Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthetic approaches of carbohydrate based self-assembling systems
Guijun
Wang
 *,
Anji
Chen
,
Pramod
Aryal
*,
Anji
Chen
,
Pramod
Aryal
 and
Jonathan
Bietsch
and
Jonathan
Bietsch
Department of Chemistry and Biochemistry, Old Dominion University, Norfolk, VA 23529, USA. E-mail: g1wang@odu.edu; Fax: +(757) 683-4628; Tel: +(757) 683-3781
First published on 25th May 2024
Abstract
Carbohydrate-based self-assembling systems are essential for the formation of advanced biocompatible materials via a bottom-up approach. The self-assembling of sugar-based small molecules has applications encompassing many research fields and has been studied extensively. In this focused review, we will discuss the synthetic approaches for carbohydrate-based self-assembling (SA) systems, the mechanisms of the assembly, as well as the main properties and applications. This review will mainly cover recent publications in the last four years from January 2020 to December 2023. We will essentially focus on small molecule self-assembly, excluding polymer-based systems, which include various derivatives of monosaccharides, disaccharides, and oligosaccharides. Glycolipids, glycopeptides, and some glycoconjugate-based systems are discussed. Typically, in each category of systems, the system that can function as low molecular weight gelators (LMWGs) will be discussed first, followed by self-assembling systems that produce micelles and aggregates. The last section of the review discusses stimulus-responsive self-assembling systems, especially those forming gels, including dynamic covalent assemblies, chemical-triggered systems, and photoresponsive systems. The review will be organized based on the sugar structures, and in each category, the synthesis of representative molecular systems will be discussed next, followed by the properties of the resulting molecular assemblies.
1. Introduction
Carbohydrate-based self-assembling systems are important for the formation of advanced biocompatible materials with different functions and they have numerous applications. In this review, we will discuss the recent development of sugar-based small molecules and their self-assemblies, as well as the construction of large molecular assemblies by noncovalent interactions and dynamic covalent interactions. The main sections of the review are based on the structures of carbohydrates: monosaccharides, disaccharides, oligosaccharides, and branched systems. The formation of the self-assembling carbohydrates for functional materials including organogels and hydrogels, as well as the applications for optical probes, and biomedical applications for diagnostics and therapeutics, will be discussed. In the last section, various stimulus-responsive carbohydrate-based assemblies will be reviewed, with an emphasis on dynamic covalent, chemical triggered, and photoresponsive systems. The formation of dynamic covalent bonds is often used for the preparation of functional assemblies and reversible supramolecular gels.Carbohydrate-based self-assembling systems encompass a broad range of materials and fields. Many important reviews discuss various aspects of these systems. These include, for example, carbohydrate-based macromolecular materials,1 supramolecular chemistry and glycoconjugates,2 and click chemistry and its applications in glycoscience.3,4 The preparation of glycomimetics and multivalent glycoconjugates and their applications for lectin binding have been reviewed.5–7 Carbohydrate-based chiral assemblies10 and carbohydrate-derived amphiphiles and their applications for materials and medicines have also been reviewed recently.8,9 Carbohydrate-based molecular gelators as special classes of carbohydrate-based molecular assemblies have been examined.11–13 However, the reviews for gelators typically did not include their syntheses. In this review, we wish to summarize the general methods in the preparation of carbohydrate-based self-assembling systems and aim to provide not only the structures of compounds that can form gels or other SA architectures, but also the general methods for their chemical synthesis.
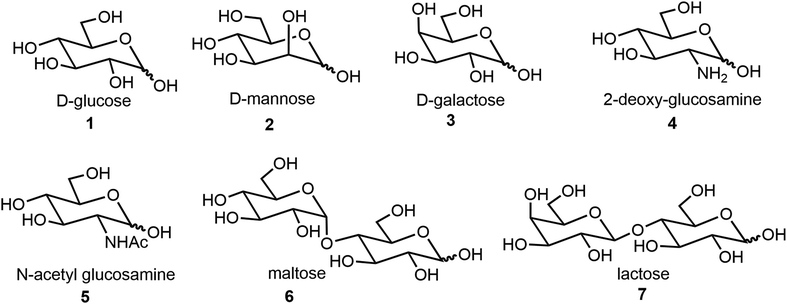
We will first discuss self-assemblies from monosaccharides, disaccharides, and oligosaccharides, and lastly stimulus-responsive sugar derivatives. Most of the synthesis starts from commercially available starting materials. The structures of sugar scaffolds that are often used for the synthesis of self-assembling systems are shown in Fig. 1. These include the common hexoses 1–5 and disaccharides 6–7. The sugars discussed in this review are D-sugars unless specifically defined. Monosaccharide derivatives are a major class of compounds that form low molecular weight gelators (LMWGs) and other assemblies, and this section will include more detailed examples.
2. Monosaccharide derivatives
Common hexoses such as glucose (Glc), galactose (Gal), mannose (Man) and glucosamine (Fig. 1), and sugar alcohols such as sorbitol (glucitol) and mannitol, as well as some pentoses, have all been utilized as building blocks for the formation of carbohydrate-based self-assembling systems. In this section, the carbohydrate derivatives are organized into three main categories based on the glycoside linkages: O-glycosides, N-glycosides, and all other types of glycoside derivative including glycopeptides and glycolipids. These will be discussed in the same order in the following sections.2.1 O-Glycosides
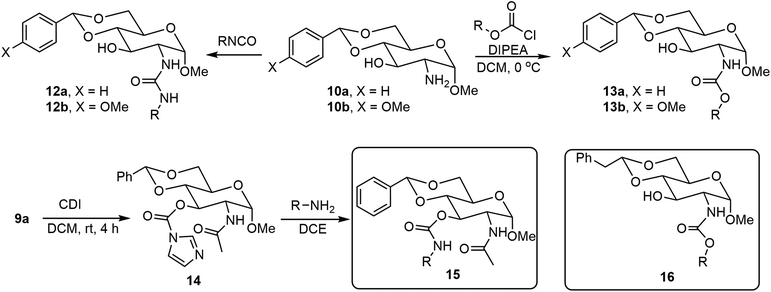
The formation of O-glycosides can be obtained by the reaction of monosaccharides with the corresponding alcohols via straightforward reactions. The synthesis of both anomers from free sugars can be achieved readily by several methods. An advantage of the formation of glycoside is that the sugar structures can be synthesized in optically pure forms and anomers can be obtained as single isomers. In addition to fixing the configuration, different functional groups can also be introduced to the anomeric positions. Many derivatives with alpha-methoxy glycosides have been synthesized due to the ease of formation, and the methoxy group is the smallest O-glycoside.11 As shown in Scheme 1, N-acetyl-D-glucosamine (NAG) 5 has been used extensively for the formation of various self-assembling systems due to the presence of the 2-amino group, which can be utilized for further functionalization.14,15 NAG 5 can be converted to glycoside 8, the 4,6-hydroxyl groups are then functionalized by the formation of the acetal 9, the pure alpha isomer can be obtained by recrystallization or chromatography, and deacetylation under alkaline conditions using a microwave synthesizer leads to the corresponding sugar amine alcohol 10. The 2-amino group can be further functionalized with different functional groups to obtain LMWGs (Scheme 2). Compound 10a can be converted to the corresponding amides 11, ureas 12, and carbamates 13. Several series of derivatives with the general structures 11a, 11b, 11c, 12a, 12b, 13a, and 13b have been synthesized and have demonstrated gelation properties in organic solvents, mixtures of alcohol with water or dimethyl sulfoxide (DMSO) with water, and water, depending on the nature of the R group.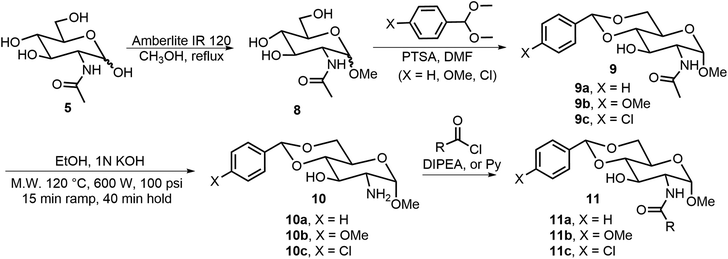 | ||
| Scheme 1 Synthesis of methyl glycoside 8, the derivatives 9 and 10 as headgroups, and the amides 11 as LMWGs. | ||
N-Acetyl-D-glucosamine-based 3-O-carbamates 15 were prepared by first acylation of 3-OH with 1,1′-carbonyldiimidazole (CDI) followed by displacing the imidazole group with a primary amine.16 Alternatively, functionalizing 3-OH with isocyanate in the presence of DBU17 in one step can also afford the 3-O-carbamates 15. Many derivatives in this series were effective LMWGs, especially for aqueous mixtures. The gelator 15 (when R′ = p-methoxyl benzyl) formed spontaneous gels when adding aqueous solutions of metal ions to the gelator solution dissolved in a small amount of DMSO. Wang's group also reported several C-2 N-linked carbamates of the 4,6-O-phenylethylidene acetal protected D-glucosamine derivatives 16,18 which were prepared by commercially available chloroformate and the previous literature-reported amine headgroup.19 Among the synthesized carbamates, the isopropyl derivative (16, R = iPr) is a hydrogelator and can form hydrogel at 0.2 wt%. This hydrogelator gels with tetra alkyl ammonium salts to create effective gel electrolytes.
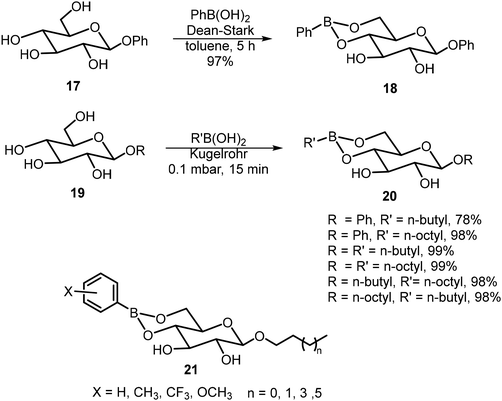
Ludwig et al. synthesized a series of boronate derivatives of O-glucosides and studied their organogelation properties.20,21 The synthesis was carried out by heating the glucosides with the corresponding boronic acids and the removal of water, as shown in Schemes 3. A few boronate derivatives were able to form gels in toluene. The 4,6-boronate group can be hydrolyzed with the addition of a small amount of water, resulting in the degradation of the corresponding organogels. The extent of the water sensitivity of the gels formed by 21 was evaluated.21 The monomethyl and p-methoxy group on the phenyl ring did not affect gelation, but dimethyl substituted and CF3 groups increased the solubility and reduced the gelation tendencies. Also, the alkyl chain lengths seem to affect the water sensitivity more significantly than the aryl substituents.
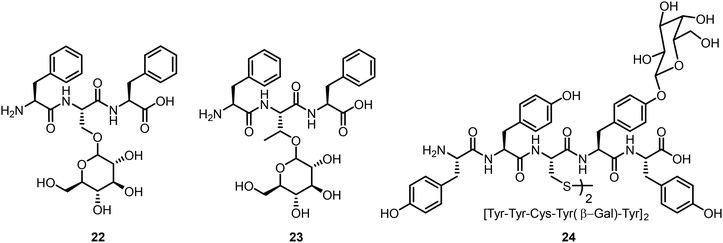
The glycosylated tripeptides 22 and 23 were synthesized from the corresponding Fmoc protected Ser or Thr glucosides via solid-phase peptide synthesis (Fig. 2).22 The presence of the glucose unit affected the tripeptides’ aggregation properties, with enhanced disorders and dynamics due to the introduced CH–π interaction. Glycopeptides containing disulfide linkages were also synthesized and the self-assembling properties were investigated.23 Compound 24 is one of these compounds from a library of glycopeptides which contain different monosaccharides; these glycopeptides were synthesized by peptide coupling reactions from glycosylated tyrosines. Various monosaccharides were attached to the tyrosine residue and certain glycopeptides were able to form hydrogels, presumably through enhanced CH–π interaction with the sugar moieties.
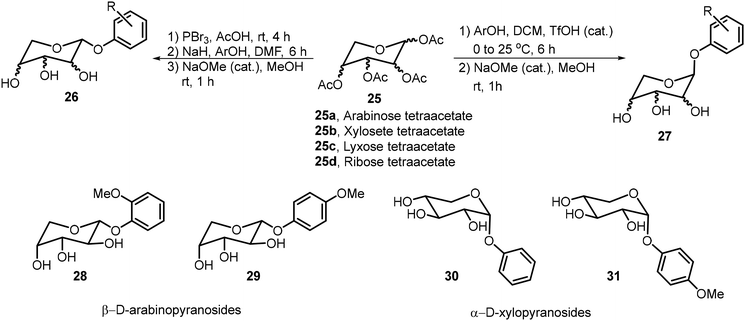
Pathak et al. synthesized a library of phenolic glycopyranosides from pentoses 25a–d and studied the structure to gelation relationships (Scheme 4).24 The sugar scaffolds used in this library included arabinose, xylose, lyxose, and ribose. Four organogelators (28–31) were obtained, two β-arabinose and two α-xylose derivatives. Compound 28 was used to entrap curcumin in a mustard oil gel and its sustained release was studied at various pH values. Slower release was observed under basic conditions.
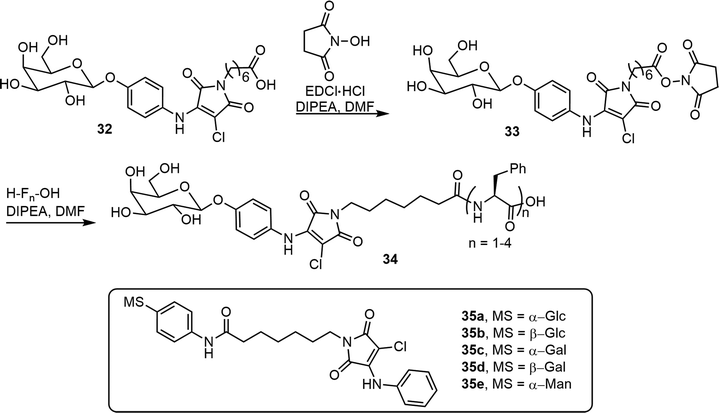
Tsutsumi et al. synthesized glycosylated N-alkyl-2-anilino-3-chloromaleimide-based chromophore (AAC) derivatives and studied their self-assembling properties.25 The synthesis of beta-D-Gal-AAC-C6-Fn 34 is shown in Scheme 5. This compound was prepared from the AAC containing bola-amphiphilic glycolipid 32. The conjugation of 32 with mono, di, tri, and tetrapeptides of phenylalanine (F) yielded the glycolipopeptides 34. The compound 34 (n = 3), betaGal-AAC-C6-F3, formed transparent gels at 0.19% in solvent 200 mM HEPES–NaOH buffer (pH 8.0). The same team also synthesized and analyzed five H-AAC-C6-Ph-MS compounds 35a–e;26 the monosaccharides used include α- and β- glucose, galactose, and α- mannose. The glucosyl and galactosyl amphiphilic compounds exhibited aggregation-induced emission (AIE) features, but the mannose derivatives did not exhibit fluorescence.
Several O-glycosides containing anthracene groups at the anomeric position were synthesized and their self-assembling properties as well as fluorescence properties were analyzed.27,28 The structures of some of these compounds are shown in Scheme 6. Glycosylation of the hydroxyl methyl anthracene derivatives 36 with tetraacetyl glucosyl bromide 37, followed by deacetylation, afforded the anthracene glycosides 38. Other sugar bromides were also used, including derivatives of D-Glc and L-Glc, Gal, and Lactose (Lac). The glucose derivative 38a formed chiral assemblies in aqueous solutions, the π–π stacking of the anthracene moiety is the driving force for the assembly and the chirality of the assemblies is dependent on the nature of the sugar structures. Irradiation at 365 nm disrupted the assembly structures due to (4π + 4π) photochemical reactions of the anthracene moieties. Circular dichroism (CD) was used to characterize the chirality of the SA structures of the anthracene glycosides; the CD spectra indicate left-handed chirality for 38a and right-handed chirality for 38e (L-Glucoside). Galactose derivative 38f exhibited right-handed chirality and lactose derivative 38g showed no or little chirality. The team also synthesized anthracene glucosides with different chain lengths of ethylene glycol linkages (38b–d) and analyzed their SA properties.28 They found that the introduction of ethylene glycol linkages changed the SA morphologies significantly, though the glucosides still produced chiral aggregates.
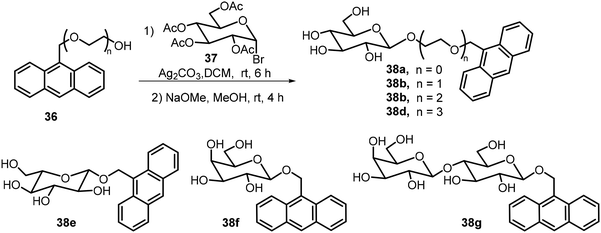 | ||
| Scheme 6 Synthesis of anthracene derivative of glycosides 38 with different linkers and sugar moieties. | ||
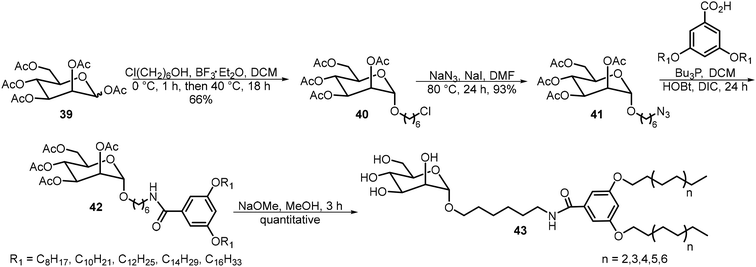
Mousavifar et al.29 synthesized and analyzed a series of mannosyl neoglycolipids with different chain lengths and evaluated their effectiveness as liposome-based drug delivery vehicles (Scheme 7). These glycolipids 43 were prepared starting from the peracetylated mannose 39. Glycosylation with 6-chloro-1-hexanol followed by azide displacement afforded intermediate 41. Staudinger reduction of the azido group followed by amide ligation with carboxylic acids afforded compound 42 as shown in Scheme 7. After de-acetylation, glycolipids 43 with lipid tails of 8–16 carbons were prepared. The alkyl groups with the formula of CnH2n+1 are all linear alkyl chains. These compounds were able to form liposomes. Liposomes formed by the C14 lipid exhibited ideal properties and were further studied for their drug delivery capabilities.
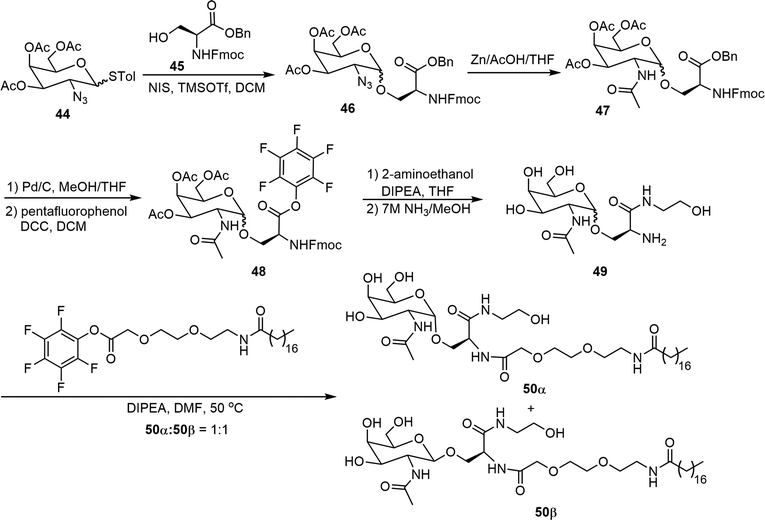
Two N-acetylgalactosamine (GalNAc) based glycolipids 50α and 50β were synthesized and evaluated as potentially useful supramolecular materials (Scheme 8).30 Preparation of the epimers 50α and 50β started with the glycosylation of 2-azido thioglycosyl donor 44 with N-Fmoc and benzyl protected serine acceptor 45. The resulting 2-azido glycoside 46 was then reduced and further acetylated to form 47. The benzyl group was then removed by catalytic hydrogenation, followed by the formation of the pentafluorophenyl ester 48. Amide coupling reactions with 2-aminoethanol were then carried out, subsequent removal of the Fmoc and Ac groups afforded intermediate 49. The amine in 49 was then reacted with a long chain lipid containing pentafluorophenyl ester to afford the glycolipids 50, which were isolated to obtain the α and β epimers. These GalNAc-based epimers on their own can self-assemble into ribbons in aqueous solutions while the mixture of the epimers (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) forms spherical micelles due to carbohydrate–carbohydrate interactions (Scheme 8).
50) forms spherical micelles due to carbohydrate–carbohydrate interactions (Scheme 8).
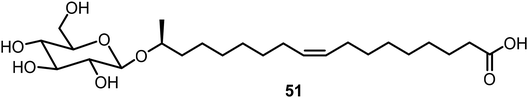
Baccile's group studied the self-assembling properties of a class of natural glycolipids and their derivatives;31,32 an example 51 is shown in Fig. 3. These compounds have been studied extensively and the glycolipids exhibited interesting properties as LMWGs, and the carboxyl substituted lipids also formed metallogels in the presence of various metals.33–35
2.2 N-Glycosides
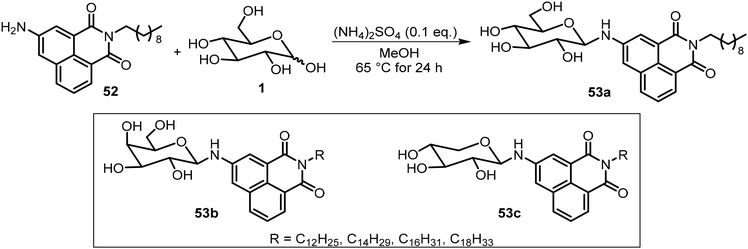
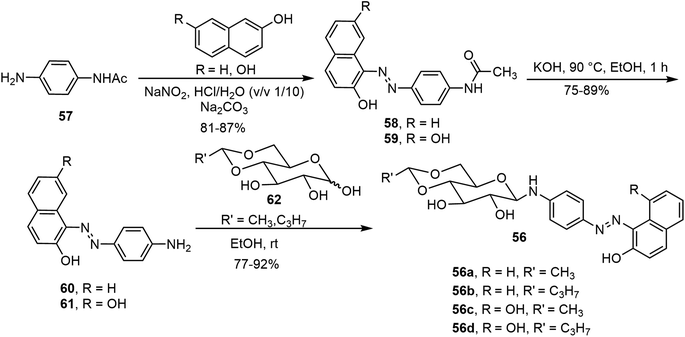
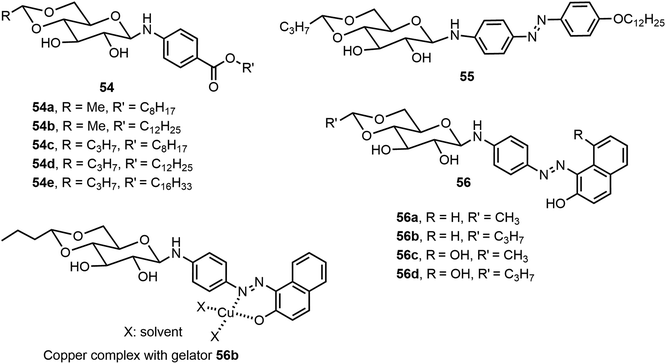
Besides the common O-glycoside derivatives, several N-glycosylated systems have been synthesized and their molecular assemblies evaluated. The synthesis of these compounds typically was done by reaction of an amine with a reducing sugar to form the N-glycosides. Several examples are discussed in the next section for monosaccharide derivatives. Rachamalla et al. explored N-glycosylation reactions under various conditions to create a library of amphiphilic N-glycosyl naphthalimides.36 The synthesis of these carbohydrate derivatives utilized ammonium sulfate as a catalyst and exclusively produced the β-anomers (Scheme 9). The scope of the reaction was explored using glucose, galactose, and xylose, along with several long chain alkyl groups, to create a library of compounds. The mechanism of the N-glycosylation reaction was proposed and supported by a relative energy profile diagram. Several glucose derivatives from the library were found to form gels in chloroform and compound 53a also formed gels in a DMSO–H2O (40% v/v) mixture. Additionally, thin drop cast films of 53a possess semiconducting behavior and it was capable of being anchored on cotton fabric, resulting in increased conducting properties.Mohan Das et al. have reported several glucose-derived gelators 54. These are 4,6-alkylidene acetal-protected glucose derivatives with various β-arylamines introduced at the anomeric positions.37 4,6-O-Ethylidene and butylidene were used as the protecting groups, whereas on anomeric positions C8, C12 and C16 chain-length ester groups were attached to the phenyl ring at the para position (Fig. 4). These compounds formed gels in several organic solvents, and they have high selectivity towards the organic phase from a mixture of aqueous and organic phases. The toluene gel formed by 54e was studied for metal ion responsiveness and a gel–sol transition was observed upon the addition of Cu2+ metal ions. The structure of coordination of Cu2+ with compound 54e was determined by NMR titration, which showed a dimeric gelator to one copper ion. The same team introduced a photoresponsive azobenzene moiety to the anomeric position and synthesized a series of derivatives, of which the best performing gelator, compound 55, was further studied.38 These compounds formed phase-selective gels in organic solvents and also exhibited the effective removal of dyes. Long aliphatic chains resulted in the formation of hydrophobic interaction and van der Waals interactions to form organogels in toluene and acetonitrile. These organogels were selective for the removal of cationic dyes from the mixture of cationic and anionic dyes. Photoisomerization of azobenzene leads to the transition of gel-to-sol when exposed to UV light. More recently azophenol and azonaphthol derivatives were also synthesized and the azonaphthol derivatives 56a–d were able to form gels in DMF/water and DMSO/water mixtures.39 The synthesis of compound 56 is described in Scheme 10. Diazotization from the amine 57 with naphthols afforded intermediates 58 and 59. Hydrolysis and glycosylation with compound 62 led to compounds 56a–d. Gelator 56b formed gels in the presence of various metal ions except copper ions. The addition of Cu2+ ions resulted in a gel-to-sol transition. This was rationalized by the formation of complexes with Cu2+ as shown in Fig. 4.
2.3 Glycolipids and other glycosides
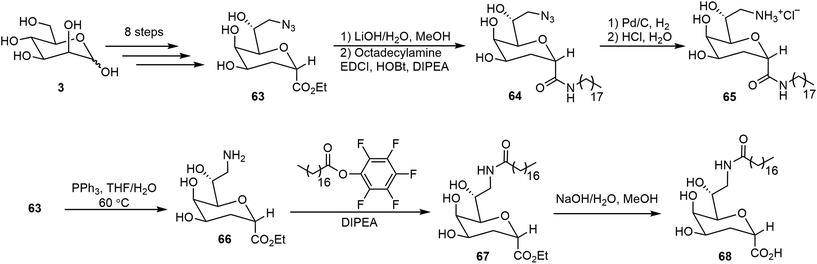
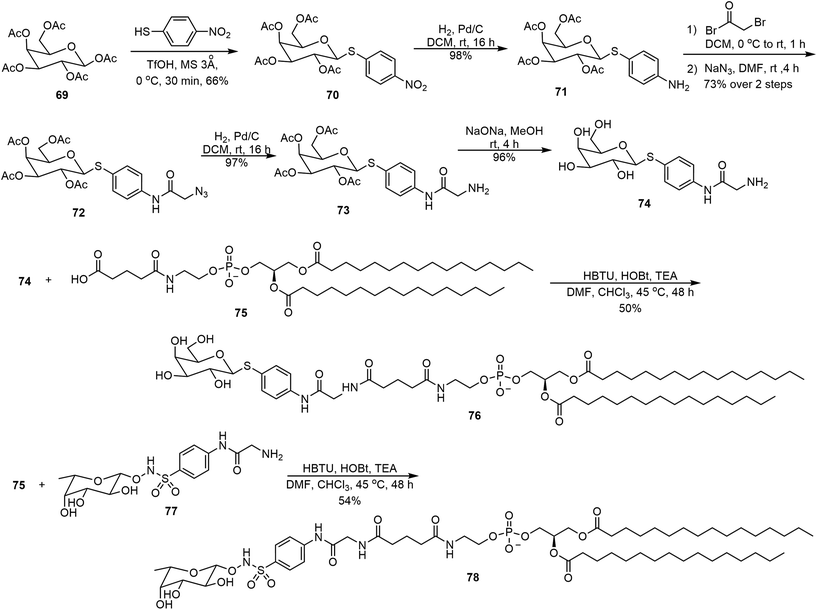
Several glycolipids derived from 3-deoxy-D-manno-2-octulosonic acid (Kdo) were prepared and their self-assembling properties were studied (Scheme 11).40 The key intermediate 63 bearing both azido and ethyl ester functional groups was prepared starting from D-mannose in 8 steps.40 The ester 63 was hydrolyzed and the resulting acid was coupled with octadecylamine to afford the amide 64. Catalytic hydrogenation then afforded the glycolipid mimic 65. In parallel, a Staudinger reduction of azide 63, followed by an amide formation and ester hydrolysis, provided glycolipid 68. Interestingly, these glycolipids formed different morphologies formed during the self-assembling process. The self-assembly of 65 resulted in thermodynamically stable bicelle formation; while the bicelle is only a kinetic intermediate of the self-assembly product with 68, which eventually transformed into ribbons.The lipid 68 formed ribbons directly by using MeOH/H2O (v/v 1 : 1) as the solvent.Metelkina et al. synthesized two lectin-targeting phospholipids for Pseudomonas aeruginosa, both containing phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) moieties.41 These lipids are LecA-targeted galactose derivative 76 and LecB-targeted fucose derivative 78 (Scheme 12). Either 1% or 15% (w/w) of these lipids was used to form liposomes together with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol. The synthesis method is shown in Scheme 12. Galactose pentaacetate 69 was glycosylated using 4-nitrobenzenethiol to afford 70. Reduction of the nitro group led to the amine 71, which was acylated with bromoacetyl bromide, followed by azide displacement of the bromide to form intermediate 72. The azido group was then reduced to an amino group, followed by deacetylation to afford compound 74. Compound 74 was then used for the amide coupling reaction with the phospholipid derivative 75 to produce the glycophospholipid 76. Alternatively, coupling of the acid 75 with the fucose derivative 77 afforded the lipid 78.
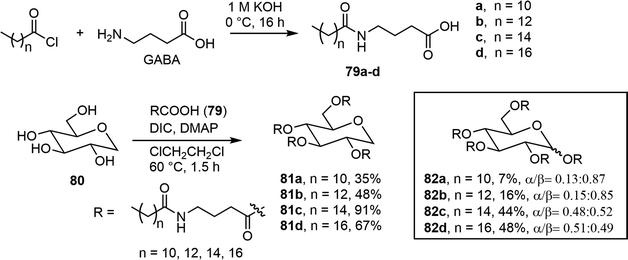
A series of long chain ester derivatives of D-glucose and 1,5-anhydro-D-glucitol (AG) was synthesized and evaluated for its organogelation properties.42 As shown in Scheme 13, γ-aminobutyric acid (GABA) was reacted with fatty acids of varying chain lengths to form the long chain acids 79a–d, which were used for the per-esterification of the sugar units to give the derivatives 81 and 82. Many of these compounds exhibited gelation properties in paraffin oil and some other organic solvents.
Heterocycle functionalized sugar derivatives are useful for the formation of self-assembled networks and for the formation of low molecular weight gelators (LMWGs). Glycoconjugates formed by sugar derivatives connected through the copper-catalyzed azide and alkyne cycloaddition reaction (CuAAC) are an effective method to generate new materials with interesting properties.
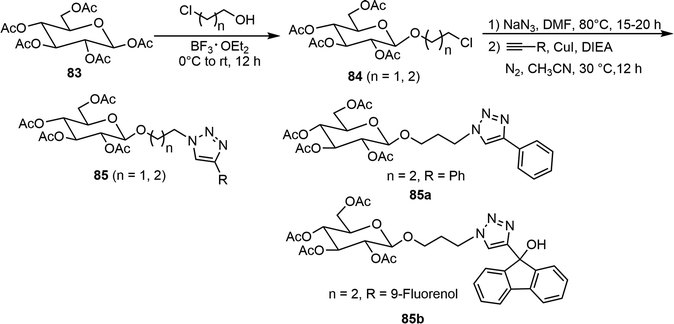
Several peracetyl β-O-alkyl triazolyl D-glucosides 85, where two or three carbon linkers are placed between the anomeric position and triazole moiety, were prepared as versatile organogelators (Scheme 14).43 Starting from pentaacetate of β-D-glucose 83, BF3·Et2O-mediated glycosylation with 2-chloroethanol or 3-chloro-1-propanol afforded the chloro intermediates 84. The chloro group underwent an azide displacement reaction, followed by reactions with various terminal alkynes under copper-catalyzed click conditions to give several glucosyl triazole derivatives 85. The gelator 85a (n = 2, R = Ph) formed metallogels with several different metal ions (Hg2+, Zn2+, Ni2+, and Pd4+). Interestingly, the co-gel of the 9-fluorenol derivative 85b with the gelator 85a showed enhanced fluorescence upon gelation. Similar triazole derivatives starting from the galactose pentaacetate 69 were also synthesized, but the galactose derivatives generally did not form gels and typically were soluble in many of the tested solvents.
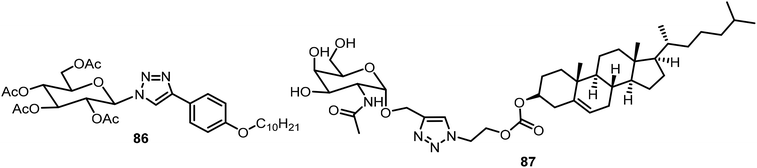
Another glucose triazole-based gelator 86 was synthesized via a CuAAC reaction recently and the compound was found to be an efficient phase selective gelator for oil spill recovery (Fig. 5).44 The combined effect of the van der Waals force of the long alkyl group and π–π stacking of the phenyl group and triazole group was the driving force for the formation of gels. Compound 86 was utilized for the selective removal of oil from a mixture of oil and water. Using click chemistry, a novel self-assembling cancer vaccine platform composed of Tn antigen Chol-GalNAc 87 and adjuvant CpG (unmethylated 5′-C-phosphate-G-3′) oligodeoxynucleotide (ODN) was developed, which is capable of inducing both humoral immune and cellular antitumor responses.45 The Tn antigen 87 was formed through CuAAC reactions between the GlcNAc-based alkyne and cholesterol-derived azide (Fig. 5). To acquire the vaccine platform, liposomes of compound 87 were prepared via the membrane-ultrasound method followed by encapsulating TLR9 agonist CpG ODN adjuvant.
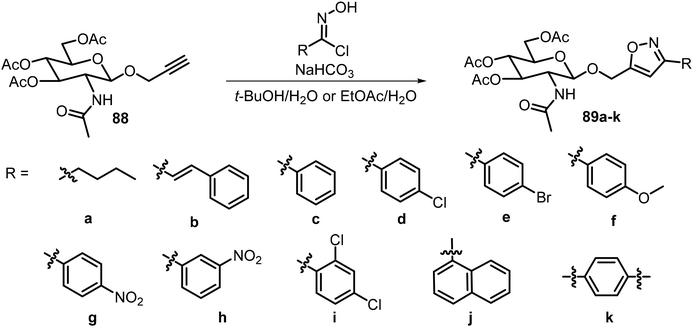
In addition to triazoles, recently a series of glucosamine derivatives containing isoxazole groups at the anomeric position were synthesized and analyzed, and many of these compounds were effective LMWGs.46 The synthesis is shown in Scheme 15. Glucosamine- and glucose-derived propargyl glycosides were used to synthesize the 3,5 disubstituted isoxazole derivatives with different chloroximes. The structures of the 5-substituents and the nature of the sugars used were important for the gelation properties. Most of the synthesized derivatives were able to form hydrogels, representing the first class of isoxazole-based molecular hydrogelators. Compounds 89d–i formed hydrogels at minimum gelation concentration (MGC) lower than 0.5 wt%. Compound 89d formed hydrogels at 1.1 mg mL−1, or 0.11 wt%, in water and also formed metallogels in the presence of earth metal ions such as Cu2+, Zn2+, and Ni2+. Both gelators 89d and 89h were able to form stable hydrogels which can remove dyes from aqueous mixtures.
2.4 Open chain sugar non-glycosylated
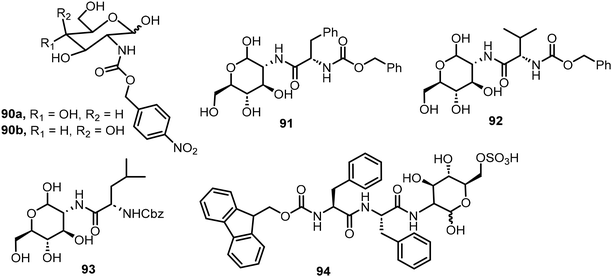
In the next section, several examples of open-chain sugar derivatives capable of self-assembly will be discussed. These include several non-glycosylated derivatives in which the anomeric position exists as an aldehyde or hemiacetal, and open-chain sugar derivatives functionalized at the anomeric carbon through oxidative amidation or by condensation with amino groups followed by reductive amination to form amines.Glucosamine and galactosamine were functionalized at the 2-amino position by reacting with p-nitrobenzyloxy carbonyl chloride to form the 4-nitrophenylmethoxycarbonyl (NPmoc) protected compounds 90a–b.47 The glucosamine derivative 90a (Fig. 6) formed a hydrogel whereas the galactose carbamate 90b only formed suspensions. Self-assembly of compound 90b was hindered by the presence of an axial OH at the C4 position and resulted in the formation of non-networking spheres. Modeling supports that the combined effect of π–π interaction of the nitrophenyl group and hydrogen bonding of the C2-amide in compound 90a was the driving force for self-assembly. The gel formed by compound 90a turned into solution upon treatment with a reductant Na2S2O4. The nitrobenzyl group can be reductively cleaved, resulting in gel disassembly. Three different glycoconjugates 91–93 were synthesized by reacting Cbz-protected amino acids (Phe, Val, Leu) with glucosamine.48 These glycoconjugates formed gels in several different solvents including water. The phenylalanine derivative 91 formed a robust transparent to opaque hydrogel upon aging at 17 mM, the critical gelation concentration (CGC). Spectroscopic methods were used to observe the nanofibers formed by twisted β-sheet secondary structures. The gel has non-cytotoxicity with self-healing properties. In a more recent study, glycopeptide 94 was synthesized by coupling Fmoc-diphenylalanine (Fmoc-FF) with glucosamine-6-sulfate (GlcN6S) using NHS and DCC as the coupling agents.49 The glycopeptide formed supramolecular hydrogels either by a thermal (heating–cooling) method or by a solvent switch (from DMSO to water). Adding cations formed salt bridges with the sulfates of GlcN6S to promote assemblies. Additionally, the gel prepared from the thermal method with supplemental protein had more fibers with a higher density of cross-linking compared with the gel made from the solvent switch method, which was confirmed by AFM, fluorescence spectroscopy and CD analyses. They found that in comparison with non-glycosylated peptide, the glycopeptide system exhibited a better performance for cell culture and was compatible with growth factors. The glycosylation is crucial for the gels’ biocompatibility and biofunctionality.
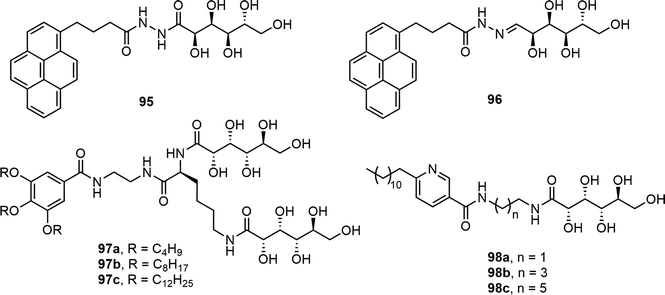
Pyrene derivatives 95 and 96 were prepared by refluxing 1-pyrenebutyryl hydrazide with D-gluconolactone and D-glucose, respectively (Fig. 7).50 The pyrene group contributed to fluorescence and hydrophobicity whereas the gluconic acid unit was responsible for the hydrophilic properties. Compound 95 formed gels in both ethanol and water, but compound 96 did not form gels. The gelation of compound 95 in ethanol was faster than in water. The gel was used for analytical study of the alcohol contents of beverages. Gluconolactone was also used for the synthesis of several amphiphilic molecules 97 which contain two gluconolactone derivatives and tris-alkoxy groups linked via an L-lysine (Fig. 7).51 The 3,4,5-tris(alkoxy) benzoic acid was coupled with ethylene diamine and the resulting compound was further reacted with L-lysine. The amino groups of the lysine were reacted with D-gluconolactone to afford the target compounds. The self-assembly properties of these compounds were analyzed in different solvents and biphasic solutions of chloroform and water. Compound 97a was able to form fibrous networks in the biphasic system, which was used for dye removal experiments and exhibited the effective removal of tartrazine. In a recent study, several single-chain amphiphilic pyridine-based gluconolactone derivatives 98 were also reported (Fig. 7). These compounds formed gels at 15–21 mg mL−1 concentrations in ethanol water (v/v, 1/9) mixture and similarly in DMSO–water mixtures. The gels were prepared in syringes and utilized for the absorption of dyes.52
2.5 Sugar alcohol derivatives
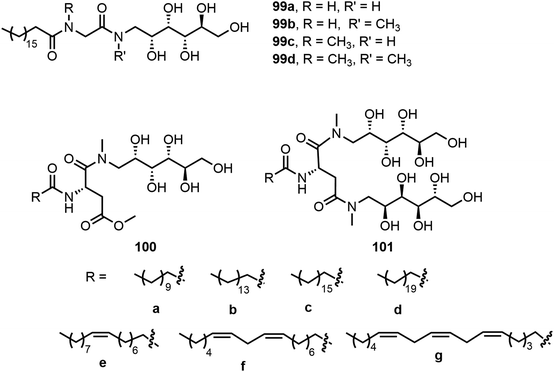
Four D-glucamine derivatives containing stearoyl and glycine groups were synthesized and analyzed.53 As shown in Fig. 8, these glucamides 99a–d were able to form hydrogels effectively. The thixotropic properties of the hydrogels were tuned via the effect of the presence or absence of a methyl group on the amide function of the molecules, which affected the molecule's ability to form a hydrogen bond. N-Methyl-D-glucamine was also used for the preparation of self-assembling glycolipids by Nayak et al.54–56 Two series of glycolipids which contain either mono or di N-methyl-D-glucamine moieties and different acyl chain lengths 100a–g and 101a–g were synthesized. Compounds with tails a, b, and d were analyzed in several solvents; 100a and 101a–b formed gels in hexanes, benzene, and other organic solvents. 101d formed emulsions in these organic solvents and was soluble in water. Compounds 100b and 100c formed hydrogels and the hydrogels formed by 100b were injectable and have thixotropic properties.55 Glycolipids with different degrees of unsaturation were prepared to analyze the impact of unsaturation in the tail groups toward molecular self-assembly.54 They have demonstrated that the increased unsaturation is correlated to enhanced critical micelle concentration (CMC) values. The lipids with one double bond (100e and 101e) were deemed more suitable for loading hydrophobic drugs and solubilization. The glycolipids with di-unsaturated lipid tails (100f and 101f) were more suitable for foamability and wettability. The team later on studied the gelation properties of gold nanocomposite gels formed by the glycolipid gelator 100e with HAuCl4.56 Compound 100e was analyzed with gold solutions of different concentrations and the maximum loading of gold without disturbing gelation was 57.60 mg g−1 (gold![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gelator).
gelator).
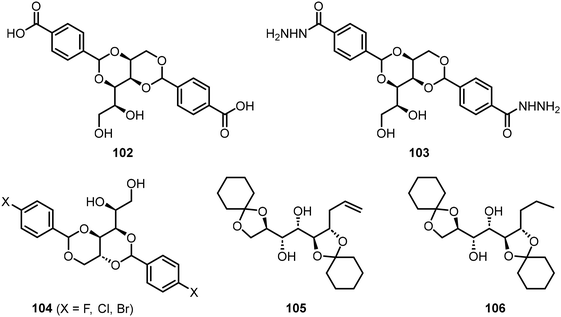
Smith et al. have studied the properties of pH-responsive gelators, the carboxylic acid of 1,3:2,4-dibenzylidenesorbitol 102 (DBS-COOH) and 103 (DBS-CONHNH2) (Fig. 9).57–59 The spatial and temporal diffusion of the resulting gels were characterized and applications of the gels for various organic reactions were studied. For instance, gelator 103 has been applied in the fabrication of gel beads which were used to catalyze the Suzuki–Miyura reaction.58 Fan et al.60 prepared supramolecular eutectogels utilizing halogenated dibenzylidene sorbitol derivatives 104 (X-DBS) and deep eutectic solvents (DESs). The gelators 104 were used to form gels with a mixture of choline chloride and alcohols or ureas. The resulting gels possessed self-healing properties and high conductivity. Two cyclohexanone acetal-protected mannose derivatives 105 and 106 were also shown to exhibit gelation properties in hexanes and related alkanes.61 Two of the compounds were found to be capable of selective gelation of hydrocarbon solvents. The terminal alkene functionality allows for further chemical modification, and thus, further customization of the gelation properties.
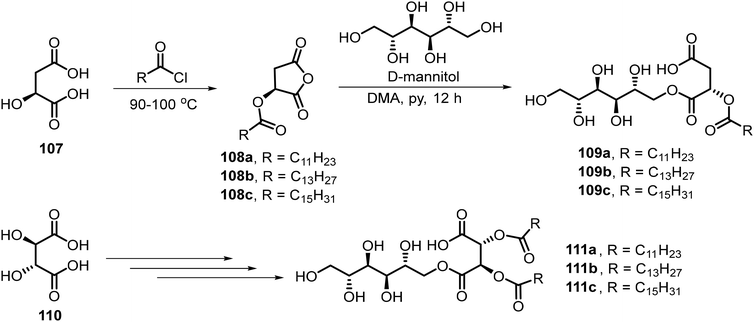
Singh et al. synthesized a series of six amphiphilic D-mannitol derivatives 109a–c and 111a–c with various lengths of aliphatic chains linked through tartaric acid or malic acid (Scheme 16).62 Most of the derivatives in the library were found to act as surfactants with low CMC and formed hydrogels. As shown in Scheme 16, among the six long chain esters except 109a, five compounds formed stable hydrogels. Longer aliphatic chains led to increased thermal stability in the same series. The hydrogel formed by 111c was used to stabilize silver nanoparticles (Ag NPs).
3. Disaccharide derivatives
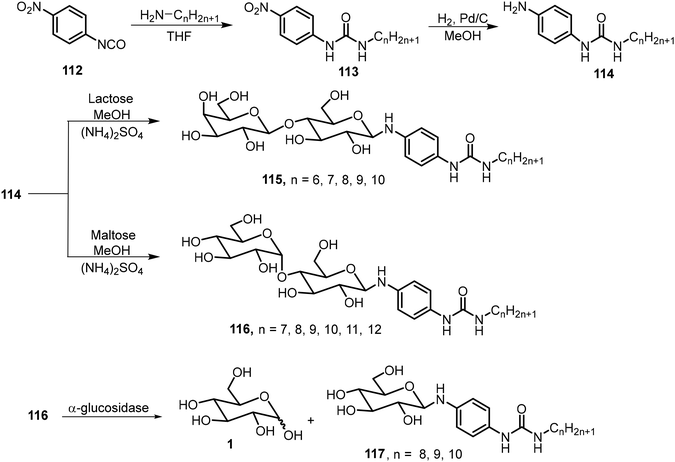
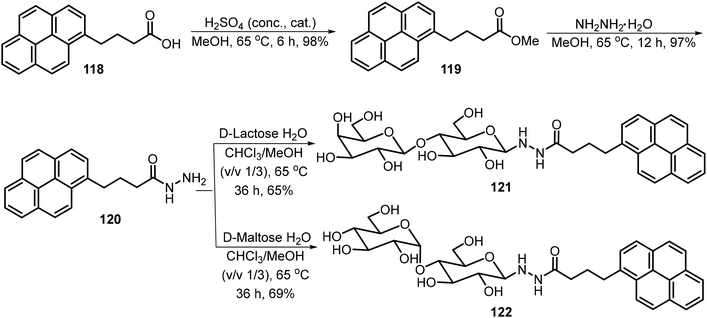
A series of amphiphilic lactose derivatives containing urea-linked aliphatic chains were reported as LMWGs.63 The gelation properties were tuned via the length of the aliphatic chain. As shown in Scheme 17, lactose urea derivatives 115 were synthesized and tested. The derivatives with n = 6, 7, 8, and 9 were gelators, with MGCs of 2.0, 1.8, 1.2 and 0.9 mM respectively. The decyl derivative was insoluble in water. The gel-to-sol transition was controlled using β-galactosidase, which cleaves the lactose to form the corresponding glucose urea derivatives. The same team also synthesized mannose derivatives 116 (n = 7–12), and the alkyl chains with greater than eight carbons were found to be effective hydrogelators too.64 The gels were found to undergo a gel–sol transition in the presence of α-glucosidase due to the hydrolysis of maltose and the formation of the glucose derivatives 117.A pyrene-based lactose derivative (PyLac) was prepared by refluxing 4-(pyren-1-yl) butanehydrazide 120 with lactose in methanol and chloroform.65 The pyrene-appended lactose derivative 121 formed a fluorescent hydrogel, while the mannose derivative 122 was not a hydrogelator (Scheme 18). Using variable temperature CD experiments, the self-assembled structures were predicted to form a bilayer structure with the pyrene π–π stacking in the middle and the polar lactose at the exterior of the vesicle. The terminal galactose residue of 121 was able to bind to cholera toxin, and therefore the system was used for chlolera toxin's fluorescence sensing. In another study, the gelator 121 formed thermo-responsive hydrogels which were utilized for the sustained delivery of the anticancer drugs mitoxantrone and doxorubicin.66 At physiological pH, the drug release amount from drug-loaded hydrogels was much higher at 37 °C than the release rate at 25 °C. The elevated temperature caused the disruption of the self-assembled networks and allowed the entrapped drug molecules to be released more rapidly.
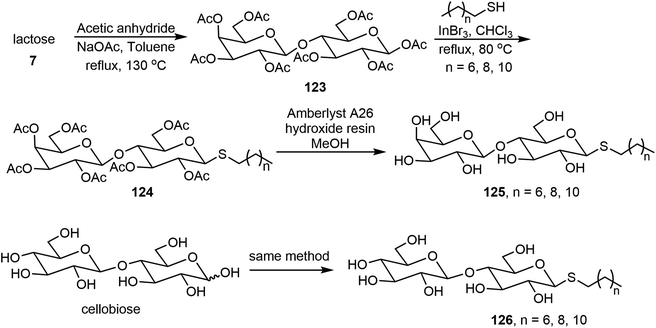
Wang et al. synthesized thioglycolipids 125 and 126 from lactose and cellobiose with three alkyl thiols (Scheme 19).67 The lactose derivatives 125 formed stable gels and the thiocellobiosides 126 formed metastable gels which precipitated over time. The thiolactoside hydrogels have thixotropic properties. The phase transitions of the gels were analyzed via fluorescence spectroscopy using a polarity sensitive fluorophore Prodan.
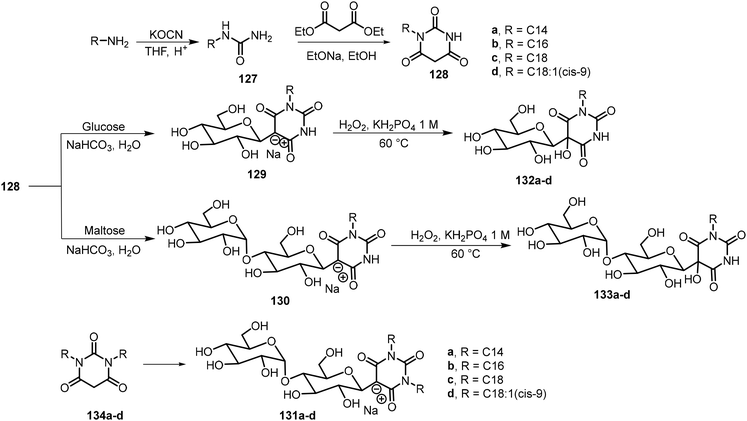
Yao et al. used Knoevenagel condensation to synthesize amphiphilic β-C-glycosyl barbiturates from unprotected glucose and maltose.68 As indicated in Scheme 20, the Knoevenagel condensation of N-substituted barbiturates 128 with glucose or maltose directly afforded the corresponding β-C-glycosylbarbiturates 129a–d or 130a–d. They found that while the glucose derivatives 129a–d were insoluble in water, the maltose derivatives 130a–d were fully soluble in water upon heating. Upon lowering the pH or the addition of a divalent metal salt such as CaCl2, 130c and 130d formed hydrogels at 2–5% MGC, and the gelators formed fibrous networks. N,N-Disubstituted β-C-glycosylbarbiturates 131a–d were also prepared similarly, and compounds 131b and 131c formed vesicle hydrogels after two heating and cooling cycles due to the lower water solubility. These compounds were found to self-assemble into percolating multilamellar vesicles and connected vesicles, which further formed vesicle hydrogels. While for the monoalkylated urea derivatives, hydrogelation only occurred under strongly acidic conditions or by adding metal ions at neutral conditions, under these conditions, the N-substituted maltosyl barbiturates 130 exist predominantly in the keto form. It was hypothesized that the keto forms would be able to self-assemble and form hydrogels. Therefore, they further modified the glycosylbarbiturate structures by oxidation using H2O2 to enable the compounds to adopt a fixed keto form to give 132 and 133,69 which were expected to enhance hydrogelation. The hydroxylated glucose and maltose derivatives were obtained in 54–72% yields, and these compounds were found to be more effective hydrogelators. The glucosyl derivatives 132b–d and maltosyl derivatives 133c–d formed stable hydrogels at neutral conditions.
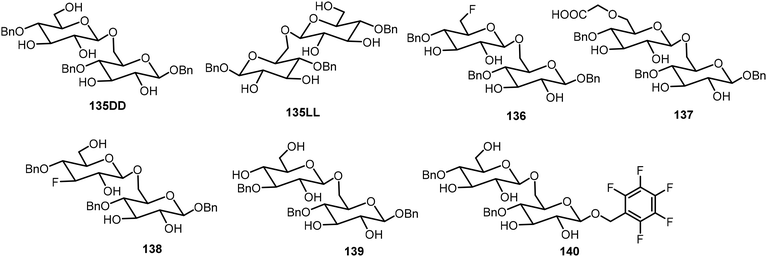
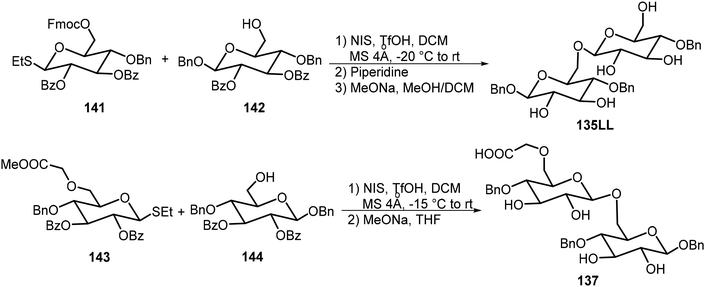
Seeberger and Delbianco et al. have synthesized a series of disaccharides with different degrees of protection and studied their self-assembling properties (Fig. 10).70,71 These disaccharides were prepared in both the 135DD and 135LL enantiomers, and interestingly, the disaccharides formed self-assembled helices that are relevant to their structures. It was found that the local crystal organization dictated the supramolecular morphology. The 135DD isomer formed left-handed helices and 135LL formed right-handed helices; however, the racemic mixture of 135DD and 135LL did not form helices; only planar sheets were observed. The synthesis of the disaccharides with the 135DD configuration was carried out by a standard glycosylation reaction. The preparation of the enantiomer 135LL is shown in Scheme 21 starting from the corresponding L sugar derivatives 141 and 142.
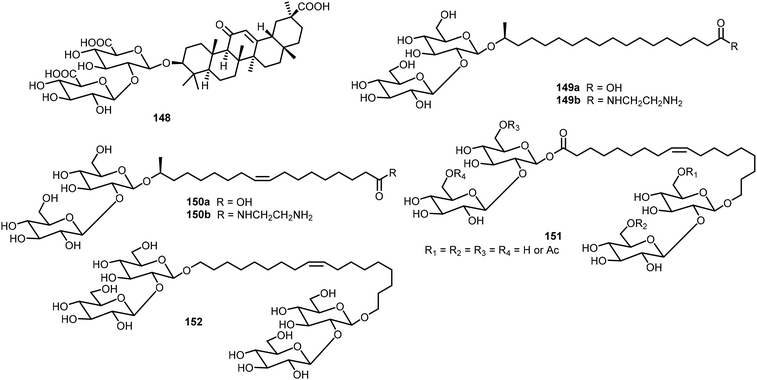
The D-glucose-based disaccharide 135DD formed highly crystalline twisted fibers. Systematic chemical modifications of 135DD can provide important information responsible for the supramolecular assembly.72 Substituting the hydroxyl group at C-6 position with F (136) or –OCH2COOH (137) preserved the self-assembly properties, while substitutions of the hydroxyl group at the C-3 position (138 and 139) or OBn group at the aromatic position with pentafluorobenzene group (140) disturbed the assembly. Structural modifications of 135DD produced new geometries, such as hollow tubular structures, spherical colloidal particles, as well as highly twisted fibers/ribbons as characterized by electron tomography and electron diffraction. The disaccharide 137 was synthesized via glycosylation reaction of monosaccharides 143 and 144 as shown in Scheme 21.
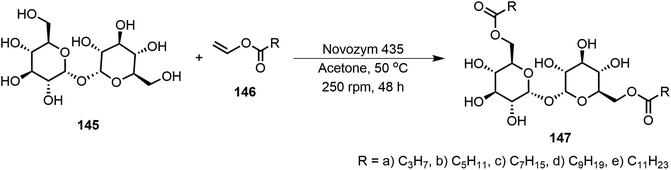
In another study, the disaccharide trehalose 145 was functionalized via regioselective transesterification using Novozym 435 (Scheme 22).73 Trehalose ester derivatives 147 at the primary hydroxyl groups with various length aliphatic chains (C3–C11) were synthesized. These derivatives formed organogels in ethyl acetate and oleogels in olive oil, canola oil, and grapeseed oil. The eight-carbon and ten-carbon fatty acid derivatives were found to be supergelators with MGC at <0.2 wt%. Powder X-ray diffraction (PXRD) studies identified the molecular assembly patterns of the gelators, and compound 147d formed monophasic hexagonal columnar packing patterns. The trehalose lipids were used for the preparation of wax-free lip balms and may be useful for cosmetics.
Zhao et al. studied the gelation properties of a naturally occurring compound, glycyrrhizic acid 148 (Fig. 11).74,75 They found that it formed hydrogels at 37 °C in a phosphate buffer solution and the gels can be injectable. The gel was found to have good biocompatibility and possess inherent antibacterial activity. Baccile's group studied the self-assembling properties of sophorolipid derivatives 149–152 systematically, and these glycolipids were isolated from microbial sources.76–78 The fatty acid formed different assemblies in the neutral and charged forms.78 The acids can be further derivatized with amines to form amides.76 Several sophorolipids were found to be able to form hydrogels and metallogels. These biobased surfactants have also been reviewed recently.79,80
4. Glycoconjugates or branched systems
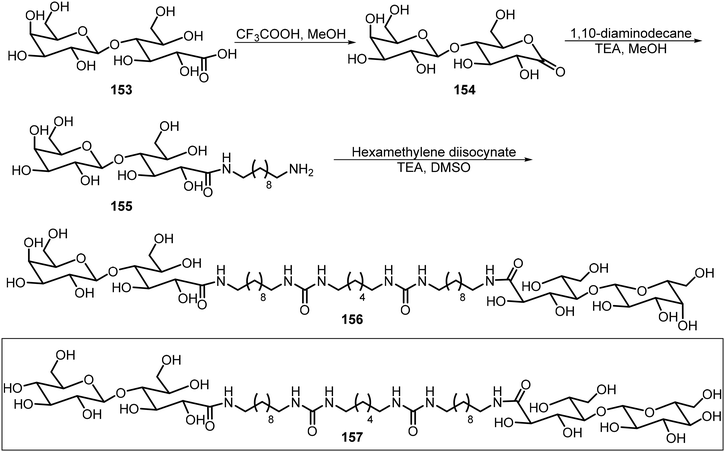
In this section, we review the recent literature on dimeric, trimeric, and multiple branched systems, and a majority of these systems employed click chemistry for the synthesis of glycoconjugates.Scheme 23 shows the synthesis of two bisurea amphiphiles of sugar derivatives 156 and 157.81 These compounds were synthesized from either lactobionic acid 153 or maltobionic acid. The reaction of intermediate lactobiono-δ-lactone 154 with a diamine afforded the monoamine derivative 155, which was further reacted with a diisocyanate to afford the corresponding bisurea 156. The maltobionic derivative 157 was synthesized by the same method. The two sugar derivatives self-assembled and formed fibers and bundles which further formed hydrogels that can mimic ECMs.
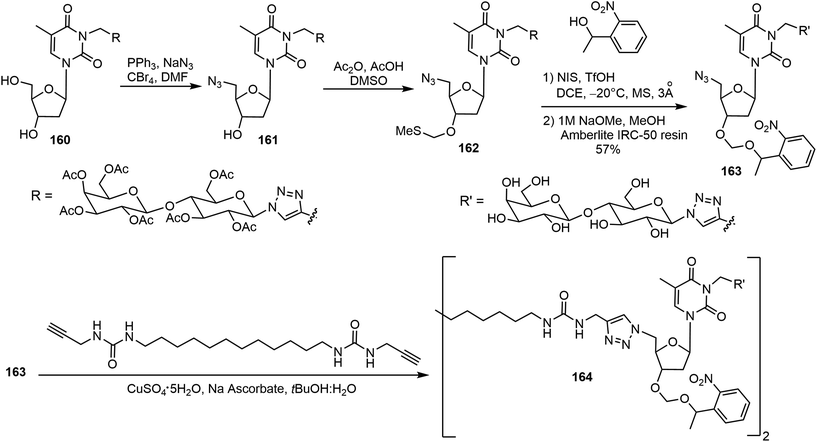
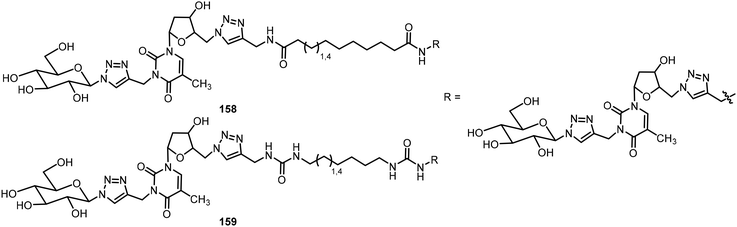
El Hamoui et al. have synthesized glyconucleo-bola-amphiphiles (GNBs) linked through amide, urea, and carbamate functions and studied their self-assembling hydrogelation properties.82,83 Some of these glyconucleoside-based LMWGs 158–159 are shown in Fig. 12. These hydrogelators were synthesized using a click reaction from the corresponding glycosyl azide and propargylated nucleoside. More recently, an O-nitrobenzyl group was attached to the nucleoside to prepare the glyconucleo-bola-amphiphile amide (GNBA) linked photocleavable gelator 164.84 The synthesis of 164 is shown in Scheme 24. A click reaction of N-propargyl thymidine with β-1-azido hepta-O-acetyl-β-D-lactopyranose afforded the intermediate 160, which was converted to the azide derivative 161. Methylthiomethylation at the 3′ position followed by introducing the nitrophenyl unit and removal of acetyl groups on the lactose unit afforded 163. A second click reaction with the bisurea bisalkyne led to the formation of the nitrophenyl functionalized GNBA 164. Compound 164 formed a hydrogel with enhanced stiffness. Upon cleavage of the nitrophenyl group, the resulting hydrogel became softer.
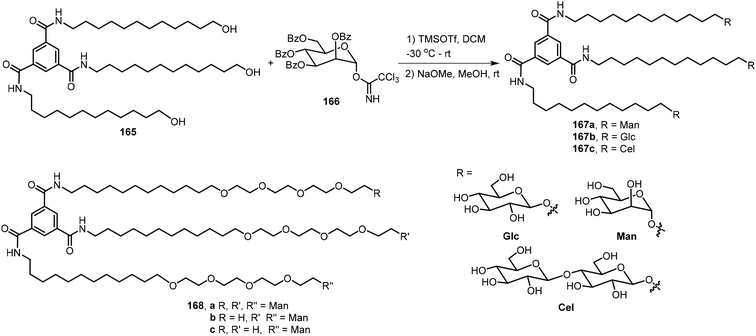
Meijer's group designed and synthesized four benzene-1,3,5-tricarboxamide (BTA) derivatives 167a–c and 168 which contain sugars at the periphery and further studied their biomaterial applications.85,86 The synthesis is shown in Scheme 25. Glycosylation of the BTA-derived trimeric alcohol 165 with corresponding protected sugar imidates followed by deprotection afforded the BTA derivatives 167. An example using the mannose derivative 166 is included, and the product 167a was obtained. Other derivatives with glucose and cellobiose at the periphery, BTA-Glc 167b and BTA-Cel 167c, and a derivative with tetraethyleneglycol (TEG) linked mannose BTA-TEG-Man 168 were synthesized by similar methods. These trimeric branched glycosides exhibited different morphologies. Cryo-TEM images indicated that the glucose and mannose derivatives 167a–b formed fibrous networks, but the cellobiose derivative 167c and compound 168a formed micellar morphologies. They concluded that the trimer 167a and 167b self-assembled and formed 1D supramolecular polymers. Co-assembly of the compounds formed supramolecular polymers which resulted in very soft hydrogels. Rijns et al. synthesized and analyzed the self-assembling properties of three 1,3,5-tricarboxamide (BTA-TEG) derivatives containing one to three mannose moieties 168a–c.87 Derivatives containing one and two mannose groups 168b–c were found to be able to form hydrogels.
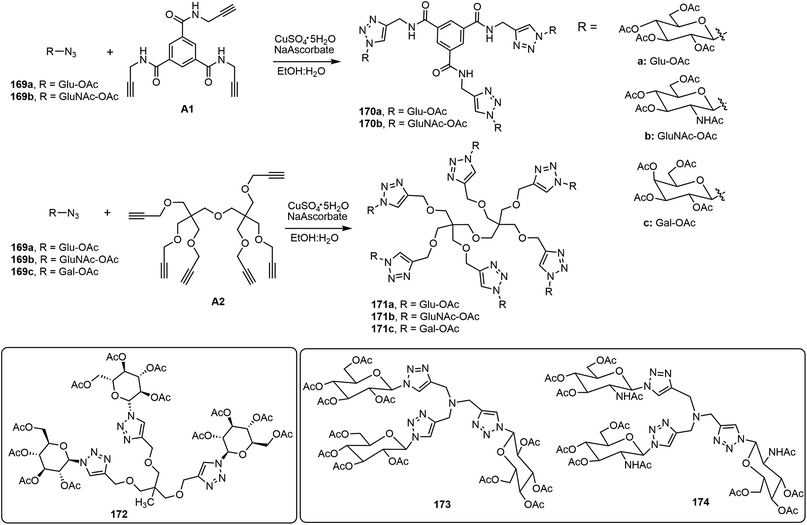
Wang's group designed, synthesized, and characterized a series of glycoconjugates via a click reaction of various trimeric, tetrameric, and hexameric alkynes with glycosyl azides (Scheme 26).88–90 Using pentaerythritol alkyne as the core structure, click reactions with azido sugars afforded a library of glycoclusters with different degrees of conjugation. The monomeric and dimeric glycosyl triazole derivatives tend to be soluble in most of the organic solvents tested, but compounds with three and four branches of sugar triazole units were found to be effective gelators for water and organic solvents.89 The glycoclusters were expanded systematically into a series of seventeen glycoconjugates of three to six arms using eight different branched alkynes as the building blocks, A1 and A2 are inlcuded in Scheme 26. The self-assembling properties of these novel molecules and their catalytic activity as ligands in copper-catalyzed azide and alkyne cycloaddition (CuAAC) reactions were characterized.88 The glycoconjugates containing six branches showed significant catalytic activities for CuAAC reactions. In an aqueous ethanol mixture, 1.0 mol% of six-armed glycocluster 171a was able to accelerate the click reaction of 169b with phenylacetylene significantly. Moreover, the co-gels formed by trimer 172 and hexamer 171a together with CuSO4 were able to form stable gels inside a column as the reaction matrix. The gel columns were used to catalyze click reactions and were reused for several cycles. The formation of triazoles using azide and alkynes, as well as the formation of isoxazoles using choroximes and alkynes, were demonstrated. These glycoconjugates can be used as supramolecular catalysts to efficiently catalyze click reactions in a flow-chemistry fashion. In a related study, eight trimeric glycoclusters from different sugar azides were prepared and analyzed.90 The resulting glycoclusters formed different SA structures depending on the sugar structures. Galactose and maltose derivatives did not form hydrogels. The glucose and glucosamine derivatives 173 and 174 both are effective LMWGs. Compound 173 was also able to form stable co-gels with CuSO4 in gel columns and was used for running flow-like click reactions.
Percec et al. have synthesized several series of glycodendrimersomes via thiourea formation and click reactions and analyzed their self-assembling properties.91–93 Janus glycodendrimers (JGDs) were synthesized via isothiocyanate-functionalized pentaerythritol derivatives and with amine-functionalized sugar derivatives (Scheme 27).91 Different oligosaccharides were synthesized first and a pentyl amino linker was attached to the anomeric position with either alpha or beta stereochemistry. The oligosaccharides were synthesized via an automated glycan assembly (AGA) method. An example using a disaccharide is shown in Scheme 27. Lactose pentaacetate 123 was converted to intermediate 175, followed by glycosylation and deprotection to afford the sugar amine 177. The Janus glycodendrimers (JGDs) 178 and 179 were prepared via isothiocyanate and amine reactions which formed thiourea linkages. These JGDs formed self-assembled vesicles that exhibited lamellar structures with the glycans on the outer surface. Glycodendrimersomes formed by the self-assembly of JGDs exhibited lamellar nanovesicles. The thiourea in the glycoclusters contributed to the molecular assembly. Compound 180 formed a lamellar assembly morphology as indicated by AFM spectroscopy. These glycoclusters were synthesized via a method that resembles the CuAAC reaction in a “click” manner. Another series of GDS formed by JGDs and their formation of membrane-mimicking vesicles was also reported (Scheme 27).92 These Janus glycoclusters were synthesized using azido alkyl sugars via click chemistry. The self-assembled vesicles of the JGDs with different sugar units at the periphery 180a–c exhibited similar types of lamellar morphology, despite the sugar structures. However, the sugar structures did influence the thickness of the bilayers formed.
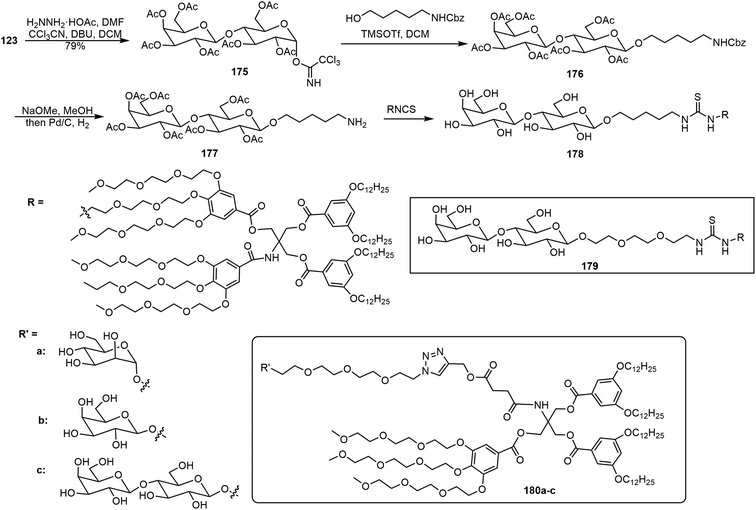 | ||
| Scheme 27 Synthesis of Janus glycodendrimer 178 and chemical structures of Janus glycodendrimers 179–180. | ||
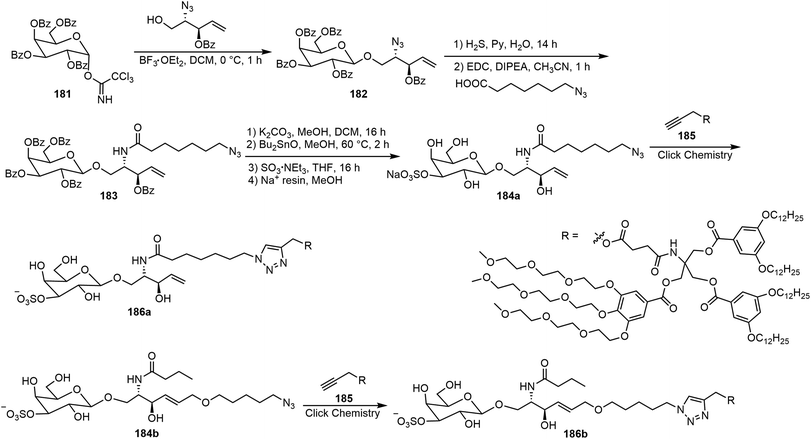
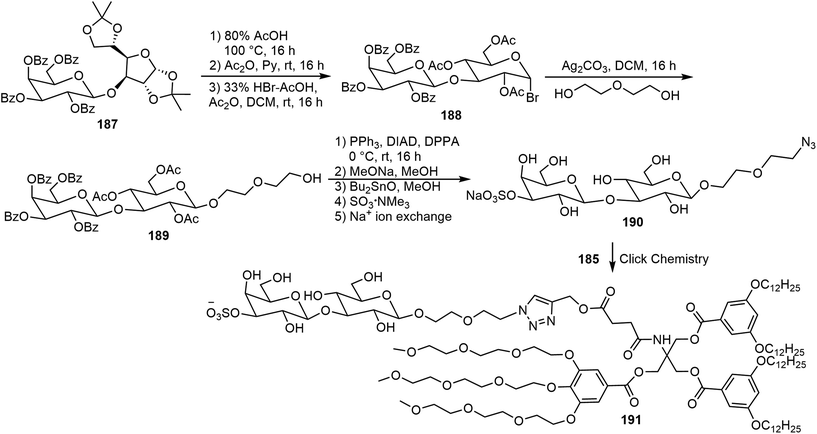
Murphy et al. synthesized three amphiphilic sulfate-substituted galactose-containing glycodendrimersomes and analyzed their interactions with galectins.93 The synthesis of the clickable headgroup is shown in Schemes 28 and 29. The JGD-sulfatide 186a and 186b and sulfatide lactose headgroup JGD derivative 191 all contain 3-sulfate groups at the terminal sugar moiety, the presence of which is important for molecular assemblies and the interactions with lectins.
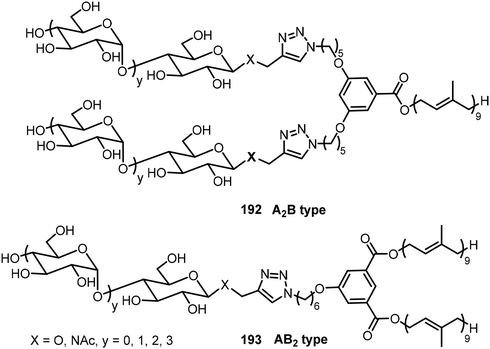
Mullerova et al. reported the preparation of carbosilane-based glycodendrimers and their application for anticancer drug delivery.94 Different sugar azides were used for the synthesis of the glycoconjugates by click chemistry. In another study, a series of branched phenyl-cored block-co-oligomers (BCOs) 192 and 193 composed of maltooligosaccharides with 1-4 glucose units (A) as the hydrophilic component and solanesol (B) as the hydrophobic component were synthesized via click chemistry (Fig. 13).95 Besides these A2B, AB2 systems, several other A2B2 systems were synthesized and studied. These BCOs self-assembled and formed different morphologies including lamellar, cylindrical, and spherical morphologies as well as double gyroid, etc.
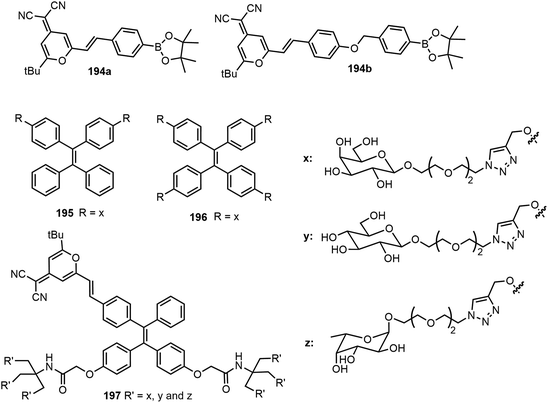
Dong et al. have synthesized a series of tetraphenylethylene (TPE)-based glycoclusters and studied the self-assembling properties and fluorescence properties.96,97 As shown in Fig. 14, water-soluble glyco-dots formed by TPE-based glycoclusters and dicyanomethylene-4H-pyran (DM) fluorescent probes (194) were shown to enhance peroxynitrite sensing.96 To take advantage of the multiple hydroxyl groups in the sugar moiety, the water-soluble TPE-cored glycoclusters were prepared from a propargylated TPE core and azido glycoside via click chemistry. When forming glycol dots with DM probes, among the synthesized glycoclusters, the galactose-appended tetrameric compound 196 exhibited a better sensitivity for peroxynitrite sensing in pure PBS buffer compared with the bivalent system using glycocluster 195. The team also reported the AIE-based glycoclusters 197 as efficient fluorogenic glycosidase probes.97 Hexavalent glycoclusters composed of a TPE core conjugated with a DM were found to be an optimal structural motif for glycosidase sensing through AIE. Structurally diverse glycoclusters with different sugars (D-galactose, D-glucose, and L-fructose) in the periphery were synthesized using click chemistry, by coupling an alkynylated TPE-DM core and various glycoside azides. Enzymatic hydrolysis of the glycoclusters by the corresponding glycosidases in water as well as within the cell produced significantly enhanced and stable fluorescence due to the formation of amphiphilic AIE-based aggregates.
BODIPY dyes have a common structure of BF2 groups joined to a dipyrromethene group. 4,4-Difluoro-4-bora-3a,4a-S-indacene represents a large family of fluorophores. Various glycoBODIPY systems have been developed in recent years, and the synthesis and applications of these systems have been an intense interest as reported in a recent review.98 We only show a few selected examples that are relevant to sugar-based molecular assemblies.
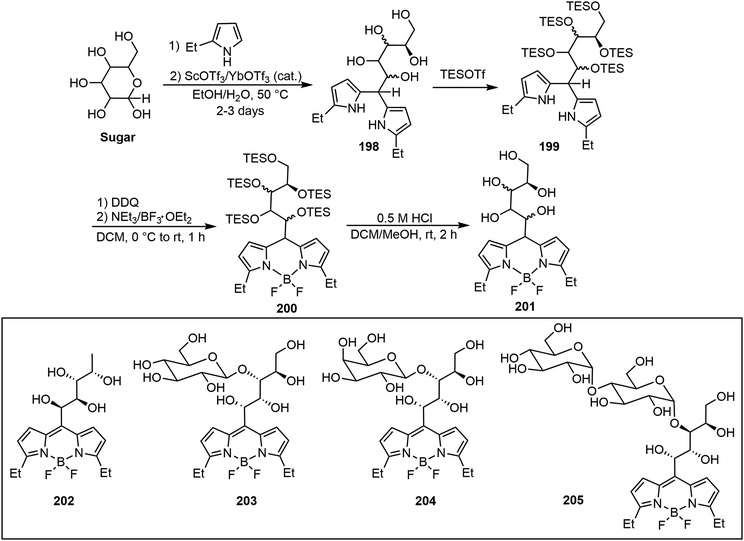
Diverse water-soluble mono-, di-, and trisaccharide BODIPY conjugates (glycoBODIPYs) with preserved chirality and high fluorescence efficiency were prepared by Werz's group.99 As shown in Scheme 30, through a straightforward four-step synthesis including (1) ethyl pyrrole condensing with the reducing end of the sugar to give intermediate 198, (2) triethyl silyl (TES) protection of the hydroxyl groups to form 199, (3) BODIPY formation with BF3·Et2O and lastly (4) TES deprotection afforded glycoBODIPYs 201 smoothly. Some representative glycoBODIPYs synthesized from various sugars, L-fucose (202), D-cellobiose (203), D-lactose (204) and D-maltotriose (205), are shown in Scheme 30. Cell staining experiments showed that compound 202 (L-Fuc) is detected in the cytoplasm as well as in the nucleus. D-Cellobiose-based glycoBODIPY 203 illustrated staining in mitochondria, specifically contrary to its epimer 204.
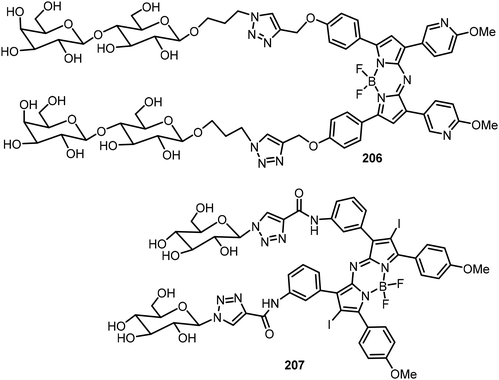
A lactosylated aza-BODIPY compound 206 was synthesized via click reactions (Fig. 15).100In vivo studies showed that the glycoconjugate 206 was capable of selectively entering HepG2 cells by forming J-aggregated nanofibers. The lactose moieties tuned the aza-BODIPY organization preciously to allow the dye to adopt the J-aggregate structures. Synergistically, these J-aggregates can generate superoxide radicals (O2˙) by photoinduced electron transfer, facilitating type I photodynamic therapy (PDT) to enhance the tumor suppression efficacy. Kamkaew et al. reported a novel glyco-BODIPY compound 207 as a promising targeted PDT agent, which is composed of two glucose motifs conjugating to iodine-functionalized NIR aza-BODIPY dye via a click reaction (Fig. 15).101 Because of the presence of overexpressed glucose transporters (GLUTs) in cancer cells, GLUTs can be used as anti-cancer targets. A higher level of glucose triazole derivative 207 was detected in breast cancer cells than in normal cells. After exposing the cancer cells targeted with glycoconjugate 207 to light, singlet oxygen was generated, which enables high cancer cell toxicity through the type II PDT mechanism.
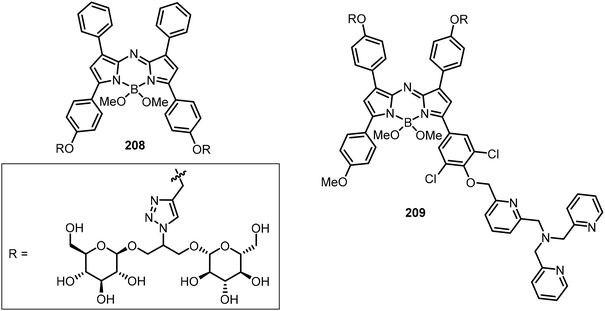
The tetravalent glucose functionalized aza-BODIPY conjugate 208 was designed to target cancer and to increase the photoacoustic (PA) imaging by addressing the following issues: (1) solubilizing effect, (2) prevention of π-stacking of the appended dye, and (3) cancer cell targeting compared with a single glucose moiety (Fig. 16).102 Based on a similar scaffold, affixing tri[(2-pyridyl)-methyl]amine (TPA) generated a copper(I) detection probe 209 (Fig. 16). Upon coordination of compound 209 with Cu(I), the triggered oxidative cleavage releases the dye, causing a significant PA signal absorption peak shift from 676 nm to 754 nm.
5. Oligosaccharides
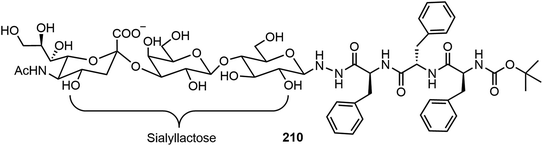
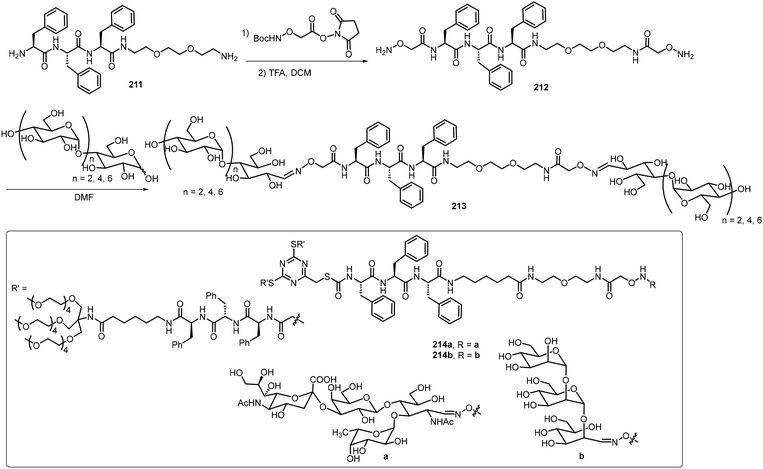
The self-assembling properties of oligosaccharides either on their own or as part of glycopeptides have also been studied. In this section, several recent reports are included. Chen et al. reported two different series of glycopeptides and their self-assembling properties.103 These include glycopeptide 210 composed of sialyllactose, a trisaccharide bearing a negative charged COO− group, and triphenylalanine (Fig. 17).103 The compound was prepared by an ytterbium(III) trifluoromethanesulfonate catalyzed N-glycosylation of sialyllactose with the peptide triphenylalanine hydrazide. The self-assembly of glycopeptide 210 formed double-strand fibrils, which were fully characterized by TEM and AFM. The self-assembling behaviors of several glycopeptide analogs were also studied via computational modeling. The sialyllactose portion contributed to hydrogen bonding and the peptide portion self-assembled through π–π stacking and hydrogen bonding.Chen's team also synthesized several linear dimeric sugar peptide conjugates 213 as well as two amphiphilic dendritic glycopeptide molecules 214a–b with oligosaccharides in the periphery via an oxime-mediated strategy (Scheme 31).104 They were synthesized by using hydroxyl amine reacting with the corresponding sugar aldehydes. The linear dimeric compounds self-assembled and formed spherical nanoparticles. The dendritic oligosaccharide-tripeptide monomers self-assembled to form uniform supramolecular nanorods in an aqueous solution. In vitro studies demonstrated that these supramolecular nano-rod like polymers have no cytotoxicity to macrophages, but can significantly affect the production of proinflammatory cytokines.
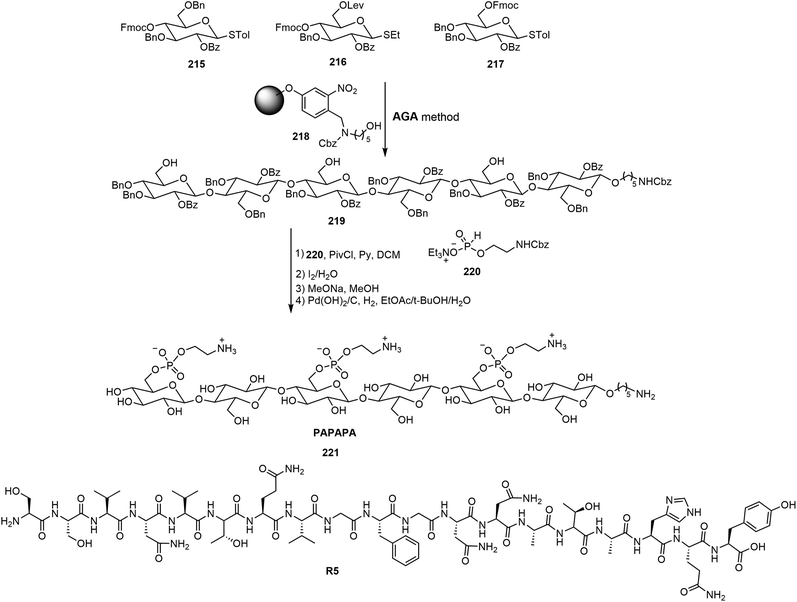
Delbianco et al. have synthesized oligosaccharides as mimics of cellulose and other polysaccharides by automated synthesis and studied the self-assembling properties of those oligosaccharides.105–107 A series of phosphoethanolamine (pEtN)-modified oligosaccharides with defined sequences and structures were synthesized via the automated glycan assembly (AGA) method.105 The synthesis of one oligosaccharide by AGA is shown in Scheme 32, where the suitably protected monosaccharide derivatives 215–217 are the building blocks for the automated synthesis. In order to elucidate the glycan–protein interactions, the synthetic oligosaccharides were co-assembled with a polypeptide R5, and the assemblies were analyzed using AFM. These pEtN-modified oligomers can form biofilm-inspired assemblies with synthetic polypeptide R5. It was found that mono-, di- and trisaccharide did not influence the secondary structure transition rate of peptide R5 while the longer hexasaccharides such as 221 hindered the structural transition into β-sheets of the peptide.
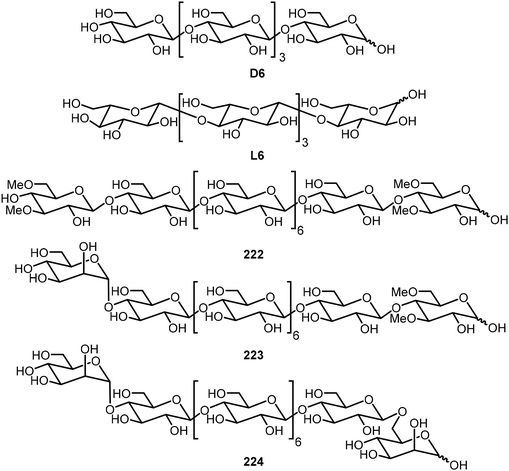
Cellulose oligomers with defined sequence and chirality were also synthesized by the AGA method and their self-assembly was investigated.106 Hexasaccharides composed of D- and L-glucose derivatives were prepared; these are denoted as D6 and L6, and these oligomers formed self-assembled platelets and further assembled into bundles with a chirality directly related to the structures of the monosaccharides (Fig. 18). D6 exhibited right-handed bundles and L6 formed left-handed bundles. Oligomers composed of both D- and L-glucose were also synthesized; the hybrid DL-oligomers were prepared to help elucidate the assembling mechanism. The team also prepared six cellulose-based oliogosaccharides by the AGA method and studied their self-assemblies.107 The synthetic oligomers formed different self-assembled aggregates depending on the sequence of the sugars. Modification of the glucose unit resulted in the formation of hydrogels. Modification at the terminal position with a 3,6-methylated D-glucose unit (222) or a mannose unit (223) resulted in self-healing supramolecular hydrogels, while the bis-mannose (224) compound failed to gelate in water, indicating that 3,6-methylated glucose unit is accountable for hydrogel formation.
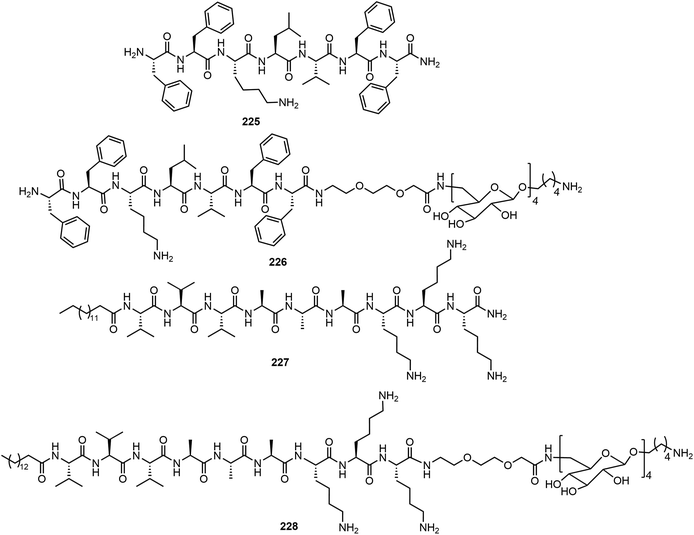
Combining solid phase peptide synthesis (SPPS) with AGA, a series of peptides and glycans 225–228 were synthesized and their self-assemblies were analyzed (Fig. 19).108 The peptides 225 FFKLVFF formed ribbon-like nanofibers and the lipid peptide 227 (palmitoyl-VVVAAAKKK) formed self-assembled fiberous aggregates observed on AFM. The complex peptide–glycans 226 and 228 were prepared efficiently and depending on the structures of the peptide, the chimera exhibited different self-assembled network structures. The glycopeptide chimera 226 did not form self-assembled fibers, even upon mixing the peptide 225 with glycopeptide 226. Further dilution of the mixture resulted in the formation of micelles. The presence of glycans in the chimera interferes with the self-assembling of the peptides into fibers, and this may have implication for application in preventing amyloid fiber formation. Compound 228 did not form well-defined fibers but formed short fibers on its own, and the glycan did not block the self-assembly as in the case of 226. Mixing 227 and 228 in different ratios resulted in well-defined fibers, as indicated by AFM experiment.
6. Stimulus-responsive carbohydrate derivatives
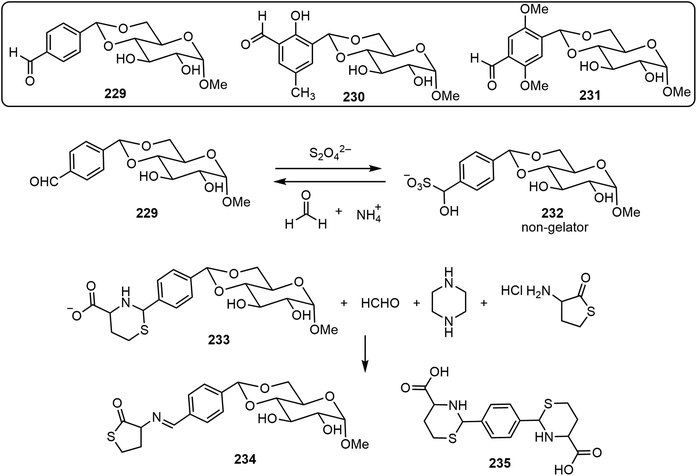
An advantage of the bottom-up molecular self-assemblies formed by non-covalent interactions of small molecules is that various functions can be designed into the molecular systems to achieve control of the resulting assemblies. In the field of LMWGs, this has been demonstrated in many examples. The stimuli or triggers in the various stimulus-responsive systems are photo, enzyme, pH, metal ion, chemical, etc. These are also utilized for carbohydrate-based assemblies. In this section, we briefly review a few recent examples, with the following systems: chemical-triggered, boronic acid dynamic covalant systems, and photoresponsive systems.Several glucose derivatives containing an aldehyde functional group are shown in Scheme 33, and the 4,6-p-formyl-bezyldiene glucose derivative 229 is an organogelator.109 Upon the addition of dithionite, gelator 229 was converted to the sulfonated derivative GluSO3−232, which is not a gelator.110 Therefore, the addition of dithionite can trigger the gel to solution transition. The solution can then be converted back to a gel upon the addition of formaldehyde (fuel), which can be prepared from hexamethylenetetramine (HMTA) and gluconolactone in situ. This mechanistic cause and effect was termed chemical-fueled gelation/supramolecular polymerization. In addition, two other glucose and formyl-benzaldehyde derivatives 230 and 231 were synthesized and studied. These glucose derivatives were mixed to obtain molecular assembling information on the co-assembly of either two or three components.111 The three-component (229 + 230 + 231) system formed self-sorting gels and exhibited self-healing properties. More recently, a chemical triggered sol–gel–sol–gel–sol autonomous system was reported.112 Compound 229 was condensed with homocysteine to form the sugar-thiaz derivative 233, and by adding suitable reagents, the gel to sol transition was tuned (Scheme 33). At time zero, HCHO, piperazine, and homocysteine thiolactone (HCT) were added to the clear solution of 233. The sequence of reactions then took place and the solution turned turbid and resulted in gel formation (G1) in one minute. The gel (G1) was formed by a mixture of gelator 229 and the imine derivative 234. The gel (G1) turned into a clear solution after 10 minutes due to the disappearance of imine 234 and formation of aldehyde 229. After a few minutes, this turned to the formation of another gel (G2) which was formed by 229. The gel (G2) turned into solution after 3 days due to the reformation of compound 233. Overall, the system represents chemical-fueled autonomous sol–gel transitions.
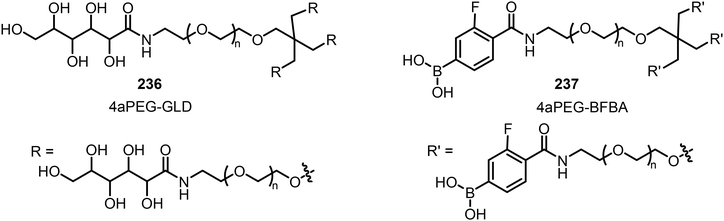
The dynamic covalent interaction between phenyl boronic acid and diols has been utilized in the design of self-healing hydrogels. For example, Webber's team has reported the preparation of 4-armed polyethylene glycol (4aPEG) hydrogels with different stiffnesses (Fig. 20).113 The hydrogel's stiffness can be controlled by dynamic covalent PBA–diol interactions which are temperature, pH, and concentration dependent. The diol from the 4-arm PEG glucose derivative (4aPEG-GLD) 236 acts as a crosslinking component with 4-arm PEG 4-borono-2-fluorobenzoic acid derivative (4aPEG-BFBA) 237.
Due to space limitations, we did not include a separate section for nucleoside- and nucleotide-based self-assembling systems and gelators, but these important classes of compounds were discussed in several recent reviews.11,114–116 Recently guanosine and borate hydrogels were also used as organocatalysts for aldol reactions,117 and the hydrogel formed by a GMP mixture with 1,4,5,8-naphthalene tetracarboxylic acid was used for controlled release of the anticancer drug doxorubicin.118
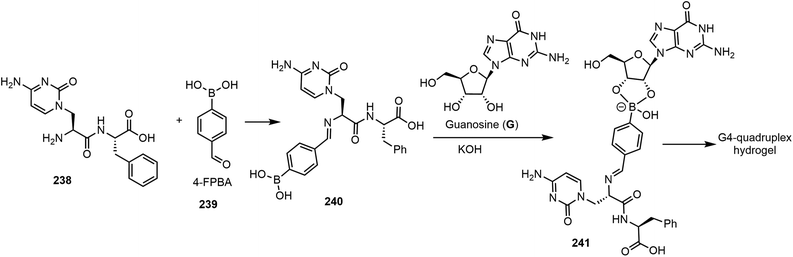
The next section will focus on a few recent examples of nucleoside-based gelators via chemical-triggered systems. Ghosh et al. reported a G-quadruplex hydrogel formed by guanosine (G), nucleopeptide, and 4-formylphenylboronic acid (4-FPBA).119 The combination of these compounds was found to form stable hydrogels in the presence of potassium ions in water. The hydrogel system was named G4NP. Gelation is achieved through a combination of self-assembly and bioconjugation, as shown in Scheme 34. The nucleopeptide reaction with 4-FPBA (239) led to the formation of the imino adduct 240, which then formed a conjugate with guanosine to afford the intermediate 241. The formation of G4-quadruplex from this compound resulted in the formation of a hydrogel system which is injectable and capable of self-healing. The hydrogel system possessed inherent antibacterial activity and antifungal activity.
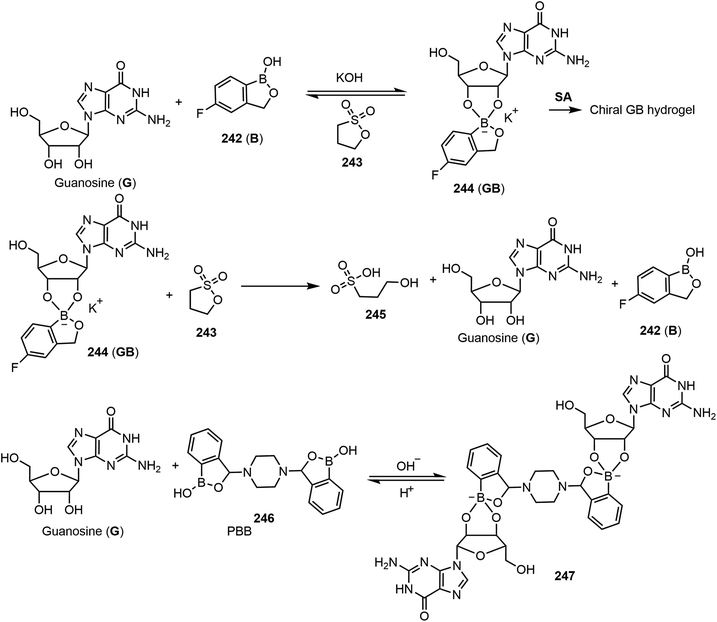
Tuning the G-quadruplex hydrogel through controlling the reaction of the diol and boric acid derivatives can be a useful strategy for creating transient hydrogels and new types of material. Using different boronic acid derivatives in combination with guanosine for the formation of G-quadruplex hydrogels, several other functional supramolecular gels with different properties have been reported.120,121 As shown in Scheme 35, the self-assembling and chirality properties of the G-quadruplex hydrogels formed by guanosine and 5-fluorobenzoxaborole 242 (B) complex have been studied. The gel to sol transition can be controlled by adding various reagents (termed fuels). Under basic conditions by adding KOH, the resulting GB complex self-assembled and formed hydrogels. Under acidic conditions, the gels return to solution. The formation of dynamic covalent bonds between the cis-diols of guanosine with boric acid derivatives led to the formation of supramolecular gels under thermodynamic stable conditions by SA and forming a G-quadruplex. In a related study, acidic and basic conditions were used as chemical triggers or fuels to adjust the state of the molecular assemblies. KOH and 1,3-propanesultone (243) were utilized as the chemical fuels. Compound 243 was hydrolyzed in water to form the acidic 3-hydroxypropanesulfonic acid 245 (HPSA), which in turn reduces the pH values and breaks down the G-quadruplex back to the guanosine (G) and 5-fluorobenzoxaborole mixture. These cycles could be tuned multiple times using these two reagents. More recently, the team reported self-assembling properties of guanosine with 3,3′-piperazine-bis(benzoxaborol) (PBB) 246 and found that the supramolecular chirality can be programmed at different pH values, allowing the system to act as a multistate chiral optical switch.121 A mixture of G and PBB (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in the presence of potassium ions formed gels at a broad range of pH 1–14, and the formation of the G-PBB-G diester complex 247 resulted in the formation of stable hydrogels. At different pH values, the gelation and self-assembly were analyzed. The chiroptical properties of the assemblies under acidic conditions were deemed left-handed helical through a G⋯PBB quadruplex, and right-handed at basic conditions through N–H⋯O hydrogen bonding between G-PBB-G. They concluded that chemical fuels can be used to regulate the self-assembling pathways and lead to different supramolecular chiralities. 1,3-Propanesultone (PrS) was used to adjust the pH. The reaction of the G-PBB-G diester with PrS produces 3-hydroxylpropanesulfonic acid, which decreases the pH and increases the acidity, which results in the cleavage of the diester back to the G and PBB starting materials.
1 ratio) in the presence of potassium ions formed gels at a broad range of pH 1–14, and the formation of the G-PBB-G diester complex 247 resulted in the formation of stable hydrogels. At different pH values, the gelation and self-assembly were analyzed. The chiroptical properties of the assemblies under acidic conditions were deemed left-handed helical through a G⋯PBB quadruplex, and right-handed at basic conditions through N–H⋯O hydrogen bonding between G-PBB-G. They concluded that chemical fuels can be used to regulate the self-assembling pathways and lead to different supramolecular chiralities. 1,3-Propanesultone (PrS) was used to adjust the pH. The reaction of the G-PBB-G diester with PrS produces 3-hydroxylpropanesulfonic acid, which decreases the pH and increases the acidity, which results in the cleavage of the diester back to the G and PBB starting materials.
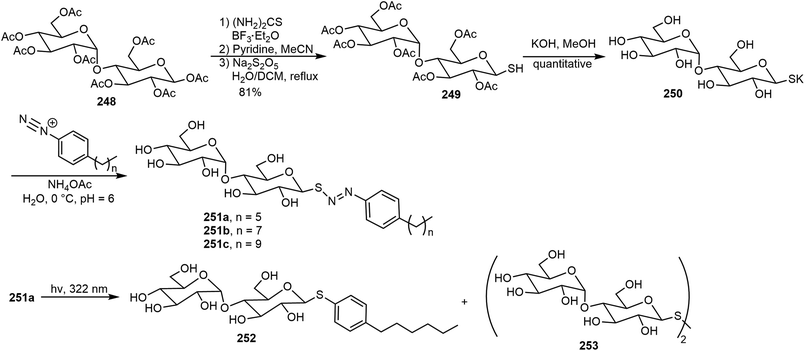
Photoresponsive carbohydrate self-assembling systems have also been investigated. These include azobenzene, diacetylene, and diarylethene based systems. In the next section, a few recent examples will be discussed. Several maltose-based nonionic surfactants 251a–c containing a photocleavable azo-sulfide linker were synthesized and the self-assembling properties of these surfactants were explored (Scheme 36).122 β-D-Maltose octaacetate 248 was converted to the corresponding thiolglycoside 249 through glycosylation using thiourea followed by reduction. The coupling reaction of the hydrolyzed product 250 with diazonium ions led to the formation of the maltose azo sulfide compounds 251. Transmission electron microscopy (TEM) illustrated that the supramolecular assemblies formed by the synthesized surfactants 251 have micelle morphology. Upon UV light exposure, the light-switchable surfactant 251a can rapidly degrade to photostable surfactant 252 indicated by surface tension measurements and LCMS. Biological applications using these surfactants for protein extraction were demonstrated. Surfactant 251b (n = 7) provided the best protein solubility, while surfactants 251a and 251c exhibited slightly less effective results.
Wang et al.123 synthesized glucoside or mannoside coumarin conjugates 256 from 2-allylethoxyl glucoside or mannoside 254 with 7-mercaptohexyloxy-4-methylcoumarin 255 through thiol–ene reactions under UV irradiation. The coumarin moiety in the resulting conjugate of 254 with 255 simultaneously dimerized through [2 + 2] photocyclization to form the sugar-based bolaamphiphiles 256. This is illustrated in Scheme 37. Interestingly, these water-soluble compounds exhibited temperature-responsive properties as indicated by 1H NMR spectroscopy in D2O. At 30 °C, the aromatic signals were invisible, and the aliphatic signals were diminished. When increasing the temperature to 60 °C, both regions became sharp, which indicates that at lower temperatures strong intermolecular interactions exist, which diminish with increasing temperatures. They also observed a reversible transparent–turbid transition upon cooling and heating, which indicates micelle to vesicle transition.
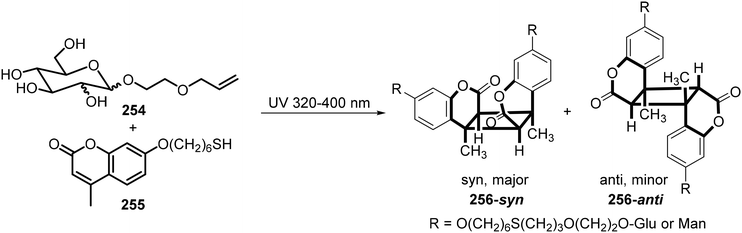 | ||
| Scheme 37 Synthesis of sugar-based bolaamphiphiles 256 by thiol–ene addition and photodimerization of coumarin. | ||
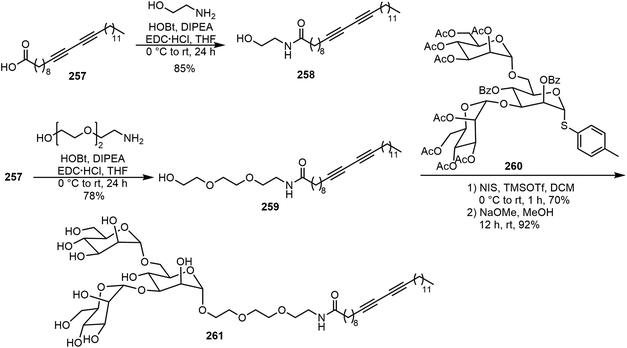
Diacetylene functional groups have also been introduced to carbohydrate derivatives for the formation of glycolipids which can result in a variety of different molecular assemblies and applications. Yadav et al. synthesized glycovesicles prepared from diacetylene-containing glycolipid, as shown in Scheme 38.124 Pentacosadiynoic acid (PCDA) 257 was converted to diacetylene lipids 258 and 259 by amide formation. The manno-triose headgroup 260 was coupled with the diacetylene lipid 259 through an alpha-glycoside linkage to afford the glycolipid 261. The 258–Con A interaction was analyzed as a function of the ligand density at inter- and intra-vesicular ligand–lectin interactions. For the tris-mannose lipid 261, it was found that the intra-vesicular lectin interaction was favored over the inter-vesicular interactions.
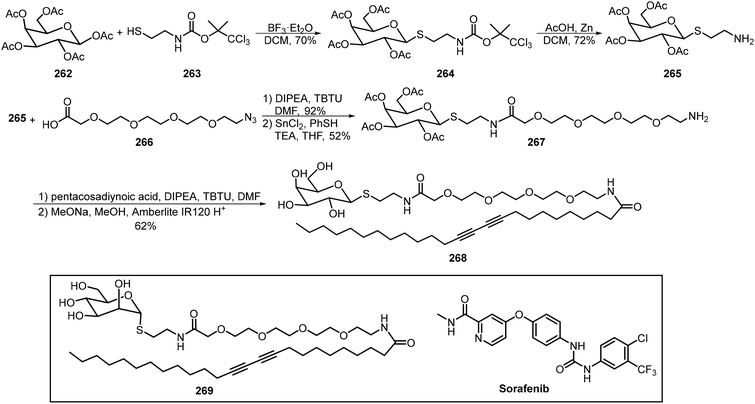
Pentacosadiynoic acid has been incorporated into the anomeric positions of thioglycoside through amide coupling reactions. The resulting amphiphilic glycolipids formed micelles which were then crosslinked with UV light (254 nm) to form the photopolymerized vesicles. The diacetylene-containing galactose-based amphiphile 268 and mannose-based amphiphile 269 were synthesized and their self-assembling ability to form micelles was studied (Scheme 39).125 The polydiacetylenic-based micelles were formed by dispersing the monomers 268 and 269 in distilled water at concentrations higher than their critical micelle concentration (CMC), followed by removing the excess monomers via dialysis and photopolymerization. The prepared micelles served as drug carriers to trap sorafenib, a drug to treat hepatocellular carcinoma (HCC). These nanomicelles-sorafenib carriers can target mannose and asialoglycoprotein receptors (MR and ASGPR) to induce cell apoptosis and reduce cell proliferation. It was found that the mannose-based micelles incorporating sorafenib were superior to galactose-based micelles incorporating sorafenib in treating hepatocellular carcinoma (HCC).
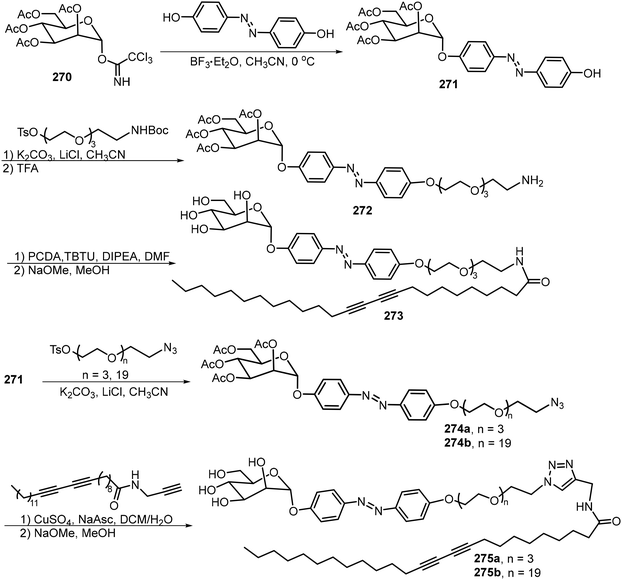
The preparation of glycoamphiphiles 273 and 275 started from the glycosylation of tetra-O-acetyl-α-D-mannopyranosyl trichloroacetimidate 270 with 4,4-dihydroxylazobenzene to afford the azobenzene-containing glycoside 271, which serves as the common intermediate (Scheme 40).126 The hydrophilic tail was introduced by first functionalizing the phenyl OH in the azobenzene glycoside with a bifunctional spacer bearing tosyl and NHBoc or azido groups to form 272, followed by amidation with diacetylene-containing acid or click reactions with diacetylene-containing amido alkyne. Then global deacetylation gave the targeted compounds. Glycolipid 273 formed gels in alcohol and aqueous mixture and the resulting gels underwent photoresponsive gel–sol transition upon UV irradiation. Glycoamphiphile 275b can photopolymerize to create spherical micelles, the cavity of which can trap hydrophobic dye like Nile Red or the drug molecule docetaxel.
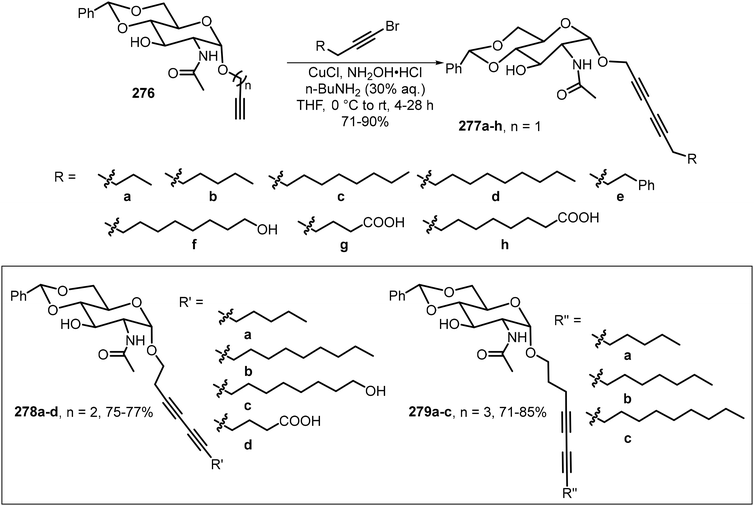
A library of novel α-anomeric diacetylene-containing glycosides 277–279 was designed and synthesized, in which one, two, and three methylene spacers were inserted between the anomeric oxygen and diacetylene group (Scheme 41).127 Glycosylation of N-acetyl-D-glucosamine with terminal alkynyl alcohols furnished α-alkynyl glycosides 276. Subsequent CuCl-catalyzed Cadiot–Chodkiewicz coupling completed the synthesis of the glycosides 277–279. A majority of the prepared diacetylene glycosides are effective gelators and formed photopolymerizable red- and purple-colored gels under UV irradiation.
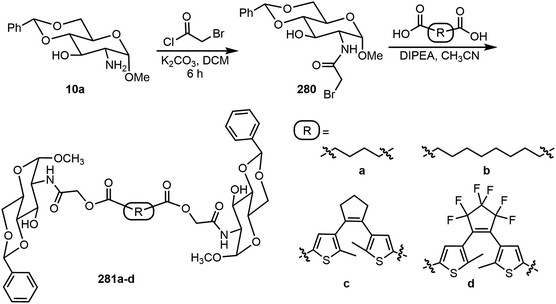
Recently, diarylethene (DAE) or dithienylethene (DTE) photoresponsive functional groups were incorporated into a sugar-based self-assembling system.128 The structures and synthesis are shown in Scheme 42. Two linear dimeric glycolipid-based gelators 281a–b were designed to be responsive to chemical stimuli. The two DTE-based photoswitchable derivatives 281c–d were designed to be responsive to UV and visible light. Compound 281c formed gels in DMSO aqueous solutions at low concentrations. The compound formed gels in nine different solvents and the gel formed by a DMSO and water mixture was shown to absorb rhodamine B dye or toluidine blue. The gel of 281c in DMSO water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) exhibited reversible opaque white to deep purple color transitions upon UV irradiation and visible light treatment. The compound 281d formed fewer gels in the tested solvents, and the ethanol gels exhibited a transparent yellow to dark blue color change when irradiated with UV light.
2 v/v) exhibited reversible opaque white to deep purple color transitions upon UV irradiation and visible light treatment. The compound 281d formed fewer gels in the tested solvents, and the ethanol gels exhibited a transparent yellow to dark blue color change when irradiated with UV light.
7. Concluding remarks
In summary, the most recent developments of 2020 through 2023 in the field of carbohydrate-based self-assembling systems have been discussed. The review has an emphasis on the synthesis of the various carbohydrate derivatives, with a special focus on monosaccharide derivatives that can form supramolecular gels. The factors that determine the molecular assemblies are also discussed in the pertinent section. The monosaccharide derivatives that form micelles or vesicles instead of gels are also reviewed. Similar types of reaction can be used for the preparation of disaccharide derivatives, including the formation of glycosides, glycolipids, and glycopeptides. A few oligosaccharide-based systems that can be prepared by automated synthesis are also discussed. A useful reaction in the construction of sugar-based supramolecular assemblies is the click reaction, which has been employed extensively in the synthesis of various glycoclusters and glycoconjugates. These branched compounds often have additional properties depending on what functional groups are being incorporated. Finally, several stimulus-responsive carbohydrate-based self-assembling systems have been reviewed, and this section includes several dynamic covalent bonds which are reversible and can be controlled by adding different reagents; lastly, several photoresponsive molecular assemblies are also discussed. These represent interesting functional materials.Carbohydrate-based self-assembling systems are an interesting and important class of compounds which will play a more crucial role in future multidisciplinary research fields. There are several advantages using sugars as the sources for the preparation of new functional materials. These starting materials are structurally diverse, sustainable, renewable feedstocks, and most of their derivatives are biocompatible and biodegradable. Many methods have been developed to convert the diverse classes of sugars into useful molecular systems including those that can form supramolecular gels. Readily available monosaccharides and disaccharides can be modified to form self-assembling systems. In this review, we summarize the synthetic approaches for different classes of sugar derivatives, which are typically carried out in a few steps. It is necessary to be able to prepare these self-assemblies in short steps for the sake of efficiency and practicality. For example, the glycosylation reaction is one of the most frequently employed reactions for mono- and disaccharide modification, and many synthetic methods are available. Modifications at the hydroxyl groups, especially primary hydroxyl groups selectively, can also lead to specifically functionalized sugars with simple reactions. Natural products such as lipids, amino acids and nucleobases have been utilized for the formation of various sugar self-assembling systems. The self-assembled gels typically are not as stable as polymer gels; however the branched systems and polymerizable systems can be utilized to enhance the stability.
In comparison with other classes of natural product-based molecular self-assemblies, including peptide and nucleic acids etc., carbohydrate-based self-assembling systems are inherently more complex and underexplored. Sugars are unique in their structures, and they are the only naturally available compounds containing multiple and redundant functional groups while being optically pure with great diversity. We hope to address some of these complexities and deliver the message that sugar derivatives have great potentials for the creation of soft materials and nanostructures that are applicable for catalysis and in biomedicines. Though some steps are complex in certain examples, the structures of the systems with built-in design elements are important and the knowledge gained from existing systems can be used to guide future studies. There is great promise for these classes of materials in various research fields as new biocompatible, biodegradable materials.
It is also necessary to develop new methods of designing self-assembling systems with the desired properties by introducing functional groups or scaffolds that can have a controllable response to various stimuli such as light, mechanical forces, temperature, magnetic and electrical fields, and chemical environment such as pH, ligand binding, and reactions. These unique systems with an optimal function and structure correlation are likely to have important future applications for advanced materials. The creation of these sugar derivatives has potential applications in drug delivery, therapeutic agents, tissue engineering, 3D printing, fluorescent sensors, etc.
Sugar-based molecular gels and new materials have endless potential and real-world utilities, they must be easy to prepare, and their properties need to be readily predicable. The future direction can focus on the element of design and achieving multifunctional stimulus-responsive molecular entities. Utilizing new catalytic reactions and incorporating automated synthesis will increase efficiency and reduce the synthetic difficulties. The advancement of machine learning and AI technology may also assist the development of novel materials and molecular assemblies. This technology may one day predict the structural requirements and functionalities needed for optimal supramolecular interactions and for binding to metal ions or ligands. Finally, collaborations of researchers from different fields including chemistry, materials sciences, engineering, and biology will ultimately lead to more impactful research discoveries that allow the carbohydrate-based self-assemblies to have practical applications.
Conflicts of interest
There are no conflicts of interest to declare.References
- L. Su, Y. Feng, K. Wei, X. Xu, R. Liu and G. Chen, Chem. Rev., 2021, 121, 10950–11029 CrossRef CAS PubMed.
- M. Delbianco, P. Bharate, S. Varela-Aramburu and P. H. Seeberger, Chem. Rev., 2016, 116, 1693–1752 CrossRef CAS PubMed.
- V. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh and X. Chen, Chem. Rev., 2016, 116, 3086–3240 CrossRef CAS PubMed.
- A. K. Agrahari, P. Bose, M. K. Jaiswal, S. Rajkhowa, A. S. Singh, S. Hotha, N. Mishra and V. K. Tiwari, Chem. Rev., 2021, 121, 7638–7956 CrossRef CAS PubMed.
- S. Cecioni, A. Imberty and S. Vidal, Chem. Rev., 2015, 115, 525–561 CrossRef CAS PubMed.
- S. Leusmann, P. Menova, E. Shanin, A. Titz and C. Rademacher, Chem. Soc. Rev., 2023, 52, 3663–3740 RSC.
- C. Mueller, G. Despras and T. K. Lindhorst, Chem. Soc. Rev., 2016, 45, 3275–3302 RSC.
- A. Brito, S. Kassem, R. L. Reis, R. V. Ulijn, R. A. Pires and I. Pashkuleva, Chem, 2021, 7, 2943–2964 CAS.
- A. Mittal, Krishna, Aarti, S. Prasad, P. K. Mishra, S. K. Sharma and B. Parshad, Mater. Adv., 2021, 2, 3459–3473 RSC.
- Y. Yao, X. Meng, C. Li, K. V. Bernaerts and K. Zhang, Small, 2023, 19, e2208286 CrossRef PubMed.
- J. Morris, J. Bietsch, K. Bashaw and G. Wang, Gels, 2021, 7, 24 CrossRef CAS PubMed.
- R. Tyagi, K. Singh, N. Srivastava and R. Sagar, Mater. Adv., 2023, 4, 3929–3950 RSC.
- S. Datta and S. Bhattacharya, Chem. Soc. Rev., 2015, 44, 5596–5637 RSC.
- J. Bietsch, M. Olson and G. Wang, Gels, 2021, 7, 134 CrossRef CAS PubMed.
- J. Bietsch, L. Baker, A. Duffney, A. Mao, M. Foutz, C. Ackermann and G. Wang, Gels, 2023, 9, 445 CrossRef CAS PubMed.
- D. Wang, A. Chen, J. Morris and G. Wang, RSC Adv., 2020, 10, 40068–40083 RSC.
- A. J. Chen, D. Wang, L. P. Samankumara and G. J. Wang, Synthesis, 2019, 2897–2908 CAS.
- P. Sharma and G. Wang, Gels, 2022, 8, 191 CrossRef CAS PubMed.
- A. Chen, S. B. Adhikari, K. Mays and G. Wang, Langmuir, 2017, 33, 8076–8089 CrossRef CAS PubMed.
- A. D. Ludwig, V. Gorbunova, A. Saint-Jalmes, F. Berrée and L. Lemiègre, ChemistrySelect, 2023, 8, e202300213 CrossRef CAS.
- A. D. Ludwig, N. Ourvois-Maloisel, A. Saint-Jalmes, F. Artzner, J. P. Guegan, O. Tasseau, F. Berree and L. Lemiegre, Soft Matter, 2022, 18, 9026–9036 RSC.
- A. Brito, D. Dave, A. Lampel, V. I. B. Castro, D. Kroiss, R. L. Reis, T. Tuttle, R. V. Ulijn, R. A. Pires and I. Pashkuleva, J. Am. Chem. Soc., 2021, 143, 19703–19710 CrossRef CAS PubMed.
- C. He, S. Wu, D. Liu, C. Chi, W. Zhang, M. Ma, L. Lai and S. Dong, J. Am. Chem. Soc., 2020, 142, 17015–17023 CrossRef CAS PubMed.
- N. P. Pathak, A. Sengupta and S. Yadav, Mater. Adv., 2022, 3, 3906–3914 RSC.
- N. Tsutsumi, A. Ito, A. Ishigamori, M. Ikeda, M. Izumi and R. Ochi, Int. J. Mol. Sci., 2021, 22, 1860 CrossRef CAS PubMed.
- N. Tsutsumi, A. Ito, Y. Niko, Y. Bando, K. Takahashi, M. Ikeda, K. Yoneyama, T. Nakamura, M. Izumi and R. Ochi, ChemistrySelect, 2022, 7, e202202559 CrossRef CAS.
- A. George and N. Jayaraman, ACS Omega, 2023, 8, 16927–16934 CrossRef CAS PubMed.
- A. George and N. Jayaraman, Carbohydr. Res., 2023, 533, 108933 CrossRef CAS PubMed.
- L. Mousavifar, J. D. Lewicky, A. Taponard, R. Bagul, M. Rivat, S. Abdullayev, A. L. Martel, N. L. Fraleigh, A. Nakamura, F. J. Veyrier, H.-T. Le and R. Roy, Pharmaceutics, 2022, 14, 2300 CrossRef CAS PubMed.
- X. Xu, L. Li, L. Ye, X. Liu, Y. Feng and G. Chen, Macromol. Rapid Commun., 2023, 44, 2300359 CrossRef CAS PubMed.
- N. Baccile, A. Poirier, C. Seyrig, P. Le Griel, J. Perez, D. Hermida-Merino, P. Pernot, S. L. K. W. Roelants and W. Soetaert, J. Colloid Interface Sci., 2023, 630, 404–415 CrossRef CAS PubMed.
- G. Ben Messaoud, P. Le Griel, S. Prevost, D. Hermida-Merino, W. Soetaert, S. L. K. W. Roelants, C. V. Stevens and N. Baccile, Soft Matter, 2020, 16, 2528–2539 RSC.
- A. Poirier, P. Le Griel, T. Bizien, T. Zinn, P. Pernot and N. Baccile, Soft Matter, 2023, 19, 366–377 RSC.
- A. Poirier, P. Le Griel, I. Hoffmann, J. Perez, P. Pernot, J. Fresnais and N. Baccile, Soft Matter, 2023, 19, 378–393 RSC.
- A. Poirier, P. Le Griel, J. Perez, D. Hermida-Merino, P. Pernot and N. Baccile, ACS Sustainable Chem. Eng., 2022, 10, 16503–16515 CrossRef CAS.
- A. K. Rachamalla, V. P. Rebaka, T. Banoo, R. Pawar, M. Faizan, K. Lalitha and S. Nagarajan, Green Chem., 2022, 24, 2451–2463 RSC.
- P. V. Bhavya, K. Soundarajan, J. G. Malecki and T. Mohan Das, ACS Omega, 2022, 7, 39310–39324 CrossRef CAS PubMed.
- V. Rabecca Jenifer and T. Mohan Das, Soft Matter, 2022, 18, 9017–9025 RSC.
- R. J. Vasanthan, J. G. Malecki and T. M. Das, Ind. Eng. Chem. Res., 2023, 62, 13034–13045 CrossRef CAS.
- Y. Feng, L. Li, Q. Du, L. Gou, L. Zhang, Y. Chai, R. Zhang, T. Shi and G. Chen, CCS Chem., 2022, 4, 2228–2238 CrossRef CAS.
- O. Metelkina, B. Huck, J. S. O'Connor, M. Koch, A. Manz, C.-M. Lehr and A. Titz, J. Mater. Chem. B, 2022, 10, 537–548 RSC.
- S. Komba and R. Iwaura, ACS Omega, 2021, 6, 20912–20923 CrossRef CAS PubMed.
- P. Sharma, A. Chen, D. Wang and G. Wang, Chemistry, 2021, 3, 935–958 CrossRef CAS.
- G.-R. Huang, X.-W. Shi, Y.-M. Wu, B.-P. Cao, H. Okamoto and Q. Xiao, New J. Chem., 2023, 47, 84–91 RSC.
- L. Yao, L. Wu, R. Wang, Y. Liu, F. Luo, Y. Zhang and G. Chen, ACS Macro Lett., 2022, 11, 975–981 CrossRef CAS PubMed.
- P. Aryal, S. B. Adhikari, A. Duffney and G. Wang, New J. Chem., 2023, 47, 17224–17228 RSC.
- S. L. Higashi and M. Ikeda, JACS Au, 2021, 1, 1639–1646 CrossRef CAS PubMed.
- A. Ghosh, S. K. Dubey, M. Patra, J. Mandal, N. N. Ghosh, P. Das, A. Bhowmick, K. Sarkar, S. Mukherjee, R. Saha and S. Bhattacharjee, Chem. – Eur. J., 2022, 28, e202201621 CrossRef CAS PubMed.
- V. I. B. Castro, A. R. Araujo, F. Duarte, A. Sousa-Franco, R. L. Reis, I. Pashkuleva and R. A. Pires, ACS Appl. Mater. Interfaces, 2023, 15, 29998–30007 CrossRef CAS PubMed.
- D. Biswakarma, N. Dey and S. Bhattacharya, J. Colloid Interface Sci., 2022, 615, 335–345 CrossRef CAS PubMed.
- Y. Sasaoka and K. Ito, Chem. Lett., 2022, 51, 1049–1053 CrossRef CAS.
- M. Khan, S. Das, A. Roy and S. Roy, Langmuir, 2023, 39, 899–908 CrossRef CAS PubMed.
- Y. Ohsedo, Chem. – Asian J., 2022, 17, e202200461 CrossRef CAS PubMed.
- K. P. C. Sekhar, D. Patel, S. A. Holey, S. Kanjilal and R. R. Nayak, J. Mol. Liq., 2022, 351, 118585 CrossRef CAS.
- S. A. Holey, K. P. C. Sekhar, D. K. Swain, S. Bojja and R. R. Nayak, ACS Biomater. Sci. Eng., 2022, 8, 1103–1114 CrossRef CAS PubMed.
- S. A. Holey, P. Basak, S. Bojja and R. R. Nayak, Soft Matter, 2023, 19, 6305–6313 RSC.
- H. S. Cooke, L. Schlichter, C. C. Piras and D. K. Smith, Chem. Sci., 2021, 12, 12156–12164 RSC.
- M. Albino, T. J. Burden, C. C. Piras, A. C. Whitwood, I. J. S. Fairlamb and D. K. Smith, ACS Sustainable Chem. Eng., 2023, 11, 1678–1689 CrossRef CAS PubMed.
- L. Schlichter, C. C. Piras and D. K. Smith, Chem. Sci., 2021, 12, 4162–4172 RSC.
- K. Fan, L. Wang, W. Wei, F. Wen, Y. Xu, X. Zhang and X. Guan, Chem. Eng. J., 2022, 441, 136026 CrossRef CAS.
- I. Mattsson, M. Lahtinen, R. Sitdikov, B. Wank, T. Saloranta-Simell and R. Leino, Carbohydr. Res., 2022, 518, 108596 CrossRef CAS PubMed.
- S. K. Singh, S. Dey, M. P. Schneider and S. Nandi, New J. Chem., 2022, 46, 6193–6200 RSC.
- T. Maki, R. Yoshisaki, S. Akama and M. Yamanaka, Polym. J., 2020, 52, 931–938 CrossRef CAS.
- R. Yoshisaki, S. Kimura, M. Yokoya and M. Yamanaka, Chem. – Asian J., 2021, 16, 1937–1941 CrossRef CAS PubMed.
- D. Biswakarma, N. Dey and S. Bhattacharya, Chem. Commun., 2020, 56, 7789–7792 RSC.
- D. Biswakarma, N. Dey and S. Bhattacharya, J. Chem. Sci., 2023, 135, 7 CrossRef CAS.
- Y.-C. Wang, L. L. Kegel, D. S. Knoff, B. S. Deodhar, A. V. Astashkin, M. Kim and J. E. Pemberton, J. Mater. Chem. B, 2022, 10, 3861–3875 RSC.
- S. Yao, R. Brahmi, F. Portier, J.-L. Putaux, J. Chen and S. Halila, Chem. – Eur. J., 2021, 27, 16716–16721 CrossRef CAS PubMed.
- S. Yao, R. Brahmi, A. Bouschon, J. Chen and S. Halila, Green Chem., 2023, 25, 330–335 RSC.
- Y. Yu, S. Gim, D. Kim, Z. A. Arnon, E. Gazit, P. H. Seeberger and M. Delbianco, J. Am. Chem. Soc., 2019, 141, 4833–4838 CrossRef CAS PubMed.
- S. Gim, G. Fittolani, Y. Nishiyama, P. H. Seeberger, Y. Ogawa and M. Delbianco, Angew. Chem., Int. Ed., 2020, 59, 22577–22583 CrossRef CAS PubMed.
- S. Gim, G. Fittolani, Y. Yu, Y. Zhu, P. H. Seeberger, Y. Ogawa and M. Delbianco, Chemistry, 2021, 27, 13139–13143 CrossRef CAS PubMed.
- P. Tsupko, S. S. Sagiri, M. Samateh, S. Satapathy and G. John, J. Surfactants Deterg., 2023, 26, 369–385 CrossRef CAS PubMed.
- X. Zhao, H. Zhang, Y. Gao, Y. Lin and J. Hu, ACS Appl. Bio Mater., 2020, 3, 648–653 CrossRef CAS PubMed.
- Q. Li, Z. L. Wan and X. Q. Yang, Curr. Opin. Food Sci., 2022, 43, 107–113 CrossRef CAS.
- A. A. Ba, J. Everaert, A. Poirier, P. Le Griel, W. Soetaert, S. L. K. W. Roelants, D. Hermida-Merino, C. V. Stevens and N. Baccile, Green Chem., 2020, 22, 8323–8336 RSC.
- A. Poirier, P. Le Griel, T. Zinn, P. Pernot, S. L. K. W. Roelants, W. Soetaert and N. Baccile, Chem. Mater., 2022, 34, 5546–5557 CrossRef CAS.
- N. Baccile, A. Poirier, P. Le Griel, P. Pernot, M. Pala, S. Roelants, W. Soetaert and C. V. Stevens, Colloids Surf., A, 2023, 679, 132518 CrossRef CAS.
- I. Russo Krauss, R. Esposito, L. Paduano and G. D’Errico, Curr. Opin. Colloid Interface Sci., 2024, 70, 101792 CrossRef CAS.
- G. Ben Messaoud, Curr. Opin. Colloid Interface Sci., 2024, 71, 101805 CrossRef CAS.
- J. Liu, Y. Zhang, K. van Dongen, C. Kennedy, M. J. G. Schotman, P. P. Marin San Roman, C. Storm, P. Y. W. Dankers and R. P. Sijbesma, Biomacromolecules, 2023, 24, 2447–2458 CrossRef CAS PubMed.
- O. El Hamoui, K. Gaudin, S. Battu, P. Barthelemy, G. Lespes and B. Alies, Langmuir, 2021, 37, 297–310 CrossRef CAS PubMed.
- O. El Hamoui, T. Sayde, I. Svahn, A. Gudin, E. Gontier, P. Le Coustumer, J. Verget, P. Barthelemy, K. Gaudin, S. Battu, G. Lespes and B. Alies, ACS Biomater. Sci. Eng., 2022, 8, 3387–3398 CrossRef CAS PubMed.
- N. Bansode, J. Verget and P. Barthelemy, Soft Matter, 2023, 19, 6867–6870 RSC.
- S. I. S. Hendrikse, L. Su, T. P. Hogervorst, R. P. M. Lafleur, X. Lou, G. A. van der Marel, J. D. C. Codee and E. W. Meijer, J. Am. Chem. Soc., 2019, 141, 13877–13886 CrossRef CAS PubMed.
- S. Varela-Aramburu, G. Morgese, L. Su, S. M. C. Schoenmakers, M. Perrone, L. Leanza, C. Perego, G. M. Pavan, A. R. A. Palmans and E. W. Meijer, Biomacromolecules, 2020, 21, 4105–4115 CrossRef CAS PubMed.
- L. Rijns, L. Su, K. Maxeiner, G. Morgese, D. Y. W. Ng, T. Weil and P. Y. W. Dankers, Chem. Commun., 2023, 59, 2090–2093 RSC.
- G. Wang, D. Wang, J. Bietsch, A. Chen and P. Sharma, J. Org. Chem., 2020, 85, 16136–16156 CrossRef CAS PubMed.
- A. Chen, D. Wang, J. Bietsch and G. Wang, Org. Biomol. Chem., 2019, 17, 6043–6056 RSC.
- J. Bietsch, A. Chen, D. Wang and G. Wang, Molecules, 2023, 28, 6056 CrossRef CAS PubMed.
- Q. Xiao, M. Delbianco, S. E. Sherman, A. M. R. Perez, P. Bharate, A. Pardo-Vargas, C. Rodriguez-Emmenegger, N. Y. Kostina, K. Rahimi, D. Soder, M. Moller, M. L. Klein, P. H. Seeberger and V. Percec, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 11931–11939 CrossRef CAS PubMed.
- N. Yu Kostina, D. Soder, T. Haraszti, Q. Xiao, K. Rahimi, B. E. Partridge, M. L. Klein, V. Percec and C. Rodriguez-Emmenegger, Angew. Chem., Int. Ed., 2021, 60, 8352–8360 CrossRef CAS PubMed.
- P. V. Murphy, A. Romero, Q. Xiao, A. K. Ludwig, S. Jogula, N. V. Shilova, T. Singh, A. Gabba, B. Javed, D. Zhang, F. J. Medrano, H. Kaltner, J. Kopitz, N. V. Bovin, A. M. Wu, M. L. Klein, V. Percec and H. J. Gabius, iScience, 2021, 24, 101919 CrossRef CAS PubMed.
- M. Mullerova, D. Maciel, N. Nunes, D. Wrobel, M. Stofik, L. Cervenkova Stastna, A. Krupkova, P. Curinova, K. Novakova, M. Bozik, M. Maly, J. Maly, J. Rodrigues and T. Strasak, Biomacromolecules, 2022, 23, 276–290 CrossRef CAS PubMed.
- T. Isono, R. Komaki, N. Kawakami, K. Chen, H.-L. Chen, C. Lee, K. Suzuki, B. J. Ree, H. Mamiya, T. Yamamoto, R. Borsali, K. Tajima and T. Satoh, Biomacromolecules, 2022, 23, 3978–3989 CrossRef CAS PubMed.
- L. Dong, M. Fu, L. Liu, H.-H. Han, Y. Zang, G.-R. Chen, J. Li, X.-P. He and S. Vidal, Chem. – Eur. J., 2020, 26, 14445–14452 CrossRef CAS PubMed.
- L. Dong, M.-Y. Zhang, H.-H. Han, Y. Zang, G.-R. Chen, J. Li, X.-P. He and S. Vidal, Chem. Sci., 2022, 13, 247–256 RSC.
- A. Barattucci, C. M. A. Gangemi, A. Santoro, S. Campagna, F. Puntoriero and P. Bonaccorsi, Org. Biomol. Chem., 2022, 20, 2742–2763 RSC.
- L. J. Patalag, S. Ahadi, O. Lashchuk, P. G. Jones, S. Ebbinghaus and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 8766–8771 CrossRef CAS PubMed.
- Y.-c. Liu, G.-j. Liu, W. Zhou, G.-l. Feng, Q.-y. Ma, Y. Zhang and G.-w. Xing, Angew. Chem., Int. Ed., 2023, 62, e202309786 CrossRef CAS PubMed.
- J. Treekoon, T. Pewklang, K. Chansaenpak, J. N. Gorantla, S. Pengthaisong, R.-Y. Lai, J. R. Ketudat-Cairns and A. Kamkaew, Org. Biomol. Chem., 2021, 19, 5867–5875 RSC.
- A. K. East, M. C. Lee, C. Jiang, Q. Sikander and J. Chan, J. Am. Chem. Soc., 2023, 145, 7313–7322 CrossRef CAS PubMed.
- R. Liu, R. Zhang, L. Li, Z. Kochovski, L. Yao, M.-P. Nieh, Y. Lu, T. Shi and G. Chen, J. Am. Chem. Soc., 2021, 143, 6622–6633 CrossRef CAS PubMed.
- L. Li, L. Wu, M. Urschbach, D. Strassburger, X. Liu, P. Besenius and G. Chen, ACS Polym. Au, 2022, 2, 478–485 CrossRef CAS PubMed.
- T. Tyrikos-Ergas, S. Gim, J. Y. Huang, S. Pinzon Martin, D. Varon Silva, P. H. Seeberger and M. Delbianco, Nat. Commun., 2022, 13, 3954 CrossRef CAS PubMed.
- G. Fittolani, D. Vargova, P. H. Seeberger, Y. Ogawa and M. Delbianco, J. Am. Chem. Soc., 2022, 144, 12469–12475 CrossRef CAS PubMed.
- N. Hribernik, D. Vargova, M. C. S. Dal Colle, J. H. Lim, G. Fittolani, Y. Yu, J. Fujihara, K. Ludwig, P. H. Seeberger, Y. Ogawa and M. Delbianco, Angew. Chem., Int. Ed., 2023, 62, e202310357 CrossRef CAS PubMed.
- M. G. Ricardo and P. H. Seeberger, Chem. – Eur. J., 2023, 29, e202301678 CrossRef CAS PubMed.
- Q. Chen, Y. Lv, D. Zhang, G. Zhang, C. Liu and D. Zhu, Langmuir, 2010, 26, 3165–3168 CrossRef CAS PubMed.
- N. Singh, B. Lainer, G. J. M. Formon, S. De Piccoli and T. M. Hermans, J. Am. Chem. Soc., 2020, 142, 4083–4087 CrossRef CAS PubMed.
- N. Singh, A. Lopez-Acosta, G. J. M. Formon and T. M. Hermans, J. Am. Chem. Soc., 2022, 144, 410–415 CrossRef CAS PubMed.
- T. M. Hermans and N. Singh, Angew. Chem., Int. Ed., 2023, 62, e202301529 CrossRef CAS PubMed.
- R. C. Ollier, Y. Xiang, A. M. Yacovelli and M. J. Webber, Chem. Sci., 2023, 14, 4796–4805 RSC.
- G. M. Peters and J. T. Davis, Chem. Soc. Rev., 2016, 45, 3188–3206 RSC.
- F. Pu, J. Ren and X. Qu, Chem. Soc. Rev., 2018, 47, 1285–1306 RSC.
- T. Giraud, P. Hoschtettler, G. Pickaert, M.-C. Averlant-Petit and L. Stefan, Nanoscale, 2022, 14, 4908–4921 RSC.
- Z. Chen, P. Zhou, Y. Guo, Anna, J. Bai, R. Qiao and C. Li, J. Org. Chem., 2022, 87, 2624–2631 CrossRef CAS PubMed.
- Umesh, S. Sarkar, S. Bera, P. Moitra and S. Bhattacharya, Mater. Today Chem., 2023, 30, 101554 CrossRef CAS.
- S. Ghosh, T. Ghosh, S. Bhowmik, M. K. Patidar and A. K. Das, ACS Appl. Bio Mater., 2023, 6, 640–651 CrossRef CAS PubMed.
- X.-Q. Xie, Y. Zhang, Y. Liang, M. Wang, Y. Cui, J. Li and C.-S. Liu, Angew. Chem., Int. Ed., 2022, 61, e202114471 CrossRef CAS PubMed.
- J. Li, Y. Cui, Y.-L. Lu, Y. Zhang, K. Zhang, C. Gu, K. Wang, Y. Liang and C.-S. Liu, Nat. Commun., 2023, 14, 5030 CrossRef CAS PubMed.
- K. A. Brown, M. K. Gugger, D. S. Roberts, D. Moreno, P. S. Chae, Y. Ge and S. Jin, Langmuir, 2023, 39, 1465–1473 CrossRef CAS PubMed.
- S. Wang, M. C. Forster, K. Xue, F. Ehlers, B. Pang, L. B. Andreas, P. Vana and K. Zhang, Angew. Chem., Int. Ed., 2021, 60, 9712–9718 CrossRef CAS PubMed.
- S. Yadav, K. Naresh and N. Jayaraman, ChemBioChem, 2021, 22, 3075–3081 CrossRef CAS PubMed.
- M. Negrete, E. Romero-Ben, A. Gutierrez-Valencia, C. Rosales-Barrios, E. Ales, T. Mena-Barragan, J. A. Flores, M. C. Castillejos, P. de la Cruz-Ojeda, E. Navarro-Villaran, C. Cepeda-Franco, N. Khiar and J. Muntane, ACS Appl. Bio Mater., 2021, 4, 4789–4799 CrossRef CAS PubMed.
- E. Romero-Ben, M. C. Castillejos, C. Rosales-Barrios, M. Exposito, P. Ruda, P. M. Castillo, S. Nardecchia, J. de Vicente and N. Khiar, J. Mater. Chem. B, 2023, 11, 10189–10205 RSC.
- G. Wang, D. Wang, A. Chen, I. S. Okafor and L. P. Samankumara, ACS Omega, 2022, 7, 11330–11342 CrossRef CAS PubMed.
- P. Aryal, J. Morris, S. B. Adhikari, J. Bietsch and G. Wang, Molecules, 2023, 28, 6228 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |