Open Access Article
Open Access ArticleNanomaterial journey in the gut: from intestinal mucosal interaction to systemic transport
Xin
Qiao
 abc,
Lin
Bao
abc,
Lin
Bao
 abc,
Guanyu
Liu
abc and
Xuejing
Cui
abc,
Guanyu
Liu
abc and
Xuejing
Cui
 *abc
*abc
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety & CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China. E-mail: cuixj@nanoctr.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cNew Cornerstone Science Laboratory, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety and CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China
First published on 26th September 2024
Abstract
Engineered nanomaterials (NMs) are commonly utilized in food additives, cosmetics, and therapeutic applications due to their advantageous properties. Consequently, humans are frequently exposed to exogenous nanomaterials through oral ingestion, thus making the intestinal mucosal system a primary site for these particles. Understanding the interactions between nanomaterials and the intestinal mucosal system is crucial for harnessing their therapeutic potential and mitigating potential health risks from unintended exposure. This review aims to elucidate recent advancements in the dual effects of nanomaterials on the intestinal mucosal system. Upon entering the gut lumen, nanomaterials will interact with diverse intestinal components, including trillions of gut microbiota, mucus layer, intestinal epithelial cells (IECs), and the intestinal immune system. Additionally, the systemic fate and transportation of nanomaterials to distal organs, such as central nervous system, are also highlighted. These interactions result in a distinct biological effect of nanomaterials on the multilayer structure of intestine, thus displaying complex journeys and outcomes of nanomaterials in the living body. This in-depth exploration of the in vivo destiny and immunological implications of nanomaterials encountering the intestine has the potential to propel advancements in oral drug delivery techniques and motivate future investigations in novel toxicology research.
1. Introduction
Engineered nanomaterials (NMs) have been extensively employed in a range of consumer products, such as food additives, cosmetics, and therapeutic agents, owing to their unique physicochemical properties.1–3 This increased consumption has raised concerns about elevated human exposure to NMs through combustion processes, industrial activities, and environmental contamination,4,5 emphasizing the need to address potential risks associated with their usage. In recent decades, great endeavors have been made to comprehend the in vivo side effects of nanomaterials, primarily centered on the toxic impact on recognized organs including liver, lung, and kidney.6 Nonetheless, the potential toxicity of nanomaterials on the gastrointestinal tract (GIT) has been left behind. Given the important role of NMs, understanding the interactions between nanomaterials and the GIT, as well as their underlying mechanisms, is essential for both nanosafety and nanomedicine. This knowledge is critical for mitigating risks and enhancing the applications of nanomaterials.The GIT serves as a central site for the exposure of NMs that are either ingested directly or transported from the respiratory tract to the oral cavity before being swallowed.7 Within the GIT, NMs will interact with a complex intestinal microenvironment, especially the mucosal system. While a significant portion of these NMs are eliminated through fecal excretion, aided by mucus shedding and intestinal peristalsis, a considerable quantity persists, potentially affecting the resident microbiota and compromising the mucosal barrier.8 The subsequent penetration to the lamina propria and interplay with immune cells can disrupt mucosal immunity, potentially triggering adverse immune responses and exacerbating intestinal inflammation.9,10 The unique feature of the intestinal mucosal immune system is its exposure to a diverse range of antigens, necessitating the development of sophisticated mechanisms to defend against pathogenic invasion while maintaining tolerance towards commensal bacteria.11 Multiple studies have revealed that NMs from vegetables and those endogenously produced in the body can leverage the distinctive characteristics of intestinal mucosal immunity to promote mucosal immune tolerance.12,13 This dual role underscores the delicate impact of NMs on mucosal immunity, offering promising avenues for novel therapies for intestinal inflammatory disorders. However, current research on the intestinal mucosal effects of NMs is still in its early stage. A comprehensive understanding of the underlying immune interactions and mechanisms remains largely unexplored.
This review aims to delineate the multiple interactions between NMs and the intestinal mucosal system, examining the implications for gut microbiota, mucus layer, and intestinal epithelial barrier. We also evaluate the beneficial and detrimental effects of NMs on intestinal immunity. Furthermore, we elucidate the subsequent fates of NMs in intestine, differentiating between the immune responses elicited by degradable versus non-degradable nanomaterials and mapping the diverse systemic transport pathways these particles may undertake. These pathways include direct entry into the circulatory system via the portal vein, lymphatic transport through mesenteric lymph nodes circumventing hepatic filtration, and neural transport to the central nervous system, each pathway presenting unique implications for NMs-mediated effects on human health.
2. Interaction of NMs with intestinal mucosal system
2.1. The structure of intestinal mucosal system
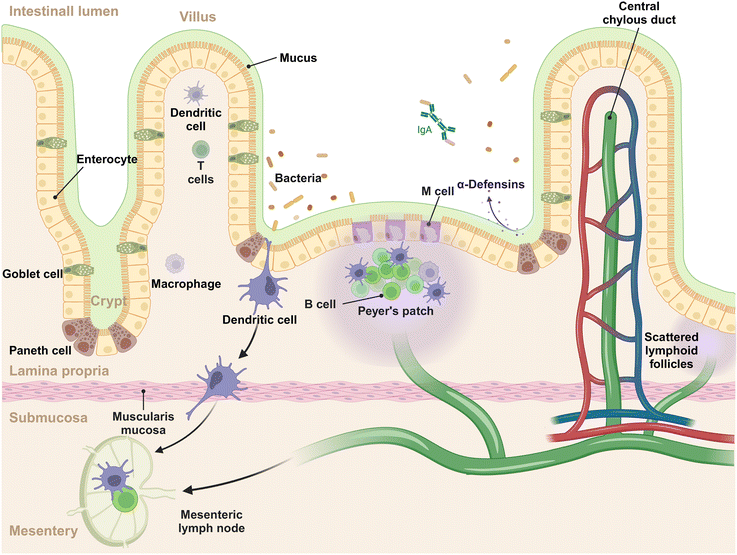
The intestinal mucosal system is a multilayer structure that serves as the first line of defense against pathogens and simultaneously tolerates harmless microbes and dietary antigens within the GIT (Fig. 1).14 In this system, the outermost layer is the gut microbiota, a diverse array of microbial communities that exert the functions of immune regulation and nutrient absorption.15,16 The mucus layer, produced by goblet cells, acts as the initial barrier to intestinal antigens, microorganisms, and other substances, capturing the invading foreign materials and aiding in their clearance.17 The intestinal epithelial cells (IECs) lie beneath the mucus layer, comprising the second tier of the mucosal barrier. This epithelial stratum is composed of various cell types, each with distinct functions. These include: (1) absorptive epithelial cells, which facilitate the uptake of nutrients; (2) goblet cells, pivotal in the production of mucus; (3) Paneth cells, known for their secretion of antimicrobial peptides; (4) tuft cells, which are sensitive to the presence of parasites; (5) enteroendocrine cells, responsible for the secretion of hormones; (6) M cells, specialized for antigen sampling and presentation. This intricate arrangement of cell types underscores the complexity and multifunctionality of the intestinal epithelium, which is essential for maintaining the mucosal barrier's integrity and conducting immune surveillance.18 The lamina propria, situated beneath the epithelial layer, forms the third tier of the mucosal immune system, often referred to as the immune barrier.19 This layer is rich in immune cells, including dendritic cells (DCs), macrophages, B cells, T cells, and plasma cells, which are instrumental in mounting a robust immune response to pathogens and promoting immunological tolerance. Within the epithelium and lamina propria, Peyer's patches are strategically positioned and integral to the gut-associated lymphoid tissue (GALT), which are teeming with immune cells such as B cells in germinal centers, T cells, DCs, and macrophages, contribute to initiating the mucosal immune response.20 Beyond the lamina propria, the mucosal system includes scattered lymphoid follicles throughout the intestine, which are vital for localized immune surveillance and reaction.21 Of note, the intestine is connected to the mesentery (a new organ identified in the year of 2016) that offers structural support.22 Within the mesentery lie mesenteric lymph nodes, which function as a conduit for lymphatic drainage from the intestinal mucosa to the systemic immune system.23 These lymph nodes are crucial for initiating and regulating immune responses, acting as filters that identify and process pathogens and antigens from the intestine.24 The complex interplay of cells and structures is designed to ensure a balanced immune response, protecting the host from infections while avoiding overreactions to benign environmental elements.2.2 The interaction of NMs with gut microbiota and luminal contents
Upon entering the gut lumen, nanomaterials interact with the vast population of gut microbiota, potentially influencing its composition, diversity, and functionality, these changes can subsequently impact the host's metabolic processes and immune responses.25,26 For instance, numerous studies have demonstrated that NMs possess antibacterial properties through various mechanisms, such as membrane disruption,27 interference with the electron transport chain,28 catalytic bactericidal activity,29 inhibition of bacterial division,30 toxicity from metal ions,31 and bacterial entrapment mediated by aggregation.32 To be noticed, the antibacterial properties of NMs can disrupt the normal structure of gut microbiota.33 Our recent finding indicate that following a short-term (14-day) administration, both silver NMs and silver nanowires suppressed the proliferation of Gram-negative bacteria.34 This inhibitory effect may be attributed to the presence of hydrophilic lipopolysaccharide (LPS) on the surface of Gram-negative bacteria, thus facilitating the adsorption of silver nanomaterials.34 As a result, the α-diversity of the gut microbiota gradually changed.34,35 Apart from the antibacterial properties, certain bacteria can in turn utilize the NMs as available carbon source to change the structure of gut microbiota. For example, we have shown that butyrate-producing bacteria can ferment carbon NMs as a carbon source, which shift the overall bacterial compositions.36 Interestingly, a recent study found that fullerol NMs could be degraded by gut microbiota, exhibiting a similar ability as inulin to promote short-chain fatty acid (SCFA) production.37 Additionally, NMs can modulate reactive oxygen species (ROS) levels in the intestine, impacting the composition of the intestinal microbiota. Wang et al. devised a carbon nanoparticle suspension injection to counteract radiation-induced ROS within the gut microenvironment, thereby preserving the equilibrium of the gut microbiota.38 They also suggested that these nanoparticles attenuated harm to the intestinal mechanical barrier, consequently impeding pathogenic bacteria from reaching epithelial cells and dominating the microbiota through unbridled proliferation.38 In summary, despite progress in elucidating the mechanisms through which NMs influence the gut microbiota, the fundamental principles governing these interactions are still incipient and necessitate further exploration.Alterations in the gut microbiota induced by NMs can significantly impact gastrointestinal and overall health. The Firmicutes to Bacteroidetes (F/B) ratio is commonly associated with intestinal health, and deviations in this ratio have been linked to obesity.39 For example, Chen et al. demonstrated that exposure of mice to silver NMs at a dose of 2.5 mg kg−1 for 7 days led to a decreased F/B ratio and subsequent weight loss.40 Similarly, Li et al. revealed that TiO2 NMs can reduce the population of Bifidobacterium, leading to the degradation of the mucus layer.41 Moreover, TiO2 NMs have been found to diminish beneficial probiotics like Bifidobacterium and Lactobacillus, potentially contributing to weight loss and inflammation.42
Currently, most studies have been focused on the effects of nanomaterials on the intestine.10,34,38,43,44 However, limited research has been conducted on the effects of the intestinal components on the NMs, especially on the biodegradable ones. Of note, several pieces of evidence indicate that the NMs, such as those made from engineered carbon-based materials and polylactic acid polymers, are capable of degrading through enzymatic activity and microbial interactions in the GIT.36,45 Our recent study has demonstrated that gut microbiota can decompose single-walled carbon nanotubes (SWCNTs) and graphene oxides (GO) nanosheets into smaller fragments, which are further metabolized into organic butyrate through microbial fermentation.36 This degradation process appears to be particularly prevalent among graphene-based nanoparticles, likely attributable to the ample oxidation and defect sites in SWCNTs and GO. These sites provide reactive targets for bacterial action through their dangling bonds and surface groups such as carboxyl, hydroxyl, and epoxy. Additionally, some butyrate-producing bacterial genera such as Roseburia and Odoribacter, along with members of the Ruminococcaceae family, utilize these degradation products to synthesize organic butyrate via the central pyruvate metabolic pathway. This finding highlights the potential for gut microbiota to metabolize NMs as carbon sources, altering the microbiome. Moreover, increased butyrate production through microbial fermentation can inhibit stem cell proliferation, enhance the intestinal barrier, regulate immune responses in the gut, and provide energy substrates for colonocytes.46 In contrast to the engineered nanoparticles, an interesting study has shown that biodegradable polylactic acid (PLA) microplastics can be enzymatically cleaved into oligomers by commercial lipases, and these oligomers then self-assemble into nanoplastic particles through hydrophobic interactions.45 The accumulation of these oligomers and resulting nanoplastic led to acute inflammatory reactions in the intestine of mice.45 Particularly, smaller nanoplastics (∼50 nm) produced during digestion showed a tendency to penetrate the GIT and spread throughout the body via the bloodstream, thus posing systemic risks.47
The breakdown of degradable NMs into smaller fragments or precursor forms leads to distinct patterns of distribution, cellular uptake, elimination, and immune reactions compared to non-degradable counterparts. For instance, the degradation of microplastics into nanoplastics altered their biological distribution, with smaller dimensions correlating with a broader dissemination scope.48,49 Typically, the degradation of NMs speeds up their elimination, with NMs or their degradation by-products smaller than 5.5 nanometers being quickly excreted through the renal system.50 In contrast, particles larger than 6 nanometers are mainly eliminated through the hepatobiliary pathway.50 Although this degradation process may reduce the risk of accumulation, it introduces new complexities to the interaction between gut microbiota and host metabolism, potentially impacting gut health and disease development. Notably, certain types of NMs previously considered non-biodegradable, such as gold, silicon dioxide, and iron oxide NMs, have been found to undergo structural changes or degradation in the body.51 Therefore, a comprehensive understanding of the reciprocal influences between these degradable particles and the intestinal environment is crucial for fully assessing their long-term effects on human health.
2.3. Interaction of NMs with mucus
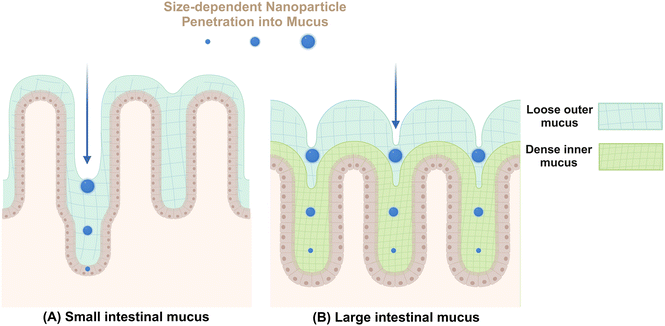
Mucus secreted by epithelial goblet cells forms a vital defensive barrier that traps invading exogenous substances, preventing their deeper penetration into intestinal tissues (Fig. 2). In the small intestine, a thin layer of mucus aids in nutrient absorption and digestion.52 In contrast, the colonic mucus consists of an inner compact layer and an outer fluidic layer. This stratification, approximately 50 μm in murine models and 200 μm in human from the epithelial surface, allows endogenous proteases to convert the inner layer to the outer layer, creating a distinct boundary.53 The formation of this mucus barrier primarily involves mucoprotein polymerization through disulfide linkages, forming a molecular sieve-like network.54 The outer colonic mucus layer contains larger pores, enabling the passage of particles up to 0.5 μm, including bacteria.52 In addition to the size, various intrinsic properties of NMs including shape, surface charge, chemical composition, hydrophilicity, and ligand density, can also influence their permeability within mucus.33In general, NMs can interact with mucin in various ways to alter their physicochemical properties. For example, Ag NMs have been found to stimulate goblet cells in rats, leading to increased mucin production and changes in mucin composition characterized by elevated salivary mucin levels and decreased thiomucin content.55 Furthermore, gut microbiota are important contributors to mucin production,54 The presence of bacteria, including probiotic strains like Lactobacillus rhamnosus and opportunistic pathogens like Gram-negative Escherichia coli, can stimulate mucus production.56 Nonetheless, the simultaneous exposure to titanium dioxide NMs (TiO2 NMs) and E. coli does not impact mucus secretion, whereas co-exposure with TiO2 NMs and L. rhamnosus markedly diminishes mucus production. These findings suggest that NMs not only directly influence the mucus layer but also potentially modulate its thickness and composition through the interactions with bacterial populations.
The interaction between intestinal mucin and NMs significantly dictates the trajectory of nanoparticles in the body, beyond their impact on mucosal mucin. For instance, intestinal mucin facilitates the aggregation of NMs, leading to increased endocytosis, decreased transcytosis, and enhanced retrograde transport pathways.57 Furthermore, mucus-adherent NMs, characterized by poor permeability and features such as positive surface charge or hydrophobic characteristics, strongly adhere to the mucus layer through electrostatic and hydrophobic interactions, resulting in their entrapment.58 These trapped particles are generally eliminated from the mucosal tissue along with the shedding of the outer mucus layer, a process that can occur within seconds to several hours based on their specific locations.59 This elimination is facilitated by peristaltic movements and culminates in their excretion via fecal matter.60 Conversely, smaller NMs61 or those with tailored surface modifications, such as neutral or slightly negative surface charge,62,63 hydrophilicity,62,64 rod-like shape,65,66 and moderate rigidity,67,68 may penetrate this barrier and reach deeper underlying tissues.
2.4. Interaction of NMs with intestinal epithelial cells
The intestinal epithelial barrier comprises a monolayer of cellular structures that serve not only as a physical barrier but also play a crucial role in immune protection and mediating interactions between microbial entities and immune cells. Within the intercellular spaces of adjacent epithelial cells lie essential components such as occludins and claudins, which are indispensable components in the formation of tight junctions.69 These structures cooperate with each other to prevent the translocation of bacteria and pathogen-associated molecular patterns (PAMPs) larger than 0.6–1.2 nm in diameter from breaching the intercellular space into the lamina propria.70A recent study has demonstrated the effects of nanomaterials on the epithelial layer, revealing their potential to induce epithelial cell death and compromise epithelial barrier integrity.33 The intracellular accumulation of NMs leads to increased production of ROS, a key factor in NM-induced cytotoxicity.71 NMs exposure elevates ROS levels, causing DNA damage, mitochondrial dysfunction, increased intracellular calcium levels, cellular structure impairment, and activation of inflammatory signaling pathways such as NF-κB and MAPK.72 This oxidative stress particularly affects the intestinal epithelial barrier, resulting in cell death through apoptosis, necrosis, and pyroptosis, as well as the release of pro-inflammatory mediators (e.g., mtDNA, ATP, IL-1β, TNF-α) from compromised cells and organelles.73 However, it is important to note that not all nanomaterials have detrimental effects on epithelial cells. For example, our research has shown that carbon nanotubes can enhance intestinal organoid growth by modulating the viscoelastic properties of the extracellular matrix and intracellular energy metabolic processes.74
In contrast to the direct effect of NMs on IECs, NMs might indirectly impact the IECs through influencing gut microbial metabolism that are essential for intestinal homeostasis.34 To be noticed, SCFAs, especially butyrate, are important metabolic products of gut microbiota in the colon produced mainly through dietary fiber fermentation, which is vital for supporting intestinal epithelial cells and maintaining colonic stability.75,76 The intestinal epithelial cells at the crypt surface utilize butyrate as a primary energy source through β-oxidation, depleting local oxygen levels from nearby blood vessels.14 This process creates a gradient of butyrate and oxygen along the crypt axis, establishing an anaerobic, butyrate-rich environment at the luminal surface while maintaining an oxygenated environment at the crypt base to protect stem cells from the inhibitory effects of butyrate on cell proliferation.14 Furthermore, our research indicates that gut microbiota can convert inorganic carbon NMs into organic butyrate, potentially disrupting epithelial homeostasis and impacting intestinal stem cell function.36 Moreover, SCFAs, particularly butyrate, play a crucial role in regulating tight junction proteins through various signaling pathways, including stabilization of hypoxia-inducible factor-1 (HIF-1) and inhibition of histone deacetylases (HDACs). These findings imply that carbon NMs could potentially compromise the integrity of the intestinal epithelial barrier by altering the interactions with gut microbiota. Additionally, a recent study has shown that the reduction in bacterial populations caused by microplastics and nanoplastics predominantly impacts beneficial microorganisms, which are known to enhance tight junction functionality.77 This indicates that alterations in the gut microbiome induced by these particles substantially contribute to the indirect toxicological effects that lead to the deterioration of intestinal barrier functions.77
2.5. Translocation mechanisms of NMs across IEC
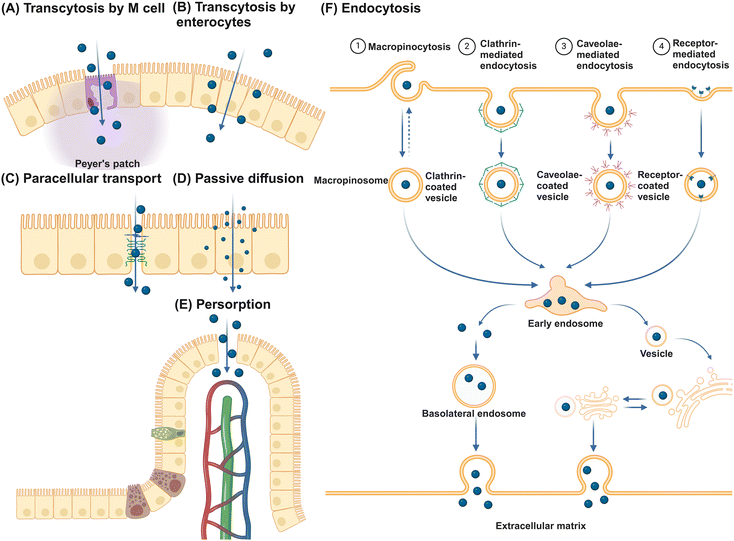
The primary function of the intestinal epithelial barrier is to segregate the luminal contents of the intestine from the host, thereby protecting against foreign substances.78 NMs can translocate the epithelial barrier through various pathways (Fig. 3). Initially, NMs can traverse the epithelium and undergo secretion on the opposite side via transcytosis, with transport through M cells being particularly efficient.79 Alternatively, NMs may potentially traverse the epithelial layer by utilizing gaps in the tight junctions of IECs through a paracellular pathway. However, given that tight junctions, consisting of transmembrane integral proteins, typically have a span of only 0.3 to 1 nm, the likelihood of NMs passing through this route is minimal.80 Therefore, the use of permeation enhancers is often necessary to augment the permeability of these junctions, thereby facilitating the passive transportation of NMs. Substances such as EDTA, chitosan, sodium caprate, bile salts, and specific synthetic peptides have demonstrated efficacy in transiently widening these junctions to enable passive transport.81 Despite these interventions, studies indicate that even with permeation enhancers, the enlargement of tight junctions seldom surpasses 20 nm, leading to unresolved debates regarding the feasibility of transporting larger NMs via the paracellular route.82 Interestingly, small NMs such as quantum dots and nanotubes with diameters of just several nanometers can pass directly through gaps in the cellular membrane phospholipid bilayer, facilitating passive diffusion across epithelial cells.83,84 Persorption is a mechanism where solid particles up to 130 μm pass into the lamina propria by mechanical kneading at the epithelial gap of the GIT villus tip, particularly at desquamation zones, before entering the circulatory system.85 This phenomenon is attributed to the cellular turnover at these sites, resulting in transient openings that permit particle translocation.86 Furthermore, receptor-mediated endocytosis allows for NMs internalization through interactions with specific receptors and cell surface molecules, with NMs endocytosis categorized into macropinocytosis, clathrin-mediated, caveolae-mediated, and other receptor-mediated endocytosis. Caveolin-mediated endocytosis predominantly facilitates the uptake of NMs ranging from 20 to 100 nm, whereas clathrin-mediated endocytosis mainly handles the uptake of submicron particles between 100 and 350 nm.87 Macropinocytosis is especially vital for internalizing larger NMs, as it allows for the formation of large vesicles from 0.2 to 5 μm.88 Engineered NMs, designed with specific bioactive compounds such as vitamins, oligopeptides, fatty acids, and antibody fragments, leverage receptor-mediated endocytosis for the targeted delivery of active pharmaceutical agents.89 The key receptors targeted in this process include the folate receptor,90 transferrin receptor,91 vitamin B12 receptor,92 and neonatal Fc receptor.93M cells, along with goblet cells among the various types of IECs, are of great importance to promote intestinal tolerance and initiate mucosal immune responses.94,95 These cells sample luminal contents indiscriminately and facilitate the transport of intact antigens to underlying DCs for antigen processing and presentation. Located in the follicle-associated epithelium (FAE) covering Peyer's patches, M cells are characterized by unique morphology, including short, folded apical invaginations and a significant basolateral pocket that allows close interaction with antigen-presenting cells (APCs) or lymphocytes.96,97 This special structure enables M cells to capture particulate matter through receptor-dependent pathways, involving cytoskeletal proteins such as glycoprotein 2, β1-integrin, prion protein PrP, dectin-1, claudin-4, and CD155.14 After antigen uptake, M cells efficiently deliver them to neighboring immune cells through processes like transcytosis, phagocytosis, or the release of microvesicles.98 Despite constituting only about 5% of FAE cells and 1% of the total absorptive surface area of IECs in humans,99,100 M cells face challenges in transporting significant amounts of nanoparticles.101 Studies in rodents suggest that villous cells are primarily responsible for absorbing 2 μm polystyrene particles, accounting for 93% of absorption, while FAE is associated with a minimal 4%.102 This evidence indicates that IECs rather than M cells are the main players in NM translocation.103–105 Furthermore, goblet cells are implicated in enhancing the transport of luminal antigens to DCs beneath the epithelium.106,107 However, research on goblet cell-mediated NM transport is limited, highlighting a significant gap in our understanding of mucosal NM delivery mechanisms.
3. The mucosal immune effects of NMs on intestine
3.1. The innate immune effects of NMs
Upon breaching the mucus layer and entering the epithelial layer and lamina propria, NMs are known to elicit innate immune responses. In 1996, Powell et al. identified conglomerations of TiO2 NMs within M cells and adjacent macrophages in the GALT of intestinal biopsy specimens from individuals with inflammatory bowel disease (IBD).108 Similarly, a variety of NMs such as TiO2, ZnO, SiO2, Fe3O4, aluminosilicates, and Ag have been observed to accumulate on the cell surface and within the cytoplasm of IECs, M cells, Peyer's patches, and macrophages in the GI tract.108–112 The intracellular accumulation of these particles is widely known to catalyze the overproduction of ROS like superoxide (O2˙−), hydroxyl radicals (OH˙), and hydrogen peroxide (H2O2), which are recognized as primary contributors to cytotoxicity.113–116 In addition, the surface chemistry of NMs can also contribute to ROS formation.113,117 For example, the prooxidant functional groups on the surfaces of NMs, such as surface-bound radicals found on quartz particles like SiO˙ and SiO2˙, plays a crucial role in ROS generation.113,118,119 Notably, ZnO NMs have shown to induce more pronounced ROS levels compared to SiO2, likely due to their heightened chemical reactivity.113,115 Furthermore, the intrusion of NMs into mitochondria can stimulate the endogenous generation of ROS by disrupting the electron transport chain, leading to structural impairment, activation of NADPH-like enzyme systems, and mitochondrial membrane depolarization.115 This oxidative stress subsequently prompts the release of pro-inflammatory mediators through key signaling pathways such as Nuclear Factor-κB (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K).120The innate immune system employs various pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) to recognize PAMPs and damage-associated molecular patterns (DAMPs).121 The interactions between NMs interaction and macrophages, along with the ensuing cellular immune responses, are complicated, involving various pathways that lead to inflammasome activation through PRRs. This activation process generally involves the identification of NMs as exogenous entities, the perturbation of cellular equilibrium, and the release of danger signals (e.g., mitochondrial DNA122 and ATP123), facilitated by both direct and indirect NMs interactions with cellular components. These interactions can cause disturbances in ion levels, destabilization of lysosomes, and ROS production.124 Among inflammasomes, the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome is particularly notable for its responsiveness to a diverse array of stimuli, including silica and aluminum crystals, asbestos, and diverse NMs.125–128 In an acute colitis mouse model induced by dextran sodium sulfate (DSS), oral administration of TiO2 NMs was found to induce the formation of the NLRP3 inflammasome.9 This led to the release of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18, exacerbating intestinal inflammation. Interestingly, TiO2 NMs did not provoke the same colitis pathology in NLRP3 knockout mice, underscoring the critical role of the NLRP3 inflammasome in mediating intestinal inflammation. A similar study demonstrated that oral administration of small SiO2 NMs also worsens DSS-induced colitis by activating the apoptosis-associated speck-like protein containing a CARD (ASC)-mediated inflammasome in immune cells of the colonic lamina propria.10 This potential influence on innate immunity by NMs may thus lead to macroscopic mucosal immune effects and clinical symptoms associated with inflammation. A recent study has demonstrated that administering Ag NMs orally to mice significantly increased levels of IL-1β, IL-6, and TNF-α, leading to classic symptoms of ulcerative colitis including elevated disease activity index and decreased colon length.40 Furthermore, exposure to SWCNTs has been shown to cause substantial intestinal damage, marked by increased histological lesion scores, heightened intestinal permeability, and elevated secretion of proinflammatory cytokines.129 Further investigations have revealed that the administration of 150 mg kg−1 of nano-MoS2 resulted in mucosal hemorrhage, villus shortening in the small intestine, and edema in the intestinal wall of the large intestine, alongside a notable increase in TNF-α gene expression.130
A question arises as to whether nanoparticles are directly recognized by innate immune cells or if proteins and other biomolecules adsorbed on the surface of NMs act as “NM-associated molecular patterns” (NAMPs), triggering an immune response.131 The concept of NAMPs was introduced by Gallo and Gallucci in 2013, based on the evidence of immune cell detection of NMs.132 However, recent studies suggest that the diverse signal responses of the NLRP3 inflammasome are more appropriately activated in response to “homeostasis-altering molecular processes” (HAMPs) rather than specific molecular patterns.133 Nonetheless, in the case of NMs such as SiO2 NMs and carbon nanotubes, a distinct identification mechanism involving NAMPs has been observed. In 2017, Nakayama et al. revealed that SiO2 NMs primarily interact with the class B scavenger receptor (SR-B1), identifying it as the main receptor for silica.134 This interaction is specific to SR-B1 and both amorphous and crystalline silica, with no corresponding recognition observed for TiO2 NMs, latex NMs, or monosodium urate crystals. Subsequently, they demonstrated that the extracellular domain of murine T cell mucin immunoglobulin 4 (Tim4) contains a specific cluster of aromatic residues that facilitate binding to multi-walled carbon nanotubes (MWCNTs).135 Similarly, they found that sialic acid binding immunoglobulin-like lectin-14 (Siglec-14), highly expressed on human macrophages, binds to carbon nanotubes (CNTs), thereby initiating pro-inflammatory signaling.136 In particular, they observed that Tim4 engages with polystyrene (PS) microplastics through aromatic–aromatic interactions, thus facilitating the macrophage uptake of these microplastics.137 Despite these interactions, PS microplastics do not induce the release of inflammatory cytokines from macrophages.
In addition to the immune recognition, NMs have been found to contribute to the modulation of macrophage activation, differentiation, and polarization. An example for this phenomenon was that TiO2 NMs instigate an aberrant activation of macrophages, as manifested by the amplified pro-inflammatory M1 phenotype coupled with TLR4-mediated suppression of innate immune.138 Furthermore, TiO2 NMs have been implicated in inducing significant mitochondrial dysfunction and attenuating macrophage phagocytic ability, showing increased mitochondrial ROS levels, diminished ATP synthesis, and reduced metabolic activity within the tricarboxylic acid (TCA) cycle.139 Notably, NMs in the GIT also exhibit an adjuvant effect by enhancing the immune response to biomolecules adsorbed on their surfaces.112 The interaction between macrophages and antigens or toxins attached to particles leads to increased T-cell proliferation compared to soluble forms.140 Moreover, studies have shown that TiO2 NMs induce increased inflammatory response of primary human mononuclear phagocytes to endotoxin lipopolysaccharides (LPS), which is enhanced in cell culture media containing calcium that promotes the binding of biomolecules to intestinal proteins and calcium precipitates.141,142 These findings exhibit the intricate interplay between NMs, cellular immune responses, and the local microenvironment.
3.2. The immune tolerance mediated by NMs
The intricate interplay between endogenous NMs and immune tolerance in the GIT is essential for maintaining intestinal health.143 In particular, amorphous magnesium-substituted calcium phosphate (AMCP) NMs, formed in the gut from secreted calcium and phosphate ions, participate in the regulation of immune responses. These NMs facilitate the capture of soluble macromolecules such as proteins and bacterial peptidoglycan, facilitating their transport across the epithelial barrier to APCs located in Peyer's patches (Fig. 4).12 This process not only shields protein antigens from enzymatic degradation en route but also modulates the intestinal immune equilibrium by promoting immune tolerance.12 Of note, the simultaneous co-transportation of peptidoglycan alongside AMCP NMs is vital, as it influences the APCs phenotype by upregulating PD-L1, an immunosuppressive molecule that increases during inflammation to mitigate tissue damage.144 The complex interplay emphasizes the vital role of AMCP NMs in coordinating the intestinal immune response, elucidating a fundamental strategy by which the body preserves tolerance to external antigens while combating pathogenic challenges.Thus far, investigations have also illuminated the contributory role of NMs present in edible plants towards the regulation of intestinal immune balance. For instance, Zhang et al. demonstrated that sulforaphane (SFN), delivered by nanoparticles derived from broccoli (BDNs), effectively alleviated mouse colitis by promoting tolerogenic DCs through AMP-activated protein kinase (AMPK) activation.13 They revealed that BDN treatment not only reduced the levels of TNF-α, IL-17A, and IFN-γ, which were elevated by DSS, but also enhanced the expression of IL-10. Furthermore, BDN administration prevented weight loss associated with DSS, reduced inflammatory infiltrates in the mucosa, preserved a higher number of colonic goblet cells, and significantly mitigated colonic shortening compared to mice that received only DSS. This study highlights the importance of nanoparticle-mediated SFN delivery to DCs in the lamina propria, compared to the free form of SFN, which lacks DC-targeting specificity.145 In addition to broccoli, numerous edible plants, including grapes, grapefruits, ginger, and tomatoes, are sources of NMs.146 Analogous to BDNs SFN, NMs from these dietary sources may harbor molecules with similar functions, capable of fostering intestinal immune tolerance. Various edible plants such as grapes, grapefruits, ginger, and tomatoes also contain nanoparticles that may possess similar immune-modulating properties as BDNs SFN, potentially promoting intestinal immune tolerance. Therefore, nanoparticles in edible plants play a significant role in maintaining intestinal immune homeostasis.
Leveraging the concept of NMs shielding antigens to promote immune tolerance, various immunotherapeutic strategies have been proposed.145 The GIT plays a crucial role in immune responses by distinguishing harmless dietary antigens from harmful pathogens, maintaining a delicate balance between immune tolerance and activation.147 NMs, with their unique physicochemical properties, have emerged as effective tools for modulating this balance. By delivering antigens, immune regulatory signals, or therapeutic agents to the GALT, NMs promote the generation of regulatory T cells (Tregs) and facilitate local or systemic immune tolerance.12,148,149 This novel approach overcomes the limitations of traditional immunotherapy, offering a precise method to modulate the immune system. The application of NMs to induce intestinal immune tolerance represents a promising advancement in the management of inflammatory bowel disease (IBD), food allergies, and other gastrointestinal immune disorders. However, further research is warranted to elucidate the intricate interactions and long-term impacts of NMs within the human body to ensure the safety and efficacy of clinical interventions.
4. Systemic fate of NMs in the intestine
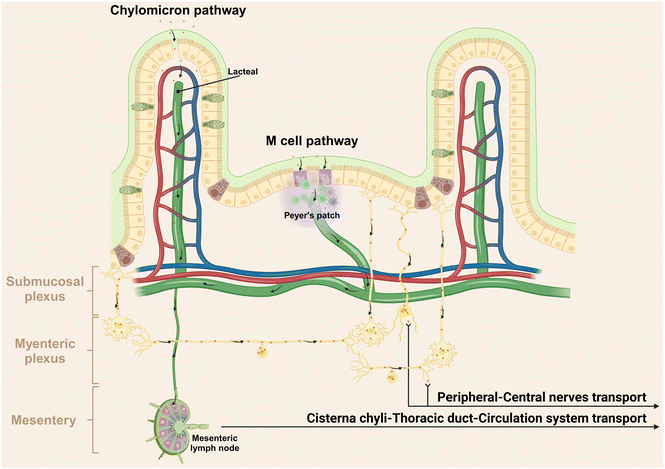
Following translocation across the epithelial barrier, non-degradable particles typically undergo two primary pathways including lymphatic transport and circulation transport via the portal vein.150 For instance, Volkheimer et al. investigated the behaviors of non-degradable microparticles, using polyvinyl chloride (PVC) particles ranging from 5 to 110 μm as a model.150 Initially, these PVC particles traverse the intestinal wall, entering the chyle in lymph vessels below, thereby facilitating lymphatic transport. Alternatively, these particles can translocate through the portal vein circulation, as evidenced by elevated particle levels in blood from mesenteric veins after PVC particle ingestion. Actually, the effective lymphatic transport is supported by chylomicrons (dietary lipids encapsulated into NMs by intestinal epithelial cells) and M cells in Peyer's patches, predominantly through lacteals (Fig. 5).151,152 Within the villus core connective tissue, one to two longitudinal lymphatic capillaries, referred to as the central lacteal, originate at the villus apex, descend through the mucosal muscle layer into the submucosa, and give rise to a lymphatic plexus. The lymphatic journey of NMs involves passage through pre-collecting lymphatics, collecting lymphatics, lymphatic trunks, and ducts, culminating in arrival at the thoracic duct via mesenteric lymph nodes and the lacteal pool (Fig. 5).79 Consequently, these substances evade first-pass metabolism in the liver.153,154Apart from the blood and lymphatic vessels, our recent report has unveiled a novel pathway for the transportation of orally administered NMs to the central nervous system via peripheral nerves, specifically demonstrating the direct transportation of Ag NMs in the GI tract to the brain and spinal cord through the vagus and spinal nerves, bypassing the traditional blood circulation route.155 This transport mechanism is probably facilitated by the selective uptake of Ag NMs by neurons, due to their argyrophilic properties.156 It is well established that a complex and dynamic interplay exists between the nervous system and the immune system. The nervous system regulates immune functions by directly innervating lymphoid organs and releasing neurotransmitters that modulate immune cell activities.157 Conversely, immune cells can influence neuronal function through the secretion of cytokines.158 Hence, the discovery of this novel neural pathway for NMs transportation from gut presents a significant opportunity to modulate neural immunity. It provides innovative approaches for delivering therapeutic agents directly to the central nervous system, potentially impacting immune responses associated with neurological disorders.
5. Conclusions and perspectives
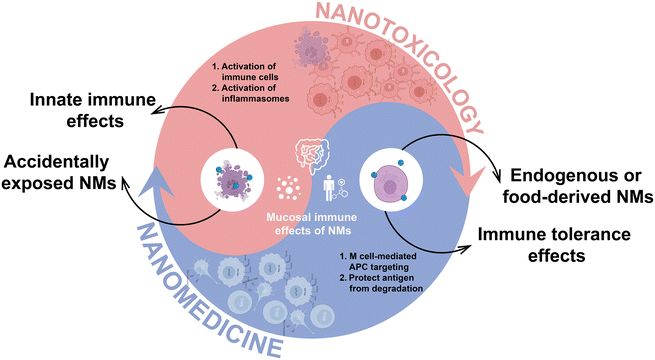
The intersection of nanotechnology and intestinal health has sparked interdisciplinary research into NMs and their impact on the gut.33 This review explores the complex journey of NMs in the intestinal environment, including: (1) interactions with enzymes and microbes in the gut lumen, which can affect the microbiome and overall intestinal health; (2) passage through the intestinal mucus layer, where NMs may be eliminated or penetrate deeper; (3) interaction with intestinal epithelial cells and potential translocation across the epithelial barrier; (4) involvement with the mucosal immune system, leading to activation of innate immune responses or induction of immune tolerance, thus influencing mucosal immunity; and (5) transportation to distant organs through circulatory, lymphatic, or neural pathways.It is essential to recognize that the interaction between NMs and the gut environment is inherently bidirectional. Enzymes and microorganisms within the gut lumen can catalyze the degradation of biodegradable NMs, altering their physical and chemical properties. Concurrently, the NMs and their degradation products can significantly influence the structure and function of these initiating microbial communities, with the potential to induce extensive systemic physiological changes. After these initial interactions, NMs progress to the intestinal mucus layer, which serves as a critical selective barrier. Depending on their altered physicochemical properties, NMs may be either retained and eliminated or penetrate deeper, reaching the epithelial cells beneath. This pivotal interaction with the intestinal epithelium not only determines the potential for NMs to translocate across the epithelial barrier but also significantly impacts intestinal health. Understanding these initial stages of NM interactions within the gut is crucial for setting the stage for their subsequent engagement with the mucosal immune system and systemic transport, thereby influencing broader physiological effects. This comprehensive view underscores the importance of considering both the local level interactions and the broader systemic implications when studying the impact of NMs on intestinal health.
The dual functionality of NMs concerning mucosal immunity has been a focal point of this review. While some studies have begun to explore the activation of innate immune responses and the promotion of mucosal tolerance by NMs,9,12,13 a comprehensive understanding of the underlying mechanisms remains elusive. The current literature primarily observes the impact of NMs on macrophages and DCs, particularly in the context of inflammation. However, there is a notable gap in our knowledge regarding the direct effects of NMs on macrophage polarization and the molecular mechanisms. Future researches are warranted to delve deeper into the specificity of immune activation by NMs, identifying the targeted immune cell types (e.g., macrophages, DCs, T cells) and elucidating the modulation of activation or suppression pathways within these cells. In addition, unraveling the precise mechanisms of NM interaction with cell surface receptors and the subsequent intracellular signaling pathways is critical for advancing our understanding of NMs’ impact on mucosal immunity.
The convenience and high patient compliance associated with oral drug delivery have garnered significant interest in the transport of NMs across the intestinal epithelium. Although we have outlined three primary pathways for the systemic circulation of orally administered NMs, a thorough analysis of the transport mechanisms, especially through mesenteric routes, is still lacking. The processes by which NMs transition from the intestinal lumen to systemic circulation and the influence of their physicochemical properties on this translocation are areas that require further investigation. Additionally, the variability in research findings, attributed to differences in experimental models and individual variances, underscores the complexity of NMs and the need for a more nuanced understanding of their transport dynamics.
In conclusion, the field of nano-intestine research has made substantial strides in enhancing our knowledge of NMs’ biosafety, offering valuable insights that promote human health and societal advancement. Despite the significant progress, there is an ongoing need for research to address the gaps identified in this review. Understanding the specificity of immune activation by NMs, the detailed mechanisms of their interaction with the mucosal immune system, and the pathways facilitating their passage through the intestinal epithelium are essential for harnessing their therapeutic potential while mitigating potential health risks.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFC2409701, 2021YFA1200900), the National Natural Science Foundation of China (32271460, 22388101), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2023042), Beijing Nova Program (20240484663), and the New Cornerstone Science Foundation (NCI202318). All figures were retrieved from https://app.biorender.com.References
- H. Zhao, J. B. Xu, C. Feng, J. Y. Ren, L. Bao, Y. B. Zhao, W. Tao, Y. L. Zhao and X. L. Yang, Adv. Mater., 2022, 34, 2106390 CrossRef CAS PubMed.
- X. Y. Wang, X. J. Cui, Y. L. Zhao and C. Y. Chen, Sci. China: Life Sci., 2020, 63, 1168–1182 CrossRef CAS PubMed.
- Y. Nazarenko, T. W. Han, P. J. Lioy and G. Mainelis, J. Exposure Sci. Environ. Epidemiol., 2011, 21, 515–528 CrossRef CAS PubMed.
- K. Donaldson, L. Tran, L. A. Jimenez, R. Duffin, D. E. Newby, N. Mills, W. MacNee and V. Stone, Part. Fibre Toxicol., 2005, 2, 10 CrossRef PubMed.
- M. R. Gwinn and V. Vallyathan, Environ. Health Perspect., 2006, 114, 1818–1825 CrossRef CAS PubMed.
- E. Valsami-Jones and I. Lynch, Science, 2015, 350, 388–389 CrossRef CAS PubMed.
- E. A. Mutlu, P. A. Engen, S. Soberanes, D. Urich, C. B. Forsyth, R. Nigdelioglu, S. E. Chiarella, K. A. Radigan, A. Gonzalez, S. Jakate, A. Keshavarzian, G. S. Budinger and G. M. Mutlu, Part. Fibre Toxicol., 2011, 8, 19 CrossRef CAS PubMed.
- M. Yang, S. K. Lai, Y. Y. Wang, W. X. Zhong, C. Happe, M. Zhang, J. Fu and J. Hanes, Angew. Chem., Int. Ed., 2011, 50, 2597–2600 CrossRef CAS PubMed.
- P. A. Ruiz, B. Morón, H. M. Becker, S. Lang, K. Atrott, M. R. Spalinger, M. Scharl, K. A. Wojtal, A. Fischbeck-Terhalle, I. Frey-Wagner, M. Hausmann, T. Kraemer and G. Rogler, Gut, 2017, 66, 1216–1224 CrossRef CAS PubMed.
- T. Ogawa, R. Okumura, K. Nagano, T. Minemura, M. Izumi, D. Motooka, S. Nakamura, T. Iida, Y. Maeda, A. Kumanogoh, Y. Tsutsumi and K. Takeda, Biochem. Biophys. Res. Commun., 2021, 534, 540–546 CrossRef CAS PubMed.
- K. Honda and K. Takeda, Mucosal Immunol., 2009, 2, 187–196 CrossRef CAS PubMed.
- J. J. Powell, E. Thomas-McKay, V. Thoree, J. Robertson, R. E. Hewitt, J. N. Skepper, A. Brown, J. C. Hernandez-Garrido, P. A. Midgley, I. Gomez-Morilla, G. W. Grime, K. J. Kirkby, N. A. Mabbott, D. S. Donaldson, I. R. Williams, D. Rios, S. E. Girardin, C. T. Haas, S. F. A. Bruggraber, J. D. Laman, Y. Tanriver, G. Lombardi, R. Lechler, R. P. H. Thompson and L. C. Pele, Nat. Nanotechnol., 2015, 10, 361–369 CrossRef CAS PubMed.
- Z. B. Deng, Y. Rong, Y. Teng, J. Y. Mu, X. Zhuang, M. Tseng, A. Samykutty, L. F. Zhang, J. Yan, D. Miller, J. Suttles and H. G. Zhang, Mol. Ther., 2017, 25, 1641–1654 CrossRef CAS PubMed.
- J. M. Allaire, S. M. Crowley, H. T. Law, S.-Y. Chang, H.-J. Ko and B. A. Vallance, Trends Immunol., 2018, 39, 677–696 CrossRef CAS PubMed.
- H. J. Flint, K. P. Scott, P. Louis and S. H. Duncan, Nat. Rev. Gastroenterol. Hepatol., 2012, 9, 577–589 CrossRef CAS PubMed.
- L. V. Hooper and A. J. Macpherson, Nat. Rev. Immunol., 2010, 10, 159–169 CrossRef CAS PubMed.
- G. M. H. Birchenough and M. E. V. Johansson, Science, 2020, 370, 402–403 CrossRef PubMed.
- L. W. Peterson and D. Artis, Nat. Rev. Immunol., 2014, 14, 141–153 CrossRef CAS PubMed.
- E. Moens and M. Veldhoen, Immunology, 2012, 135, 1–8 CrossRef CAS PubMed.
- C. Jung, J.-P. Hugot and F. Barreau, Int. J. Inflamm., 2010, 2010, 823710 Search PubMed.
- K. Murphy, P. Travers, M. Walport and C. Janeway, Janeway's immunobiology, Garland Science, New York, 8th edn, 2012 Search PubMed.
- J. C. Coffey and D. P. O'Leary, Lancet Gastroenterol. Hepatol., 2016, 1, 238–247 CrossRef PubMed.
- G. Y. Liu, L. Bao, C. Y. Chen, J. F. Xu and X. J. Cui, Nanoscale, 2023, 15, 12868–12879 RSC.
- S. Voedisch, C. Koenecke, S. David, H. Herbrand, R. Förster, M. Rhen and O. Pabst, Infect. Immun., 2009, 77, 3170–3180 CrossRef CAS PubMed.
- B. Lamas, N. M. Breyner and E. Houdeau, Part. Fibre Toxicol., 2020, 17, 19 CrossRef PubMed.
- M. Ghebretatios, S. Schaly and S. Prakash, Int. J. Mol. Sci., 2021, 22, 1942 CrossRef CAS PubMed.
- Y. Wang, Y. Yang, Y. Shi, H. Song and C. Yu, Adv. Mater., 2020, 32, 1904106 CrossRef CAS PubMed.
- J. Li, W. Liu, X. Zhang, P. K. Chu, K. M. C. Cheung and K. W. K. Yeung, Mater. Today, 2019, 22, 35–49 CrossRef CAS.
- Y. Liu, L. Shi, L. Su, H. C. van der Mei, P. C. Jutte, Y. Ren and H. J. Busscher, Chem. Soc. Rev., 2019, 48, 428–446 RSC.
- S. Sanyasi, R. K. Majhi, S. Kumar, M. Mishra, A. Ghosh, M. Suar, P. V. Satyam, H. Mohapatra, C. Goswami and L. Goswami, Sci. Rep., 2016, 6, 24929 CrossRef PubMed.
- P. Makvandi, C. Wang, E. N. Zare, A. Borzacchiello, L. Niu and F. R. Tay, Adv. Funct. Mater., 2020, 30, 1910021 CrossRef CAS.
- B. Sun, Y. Zhang, W. Chen, K. Wang and L. Zhu, Environ. Sci. Technol., 2018, 52, 7212–7219 CrossRef CAS PubMed.
- X. J. Cui, L. Bao, X. Y. Wang and C. Y. Chen, Small, 2020, 16, 1907665 CrossRef CAS PubMed.
- X. Y. Wang, X. J. Cui, J. G. Wu, L. Bao and C. Y. Chen, J. Mater. Chem. B, 2023, 11, 1904–1915 RSC.
- X. L. Wang, N. Yu, C. Wang, H. R. Zhou, C. Wu, L. Yang, S. Wei and A.-J. Miao, ACS Nano, 2022, 16, 19002–19012 CrossRef CAS PubMed.
- X. J. Cui, X. Y. Wang, X. L. Chang, L. Bao, J. G. Wu, Z. Q. Tan, J. M. Chen, J. Y. Li, X. F. Gao, P. C. Ke and C. Y. Chen, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2218739120 CrossRef CAS PubMed.
- J. Li, R. Lei, X. Li, F. Xiong, Q. Zhang, Y. Zhou, S. Yang, Y. Chang, K. Chen, W. Gu, C. Wu and G. Xing, Part. Fibre Toxicol., 2018, 15, 5 CrossRef PubMed.
- C. Y. Wang, J. N. Xie, X. H. Dong, L. Q. Mei, M. R. Zhao, Z. W. Leng, H. X. Hu, L. L. Li, Z. J. Gu and Y. L. Zhao, Small, 2020, 16, 1906915 CrossRef CAS PubMed.
- F. J. Verdam, S. Fuentes, C. de Jonge, E. G. Zoetendal, R. Erbil, J. W. Greve, W. A. Buurman, W. M. de Vos and S. S. Rensen, Obesity, 2013, 21, E607–E615 CrossRef CAS PubMed.
- H. Chen, R. Zhao, B. Wang, C. Cai, L. Zheng, H. Wang, M. Wang, H. Ouyang, X. Zhou, Z. Chai, Y. Zhao and W. Feng, NanoImpact, 2017, 8, 80–88 CrossRef.
- X. Li, Y. Zhang, B. Li, J. Cui, N. Gao, H. Sun, Q. Meng, S. Wu, J. Bo, L. Yan, J. Wu and R. Chen, NanoImpact, 2019, 14, 100164 CrossRef.
- W. Mu, Y. Wang, C. Huang, Y. Fu, J. Li, H. Wang, X. Jia and Q. Ba, J. Agric. Food Chem., 2019, 67, 9382–9389 CrossRef CAS PubMed.
- R. Cornu, C. Chrétien, Y. Pellequer, H. Martin and A. Béduneau, Arch. Toxicol., 2020, 94, 1191–1202 CrossRef CAS PubMed.
- T. T. Zhang, X. Zhu, J. H. Guo, A. Z. Gu, D. Li and J. M. Chen, Environ. Sci. Technol., 2021, 55, 6884–6896 CrossRef CAS PubMed.
- M. J. Wang, Q. Q. Li, C. Z. Shi, J. Lv, Y. D. Xu, J. J. Yang, S. L. Chua, L. R. Jia, H. W. Chen, Q. Liu, C. J. Huang, Y. C. Huang, J. M. Chen and M. L. Fang, Nat. Nanotechnol., 2023, 18, 403–411 CrossRef CAS PubMed.
- L. Zhang, C. D. Liu, Q. Y. Jiang and Y. L. Yin, Trends Endocrinol. Metab., 2021, 32, 159–169 CrossRef CAS PubMed.
- S. L. Wright and F. J. Kelly, Environ. Sci. Technol., 2017, 51, 6634–6647 CrossRef CAS PubMed.
- B. X. Liang, Y. Z. Zhong, Y. J. Huang, X. Lin, J. Liu, L. Lin, M. J. Hu, J. Y. Jiang, M. Z. Dai, B. Wang, B. L. Zhang, H. Meng, J. J. J. Lelaka, H. X. Sui, X. F. Yang and Z. L. Huang, Part. Fibre Toxicol., 2021, 18, 20 CrossRef CAS PubMed.
- C. Z. Ma, Q. Q. Chen, J. W. Li, B. W. Li, W. W. H. Liang, L. Su and H. H. Shi, Crit. Rev. Toxicol., 2021, 51, 740–753 CrossRef CAS PubMed.
- G. B. Yang, S. Z. F. Phua, A. K. Bindra and Y. Zhao, Adv. Mater., 2019, 31, 1805730 CrossRef PubMed.
- N. Feliu, D. Docter, M. Heine, P. del Pino, S. Ashraf, J. Kolosnjaj-Tabi, P. Macchiarini, P. Nielsen, D. Alloyeau, F. Gazeau, R. H. Stauber and W. J. Parak, Chem. Soc. Rev., 2016, 45, 2440–2457 RSC.
- P. Paone and P. D. Cani, Gut, 2020, 69, 2232–2243 CrossRef CAS PubMed.
- G. M. H. Birchenough, M. E. Johansson, J. K. Gustafsson, J. H. Bergström and G. C. Hansson, Mucosal Immunol., 2015, 8, 712–719 CrossRef CAS PubMed.
- M. E. V. Johansson and G. C. Hansson, Nat. Rev. Immunol., 2016, 16, 639–649 CrossRef CAS PubMed.
- G. N. Jeong, U. B. Jo, H. Y. Ryu, Y. S. Kim, K. S. Song and I. J. Yu, Arch. Toxicol., 2010, 84, 63–69 CrossRef CAS PubMed.
- R. Limage, E. Tako, N. Kolba, Z. Y. Guo, A. García-Rodríguez, C. N. H. Marques and G. J. Mahler, Small, 2020, 16, 2000601 CrossRef CAS PubMed.
- D. Yang, D. C. Liu, M. M. Qin, B. L. Chen, S. Y. Song, W. B. Dai, H. Zhang, X. Q. Wang, Y. G. Wang, B. He, X. Tang and Q. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 11443–11456 CrossRef CAS PubMed.
- X. Tan, N. Yin, Z. Liu, R. Sun, J. Gou, T. Yin, Y. Zhang, H. He and X. Tang, Mol. Pharm., 2020, 17, 3177–3191 CrossRef CAS PubMed.
- A. McShane, J. Bath, A. M. Jaramillo, C. Ridley, A. A. Walsh, C. M. Evans, D. J. Thornton and K. Ribbeck, Curr. Biol., 2021, 31, R938–R945 CrossRef CAS PubMed.
- K. Bergstrom, X. D. Shan, D. Casero, A. Batushansky, V. Lagishetty, J. P. Jacobs, C. Hoover, Y. J. Kondo, B. J. Shao, L. Gao, W. Zandberg, B. Noyovitz, J. M. McDaniel, D. L. Gibson, S. Pakpour, N. Kazemian, S. McGee, C. W. Houchen, C. V. Rao, T. M. Griffin, J. L. Sonnenburg, R. P. McEver, J. Braun and L. J. Xia, Science, 2020, 370, 467–472 CrossRef CAS PubMed.
- S. P. Bandi, Y. S. Kumbhar and V. V. K. Venuganti, J. Nanopart. Res., 2020, 22, 62 CrossRef CAS.
- Y. Gao, Y. He, H. Zhang, Y. Zhang, T. Gao, J.-H. Wang and S. Wang, J. Colloid Interface Sci., 2021, 582, 364–375 CrossRef CAS PubMed.
- Y. Zhou, Z. Chen, D. Zhao, D. Li, C. He and X. Chen, Adv. Mater., 2021, 33, 2102044 CrossRef CAS PubMed.
- Y. Guo, Y. Ma, X. Chen, M. Li, X. Ma, G. Cheng, C. Xue, Y. Y. Zuo and B. Sun, ACS Nano, 2023, 17, 2813–2828 CrossRef CAS PubMed.
- M. Yu, J. Wang, Y. Yang, C. Zhu, Q. Su, S. Guo, J. Sun, Y. Gan, X. Shi and H. Gao, Nano Lett., 2016, 16, 7176–7182 CrossRef CAS PubMed.
- M. R. Guo, M. D. Wei, W. Li, M. C. Guo, C. L. Guo, M. C. Ma, Y. Wang, Z. M. Yang, M. Li, Q. Fu, L. Yang and Z. G. He, J. Controlled Release, 2019, 307, 64–75 CrossRef CAS PubMed.
- M. Yu, L. Xu, F. Tian, Q. Su, N. Zheng, Y. Yang, J. Wang, A. Wang, C. Zhu, S. Guo, X. Zhang, Y. Gan, X. Shi and H. Gao, Nat. Commun., 2018, 9, 2607 CrossRef PubMed.
- M. Yu, W. Song, F. Tian, Z. Dai, Q. Zhu, E. Ahmad, S. Guo, C. Zhu, H. Zhong, Y. Yuan, T. Zhang, X. Yi, X. Shi, Y. Gan and H. Gao, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 5362–5369 CrossRef CAS PubMed.
- C. Zihni, C. Mills, K. Matter and M. S. Balda, Nat. Rev. Mol. Cell Biol., 2016, 17, 564–580 CrossRef CAS PubMed.
- K. Matter and M. S. Balda, Cell, 2014, 157, 992–992 CrossRef CAS PubMed.
- Y. Liu, Y. L. Zhao, B. Y. Sun and C. Y. Chen, Acc. Chem. Res., 2013, 46, 702–713 CrossRef CAS PubMed.
- C. C. Huang, R. S. Aronstam, D. R. Chen and Y. W. Huang, Toxicol. In Vitro, 2010, 24, 45–55 CrossRef CAS PubMed.
- A. A. Vita, E. A. Royse and N. A. Pullen, J. Leukocyte Biol., 2019, 106, 95–103 CrossRef CAS PubMed.
- L. Bao, X. J. Cui, X. Y. Wang, J. G. Wu, M. Y. Guo, N. Yan and C. Y. Chen, ACS Nano, 2021, 15, 15858–15873 CrossRef CAS PubMed.
- D. R. Donohoe, N. Garge, X. Zhang, W. Sun, T. M. O'Connell, M. K. Bunger and S. J. Bultman, Cell Metab., 2011, 13, 517–526 CrossRef CAS PubMed.
- A. L. Kau, P. P. Ahern, N. W. Griffin, A. L. Goodman and J. I. Gordon, Nature, 2011, 474, 327–336 CrossRef CAS PubMed.
- J. Y. Qiao, R. Chen, M. J. Wang, R. Bai, X. J. Cui, Y. Liu, C. M. Wu and C. Y. Chen, Nanoscale, 2021, 13, 8806–8816 RSC.
- S. S. Ghosh, J. Wang, P. J. Yannie and S. Ghosh, J. Endocr. Soc., 2020, 4, bvz039 CrossRef PubMed.
- Z. C. Zhang, Y. Lu, J. P. Qi and W. Wu, Acta Pharm. Sin. B, 2021, 11, 2449–2468 CrossRef CAS PubMed.
- H. N. Nellans, Adv. Drug Delivery Rev., 1991, 7, 339–364 CrossRef CAS.
- H. J. Lemmer and J. H. Hamman, Expert Opin. Drug Delivery, 2012, 10, 103–114 CrossRef PubMed.
- L. Orci, F. Malaisse-Lagae, M. Ravazzola, D. Rouiller, A. E. Renold, A. Perrelet and R. Unger, J. Clin. Invest., 1975, 56, 1066–1070 CrossRef CAS PubMed.
- L. Bao, X. J. Cui, M. Mortimer, X. Y. Wang, J. G. Wu and C. Chen, Nano Today, 2023, 49, 101784 CrossRef CAS.
- E. Hinde, K. Thammasiraphop, H. T. T. Duong, J. Yeow, B. Karagoz, C. Boyer, J. J. Gooding and K. Gaus, Nat. Nanotechnol., 2017, 12, 81–89 CrossRef CAS PubMed.
- K. J. Steffens, in Absorption of Orally Administered Enzymes, ed. M. L. G. Gardner and K. J. Steffens, Springer, Berlin, Heidelberg, 1995, pp. 9–21 Search PubMed.
- Y. Kai, Biophys. J., 2021, 120, 699–710 CrossRef CAS PubMed.
- R. Augustine, A. Hasan, R. Primavera, R. J. Wilson, A. S. Thakor and B. D. Kevadiya, Mater. Today Commun., 2020, 25, 101692 CrossRef CAS.
- S. Behzadi, V. Serpooshan, W. Tao, M. A. Hamaly, M. Y. Alkawareek, E. C. Dreaden, D. Brown, A. M. Alkilany, O. C. Farokhzad and M. Mahmoudi, Chem. Soc. Rev., 2017, 46, 4218–4244 RSC.
- X. Li, M. Yu, W. Fan, Y. Gan, L. Hovgaard and M. Yang, Expert Opin. Drug Delivery, 2014, 11, 1435–1447 CrossRef CAS PubMed.
- Y. Yang, Y. Yin, J. Zhang, T. Zuo, X. Liang, J. Li and Q. Shen, Pharmaceutics, 2018, 10, 146 CrossRef CAS PubMed.
- X. Zhu, J. Wu, W. Shan, W. Tao, L. Zhao, J.-M. Lim, M. D'Ortenzio, R. Karnik, Y. Huang, J. Shi and O. C. Farokhzad, Angew. Chem., Int. Ed., 2016, 55, 3309–3312 CrossRef CAS PubMed.
- R. Fowler, D. Vllasaliu, F. F. Trillo, M. Garnett, C. Alexander, H. Horsley, B. Smith, I. Whitcombe, M. Eaton and S. Stolnik, Small, 2013, 9, 3282–3294 CrossRef CAS PubMed.
- E. M. Pridgen, F. Alexis, T. T. Kuo, E. Levy-Nissenbaum, R. Karnik, R. S. Blumberg, R. Langer and O. C. Farokhzad, Sci. Transl. Med., 2013, 5, 213ra167 Search PubMed.
- K. A. Knoop and R. D. Newberry, Mucosal Immunol., 2018, 11, 1551–1557 CrossRef CAS PubMed.
- M. R. Neutra, A. Frey and J.-P. Kraehenbuhl, Cell, 1996, 86, 345–348 CrossRef CAS PubMed.
- N. A. Mabbott, D. S. Donaldson, H. Ohno, I. R. Williams and A. Mahajan, Mucosal Immunol., 2013, 6, 666–677 CrossRef CAS PubMed.
- J. Campbell, J. Berry and Y. Liang, in Shackelford's Surgery of the Alimentary Tract, 2 Volume Set (Eighth Edition), ed. C. J. Yeo, Elsevier, Philadelphia, 8th edn, 2019, vol. 71, pp. 817–841 Search PubMed.
- O. S. Sakhon, B. Ross, V. Gusti, A. J. Pham, K. Vu and D. D. Lo, Tissue Barriers, 2015, 3, e1004975 CrossRef PubMed.
- H. Ohno, J. Biochem., 2016, 159, 151–160 CrossRef CAS PubMed.
- A. Gebert, I. Steinmetz, S. Fassbender and K. H. Wendlandt, Am. J. Pathol., 2004, 164, 65–72 CrossRef PubMed.
- S. H. Smyth, S. Feldhaus, U. Schumacher and K. E. Carr, Int. J. Pharm., 2008, 346, 109–118 CrossRef CAS PubMed.
- K. E. Carr, S. H. Smyth, M. T. McCullough, J. F. Morris and S. M. Moyes, Prog. Histochem. Cytochem., 2012, 46, 185–252 CrossRef PubMed.
- L. Delon, R. J. Gibson, C. A. Prestidge and B. Thierry, J. Controlled Release, 2022, 343, 584–599 CrossRef CAS PubMed.
- J. J. Reineke, D. Y. Cho, Y.-T. Dingle, A. P. Morello, J. Jacob, C. G. Thanos and E. Mathiowitz, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 13803–13808 CrossRef CAS PubMed.
- J. Limpanussorn, L. Simon and A. D. Dayan, J. Pharm. Pharmacol., 1998, 50, 745–751 CrossRef CAS PubMed.
- K. A. Knoop, K. G. McDonald, S. McCrate, J. R. McDole and R. D. Newberry, Mucosal Immunol., 2015, 8, 198–210 CrossRef CAS PubMed.
- J. R. McDole, L. W. Wheeler, K. G. McDonald, B. Wang, V. Konjufca, K. A. Knoop, R. D. Newberry and M. J. Miller, Nature, 2012, 483, 345–349 CrossRef CAS PubMed.
- J. J. Powell, C. C. Ainley, R. S. Harvey, I. M. Mason, M. D. Kendall, E. A. Sankey, A. P. Dhillon and R. P. Thompson, Gut, 1996, 38, 390–395 CrossRef CAS PubMed.
- E. Brun, F. Barreau, G. Veronesi, B. Fayard, S. Sorieul, C. Chanéac, C. Carapito, T. Rabilloud, A. Mabondzo, N. Herlin-Boime and M. Carrière, Part. Fibre Toxicol., 2014, 11, 13 CrossRef PubMed.
- A. Georgantzopoulou, T. Serchi, S. Cambier, C. C. Leclercq, J. Renaut, J. Shao, M. Kruszewski, E. Lentzen, P. Grysan, S. Eswara, J.-N. Audinot, S. Contal, J. Ziebel, C. Guignard, L. Hoffmann, A. J. Murk and A. C. Gutleb, Part. Fibre Toxicol., 2016, 13, 9 CrossRef PubMed.
- D. Lichtenstein, J. Ebmeyer, T. Meyer, A.-C. Behr, C. Kästner, L. Böhmert, S. Juling, B. Niemann, C. Fahrenson, S. Selve, A. F. Thünemann, J. Meijer, I. Estrela-Lopis, A. Braeuning and A. Lampen, Eur. J. Pharm. Biopharm., 2017, 118, 21–29 CrossRef CAS PubMed.
- J. J. Powell, N. Faria, E. Thomas-McKay and L. C. Pele, J. Autoimmun., 2010, 34, J226–J233 CrossRef CAS PubMed.
- M. I. Setyawati, C. Y. Tay and D. T. Leong, Small, 2015, 11, 3458–3468 CrossRef CAS PubMed.
- K. Gerloff, C. Albrecht, A. W. Boots, I. Förster and R. P. F. Schins, Nanotoxicology, 2009, 3, 355–364 CrossRef CAS.
- A. Manke, L. Wang and Y. Rojanasakul, BioMed Res. Int., 2013, 2013, 942916 Search PubMed.
- N. Andre, X. Tian, M. Lutz and N. Li, Science, 2006, 311, 622–627 CrossRef PubMed.
- A. Datta, S. Mishra, K. Manna, K. D. Saha, S. Mukherjee and S. Roy, ACS Omega, 2020, 5, 9714–9723 CrossRef CAS PubMed.
- B. Fubini and A. Hubbard, Free Radicals Biol. Med., 2003, 34, 1507–1516 CrossRef CAS PubMed.
- S. Lee, H. S. Yun and S. H. Kim, Biomaterials, 2011, 32, 9434–9443 CrossRef CAS PubMed.
- P. Khanna, C. Ong, B. H. Bay and G. H. Baeg, Nanomaterials, 2015, 5, 1163–1180 CrossRef CAS PubMed.
- N. Yan, J. C. Xu, G. L. Liu, C. Ma, L. Bao, Y. L. Cong, Z. Y. Wang, Y. L. Zhao, W. H. Xu and C. Y. Chen, ACS Nano, 2022, 16, 18253–18265 CrossRef CAS PubMed.
- X. Wei, B. Shao, Z. He, T. Ye, M. Luo, Y. Sang, X. Liang, W. Wang, S. Luo, S. Yang, S. Zhang, C. Gong, M. Gou, H. Deng, Y. Zhao, H. Yang, S. Deng, C. Zhao, L. Yang, Z. Qian, J. Li, X. Sun, J. Han, C. Jiang, M. Wu and Z. Zhang, Cell Res., 2015, 25, 237–253 CrossRef CAS PubMed.
- L. Baron, A. Gombault, M. Fanny, B. Villeret, F. Savigny, N. Guillou, C. Panek, M. Le Bert, V. Lagente, F. Rassendren, N. Riteau and I. Couillin, Cell Death Dis., 2015, 6, e1629–e1629 CrossRef CAS PubMed.
- R. Mohammapdour and H. Ghandehari, Adv. Drug Delivery Rev., 2022, 180, 114022 CrossRef CAS PubMed.
- C. Dostert, V. Pétrilli, R. Van Bruggen, C. Steele, B. T. Mossman and J. Tschopp, Science, 2008, 320, 674–677 CrossRef CAS PubMed.
- V. Hornung, F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald and E. Latz, Nat. Immunol., 2008, 9, 847–856 CrossRef CAS PubMed.
- S. C. Eisenbarth, O. R. Colegio, W. O'Connor, F. S. Sutterwala and R. A. Flavell, Nature, 2008, 453, 1122–1126 CrossRef CAS PubMed.
- B. B. Sun, X. Wang, Z. X. Ji, R. B. Li and T. Xia, Small, 2013, 9, 1595–1607 CrossRef CAS PubMed.
- H. Chen, R. Zhao, B. Wang, L. Zheng, H. Ouyang, H. Wang, X. Zhou, D. Zhang, Z. Chai, Y. Zhao and W. Feng, Adv. Healthcare Mater., 2018, 7, 1701313 CrossRef PubMed.
- B. Wu, L. Chen, X. Wu, H. Hou, Z. Wang and S. Liu, Environ. Sci. Nano, 2019, 6, 1594–1606 RSC.
- D. Boraschi, P. Italiani, R. Palomba, P. Decuzzi, A. Duschl, B. Fadeel and S. M. Moghimi, Semin. Immunol., 2017, 34, 33–51 CrossRef CAS PubMed.
- P. M. Gallo and S. Gallucci, Front. Immunol., 2013, 4, 10 Search PubMed.
- A. Liston and S. L. Masters, Nat. Rev. Immunol., 2017, 17, 208–214 CrossRef CAS PubMed.
- M. Tsugita, N. Morimoto, M. Tashiro, K. Kinoshita and M. Nakayama, Cell Rep., 2017, 18, 1298–1311 CrossRef CAS PubMed.
- S. Omori, M. Tsugita, Y. Hoshikawa, M. Morita, F. Ito, S.-I. Yamaguchi, Q. L. Xie, O. Noyori, T. Yamaguchi, A. Takada, T. Saitoh, S. Toyokuni, H. Akiba, S. Nagata, K. Kinoshita and M. Nakayama, Cell Rep., 2021, 34, 108734 CrossRef CAS PubMed.
- M. K. Greene and C. J. Scott, Nat. Nanotechnol., 2023, 18, 544–545 CrossRef CAS PubMed.
- M. Kuroiwa, S.-I. Yamaguchi, Y. Kato, A. Hori, S. Toyoura, M. Nakahara, N. Morimoto and M. Nakayama, Sci. Total Environ., 2023, 875, 162586 CrossRef CAS PubMed.
- C. Huang, M. Y. Sun, Y. Yang, F. Wang, X. Q. Ma, J. Q. Li, Y. L. Wang, Q. R. Ding, H. Ying, H. Y. Song, Y. N. Wu, Y. G. Jiang, X. D. Jia, Q. Ba and H. Wang, Nanotoxicology, 2017, 11, 737–750 Search PubMed.
- Q. Chen, N. N. Wang, M. J. Zhu, J. H. Lu, H. Q. Zhong, X. L. Xue, S. Y. Guo, M. Li, X. B. Wei, Y. Z. Tao and H. Y. Yin, Redox Biol., 2018, 15, 266–276 CrossRef CAS PubMed.
- M. Kovacsovics-Bankowski, K. Clark, B. Benacerraf and K. L. Rock, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 4942–4946 CrossRef CAS PubMed.
- P. Ashwood, R. P. H. Thompson and J. J. Powell, Exp. Biol. Med., 2007, 232, 107–117 CAS.
- S. M. Evans, P. Ashwood, A. Warley, F. Berisha, R. P. H. Thompson and J. J. Powell, Gastroenterology, 2002, 123, 1543–1553 CrossRef CAS PubMed.
- J. D. Söderholm, Nat. Nanotechnol., 2015, 10, 298–299 CrossRef PubMed.
- L. M. Francisco, P. T. Sage and A. H. Sharpe, Immunol. Rev., 2010, 236, 219–242 CrossRef CAS PubMed.
- A. Cifuentes-Rius, A. Desai, D. Yuen, A. P. R. Johnston and N. H. Voelcker, Nat. Nanotechnol., 2021, 16, 37–46 CrossRef CAS PubMed.
- J. Y. Mu, X. Y. Zhuang, Q. L. Wang, H. Jiang, Z. B. Deng, B. M. Wang, L. F. Zhang, S. Kakar, Y. Jun, D. Miller and H. G. Zhang, Mol. Nutr. Food Res., 2014, 58, 1561–1573 CrossRef CAS PubMed.
- B. Ahluwalia, M. K. Magnusson and L. Öhman, Scand. J. Gastroenterol., 2017, 52, 1185–1193 CrossRef CAS PubMed.
- I. Belyakov, E. Zimmermann, D. Isakov, E. Fisk, N. Orr, L. Schmiedlin-Ren, S. K. Choi and J. Baker, J. Immunol., 2012, 188, 52.1 CrossRef.
- J. J. Li, W. L. Hou, S. Lin, L. Wang, C. Pan, F. Wu and J. Y. Liu, Adv. Sci., 2022, 9, 2104006 CrossRef CAS PubMed.
- G. Volkheimer, Ann. N. Y. Acad. Sci., 1975, 246, 164–171 CrossRef CAS PubMed.
- N. L. Trevaskis, L. M. Kaminskas and C. J. H. Porter, Nat. Rev. Drug Discovery, 2015, 14, 781–803 CrossRef CAS PubMed.
- S. H. Kim and Y. S. Jang, Exp. Mol. Med., 2014, 46, e85–e85 CrossRef CAS PubMed.
- S. A. Ejazi, R. Louisthelmy and K. Maisel, ACS Nano, 2023, 17, 13044–13061 CrossRef CAS PubMed.
- J. Bernier-Latmani and T. V. Petrova, Nat. Rev. Gastroenterol. Hepatol., 2017, 14, 510–526 CrossRef CAS PubMed.
- X. Y. Wang, X. J. Cui, J. G. Wu, L. Bao, Z. Q. Tan and C. Chen, Sci. Adv., 2023, 9, eadg2252 CrossRef CAS PubMed.
- G. Grant, Brain Res. Rev., 2007, 55, 490–498 CrossRef CAS PubMed.
- E. M. Sternberg, Nat. Rev. Immunol., 2006, 6, 318–328 CrossRef CAS PubMed.
- N. J. Rothwell and S. J. Hopkins, Trends Neurosci., 1995, 18, 130–136 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |