Graphene quantum dot-mediated anchoring of highly dispersed bismuth nanoparticles on porous graphene for enhanced electrocatalytic CO2 reduction to formate†
Yi
Cheng
ab,
Ruizhe
Yang
ab,
Lu
Xia
c,
Xiaoli
Zhao
*d,
Yuwei
Tan
ab,
Ming
Sun
ab,
Suming
Li
ab,
Fei
Li
*e and
Ming
Huang
 *ab
*ab
aInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 611731, China. E-mail: huangming@uestc.edu.cn
bYangtze Delta Region Institute (Huzhou), University of Electronic Science and Technology of China, Huzhou 313001, China
cICFO–Institut de Ciències Fotòniques, The Barcelona Institute of Science and Technology, Barcelona 08860, Spain
dSchool of Materials Science and Engineering, Xihua University, Chengdu, 610039, China. E-mail: zhaoxl@mail.xhu.edu.cn
eSchool of Materials and Energy, University of Electronic Science and Technology of China, Chengdu 611731, China. E-mail: feili@uestc.edu.cn
First published on 21st December 2023
Abstract
The electrocatalytic reduction of CO2 to produce formic acid is gaining prominence as a critical technology in the pursuit of carbon neutrality. Nonetheless, it remains challenging to attain both substantial formic acid production and high stability across a wide voltage range, particularly when utilizing bismuth-based catalysts. Herein, we present a novel graphene quantum dot-mediated synthetic strategy to achieve the uniform deposition of highly dispersed bismuth nanoparticles on porous graphene. This innovative design achieves an elevated faradaic efficiency for formate of 87.0% at −1.11 V vs. RHE with high current density and long-term stability. When employing a flow cell, a maximum FEformate of 80.0% was attained with a total current density of 156.5 mA cm−2. The exceptional catalytic properties can be primarily attributed to the use of porous graphene as the support and the auxiliary contribution of graphene quantum dots, which enhance the dispersion of bismuth nanoparticles. This improved dispersion, in turn, has a significantly positive impact on CO2 activation and the generation of *HCOO intermediates to facilitate the formation of formate. This work presents a straightforward technique to create uniform metal nanoparticles on carbon materials for advancing various electrolytic applications.
Introduction
The efficient conversion of the greenhouse gas CO2 into value-added fuels or chemicals is a significant approach for addressing CO2 emissions while simultaneously mitigating the increasing scarcity of non-renewable fossil fuels.1–10 Currently, the electrocatalytic CO2 reduction reaction (CO2RR) stands as one of the most promising avenues, owing to its mild reaction conditions and high energy efficiency. However, its practical realization and implementation pose a formidable challenge due to the inherent thermodynamic stability of CO2 molecules and the multitude of reaction pathways for CO2 reduction, which limit reaction selectivity. On the other hand, within the reaction pathway, the hydrogen evolution reaction (HER) in an aqueous electrolyte acts as a competing side reaction, making it challenging for the CO2RR to generate the desired product.11–17 Hence, there is a pressing demand to develop electrocatalysts with high efficiency for the CO2RR that can enhance both the reaction rate and the selectivity toward the target product.Among the products resulting from the CO2RR, formic acid or formate has garnered substantial interest due to its numerous potential applications and promising economic benefits. It can be utilized in formic acid fuel cells, hydrogen storage, and as a raw material in various industrial processes.18–23 In recent decades, researchers have explored various metal-based catalysts like Pb, In, Cd, Sn, and Co for the CO2RR to generate formate in aqueous solutions. Unfortunately, these catalysts suffer from drawbacks such as high cost, poor selectivity, limited availability, and low durability, posing significant obstacles to their widespread practical applications.24–26 In contrast, bismuth (Bi) is renowned for its excellent formate selectivity, low toxicity, and cost-effectiveness compared to pricier alternatives like Sn and In.13,27–31 Nevertheless, certain key issues persist, including low current density and poor catalytic stability arising from its chemically unstable and prone-to-agglomeration nature.32 To address these issues, a crucial strategy involves immobilizing Bi on the support material to enhance stability and regulate spatial distribution. The strong interaction between the metal and support, termed metal–support interaction, provides substantial opportunities for enhancing electrocatalytic CO2RR performance.
Various catalyst supports, including carbon materials, oxides, and nitrides, have been developed for the immobilization of metal nanoparticles.33–36 Notably, porous graphene stands out as an ideal platform for metal catalysts owing to its expansive surface area, high conductivity, and chemical stability.37,38 Confining metal nanoparticles onto graphene hinders particle aggregation by regulating their spatial distribution. The open structure of graphene layers facilitates the exposure of active sites, enhancing the adsorption and activation of CO2 molecules for improved electroactivity and durability.39,40 Additionally, the interface between metal nanoparticles and the support induces a noticeable redistribution of electrons. Combining Bi nanoparticles with a porous graphene support has the potential to improve both catalytic activity and stability in the electrochemical CO2 reduction process. It is worth noting that the CO2RR-to-formate activity is easily influenced by the particle size and mass loading of Bi on the graphene support. Therefore, there is a high desire, albeit a challenge, to devise a novel and efficient approach that can uniformly deposit Bi nanoparticles with controlled particle size.
Herein, we demonstrate a graphene quantum dot-assisted deposition strategy to synthesize uniformly loaded Bi nanoparticles on porous graphene (Bi NPs@PG). The abundant oxygenated groups and numerous defective sites of graphene quantum dots (GQDs) furnish porous graphene (PG) supports with high-density coordination sites. This facilitates the anchoring of Bi ions during the mixing process, resulting in the uniform deposition of Bi nanoparticles on PG after the pyrolysis process. As a result, the formate faradaic efficiency (FEformate) for Bi NPs@PG demonstrates a notable value of 87.0% at −1.11 V (vs. RHE) with remarkable durability evidenced by long-term electrolysis. In addition, it exhibits high CO2RR activity in a flow cell, achieving an FEformate of 80% and a partial current density of 156.5 mA cm−2. Detailed theoretical calculations reveal that the interaction between Bi and PG leads to increased electron transfer toward *CO2 and *HCOO. This establishes a favorable local coordination environment for CO2 activation, reducing the energy barrier for *HCOO intermediate formation and ultimately enhancing formate formation. This study presents an accessible strategy to achieve uniform metal nanoparticle deposition on porous graphene and gain insights into the synergistic effect of metal and porous graphene in influencing the selectivity and activity in the CO2RR.
Results and discussion
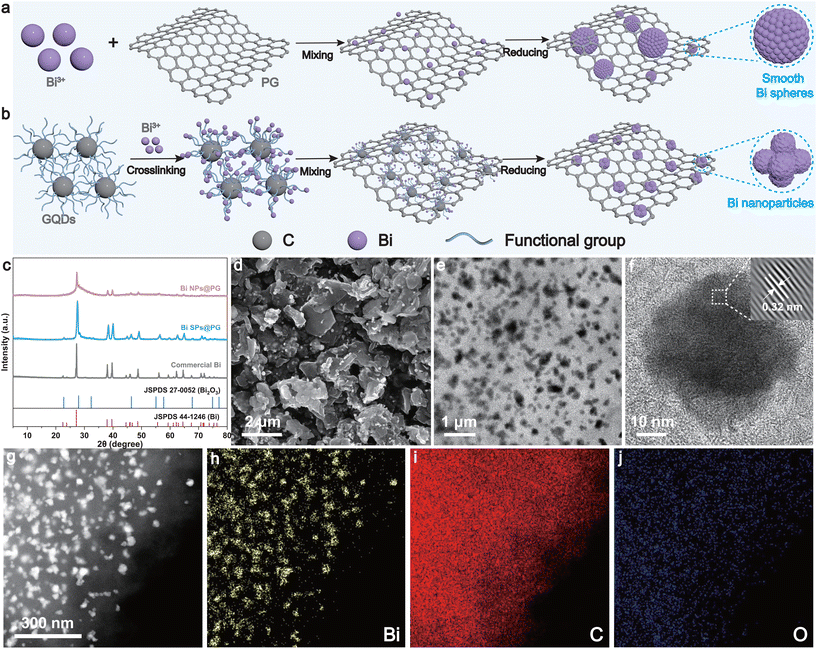
Fig. 1a and b show the preparation processes of the Bi nanoparticles on porous graphene (Bi NPs@PG) and Bi spheres on porous graphene (Bi SPs@PG) catalysts using PG as the carbon support. First, PG and GQDs were synthesized by a template-assisted chemical vapor deposition method and a refluxing method, respectively (detailed procedures are shown in the Experimental section in the ESI†). Characterization by X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) validates the successful preparation of the materials (Fig. S1 and S2†). Subsequently, Bi NPs@PG and Bi SPs@PG were synthesized using the same method, differing only in the presence or absence of GQDs. In the presence of GQDs, the Bi cation initially forms a stable GQD–Bi coordination composite through complexation or crosslinking. The robust interaction between the Bi cation and O-containing groups in GQDs effectively prevents metal precursor aggregation. Therefore, achieving a highly dispersed metal precursor is a crucial step in the synthesis of uniform Bi NPs. Subsequently, the resulting metal complex is combined with PG via π–π interactions during a mixing process. The π-conjugated structure of GQDs ensures a strong interaction between the PG support and the GQD. Finally, Bi NPs@PG is obtained after pyrolysis in a reducing environment (Fig. 1b). Without GQDs, the Bi cations cannot be well dispersed on PG and, as a result, easily aggregate into nonuniform and irregular Bi spheres (Fig. 1a). The prominent peaks in the XRD patterns of the synthesized samples closely match the characteristic peaks of Bi (Fig. 1c). The lack of diffraction peaks for PG can be attributed to its inferior crystallinity in comparison with the pronounced intensity exhibited by the Bi nanoparticles. SEM and TEM images (Fig. 1d and e) reveal that Bi nanoparticles with sizes ranging from 30 to 40 nm are uniformly dispersed on the PG layer. The corresponding SEM images of commercial Bi and Bi SPs@PG are shown in Fig. S3.† The results indicate that commercial Bi exhibits a blocky morphology, while Bi SPs@PG adopts the shape of a smooth sphere with nonuniform sizes, revealing limitations in the surface area or active sites for subsequent CO2 reduction reactions. The high-resolution transmission electron microscopy (HRTEM) image displays clear lattice fringes with an interplanar space of 0.32 nm, corresponding to the (012) plane of Bi and agreeing with the XRD findings (Fig. 1f). Additionally, the energy dispersive spectroscopy (EDS) mapping images (Fig. 1g–j) show a uniform distribution of Bi, C, and O within the Bi NPs@PG, affirming the consistent integration of Bi NPs within the graphene composite matrix.X-ray photoelectron spectroscopy (XPS) measurement of the Bi NPs@PG reveals the presence of Bi, C, O, and N elements. In the Bi 4f spectrum, two peaks at 162.7 and 157.4 eV are assigned to Bi0 4f5/2 and Bi0 4f7/2, respectively. Additionally, the peaks located at 164.7 and 159.4 eV correspond to Bi3+ 4f5/2 and 4f7/2, respectively (Fig. S4a†).41–43 The presence of Bi3+ peaks can be attributed to inevitable surface oxidation during sample preparation or characterization. The results from XRD and TEM indicate that the oxidation occurred only on the surface area, while the bulk material remains as metallic Bi. This surface oxidation was also observed in the Bi SP@PG sample (Fig. S5a†). It is noteworthy that the proportion of elemental metal Bi in Bi NPs@PG is higher than that in Bi SPs@PG. This is likely due to the former having a more uniform and smaller size, resulting in better encapsulation by PG and, thus, a slower rate of oxidation. The high-resolution C 1s spectrum exhibits three decoupled peaks corresponding to C–C (284.8 eV), C–N (285.9 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV), respectively (Fig. S4b†).44,45 The prevalence of sp2-bonded carbon atoms indicates a relatively high degree of carbonization for Bi NPs@PG, suggesting excellent conductivity for the catalysts. Peaks at 530.7 eV and 533.4 eV in the O 1s spectrum are ascribed to the Bi–O bonds and chemisorbed oxygen or OH species, respectively (Fig. S4c†).11,46–48 The N 1s XPS spectrum (Fig. S4d†) of Bi NPs@PG is fitted into three typical peaks corresponding to graphitic N (402.6 eV), pyrrolic N (400.7 eV), and pyridinic N (399.2 eV).49 The XPS results above indicate the successful incorporation of Bi nanoparticles into the porous graphene support, leading to a rearrangement of electrons at the interface. This could further enhance the conductivity and activity of the catalyst. In addition, the high-resolution C 1s, O 1s and N 1s spectra of Bi SPs@PG are shown in Fig. S5.†
O (288.1 eV), respectively (Fig. S4b†).44,45 The prevalence of sp2-bonded carbon atoms indicates a relatively high degree of carbonization for Bi NPs@PG, suggesting excellent conductivity for the catalysts. Peaks at 530.7 eV and 533.4 eV in the O 1s spectrum are ascribed to the Bi–O bonds and chemisorbed oxygen or OH species, respectively (Fig. S4c†).11,46–48 The N 1s XPS spectrum (Fig. S4d†) of Bi NPs@PG is fitted into three typical peaks corresponding to graphitic N (402.6 eV), pyrrolic N (400.7 eV), and pyridinic N (399.2 eV).49 The XPS results above indicate the successful incorporation of Bi nanoparticles into the porous graphene support, leading to a rearrangement of electrons at the interface. This could further enhance the conductivity and activity of the catalyst. In addition, the high-resolution C 1s, O 1s and N 1s spectra of Bi SPs@PG are shown in Fig. S5.†
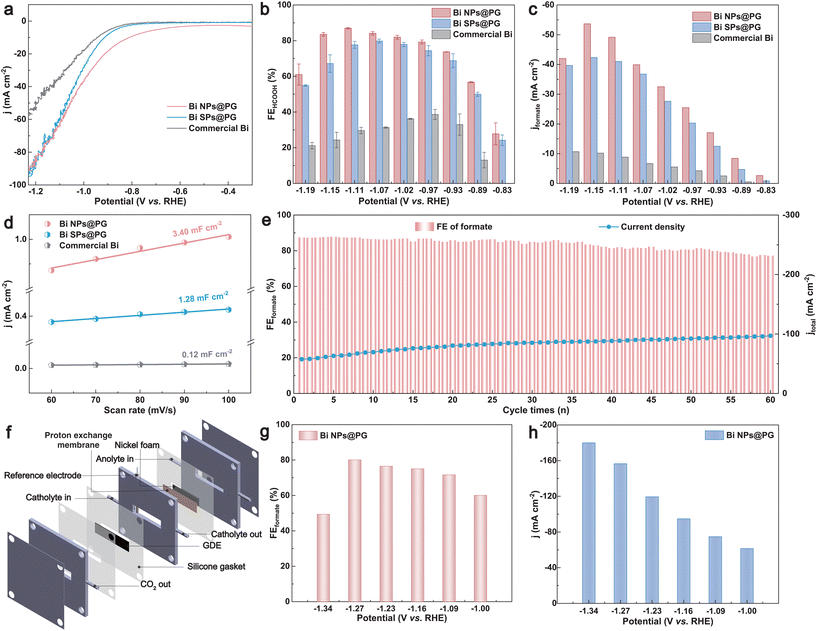
The electrocatalytic CO2 reduction performance was assessed in a gastight two-compartment H-cell using 0.1 M KHCO3 (CO2-saturated) as the electrolyte. Linear sweep voltammetry (LSV) curves were recorded at a scan rate of 5 mV s−1 (Fig. 2a). The current density of the Bi NPs@PG, characterized by the lowest onset potential, sharply increases under a CO2 flow, suggesting its superior CO2 reduction activity in comparison with Bi SPs@PG and commercial Bi catalysts. To assess the CO2 reduction efficiency at different potentials, we conducted a chronoamperometry test in a potential range of −0.83 to −1.19 V vs. RHE (Fig. S6†). Gaseous and liquid products were periodically analyzed using gas chromatography and ion chromatography, respectively (Fig. S7 and S8†). The faradaic efficiencies of Bi NPs@PG, Bi SPs@PG, and commercial Bi are presented in Fig. 2b and Fig. S9.† Notably, formate predominated as the product for Bi NPs@PG, accompanied by small amounts of CO and H2 (Fig. S10†), demonstrating the highest faradaic efficiency of formate (FEformate) of 87.0% at −1.11 V (vs. RHE) (Fig. 2b). This performance surpasses that of Bi SPs@PG (79.8%), commercial Bi (38.6%) and PG (<10%, Fig. S11†). Additionally, the FEformate remains >80% across a wide potential window (from −0.97 to −1.15 V vs. RHE) for Bi NPs@PG. The improved CO2RR activity can be attributed to the introduction of GQDs, which offer numerous anchoring sites for Bi cations. GQDs combine with Bi cations through many functional groups, thereby facilitating the uniform deposition of Bi nanoparticles on the porous graphene support, resulting in more accessible sites for the CO2RR. The partial formate current density (jformate) of these catalysts in the corresponding potential range is shown in Fig. 2c. Bi NPs@PG exhibits the highest jformate of 53.7 mA cm−2 at −1.15 V, surpassing those of Bi SPs@PG (42.3 mA cm−2) and commercial Bi (10.2 mA cm−2), consistent with the LSV results. Faradaic efficiencies and partial current densities of CO and H2 for these catalysts are also evaluated and presented in Fig. S9.† The electrochemical double-layer capacitance (Cdl) was measured through cyclic voltammetry (CV) to assess the electrochemically active surface area (ECSA) (Fig. S12†). As depicted in Fig. 2d, the Cdl value of the Bi NP@PG catalyst is 3.40 mF cm−2, exceeding that of the other two catalysts. This is attributed to the uniform Bi NPs providing a large number of catalytically active sites, indicating their intrinsically superior catalytic activity for CO2 reduction. Additionally, the stability of Bi NPs@PG was determined through long-term electrolysis at −1.11 V. After 60 cycles of electrolysis (each cycle lasting 1000 s), there was no significant decrease in FEformate, demonstrating the high stability of Bi NPs@PG (Fig. 2e). For comparison, the stability of Bi SPs@PG and commercial Bi was also measured under the same electrolysis conditions, revealing lower current densities and a rapid decay in FEformate (Fig. S13†). These results indicate that the introduction of GQDs during the synthesis of Bi NPs@PG promotes the uniform deposition of Bi NPs and thus improves the catalyst stability. To address the low solubility limitation of CO2 in an H-cell and showcase the feasibility of the CO2RR for high-current-density practical application, the performance of Bi NPs@PG was further evaluated in a flow cell system using 0.5 M KHCO3 as the cathode electrolyte (Fig. 2f). With testing conducted at potentials ranging from −1.00 V to −1.34 V (Fig. S14†), a peak FEformate of 80% was achieved at −1.27 V (Fig. 2g) with a total current density of 156.5 mA cm−2 (Fig. 2h), significantly exceeding the values obtained in the H-cell setup. At −1.34 V, an additional current increment was observed, highlighting the exceptional electrocatalytic performance of Bi NPs@PG in the CO2RR process.
To probe the reaction intermediates and elucidate the reaction pathways of the CO2RR to formate, in situ attenuated total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) was performed during the electrolysis process at potentials ranging from 0.10 V to −1.90 V vs. RHE. As shown in Fig. 3a and c, the peaks at 1059 cm−1 and 1108 cm−1 are attributed to CO32− species.50 A peak observed at approximately 1645 cm−1 is assigned to the in-plane bending of water molecules. The band centers at 1239 cm−1 and 1274 cm−1 in the spectra of the two samples were assigned to the formation of *CO2−, ascribing to the CO2 activation on the surface of the catalyst.51 Two peaks located at ∼1390 cm−1 and ∼1580 cm−1 are attributed to the *HCOO species (vibration of O–C–O in formic acid).52–54 The intensity of these two peaks increases with the applied potentials, indicating the gradual generation and accumulation of *HCOO during formate formation. These results reveal that *CO2− and *HCOO are the key reaction intermediates, providing further insights into the reaction pathways of the formation of formate during the CO2RR. It is worth noting that the intensity of *HCOO over Bi NPs@PG was significantly higher than that over Bi SPs@PG, indicating heightened activity and selectivity for formate formation.55 These pieces of evidence suggest that the uniform deposition of Bi NPs on PG significantly promotes the activation of CO2 molecules and enhances the generation of the key reaction intermediate (*HCOO), leading to improved CO2 performance.
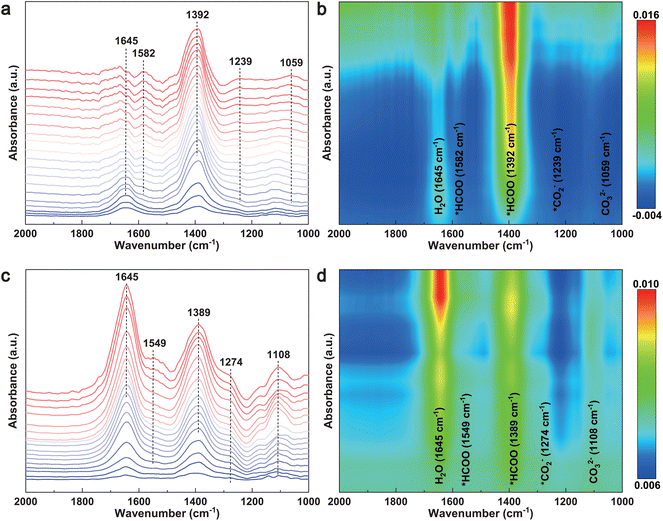 | ||
| Fig. 3 In situ ATR-SEIRAS spectra of (a and b) Bi NPs@PG and (c and d) Bi SPs@PG in CO2-saturated 0.1 M KHCO3 solution from 0.1 V to −1.9 V vs. RHE. | ||
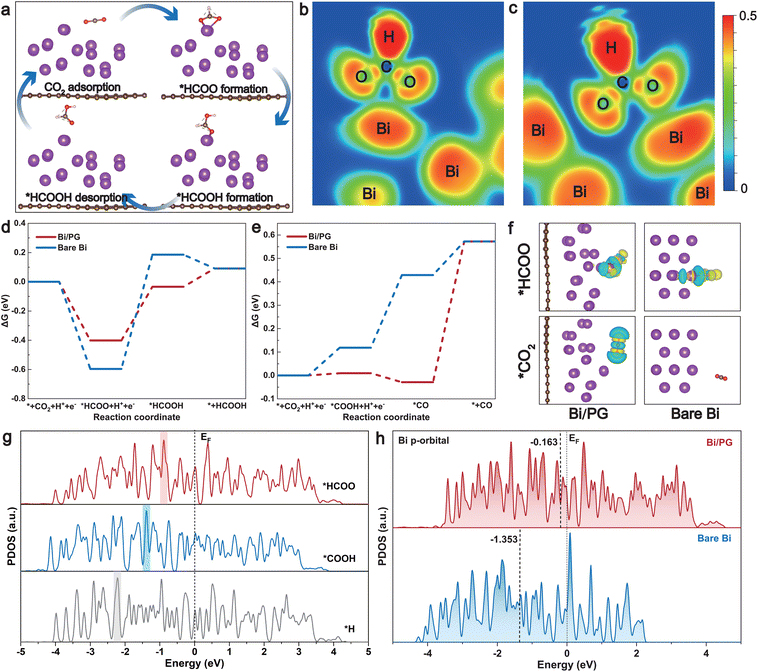
Density functional theory (DFT) calculations were further applied to simulate and compare the CO2RR pathways on both bare and PG-supported Bi surfaces (see details in the Method section in the ESI†). Referring to the experimental data, various adsorption models were generated through structure optimization to simulate the CO2RR process on the surface of Bi NPs@PG and commercial Bi. We constructed representative Bi/PG and bare Bi models, which are shown in Fig. S15 and S16,† highlighting the distinction between the cases with and without PG. The adsorption energy of CO2 on the surface of Bi/PG is lower (more negative) than that on bare Bi. This suggests that the PG substrate enhances the adsorption of CO2, thereby promoting its activation. The adsorption energy of *HCOO on Bi/PG is also lower than that on the bare Bi surface (Fig. S15†). In light of the aforementioned results, the proposed reaction pathway for the CO2RR to formic acid/formate on the catalyst surface is depicted in Fig. 4a. It underscores that the formation of the *HCOO intermediate is deemed the rate-determining step critical to the activity of formate. Electron localization function (ELF) maps clearly demonstrate that PG, as the substrate, significantly enhances the interaction of the *HCOO intermediate with Bi atoms (Fig. 4b and c).11,56 In addition, the Gibbs free-energy diagrams for the CO2RR on the two catalyst surfaces were constructed (Fig. 4d and e and Table S1†). As shown in Fig. 4d, the initial protonation of CO2 to form *HCOO is exothermic on both the catalyst surfaces. In the second step, the free-energy change (ΔG) for further protonation of *HCOO to form *HCOOH on Bi/PG (0.37 eV) is much lower than that on bare Bi (0.78 eV). In addition, the Gibbs free-energy diagrams for competitive reaction pathways to generate CO and H2 are also considered. The ΔG values for the formation of CO and H2 are 0.59 and 4.97 eV on the Bi/PG surface, respectively, showing much larger energy barriers compared to the formation of HCOOH (Fig. 4e and Fig. S17†). It is evident from the calculations that Bi/PG is more favored for HCOOH formation, indicating that the introduction of PG promotes the reduction of CO2 to HCOOH. The charge difference diagrams of the optimized adsorption configurations of *HCOO and *CO2 on the two catalyst surfaces are displayed in Fig. 4f, Fig. S18 and S19.† Compared to the bare Bi surface, the electron accumulation at the interface where *HCOO and *CO2 are absorbed is more significant on Bi/PG, enhancing the CO2 reduction to formate.57 The binding strength of various reaction intermediates could be deduced by comparing the projected density of states (PDOS) of the active Bi site with adsorbates (Fig. 4g). It has been reported that the position of the highest peak (Ep) of active site DOS with adsorbates was closer to the Fermi level, the stronger the adsorption strength.58 In the model of Bi/PG, the Ep of *HCOO is closest to the Fermi level (Ef) compared to that of *COOH and *H, indicating the lowest filling of anti-bonding states and, consequently, stronger adsorbate binding.59,60 In addition, the d-band center of Bi/PG (−0.163 eV) upshifts significantly compared to bare Bi (−1.353 eV) in the Bi p-orbital, following the general rule that a higher d-band center leads to a more reactive active site (lower transition state energy) (Fig. 4h),12,61–63 which is also applicable to the Bi s-orbital and d-orbital (Fig. S20†). These results suggest that utilizing PG as the substrate modifies the electronic structure of Bi metal, boosting its intrinsic activity. The collaboration in the Bi NP@PG catalyst not only disperses and stabilizes Bi NPs but also promotes the formation of the *HCOO intermediate, thereby enhancing CO2RR activity toward formate.
Conclusions
In summary, we established a cost-effective and straightforward synthesis route by leveraging graphene quantum dots to efficiently anchor Bi nanoparticles on porous graphene. The designed catalyst demonstrates remarkable efficiency in the electrolytic CO2RR to formate, achieving an FEformate of 87.0% and a formate current density of 49.2 mA cm−2 at −1.11 V vs. RHE. The performance remains impressive in a flow cell, showing an FEformate > 80% at a current density of 156.5 mA cm−2. In situ ATR-SEIRAS and DFT calculations indicate that porous graphene modifies the electronic structure of Bi metal, enhancing CO2 adsorption energy, lowering the energy barrier for *HCOO intermediate formation, and thereby increasing activity. This work provides insights for the rational design of carbon-supported metal catalysts for wide electrocatalytic applications.Experimental section
The experimental details are given in the ESI.†Author contributions
Ming Huang conceived the original concept and initiated the project. Yi Cheng carried out the experiments, data analysis, and wrote the draft manuscript. Ruizhe Yang, Lu Xia, Yuwei Tan, Ming Sun and Suming Li participated in material characterization and data analysis. Xiaoli Zhao, Fei Li and Ming Huang revised the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52373223 and 52202215), Sichuan Science and Technology Program (2023NSFSC0434 and 2023NSFSC0956), and the China Postdoctoral Science Foundation (2022M720652).References
- P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo and E. H. Sargent, Science, 2019, 364, eaav3506 CrossRef CAS PubMed.
- J. Zhu, J. Li, R. Lu, R. Yu, S. Zhao, C. Li, L. Lv, L. Xia, X. Chen, W. Cai, J. Meng, W. Zhang, X. Pan, X. Hong, Y. Dai, Y. Mao, J. Li, L. Zhou, G. He, Q. Pang, Y. Zhao, C. Xia, Z. Wang, L. Dai and L. Mai, Nat. Commun., 2023, 14, 4670 CrossRef CAS PubMed.
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, Joule, 2018, 2, 825–832 CrossRef CAS.
- B. Deng, M. Huang, X. Zhao, S. Mou and F. Dong, ACS Catal., 2021, 12, 331–362 CrossRef.
- X. Wang, P. Ou, J. Wicks, Y. Xie, Y. Wang, J. Li, J. Tam, D. Ren, J. Y. Howe, Z. Wang, A. Ozden, Y. Z. Finfrock, Y. Xu, Y. Li, A. S. Rasouli, K. Bertens, A. H. Ip, M. Graetzel, D. Sinton and E. H. Sargent, Nat. Commun., 2021, 12, 3387 CrossRef CAS PubMed.
- Z. Li, X. Qi, J. Wang, Z. Zhu, J. Jiang, X. Niu, A. Cabot, J. S. Chen and R. Wu, SusMat, 2023, 3, 498–509 CrossRef CAS.
- T. Tang, Z. Wang and J. Guan, Exploration, 2023, 3, 20230011 CrossRef PubMed.
- J. H. Cho, J. Ma and S. Y. Kim, Exploration, 2023, 3, 20230001 CrossRef PubMed.
- Y. Zhai, P. Han, Q. Yun, Y. Ge, X. Zhang, Y. Chen and H. Zhang, eScience, 2022, 2, 467–485 CrossRef.
- A. Conte, M. Baron, S. Bonacchi, S. Antonello and A. Aliprandi, Nanoscale, 2023, 15, 3693–3703 RSC.
- M. Huang, B. Deng, X. Zhao, Z. Zhang, F. Li, K. Li, Z. Cui, L. Kong, J. Lu, F. Dong, L. Zhang and P. Chen, ACS Nano, 2022, 16, 2110–2119 CrossRef CAS PubMed.
- G. Wen, D. U. Lee, B. Ren, F. M. Hassan, G. Jiang, Z. P. Cano, J. Gostick, E. Croiset, Z. Bai, L. Yang and Z. Chen, Adv. Energy Mater., 2018, 8, 1802427 CrossRef.
- N. Han, P. Ding, L. He, Y. Li and Y. Li, Adv. Energy Mater., 2019, 10, 1902338 CrossRef.
- Y. Zhang, V. Sethuraman, R. Michalsky and A. A. Peterson, ACS Catal., 2014, 4, 3742–3748 CrossRef CAS.
- Q. Li, Y. Zhang, L. Shi, M. Wu, Y. Ouyang and J. Wang, InfoMat, 2021, 3, 1285–1294 CrossRef CAS.
- F. Chen, Z. Yao, Z. Lyu, J. Fu, X. Zhang and J. Hu, eScience, 2023 DOI:10.1016/j.esci.2023.100172.
- J. Yin, J. Jin, Z. Yin, L. Zhu, X. Du, Y. Peng, P. Xi, C. Yan and S. Sun, Nat. Commun., 2023, 14, 1724 CrossRef CAS PubMed.
- L. C. Pardo Pérez, D. Teschner, E. Willinger, A. Guiet, M. Driess, P. Strasser and A. Fischer, Adv. Funct. Mater., 2021, 31, 2103601 CrossRef.
- L. Jia, M. Sun, J. Xu, X. Zhao, R. Zhou, B. Pan, L. Wang, N. Han, B. Huang and Y. Li, Angew. Chem., Int. Ed., 2021, 60, 21741–21745 CrossRef CAS PubMed.
- G. Wang, F. Wang, P. Deng, J. Li, C. Wang, Y. Hua, Y. Shen and X. Tian, Mater. Rep.: Energy, 2023, 3, 100181 CAS.
- X. Liu, K. Zhang, Y. Sun, S. Zhang, Z. Qiu, T. Song, J. Xie, Y. Wu and Y. Chen, SusMat, 2023, 3, 235–247 CrossRef CAS.
- L. Liu, X. Li, Y. Cai, H. Du, F. Liu, J. Zhang, J. Fu and W. Zhu, Nanoscale, 2022, 14, 13679–13688 RSC.
- B. Miao, W. Fang, B. Sun, F. Li, X. Wang, B. Xia and Y. Chen, Chin. J. Struct. Chem., 2023, 42, 100095 CrossRef.
- F. Yang, A. O. Elnabawy, R. Schimmenti, P. Song, J. Wang, Z. Peng, S. Yao, R. Deng, S. Song, Y. Lin, M. Mavrikakis and W. Xu, Nat. Commun., 2020, 11, 1088 CrossRef CAS PubMed.
- L. Dai, Q. Qin, P. Wang, X. Zhao, C. Hu, P. Liu, R. Qin, M. Chen, D. Ou, C. Xu, S. Mo, B. Wu, G. Fu, P. Zhang and N. Zheng, Sci. Adv., 2017, 3, e1701069 CrossRef PubMed.
- T. Wang, W.-S. Fang, Y. Liu, F. Li, P. Chen and Y. Chen, J. Energy Chem., 2022, 70, 407–413 CrossRef CAS.
- E. Zhang, T. Wang, K. Yu, J. Liu, W. Chen, A. Li, H. Rong, R. Lin, S. Ji, X. Zheng, Y. Wang, L. Zheng, C. Chen, D. Wang, J. Zhang and Y. Li, J. Am. Chem. Soc., 2019, 141, 16569–16573 CrossRef CAS PubMed.
- X. Liang, N. Tian, S. Hu, Z. Zhou and S. Sun, Mater. Rep.: Energy, 2023, 3, 100191 CAS.
- Y. Zhang, Y. Chen, R. Liu, X. Wang, H. Liu, Y. Zhu, Q. Qian, Y. Feng, M. Cheng and G. Zhang, InfoMat, 2022, 5, e12375 CrossRef.
- J. Yin, Z. Yin, J. Jin, M. Sun, B. Huang, H. Lin, Z. Ma, M. Muzzio, M. Shen, C. Yu, H. Zhang, Y. Peng, P. Xi, C.-H. Yan and S. Sun, J. Am. Chem. Soc., 2021, 143, 15335–15343 CrossRef CAS PubMed.
- S. Wu, M. Tian, Y. Hu, N. Zhang, W. Shen, J. Li, L. Guo, P. Da, P. Xi and C.-H. Yan, Inorg. Chem., 2023, 62, 4088–4096 CrossRef CAS PubMed.
- Q. Gong, P. Ding, M. Xu, X. Zhu, M. Wang, J. Deng, Q. Ma, N. Han, Y. Zhu, J. Lu, Z. Feng, Y. Li, W. Zhou and Y. Li, Nat. Commun., 2019, 10, 2807 CrossRef PubMed.
- Y. Xu, X. Shi, R. Hua, R. Zhang, Y. Yao, B. Zhao, T. Liu, J. Zheng and G. Lu, Appl. Catal., B, 2020, 260, 118142 CrossRef CAS.
- R. Ryoo, J. Kim, C. Jo, S. W. Han, J. Kim, H. Park, J. Han, H. S. Shin and J. W. Shin, Nature, 2020, 585, 221–224 CrossRef CAS PubMed.
- Z. Wu, C. Li, Z. Li, K. Feng, M. Cai, D. Zhang, S. Wang, M. Chu, C. Zhang, J. Shen, Z. Huang, Y. Xiao, G. A. Ozin, X. Zhang and L. He, ACS Nano, 2021, 15, 5696–5705 CrossRef CAS PubMed.
- P. Zhou, N. Li, Y. Chao, W. Zhang, F. Lv, K. Wang, W. Yang, P. Gao and S. Guo, Angew. Chem., Int. Ed., 2019, 58, 14184–14188 CrossRef CAS PubMed.
- X. Jing, Z. Zhu, L. Chen, D. Liu, H. Huang, W. Tian and A. Yin, ACS Appl. Mater. Interfaces, 2023, 15, 20317–20324 CrossRef CAS PubMed.
- C. Rogers, W. S. Perkins, G. Veber, T. E. Williams, R. R. Cloke and F. R. Fischer, J. Am. Chem. Soc., 2017, 139, 4052–4061 CrossRef CAS PubMed.
- P. Su, W. Pei, X. Wang, Y. Ma, Q. Jiang, J. Liang, S. Zhou, J. Zhao, J. Liu and G. Q. Lu, Angew. Chem., Int. Ed., 2021, 60, 16044–16050 CrossRef CAS PubMed.
- J. Wang, X. Huang, S. Xi, J. M. Lee, C. Wang, Y. Du and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 13532–13539 CrossRef CAS PubMed.
- P. Xiong, P. Bai, A. Li, B. Li, M. Cheng, Y. Chen, S. Huang, Q. Jiang, X. H. Bu and Y. Xu, Adv. Mater., 2019, 31, 1904771 CrossRef CAS PubMed.
- Y. Liang, N. Song, Z. Zhang, W. Chen, J. Feng, B. Xi and S. Xiong, Adv. Mater., 2022, 34, e2202673 CrossRef PubMed.
- G. Li, W. Qiu, W. Gao, Y. Zhu, X. Zhang, H. Li, Y. Zhang, X. Wang and Z. Chen, Adv. Funct. Mater., 2022, 32, 2202853 CrossRef CAS.
- K. Zhou, S. Wang, X. Guo, G. Zhong, Z. Liu, Y. Ma, H. Wang, Y. Bao, D. Han and L. Niu, Small, 2022, 18, e2105770 CrossRef PubMed.
- Q. Zhang, J. Zhang, X. Wang, L. Li, Y. Li and W.-L. Dai, ACS Catal., 2021, 11, 6276–6289 CrossRef CAS.
- H. Wang, D. Wei, Y. He, H. Deng, B. Wu, L. Yan, H. Gang, Y. Cao, L. Jin and L. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 13177–13185 CrossRef CAS PubMed.
- B. Pu, Y. Liu, J. Bai, X. Chu, X. Zhou, Y. Qing, Y. Wang, M. Zhang, Q. Ma, Z. Xu, B. Zhou and W. Yang, ACS Nano, 2022, 16, 18746–18756 CrossRef CAS PubMed.
- P. Xiong, J. Wu, M. Zhou and Y. Xu, ACS Nano, 2020, 14, 1018–1026 CrossRef CAS PubMed.
- J. Liu, S. Zhao, C. Li, M. Yang, Y. Yang, Y. Liu, Y. Lifshitz, S. Lee and Z. Kang, Adv. Energy Mater., 2016, 6, 1502039 CrossRef.
- Y. Wang, S. Kattel, W. Gao, K. Li, P. Liu, J. G. Chen and H. Wang, Nat. Commun., 2019, 10, 1166 CrossRef PubMed.
- Y. Liu, Z. X. Lou, X. Wu, B. Mei, J. Chen, J. Y. Zhao, J. Li, H. Y. Yuan, M. Zhu, S. Dai, C. Sun, P. F. Liu, Z. Jiang and H. G. Yang, Adv. Mater., 2022, 34, 2202568 CrossRef CAS PubMed.
- Z. Wu, H. Wu, W. Cai, Z. Wen, B. Jia, L. Wang, W. Jin and T. Ma, Angew. Chem., Int. Ed., 2021, 60, 12554–12559 CrossRef CAS PubMed.
- Y. Shi, Y. Ji, J. Long, Y. Liang, Y. Liu, Y. Yu, J. Xiao and B. Zhang, Nat. Commun., 2020, 11, 3415 CrossRef CAS PubMed.
- C. Cao, D. D. Ma, J. F. Gu, X. Xie, G. Zeng, X. Li, S. G. Han, Q. L. Zhu, X. T. Wu and Q. Xu, Angew. Chem., Int. Ed., 2020, 59, 15014–15020 CrossRef CAS PubMed.
- X. Zhao, M. Huang, B. Deng, K. Li, F. Li and F. Dong, Chem. Eng. J., 2022, 437, 135114 CrossRef CAS.
- Q. Cheng, M. Huang, L. Xiao, S. Mou, X. Zhao, Y. Xie, G. Jiang, X. Jiang and F. Dong, ACS Catal., 2023, 13, 4021–4029 CrossRef CAS.
- S. Liu, Y. Fan, Y. Wang, S. Jin, M. Hou, W. Zeng, K. Li, T. Jiang, L. Qin, Z. Yan, Z. Tao, X. Zheng, C. Shen, Z. Liu, T. Ahmad, K. Zhang and W. Chen, Nano Lett., 2022, 22, 9107–9114 CrossRef CAS PubMed.
- Y. Jiao, Y. Zheng, K. Davey and S. Qiao, Nat. Energy, 2016, 1, 16130 CrossRef CAS.
- N. Han, Y. Wang, H. Yang, J. Deng, J. Wu, Y. Li and Y. Li, Nat. Commun., 2018, 9, 1320 CrossRef PubMed.
- Y. Zhao, X. Liu, Z. Liu, X. Lin, J. Lan, Y. Zhang, Y. R. Lu, M. Peng, T. S. Chan and Y. Tan, Nano Lett., 2021, 21, 6907–6913 CrossRef CAS PubMed.
- Y. X. Duan, Y. T. Zhou, Z. Yu, D. X. Liu, Z. Wen, J. M. Yan and Q. Jiang, Angew. Chem., Int. Ed., 2021, 60, 8798–8802 CrossRef CAS PubMed.
- B. Hammer, J. K. Norskov, B. C. Gates and H. Knozinger, Adv. Catal., 2000, 45, 71–129 CAS.
- G. Jia, Y. Wang, M. Sun, H. Zhang, L. Li, Y. Shi, L. Zhang, X. Cui, T. W. B. Lo, B. Huang and J. C. Yu, J. Am. Chem. Soc., 2023, 145, 14133–14142 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details and computational methods, XRD patterns, SEM images, XPS spectra, GC calibration curves, additional electrochemical test results, and DFT calculation results. See DOI: https://doi.org/10.1039/d3nr05853k |
| This journal is © The Royal Society of Chemistry 2024 |