Open Access Article
Open Access ArticleEnabling white color tunability in complex 3D-printed composites by using lead-free self-trapped exciton 2D perovskite/carbon quantum dot inks†
Tawanwit
Luangwanta
 ab,
Silver-Hamil
Turren-Cruz
ab,
Silver-Hamil
Turren-Cruz
 *ac,
Sofia
Masi
*ac,
Sofia
Masi
 a,
Samrat
Das Adhikari
a,
Samrat
Das Adhikari
 a,
Ileana B.
Recalde
a,
Ileana B.
Recalde
 a,
Marcileia
Zanatta
a,
Marcileia
Zanatta
 a,
Diego
Iglesias
a,
Jhonatan
Rodríguez-Pereira
a,
Diego
Iglesias
a,
Jhonatan
Rodríguez-Pereira
 d,
Santi
Gené-Marimon
d,
Santi
Gené-Marimon
 e,
Eugenia
Martinez-Ferrero
e,
Eugenia
Martinez-Ferrero
 e,
Sulawan
Kaowphong
e,
Sulawan
Kaowphong
 b,
Emilio
Palomares
b,
Emilio
Palomares
 ef,
Victor
Sans
ef,
Victor
Sans
 a,
Andrés F.
Gualdrón-Reyes
a,
Andrés F.
Gualdrón-Reyes
 *ag and
Iván
Mora-Seró
*ag and
Iván
Mora-Seró
 *a
*a
aInstitute of Advanced Materials (INAM), Universitat Jaume I (UJI), Avenida de Vicent Sos Baynat, s/n, 12071 Castelló de la Plana, Castellón, Spain. E-mail: silver.turren@gmail.com; andres.gualdron@uach.cl; sero@uji.es
bDepartment of Chemistry, Center of Excellence in Materials Science and Technology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
cDepartment of Physical Chemistry, Polish Academy of Sciences, Warsaw 01-224, Poland
dCenter of Materials and Nanotechnologies, Faculty of Chemical Technology, University of Pardubice, Nam. Cs. Legii 565, 53002 Pardubice, Czech Republic
eThe Institute of Chemical Research of Catalonia – CERCA (ICIQ-CERCA), Tarragona 43007, Spain
fICREA, Passeig Lluís Companys, 28, 08010 Barcelona, Spain
gFacultad de Ciencias, Instituto de Ciencias Químicas, Universidad Austral de Chile, Isla Teja, Valdivia, 5090000, Chile
First published on 22nd April 2024
Abstract
The generation of stable white light emission using lead-free perovskites remains a huge challenge in the development of future display and lighting technologies, due to fast material deterioration and the decrease of the color quality. In this work, we report a combination of diverse types of 2D A2SnX4 (A = bulky cation, X = Br, I) perovskites exhibiting self-trapped exciton (STE) emission and blue luminescent carbon quantum dots (CQDs), with the purpose of generating A2SnX4/CQD inks with a broadband emission in the visible region and a tunable white light color. By varying the concentration of the 2D perovskite, the white emission of the mixtures is modulated to cool, neutral, and warm tonalities, with a PL quantum yield up to 45%. From the combinations, the PEA2SnI4/CQD-based ink shows the longest stability, due to suitable surface ligand passivation provided by the capping ligands covering the CQDs, compensating the defect sites in the perovskite. Then, by incorporating the PEA2SnI4/CQDs inks into an acrylate polymer matrix, the quenching of the PL component from the perovskite was restrained, being stable for >400 h under ambient conditions and at a relative humidity of ∼50%, and allowing the preparation of complex 3D-printed composites with stable white emission tonalities. This contribution offers an application of STE-based Sn-perovskites to facilitate the future fabrication of lead-free white-light optoelectronic devices.
1. Introduction
For several years, halide perovskites (HPs) have favored the fast development of efficient photovoltaic and optoelectronic devices due to their fascinating intrinsic properties, including good light-absorption ability, suitable electronic properties, versatile surface chemistry, and high color purity.1–3 Therefore, these materials have been considered the most revolutionary semiconductors, reaching breakthroughs in operational efficiency in the above technologies. Recently, they have been good candidates for the fabrication of the next generation of liquid crystal display (LCD) prototypes,4,5 being able to produce high-quality white emission, with a better color rendering index (CRI) and a wide color gamut compared to commercial phosphor-based LCDs available on the market. However, the ionic nature of the HPs, especially those with a 3D structure, makes them prone to progressive degradation under moisture6 or photoirradiation,7 deteriorating their structural integrity. In addition, the presence of Pb is one of the most pivotal obstacles to the commercialization of these innovative materials due to the growing demand for the use of non-toxic elements and partial replacement of Pb from the HP structure.8 Among the most prominent candidates, Sn2+ is a suitable metal cation to substitute Pb2+, promoting the fabrication of potential lead-free Sn-HP-based devices.9–11 Unfortunately, Sn2+ is easily oxidized to Sn4+, generating Sn-vacancies and a high density of defects in the perovskite.12,13 These defect sites induce the formation of carrier traps that decrease the performance of the Sn-HP devices.14,15 Therefore, the incorporation of lead-free perovskites into the LCD technology to generate white-light emission is hindered by the fast deterioration of the structural integrity of the material, which would decrease the quality of the color, leading to low CRI values. Some alternative lead-free structures such as vacancy ordered double perovskites have been studied to generate stable white light through the appearance of a single broadband emission, where the color quality can be modulated as a function of the chemical composition.16,17 However, this feature would not allow the facile emergence of white color tonalities, which is a practical drawback, for instance, in image processing applications where an adequate color balance needs to be achieved.Therefore, the study of A2SnX4 structures (A = bulky alkylammonium cations, X = Br, I) has been highlighted to overcome the limitations in the use of Sn-HPs, attending to the fact that the organic species are located on the perovskite surface to give stability against moisture and water.18–20 In a typical 2D structure, bulky organic cations are beyond the tolerance limit, acting as spacers between the inorganic [SnX6]4− octahedral layers. This feature defines the optical, electronic, and carrier mobility properties of the 2D HPs.21,22 Although composition engineering can modify the band gap of the photomaterial (by varying the type of halide), the intercalation of the organic spacers with diverse geometries or organic functional moieties through the stacking layers can also favor the octahedral distortion,23 which is another way to control the photophysical properties. Interestingly, some A2SnX4 perovskites exhibit two kinds of emission features: (i) band-to-band (b–b) PL,24,25 where the electrons accumulated in the conduction band (CB) can recombine with photogenerated holes in the valence band (VB), making the absorption edge and PL spectrum with narrow full width at half maximum (FWHM) get close together (small Stokes shifted PL), and (ii) a broadband emission (wide FWHM) which emerges in the visible region even though the absorption edge of the 2D HPs is within the UV range. This is a characteristic of the formation of non-accessible located states in the wide bandgap of the perovskite, where photons are emitted with a lower energy than the band gap (large Stokes shifted PL). This phenomenon is called self-trapped exciton (STE),25–27 which has been used to prepare promising materials for solid-state lighting. Under this concept, the synthesis of alkylammonium tin bromide (OLA2SnBr4) microplates with a high PL quantum yield (PLQY) of ∼88% and the fabrication of orange-emitting LEDs with external quantum efficiencies of ∼0.1% have been reported.28 Other potential applications include the fabrication of luminescent solar concentrators, where the STE-based perovskites can absorb UV light to emit photons in the visible range, minimizing the self-absorption and providing transparency,29 adequate for solar windows. However, most applications involving 2D Sn-HPs require materials with optical features based on b–b transitions, ideal for charge transport and light emission, taking advantage of the fast emission from the band-edge carrier radiative relaxation.
Nevertheless, the appearance of a broadband emission from STE-based Sn-HPs would allow them to cover a wide range of the yellow-orange-red region from the energy spectrum, making them suitable candidates for the generation of white light emission in combination with other light-emitting nanomaterials. In a previous study, we used carbon quantum dots (CQDs) as suitable materials to combine with Pb-HPs (red-emitting CsPbI3), providing a broadband emission in the visible spectrum and favoring the generation of high-quality white color tonalities (cool-white, CW; neutral white, NW; and warm white, WW).30 In this work, we have combined STE-based A2SnX4 microcrystals with blue-emitting CQDs to obtain inks with modifiable white color emission. The variation of the Sn-HP content in the mixture allowed us to achieve CW, NW, and WW tonalities with a PLQY of up to 45%. Interestingly, by varying the separation distance between the [SnX6]4− layers composing the Sn-HPs, depending on the A-site cation, the modification of the emission mechanism can occur as well due to a dynamic interaction between the perovskite organic cation and the CQD organic shell.31 This premise can describe the fast or delayed deterioration of the white color quality in the prepared mixtures. After adding the A2SnX4/CQDs inks to an acrylate polymer matrix, composites were stable for >400 h under ambient conditions, at a relative humidity (RH) of ∼50%, allowing the fabrication of 3D-printed white light-emitting solids with complex shapes. These findings open the door to the utilization of STE-based 2D Sn-perovskites as a step forward to produce scalable lead-free white-light optoelectronic devices with desirable color tunability and quality.
2. Results and discussion
The nature of the organic spacer and halides influences the photophysical features of the perovskite samples. For this reason, two different 2D A2SnX4 perovskites through the colloidal hot-injection have been synthesized by modifying a procedure reported elsewhere.28 Using oleylammonium (OLA+) and phenethylammonium (PEA+) as A-site cations and bromine and iodine as the X halides, the following perovskite systems are obtained: OLA2SnBr4, OLA2SnI4 and PEA2SnI4. To obtain information about the morphology of the as-prepared perovskites, transmission electron microscopy (TEM) images were recorded (Fig. 1A–C). We observed a microplate-type shape in OLA2SnX4, with diameters of 4.1 ± 1.7 μm (OLA2SnI4) and 6.5 ± 2.5 μm (OLA2SnBr4), while PEA2SnI4 exhibited a microsheet-type form with a size of 4.9 ± 2.0 μm. The corresponding particle size distribution is shown in Fig. S1.† Then, as seen in the insets of Fig. 1A–C, the corresponding as-prepared perovskite dispersions generate a yellow-orange-red emission under UV irradiation. By measuring the optical properties of these materials (Fig. 1D), we evidenced the absorption edge/PL peak position at 316/620 nm, 330/640 nm, and 357/671 nm for OLA2SnBr4, PEA2SnI4, and OLA2SnI4, respectively, confirming the appearance of the intrinsic STE phenomenon as the main radiative recombination pathway for the 2D Sn-perovskites.25,28 This PL feature is maintained for 15 days under ambient air before the quenching starts (Fig. S2†). Additionally, the nature of the organic spacer and halides influences the perovskite samples’ photophysical features. The substitution of Br by I in OLA2SnX4 produces a redshift in the absorption edge and the PL peak position of the perovskite, narrowing its band gap.32 Due to the fact that halide substitution generates mainly a variation of electronic states coming from the VB (due to the replacement of Br 4p by I 5p orbitals),33 the VBM is closer to the STE energy levels, which would explain the displacement of the broadband emission to higher wavelengths. Furthermore, OLA2SnBr4 achieved a PLQY of 100%, while OLA2SnI4 exhibited a PLQY of 53% (Fig. 1E). This change is attributed to the emergence of defect sites in the [SnI6]4− octahedra,27 considering that the iodide species show a higher lability than that of Br ones to diffuse out from the perovskite.33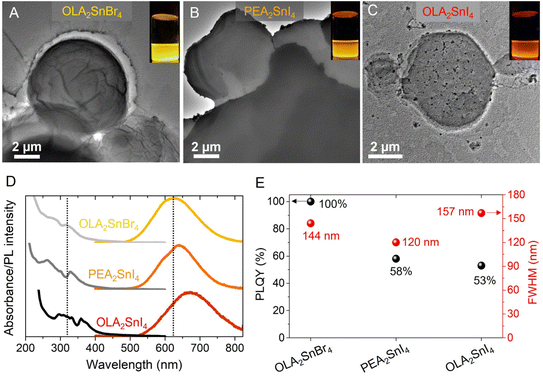 | ||
| Fig. 1 TEM images of the as-prepared (A) OLA2SnBr4, (B) PEA2SnI4, and (C) OLA2SnI4. (D) Typical absorption/PL spectra and (E) PLQY and PL FWHM of the corresponding A2SnX4 perovskite microcrystals. The insets of Fig. 1A–C show the STE-based emission of the 2D perovskites under 365 nm-UV illumination. | ||
On the other hand, substituting the OLA+ cation with PEA+ to prepare the PEA2SnI4 perovskite induces a slight blueshift of the Abs/PL emission features, distinguishing two different contributions in the PL spectrum. Even though OLA+ is a larger cation than PEA+, this aromatic cation has been reported to produce high octahedral distortion in [MX6]4− units, for instance, in the [PbI6]4− layers from PEA2PbI4, when the length of the equatorial Pb–I bonds is elongated.23 This fact extends the atomic overlapping between the metal and I orbitals, increasing the energy separation between the VB and the CB.34 The distortion of the inorganic Sn–I layers in the presence of PEA+ can explain the emergence of the two PL signals associated with isolated [SnI6]4− units (shorter wavelengths) and the interconnection of octahedra through the crystal edge (longer wavelengths).18 Then, PEA2SnI4 showed a PLQY of 58%, quite similar to that of OLA2SnI4. Lastly, the FWHM values of the 2D perovskites were determined to be 144, 157, and 120 nm for OLA2SnBr4, OLA2SnI4, and PEA2SnI4, respectively (Fig. 1E). This feature is very useful to cover a wide range of the yellow-orange-red region of the energy spectrum (Fig. 1D), mediating the generation of a broadband emission in all the visible region, in combination with luminescent CQDs, to produce white light (see below). From the XRD patterns of OLA2SnX4 and PEA2SnI4 perovskites, provided in Fig. S3 of the ESI,† a periodicity in the XRD peaks can be observed. This is the typical feature of a layered 2D perovskite structure (n = 1). By calculating the d-spacing through their corresponding periodicity values,21 we evidenced that the separation between the inorganic octahedral layers from OLA2SnI4 is smaller (2.8 nm) than that of OLA2SnBr4 (4.4 nm), ascribed to (i) the bigger size of [SnI6]4− than [SnBr6]4− units and (ii) the appearance of an A-site deficiency in the iodide perovskite. For PEA2SnI4, a d-spacing of 1.8 nm was achieved, larger than the reported value for red-emitting PEA2SnI4.35 We associate this discrepancy to some modifications in the intramolecular packing of the PEA+ cations from the 2D structure of the perovskite, which generates the octahedral distortion. The fact that OLA+ species induces a higher [SnX6]4− separation than PEA+ would give the possibility to an accelerated deterioration of the structural integrity of the OLA2SnX4 perovskites, which could explain the presence of cracks and black dots in their surface (Fig. 1A and C) and the fast quenching of the STE-PL intensity, compared to PEA2SnI4 (Fig. S2†).
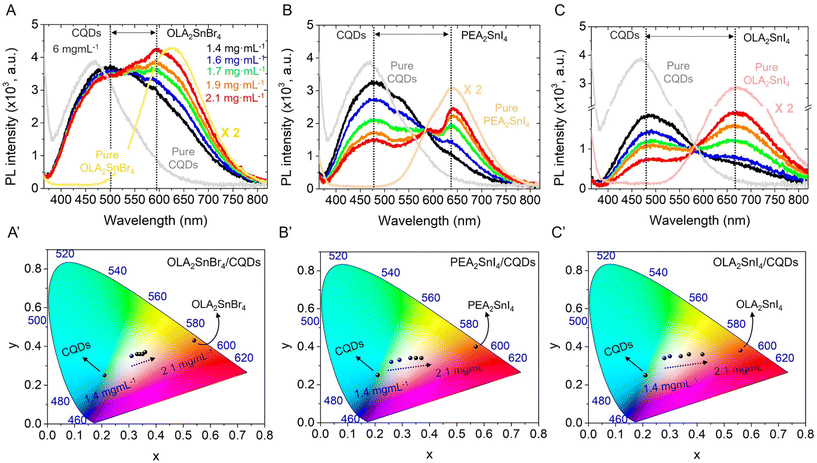
After studying the photophysical properties of the as-synthesized A2SnX4 perovskites, we proceeded to add different contents of these materials to a fixed concentration of the CQD colloidal solution (6 mg mL−1 in hexane), achieving the corresponding mixtures with perovskite concentrations varying from 1.4 to 2.1 mg mL−1. The morphology and optical properties of the CQDs are shown in Fig. S4 in the ESI†. Fig. 2A–C exhibits the PL spectra of the A2SnX4/CQDs inks (λexc = 350 nm), for which two main PL signals were achieved. A broad PL emission associated with the CQDs was centered at 480 nm, showing a redshift compared to the PL feature obtained for the solution of carbon nanoparticles. Meanwhile, the broadband emissions attributed to the presence of OLA2SnBr4 and OLA2SnI4 are located at ∼597 and 668 nm, respectively, presenting a slight blueshift in comparison with the PL emission of the pure perovskites. The variation of the optical features for CQDs and Sn-HPs before and after the combination indicates their dynamic interaction in the prepared mixtures. The displacement of the STE-based broadband emission to smaller wavelengths could result from octahedral distortion in the OLA2SnX4 systems,23 by the loss of labile OLA+ cations. In the case of PEA2SnI4, the negligible shift of the PL emission indicates the presence of more stable structural integrity in the perovskite during the preparation of mixtures.
Then, the increase in the perovskite content generates a higher STE-PL intensity in all the mixtures, allowing the modulation of the white-light emission in the CW, NW, and WW tonalities, as is evidenced in the Commission Internationale de l'Eclairage (CIE) color space (Fig. 2A′–C′) and the corresponding color temperature obtained from CIE coordinates (Fig. S5A, ESI†). However, the PL intensity of CQDs is slightly/strongly quenched depending on the nature of the perovskites. After adding the lowest content of the A2SnX4 microcrystals to the mixture (1.4 mg mL−1), we noted that the PL emission of CQDs decreases in the following order: OLA2SnI4/CQDs > PEA2SnI4/CQDs > OLA2SnBr4 (Fig. 2A–C). This trend agrees with the separation between the PL peak positions of CQDs and Sn-HPs, which the OLA2SnI4/CQD system depicts as the largest. In this scenario, we suggest the charge transfer from energy levels contained from CQDs (from larger wavelengths) to the perovskite after photon absorption,30 which mediates the decrease of PL in CQDs and favors the STE mechanism in the 2D perovskites. This process tends to increase the PLQY in the OLA2SnX4/CQD inks, independently of the bare CQD PL (Fig. S5B, ESI†). Surprisingly, for the PEA2SnI4/CQD system, the PLQY value is almost similar even when more perovskite was added to the ink, deducing that a different interaction between CQDs and PEA2SnI4 was occurring in the mixture. At this point, we can conclude that white light emission coordinates obtained from the combination between CQDs and A2SnX4 can be controlled, suggesting that the charge transfer towards the perovskite improves the STE pathway. This fact preserves or increases the white color quality of the ink.
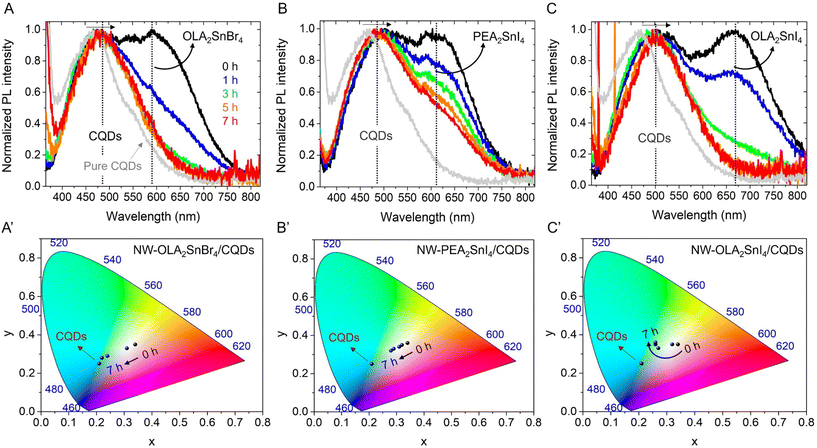
To provide a wide analysis of the stability of the different A2SnX4/CQDs inks, we measured the PL properties of the corresponding mixtures with the NW light emission as a function of time (after 7 h) under ambient air and RH ∼ 50%. We have chosen this color tonality as both the PL peaks for the CQDs and Sn-HP components show a similar initial intensity. As seen in Fig. 3A and C, the PL intensity of OLA2SnX4 rapidly quenches after 3 h under exposure to the environment. Taking into account that OLA+ cations exhibit a weak binding capability to the perovskite structure36 and also produce a significant separation between the inorganic [SnX6]4− octahedral layers (observed from the XRD results), we suggest that the 2D structure can eventually deteriorate, promoting the loss of the STE feature in the material. Simultaneously, the PL emission intensity of the CQDs also decreased in the presence of these OLA2SnX4 perovskites (Fig. S6A and C, ESI†), influencing the PLQY of the samples after 7 h. For OLA2SnBr4/CQDs and OLA2SnI4/CQD systems, the PLQY values decreased from 39 and 22%, respectively, to ∼6% (Fig. S6D, ESI†). Considering that the pure CQDs show a stable PLQY of ∼33% after 7 h, the PL of the above mixtures is quenched due to defect states from the Sn-HPs, and the CIE color did not reach the respective feature of the pure carbon nanoparticles (Fig. 3A′ and C′). This means that the optical performance of the CQDs has been modified by a weakened degree of the ligand interaction with the perovskite system (organic shell affects the carrier recombination mechanism in the CQDs and passivates or not possible in-gap states on the surface of the emitting core).31,37 We hypothesize a direct interaction between OLA2SnX4 perovskites and the hexadecylamine (HDA)/citric acid (CA) shell covering the CQD surface, considering the redshift observed for the PL component of the carbon nanoparticles (Fig. 3A and C). Interestingly, for PEA2SnI4/CQDs, the STE-PL quenching and the shift in the CIE tonality are delayed (Fig. 3B and B′), observing a gradual improvement of the PL contribution from CQDs (Fig. S6B, ESI†) and a higher PLQY than that of the OLA2SnX4/CQD mixtures over time (Fig. S6D, ESI†). This is an indication of better stability of this type of perovskite–CQD combination for a long time. Thus, HDA and CA and PEA+ can favor the stabilization/passivation of the PEA2SnI4–CQD system, maintaining the STE features for a longer time.
In this scenario, it has been reported that the PL mechanism of CQDs is mainly dominated by the surface states with different energy levels (some of them closer to the ground state from CQDs), provided by surface functional groups such as –COOH and –NH2, to lead the electron radiative relaxation.38,39 In this context, the higher the density of surface oxygenated groups in the CQDs, the higher the density of surface states contained in the nanoparticles to capture excitons.39 The presence of these states favors the electron transfer from the carbon core, where direct radiative recombination occurs. In the case of a high density of surface NH2 groups, lone pairs in N atoms can fill surface states and reduce their energy separation (emergence of continuous energy levels), causing the energy gap to narrow between the VB and the CB of CQDs.37,38 Accordingly, in addition to both of the possibilities for the carrier recombination in CQDs under a high density of (i) –COOH and (ii) –NH2, (our CQDs contain HDA and CA on the surface), a charge transfer process could be promoted from CQDs to STE levels from the A2SnX4 perovskites to increase their PL properties (Scheme S1, ESI†). Therefore, we infer that the lowering and redshift of the PL contribution from the CQDs in the presence of OLA2SnX4 would lead to a decrease in the density of surface –COOH and –NH2 functionalities in the CQDs–OLA2SnX4 interaction, reducing the content of surface states and electron density from N atoms for charge transfer. Conversely, the eventual increase of the PL emission of CQDs in the presence of PEA2SnI4 over time would indicate that the radiative relaxation into CQDs is gradually facilitated by the deterioration of the PL properties in the perovskite, without affecting widely the CQD surface.
With the purpose of obtaining valuable information on the interaction between CQDs and the PEA2SnI4 perovskite, we recorded the PL spectra of the PEA2SnI4/CQDs inks showing CW, NW, and WW light emission for 48 h under air and RH ≈ 50% (Fig. S7A–C, ESI†). Similar to the result shown in Fig. 3B, the PL contribution of the Sn-HPs was progressively decreased in all the mixtures, while the PL contribution of the CQDs eventually increased. A CW tonality with a different CIE color from the one generated by pure CQDs was obtained in the inks (Fig. S7A′–C′†), with a higher PLQY along the time (Fig. S5D, ESI†). Independent of the perovskite content added to the mixture, the PL emission of the CQDs improves, deducing that PEA2SnI4 does not alter largely the CQD surface. Then, through time-resolved PL (TRPL) measurements, we analyze the carrier recombination dynamics of the PEA2SnI4/CQD inks by varying the aging time. For this analysis, we have chosen the WW-light emitting ink as a representative sample; see Fig. S8, ESI.† A biexponential equation y fitted all the TRPL curves obtained in the function of aging time 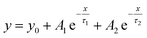 , to determine the corresponding PL lifetimes, τavg30 (Table S1, ESI†). The sample was irradiated with a 405 nm pulsed laser, fixing the PL emission of the CQDs at 480 nm. In this way, we only guarantee the abs/emission process of the CQDs. Typically, for pure CQDs, τ1 and τ2 are associated with the direct radiative recombination at the CQD surface and the carrier recombination from the CQD core to the surface, respectively.40,41 However, considering the CQD–perovskite interaction, we have assigned τ2 to the charge transfer from the carbon nanoparticles to PEA2SnI4. Accordingly, the longer the aging time, the higher the τ1, with a higher weight of the first component (A1). This behavior indicates that the carrier recombination in the CQD surface is progressively favored once the STE emission of the perovskite microcrystals decreases over time, which can explain the increase of the PL emission and PLQY from CQDs (Fig. S7, ESI†).
, to determine the corresponding PL lifetimes, τavg30 (Table S1, ESI†). The sample was irradiated with a 405 nm pulsed laser, fixing the PL emission of the CQDs at 480 nm. In this way, we only guarantee the abs/emission process of the CQDs. Typically, for pure CQDs, τ1 and τ2 are associated with the direct radiative recombination at the CQD surface and the carrier recombination from the CQD core to the surface, respectively.40,41 However, considering the CQD–perovskite interaction, we have assigned τ2 to the charge transfer from the carbon nanoparticles to PEA2SnI4. Accordingly, the longer the aging time, the higher the τ1, with a higher weight of the first component (A1). This behavior indicates that the carrier recombination in the CQD surface is progressively favored once the STE emission of the perovskite microcrystals decreases over time, which can explain the increase of the PL emission and PLQY from CQDs (Fig. S7, ESI†).
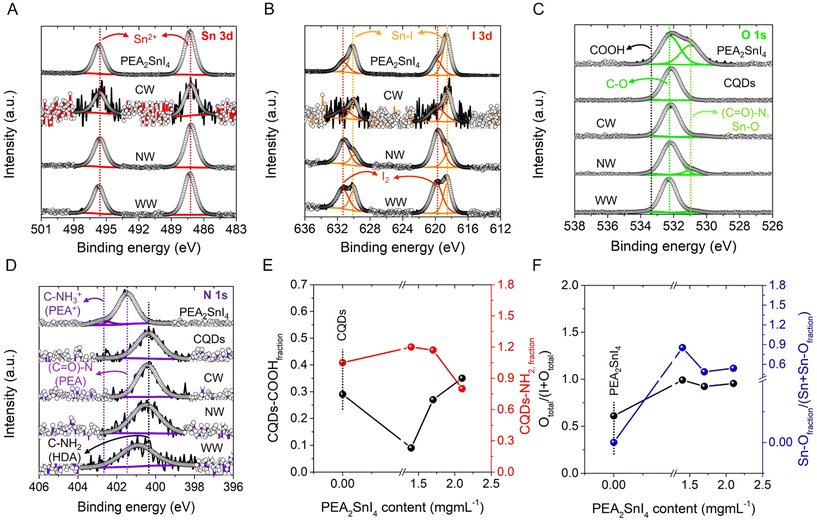
To understand the surface chemical environment and composition of the PEA2SnI4/CQDs inks, we carried out X-ray photoelectron spectroscopy (XPS). In all the mixtures and pure PEA2SnI4 microcrystals, we identified the co-existence of C, O, N, Sn, and I; see XPS survey spectra in Fig. S9, ESI.† The surface chemistry of CQDs was also analyzed for comparative purposes. The corresponding speciation of each sample is shown in Table S2, ESI.†Fig. 4A shows the high-resolution (HR) XPS Sn 3d spectra of the PEA2SnI4/CQD inks with different white light tonalities and the individual perovskite, where a doublet centered at 487/496 eV was observed. These signals are ascribed to the presence of Sn2+ from the [SnI6]4− octahedra composing the 2D perovskite.42 On the other hand, two kinds of contributions are observed from the HR XPS I 3d spectra of the pure PEA2SnI4 and PEA2SnI4/CQDs samples, as exhibited in Fig. 4B. The first I 3d doublet at 618/630 eV is associated with the iodide species from the inorganic octahedral units, forming Sn–I bonds, while the couple centered at 620/631 eV is attributed to the existence of molecular iodine (I2).42 The appearance of this species indicates that a part of the iodide fraction is released from the inorganic layers, generating halide vacancies in the perovskite. Then, according to Table S2,† the I2 fraction (described as the (I2/Sn + I2) ratio) is lower for the inks than that of the pure PEA2SnI4 perovskite, deducing that the presence of CQDs is pivotal to keeping the structural integrity of the Sn-HPs. By comparing the PEA2SnI4/CQD mixtures, the NW light-emitting one shows the lowest I2 fraction, suggesting this combination as the scenario where CQDs promote a suitable protective effect to prevent the formation of iodide vacancies. In contrast, the WW-light-emitting ink exhibits a higher I2 fraction than the NW light-emitting one, inferring that more iodide was available to diffuse out from the perovskite structure and produce defect sites.
Fig. 4C shows the HR XPS O 1s spectra of PEA2SnI4, CQD, and the PEA2SnI4/CQD inks, with some critical differences. While pure PEA2SnI4 depicts two contributions at 531 and 532 eV, ascribed to the formation of (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N or Sn–O (these signals can be overlapped) and C
O)–N or Sn–O (these signals can be overlapped) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively,43 CQDs exhibit the co-existence of C
O bonds, respectively,43 CQDs exhibit the co-existence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH moieties, at 532 and 533 eV,44,45 respectively. In the case of CQDs, the obtained signals are attributed to the presence of a carboxyl functional group (R-COOH) provided by CA, used to synthesize the nanoparticles. Then, the contributions of C
O and COOH moieties, at 532 and 533 eV,44,45 respectively. In the case of CQDs, the obtained signals are attributed to the presence of a carboxyl functional group (R-COOH) provided by CA, used to synthesize the nanoparticles. Then, the contributions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH bonds were also detected for all the inks, with the appearance of a new signal ∼530 eV corresponding to the Sn–O bond46 and without the (C
O and COOH bonds were also detected for all the inks, with the appearance of a new signal ∼530 eV corresponding to the Sn–O bond46 and without the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N interaction. Lastly, we revealed the typical XPS N 1s spectra for PEA2SnI4, CQDs and the inks (Fig. 4D), detailing two prominent peaks for the pure perovskite located at 401 and 403 eV. Taking into account the XPS N 1s assignations for another type of alkylammonium species, for instance, octylammonium cations,25 these peaks can be associated with (C
O)–N interaction. Lastly, we revealed the typical XPS N 1s spectra for PEA2SnI4, CQDs and the inks (Fig. 4D), detailing two prominent peaks for the pure perovskite located at 401 and 403 eV. Taking into account the XPS N 1s assignations for another type of alkylammonium species, for instance, octylammonium cations,25 these peaks can be associated with (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N and C–NH3+ species, coming from the PEA+ cations composing the 2D perovskite. Nevertheless, only a single peak for the CQDs and the PEA2SnI4/CQD mixtures was noted centered at ∼400 eV, attributed to C–NH2 from the hexadecylamine (HDA) ligand covering the carbon nanoparticles.47 The co-existence of (C
O)–N and C–NH3+ species, coming from the PEA+ cations composing the 2D perovskite. Nevertheless, only a single peak for the CQDs and the PEA2SnI4/CQD mixtures was noted centered at ∼400 eV, attributed to C–NH2 from the hexadecylamine (HDA) ligand covering the carbon nanoparticles.47 The co-existence of (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N, C
O)–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH3+ species in the pure perovskite indicates the generation of phenethylammonium oleate (PLM). As we have reported previously, by using high temperatures to prepare perovskites through colloidal synthesis in the presence of an amine and a carboxylic acid, the corresponding alkylammonium carboxylate species is formed, being the primary ligand to stabilize the final product.48 Then, from the 1H NMR spectra recorded for the pure PEA2SnI4 and the PEA2SnI4/CQD mixtures (Fig. S10, ESI†), a triplet at δ ∼ 2.08 ppm was observed, associated with CH2– near the COOH functionality from free OA.36,49 Additionally, a signal is observed at a similar chemical shift for pure CQDs, attributed to the presence of CH2– coming from the R-COOH moiety provided by CA. Simultaneously, the signals at δ ∼ 3.01 ppm and 1.55 ppm are assigned to CH2– and the proton bonded to NH2 from phenethylamine (PEA), respectively. The co-existence of OA and PEA reinforces the hypothesis about the formation of PLM during the perovskite synthesis (see explanation below).
O and NH3+ species in the pure perovskite indicates the generation of phenethylammonium oleate (PLM). As we have reported previously, by using high temperatures to prepare perovskites through colloidal synthesis in the presence of an amine and a carboxylic acid, the corresponding alkylammonium carboxylate species is formed, being the primary ligand to stabilize the final product.48 Then, from the 1H NMR spectra recorded for the pure PEA2SnI4 and the PEA2SnI4/CQD mixtures (Fig. S10, ESI†), a triplet at δ ∼ 2.08 ppm was observed, associated with CH2– near the COOH functionality from free OA.36,49 Additionally, a signal is observed at a similar chemical shift for pure CQDs, attributed to the presence of CH2– coming from the R-COOH moiety provided by CA. Simultaneously, the signals at δ ∼ 3.01 ppm and 1.55 ppm are assigned to CH2– and the proton bonded to NH2 from phenethylamine (PEA), respectively. The co-existence of OA and PEA reinforces the hypothesis about the formation of PLM during the perovskite synthesis (see explanation below).
Having described the chemical environment of the samples and according to Table S2,† it is notable that adding PEA2SnI4 significantly decreases the –COOH content in the CQDs. However, once the perovskite content is higher in the mixture, the –COOH content is increased, and simultaneously, the C–NH2 content is decreased; see Fig. 4E. The formation of phenethylammonium oleate can be promoted through an acid–base reaction between PEA and OA as given in eqn (1):
| C8H9NH2 + C17H33COOH ⇌ C8H9NH3+ + C17H33COO−. | (1) |
We propose that carboxyl ligands covering the CQDs can react with PEA species to favor the generation of carboxylate anions (R-COO−) and the formation of PEA+, see eqn (2). This can elucidate the marked decrease in the initial COOH fraction in the presence of the lowest perovskite content. However, a higher free OA fraction would also be achieved, explaining the eventual increase in the –COOH content in the mixtures even higher than that of the individual CQDs. This –COOH species could be responsible for promoting the progressive increase in the PL emission of CQDs after the delayed deterioration of PEA2SnI4.
| C8H9NH2 + CQDs–COOH ⇌ C8H9NH3+ + CQDs–COO−. | (2) |
Simultaneously, some OA fractions can react with HDA ligands to induce the formation of HDA+ species, see eqn (3), which are available to fill/replace some A-site cations from the 2D perovskite:
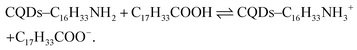 | (3) |
Taking into account the particle size of CQDs (3.4 ± 0.7 nm, estimated by TEM), this is a bigger nanoparticle in comparison to the separation between the [SnI6]4− layers from PEA2SnI4 (1.8 nm, obtained from XRD). Therefore, we conclude that CQDs can promote the surface passivation of defect sites in the PEA2SnI4 perovskite, providing a low fraction of –COOH and –NH2 moieties without significantly compromising their PL properties. This deduction can also explain the interaction between the OLA2SnX4 systems and CQDs. Considering that the d-spacings for OLA2SnBr4 and OLA2SnI4 systems are 4.4 and 2.8 nm, respectively (Fig. S3†), these values are larger than the size of CQDs, with the integration of CQDs between the OLA2SnX4 inorganic layers being plausible. This fact leads to the deterioration of the 2D structure and PL quenching of CQDs. Therefore, we conclude that the PEA2SnI4 surface is covered by CQDs, protecting the structural integrity for a longer time. At this point, we deduce that the nature of the A-site cation is the most important parameter that affects the stability of the ink, mediating the internal interaction between the 2D structure and CQDs or externally through passivation of surface defects in the perovskite.
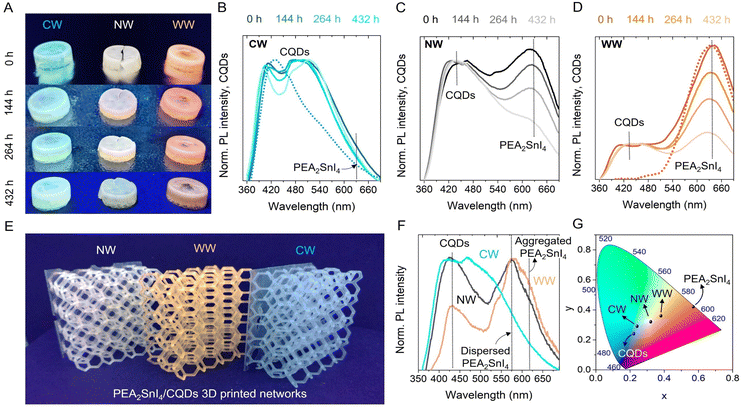
Once the explanation about the effect of CQDs on the defect passivation of Sn-HPs was provided, we analyzed the possibility of combining the CW, NW and WW light-emitting PEA2SnI4/CQDs inks with a polymeric matrix and prepared 3D printed solid composites with geometrical complex shapes and films. To this end, we introduced the luminescent inks into a mixture of acrylate monomers such as butylacrylate (BA) and isobornyl acrylate (IBA) (using 15 mol% BA and 85 mol% IBA), using 1,4-butanediol diacrylate and diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide as the crosslinker agent and the UV-initiator, respectively. This allowed suitable plasticization of the final polymeric composite, as we have reported previously for other kinds of luminescent perovskite-based 3D solids.36 As seen in Fig. S11,† both pure CQDs and PEA2SnI4 were embedded into the acrylate matrix, producing the corresponding blue- and orange-emitting 3D solids. In this context, with the aim to study the changes in the PL intensity coming from the mixture between CQDs and the different contents of Sn-perovskite in the polymeric matrix, the PL properties of the PEA2SnI4/CQD acrylate composites in the form of pellets were obtained over time (Fig. 5A). In this way, the PLQY values of the CW-, NW- and WW-emitting PEA2SnI4/CQDs pellets were estimated to be 6%, 10% and 8%, respectively.
Unlike the previous discussion of the inks, where the PL emission associated with the STE properties of the perovskite is quenched after 48 h, the presence of the acrylate matrix extends the appearance of the STE emission up to 436 h under ambient air and HR ≈ 50%. This fact causes a slower modification in the PL feature, see Fig. 5B–D, and the CIE color of each ink, see Fig. S12A–C, ESI.† Although CQDs can delay the progressive deterioration of the STE emission by passivating defects in the structure of PEA2SnI4, the Sn2+-to-Sn4+ oxidation cannot be totally avoided. Accordingly, the acrylate-based polymer protects the perovskite structure against oxygen and moisture,50 hindering the fast metal oxidation and favoring the preservation of the white color emission. Interestingly, the CW-light emitting ink in both inks and composite systems shows the most stable color tonality, deducing that at the lowest perovskite content, CQDs can efficiently protect their integrity and intrinsic properties, as we observed the highest Sn–O fraction in this material through the XPS. Then, NW and WW-light emitting inks show a lower iodide defect content, achieving a decrease in the Sn–O fraction, but it is more likely to facilitate the I2 release (being a pivotal reason to quench the PL of the perovskite). Lastly, it is possible to prepare more complex and well-defined luminescent 3D-printed shapes, as is the case of small and big polymeric networks; see Fig. S13, ESI† and Fig. 5E. Here, the prepared emissive inks are homogeneously distributed along the entire 3D composite to provide the three white-light tonalities (Fig. 5F and G), hindering the agglomeration of the perovskite. In conclusion, the combination of CQDs and Sn-HPs, such as PEA2SnI4, can mediate the preparation of PEA2SnI4/CQD mixtures, ideal for preparing suitable inks for the fabrication of luminescent 3D printed polymeric composites with tunable white-light emission.
3. Conclusions
In this work, we have shown the combination of different kinds of Sn-HP microcrystals with CQD colloidal solutions to generate a series of white-light emitting inks with modifiable color tonality. By studying the nature of the A-site cation in the A2SnX4/CQDs inks, we observed that the Sn-HPs based on OLA2SnX4 exhibit a fast quenching of their PL emission, deteriorating the white light color stability. Taking into account the weak binding capability of OLA+ and the large separation between the inorganic [SnX6]4− layers provided by this ligand, CQDs are prone to access and interact with the internal octahedral units coming from the OLA2SnX4 perovskites, through a high density of surface functional groups such as –COOH and –NH2. This promotes the loss of structural integrity and STE features in the perovskite and PL quenching of CQDs. In contrast, Sn-HPs with smaller d-spacing such as PEA2SnI4 depict a more stable white-light emission, helping a low fraction of –COOH and –NH2 moieties covering CQDs mediate the surface passivation of the perovskite. In this way, the structure and STE properties are maintained for a longer time, without altering the PL properties of CQDs. On comparison of the prepared PEA2SnI4/CQD inks, the NW-light emitting one is found to exhibit the lowest halide defect content in the perovskite, carrying out an efficient ligand passivation process. By increasing the PEA2SnI4 microcrystal content to obtain the WW light-emitting ink, the iodide fraction is increased, being more likely to favor the I2 release and the deterioration of the STE features in the perovskite. Interestingly, although the highest defect site content is shown in the CW-light emitting ink, this combination provides the most stable white color tonality, associated with a full compensation of halide vacancies through the formation of Sn–O bonds from R-COO− moieties covering the CQDs. To preserve the intrinsic properties of the inks, an acrylate based polymeric matrix is pivotal, preventing the Sn oxidation by ligand detachment and facilitating the fabrication of white color luminescent 3D-printed composites with complex shapes. Therefore, we highlight an interesting strategy for the processability of lead-free perovskites with STE emission, being prominent for promoting the fabrication of scalable white light emitting devices.Author contributions
A. F. G.-R. and S.-H. T.-C. designed the experiments. T. L. and A. F. G.-R. synthesized and characterized the A2SnX4 perovskites and CQDs. S. G.-M., E. M.-F. and E. P. synthesized and characterized the CQDs. S.-H. T.-C., S. M. and S. D. A. contributed to the analysis of materials characterization. J. R.-P. contributed to the XPS measurements and analysis. M. Z. designed and performed the NMR analysis. V. S. coordinated the experiments with polymeric matrices. I. R. formulated and stabilized the polymeric composite, prepared the 3D composite structures and performed the corresponding characterization studies of all the composites. I. R. and D. I. fabricated the PNC-composites. D. I. designed the 3D structures and optimized the printing parameters. S.-H. T.-C., A. F. G.-R. and I. M.-S. conceived the idea and oversaw the project. All authors contributed to writing the manuscript and the discussions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Innovation of Spain (MCIN/AEI/10.13039/501100011033/), the FEDER “Una manera de hacer Europa” under Projects She-LED (PID2021-122960OA-I00), Step-Up (TED2021-131600B-C31), project ELECTROVOLT (PID2022-139866NB-I00) and by Generalitat Valenciana via the PROMETEO project Q-Solutions (CIPROM/2021/078). We thank the European Union's Horizon 2020 research and the Ministry of Education, Youth and Sports of the Czech Republic for the financial support of XPS measurements using the CEMNAT infrastructure (projects LM2018103 and LM2023037). S.-H. T. C. would like to thank the Spanish Ministry of Economy, Industry and Competitiveness (postdoctoral contract Juan de la Cierva Formación FJC2019-041835-I) and The National Science Centre-POLONES BIS 1 (DEC-2021/43/P/ST5/01780) for the financial support during this work. S. M. acknowledges financial support from MICINN (Spain) through the program Juan de la Cierva-Incorporatión (IJC2020-042618-I). M. Z. acknowledges the financial support from the innovation programme under the Marie Skłodowska-Curie Individual Fellowships (GA no. 101026335). V. S. thanks Generalitat Valenciana (CIDEGENT 2018/036) and UJI (B-2020-44) for funding. T. L. and S. K. thank the Institute for the Promotion of Teaching Science and Technology (IPST) and Chiang Mai University. A. F. G.-R. acknowledges to ANID through the FONDECYT Iniciación Project (Grant no. 11240161). The authors are very grateful to the “Serveis Centrals d'Instrumentació Científica (SCIC)” of the Universitat Jaume I.References
- J. S. Kim, J.-M. Heo, G.-S. Park, S.-J. Woo, C. Cho, H. J. Yun, D.-H. Kim, J. Park, S.-C. Lee, S.-H. Park, E. Yoon, N. C. Greenham and T.-W. Lee, Nature, 2022, 611, 688–694 CrossRef CAS PubMed.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- A. Dey, J. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Gao, X. Li, J. Shamsi, T. Debnath, M. Cao, M. A. Scheel, S. Kumar, J. A. Steele, M. Gerhard, L. Chouhan, K. Xu, X.-g. Wu, Y. Li, Y. Zhang, A. Dutta, C. Han, I. Vincon, A. L. Rogach, A. Nag, A. Samanta, B. A. Korgel, C.-J. Shih, D. R. Gamelin, D. H. Son, H. Zeng, H. Zhong, H. Sun, H. V. Demir, I. G. Scheblykin, I. Mora-Seró, J. K. Stolarczyk, J. Z. Zhang, J. Feldmann, J. Hofkens, J. M. Luther, J. Pérez-Prieto, L. Li, L. Manna, M. I. Bodnarchuk, M. V. Kovalenko, M. B. J. Roeffaers, N. Pradhan, O. F. Mohammed, O. M. Bakr, P. Yang, P. Müller-Buschbaum, P. V. Kamat, Q. Bao, Q. Zhang, R. Krahne, R. E. Galian, S. D. Stranks, S. Bals, V. Biju, W. A. Tisdale, Y. Yan, R. L. Z. Hoye and L. Polavarapu, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS PubMed.
- Z. Liu, L. Sinatra, M. Lutfullin, Y. P. Ivanov, G. Divitini, L. De Trizio and L. Manna, Adv. Energy Mater., 2022, 12, 2201948 CrossRef CAS.
- Q. Pan, J. Fu, S. Liu, J. Zhou, B. Ma, S. Chen, Y. Qiu, Y. Lin, Y. Hu, D. Yang, J. Chen, M.-K. Fung, Y. Wang, Q. Zhang, L. Wang and M. Cao, Cell Rep. Phys. Sci., 2023, 4, 101275 CrossRef CAS.
- Z. Zheng, L. Liu, F. Yi and J. Zhao, J. Lumin., 2019, 216, 116722 CrossRef CAS.
- R. An, F. Zhang, X. Zou, Y. Tang, M. Liang, I. Oshchapovskyy, Y. Liu, A. Honarfar, Y. Zhong, C. Li, H. Geng, J. Chen, S. E. Canton, T. Pullerits and K. Zheng, ACS Appl. Mater. Interfaces, 2018, 10, 39222–39227 CrossRef CAS PubMed.
- F. Gao, X. Zhu, Q. Feng, W. Zhong, W. Liu, H. Xu and Y. Liu, Nano Energy, 2022, 98, 107270 CrossRef CAS.
- Y. Wang, R. Zou, J. Chang, Z. Fu, Y. Cao, L. Zhang, Y. Wei, D. Kong, W. Zou, K. Wen, N. Fan, N. Wang, W. Huang and J. Wang, J. Phys. Chem. Lett., 2019, 10, 453–459 CrossRef CAS PubMed.
- F. Yuan, X. Zheng, A. Johnston, Y.-K. Wang, C. Zhou, Y. Dong, B. Chen, H. Chen, J. Z. Fan, G. Sharma, P. Li, Y. Gao, O. Voznyy, H.-T. Kung, Z.-H. Lu, O. M. Bakr and E. H. Sargent, Sci. Adv., 2020, 6, eabb0253 CrossRef CAS PubMed.
- J. Sanchez-Diaz, R. S. Sánchez, S. Masi, M. Kreĉmarová, A. O. Alvarez, E. M. Barea, J. Rodriguez-Romero, V. S. Chirvony, J. F. Sánchez-Royo, J. P. Martinez-Pastor and I. Mora-Seró, Joule, 2022, 6, 861–883 CrossRef CAS PubMed.
- T. Mahmoudi, W.-Y. Rho, M. Kohan, Y. H. Im, S. Mathur and Y.-B. Hahn, Nano Energy, 2021, 90, 106495 CrossRef CAS.
- H. Cao, Z. Zhang, M. Zhang, A. Gu, H. Yu, H. Ban, Q. Sun, Y. Shen, X. L. Zhang, J. Zhu and M. Wang, Mater. Today Phys., 2021, 21, 100513 CrossRef CAS.
- Z. Zhang, Y. Huang, J. Jin, Y. Jiang, Y. Xu, J. Zhu and D. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202308093 CrossRef CAS PubMed.
- P. Li, X. Cao, J. Li, B. Jiao, X. Hou, F. Hao, Z. Ning, Z. Bian, J. Xi, L. Ding, Z. Wu and H. Dong, Nano-Micro Lett., 2023, 15, 167 CrossRef CAS PubMed.
- C. Chen, J. Xiang, Y. Chen, M. Jin, J. Zheng, N. Zhang and C. Guo, Ceram. Int., 2022, 48, 1851–1856 CrossRef CAS.
- F. Zhang, Z. Chen, Z. Liu, M. Jia, X. Chen, D. Wu, X. Li and Z. Shi, J. Lumin., 2022, 251, 119150 CrossRef CAS.
- V. V. Nawale, T. Sheikh and A. Nag, J. Phys. Chem. C, 2020, 124, 21129–21136 CrossRef CAS.
- E. Jokar, P.-Y. Cheng, C.-Y. Lin, S. Narra, S. Shahbazi and E. Wei-Guang Diau, ACS Energy Lett., 2021, 6, 485–492 CrossRef CAS.
- C. Gao, Z. Hu, C. Yang, H. Xu, Y. Wang, J. Zhang and Y. Zhu, Org. Electron., 2019, 74, 126–134 CrossRef CAS.
- M. C. Weidman, M. Seitz, S. D. Stranks and W. A. Tisdale, ACS Nano, 2016, 10, 7830–7839 CrossRef CAS PubMed.
- T. Zhang, Q. Sun, X. Zhang, Y. Shen and M. Wang, APL Mater., 2021, 9, 020906 CrossRef CAS.
- E. Mahal, S. C. Mandal and B. Pathak, Mater. Adv., 2022, 3, 2464–2474 RSC.
- S. Li, J. Luo, J. Liu and J. Tang, J. Phys. Chem. Lett., 2019, 10, 1999–2007 CrossRef CAS PubMed.
- S. Ghimire, K. Oldenburg, S. Bartling, R. Lesyuk and C. Klinke, ACS Energy Lett., 2022, 7, 975–983 CrossRef CAS.
- Y. Han, X. Cheng and B.-B. Cui, Mater. Adv., 2023, 4, 355–373 RSC.
- Y. Chen, Z. Wang, Y. Wei, Y. Liu and M. Hong, Angew. Chem., Int. Ed., 2023, 62, e2023016 Search PubMed.
- X. Zhang, C. Wang, Y. Zhang, X. Zhang, S. Wang, M. Lu, H. Cui, S. V. Kershaw, W. W. Yu and A. L. Rogach, ACS Energy Lett., 2018, 4, 242–248 CrossRef.
- L. Zdražil, S. Kalytchuk, M. Langer, R. Ahmad, J. Pospíšil, O. Zmeškal, M. Altomare, A. Osvet, R. Zbořil, P. Schmuki, C. J. Brabec, M. Otyepka and Š. Kment, ACS Appl. Energy Mater., 2021, 4, 6445–6453 CrossRef.
- R. R. Rad, A. F. Gualdrón-Reyes, S. Masi, B. A. Ganji, N. Taghavinia, S. Gené-Marimon, E. Palomares and I. Mora-Seró, Adv. Opt. Mater., 2020, 9, 2001508 CrossRef.
- S. Tao, C. Zhou, C. Kang, S. Zhu, T. Feng, S.-T. Zhang, Z. Ding, C. Zheng, C. Xia and B. Yang, Light: Sci. Appl., 2022, 11, 56 CrossRef CAS PubMed.
- L. Lanzetta, J. M. Marin-Beloqui, I. Sanchez-Molina, D. Ding and S. A. Haque, ACS Energy Lett., 2017, 2, 1662–1668 CrossRef CAS.
- A. F. Gualdrón-Reyes, J. Rodríguez-Pereira, E. Amado-González, J. Rueda-P, R. Ospina, S. Masi, S. J. Yoon, J. Tirado, F. Jaramillo, S. Agouram, V. Muñoz-Sanjosé, S. Giménez and I. Mora-Seró, ACS Appl. Mater. Interfaces, 2020, 12, 914–924 CrossRef PubMed.
- M. Javed, A. Noureddine and M. Benkraouda, Mater. Sci. Semicond. Process., 2023, 162, 107490 CrossRef CAS.
- Z. Wang, F. Wang, B. Zhao, S. Qu, T. Hayat, A. Alsaedi, L. Sui, K. Yuan, J. Zhang, Z. Wei and Z. a. Tan, J. Phys. Chem. Lett., 2020, 11, 1120–1127 CrossRef CAS PubMed.
- I. Recalde, A. F. Gualdrón-Reyes, C. Echeverría-Arrondo, A. Villanueva-Antolí, J. Simancas, J. Rodriguez-Pereira, M. Zanatta, I. Mora-Seró and V. Sans, Adv. Funct. Mater., 2023, 33, 2210802 CrossRef CAS.
- X. Li, S. Zhang, S. A. Kulinich, Y. Liu and H. Zeng, Sci. Rep., 2014, 4, 4976 CrossRef CAS.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, Microchim. Acta, 2019, 186, 583 CrossRef PubMed.
- H. Ding, S.-B. Yu, J.-S. Wei and H.-M. Xiong, ACS Nano, 2015, 10, 484–491 CrossRef PubMed.
- V. Nguyen, J. Si, L. Yan and X. Hou, Carbon, 2015, 95, 659–663 CrossRef CAS.
- S. Paulo-Mirasol, S. Gené-Marimon, E. Martínez-Ferrero and E. Palomares, ACS Appl. Electron. Mater., 2020, 2, 1388–1394 CrossRef CAS.
- Y. J. Heo, H. J. Jang, J. H. Lee, S. B. Jo, S. Kim, D. H. Ho, S. J. Kwon, K. Kim, I. Jeon, J. M. Myoung, J. Y. Lee, J. W. Lee and J. H. Cho, Adv. Funct. Mater., 2021, 31, 2106974 CrossRef CAS.
- P. G. Rouxhet and M. J. Genet, Surf. Interface Anal., 2011, 43, 1453–1470 CrossRef CAS.
- D. Stefanakis, A. Philippidis, L. Sygellou, G. Filippidis, D. Ghanotakis and D. Anglos, J. Nanopart. Res., 2014, 16, 2646 CrossRef.
- M. Vedamalai, A. P. Periasamy, C.-W. Wang, Y.-T. Tseng, L.-C. Ho, C.-C. Shih and H.-T. Chang, Nanoscale, 2014, 6, 13119–13125 RSC.
- H. B. Lee, N. Kumar, M. M. Ovhal, Y. J. Kim, Y. M. Song and J. W. Kang, Adv. Funct. Mater., 2020, 30, 2001559 CrossRef CAS.
- Z. Liang, B. Wang, M. Luo and H. Lu, Diamond Relat. Mater., 2021, 112, 108238 CrossRef CAS.
- E. Hassanabadi, M. Latifi, A. F. Gualdrón-Reyes, S. Masi, S. J. Yoon, M. Poyatos, B. Julián-López and I. Mora-Seró, Nanoscale, 2020, 12, 14194–14203 RSC.
- R. Grisorio, M. E. Di Clemente, E. Fanizza, I. Allegretta, D. Altamura, M. Striccoli, R. Terzano, C. Giannini, M. Irimia-Vladu and G. P. Suranna, Nanoscale, 2019, 11, 986–999 RSC.
- A. Pan, Y. Zhou, C. Zhao, C. Shi, Y. Wu, Y. Zhang, Y. Liu and L. He, Chem. Eng. J., 2022, 433, 133590 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup, morphology, and optical properties of CQDs, XRD patterns of the as-prepared A2SnX4 perovskites, PLQY measurements of A2SnX4/CQDs inks, PL spectra with the corresponding CIE diagrams, XPS spectra, surface chemical composition, 1H NMR spectra and TRPL decay curves for white-light emitting PEA2SnI4/CQD samples. See DOI: https://doi.org/10.1039/d4nr00707g |
| This journal is © The Royal Society of Chemistry 2024 |