* antibonding region, as shown in Fig. 5j–m. Obviously, for all these 1D TMN4@CNT and TMN3@CNT structures, the valence electrons of the TM can play a crucial role in determining the overpotential η by effectively influencing the adsorption state of O*, regardless of the number of N atoms.
Obviously, for a series of TMNx@CNT systems, the adsorption state of O* (ΔGO*) as an effective descriptor can be not only affected by the number of valence electrons in the TM atom, but also by the chemical environment surrounding the TM atom (such as the coordinated N atoms and the possible adsorbed species, including OH*, O*, and H*). The effective synergy of these factors will induce the appropriate adsorption state of O* (usually exhibiting a certain antibonding characteristic), which can lead to a high OER catalytic performance with a low overpotential η.
Finally, based on the linear relationship in Fig. S11, Tables S2, 3,† and eqn (3)–(7), a two-dimensional volcano diagram was constructed to evaluate the limiting overpotential η of the TMN4@CNT and TMN3@CNT series. A similar approach has also been successfully employed in previous works.75–77Fig. 6 shows that the 2D volcano map could be divided into four regions according to the different potential determination steps (PDSs). Most the TMN4@CNT and TMN3@CNT samples were distributed in the PDS3 region (O* → OOH*). We can find that the adsorption strength of O* is an important factor to determine the PDS step of the OER reaction. The TMN4@CNT and TMN3@CNT systems with weaker O* absorption were located in the PDS1 and PDS2 regions, while the related systems with stronger O* absorption were located in the PDS3 and PDS4 regions for TMN4@CNT and PDS2 and PDS3 regions for TMN3@CNT, respectively. Especially, the structures located at the boundary between PDS2 and PDS3 regions could display the best OER catalytic activity, including the systems doped by Ti, Fe, Ru, Co, Rh, and Ir. Here, the red area at the center of these two volcano plots predicts the limiting overpotential η of the TMN4@CNT and TMN3@CNT systems, both of which could uniformly be as small as 0.27 V. All of these provide good in-depth insights for designing SACs with high OER activity within the frameworks of TMN4@CNT and TMN3@CNT.
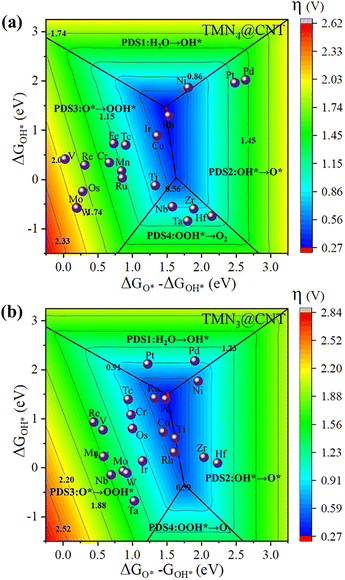 | ||
| Fig. 6 The 2D colored contour plots of OER activity volcanos for (a) TMN4@CNT and (b) TMN3@CNT by presenting the η value as a function of the Gibbs free energy. | ||
Conclusions
In summary, high-throughput DFT calculations were carried out to systematically investigate the structures and OER catalytic activities of a total of 42 1D CNT-based systems with the TMN3 or TMN4 functional units. The following interesting findings can be noted:(1) All these TMNx@CNT (x = 3 and 4) systems possessed high structural stability, in which TM atoms were stably anchored to form the TMN3 or TMN4 units. In addition, the DOS calculations indicated that they would exhibit metallic conductivity. All of these advantages are beneficial for the catalytic performance of the OER.
(2) By screening a series of 3d/4d/5d TM atoms, we found that when employing Co, Rh, and Ir atoms in Group VIII, the overpotential η values of the TMN4@CNT systems could be as low as 0.29–0.51 V, which is comparable to or even lower than the state-of-the-art IrO2 (0.56 V), suggesting their considerably high OER catalytic activity.
(3) In contrast, a high OER activity could also be observed in the TMN3@CNT framework systems, which were doped not only with Co and Rh atoms, but also with Ti, Fe, and Ru atoms, as revealed by the corresponding small η values (0.35–0.48 V). In particular, different from the general situation of the OER, the pre-adsorption of some species from water dissociation on the TM centers could play a key role in realizing the high OER activity within the framework of TMN3@CNT (TM = Ti, Fe, and Ru).
(4) ΔGO* can be considered to be a good descriptor for the OER catalytic activity of TMNx@CNT (x = 3 and 4) systems. The filling of the valence electrons of the TM atom can have an important impact on the ΔGO* value, and the appropriate ΔGO* values led to the low overpotentials for all these eight TMN3@CNT or TMN4@CNT systems, showing excellent OER catalytic activity.
Clearly, introducing the functional units TMN3 or TMN4 into 1D CNTs can be regarded as an effective strategy for obtaining nonprecious and highly efficient SACs for the OER. This study can offer in-depth theoretical insights into the OER activity and mechanism of CNT-based SACs, which is advantageous for promoting the practical application of important CNT-based nanomaterials in OER catalytic processes.
Author contributions
Xia Yang performed investigation, data curation and writing – original draft preparation. Guangtao Yu and Wei Chen performed conceptualization, investigation, writing – review and editing, supervision, project administration and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Fujian Province (2020J01147 and 2022J01167), Research Foundation of Academy of Carbon Neutrality of Fujian Normal University (TZH2022-05), Minjiang Scholar and startup fund for high-level talent at Fujian Normal University, and Fujian-Taiwan Science and Technology Cooperation Base of Biomedical Materials and Tissue Engineering (2021D039). We acknowledge the Computing Center of Jilin Province for supercomputer time.References
- Z. G. Yang, J. L. Zhang, M. C. W. Kintner-Meyer, X. C. Lu, D. W. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- G. S. Liu, S. J. You, Y. Tan and N. Q. Ren, Environ. Sci. Technol., 2017, 51, 2339–2346 CrossRef CAS.
- N. L. Panwar, S. C. Kaushik and S. Kothari, Renewable Sustainable Energy Rev., 2011, 15, 1513–1524 CrossRef.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- G. W. K. Moore, S. E. L. Howell, M. Brady, X. Xu and K. McNeil, Nat. Commun., 2021, 12, 1 CrossRef CAS.
- L. Schlapbach, Nature, 2009, 460, 809–811 CrossRef CAS PubMed.
- J. Li, C. A. Triana, W. Wan, D. P. Adiyeri Saseendran, Y. Zhao, S. E. Balaghi, S. Heidari and G. R. Patzke, Chem. Soc. Rev., 2021, 50, 2444–2485 RSC.
- G. Qian, G. Yu, J. Lu, L. Luo, T. Wang, C. Zhang, R. Ku, S. Yin, W. Chen and S. Mu, J. Mater. Chem. A, 2020, 8, 14545–14554 RSC.
- H. Chang, Z. Liang, L. Wang and C. Wang, Nanoscale, 2022, 14, 5639–5656 RSC.
- H. Dau, C. Limberg, T. Reier, M. Risch, S. Roggan and P. Strasser, ChemCatChem, 2010, 2, 724–761 CrossRef CAS.
- M. K. Debe, Nature, 2012, 486, 43–51 CrossRef CAS.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- X. Yin, L. Yang and Q. Gao, Nanoscale, 2020, 12, 15944–15969 RSC.
- L.-L. Feng, G. Yu, Y. Wu, G.-D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS.
- L. Yang, G. T. Yu, X. Ai, W. S. Yan, H. L. Duan, W. Chen, X. T. Li, T. Wang, C. H. Zhang, X. R. Huang, J. S. Chen and X. X. Zou, Nat. Commun., 2018, 9, 5236 CrossRef CAS PubMed.
- K. Sathiyan, T. Mondal, P. Mukherjee, S. G. Patra, I. Pitussi, H. Kornweitz, R. Bar-Ziv and T. Zidki, Nanoscale, 2022, 14, 16148–16155 RSC.
- B. T. Qiao, A. Q. Wang, X. F. Yang, L. F. Allard, Z. Jiang, Y. T. Cui, J. Y. J. Y. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- X. J. Cui, W. Li, P. Ryabchuk, K. Junge and M. Beller, Nat. Catal., 2018, 1, 385–397 CrossRef CAS.
- X. N. Li, Y. Q. Huang and B. Liu, Chem, 2019, 5, 2733–2735 CAS.
- X. Gong, Z. Jiang, W. Zeng, C. Hu, X. Luo, W. Lei and C. Yuan, Nano Lett., 2022, 22, 9411–9417 CrossRef CAS.
- X. Liang, F.-K. Chiang, L. Zheng, H. Xiao, T. Zhang, F. Zhang and Q. Gao, Chem. Commun., 2022, 58, 7678–7681 RSC.
- R. Zhang, W. Liu, F.-M. Zhang, Z.-D. Yang, G. Zhang and X. C. Zeng, Appl. Catal., B, 2023, 325, 122366 CrossRef CAS.
- T. Zhang, B. Zhang, Q. Peng, J. Zhou and Z. Sun, J. Mater. Chem. A, 2021, 9, 433–441 RSC.
- L. Cao, Q. Luo, J. Chen, L. Wang, Y. Lin, H. Wang, X. Liu, X. Shen, W. Zhang, W. Liu, Z. Qi, Z. Jiang, J. Yang and T. Yao, Nat. Commun., 2019, 10, 4849 CrossRef PubMed.
- H. Fei, J. Dong, Y. Feng, C. S. Allen, C. Wan, B. Volosskiy, M. Li, Z. Zhao, Y. Wang, H. Sun, P. An, W. Chen, Z. Guo, C. Lee, D. Chen, I. Shakir, M. Liu, T. Hu, Y. Li, A. I. Kirkland, X. Duan and Y. Huang, Nat. Catal., 2018, 1, 63–72 CrossRef CAS.
- X. L. Zhang, Z. X. Yang, Z. S. Lu and W. C. Wang, Carbon, 2018, 130, 112–119 CrossRef CAS.
- K. Wang, Z. Lu, J. Lei, Z. Liu, Y. Li and Y. Cao, ACS Nano, 2022, 16, 11944–11956 CrossRef CAS PubMed.
- J. Liu, J. Xiao, B. Luo, E. Tian and G. I. N. Waterhouse, Chem. Eng. J., 2022, 427, 132038 CrossRef CAS.
- M. Guo, M. Ji and W. Cui, Appl. Surf. Sci., 2022, 592, 153237 CrossRef CAS.
- S.-L. Li, Q. Li, Y. Chen, Y. Zhao and L.-Y. Gan, Appl. Surf. Sci., 2022, 605, 154828 CrossRef CAS.
- H. Zhang, W. Wei, S. Wang, H. Wang, B. Huang and Y. Dai, J. Mater. Chem. A, 2021, 9, 4082–4090 RSC.
- Z. Zhang, S. Qi, X. Song, J. Wang, W. Zhang and M. Zhao, Appl. Surf. Sci., 2021, 553, 149464 CrossRef CAS.
- Z. Zhao, J. Hao, B. Jia, X. Zhang, G. Wu, C. Zhang, L. Li, S. Gao, Y. Ma, Y. Li and P. Lu, J. Energy Chem., 2023, 83, 79–89 CrossRef CAS.
- H. Xiao, H. Li, X. Li and J. Jiang, J. Phys. Chem. Lett., 2022, 13, 7392–7397 CrossRef CAS PubMed.
- C. J. Shearer, A. Cherevan and D. Eder, Adv. Mater., 2014, 26, 2295–2318 CrossRef CAS PubMed.
- D. M. Sun, C. Liu, W. C. Ren and H. M. Cheng, Small, 2013, 9, 1188–1205 CrossRef CAS PubMed.
- D. He, S. Mu and M. Pan, Carbon, 2011, 49, 82–88 CrossRef CAS.
- D. P. He, C. Zeng, C. Xu, N. C. Cheng, H. G. Li, S. C. Mu and M. Pan, Langmuir, 2011, 27, 5582–5588 CrossRef CAS.
- T. Li, Y. Chen, W. Hu, W. Yuan, Q. Zhao, Y. Yao, B. Zhang, C. Qiu and C. M. Li, Nanoscale, 2021, 13, 4444–4450 RSC.
- G.-L. Chai, Z. Hou, D.-J. Shu, T. Ikeda and K. Terakura, J. Am. Chem. Soc., 2014, 136, 13629–13640 CrossRef CAS.
- O. Gülseren, T. Yildirim and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 153405 CrossRef.
- H. J. Liu, L. M. Zhao, Y. H. Liu, J. Xu, H. Y. Zhu and W. Y. Guo, Catal. Sci. Technol., 2019, 9, 5301–5314 RSC.
- C. J. Oluigbo, Y. G. Xu, H. Louis, A. B. Yusuf, W. Yaseen, N. Ullah, K. J. Alagarasan, M. Xie, E. E. Ekpenyong and J. M. Xie, Appl. Surf. Sci., 2021, 562, 150161 CrossRef CAS.
- L. M. Zhao, S. Guo, H. J. Liu, H. Y. Zhu, S. F. Yuan and W. Y. Guo, ACS Appl. Nano Mater., 2018, 1, 6258–6268 CrossRef CAS.
- S. Aoyama, J. Kaiwa, P. Chantngarm, S. Tanibayashi, H. Saito, M. Hasegawa and K. Nishidate, AIP Adv., 2018, 8, 115113 CrossRef.
- H. Hu, P. Zhang, B. B. Xiao and J. L. Mi, ACS Appl. Mater. Interfaces, 2023, 15, 23170–23184 CrossRef CAS PubMed.
- A. V. Kuzmin and B. A. Shainyan, ACS Omega, 2021, 6, 374–387 CrossRef CAS.
- J. T. Niu, W. J. Qi, C. Li, M. Mao, Z. G. Zhang, Y. Chen, W. L. Li and S. S. Ge, Int. J. Energy Res., 2021, 45, 8524–8535 CrossRef CAS.
- Y. Zheng, Y. Jiao, Y. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S.-Z. Qiao, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef CAS.
- Y. Zhang, B. Ouyang, G. Long, H. Tan, Z. Wang, Z. Zhang, W. Gao, R. S. Rawat and H. J. Fan, Sci. China: Chem., 2020, 63, 890–896 CrossRef CAS.
- D. Huang, Q. Wang, W. Dong, L. Liao, H. Chen and J. Ye, Energy Fuels, 2022, 36, 11601–11608 CrossRef CAS.
- Y. Li, J. Tan, X. Zhao, L. Zhao and X. Chen, Mater. Today Commun., 2023, 37, 107157 CrossRef CAS.
- T. Wang, Y. Wu, Y. Han, P. Xu, Y. Pang, X. Feng, H. Yang, W. Ji and T. Cheng, ACS Appl. Nano Mater., 2021, 4, 14161–14168 CrossRef CAS.
- K. Adpakpang, L. Pukdeejorhor, L. Ngamwongwan, S. Suthirakun, S. Impeng, S. Wannapaiboon, P. Chakthranont, K. Faungnawakij and S. Bureekaew, Chem. Commun., 2022, 58, 7124–7127 RSC.
- B. Boro, M. K. Adak, S. Biswas, C. Sarkar, Y. Nailwal, A. Shrotri, B. Chakraborty, B. M. Wong and J. Mondal, Nanoscale, 2022, 14, 7621–7633 RSC.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- X. Li, Z. Su, Z. Zhao, Q. Cai, Y. Li and J. Zhao, J. Colloid Interface Sci., 2022, 607, 1005–1013 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS.
- G. Gao, E. R. Waclawik and A. Du, J. Catal., 2017, 352, 579–585 CrossRef CAS.
- M. Li, L. Zhang, Q. Xu, J. Niu and Z. Xia, J. Catal., 2014, 314, 66–72 CrossRef CAS.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martinez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Norskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- L. Wu, T. Guo and T. Li, Adv. Funct. Mater., 2022, 32, 2203439 CrossRef CAS.
- Y. Zhu, Z. He, Y. Choi, H. Chen, X. Li, B. Zhao, Y. Yu, H. Zhang, K. A. Stoerzinger, Z. Feng, Y. Chen and M. Liu, Nat. Commun., 2020, 11, 4299 CrossRef CAS PubMed.
- C. Xuan, Q. Xu, L. Han and B. Hou, Chem. Eng. J., 2023, 464, 142620 CrossRef CAS.
- X. Zhai, L. Li, X. Liu, Y. Li, J. Yang, D. Yang, J. Zhang, H. Yan and G. Ge, Nanoscale, 2020, 12, 10035–10043 RSC.
- Y. Ying, K. Fan, X. Luo, J. Qiao and H. Huang, J. Mater. Chem. A, 2021, 9, 16860–16867 RSC.
- Q.-J. Fang, J.-k. Pan, W. Zhang, F.-l. Sun, W.-x. Chen, Y.-f. Yu, A.-F. Hu and G.-l. Zhuang, J. Colloid Interface Sci., 2022, 617, 752–763 CrossRef CAS PubMed.
- F.-l. Sun, Q.-j. Fang, Y.-f. Yu, W. Zhang, J.-k. Pan, W.-X. Chen and G.-l. Zhuang, J. Mater. Chem. A, 2022, 10, 9565–9574 RSC.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Nørskov, Chem. Rev., 2018, 118, 2302–2312 CrossRef CAS.
- Z. Xue, X. Zhang, J. Qin and R. Liu, J. Mater. Chem. A, 2019, 7, 23091–23097 RSC.
- Z. Xue, X. Zhang, J. Qin and R. Liu, J. Energy Chem., 2021, 55, 437–443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr04396g |
| This journal is © The Royal Society of Chemistry 2024 |