Open Access Article
Open Access ArticleIncreased thermal stability and retained antibacterial properties in a sulbactam and amantadine salt: towards effective antibacterial–antiviral combination therapies†
Josephine
Bicknell
 a,
Ivan
Bondarenko
a,
Ivan
Bondarenko
 a,
Alice
Colatrella
a,
Alice
Colatrella
 a,
Elani J.
Cabrera-Vega
a,
Elani J.
Cabrera-Vega
 b,
Jesus Daniel
Loya
b,
Jesus Daniel
Loya
 a,
Delbert S.
Botes
a,
Delbert S.
Botes
 c,
Jay L.
Mellies
c,
Jay L.
Mellies
 *d and
Gonzalo
Campillo-Alvarado
*d and
Gonzalo
Campillo-Alvarado
 *a
*a
aDepartment of Chemistry, Reed College, Portland, OR 97202, USA. E-mail: gcampillo@reed.edu; Tel: +1 (503) 517 7469
bDepartment of Chemistry, Portland State University, OR 97201, USA
cDepartment of Chemistry, University of Missouri, Columbia, MO 65211, USA
dDepartment of Biology, Reed College, Portland, OR 97202, USA
First published on 19th November 2024
Abstract
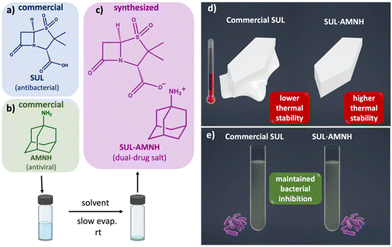
We describe the formation of a multidrug salt comprising sulbactam (SUL, β-lactamase inhibitor) and amantadine (AMNH, antiviral). Physicochemical investigation of the SUL·AMNH salt revealed enhanced thermal stability compared to pristine starting materials. In vitro studies found that salt formation in SUL·AMNH does not disrupt antibacterial activity against model organisms Escherichia coli and Staphylococcus epidermidis. To our knowledge, we show the first β-lactamase inhibitor-antiviral salt where both components have been approved by the U.S. Food and Drug Administration (FDA), and the first multicomponent solid containing SUL. We envisage our strategy could inspire the design of multicomponent solids for antimicrobial combination therapies.
The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) warn that without the rapid development of new antimicrobial formulations, treatments for bacterial infections will become obsolete due to antimicrobial resistance (AMR).1–3 Bacterial–viral coinfections are an important contributor to the anticipated medical and economic burden of AMR.4 Gaining widened attention during the COVID-19 pandemic, bacterial–viral coinfections impacted nearly 10% of all critically ill COVID-19 patients, such that patients would receive antibiotic regimens in addition to available antiviral medications.5 The reduced efficacy of single-drug therapies and exacerbation of antimicrobial comorbidities demand immediate dual-pharmaceutical strategies to alleviate the toll of AMR.6
An emerging route for pharmaceutical innovation is pharmaceutical drug–drug cocrystallization (e.g., Entresto, Seglentis) or salt formation (i.e., multicomponent solids).7–10 In the past five years, multicomponent solids with antibiotics have demonstrated marked improvement in the antibacterial activity of antimicrobial compounds.11–13 Inspired by these recent advances, we asked whether forming a multicomponent salt with sulbactam would retain its antibacterial properties. This study presents a dual-drug salt comprising antibacterial sulbactam (SUL) and antiviral amantadine (AMNH) (Scheme 1).
The multicomponent solid SUL·AMNH shows sustained antibacterial activity and improved thermal stabilities compared to pristine SUL. SUL is an antibacterial compound and irreversible β-lactamase inhibitor (BLI) that prevents β-lactamase-positive bacteria from hydrolyzing β-lactam antibiotics.14 Because SUL targets resistance mechanisms rather than acting with explicit bactericidal effect, in clinical practice, it is typically administered in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with a primary β-lactam antibiotic, such as Ampicillin. AMNH is an antiviral used for treating influenza A subtypes that has also demonstrated in vitro inhibition of SARS-CoV-2.15 Given that both SUL and AMNH are seminal active pharmaceutical ingredients (APIs) approved by the U.S. Food and Drug Administration (FDA), this work explores the SUL·AMNH salt as an initial approach toward developing effective combination therapies against bacterial–viral coinfections.16,17 As the first dual-drug solid with SUL and the first BLI-antiviral salt, our work further demonstrates how solid-state engineering can integrate current FDA-approved BLIs, creating novel BLI-antiviral multicomponent solids that retain antimicrobial properties upon salt formation.
2 with a primary β-lactam antibiotic, such as Ampicillin. AMNH is an antiviral used for treating influenza A subtypes that has also demonstrated in vitro inhibition of SARS-CoV-2.15 Given that both SUL and AMNH are seminal active pharmaceutical ingredients (APIs) approved by the U.S. Food and Drug Administration (FDA), this work explores the SUL·AMNH salt as an initial approach toward developing effective combination therapies against bacterial–viral coinfections.16,17 As the first dual-drug solid with SUL and the first BLI-antiviral salt, our work further demonstrates how solid-state engineering can integrate current FDA-approved BLIs, creating novel BLI-antiviral multicomponent solids that retain antimicrobial properties upon salt formation.
Our design strategy involved the selection of SUL (BLI) as a hydrogen bond donor and AMNH (antiviral) as a hydrogen bond acceptor. Similar strategies have been employed to synthesize pharmaceutical salts and cocrystals of norfloxacin-sulfathiazole and resveratrol-amantadine hydrochloride.18,19 Here, powders of SUL (25.0 mg, 0.107 mmol) and AMNH (16.2 mg, 0.107 mmol) were combined using liquid-assisted grinding (LAG, 3 drops methanol) in a mechanochemical ball mill (Retsch 400 MM, 1 stainless steel ball, 7 mm, 10 ml stainless steel jar) for 30 minutes. The resulting solid was examined by powder X-ray diffraction (PXRD), which revealed the formation of a new phase (Fig. S1, ESI†). The ground solids were dissolved and sonicated in methanol (2 mL), and the solution was incubated at room temperature. White, needle-like single crystals formed after five days of slow evaporation. The composition of SUL·AMNH single crystals was determined to be a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the pharmaceutical components by 1H NMR spectroscopy (Fig. S2, ESI†).
1 ratio of the pharmaceutical components by 1H NMR spectroscopy (Fig. S2, ESI†).
Single crystal X-ray diffraction (SCXRD) confirmed the formation of a multicomponent salt between SUL and AMNH. Specifically, the components in SUL·AMNH crystallize in the monoclinic space group P21 to form a two-component drug–drug ionic assembly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) sustained by [N+–H⋯O−] ionic interactions (dD⋯A = 2.743 Å) (Fig. 1a).20,21 The pKa rule supported the favorability of the ionized complexes (see ESI† for calculation).22 The β-lactam ketone exhibits hydrogen bonding (dN–H⋯O = 2.879 Å) with the ammonium hydrogen, orienting AMNH molecules to form a lipophilic aggregate with adamantyl motifs (Fig. 1b).23 Additional evidence for the formation of the drug–drug salt was provided by Fourier transform infrared (FT-IR) spectroscopy, which showed the formation of the carboxylate anion and ammonium cation (Fig. S3, ESI†). The adamantyl groups in AMNH aggregate in an interlocking pattern, creating a tightly organized hydrophobic layer in the bc-plane (Fig. 1c). The hydrophilic regions occupied by SUL molecules are supported by weak hydrogen bonds between sulbactam's β-lactam hydrogen and deprotonated carboxylic acid [dC–H⋯O = 3.233 Å]. The simulated PXRD pattern from the crystal structure of SUL·AMNH matches the experimental pattern from mechanochemical experiments.
1 ratio) sustained by [N+–H⋯O−] ionic interactions (dD⋯A = 2.743 Å) (Fig. 1a).20,21 The pKa rule supported the favorability of the ionized complexes (see ESI† for calculation).22 The β-lactam ketone exhibits hydrogen bonding (dN–H⋯O = 2.879 Å) with the ammonium hydrogen, orienting AMNH molecules to form a lipophilic aggregate with adamantyl motifs (Fig. 1b).23 Additional evidence for the formation of the drug–drug salt was provided by Fourier transform infrared (FT-IR) spectroscopy, which showed the formation of the carboxylate anion and ammonium cation (Fig. S3, ESI†). The adamantyl groups in AMNH aggregate in an interlocking pattern, creating a tightly organized hydrophobic layer in the bc-plane (Fig. 1c). The hydrophilic regions occupied by SUL molecules are supported by weak hydrogen bonds between sulbactam's β-lactam hydrogen and deprotonated carboxylic acid [dC–H⋯O = 3.233 Å]. The simulated PXRD pattern from the crystal structure of SUL·AMNH matches the experimental pattern from mechanochemical experiments.
The electronic properties of antibacterial–antiviral salt were rationalized by density functional theory (DFT) electrostatic potential calculations (ωB97X-D/cc-pVTZ basis set) based on molecular coordinates from SCXRD analysis. Analysis of SUL highlighted the Lewis acidity of the carboxylic acid with a positive band of 303.9 kJ mol−1 (Fig. 1d). The AMNH interactive counterpart, the primary amine, showed high electron-donating capacity at 192.8 kJ mol−1 (Fig. 1e). The resulting proton transfer yields strong polarity in the SUL·AMNH electron density, with SUL carboxylate at 262.0 kJ mol−1 and AMNH ammonium cation at 373.6 kJ mol−1 (Fig. 1f).
The solid of SUL·AMNH showed increased thermal stability, as observed by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) analyses, and hot-stage polarized optical microscopy (POM) (Fig. 2). For SUL·AMNH, TGA showed a decomposition point onset at ca. 178 °C, which was associated by the observation of an exotherm followed by an endotherm in the DSC trace. SUL and AMNH showed decomposition onsets at ca. 137 °C and 61.3 °C, respectively. Notably, the results demonstrated increased thermal stability of SUL·AMNH in the solid state compared to the pristine starting materials (SUL and AMNH), suggesting the formation of stronger intermolecular forces (Fig. 2a and b).24 The increase in thermal stability observed in SUL·AMNHvia salt formation is comparable to that of commercial salts, amantadine hydrochloride and sulbactam sodium (Fig. S4–S13†), though moderately lower. Hot-stage POM showed a SUL·AMNH crystal to maintain structural integrity up to 160 °C, exhibiting a disintegrative fracture after this point. The disintegrated crystal melts at ca. 200 °C followed by decomposition into a dark solid (Fig. 2c).
 | ||
| Fig. 2 Thermal properties of SUL·AMNH solid. (a) TGA and (b) DSC traces of SUL·AMNH, SUL, and AMNH. (c) Hot-stage POM of a single crystal of SUL·AMNH. | ||
The favorable intermolecular interactions in SUL·AMNH were further evidenced with 1H NMR spectroscopy studies, which showed protons in SUL·AMNH to have distinct chemical shifts compared to pristine components (Fig. S2, ESI†) due to protonation.25,26 Dynamic light scattering (DLS) confirmed the formation of larger aggregates (average particle diameter of 4.86 μm ± 0.71 μm) in solution (50 mg in 3 mL of water) compared to individual components, which showed average diameters of 2.81 μm ± 0.61 μm, and 2.37 μm ± 0.65 μm for SUL and AMNH, respectively. The observations prompted us to evaluate the applicability of the SUL·AMNH solid aggregate in biological assays to assess antimicrobial activity.
The SUL·AMNH dual-drug salt showed antibacterial effect of SUL in standard clinical 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combination with β-lactam antibiotic ampicillin (AMP) (Table 1). The synergistic effect of combined SUL-AMP treatment is well established, with the bactericidal activity of AMP bolstered by the innate antibacterial and β-lactamase inhibition activity of SUL. Zone of inhibition analysis performed through disk diffusion evaluated the bacterial inhibition of SUL·AMNH, pristine SUL, and PM against Escherichia coli and Staphylococcus epidermidis at five Clinical & Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommended concentration breakpoints (Table 1).27 Fresh and actively dividing E. coli and S. epidermidis microbial cultures (1.5 × 108 CFU mL−1) were plated on Mueller Hinton agar via a non-toxic sterile swab. Sterile paper disks (7 mm) were impregnated with the antibacterial components, placed on the plates, and incubated for 24 h at 37 °C. Correlated CLSI inhibition standards and ANOVA analysis found that E. coli showed statistically equivalent resistance against AMP-SUL, AMP-PM, and AMP-SUL·AMNH at lower tested concentrations (2
1 combination with β-lactam antibiotic ampicillin (AMP) (Table 1). The synergistic effect of combined SUL-AMP treatment is well established, with the bactericidal activity of AMP bolstered by the innate antibacterial and β-lactamase inhibition activity of SUL. Zone of inhibition analysis performed through disk diffusion evaluated the bacterial inhibition of SUL·AMNH, pristine SUL, and PM against Escherichia coli and Staphylococcus epidermidis at five Clinical & Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommended concentration breakpoints (Table 1).27 Fresh and actively dividing E. coli and S. epidermidis microbial cultures (1.5 × 108 CFU mL−1) were plated on Mueller Hinton agar via a non-toxic sterile swab. Sterile paper disks (7 mm) were impregnated with the antibacterial components, placed on the plates, and incubated for 24 h at 37 °C. Correlated CLSI inhibition standards and ANOVA analysis found that E. coli showed statistically equivalent resistance against AMP-SUL, AMP-PM, and AMP-SUL·AMNH at lower tested concentrations (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μg mL−1 and 8
1 μg mL−1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 μg mL−1).28 At higher tested concentrations (32
4 μg mL−1).28 At higher tested concentrations (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 μg mL−1, 128
16 μg mL−1, 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 μg mL−1, 512
64 μg mL−1, 512![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 μg mL−1), increased zones of inhibition presented marked antibacterial sensitivity (Fig. 3). This trend is in agreement with established susceptibility concentrations of BSL-1 E. coli for ampicillin-sulbactam treatment.29 Susceptibility of S. epidermidis found similar statistical equivalence in AMP-SUL, AMP-PM, and AMP-SUL·AMNH antibacterial activity. As predicted by CLSI values, S. epidermidis showed resistance at 2
256 μg mL−1), increased zones of inhibition presented marked antibacterial sensitivity (Fig. 3). This trend is in agreement with established susceptibility concentrations of BSL-1 E. coli for ampicillin-sulbactam treatment.29 Susceptibility of S. epidermidis found similar statistical equivalence in AMP-SUL, AMP-PM, and AMP-SUL·AMNH antibacterial activity. As predicted by CLSI values, S. epidermidis showed resistance at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 μg mL−1 and intermediate susceptibility 8
1 μg mL−1 and intermediate susceptibility 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 μg mL−1 concentrations.28 At the mid-concentration breakpoint of 32
4 μg mL−1 concentrations.28 At the mid-concentration breakpoint of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 μg mL−1, increasing zones of bacterial inhibition corresponded to increased sensitivity that became high sensitivity for the remaining tested concentrations (128
16 μg mL−1, increasing zones of bacterial inhibition corresponded to increased sensitivity that became high sensitivity for the remaining tested concentrations (128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 μg mL−1 and 512
64 μg mL−1 and 512![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 μg mL−1). These findings were corroborated with additional investigations of growth analysis by broth microdilution (5 × 105 CFU mL−1), which found enhanced and immediate inhibition at even the lowest concentrations over a 14 h period of monitored growth (Fig. S17, ESI†).27,29 The retention of antibacterial susceptibility among SUL, PM, and SUL·AMNH alongside AMP demonstrates that the addition of API AMNH does not disrupt the antibacterial activity in SUL·AMNH and performs as effectively as pristine SUL.
256 μg mL−1). These findings were corroborated with additional investigations of growth analysis by broth microdilution (5 × 105 CFU mL−1), which found enhanced and immediate inhibition at even the lowest concentrations over a 14 h period of monitored growth (Fig. S17, ESI†).27,29 The retention of antibacterial susceptibility among SUL, PM, and SUL·AMNH alongside AMP demonstrates that the addition of API AMNH does not disrupt the antibacterial activity in SUL·AMNH and performs as effectively as pristine SUL.
 | ||
| Fig. 3 Bacterial zones of inhibition at five concentrations of AMP-SUL·AMNH, AMP-SUL, AMP-PM, and Milli-Q water-sodium phosphate buffer (blank) against S. epidermidis (a) and E. coli (b). | ||
| Diameter (mm) | ||||||
|---|---|---|---|---|---|---|
| E. coli | S. epidermidis | |||||
| [C] (μg mL−1) | SUL | Physical | Physical | |||
| Mixturea | SUL·AMNHa | SUL | Mixturea | |||
| SUL·AMNHa | ||||||
a SUL, physical mixture, and SUL·AMNH treatments were each tested in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio with antibiotic Ampicillin. 2 ratio with antibiotic Ampicillin.
|
||||||
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 0 | 0 | 10.45 ± 3.1 | 15.3 ± 1.3 | 15.8 ± 1.3 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0 | 0 | 0 | 23.0 ± 3.3 | 22.9 ± 3.1 | 22.7 ± 3.2 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
9.0 ± 0.9 | 11.4 ± 0.9 | 10.1 ± 0.6 | 29.4 ± 2.0 | 29.8 ± 3.1 | 29.2 ± 2.1 |
128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
17.7 ± 0.7 | 19.6 ± 0.2 | 18.7 ± 0.4 | 41.7 ± 3.2 | 42.5 ± 4.6 | 41.6 ± 4.5 |
512![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256 256 |
24.6 ± 4 | 25.0 ± 0.7 | 24.4 ± 0.2 | 48.6 ± 1.2 | 49.8 ± 0.4 | 50.6 ± 2.4 |
Conclusions
In summary, we successfully synthesized the multicomponent solid SUL·AMNH comprising BLI and antiviral APIs. To our knowledge, SUL·AMNH is the first BLI-antiviral salt, and multicomponent solid to feature SUL, where both APIs are FDA-approved. SUL·AMNH showed improved thermal stability compared to pristine SUL and AMNH, and showed antibacterial properties against Escherichia coli and Staphylococcus epidermidis. The addition of AMNH did not compromise the antibacterial activity despite the strong interaction observed in 1H NMR and DFT calculations. We envisage the design strategy involving multidrug solids (e.g., antibacterial–antiviral) could accelerate the approval of effective pharmaceutical formulations for co-infections (e.g., viral, bacteria) and combination therapies.Author contributions
J. B. carried out investigation, methodology, data curation, validation, writing and reviewing the original draft. I. B., A. C., E. J. C.-V., J. D. L., D. S. B., carried out formal analysis, data curation, investigation and visualization. J. L. M. and G. C.-A. carried out conceptualization, funding acquisition, formal analysis, project administration, writing, and reviewing the original draft.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the M. J. Murdock Charitable Trust (FSU-202118942), the National Science Foundation (CHE-2319929), and Reed College (start-up, Stillman Drake and summer funds). J. D. L. appreciates support from the Consortium of Faculty Diversity Postdoctoral Fellowship. I. B. appreciates support from the Center of Life Beyond Reed through a Climate Change Educational Fund Fellowship. We acknowledge Prof. Kristin Hutchins for access to TGA and DSC instruments, and Prof. Andrea M. Goforth for access to dynamic light scattering instrument. We thank Prof. Kelly Chacón for their helpful discussions and suggestions on this work. This study was supported in part by the Betty Moore Foundation.References
- D. G. J. Larsson and C.-F. Flach, Antibiotic Resistance in the Environment, Nat. Rev. Microbiol., 2022, 20(5), 257–269, DOI:10.1038/s41579-021-00649-x
.
-
World Health Organization, Antimicrobial Resistance: Global Report on Surveillance, World Health Organization, Geneva, 2014 Search PubMed
.
-
Centers for Disease Control and Prevention (U.S.), Antibiotic Resistance Threats in the United States, 2019, Centers for Disease Control and Prevention (U.S.), 2019. DOI:10.15620/cdc:82532
.
- T. M. Rawson, D. Ming, R. Ahmad, L. S. P. Moore and A. H. Holmes, Antimicrobial Use, Drug-Resistant Infections and COVID-19, Nat. Rev. Microbiol., 2020, 18(8), 409–410, DOI:10.1038/s41579-020-0395-y
.
- J. Oliva and O. Terrier, Viral and Bacterial Co-Infections in the Lungs: Dangerous Liaisons, Viruses, 2021, 13(9), 1725, DOI:10.3390/v13091725
.
- M. Baym, L. K. Stone and R. Kishony, Multidrug Evolutionary Strategies to Reverse Antibiotic Resistance, Science, 2016, 351(6268), aad3292, DOI:10.1126/science.aad3292
.
- M. Bashimam and H. El-Zein, Pharmaceutical Cocrystal of Antibiotic Drugs: A Comprehensive Review, Heliyon, 2022, 8(12), e11872, DOI:10.1016/j.heliyon.2022.e11872
.
- G. Campillo-Alvarado, E. A. Keene, D. C. Swenson and L. R. MacGillivray, Repurposing of the Anti-HIV Drug Emtricitabine as a Hydrogen-Bonded Cleft for Bipyridines via Cocrystallization, CrystEngComm, 2020, 22(21), 3563–3566, 10.1039/D0CE00474J
.
- G. Campillo-Alvarado, C. A. Staudt, M. J. Bak and L. R. MacGillivray, Generation of Cocrystals of Tavaborole (AN2690): Opportunities for Boron-Containing APIs, CrystEngComm, 2017, 19(22), 2983–2986, 10.1039/C7CE00314E
.
- G. Campillo-Alvarado, T. D. Didden, S. M. Oburn, D. C. Swenson and L. R. MacGillivray, Exploration of Solid Forms of Crisaborole: Crystal Engineering Identifies Polymorphism in Commercial Sources and Facilitates Cocrystal Formation, Cryst. Growth Des., 2018, 18(8), 4416–4419, DOI:10.1021/acs.cgd.8b00375
.
- A. Bonetti, B. Tugnoli, A. Piva and E. Grilli, Thymol as an Adjuvant to Restore Antibiotic Efficacy and Reduce Antimicrobial Resistance and Virulence Gene Expression in Enterotoxigenic Escherichia Coli Strains, Antibiotics, 2022, 11(8), 1073, DOI:10.3390/antibiotics11081073
.
- X.-N. Hua, X. Pan, Y. Zhu, Z. Cai, Q. Song, Y. Li, W. Feng, X. Chen, H. Zhang and B. Sun, Novel Pharmaceutical Salts of Cephalexin with Organic Counterions: Structural Analysis and Properties, RSC Adv., 2022, 12(54), 34843–34850, 10.1039/D2RA05565A
.
- L.-Y. Wang, F.-Z. Bu, Y.-T. Li, Z.-Y. Wu and C.-W. Yan, A Sulfathiazole–Amantadine Hydrochloride Cocrystal: The First Codrug Simultaneously Comprising Antiviral and Antibacterial Components, Cryst. Growth Des., 2020, 20(5), 3236–3246, DOI:10.1021/acs.cgd.0c00075
.
- D. Carcione, C. Siracusa, A. Sulejmani, V. Leoni and J. Intra, Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use, Antibiotics, 2021, 10(8), 995, DOI:10.3390/antibiotics10080995
.
- K. Fink, A. Nitsche, M. Neumann, M. Grossegesse, K.-H. Eisele and W. Danysz, Amantadine Inhibits SARS-CoV-2 In Vitro, Viruses, 2021, 13(4), 539, DOI:10.3390/v13040539
.
- D. Wong and D. Van Duin, Novel Beta-Lactamase Inhibitors: Unlocking Their Potential in Therapy, Drugs, 2017, 77(6), 615–628, DOI:10.1007/s40265-017-0725-1
.
- E. De Clercq, Antiviral Agents Active against Influenza A Viruses, Nat. Rev. Drug Discovery, 2006, 5(12), 1015–1025, DOI:10.1038/nrd2175
.
- S. P. Gopi, S. Ganguly and G. R. Desiraju, A Drug–Drug Salt Hydrate of Norfloxacin and Sulfathiazole: Enhancement of in Vitro Biological Properties via Improved Physicochemical Properties, Mol. Pharm., 2016, 13(10), 3590–3594, DOI:10.1021/acs.molpharmaceut.6b00320
.
- L.-Y. Wang, M.-Y. Zhao, F.-Z. Bu, Y.-Y. Niu, Y.-M. Yu, Y.-T. Li, C.-W. Yan and Z.-Y. Wu, Cocrystallization of Amantadine Hydrochloride with Resveratrol: The First Drug–Nutraceutical Cocrystal Displaying Synergistic Antiviral Activity, Cryst. Growth Des., 2021, 21(5), 2763–2776, DOI:10.1021/acs.cgd.0c01673
.
- A. Gunnam, K. Suresh and A. Nangia, Salts and Salt Cocrystals of the Antibacterial Drug Pefloxacin, Cryst. Growth Des., 2018, 18(5), 2824–2835, DOI:10.1021/acs.cgd.7b01600
.
- P. P. Bag, S. Ghosh, H. Khan, R. Devarapalli and C. Malla Reddy, Drug–Drug Salt Forms of Ciprofloxacin with Diflunisal and Indoprofen, CrystEngComm, 2014, 16(32), 7393–7396, 10.1039/C4CE00631C
.
- A. J. Cruz-Cabeza, Acid–Base Crystalline Complexes and the pKa Rule, CrystEngComm, 2012, 14(20), 6362, 10.1039/c2ce26055g
.
- J. Bicknell, S. A. Agarwal, K. J. Petersen, J. D. Loya, N. Lutz, P. M. Sittinger, S. J. Teat, N. S. Settineri and G. Campillo-Alvarado, Engineering Lipophilic Aggregation of Adapalene and Adamantane-Based Cocrystals via van Der Waals Forces and Hydrogen Bonding, Cryst. Growth Des., 2024, 24(12), 5222–5230, DOI:10.1021/acs.cgd.4c00457
.
- S. Aitipamula, A. B. H. Wong, P. S. Chow and R. B. H. Tan, Novel Solid Forms of Oxaprozin: Cocrystals and an Extended Release Drug–Drug Salt of Salbutamol, RSC Adv., 2016, 6(41), 34110–34119, 10.1039/C6RA01802E
.
- I. Wawer, M. Pisklak and Z. Chilmonczyk, 1H, 13C, 15N NMR Analysis of Sildenafil Base and Citrate (Viagra) in Solution, Solid State and Pharmaceutical Dosage Forms, J. Pharm. Biomed. Anal., 2005, 38(5), 865–870, DOI:10.1016/j.jpba.2005.01.046
.
- H. Kim, C. R. Babu and D. J. Burgess, Quantification of Protonation in Organic Solvents Using Solution NMR Spectroscopy: Implication in Salt Formation, Int. J. Pharm., 2013, 448(1), 123–131, DOI:10.1016/j.ijpharm.2013.03.040
.
- I. Wiegand, K. Hilpert and R. E. W. Hancock, Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances, Nat. Protoc., 2008, 3(2), 163–175, DOI:10.1038/nprot.2007.521
.
-
CLSI, Performance Standards for Antimicrobial Susceptibility Testing, 30th edn, CSI supplement M100, Clinical and Laboratory Standards Institute, Wayne, PA, 2020 Search PubMed
.
- K. C. Lamp and M. K. Vickers, Pharmacodynamics of Ampicillin-Sulbactam in an In Vitro Infection Model against Escherichia Coli Strains with Various Levels of Resistance, Antimicrob. Agents Chemother., 1998, 42(2), 231–235, DOI:10.1128/AAC.42.2.231
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental conditions, additional SCXRD data, PXRD data, NMR data, FT-IR data, thermal analyses, pKa calculations, DLS data and additional biological studies. CCDC 2375807. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4pm00247d |
| This journal is © The Royal Society of Chemistry 2024 |