Bi(OTf)3-promoted cascade annulation of hydroxy-pyranones and unsaturated γ-ketoesters for the construction of polycyclic bridged pyrano-furopyranones†
Akshay B.
Rathod
ab,
Balasaheb R.
Borade
ab,
Pooja I.
Sambherao
ab and
Ravindar
Kontham
 *ab
*ab
aOrganic Chemistry Division, CSIR-National Chemical Laboratory Dr Homi Bhabha Road, Pune-411008, India. E-mail: k.ravindar@ncl.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
First published on 20th December 2023
Abstract
An efficient protocol for constructing complex three dimensional polycyclic bridged chromano-furopyranones and pyrano-furopyranones (closely related to bioactive natural products) via bismuth(III)-catalyzed cascade annulation of hydroxy-pyranones and unsaturated γ-ketoesters is presented. This process involves intermolecular Michael addition, intramolecular hemiketalization, lactonization, formation of one C–C bond and two C–O bonds, rings, and contiguous stereocenters.
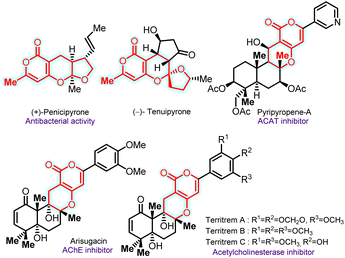
Chromane and pyrone-fused furo-pyranones are found in natural products and pharmaceuticals, with various applications, including cellular imaging and solar cells.1 For instance, (+)-penicipyrone, isolated from the fungus Penicillium sp. PSU-F44, exhibits antibacterial activity.2 On the other hand, (−)-tenuipyrone was isolated from the entomopathogenic fungus Isaria tenuipes in the presence of epigenetic modifying agents, including a histone deacetylase inhibitor and a DNA methyltransferase inhibitor.3 Pyripyropenes-A–D, isolated from Aspergillus fumigatus FO-1289, are potent acyl-CoA inhibitors and stand out as the most potent naturally derived ACAT inhibitors, with nanomolar IC50 values in rat liver microsomes.4 Arisugacin functions as an acetylcholinesterase (AChE) inhibitor, while territrems A–C, with a pyrano-pyran skeleton, selectively inhibit human AChE (Fig. 1).5,6 The intriguing aspects of these features have led to a sustained emphasis on developing efficient methodologies for synthesizing chroman/pyrone-derived scaffolds in synthetic chemistry.7
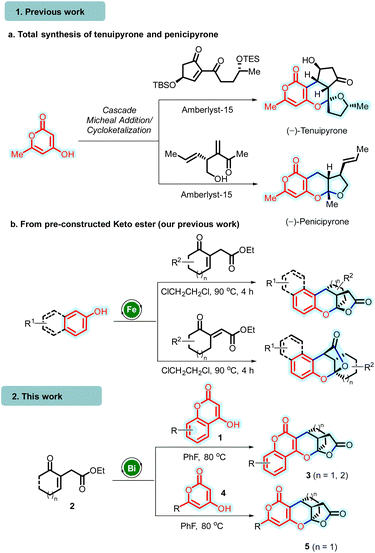
In this context, Tong and co-workers disclosed an expedited strategy for constructing pyrone-tethered [5,6]-spiroketals through amberlyst-15 promoted intermolecular annulative cyclo-ketalization (proceeds through Michael addition/hemiketolization and spiroketalization sequence) of 4-hydroxy 6-methyl-2-pyrone with α,β-unsaturated 1,3-diketones. This strategy was successfully employed in their biomimetic total synthesis of (−)-penicipyrone and (−)-tenuipyrone (entry 1a, Scheme 1).8 In 2020, Zhang's group reported an organocatalytic asymmetric reaction involving 4-hydroxycoumarins and 2-hydroxy cinnamaldehydes. This reaction proceeded via conjugate addition, facilitating the construction of chiral bridged acetals (Scheme 1).9
In continuation of our interest in developing atom and step-efficient cascade annulation reactions utilizing Lewis acid catalysis,10 recently, we unveiled a Fe(III)-catalyzed cascade annulation involving electron-rich hydroxyarenes and suitably functionalized unsaturated γ-ketoesters.11 This approach enabled the synthesis of polycyclic bridged/fused 2-chromanol lactones, introducing three new bonds, stereocenters, and new rings into the molecular framework (entry 1b, Scheme 1).12 Herein, we report the unprecedented synthesis of polycyclic bridged chromano (pyrano)-furopyranones 3/5 (which represent lactone analogs akin to penicipyrone and tenuipyrone) through bismuth(III)-catalyzed10,12 cascade annulation of chromenones/hydroxy-pyranones 1/4 and unsaturated γ-ketoesters 2 (entry 2, Scheme 1).
We initiated the reaction optimization studies by selecting commercially available 4-hydroxycoumarin (1a) and known12 unsaturated γ-ketoester 2a (featuring cyclohexenone Michael acceptor) as substrates (Table 1). Drawing from our previous research and guided by literature examples involving Brønsted acid catalysis in Michael addition-induced cascade processes, we began by assessing various catalysts such as TfOH, TFA, p-TSA, PPTS, and Amberlyst-15 (used at 20 mol%) in combination with DCE as the reaction medium. These initial reactions did not progress at room temperature (27 °C). Encouragingly, we found that TfOH, TFA, and amberlyst-15 demonstrated varying degrees of activity, leading to the formation of the desired annulation product 3aa with isolated yields of 41%, 24%, and 17% for product 3aa, respectively at 80 °C (entries 1–5 in Table 1). The product 3aa was confirmed through 1H and 13C NMR (DEPT) and HRMS analyses and further verified by comparing the obtained data to our previously reported findings for similar bridged ketal-lactones (Table 1).12
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise specified the reaction was performed with 1a (0.55 mmol), 2a (0.55 mmol), catalyst (20 mol%), and in indicated solvent (anhydrous, 2 mL) at 80 °C. b Isolated yield of 3aa. c No conversion was observed. | |||
| 1 | TfOH | DCE | 41 |
| 2 | TFA | DCE | 24 |
| 3 | PTSA | DCE | –c |
| 4 | PPTS | DCE | –c |
| 5 | Amberlyst-15 | DCE | 17 |
| 6 | Fe(OTf)3 | DCE | 51 |
| 7 | AgOTf | DCE | 24 |
| 8 | Cu(OTf)2 | DCE | 37 |
| 9 | Sc(OTf)3 | DCE | 20 |
| 10 | BF3·Et2O | DCE | 18 |
| 11 | Bi(OTf)3 | DCE | 70 |
| 12 | Bi(OTf) 3 | PhF | 76 |
| 13 | No catalyst | PhF | –c |
Subsequently, our focus shifted towards investigating the impact of various Lewis acids on this annulation process.14,15 To this end, we initially employed the conditions we had previously identified12 20 mol% of Fe(OTf)3 in DCE at 80 °C. Under these conditions, 3aa was obtained in an improved yield of 51% in an 8-hour reaction (entry 6, Table 1). Expanding our exploration, we subjected the reaction to different metal triflates catalysts including AgOTf, Cu(OTf)2, Sc(OTf)3, and BF3·Et2O. However, these alternative Lewis acids resulted in comparably lower yields of 3aa when compared to Fe(OTf)3 (entries 7–10). The reaction using 20 mol% of Bi(OTf)3 in DCE at 80 °C resulted in an improved yield of 70% (entry 11). Interestingly, when employing PhF as the solvent, the reaction furnished 3aa with a favorable outcome of 76% and exhibited a clean thin-layer chromatography (TLC) profile (entry 12).
As anticipated, the reaction failed to progress in the absence of the catalyst, leading to full recovery of both annulation partners 1a and 2a (entry 13) (Table 1). Notably, Bi(OTf)3 displayed moderate activity when PhCl, THF, and CH3CN were used as solvents (entries 1–3, Table S1†), while its activity ceased when solvents like DMF, toluene, MeOH, and EtOH were employed (entries 4–7, Table S1†).13 Further alteration of reaction parameters like molar ratios of substrates and catalyst (Bi(OTf)3) loading (5 and 10 mol%, entries 8 and 9, Table S1†) did not lead to discernible improvement.13 Ultimately, it was determined that the ideal conditions for this cascade annulation reaction were the use of Bi(OTf)3 (20 mol%) in PhF at 80 °C (entry 12, Table 1).
With the optimal reaction condition in hand, we next evaluated the scope and generality of this cascade reaction concerning the 4-hydroxy pyranones (1) and unsaturated γ-ketoesters 2 possessing diverse substituents (Scheme 2).
 | ||
| Scheme 2 Scope of the cascade annulation of hydroxy-chromenones/hydroxy-pyranones (1/4) with cycloalkenone-tethered unsaturated γ-ketoesters (2). | ||
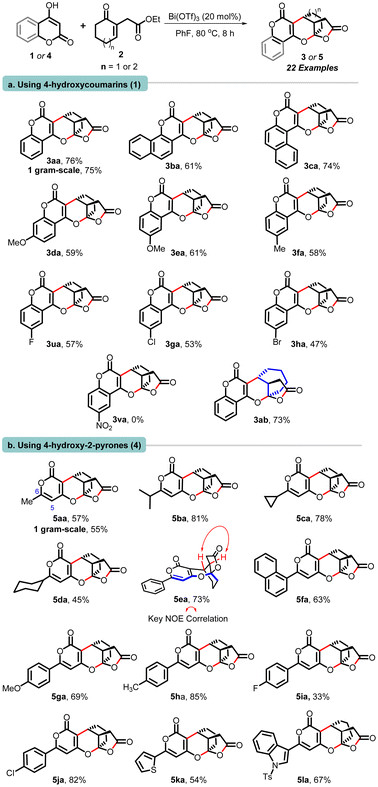
The reaction involving 4-hydroxy-2H-chromen-2-ones possessing phenyl, α-naphthyl, and β-naphthyl segments (1a–1c) proceeded well with cyclohexenone-tethered ketoester 2a, and delivered corresponding polycyclic adducts 3aa−3ca in good yields ranging from 61% to 76%. Moving forward, hydroxy-chromenones containing electron-donating substituents (–OMe, –Me) 1d, 1e and 1f were treated with 2a, which furnished products 3da, 3ea, and 3fa. Halogenated substrates 1 also reacted well and delivered adducts 3ua, 3ga, and 3ha in good yields. Conversely, hydroxy-chromenones having electron-withdrawing substituents (–NO2) did not engage in the reaction with 2a, and both starting materials were recovered. Interestingly, cycloheptenone bearing ketoester 2b also participated well in the annulation with hydroxy-2H-chromen-2-one (1a), culminating in the formation of product 3ab with a yield of 73% (entry a, Scheme 3). Whereas cyclopentenone-bearing ketoester 2c failed to participate in the annulation.13
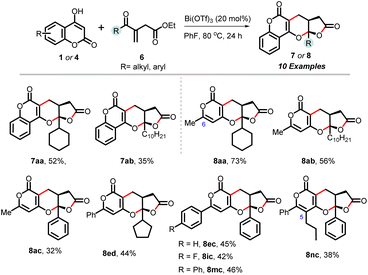 | ||
| Scheme 3 Scope of the cascade annulation of hydroxy-chromenones/hydroxy-pyranones (1/4) with acyclic enone-tethered unsaturated γ-ketoesters (6). | ||
Expanding on our protocol, we explored the reactions of 6-substituted hydroxy-pyranones 4 using optimized conditions. Encouragingly, diverse substituents at C-6 (-methyl, -i-Pr, -cyclopropyl, cyclohexyl, phenyl, α-naphthyl, anisyl, tolyl, p-fluoro-phenyl, p-chloro-phenyl) successfully reacted with cyclohexenone-tethered ketoester 2a, yielding (pyrano)-furopyranones adducts 5aa–5ja in yields ranging from 45% to 85%. Additionally, pyranones derived from heteroarenes (thiophenyl and N-tosyl-indolyl) produced 5ka and 5la in yields of 54% and 67%, respectively. Notably, C-5 substituted pyrones did not engage in this annulation (entry b, Scheme 2).13 Next, we demonstrated the practicality and scalability of this protocol by conducting reactions on a 1.0-gram scale of 1a and 4a, resulting in good yields of 3aa and 5aa. The relative stereochemistry of these adducts was assigned based on our previous report,11 NOE correlations of 5ea, and analogy.13
Encouraged by these results, we investigated the reactivity of unsaturated γ-ketoesters 6, which contain an acyclic enone and diverse substituents (cyclohexyl, decyl, phenyl, cyclopentyl), with hydroxy chromenone (1a) and various pyrones (4). All these reactions proceeded well, delivering the corresponding chromenone-derived adducts (7aa and 7ab), as well as pyrone-tethered adducts (8aa–8ac, 8ed, 8ec, 8ic and 8mc), in moderate yields in 24 hours. Interestingly, the C-5 substituted pyrone (4n) also participated in this annulation, yielding 8nc in a 38% yield (Scheme 3).
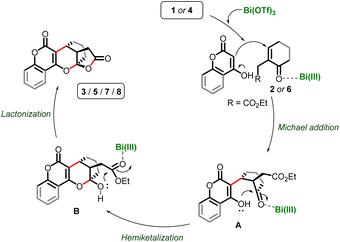
Based on previous reports from our group11 and others,8,9 as well as the results obtained in this study, we propose a plausible mechanistic sequence for this cascade annulation, outlined in Scheme 4.8,10–12 The Bi(III)-activated enone partners 2/6, trigger the Michael addition with the electron-rich hydroxy-chromen(pyran)-one 1/4, resulting in the formation of intermediate A. Subsequent intramolecular hemiketalization of A leads to the formation of intermediate B. This hemiketal intermediate B then undergoes Bi(III)-facilitated lactonization, yielding chromano(pyrano)-furopyranones 3/5/7/8.
In conclusion, we have developed a novel protocol for synthesizing intricate polycyclic bridged chromano-furopyranones and pyrano-furopyranones, which are relevant to bioactive natural compounds. This approach involves the Bi(III)-catalyzed cascade annulation of hydroxy-chromenones/hydroxy-pyranones with unsaturated γ-ketoesters. The reaction pathway encompasses a sequence of transformations, including Michael addition, hemiketalization, and lactonization. Our method has successfully yielded diverse three-dimensional polycyclic adducts akin to natural products such as tenuipyrone and penicipyrone, achieving favorable yields. Notably, the practicality of this methodology has been demonstrated through gram-scale experiments. Ongoing efforts are directed toward exploring the biological activities of these synthesized products, and we anticipate publishing these findings in due course.
Author contributions
R. K. conceived the project and directed the research work. A. B. R, B. R. B, and P. I. S conducted synthetic experiments, analyzed data, and prepared ESI. All authors commented on the manuscript and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We express our sincere gratitude for the funding received from the SERB (Science & Engineering Research Board), New Delhi, India (Grant No. CRG/2020/001875). Additionally, B. R. B. would like to thank UGC-India, and P. I. S. extends appreciation to DST-Inspire for granting the Senior Research Fellowships (SRF).References
- (a) J. R. S. Hoult and M. Payá, Gen. Pharmacol., 1996, 27, 713–722 CrossRef CAS PubMed; (b) M. Sarmah, K. Chutia, D. Dutta and P. Gogoi, Org. Biomol. Chem., 2022, 20, 55–72 RSC; (c) G. Signore, R. Nifosì, L. Albertazzi, B. Storti and R. Bizzarri, J. Am. Chem. Soc., 2010, 132, 1276–1288 CrossRef CAS PubMed; (d) K. G. Reddie, W. H. Humphries, C. P. Bain, C. K. Payne, M. L. Kemp and N. Murthy, Org. Lett., 2012, 14, 680–683 CrossRef CAS PubMed , and references cited therein.
- K. Trisuwan, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Chem. Pharm. Bull., 2009, 57, 1100–1102 CrossRef CAS PubMed.
- T. Asai, Y. M. Chung, H. Sakurai, T. Ozeki, F. R. Chang, K. Yamashita and Y. Oshima, Org. Lett., 2012, 14, 513–515 CrossRef CAS PubMed.
- T. Ohshiro, D. Matsuda, K. Sakai, C. Degirolamo, H. Yagyu, L. L. Rudel, S. Omura, S. Ishibashi and H. Tomoda, Arterioscler. Thromb. Vasc. Biol., 2011, 31, 1108–1115 CrossRef CAS PubMed.
- T. Sunazuka, M. Handa, K. Nagai, T. Shirahata, Y. Harigaya, K. Otoguro, I. Kuwajima and S. Omura, Org. Lett., 2002, 4, 367–369 CrossRef CAS PubMed.
- (a) F. C. Peng, C. M. Chiou and K. H. Ling, J. Nat. Prod., 1992, 55, 251–255 CrossRef PubMed; (b) J. Cheung, E. N. Gary, K. Shiomi and T. L. Rosenberry, ACS Med. Chem. Lett., 2013, 4, 1091–1096 CrossRef CAS PubMed.
- (a) M. Lončarić, D. G. Sokač, S. Jokić and M. Molnar, Biomolecules, 2020, 10, 151 CrossRef PubMed; (b) K. Szwaczko, Inorganics, 2022, 10, 23 CrossRef CAS; (c) S. Gulati, R. Singh and S. Sangwan, RSC Adv., 2021, 11, 29130–29155 RSC; (d) D. Dobler, M. Leitner, N. Moor and O. Reiser, Eur. J. Org. Chem., 2021, 6180–6205 CrossRef CAS, and references cited therein. (e) S. Y. Chen, Q. Li, X. G. Liu, J. Q. Wu, S. S. Zhang and H. Wang, ChemSusChem, 2017, 10, 2360–2364 CrossRef CAS PubMed; (f) Y. Liu, S. Lin, Y. Li, J. H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef CAS.
- (a) L. Song, H. Yao, L. Zhu and R. Tong, Org. Lett., 2013, 15, 6–9 CrossRef CAS PubMed; (b) H. Yao, L. Song, Y. Liu and R. Tong, J. Org. Chem., 2014, 79, 8774–8785 CrossRef CAS PubMed.
- X. Q. Zhang, X. J. Lv, J. P. Pei, R. Tan and Y. K. Liu, Org. Chem. Front., 2020, 7, 292–297 RSC.
- (a) D. A. Kambale, S. S. Thorat, M. S. Pratapure, R. G. Gonnade and R. Kontham, Chem. Commun., 2017, 53, 6641–6644 RSC; (b) S. S. Thorat, P. Kataria and R. Kontham, Org. Lett., 2018, 20, 872–875 CrossRef CAS PubMed; (c) A. K. Nakate, M. S. Pratapure and R. Kontham, Org. Biomol. Chem., 2018, 16, 3229–3240 RSC; (d) D. A. Kambale, B. R. Borade and R. Kontham, Org. Biomol. Chem., 2021, 19, 6618–6622 RSC; (e) A. K. Nakate, S. S. Thorat, S. Jain, G. Rama Krishna, K. Vanka and R. Kontham, Org. Chem. Front., 2022, 9, 802–809 RSC; (f) Y. Mankad, S. S. Thorat, P. Das, G. Rama Krishna, R. Kontham and D. S. Reddy, J. Org. Chem., 2022, 87, 3025–3041 CrossRef CAS PubMed; (g) D. A. Kambale, B. R. Borade, R. Vinodkumar and R. Kontham, J. Org. Chem., 2023, 88, 12597–12612 CrossRef CAS PubMed.
- B. R. Borade, R. Nomula, R. G. Gonnade and R. Kontham, Org. Lett., 2019, 21, 2629–2633 CrossRef CAS PubMed.
- (a) H. Gaspard-Iloughmane and C. Le Roux, Eur. J. Org. Chem., 2004, 2517–2532 CrossRef CAS; (b) T. Ollevier, Org. Biomol. Chem., 2013, 11, 2740–2755 RSC; (c) J. M. Bothwell, S. W. Krabbe and R. S. Mohan, Chem. Soc. Rev., 2011, 40, 4649–4707 RSC; (d) M. Magre and J. Cornella, J. Am. Chem. Soc., 2021, 143, 21497–21502 CrossRef CAS PubMed; (e) E. Lopez, S. C. Thorp and R. S. Mohan, Polyhedron, 2022, 222, 115765 CrossRef CAS , and references cited therein.
- See the ESI† for details.
- (a) H. Yamamoto, Lewis Acids in Organic Synthesis, Wiley-VCH, 2008 Search PubMed; (b) A. Corma and H. García, Chem. Rev., 2003, 103, 4307–4365 CrossRef CAS PubMed; (c) Y. Yamamoto, J. Org. Chem., 2008, 73, 5210 CrossRef CAS; (d) S. Kobayashi, M. Sugiura, H. Kitagawa and W. W. L. Lam, Chem. Rev., 2002, 102, 2227–2302 CrossRef CAS PubMed.
- (a) N. G. Turrini, R. C. Cioc, D. J. H. Van Der Niet, E. Ruijter, R. V. A. Orru, M. Hall and K. Faber, Green Chem., 2017, 19, 511–518 RSC; (b) P. Chen and S. Wang, Tetrahedron, 2012, 68, 5356–5362 CrossRef CAS; (c) S. Antoniotti, V. Dalla and E. Duñach, Angew. Chem., Int. Ed., 2010, 49, 7860–7888 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ob01862h |
| This journal is © The Royal Society of Chemistry 2024 |