Reversible tri-state structural transitions of hybrid copper(I) bromides toward tunable multiple emissions†
Jiajing
Wu
*,
Jing-Li
Qi
,
Yue
Guo
,
Shufang
Yan
,
Wenlong
Liu
 and
Sheng-Ping
Guo
and
Sheng-Ping
Guo
 *
*
School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, Jiangsu 225002, P. R. China. E-mail: spguo@yzu.edu.cn; jiajingw@yzu.edu.cn
First published on 17th October 2023
Abstract
Hybrid copper halides have been rapidly developed because of their high photoluminescence quantum yield (PLQY), good water resistance, and variable structures; nevertheless, the controlled synthesis and interconversion of multiple hybrid copper halides containing different coordination geometries are rarely reported. In this study, using ethyltriphenylphosphonium as the templating cation [EtPh3P]+, three stable hybrid copper bromides with diverse inorganic anion skeletons, (EtPh3P)CuBr2 (112), (EtPh3P)2Cu2Br4 (224), and (EtPh3P)2Cu4Br6 (246), were synthesized by carefully tuning the feeding ratio of CuBr and (EtPh3P)Br. The emission peaks were located at 536, 546, and 580 nm with optical absorption edges of 3.64, 2.91, and 2.82 eV, respectively, showing a gradual redshift as the inorganic units change from a monomer (112) to dimer (224) and then to a tetramer (246). More interestingly, multiple solvents, including methanol (MeOH), ethanol (EtOH), isopropanol (IPA), acetic acid, ethylene glycol, and water with heat, can induce the spontaneous conversion of 112 and 224 to 246, which is attributed to the affinity of the organic salt with the solvent. Additionally, the crystal structure can transform from 246 into 112 (224) or can undergo a reversible dynamic conversion between 112 and 224 using the crystallization method. This work not only provides a strategy to adjust the inorganic units of the crystals but also to achieve multi-state structural transitions, making them viable candidates for next-generation multifunctional optoelectronic devices.
Introduction
In recent years, three-dimensional (3D) lead halide perovskites (APbX3, A = Cs, CH3NH3, or NH2CHNH2; X = Cl, Br, or I), which exhibit high PLQY, narrow full width at half maxima (FWHM), and high defect tolerance, have become a stimulating topic for research owing to their extensive application potentials in photovoltaics, light-emitting devices (LEDs), X-ray scintillators, and anti-counterfeiting.1–4 However, the serious toxicity of Pb2+ and water stability may limit their further widespread applications.5,6 To address these issues, lead-free metal halides with low-dimensional structures were developed by substituting In3+, Sb3+, Bi3+, Mn2+, Cu+, and other heterovalent metals for Pb2+ and large-size organic cations for A-site cations.7–10 In these low-dimensional lead-free hybrid metal halides, especially 0D structures, the optically active inorganic blocks are separated and surrounded by insulating organic components with inherent quantum confinement effects, which lead to strongly bound excitons in metal halides under photoexcitation. Meanwhile, the strong electron–phonon coupling causes the excitons to be localized instantaneously, resulting in the self-trapped excitons (STE) with lower excited state energy. Thus, different from the narrow emission bands of 3D metal halides, low-dimensional metal halides always exhibit large Stokes shifts, low self-absorption, and broadband emission.11,12 In addition, the 0D metal halides are composed of different metal ions and halogen ligands with diverse coordination modes, such as the trigonal plane, tetrahedron, octahedron, and square pyramid, and thus endow them with rich physical properties, including tunable emission, nonlinear optical, and ferroelectric behaviors.13–15In the presence of 0D lead-free metal halides, copper-based halides feature abundant coordination configurations, high emission efficiencies, structural abilities, etc., enabling them to compete against optoelectronic functional materials.16–22 The research on Cu-based hybrid halides can be dated back to the 1970s.23 Since then, a large number of CuX-based (X = Cl, Br, I) hybrid structures have been reported in the CCDC database. In these hybrid copper halides, the inorganic units of the 0D structure can be categorized into CuX2, Cu2X4, Cu2X5, Cu4X6, and so on (X = Cl, Br, I).16,18,20,24 Although the synthesis of the transition metal-based binuclear and polynuclear compounds has been previously reported, there is a lack of research on synthesizing hybrid copper with different inorganic units using the same organic cations.25–28 Previous research has demonstrated that the influence of inorganic units on the optical properties of hybrid halides is crucial.16–22 However, limited research has been conducted to explore the differences in the optical properties of different Cu–X inorganic units. Moreover, switching between multiple types of inorganic units leads to changing optical properties that offer new application prospects for copper halides.29–34 This reversible transformation between two hybrid copper halides under external stimuli was recently reported, demonstrating the potential of solvent-induced phase transition behavior in anti-counterfeiting.35–37 During this submission, Lei published a reversible single-crystal to single-crystal transition between (ETPP)2Cu4Br6 and (ETPP)CuBr2 very recently.37 However, there is still not enough research focus on how to rationally design and synthesize hybrid copper halides with more than three different copper–halogen connectivity modes and their structural interconversions.
In this work, we adjusted the copper–bromine inorganic unit by reasonably controlling the feed ratio of (EtPh3P) and CuBr and synthesized three copper bromides (EtPh3P)CuBr2 (112), (EtPh3P)2Cu2Br4 (224), and (EtPh3P)2Cu4Br6 (246). In particular, they crystallize with different crystal structures; that is, 112 adopts a noncentrosymmetric (NCS) space group (P21) with a quasi-linear [CuBr2]− framework, which has been reported very recently, exhibiting encouraging nonlinear optical activity,13 while 224 and 246 are crystalized in a centrosymmetric (CS) space group (P21/c for 224 and P21/n for 246) with [Cu2Br4]2− dimers and [Cu4Br6]2− clusters, respectively. We systematically compared the structures and optical properties of three types of Cu–X units, excluding the interference of organic cations. They show distinct broad-band emission peaks at 536, 546, and 580 nm, with PLQYs of about 8.6%, 22.44%, and 76.59%, respectively. Their absorption edges show a redshift similar to that of the emission peaks from 112 to 224, then to 246, demonstrating an approach to achieve tunable bandgaps. Interestingly, the structures of 112, 224, and 246 can be interconverted by different solvent treatments or by crystallization with the aid of the precursor (EtPh3P)Br. In particular, various solvents can trigger the transformation of 112 and 224 into 246 with different stimulus-response times, which is quite different from the very recent work.37 In this work, their synthesis, structures, and optical properties have been systematically studied, demonstrating the relationship between the structure and optical properties. More importantly, tristate structural transition (112 ↔ 224 ↔ 246) accompanied by PL switching was first discovered and studied for the family of hybrid copper halides.
Experimental section
Materials and synthesis
Cuprous bromide (CuBr, 99%, Aladdin), ethyltriphenylphosphonium bromide ((EtPh3P)Br, 98%, Aladdin), ethylene glycol (≥99.5%, Sinopharm), HBr (40 wt% in H2O, AR), H3PO2 (50 wt% in H2O, Aladdin), petroleum ether (AR, General-Reagent), isopropanol (99.7%, General-Reagent), acetic acid (99.5%, Sinopharm Reagents), methanol (99.5%, General-Reagent), and ethanol (C2H5OH, 99.7%, General-Reagent), were the chemical agents that were used without further purification.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) + δ(C–H): 1584, 1485, ν(Ph–P): 1434, δ(P–C): 1107, δ(Ar–H): 732, 720, 685 (Fig. S1†). Elemental analysis by energy dispersive spectrometer (EDS), P
C) + δ(C–H): 1584, 1485, ν(Ph–P): 1434, δ(P–C): 1107, δ(Ar–H): 732, 720, 685 (Fig. S1†). Elemental analysis by energy dispersive spectrometer (EDS), P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 1
Br = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75
0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.59 (Fig. S2†).
1.59 (Fig. S2†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuBr ratio is changed to 1
CuBr ratio is changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After 1 day, colorless crystals were obtained. Yield: 0.319 g (62%). FTIR/cm−1: ν(Ar–H): 3051, νas(C–H): 2936, νs(C–H): 2894, νas(C
1. After 1 day, colorless crystals were obtained. Yield: 0.319 g (62%). FTIR/cm−1: ν(Ar–H): 3051, νas(C–H): 2936, νs(C–H): 2894, νas(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) + δ(C–H): 1586, 1480, ν(Ph–P): 1436, δ(P–C): 1109, δ(Ar–H): 735, 718, 691 (Fig. S1†). Elemental analysis by EDS, P
C) + δ(C–H): 1586, 1480, ν(Ph–P): 1436, δ(P–C): 1109, δ(Ar–H): 735, 718, 691 (Fig. S1†). Elemental analysis by EDS, P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 1
Br = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.07
1.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.81 (Fig. S3†).
1.81 (Fig. S3†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuBr ratio was changed to 1
CuBr ratio was changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After 3 days, colorless crystals were obtained. Yield: 0.328 g (58%). FTIR/cm−1: ν(Ar–H): 3053, νas(C–H): 2938, νs(C–H): 2901, νas(C
2. After 3 days, colorless crystals were obtained. Yield: 0.328 g (58%). FTIR/cm−1: ν(Ar–H): 3053, νas(C–H): 2938, νs(C–H): 2901, νas(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) + δ(C–H): 1586, 1484, ν(Ph–P): 1434, δ(P–C): 1110, δ(Ar–H): 735, 721, 685 (Fig. S1†). Elemental analysis by EDS: P
C) + δ(C–H): 1586, 1484, ν(Ph–P): 1434, δ(P–C): 1110, δ(Ar–H): 735, 721, 685 (Fig. S1†). Elemental analysis by EDS: P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu
Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 1
Br = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.51
1.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.92 (Fig. S4†).
1.92 (Fig. S4†).
Results and discussion
112, 224, and 246 were synthesized by carefully regulating the reactant ratios of cuprous bromide (CuBr) and ethyltriphenylphosphonium bromide ((EtPh3P)Br), as well as the synthetic conditions (Scheme 1). Specifically, 112 was synthesized by reacting (EtPh3P)Br and CuBr with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 in a mixture of hydrobromide acid (HBr), hypophosphorous acid (H3PO2), and ethylene glycol ((CH2OH)2) solution. The above mixture solution was heated to 80 °C under stirring to dissolve the bromide salts completely, then, the crystals were obtained after the temperature was slowly cooled to room temperature. 224 and 246 crystals were prepared following the above procedure with a feeding ratio of (EtPh3P)Br to CuBr at 1
0.5 in a mixture of hydrobromide acid (HBr), hypophosphorous acid (H3PO2), and ethylene glycol ((CH2OH)2) solution. The above mixture solution was heated to 80 °C under stirring to dissolve the bromide salts completely, then, the crystals were obtained after the temperature was slowly cooled to room temperature. 224 and 246 crystals were prepared following the above procedure with a feeding ratio of (EtPh3P)Br to CuBr at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
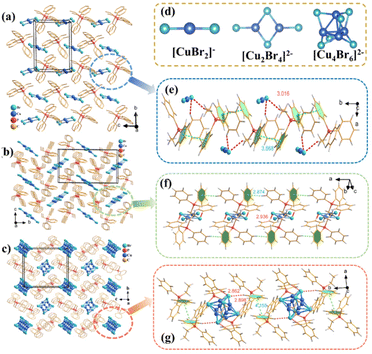
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The crystals of 112, 224, and 246 were colorless and transparent under daylight, and 112 were non-emissive under 365 nm ultraviolet (UV) light irradiation, while 224 and 246 exhibited greenish-yellow and yellow emission, respectively. Their crystal structures were determined by single-crystal X-ray diffraction (SCXRD) with refined data, as summarized in Tables S1–S7.† The measured powder XRD patterns are consistent with the simulated ones, confirming the reliability of the crystal structure analysis and phase purity (Fig. S5†). 112 adopts an NCS polar space group (P21), whereas 224 and 246 crystalize in the CS space group of P21/c and P21/n, respectively. The detailed packing structures of 112, 224, and 246 are shown in Fig. 1(a–c). They all showed typical 0D structures but possess different copper halogen units and arrangements of organic cations. The asymmetric units of the three compounds are shown in Fig. S6a–c.† The asymmetric units of 112 and 224 are composed of one [EtPh3P]+ cation and one [CuBr2]− anion, respectively, while the asymmetric units of 246 consist of one [EtPh3P]+ cation and one [Cu4Br6]2− anion. For 112, each Cu atom is coordinated with two adjacent Br atoms to form a quasi-linear [CuBr2]− framework with the average Cu–Br bond distances of 2.211(9)–2.216(1) Å and the shortest Cu⋯Cu distance of 6.47 Å. The two nearest neighboring [CuBr2]− framework is 6.470 Å (Fig. S7†). In contrast to 112, each Cu atom in the lattice of 224 and 246 is coordinated to three Br atoms forming a plane triangle [CuBr3]2−. Distinctively, in the structure of 224, two adjacent [CuBr3]− are connected forming an edge-sharing [Cu2Br4]2− dimers with Cu–Br bond distances in the range of 2.3213(6)–2.4273(7) Å and shortest Cu⋯Cu distance of 2.86 Å. The distance of the two nearest adjacent [Cu2Br4]2− dimers was 8.873 Å (Fig. S8†). In 246, four [CuBr3]2− plane triangles form a [Cu4Br6]2− cluster by corner-sharing with Cu–Br bond distances in the range of 2.308(7)–2.524(9) Å and the distance of the two adjacent [Cu4Br6]2− clusters increases to 10.506 Å (Fig. S9†). It is worth mentioning that the shortest Cu⋯Cu distance (2.71 Å) in 246 is greater than that of 112 and 224, but less than the sum of the van der Waals radius of Cu atoms (2.8 Å), suggesting that Cu–Cu bonding interactions in this 246 is stronger than 112 and 224. Fig. S10 and S11† show that the inorganic units in 112 and 224 are not entirely surrounded by organic cations, while the inorganic units in 246 are completely isolated by organic cations, which may give 246 a strong quantum confinement effect (Fig. S12†).
2, respectively. The crystals of 112, 224, and 246 were colorless and transparent under daylight, and 112 were non-emissive under 365 nm ultraviolet (UV) light irradiation, while 224 and 246 exhibited greenish-yellow and yellow emission, respectively. Their crystal structures were determined by single-crystal X-ray diffraction (SCXRD) with refined data, as summarized in Tables S1–S7.† The measured powder XRD patterns are consistent with the simulated ones, confirming the reliability of the crystal structure analysis and phase purity (Fig. S5†). 112 adopts an NCS polar space group (P21), whereas 224 and 246 crystalize in the CS space group of P21/c and P21/n, respectively. The detailed packing structures of 112, 224, and 246 are shown in Fig. 1(a–c). They all showed typical 0D structures but possess different copper halogen units and arrangements of organic cations. The asymmetric units of the three compounds are shown in Fig. S6a–c.† The asymmetric units of 112 and 224 are composed of one [EtPh3P]+ cation and one [CuBr2]− anion, respectively, while the asymmetric units of 246 consist of one [EtPh3P]+ cation and one [Cu4Br6]2− anion. For 112, each Cu atom is coordinated with two adjacent Br atoms to form a quasi-linear [CuBr2]− framework with the average Cu–Br bond distances of 2.211(9)–2.216(1) Å and the shortest Cu⋯Cu distance of 6.47 Å. The two nearest neighboring [CuBr2]− framework is 6.470 Å (Fig. S7†). In contrast to 112, each Cu atom in the lattice of 224 and 246 is coordinated to three Br atoms forming a plane triangle [CuBr3]2−. Distinctively, in the structure of 224, two adjacent [CuBr3]− are connected forming an edge-sharing [Cu2Br4]2− dimers with Cu–Br bond distances in the range of 2.3213(6)–2.4273(7) Å and shortest Cu⋯Cu distance of 2.86 Å. The distance of the two nearest adjacent [Cu2Br4]2− dimers was 8.873 Å (Fig. S8†). In 246, four [CuBr3]2− plane triangles form a [Cu4Br6]2− cluster by corner-sharing with Cu–Br bond distances in the range of 2.308(7)–2.524(9) Å and the distance of the two adjacent [Cu4Br6]2− clusters increases to 10.506 Å (Fig. S9†). It is worth mentioning that the shortest Cu⋯Cu distance (2.71 Å) in 246 is greater than that of 112 and 224, but less than the sum of the van der Waals radius of Cu atoms (2.8 Å), suggesting that Cu–Cu bonding interactions in this 246 is stronger than 112 and 224. Fig. S10 and S11† show that the inorganic units in 112 and 224 are not entirely surrounded by organic cations, while the inorganic units in 246 are completely isolated by organic cations, which may give 246 a strong quantum confinement effect (Fig. S12†).
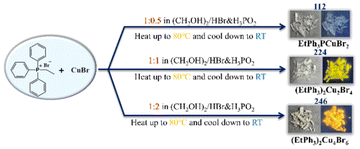 | ||
| Scheme 1 Optical photographs (under daylight and 365 nm UV light) of and synthetic conditions for 112, 224, and 246. | ||
As is known, the arrangement of the organic cations in hybrid metal halides has a great influence on their structural stability and luminescent properties.38,39 To deeply obtain an insight into the organic cation arrangement, intermolecular interactions (such as C–H⋯π, π–π interaction, and hydrogen bonds) and Hirshfeld surface analysis are depicted in Fig. 1(e–g) and Fig. S13–S15.† From Fig. 1e and g, 112 and 246 show parallelly arranged π–π interactions with a centroid-to-centroid distance of 3.868 (<4 Å) and 4.115 Å, respectively, while 224 shows a unique T-shaped conformation with an H-to-centroid distance of about 2.936 Å (Fig. 1f), indicating that 224 possesses the strongest C–H⋯π interaction, which is consistent with the result of the Hirshfeld surface analysis (Fig. S13†).40,41 The shortest hydrogen bond distances in 112, 224, and 246 were 3.016, 2.938, and 2.862 Å, respectively, suggesting that the strongest hydrogen bond existed in 246, which was also similarly observed in the Hirshfeld surface analysis (Fig. S15†). It is noteworthy that stability is an important parameter for optoelectronic applications, which led us to investigate the structural stability of 112, 224 and 246. After soaking the samples in water for 3 weeks, there was almost no change in the PXRD patterns, indicating the stable crystal lattices without structural collapse or decomposition because of their strong intermolecular interaction and large steric hindrance of organic cations (Fig. S16†).
As shown in Fig. 2(a–c), the ultraviolet-visible (UV-vis) absorption spectra of 112, 224, and 246 show strong absorbance in the UV region and weak absorbance in the visible region, which is consistent with their crystals’ transparency and colorless features. Their calculated optical absorption edges are 3.64, 2.91, and 2.82 eV, respectively (Fig. 2a). Their PL and photoluminescence excitation (PLE) spectra at room temperature are shown in Fig. 2(d–f). Under 310 nm UV light excitation, an obvious emission peak located at 536 nm with a broad full width at half-maximum (FWHM) of 100 nm, and a large Stokes shift of 225 nm was observed for 112 (Fig. 2d). The PLQY was measured as 8.67% under 310 nm excitation. The PL and PLE spectra of 224 and 246 are shown in Fig. 2e and f, respectively. Their emission peaks are located at 546 and 580 nm with an FWHM of 103 and 169 nm and a Stokes shift of 159 and 180 nm, respectively. For the greenish-yellow emissive 224, the PLQY was 22.4%; however, the PLQY of yellow-emissive 246 is as high as 76.6% probably because of the stronger quantum confinement and the longer distance between two adjacent inorganic units. To further explore more photophysical properties of these copper bromides, the time-resolved photoluminescence spectra (TRPL) and recombination dynamics were studied. As shown in Fig. 2g–i, the PL decay of 112 can be well fitted by a biexponential function with τ1 of 2.31 μs (3%) and τ2 of 232.18 μs (97%), respectively. The average lifetime of 112 was 225.66 μs, while 224 and 246 showed average lifetimes (τave) of 13.67 and 37.90 μs, respectively, based on monoexponential-fitting (Table S8†). Their lifetimes are comparable to those of (C16H36N)CuBr2 (232.05 μs), (C12H28N)CuBr2 (261.7 μs), (C8H20N)2Cu2Br4 (56 μs), and (C12H28N)2Cu4Br6 (45.12 μs) copper-bromide hybrids reported in previous studies.20–22,36 The radiative (Kr) and nonradiative (Knr) decay rates were calculated based on PLQY and τave. As listed in Table S8,† the Kr/Knr of 246 is the highest (3.273), indicating that the nonradiative pathway was greatly minimized, giving rise to the high PLQY.42–44 Combined with the crystal structure and spectroscopy analysis results, the electron transfer paths of the three copper-based halides are described as follows. The incident light was absorbed by 112 and 224, and the electrons in the valence band were elevated to the excited state. After relaxation, the free electrons fell into the low energy self-trapped excited state via ultrafast excited state structural reorganization. Thus, the trapped electrons returned to the ground state and combined with the holes, resulting in a wideband emission with a large Stokes shift.45–49 Since the Cu⋯Cu distance of 224 is close to the sum of the van der Waals radius of the two Cu atoms, the cluster-centered (CC) and metal-to-ligand charge transfer (MLCT) or halide to the ligand charge transfer (HLCT) states probably existing in 224. For 246, the Cu⋯Cu distance is shorter than the sum of the van der Waals radius of two Cu atoms (2.8 Å), the emission bands might be assigned to CC excited state, which involves mixed M/HLCT.49,50
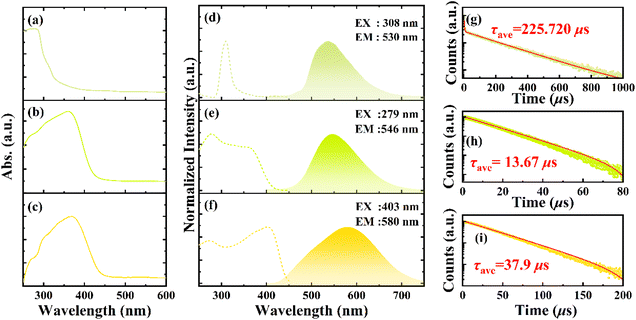 | ||
| Fig. 2 Absorption spectra, PLE and PL spectra, and PL decay of 112 (a, d and g), 224 (b, e and h), and 246 (c, f and i). | ||
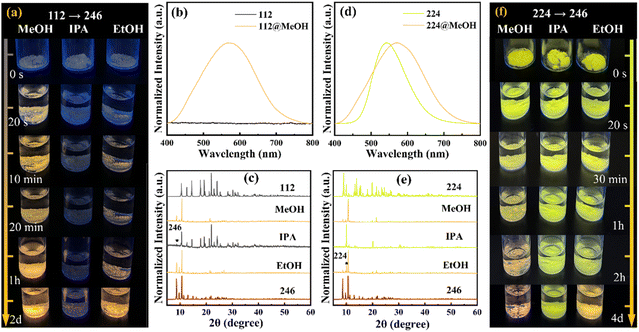
Interestingly, external stimuli induced structural transition and luminescence switching among three 0D hybrid copper bromides were observed. In Fig. 3a, an equal molar amount (0.92 mmol) of 112 powder was soaked in the same volume (1.5 mL) of methanol (MeOH), isopropanol (IPA), and ethanol (EtOH). The results showed that with the extension of soak time, the non-emissive 112 gradually evolved to yellow emission under 365 nm UV light excitation, indicating the formation of a new emissive species. Because this emissive species exhibits exactly the same emission spectrum as that of 246 (Fig. 3b), we speculate that there is a solvent-induced structural transformation from 112 to 246 through eqn (1), in which 112 is decomposed into 246 and (EtPh3P)Br. To confirm the solvent-induced structural transformation from 112 to 246, PXRD characterizations were further performed after adding solvent and the result are shown in Fig. 3c. In the PXRD pattern of the IPA-treated 112, the peaks from both 112 and 246 are clearly presented, suggesting the formation of 246 in 112. While in the PXRD patterns of MeOH- and EtOH-treated 112, characteristic peaks from 112 disappeared, indicating that 112 was completely transformed into 246 within 2 days, which is consistent with the phenomenon observed in the photographs. Notably, it can be seen from Fig. 3a and b, the rate and extent of transformation from 112 to 246 are also different among these three solvents. MeOH and EtOH solvents promoted the rate of conversion, while IPA only promoted it slightly (Fig. 3b). To explain this phenomenon, the solubility of CuBr and (EtPh3P)Br in MeOH, EtOH, and IPA were compared (Fig. S17 and S18†). The results show that CuBr is almost insoluble in these three solvents and (EtPh3P)Br has the highest solubility in MeOH, followed by EtOH, and the lowest solubility in IPA. Thus, (EtPh3P)Br from 112 is more easily stripped upon MeOH treatment, driving the decomposition of 112 to form 246 and (EtPh3P)Br. Therefore, the transformation process of 112 into 246 can be written as:
| 4(EtPh3P)CuBr2 → (EtPh3P)2Cu4Br6 + 2(EtPh3P)Br | (1) |
In addition, heating or reducing the particle size of 112 is also beneficial to accelerate the speed of transformation from 112 to 246 which is due to the increased dissolution rate of (EtPh3P)Br in the solvent according to the above equation (Fig. S19†). In addition to MeOH, EtOH, and IPA, the phase transition can be induced by other solvents, such as neutral solvent water with heating, ethylene glycol, or weakly acidic solvent acetic acid (Fig. S20†). However, no phase transformation was observed in formic acid, suggesting that the transformation can occur only at appropriate acidity (Fig. S21a–c†). A similar solvent-induced structural transformation from 224 to 246 was also observed. XRD patterns, PL spectra, and photographs indicate that MeOH and EtOH-treated 224 can be completely converted into 246 within 4 days at room temperature, as per eqn (2), while IPA did not trigger the structural transition (Fig. 3d–f). Compared with 112, the speed of transformation from 224 to 246 was slower, presumably due to the stronger hydrogen bonding of 224.
| 2(EtPh3P)2Cu2Br4 → (EtPh3P)2Cu4Br6 + 2(EtPh3P)Br | (2) |
Similarly, phase transitions from 224 to 246 can also be induced by ethylene glycol, acetic acid, and hot water, but not by formic acid (Fig. S21d,e and S22†). In addition, the stability of 246 was investigated. After one day of immersion in methanol, ethanol, and acetic acid, the powder XRD of 246 was consistent with simulations, demonstrating that 246 is a more stable phase than 112 and 224 in these solvents (Fig. S23†).
Previous studies have shown that the crystal structure and composition of metal halides are closely related to the stoichiometry of the precursors. Therefore, we expect that the crystal structure can not only transform from 112 (224) to 246 but also achieve the reversible interconversion of the three crystals by adding extra precursors. To verify these available structural transitions, their XRD patterns and spectra before and after the precursor (EtPh3P)Br were collected. The XRD patterns shown in Fig. 4a display the well-defined transformation from 246 (224) to 112. The yellow (green) light emission gradually fades when they are soaked in an EtOH solvent with the (EtPh3P)Br precursor, accompanied by the disappearance of the emission peak at 580 nm (546 nm) in accordance with the PL transition from 246 (224) to 112 (Fig. 4b). The transformation process of 246 (224) into 112 can be written as:
| (EtPh3P)2Cu4Br6 + 2(EtPh3P)Br → 4(EtPh3P)CuBr2 | (3) |
| (EtPh3P)2Cu2Br4 → 2(EtPh3P)CuBr2 | (4) |
| (EtPh3P)2Cu4Br6 + 2(EtPh3P)Br → 2(EtPh3P)2Cu2Br4 | (5) |
| 2(EtPh3P)CuBr2 → (EtPh3P)2Cu2Br4 | (6) |
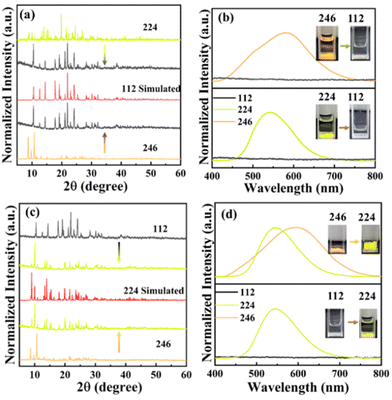 | ||
| Fig. 4 PXRD (a) and PL spectra (b) of 224 and 246 before and after adding (EtPh3P)Br precursor; PXRD (c) and PL spectra (d) of 112 and 246 before and after adding the (EtPh3P)Br precursor. | ||
Similarly, as shown in Fig. 4c and d, XRD patterns, and PL spectra confirmed that 246 (112) can be converted to 224 through eqn (5) and (6). Overall, the above results show that these three compounds can be achieved triple-mode structure interconversion by regulating the solvent, or crystallization.
Conclusions
In summary, we demonstrate here that the optical properties of hybrid Cu-based halides can be effectively regulated through rational crystal structure design. Three stable 0D hybrid copper bromides, 112, 224, and 246 with different copper halide configurations ([CuBr2]−, [Cu2Br4]2−, [Cu4Br6]2−), were synthesized by precisely adjusting the ratio of CuBr and (EtPh3P)Br. The structural and optical property analysis results showed that as the copper halide unit changed from monomeric [CuBr2]− to dimeric [Cu2Br4]2− and then to tetrametric [Cu4Br6]2−, the absorption edges and emission peaks appear to redshift. To our knowledge, the interconversion among these three crystals is firstly achieved among the family of hybrid copper halides. Specifically, 112 and 224 can be spontaneously transformed to 246 after the treatment with MeOH, EtOH, or acetic acid. This work not only provides a strategy to tune the copper halide units but also reveals multi-structural transformation behaviors, which provides a new perspective for the synthesis and design of multifunctional hybrid copper halides.Author contributions
The manuscript was written with the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071212), Qinglan Project of Jiangsu Province of China, Yangzhou University with a start-up grant (Grant No. 137012279), the Natural Science Foundation of Jiangsu Province (Grant No. BK20220558), and Lvyangjinfeng Talent Program of Yangzhou (YZLYJFJH2021YXBS085).Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed
.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites, Science, 2012, 338, 643–647 CrossRef CAS PubMed
.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang, Z. Yi, J. Li, X. Xie, Y. Wang, Y. Li, D. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. Yang, W. Huang and X. Liu, All-Inorganic Perovskite Nanocrystal Scintillators, Nature, 2018, 561, 88–93 CrossRef CAS PubMed
.
- X. Yu, L. Wu, D. Yang, M. Cao, X. Fan, H. Lin, Q. Zhong, Y. Xu and Q. Zhang, Hydrochromic CsPbBr3 Nanocrystals for Anti-Counterfeiting, Angew. Chem., Int. Ed., 2020, 59, 14527–14532 CrossRef CAS PubMed
.
- W. Ke and M. G. Kanatzidis, Prospects for Low-Toxicity Lead-Free Perovskite Solar Cells, Nat. Commun., 2019, 10, 965 CrossRef PubMed
.
- A. Abate, Perovskite Solar Cells Go Lead Free, Joule, 2017, 1, 659–664 CrossRef CAS
.
- D.-Y. Li, J.-H. Song, Y. Cheng, X.-M. Wu, Y.-Y. Wang, C.-J. Sun, C.-Y. Yue and X.-W. Lei, Ultra-Sensitive, Selective and Repeatable Fluorescence Sensor for Methanol Based on a Highly Emissive 0D Hybrid Lead-Free Perovskite, Angew. Chem., Int. Ed., 2022, 61, e202206437 CrossRef CAS PubMed
.
- Q. Kong, B. Yang, J. Chen, R. Zhang, S. Liu, D. Zheng, H. Zhang, Q. Liu, Y. Wang and K. Han, Phase Engineering of Cesium Manganese Bromides Nanocrystals with Color-Tunable Emission, Angew. Chem., Int. Ed., 2021, 60, 19653–19659 CrossRef CAS PubMed
.
- J.-J. Wu, Y. Guo, W.-D. Yao, W.-L. Liu and S.-P. Guo, Symmetry Breaking of A3M2X9-type Perovskite Derivatives Induced by Polar Quaternary Ammonium Cations: Achieving Efficient Nonlinear Optical Properties, Dalton Trans., 2022, 51, 4878–4883 RSC
.
- K.-H. Song, J.-J. Wang, L.-Z. Feng, F. He, Y.-C. Yin, J.-N. Yang, Y.-H. Song, Q. Zhang, X.-C. Ru, Y.-F. Lan, G. Zhang and H.-B. Yao, Thermochromic Phosphors Based on One-Dimensional Ionic Copper-Iodine Chains Showing Solid-State Photoluminescence Efficiency Exceeding 99%, Angew. Chem., Int. Ed., 2022, 61, e202208960 CrossRef CAS
.
- D.-Y. Li, J.-H. Song, Z.-Y. Xu, Y.-J. Gao, X. Yin, Y.-H. Hou, L.-J. Feng, C.-Y. Yue, H. Fei and X.-W. Lei, Reversible Triple-Mode Switching in Photoluminescence from 0D Hybrid Antimony Halides, Chem. Mater., 2022, 34, 6985–6995 CrossRef CAS
.
- C. Zhou, M. Worku, J. Neu, H. Lin, Y. Tian, S. Lee, Y. Zhou, D. Han, S. Chen, A. Hao, P. I. Djurovich, T. Siegrist, M.-H. Du and B. Ma, Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency, Chem. Mater., 2018, 30, 2374–2378 CrossRef CAS
.
- J.-L. Qi, J. Wu, Y. Guo, Z.-P. Xu, W. Liu and S.-P. Guo, Quasi-linear CuX2 (X = Cl, Br) Motif-Built Hybrid Copper Halides Realizing Encouraging Nonlinear Optical Activities, Inorg. Chem. Front., 2023, 10, 3319–3325 RSC
.
- F. Ge, B.-H. Li, P. Cheng, G. Li, Z. Ren, J. Xu and X.-H. Bu, Chiral Hybrid Copper(I) Halides for High Efficiency Second Harmonic Generation with a Broadband Transparency Window, Angew. Chem., Int. Ed., 2022, 61, e202115024 CrossRef CAS
.
- Z. Guo, J. Li, J. Liang, C. Wang, X. Zhu and T. He, Regulating Optical Activity and Anisotropic Second-Harmonic Generation in Zero-Dimensional Hybrid Copper Halides, Nano Lett., 2022, 22, 846–852 CrossRef CAS PubMed
.
- H. Peng, Y. Tian, X. Wang, T. Huang, Z. Yu, Y. Zhao, T. Dong, J. Wang and B. Zou, Pure White Emission with 91.9% Photoluminescence Quantum Yield of [(C3H7)4N]2Cu2I4 out of Polaronic States and Ultra-High Color Rendering Index, ACS Appl. Mater. Interfaces, 2022, 14, 12395–12403 CrossRef CAS
.
- L. Lian, P. Zhang, G. Liang, S. Wang, X. Wang, Y. Wang, X. Zhang, J. Gao, D. Zhang, L. Gao, H. Song, R. Chen, X. Lan, W. Liang, G. Niu, J. Tang and J. Zhang, Efficient Dual-Band White-Light Emission with High Color Rendering from Zero-Dimensional Organic Copper Iodide, ACS Appl. Mater. Interfaces, 2021, 13, 22749–22756 CrossRef CAS PubMed
.
- P. Fu, S. Geng, R. Mi, R. Wu, G. Zheng, B. Su, Z. Xia, G. Niu, J. Tang and Z. Xiao, Achieving Narrowed Bandgaps and Blue-Light Excitability in Zero-Dimensional Hybrid Metal Halide Phosphors via Introducing Cation–Cation Bonding, Energy Environ. Mater., 2023, e12518 CrossRef
.
- M. S. Chen, C. Y. Ye, C. Q. Dai, R. J. Qi, H. M. Fu, C. H. Luo, H. Peng and H. C. Lin, Highly Luminescent Copper(I) Halide Phosphors Encapsulated in Fumed Silica for Anti-Counterfeiting and Color-Converting Applications, Adv. Opt. Mater., 2022, 10, 2200278 CrossRef CAS
.
- L. Lian, X. Wang, P. Zhang, J. Zhu, X. Zhang, J. Gao, S. Wang, G. Liang, D. Zhang, L. Gao, H. Song, R. Chen, X. Lan, W. Liang, G. Niu, J. Tang and J. Zhang, Highly Luminescent Zero-Dimensional Organic Copper Halides for X-ray Scintillation, J. Phys. Chem. Lett., 2021, 12, 6919–6926 CrossRef CAS PubMed
.
- T. Xu, Y. Li, M. Nikl, R. Kucerkova, Z. Zhou, J. Chen, Y. Y. Sun, G. Niu, J. Tang, Q. Wang, G. Ren and Y. Wu, Lead-Free Zero-Dimensional Organic-Copper(I) Halides as Stable and Sensitive X-ray Scintillators, ACS Appl. Mater. Interfaces, 2022, 14, 14157–14164 CrossRef CAS PubMed
.
- B. Su, J. Jin, K. Han and Z. Xia, Ceramic Wafer Scintillation Screen by Utilizing Near-Unity Blue-Emitting Lead-Free Metal Halide (C8H20N)2Cu2Br4, Adv. Funct. Mater., 2023, 33, 2210735 CrossRef CAS
.
- C. L. Raston and A. H. White, Crystal structure of the copper(I) iodide–pyridine (1/1) tetramer, J. Chem. Soc., Dalton Trans., 1976, 2153–2156 RSC
.
- J. Wu, Y. Guo, J.-L. Qi, W.-D. Yao, S.-X. Yu, W. Liu and S.-P. Guo, Multi-Stimuli Responsive Luminescence and Domino Phase Transition of Hybrid Copper Halides with Nonlinear Optical Switching Behavior, Angew. Chem., Int. Ed., 2023, 62, e202301937 CrossRef CAS PubMed
.
- A. Pradhan, S. Haldar, K. B. Mallik, M. Ghosh, M. Bera, N. Sepay, D. Schollmeyer, S. K. Ghatak, S. Roy and S. Saha, Mixed phenoxo and azido bridged dinuclear nickel(II) and copper(II) compounds with N,N,O-donor schiff bases: Synthesis, structure, DNA binding, DFT and molecular docking study, Inorg. Chim. Acta, 2019, 484, 197–205 CrossRef CAS
.
- A. Patra, S. K. Saha, T. K. Sen, L. Carrella, G. T. Musie, A. R. Khuda-Bukhsh and M. Bera, Water-Soluble Heteronuclear [NaCu] Metallomacrocyclic Sandwich Complexes: Synthesis, Structure, Properties and In Vitro Biological Studies, Eur. J. Inorg. Chem., 2014, 5217–5232 CrossRef CAS
.
- G. C. Giri, S. Haldar, A. K. Ghosh, P. Chowdhury, L. Carrella, U. Ghosh and M. Bera, New cyclic tetranuclear copper(II) complexes containing quadrilateral cores: Synthesis, structure, spectroscopy and their interactions with DNA in aqueous solution, J. Mol. Struct., 2017, 1142, 175–184 CrossRef CAS
.
- S. Haldar, G. Vijaykumar, L. Carrella, S. Batha, G. T. Musie and M. Bera, Inorganic Phosphate and Arsenate within New Tetranuclear Copper and Zinc Complexes: Syntheses, Crystal Structures, Magnetic, Electrochemical, and Thermal Studies, ACS Omega, 2017, 2, 1535–1549 CrossRef CAS
.
- F. Zhang, W. Liang, L. Wang, Z. Ma, X. Ji, M. Wang, Y. Wang, X. Chen, D. Wu, X. Li, Y. Zhang, C. Shan and Z. Shi, Moisture-Induced Reversible Phase Conversion of Cesium Copper Iodine Nanocrystals Enables Advanced Anti-Counterfeiting, Adv. Funct. Mater., 2021, 31, 2105771 CrossRef CAS
.
- W. Cui, J. Zhao, L. Wang, P. Lv, X. Li, Z. Yin, C. Yang and A. Tang, Unraveling the Phase Transition and Luminescence Tuning of Pb-Free Cs–Cu–I Perovskites Enabled by Reaction Temperature and Polar Solvent, J. Phys. Chem. Lett., 2022, 13, 4856–4863 CrossRef CAS
.
- D. Lee, S.-J. Lee, J. H. Kim, J. Park, Y.-C. Kang, M. Song, H. W. Lee, H. S. Kim and J. W. Choi, Multimodal Gas Sensor Detecting Hydroxyl Groups with Phase Transition Based on Eco-Friendly Lead-Free Metal Halides, Adv. Funct. Mater., 2022, 32, 2202207 CrossRef CAS
.
- S.-L. Li, F.-Q. Zhang and X.-M. Zhang, An Organic-Ligand-Free Thermochromic Luminescent Cuprous Iodide Trinuclear Cluster: Evidence for Cluster Centered Emission and Configuration Distortion with Temperature, Chem. Commun., 2015, 51, 8062–8065 RSC
.
- Y. Li, Z. Zhou, F. K. Sheong, Z. Xing, R. Lortz, K. S. Wong, H. H. Y. Sung, I. D. Williams and J. E. Halpert, Tuning the Self-Trapped Emission: Reversible Transformation to 0D Copper Clusters Permits Bright Red Emission in Potassium and Rubidium Copper Bromides, ACS Energy Lett., 2021, 6, 4383–4389 CrossRef CAS
.
- S. Fang, B. Zhou, H. Li, H. Hu, H. Zhong, H. Li and Y. Shi, Highly Reversible Moisture-Induced Bright Self-Trapped Exciton Emissions in a Copper-Based Organic–Inorganic Hybrid Metal Halide, Adv. Opt. Mater., 2022, 10, 2200605 CrossRef CAS
.
- Y. Tian, H. Peng, Q. Wei, Y. Chen, J. Xia, W. Lin, C. Peng, X. He and B. Zou, Moisture-Induced Reversible Structure Conversion of Zero-Dimensional Organic Cuprous Bromide Hybrids for Multiple Photoluminescent Anti-Counterfeiting, Information Encryption and Rewritable Luminescent Paper, Chem. Eng. J., 2023, 458, 141436 CrossRef CAS
.
- R. An, J. Hao, S. Song, H. Zhang, J. Feng, X. Wang and H. Sun, Multi-Responsive 0D Organic-Inorganic Hybrid Cuprous Bromides Constructed via Ultra-Facile Approaches for Multimodal Luminescent Anti-Counterfeiting, Adv. Opt. Mater., 2023, 11, 2202596 CrossRef CAS
.
- D.-Y. Li, J.-H. Wu, X.-Y. Wang, X.-Y. Zhang, C.-Y. Yue and X.-W. Lei, Reversible Triple-Mode Photo- and Radioluminescence and Nonlinear Optical Switching in Highly Efficient 0D Hybrid Cuprous Halides, Chem. Mater., 2023, 35, 6598–6611 CrossRef CAS
.
- W.-T. Zhang, J.-Z. Liu, J.-B. Liu, K.-Y. Song, Y. Li, Z.-R. Chen, H.-H. Li and R. Jiang, Quaternary Phosphorus-Induced Iodocuprate(I)-Based Hybrids: Water Stabilities, Tunable Luminescence and Photocurrent Responses, Eur. J. Inorg. Chem., 2018, 4234–4244 CrossRef CAS
.
- B. Su, J. Jin, Y. Peng, M. S. Molokeev, X. Yang and Z. Xia, Zero-Dimensional Organic Copper(I) Iodide Hybrid with High Anti-Water Stability for Blue-Light-Excitable Solid-State Lighting, Adv. Opt. Mater., 2022, 10, 2102619 CrossRef CAS
.
- A. Banerjee, A. Saha and B. K. Saha, Understanding the Behavior of π–π Interactions in Crystal Structures in Light of Geometry Corrected Statistical Analysis: Similarities and Differences with the Theoretical Models, Cryst. Growth Des., 2019, 19, 2245–2252 CrossRef CAS
.
- P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, Crystalexplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed
.
- H. Luo, S. Guo, Y. Zhang, K. Bu, H. Lin, Y. Wang, Y. Yin, D. Zhang, S. Jin, W. Zhang, W. Yang, B. Ma and X. Lü, Regulating Exciton–Phonon Coupling to Achieve a Near-Unity Photoluminescence Quantum Yield in One-Dimensional Hybrid Metal Halides, Adv. Sci., 2021, 8, 2100786 CrossRef CAS PubMed
.
- G. Li, J. Huang, H. Zhu, Y. Li, J.-X. Tang and Y. Jiang, Surface Ligand Engineering for Near-Unity Quantum Yield Inorganic Halide Perovskite QDs and High-Performance QLEDs, Chem. Mater., 2018, 30, 6099–6107 CrossRef CAS
.
- J. Tong, J. Wu, W. Shen, Y. Zhang, Y. Liu, T. Zhang, S. Nie and Z. Deng, Direct Hot-Injection Synthesis of Lead Halide Perovskite Nanocubes in Acrylic Monomers for Ultrastable and Bright Nanocrystal–Polymer Composite Films, ACS Appl. Mater. Interfaces, 2019, 11, 9317–9325 CrossRef CAS
.
- S. Li, J. Xu, Z. Li, Z. Zeng, W. Li, M. Cui, C. Qin and Y. Du, One-Dimensional Lead-Free Halide with Near-Unity Greenish-Yellow Light Emission, Chem. Mater., 2020, 32, 6525–6531 CrossRef CAS
.
- X. Meng, S. Ji, Q. Wang, X. Wang, T. Bai, R. Zhang, B. Yang, Y. Li, Z. Shao, J. Jiang, K.-l. Han and F. Liu, Organic–Inorganic Hybrid Cuprous-Based Metal Halides for Warm White Light-Emitting Diodes, Adv. Sci., 2022, 9, 2203596 CrossRef CAS
.
- H. Peng, S. Yao, Y. Guo, R. Zhi, X. Wang, F. Ge, Y. Tian, J. Wang and B. Zou, Highly Efficient Self-Trapped Exciton Emission of a (MA)4Cu2Br6 Single Crystal, J. Phys. Chem. Lett., 2020, 11, 4703–4710 CrossRef CAS
.
- S. Zhou, Y. Chen, K. Li, X. Liu, T. Zhang, W. Shen, M. Li, L. Zhou and R. He, Photophysical Studies for Cu(I)-Based Halides: Broad Excitation Bands and Highly Efficient Single-Component Warm White-Light-Emitting Diodes, Chem. Sci., 2023, 14, 5415–5424 RSC
.
- J. Huang, B. Su, E. Song, M. S. Molokeev and Z. Xia, Ultra-Broad-Band-Excitable Cu(I)-Based Organometallic Halide with Near-Unity Emission for Light-Emitting Diode Applications, Chem. Mater., 2021, 33, 4382–4389 CrossRef CAS
.
- X. Liu, Y. Li, L. Zhou, M. Li, Y. Zhou and R. He, Coupling Intracompound Charge Transfer and Cluster-Centered Excited States in Cu(I) Halide Hybrids for Efficient White Light Emission, Adv. Opt. Mater., 2022, 10, 2200944 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional tables and pictures. CCDC 2257258 and 2257259. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi01739g |
| This journal is © the Partner Organisations 2024 |