A box-in-box supramolecular assembly for the highly selective recognition of natural, epigenetically and chemically modified cytosines in water†
Shu-Qin
Qin
a,
Wei
Xu
a,
Qi-Qi
Wang
a,
Run-Yi
Chen
a,
De-Zhi
Yang
 b,
Yang
Lu
b,
Wen-Cai
Ye
b,
Yang
Lu
b,
Wen-Cai
Ye
 *ac and
Ren-Wang
Jiang
*ac and
Ren-Wang
Jiang
 *ac
*ac
aState Key Laboratory of Bioactive Molecules and Druggability Assessment, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China. E-mail: trwjiang@jnu.edu.cn; chywc@aliyun.com
bInstitute of Materia Medica, Chinese Academy of Medical Sciences, 1 Xian Nong Tan Street, Beijing 100050, P. R. China
cInternational Cooperative Laboratory of Traditional Chinese Medicine Modernization and Innovative Drug Development of Ministry of Education (MOE) of China, College of Pharmacy, Jinan University, Guangzhou 510632, P. R. China
First published on 23rd November 2023
Abstract
A novel tetracationic macrocycle (1) was synthesized as a size-complementary cation for recognizing sulfonatocalix[4]arene (SC4H) anions. Complexation between 1 and SC4H resulted in the pH-responsive formation of a novel box-in-box supramolecular assembly (14+·SC44−), in which the lower rim of SC4H fits snugly into 1. Comprehensive characterization using NMR spectroscopy, isothermal titration calorimetry, and fluorescence spectroscopy revealed the high-affinity binding of 1 with SC4H in solution, which was consistent with the solid-state supramolecular structure determined by single-crystal X-ray diffraction analysis and TEM observations. Notably, the supramolecular assembly exhibited selectivity towards natural, chemically and epigenetically modified cytosines in water. In particular, it showed distinct affinities and binding modes towards cytosine (CTS, electron-rich) and 5-fluorocytosine (5F-CTS, electron-deficient), giving rise to ternary assemblies (14+·SC44−@CTS and 14+·SC44−@5F-CTS) through rare collaborative encapsulation. These three-component assemblies were characterized in the solid state through SC-XRD and in solution through NMR and ITC analyses (enthalpy-driven for CTS and entropy-driven for 5F-CTS), along with theoretical calculations. Considering their different binding affinities (ca. 103), the supramolecular assembly was utilized for the selective separation of CTS and 5F-CTS, which was found to preferentially encapsulate the natural CTS in a competitive mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), leaving the unnatural 5F-CTS (antifungal agent) with a purity significantly higher than that obtained by the previously reported procedure. In addition, the supramolecular assembly was found to bind the epigenetically and chemically modified cytosines (5-methylcytosine and 5-hydroxymethylcytosine; gemcitabine and cytarabine). The method developed here provided a powerful tool for the efficient recognition of natural, epigenetically and chemically modified cytosines and the separation of the antifungal drug.
1), leaving the unnatural 5F-CTS (antifungal agent) with a purity significantly higher than that obtained by the previously reported procedure. In addition, the supramolecular assembly was found to bind the epigenetically and chemically modified cytosines (5-methylcytosine and 5-hydroxymethylcytosine; gemcitabine and cytarabine). The method developed here provided a powerful tool for the efficient recognition of natural, epigenetically and chemically modified cytosines and the separation of the antifungal drug.
Introduction
Nucleobases (adenine, cytosine, guanine, thymine and uracil) are essential for life because they function as the fundamental units of the genetic ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) through Watson–Crick base pairs. In pathological conditions, the identification of specific nucleobases enables the diagnosis of related diseases because base mutations are usually associated with various health statuses.1 Thus, the recognition of local nucleobases is key to the diagnosis and treatment of diseases.2 Biomacromolecules, e.g., proteins,3 were reported to bind specific nucleotides; however, the delivery of macromolecules to the human body remained a significant problem. Thus, scientists turned to use synthetic receptors, e.g., naphthyridines4 and dyes,5 which provided a novel approach for targeting specific nucleotides or sequences. However, most of these detection methods depended on fluorescence signals6,7 which could be influenced by other factors and result in a false decision. Furthermore, all nucleobases were hydrophilic molecules, and it was commonly accepted that the recognition of neutral hydrophilic molecules in water is challenging for synthetic receptors because the strong solvation of hydrophilic molecules thwarted their interactions with receptor molecules.8In the past decades, macrocyclic supramolecular chemistry has attracted great interest due to its great potential in various fields such as gas storage, separation, molecular recognition, catalysis and drug delivery.9 A wide variety of macrocycles were reported, e.g., crown ethers, cyclodextrins, calixarenes, and pillararenes;9 however, most of them were seldom used for the recognition of nucleobases, because their hydrophobic cavities were not consistent with the hydrophilic nature of nucleobases. Besides these macrocycles, it was interesting to note that cucurbiturils with a large cavity could encapsulate a small hydrophilic molecule, e.g., CB[10]@CB[5],10 and the “blue box” reported by Stoddart et al. could also encapsulate hydrophilic guests,11 and even form a hierarchical Russian doll;12 however, macrocycles synthesized for the recognition of nucleobases have not been reported so far.
In order to efficiently recognize specific nucleobases, we reported herein the construction of a box-in-box supramolecular assembly through the complexation of a tetracationic macrocycle (1) and 4-sulfonatocalix[4]arene (SC4H). The macrocycle (1) with a rich π-system can form aromatic interactions with the benzene ring of nucleobases, and the SC4H moiety could form a hydrogen bond with the amine group of nucleobases. We hypothesized that the box-in-box supramolecular assembly might meet all the structural criteria of nucleobases. Among the five natural nucleobases, cytosine is the most challenging. Cytosine sequences can form four-stranded structures,13 DNA base lesions,14 bulged regions15 and epigenetic modifications.16 Besides its role as a nucleobase, cytosine has a wide range of applications in the field of medicine. For example, cytosine is used for the preparation of antiviral (lamivudine for anti-AIDS and hepatitis17) and antitumor drugs (gemcitabine18 and cytosine nucleoside19). In addition, cytosine is used as the starting material to synthesize an antifungal agent (5-flucytosine20). The market demand for cytosine increased to 3800 tons per year. To investigate the applicability of our approach, we focused on cytosine recognition, as none of the known receptors possessed sufficient selectivity and affinity.
To confirm whether our supramolecular recognition could be applied to the synthetic analogs, 5-fluorocytosine (5F-CTS) was included, which is used for the treatment of fungal infections, particularly in HIV patients as recommended by the World Health Organization (WHO).20 For a patient with a body mass of 50 kg, approximately 70 grams of 5F-CTS is required to complete the course (daily dosage of 100 mg kg−1 for a two-week program). Due to its pronounced clinical effects and high demand, several strategies for the total synthesis of 5F-CTS have been developed in recent years. Among them, a practical method involves the direct reaction of CTS with fluorine gas. However, the use of n-butanol as a solvent to precipitate the product was found to dissolve both 5F-CTS and CTS,21 resulting in the loss of the target compound and lower yields. Furthermore, removing the starting material from the product mixture to obtain highly pure antifungal 5F-CTS is a significant challenge. In addition, two antitumor drugs which are chemically modified cytosines (gemcitabine and cytarabine) were included.
To further confirm whether our supramolecular recognition could be applied to the epigenetic modifications, 5-methylcytosine (5M-CTS) and 5-hydroxymethylcytosine (5HM-CTS) were also included.
Our results showed that the supramolecular assembly could selectively recognize cytosine (CTS) and chemically and epigenetically modified derivatives (5F-CTS, 5M-CTS and 5HM-CTS) in water, and exhibit a large difference in binding modes and affinities, making it useful for the selective separation of CTS, which is electron-rich, and 5-fluorocytosine (5F-CTS), which is electron-deficient. This selective recognition is important for the identification of CTS and related epigenetic modifications, and the highly efficient separation is of great significance for the preparation of highly pure 5F-CTS for clinical applications.
Results and discussion
Synthesis of the box-in-box supramolecular assembly
Herein, we designed a supramolecular assembly that was prepared from the self-assembly of 1 and SC4H. Firstly, 2,6-dipyridyl phenylamine was synthesized by the reaction of 2,6-dibromoaniline with 4-pyridylboronic acid through Suzuki coupling (1H-, 13C-NMR and MS are shown in SI-1, Fig. S1a, b and c,† respectively).Then, 2,6-dipyridyl phenylamine was further reacted with 4,4′-bis(bromomethyl)biphenyl to afford 1 by SN2 nucleophilic substitution (Scheme 1A). The structure of 1 was characterized by NMR (1H-, 13C-NMR and MS spectra of 1 are shown in Fig. S1d, e and f,† respectively). In the 1H-NMR spectra, the signal at δ 8.97 ppm (d, J = 6.8 Hz, 8H) was assigned to Hd, which showed a 0.27 ppm down-field shift as compared to the corresponding signal of the starting material 1,3-dipyridyl phenylamine [δ 8.70 ppm (d, J = 4.6 Hz, 4H)]. The appearance of a broad singlet at 5.88 ppm (brs, 8H) was assigned to the methylene (He) of 4,4′-bis(bromomethyl)biphenyl. All these typical and proportional signals indicated the clean formation of 1, which was confirmed by high-resolution ESI-TOF-MS: C60H50N6Br4 [M + H]+m/z 1171.0909 (calcd 1171.0918) for the bromine assembly (1·Br4). It was later exchanged with a hexafluorophosphate assembly for better solubility in an organic solvent, and the composition was confirmed by high-resolution mass spectrometry C60H50N6(PF6)4 [M + H]+m/z 1435.2740 (calcd 1435.2742) (ESI, Fig. S1g†).
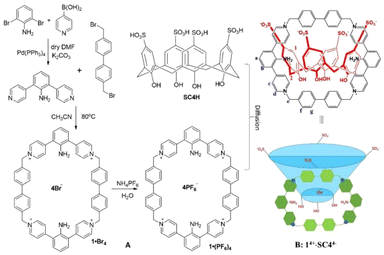 | ||
| Scheme 1 Synthetic route to supramolecular assembly of 14+·SC44−. (A) Synthesis of 1·(PF6)4; (B) recognition of SC4H by 1 to form the host–guest-like assembly. | ||
Finally, slow diffusion of 1·(PF6)4 in acetonitrile to SC4H22 in water afforded an immediate precipitate followed by the appearance of small shiny particles in the next five days, suggesting the formation of the supramolecular assembly of 14+·SC44−.
To gain insight into the morphologies of the precipitate and shiny particles, we subjected them to high-resolution transmission electron microscopy (HR-TEM). HR-TEM showed that the precipitate was amorphous (Fig. 1A) while the shiny particles were composed of small rectangular blocks (Fig. 1B and C), and even showed weak electron diffraction (Fig. 1D), indicating that the shiny particles were in the crystalline state. Further comparison of the precipitate and the shiny particles by NMR showed that they shared the same composition (1H-NMR spectrum is shown in Fig. S2†). At a high concentration (≥40 μM), the precipitate was formed, while the shiny particles appeared under dilute conditions (≤30 μM).
 | ||
| Fig. 1 HR-TEM images of the (A) precipitate; (B) shiny particles on a 5 μm scale; (C) shiny particles on a 200 nm scale; (D) selected area electron diffraction. | ||
Solid-phase characterization of the box-in-box complex
The formation of the precipitate and shining particles after mixing 1·(PF6)4 and SC4H together motivated us to investigate the mechanism of the assembly process and the detailed interactions between 1 and SC4H. Guided by the TEM images, slow diffusion of the dilute solution of 1·(PF6)4 (20 μM in acetonitrile) and SC4H (20 μM in water) for five days afforded single crystals.Then, the structure of 14+·SC44− was studied by single-crystal X-ray diffraction (for crystal data, see Table S1†). The complex crystallized in the space group Pbca. The asymmetric unit consisted of the cation of 1 and the anion of SC4H in a box-in-box manner with a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Compound 1 had a cavity size of 12.72 × 10.11 × 4.63 Å (Fig. 2A). SC4H had a height of 8.80 Å, and the sizes of the up and down rims were 17.26 and 5.04 Å (Fig. 2B), respectively. So, only the down rim could be snugly encapsulated in 1 (Fig. 2C). Intermolecular macrocycles of 1 were linked by two C–H⋯π interactions with protons to centroid distances of 2.68 and 2.85 Å (for details, see Table S2a†), respectively, and a face-to-face π–π interaction with a centroid distance of 3.63 Å (for details, see Table S2b†). SC4H was linked to the macrocycle (1) through seven C–H⋯O interactions with H⋯O distances of 2.22, 2.34, 2.41, 2.45, 2.45, 2.48 and 2.51 Å (Fig. 2E, for details, see Table S3†), respectively, and two C–H⋯π interactions with both protons to centroid distances of 2.87 Å (Fig. 2F). In the packing diagram, SC4H and 1 located in alternative layers (Fig. 2G). In addition, 1 was tetracationic with four positive charges, while SC4H had four sulfonic groups, which lose protons and carry four negative charges. The four distances between the positive nitrogen and the negative sulfonate groups were 6.40, 7.34, 7.45 and 8.13 Å (Fig. 2D), respectively. Thus, SC4H and 1 formed a stable box-in-box supramolecular assembly. Due to the balanced charge, the counterion (PF6−) of 1 was not observed in 14+·SC44−.
1. Compound 1 had a cavity size of 12.72 × 10.11 × 4.63 Å (Fig. 2A). SC4H had a height of 8.80 Å, and the sizes of the up and down rims were 17.26 and 5.04 Å (Fig. 2B), respectively. So, only the down rim could be snugly encapsulated in 1 (Fig. 2C). Intermolecular macrocycles of 1 were linked by two C–H⋯π interactions with protons to centroid distances of 2.68 and 2.85 Å (for details, see Table S2a†), respectively, and a face-to-face π–π interaction with a centroid distance of 3.63 Å (for details, see Table S2b†). SC4H was linked to the macrocycle (1) through seven C–H⋯O interactions with H⋯O distances of 2.22, 2.34, 2.41, 2.45, 2.45, 2.48 and 2.51 Å (Fig. 2E, for details, see Table S3†), respectively, and two C–H⋯π interactions with both protons to centroid distances of 2.87 Å (Fig. 2F). In the packing diagram, SC4H and 1 located in alternative layers (Fig. 2G). In addition, 1 was tetracationic with four positive charges, while SC4H had four sulfonic groups, which lose protons and carry four negative charges. The four distances between the positive nitrogen and the negative sulfonate groups were 6.40, 7.34, 7.45 and 8.13 Å (Fig. 2D), respectively. Thus, SC4H and 1 formed a stable box-in-box supramolecular assembly. Due to the balanced charge, the counterion (PF6−) of 1 was not observed in 14+·SC44−.
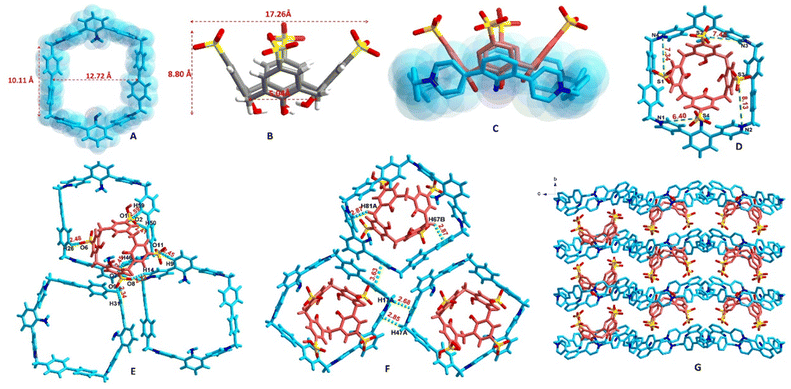 | ||
| Fig. 2 Crystal structure of 14+·SC44−. (A) The size of 1; (B) the size of SC4H; (C) the lower rim was snugly encapsulated by 1, forming a supramolecular assembly; (D) the four distances between the positive nitrogen and the negative sulfonate groups; (E) C–H⋯O interactions in 14+·SC44−; (F) C–H⋯π and π⋯π interactions in 14+·SC44−; (G) the packing diagram viewed along the a-axis (crystal morphology of 14+·SC44− and the packing diagram viewed along the b-axis are shown in Fig. S3†). | ||
To further confirm the formation of the supramolecular assembly, the infrared spectra of SC4H, 1·(PF6)4 and 14+·SC44− were compared (Fig. S4†), which showed that the intensities of 1038 and 1176 cm−1 in 14+·SC44−, due to the stretching vibration of S–O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the SO3H group,23 respectively, were significantly decreased, while the intensities of 1598 and 1634 cm−1, typical of the NH2 group, were kept unchanged, suggesting that the SO3H group was deprotonated, while there is no proton transfer to the NH2 group. The formation of the supramolecular assembly was also confirmed by theoretical calculations of the interaction energy24 (−362.09 kcal mol−1) and molecular electrostatic potential (MEP) surface (SI-5 and Fig. S5†), suggesting the formation of the supramolecular assembly rather than co-crystals.25
O in the SO3H group,23 respectively, were significantly decreased, while the intensities of 1598 and 1634 cm−1, typical of the NH2 group, were kept unchanged, suggesting that the SO3H group was deprotonated, while there is no proton transfer to the NH2 group. The formation of the supramolecular assembly was also confirmed by theoretical calculations of the interaction energy24 (−362.09 kcal mol−1) and molecular electrostatic potential (MEP) surface (SI-5 and Fig. S5†), suggesting the formation of the supramolecular assembly rather than co-crystals.25
Solution-phase characterization of the box-in-box complex
The interactions between 1·(PF6)4 and SC4H in the solution state were first evidenced by the Tyndall effect and the appearance under a UV lamp. As shown in Fig. 3A, a solution of 14+·SC44− exhibited a clear Tyndall effect (using red light of 650 nm),26 indicating the existence of abundant nanoparticles. Similar phenomena were not observed for the solutions of free 14+·SC44− and SC4H, revealing that both compounds alone could not form nanoscale aggregates under the same conditions. SC4H lowered the critical aggregation concentration of 14+·SC44− pronouncedly by a factor of ca. 200 to form binary amphiphilic aggregates.The formation of a new phase was further reinforced by the appearance under 254 (Fig. 3B) and 365 nm excitation (Fig. 3C). Under 254 nm excitation, the free SC4H, 1·(PF6)4 and 14+·SC44− appeared colourless, light yellow and yellow, respectively; in contrast, under 365 nm excitation, the free SC4H still appeared colourless and the free 1·(PF6)4 showed an orange colour; however, 14+·SC44− changed to a deep orange colour. The sharp transformation of the colourless solution into an orange colour indicates the formation of a new phase. The sharper colour changes under 365 nm excitation indicated that this wavelength was a much better excitation wavelength than 254 nm. Furthermore, adding SC4H deepened the colour of 1·(PF6)4.
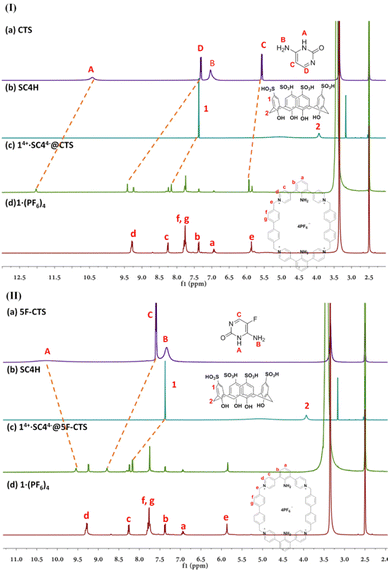
With the Tyndall effect and UV appearance in hand, we tried to use NMR27,28 to probe the host and guest interactions in solution by detecting the chemical shift changes of protons on both 1 and SC4H. At pH = 7, like the weak non-covalent interactions in GNPS29 and GBPS,30 and the electrostatic interactions in a tetraphenylethene system,31 the chemical shift of the peaks ascribed to 1 (Ha, Hb, Hc, Hd, He, Hf and Hg) showed marginal changes [up-field shifts of 0.02, 0.01, 0.01, 0.04, 0.01, 0.02 and 0.02 ppm (Fig. 4b), respectively. For details, see Table S4†]. Similarly, the protons on the benzene ring (H1) in SC4H showed slight up-field chemical shift changes (0.06 ppm, Fig. 4b), suggesting that the perturbation of chemical shifts of 1 and SC4H was very small after forming complex 14+·SC44− under neutral conditions. Then, we performed diffusion-ordered spectroscopy (DOSY)32 which confirmed the formation of a single species with a diffusion coefficient of 1.518 × 10−10 m2 s−1 (Fig. 4h). Furthermore, the formation of the supramolecular assembly was supported by the 2D NOESY spectrum, e.g., NOE correlations were observed between 1 and SC4H (Fig. S6†). Surprisingly, when increasing the pH value to 8 (Fig. 4c), all protons on 1 and SC4H continued to shift upfield with a weakened signal due to precipitation (Fig. S7†). When the pH value was increased to 10 (Fig. 4d), the signal for Hd on 1 disappeared and the signals for Hf and Hg were divided into two doublets with a much lower intensity, indicating the degradation of the complex. When the pH value was further increased to 12 (Fig. 4e), all signals for 1 and SC4H disappeared with a large amount of precipitation (Fig. S7†). The NMR study suggested that 1·(PF6)4 and SC4H form the supramolecular assembly 14+·SC44− under neutral conditions. However, it would be degraded at a pH value higher than 8. It was interesting to find that when the precipitate from pH = 12 solution was re-dissolved in pH = 7 solution, the obtained NMR spectrum (Fig. 4f) was identical to that in Fig. 4b. Thus, the formation of the supramolecular assembly 14+·SC44− was pH responsive.
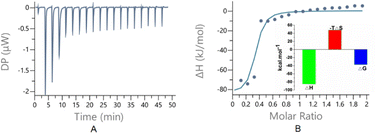
Isothermal titration calorimetry (ITC) was a powerful tool33 for determining the host–guest interactions because it not only gave the binding affinities (Ka) but also yielded their thermodynamic parameters [enthalpy (ΔH) and entropy (ΔS) changes]. To probe the thermodynamic behaviours of the host and guest interactions, ITC experiments were conducted. The thermograms obtained by titration of 1·(PF6)4 with SC4H exhibited negative peaks, implying that the host–guest interaction takes place through a favourable exothermic pathway (Fig. 5), and the larger −ΔH and smaller ΔS values suggest that the spontaneous processes were mainly due to the enthalpic driving force with an entropic compensation.
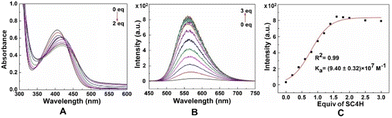
To qualitatively and quantitatively determine the host and guest interactions, UV and fluorescence spectra were obtained (Fig. 6), respectively. When SC4H was titrated into 1·(PF6)4, the UV spectrum showed a red shift of 20 nm, indicating that the formation of 14+·SC44− further increased the conjugated system. Concentration-dependent fluorescence titration experiments (based on the UV spectrum and the colours under a UV lamp, the excitation wavelength was set to 400 nm) showed increased emission intensity when a large amount of SC4H was added (0–3 eq.), which was like the deeper colour changes under the UV lamp. The fluorescence enhancement might be the result of the restriction of intramolecular motions caused by complexation with SC4H, which was like the AIE phenomena reported by Tang's team34 and Wang's group.35 The fitting plot showed a nonlinear function correlation between the emission intensity and the increasing concentration of SC4H. The fitting degree (R2) of equations can reach 0.99 with a Ka value of (9.40 ± 0.32) × 107, indicating a strong binding sensitivity.
Recognition of natural, epigenetically and chemically modified nucleobases
The supramolecular assembly 14+·SC44− bears two moieties, including the cationic macrocycle (1) which could provide rich aromatic interactions and SC4H which could form various hydrogen bonds. Thus, this assembly met the structural criterion of nucleobases. Then, we used ITC to compare the interactions of all five natural nucleobases and five cytosine derivatives with 14+·SC44−. We found that 14+·SC44− was selective (Fig. 7A and B). Adenine, guanine, thymine and uracil showed no affinities with 14+·SC44−. When cytosine (CTS) was titrated into 14+·SC44−, the subsequent fitting showed a Ka value of (5.70 ± 1.20) × 106, indicating a high affinity binding (Fig. 7C and D). In contrast, 5F-CTS only showed a weak binding affinity with a Ka value of (4.55 ± 0.78) × 103 (Fig. 7E and F), which was 1000 times lower than that of CTS. Comparison of the insets in Fig. 7D and F showed that a larger −ΔH value was observed for CTS binding, while a larger ΔS value was observed for 5F-CTS binding, suggesting that the former was mainly due to the enthalpic driving force while the latter was likely an entropic process. | ||
| Fig. 7 ITC analysis for comparing the interactions of nucleobases and cytosine derivatives with 14+·SC44−. (A) Structures of nucleobases and derivatives; (B) selectivity comparison showing that CTS and its derivatives had different affinities with 14+·SC44−; (C) ITC thermogram resulting from titrations of CTS with 14+·SC44−; (D) fitting the thermogram in Fig. 7C with a single site model; (E) ITC thermogram resulting from titrations of 5F-CTS with 14+·SC44−; (F) fitting the thermogram in Fig. 7E with a single site model. A Malvern/Micro PEAQ-ITC automated instrument was used for all ITC measurements (nucleobases and derivatives were placed in the instrument needle and 14+·SC44− was placed in the instrument cell). The nucleobases and derivatives were titrated to 14++·SC44−. The small molar ratio was due to the low solubility of 14++·SC44−. | ||
In addition, we also used ITC to compare the interactions of the epigenetically modified 5M-CTS and 5HM-CTS with 14+·SC44−. We found that 14+·SC44− showed affinities towards them with Ka values of (1.03 ± 0.22) × 105 and (2.21 ± 0.36) × 104, respectively. Similarly, we also obtained Ka values of (2.22 ± 0.73) × 105 and (3.28 ± 0.52) × 103 for cytarabine and gemcitabine, respectively (ESI, Fig. S8†). It was noteworthy that 14+·SC44− showed high selectivity towards CTS and its derivative, which might be due to the specific skeleton bearing only one aromatic ring with an NH2 group (not carbonyl in uracil and thymine) at C-4. This high selectivity was consistent with the selective recognition of methyl viologen by an endo-functionalized naphthobox.36
Formation of ternary assemblies through the collaboration of 14+ and SC44−
Bearing in mind that 1·(PF6)4 and SC4H can form a stable box-in-box supramolecular assembly in both the solid state and solution, and there is a void inside SC4H that is large enough to potentially accommodate a second guest, we thus sought out guest molecules that could occupy this cavity. The high affinity cytosine (CTS) and the low affinity antifungal drug 5-fluorocytosine (5F-CTS) discovered from ITC screening were employed as the guests in order to investigate the possibility of forming ternary assemblies. Using the same concentration of 14+·SC44−, crystals were formed within three days in the CTS tube; in contrast, crystals of the 5F-CTS complex were obtained until two weeks. Both crystals were subjected to SC-XRD analysis, which revealed the formation of 14+·SC44−@CTS and 14+·SC44−@5F-CTS inclusion complexes (Fig. 8) (the crystal data are shown in the ESI, Table S1†). CTS was found to be located in the upper cone center of 14+·SC44−@CTS (Fig. 8A). CTS was linked to SC44− through a strong N–H⋯O hydrogen bond with an H⋯O distance of 2.06 Å and a C–H⋯O interaction with an H⋯O distance of 2.89 Å (for details, see Table S3†), while CTS was linked to 14+ through a C–H⋯π interaction with an H⋯π distance of 3.54 Å (Fig. 8B, for details, see the ESI, Table S2a†).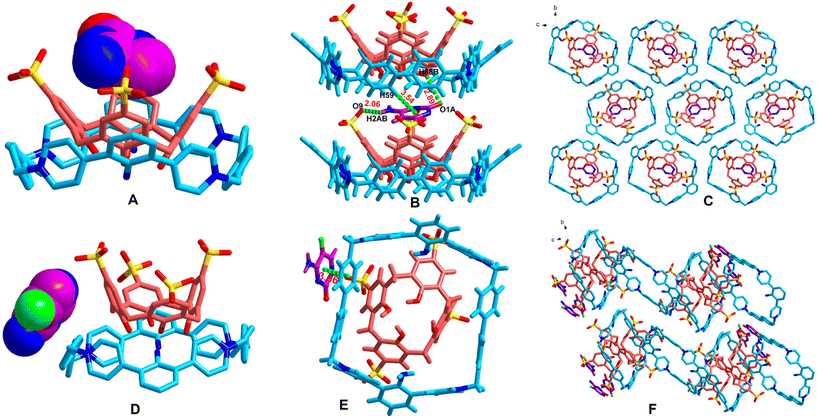 | ||
| Fig. 8 The ternary hierarchical supramolecular complexes of 14+·SC44−@CTS and 14+·SC44−@5F-CTS. (A) X-ray structure of the asymmetric unit of 14+·SC44−@CTS; (B) the interactions of CTS with 14+·SC44−; (C) the packing diagram of 14+·SC44−@CTS viewed along the a-axis; (D) X-ray structure of the asymmetric unit of 14+·SC44−@5F-CTS; (E) the interactions of 5F-CTS with 14+·SC44−; and (F) the packing diagram of 14+·SC44−@5F-CTS viewed along the a-axis. (Crystal morphologies of 14+·SC44−@CTS and 14+·SC44−@5F-CTS and packing diagram viewed along the other directions are shown in the ESI, Fig. S9.†) | ||
In contrast to the upper cone center, 5F-CTS was located at the left side in complex 14+·SC44−@5F-CTS (Fig. 8D), and the interactions were different. 5F-CTS was linked to 14+ and SC44− through a face-to-face π⋯π interaction with a distance of 3.86 Å (for details, see the ESI, Table S2b†) and an N–H⋯O hydrogen bond with an H⋯O distance of 1.86 Å (Fig. 8E, for details, see the ESI, Table S3†), respectively. It could be seen that the encapsulation of CTS or 5F-CTS by 14+·SC44− was due to the collaboration of 14+ and SC44− because the guests had interactions with both moieties. It should be noted that CTS is relatively an electron-rich molecule; in contrast, due to the electron-withdrawing properties of the fluorine atom, 5F-CTS is relatively an electron-deficient molecule. So, CTS can locate at the upper cone center of the supramolecular complex 14+·SC44−@CTS, in which the tetracationic macrocycle 1 bearing four positive charges was an electron-deficient molecule; in contrast, 5F-CTS could not locate at this position of the same supramolecular assembly 14+·SC44−.
In contrast to the collaboration of 14+ and SC44−, when we use macrocycle 1 or SC4H alone, no encapsulation of CTS or 5F-CTS could be detected.
To investigate why CTS could be encapsulated by 14+·SC44− much faster than 5F-CTS (CTS in three days, while 5F-CTS in two weeks) though they had similar volumes (ESI, Fig. S10†), we used 1H-NMR (DMSO-d6) to compare their interactions with 14+·SC44− in solution. We selected DMSO-d6 to collect the NMR spectrum because the ternary complex showed higher solubility in DMSO than in D2O; however, the complex showed a similar tendency in both solvents. It was found that the proton Hd in CTS and 5F-CTS showed downfield chemical shifts at 2.095 and 1.193 ppm, respectively, suggesting that both CTS and 5F-CTS showed interactions when complexed with 14+·SC44− in DMSO (Fig. 9) (for details, see the ESI, Tables S5 and S6†); however, the former showed larger perturbations. 2D-NOSEY spectra also showed more correlations in 14+·SC44−@CTS than 14+·SC44−@5F-CTS (ESI, Fig. S11†). There was a fluorine atom in 5T-CTS. So, we also measured the 19F-NMR spectra and found that 19F only showed a 0.423 ppm (0.2% change) downfield shift in complex 14+·SC44−@5F-CTS (Fig. S12†), indicating again a small perturbation.
The different binding affinities of CTS or 5F-CTS with 14+·SC44− were also confirmed by theoretical calculations that were carried out using the Gaussian 09 program (for calculation details, see the ESI, SI-13 and Fig. S13†), which showed that the binding energies for 14+·SC44−@CTS (−31.44 kcal mol−1) was much lower than those for 14+·SC44−@5F-CTS (−25.06 kcal mol−1). Relative to CTS, the encapsulation of 5F-CTS by 14+·SC44− increased entropy and binding energy, which sheds light on why the host dislikes unnatural molecules.
Separation of the mixture of CTS and 5F-CTS
Due to the large binding differences (ca. 103) between CTS and 5F-CTS, we envisioned that it might be possible to take advantage of this selective binding behaviour for the separation of the CTS and 5F-CTS mixture. The guest separation process is illustrated in Fig. 10(I). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CTS and 5F-CTS was added to the solution of 14+·SC44−. After incubation for a week, crystals were formed. The crystals were filtered and dried, and then extracted with (20 μL) DMSO and methanol (480 μL) under ultrasonication. The solution was filtered with a membrane filter (pore size of 0.22 μm) and then subjected to HPLC analysis (Fig. 10(II)). From Fig. 10(II), we could find that only CTS could be encapsulated. We also analysed the remaining solution after crystallization and found that 5F-CTS with a high purity was left in the solution (Fig. 10(II-D)), which was much higher than that obtained by the literature method (ESI, SI-14 and Fig. S14†).
1 mixture of CTS and 5F-CTS was added to the solution of 14+·SC44−. After incubation for a week, crystals were formed. The crystals were filtered and dried, and then extracted with (20 μL) DMSO and methanol (480 μL) under ultrasonication. The solution was filtered with a membrane filter (pore size of 0.22 μm) and then subjected to HPLC analysis (Fig. 10(II)). From Fig. 10(II), we could find that only CTS could be encapsulated. We also analysed the remaining solution after crystallization and found that 5F-CTS with a high purity was left in the solution (Fig. 10(II-D)), which was much higher than that obtained by the literature method (ESI, SI-14 and Fig. S14†).
Discussion
Despite progress in next generation sequencing (NGS), DNA damage confounded mutation detection and rendered accuracy dependent upon the sample quality, which was deeply problematic.37 Translation, along with errors in sample preparation and sequencing, contributed to an error rate of 0.1–1% in NGS. Furthermore, repetitive DNA sequences covered nearly half of the human genome. Repeats always presented technical challenges for sequence alignment and assembly processes.38 Thus, it was still necessary to recognize special nucleobases by chemical methods. In this study, a new tetracationic macrocycle (1) was synthesized and a unique box-in-box supramolecular assembly with SC4H (14+·SC44−) was formed. On one hand, this supramolecular assembly could selectively recognize cytosine from other nucleobases in water, which was generally considered a challenging environment for most host molecules;39 on the other hand, it showed large binding differences (ca. 103) between CTS and 5F-CTS, and was successfully utilized to purify 5F-CTS from the mixture.Nucleobases have been widely used in supramolecular chemistry. On one hand, they could be used as ligands for the preparation of bio-MOFs.40 For example, an adenine-based zinc-MOF was synthesized for heterogeneous catalysis.41 Hypoxanthine, an important alkaloid purine in the anticodon of tRNA, was used for the synthesis of bio-MOFs for mimicking DNA periodic docking grooves.42 On the other hand, they could also be detected as guest molecules, especially for synthetic receptors. For example, naphthyridines4 and dyes5 were designed for targeting specific nucleotides or sequences; however, the detection was based on fluorescence signals. Recently, a Mn-MOF was reported to recognize all known purine nucleobases; however, the recognition was only characterized by high-resolution synchrotron powder X-ray diffraction.43 Thus, our box-in-box supramolecular assembly represents the first synthetic receptor toward the nucleobase with detailed interactions characterized by X-ray analysis.
As the anion of 14+·SC44−, SC4H is a synthetic water-soluble receptor reported by Shinkai et al. in 1990.44 This compound consists of 4-hydroxybenzenesulfonate units linked by methylene bridges. One of the notable features of this compound is its ability to form inclusion complexes with various guests, including small molecules45 or metals,46 within their cavities. While SC4H had been previously used as a chaperone for protein crystallization,47 it primarily serves as a host molecule. In this study, for the first time, SC4H was employed as a counterion for the synthesis of the supramolecular assembly 14+·SC44−.
It was noteworthy that the size of the guest molecule played a critical role in its inclusion within the host–guest framework.48 Ideally, guest molecules that precisely fit the host cavities could be encapsulated, whereas larger or smaller compounds could not be accommodated or retained within the cavity. Rebek et al. introduced the concept of “packing coefficients” (PCs), which represented the ratio between the molecular volume and the void volume of the guest in the host cavity. A PC value of 0.55 ± 0.1 was suggested as the optimum for host–guest complexes.49 Following this rule, Cooper et al. reported the formation of isoskeletal co-crystals between a propeller-shaped organic cage and organic guests with PCs ranging from 44% to 50%.50 However, the value of the PC was still in debate. Miyata et al. analysed the crystal structures of the inclusion compounds of cholic acid with 28 monosubstituted benzene molecules and found that stable inclusion compounds had PC values ranging from 55% to 70%.51 Ward et al. reported a cubic coordination cage with packing coefficients of up to 87%,52 while Dyker et al. reported an adaptive resorcinarene hemicarcerand with packing coefficients as high as 91%.53 In this study, SC4H exhibited the highest PC (over 100%) to fit the cavity of 1·(PF6)4, with only the lower rim capable of sliding into the macrocycle.
It was noteworthy that separation played a vital role in the chemical and pharmaceutical industries. Despite significant advancements, there is growing interest in the development of improved separation technologies, particularly through the discovery of high-performance separation materials. This interest has been driven by concerns regarding the efficiency, environmental impact, and cost of existing methods. Supramolecular chemistry, characterized by excellent host–guest complexation abilities, emerged as a powerful tool for the encapsulation and separation of chemicals.54 Metal–organic frameworks (MOFs),55 covalent–organic frameworks (COFs),56 macrocycles57 and nanotubes58 had all been applied for separation. Though cytosine and 5F-cytosine were reported to form a uniform solid,59 the box-in-box supramolecular assembly 14+·SC44− was employed for their separation for the first time.
Conclusions
Several conclusions could be drawn from the current investigations. Firstly, we successfully synthesized a novel tetracationic macrocycle (1) designed to function as a size-complementary cation for recognizing sulfonatocalix[4]arene. The lower rim of the calixarene fits snugly into the macrocycle, forming a pH-responsive box-in-box supramolecular assembly. The binding affinities of 1·(PF6)4 and SC4H in solution were analysed using NMR spectroscopy, ITC and fluorescence titrations, which were consistent with the solid-state structure determined by single-crystal XRD analysis. Secondly, the most significant feature of the box-in-box supramolecular assembly was its selective recognition of cytosine (CTS) and related epigenetic analogs, and its ability to bind both electron-rich CTS and electron-deficient 5F-CTS, forming hierarchical ternary assemblies with different modes (enthalpy-driven for CTS and entropy-driven for 5F-CTS) and affinities. Such behaviour was rare in supramolecular systems. The characterization of these three-component assemblies was performed through single-crystal XRD analysis in the solid-state, and NMR and ITC analysis in solution. Thirdly, considering the substantial binding differences between CTS and 5F-CTS, the supramolecular assembly 14+·SC44− was utilized for the separation of CTS and 5F-CTS. Only natural CTS was encapsulated in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 competitive mixture, resulting in a high purity for the remaining 5F-CTS, which can be utilized to produce highly pure antifungal drugs. Furthermore, theoretical calculations demonstrated a significant interaction energy of −362.09 kcal mol−1 for 14+·SC44−, confirming the formation of a supramolecular assembly rather than a co-crystal. The binding energies of 14+·SC44−@CTS were considerably lower than those of 14+·SC44−@5F-CTS, confirming the different binding affinities between CTS and 5F-CTS with 14+·SC44−. Notably, SC4H served as the counterion of the supramolecular assembly for the first time with the highest packing coefficient to date. This study provided a novel approach for the highly selective recognition of cytosine and the epigenetic analogs in water and the separation of pharmaceutically important ingredients using a supramolecular assembly.
1 competitive mixture, resulting in a high purity for the remaining 5F-CTS, which can be utilized to produce highly pure antifungal drugs. Furthermore, theoretical calculations demonstrated a significant interaction energy of −362.09 kcal mol−1 for 14+·SC44−, confirming the formation of a supramolecular assembly rather than a co-crystal. The binding energies of 14+·SC44−@CTS were considerably lower than those of 14+·SC44−@5F-CTS, confirming the different binding affinities between CTS and 5F-CTS with 14+·SC44−. Notably, SC4H served as the counterion of the supramolecular assembly for the first time with the highest packing coefficient to date. This study provided a novel approach for the highly selective recognition of cytosine and the epigenetic analogs in water and the separation of pharmaceutically important ingredients using a supramolecular assembly.
Author contributions
Prof. Jiang and Prof. Ye conceived the research project. S.-Q. Qin performed the chemical synthesis, NMR, UV, and fluorescence titration. Q. Wang was responsible for the HPLC analysis. R.-Y. Chen carried out the IR measurement. Dr Xu performed the X-ray analyses. Dr Yang and Prof. Lu performed theoretical calculations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China [82293681 (82293680)] to W. C. Ye, and the Natural Science Foundation of Guangdong Province (2021A11515011251) and the Guangdong Scientific Scheme (2021A0505030032) to R. W. Jiang. Special thanks to the public research platform of the College of Pharmacy, Jinan University and the X-ray diffraction facility at the South China Sea Institute of Oceanology, Chinese Academy of Sciences.References
- L. M. Zahn and J. Travis, Mutation and Human Disease. Hunting Mutations, Targeting Disease. Introduction, Science, 2015, 349, 1470–1471 CrossRef CAS PubMed.
- H. Tateishi-Karimata and N. Sugimoto, Roles of non-canonical structures of nucleic acids in cancer and neurodegenerative diseases, Nucleic Acids Res., 2021, 49, 7839–7855 CrossRef CAS PubMed.
- R. Rohs, S. M. West, A. Sosinsky, P. Liu, R. S. Mann and B. Honig, The role of DNA shape in protein-DNA recognition, Nature, 2009, 461, 1248–1253 CrossRef CAS PubMed.
- C. Dohno, I. Kohyama, C. F. Hong and K. Nakatani, Naphthyridine tetramer with a pre-organized structure for 1:1 binding to a CGG/CGG sequence, Nucleic Acids Res., 2012, 40, 2771–2781 CrossRef CAS PubMed.
- B. S. Morozov, A. S. Oshchepkov, I. Klemt, A. M. Agafontsev, S. Krishna, F. Hampel, H. G. Xu, A. Mokhir, D. Guldi and E. Kataev, Supramolecular Recognition of Cytidine Phosphate in Nucleotides and RNA Sequences, JACS Au, 2023, 3, 964–977 CrossRef CAS PubMed.
- A. Karimi, R. Börner, G. Mata and N. W. Luedtke, A Highly Fluorescent Nucleobase Molecular Rotor, J. Am. Chem. Soc., 2020, 142, 14422–14426 CrossRef CAS PubMed.
- A. Shibata, S. L. Higashi and M. Ikeda, Nucleic acid-based fluorescent sensor systems: a review, Polym. J., 2022, 54, 751–766 CrossRef CAS.
- G. B. Huang, S. H. Wang, H. Ke, L. P. Yang and W. Jiang, Selective Recognition of Highly Hydrophilic Molecules in Water by Endo-Functionalized Molecular Tubes, J. Am. Chem. Soc., 2016, 138, 14550–14553 CrossRef CAS PubMed.
- Y. Zhou, K. Jie, R. Zhao and F. Huang, Supramolecular-Macrocycle-Based Crystalline Organic Materials, Adv. Mater., 2020, 32, e1904824 CrossRef PubMed.
- A. I. Day, R. J. Blanch, A. P. Arnold, S. Lorenzo, G. R. Lewis and I. Dance, A cucurbituril-based gyroscane: a new supramolecular form, Angew. Chem., Int. Ed., 2002, 41, 275–277 CrossRef CAS PubMed.
- X. Y. Chen, H. Chen and J. F. Stoddart, The Story of the Little Blue Box: A Tribute to Siegfried Hünig, Angew. Chem., Int. Ed., 2023, 62, e202211387 CrossRef CAS PubMed.
- K. Cai, M. C. Lipke, Z. Liu, J. Nelson, T. Cheng, Y. Shi, C. Cheng, D. Shen, J. M. Han, S. Vemuri, Y. Feng, C. L. Stern, W. A. Goddard, 3rd, M. R. Wasielewski and J. F. Stoddart, Molecular Russian dolls, Nat. Commun., 2018, 9, 5275 CrossRef PubMed.
- I. Berger, M. Egli and A. Rich, Inter-strand C-H⋯O hydrogen bonds stabilizing four-stranded intercalated molecules: stereoelectronic effects of O4′ in cytosine-rich DNA, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 12116–12121 CrossRef CAS PubMed.
- Z. Dvoráková, D. Renciuk, I. Kejnovská, P. Školáková, K. Bednárová, J. Sagi and M. Vorlícková, , I-Motif of cytosine-rich human telomere DNA fragments containing natural base lesions, Nucleic Acids Res., 2018, 46, 1624–1634 CrossRef PubMed.
- I. Carter-O'Connell, D. Booth, B. Eason and N. Grover, Thermodynamic examination of trinucleotide bulged RNA in the context of HIV-1 TAR RNA, RNA, 2008, 14, 2550–2556 CrossRef PubMed.
- G. Kubik and D. Summerer, Deciphering Epigenetic Cytosine Modifications by Direct Molecular Recognition, ACS Chem. Biol., 2015, 10, 1580–1589 CrossRef CAS PubMed.
- A. Lukhwareni, M. P. Gededzha, E. Amponsah-Dacosta, J. T. Blackard, R. J. Burnett, S. G. Selabe, T. Kyaw and M. J. Mphahlele, Impact of Lamivudine-Based Antiretroviral Treatment on Hepatitis B Viremia in HIV-Coinfected South Africans, Viruses, 2020, 12, 634 CrossRef CAS PubMed.
- M. Roy-Luzarraga, L. E. Reynolds, B. de Luxán-Delgado, O. Maiques, L. Wisniewski, E. Newport, V. Rajeeve, R. J. G. Drake, J. Gómez-Escudero, F. M. Richards, C. Weller, C. Dormann, Y. M. Meng, P. B. Vermeulen, D. Saur, V. Sanz-Moreno, P. P. Wong, C. Géraud, P. R. Cutillas and K. Hodivala-Dilke, Suppression of Endothelial Cell FAK Expression Reduces Pancreatic Ductal Adenocarcinoma Metastasis after Gemcitabine Treatment, Cancer Res., 2022, 82, 1909–1925 CrossRef CAS PubMed.
- W. Kern and E. H. Estey, High-dose cytosine arabinoside in the treatment of acute myeloid leukemia: Review of three randomized trials, Cancer, 2006, 107, 116–124 CrossRef CAS PubMed.
- E. Temfack and O. Lortholary, Access to flucytosine for the treatment of HIV-associated cryptococcal meningitis in Africa, Lancet Infect. Dis., 2022, 22, 1262–1264 CrossRef CAS PubMed.
- A. Harsanyi, A. Conte, L. Pichon, A. Rabion, S. Grenier and G. Sandford, One-Step Continuous Flow Synthesis of Antifungal WHO Essential Medicine Flucytosine Using Fluorine, Org. Process Res. Dev., 2017, 21, 273–276 CrossRef CAS.
- S. Shinkai, K. Araki, T. Tsubaki, T. Arimura and O. Manabe, New syntheses of calixarene-p-sulphonates and p-nitrocalixarenes, J. Chem. Soc., Perkin Trans. 1, 1987, 2297–2299 RSC.
- Y. Tian, R. Zhang, W. Zhao, S. Wen, Y. Xiang and X. Liu, A New Sulfonic Acid-Functionalized Organic Polymer Catalyst for the Synthesis of Biomass-Derived Alkyl Levulinates, Catal. Lett., 2020, 150, 3553–3560 CrossRef CAS.
- L. M. Mentel and E. J. Baerends, Can the Counterpoise Correction for Basis Set Superposition Effect Be Justified?, J. Chem. Theory Comput., 2014, 10, 252–267 CrossRef CAS PubMed.
- Y. Xie, P. Yuan, T. Heng, L. Du, Q. An, B. Zhang, L. Zhang, D. Yang, G. Du and Y. Lu, Insight into the Formation of Cocrystal and Assembly of Tenoxicam from the Isomer and Conformation, Pharmaceutics, 2022, 14, 1968 CrossRef CAS PubMed.
- Y. Sun, K. Yuan, X. Mo, X. Chen, Y. Deng, C. Liu, Y. Yuan, J. Nie and Y. Zhang, Tyndall-Effect-inspired assay with gold nanoparticles for the colorimetric discrimination and quantification of mercury ions and glutathione, Talanta, 2022, 238, 122999 CrossRef CAS PubMed.
- S. Q. Qin, J. Ma, Q. Q. Wang, W. Xu, W. C. Ye and R. W. Jiang, Identification of Photocatalytic Alkaloids from Coptidis Rhizome by an Offline HPLC/CC/SCD Approach, Molecules, 2022, 27, 6179 CrossRef CAS PubMed.
- S. Shinkai, K. Araki and O. Manabe, NMR Determination of Association Constants for Calixarene Complexes. Evidence for the Formation of a 1:2 Complex with Calix[8]arene, J. Am. Chem. Soc., 1988, 110, 7215–7217 CrossRef.
- S. Q. Qin, W. Xu, W. C. Ye and R. W. Jiang, Structure determination of liquid molecules by encapsulation in an aromatic cavity with hydrogen bonding and enhanced C–H⋯π interactions, CrystEngComm, 2022, 24, 8060–8069 RSC.
- S. Q. Qin, Q. Y. Gan, W. Xu and R. W. Jiang, Hybrid interaction network of guanidinium-biphenyldisulfonic acid for the structure determination of liquid molecules, CrystEngComm, 2022, 24, 4144–4154 RSC.
- L. Cheng, P. Tian, H. Duan, Q. Li, X. Song, A. Li and L. Cao, Chiral adaptive recognition with sequence specificity of aromatic dipeptides in aqueous solution by an achiral cage, Chem. Sci., 2023, 14, 833–842 RSC.
- L. X. Cai, S. C. Li, D. N. Yan, L. P. Zhou, F. Guo and Q. F. Sun, Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis, J. Am. Chem. Soc., 2018, 140, 4869–4876 CrossRef CAS PubMed.
- F. Sha, T. Y. Tai, M. A. Gaidimas, F. A. Son and O. K. Farha, Leveraging Isothermal Titration Calorimetry to Obtain Thermodynamic Insights into the Binding Behavior and Formation of Metal-Organic Frameworks, Langmuir, 2022, 38, 6771–6779 CrossRef CAS PubMed.
- Y. Hong, J. W. Lam and B. Z. Tang, Aggregation-induced emission, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Dai, H. Xue, D. Chen, X. Lou, F. Xia and S. Wang, Aggregation-induced emission luminogens for assisted cancer surgery, Coord. Chem. Rev., 2022, 464, 214552 CrossRef CAS.
- W. Liu, L. Kong, M. Quan, H. Yao, L. Yang, H. Y. Au-Yeung and W. Jiang, Selective recognition of methyl viologen by an endo-functionalized naphthobox, Chin. Chem. Lett., 2022, 33, 4896–4899 CrossRef CAS.
- T. Reangen and S. Salzberg, Repetitive DNA and next-generation sequencing: computational challenges and solutions, Nat. Rev. Genet., 2012, 13, 36–46 CrossRef PubMed.
- K. Xiong, D. Shea, J. Rhoades, T. Blewett, R. Liu, J. H. Bae, E. Nguyen, G. M. Makrigiorgos, T. R. Golub and V. A. Adalsteinsson, Duplex-Repair enables highly accurate sequencing, despite DNA damage, Nucleic Acids Res., 2022, 50, e1 CrossRef CAS PubMed.
- H. Yao, H. Ke, X. Zhang, S. J. Pan, M. S. Li, L. P. Yang, G. Schreckenbach and W. Jiang, Molecular Recognition of Hydrophilic Molecules in Water by Combining the Hydrophobic Effect with Hydrogen Bonding, J. Am. Chem. Soc., 2018, 140, 13466–13477 CrossRef CAS PubMed.
- H. S. Wang, Y. H. Wang and Y. Ding, Development of biological metal–organic frameworks designed for biomedical applications: from bio-sensing/bio-imaging to disease treatment, Nanoscale Adv., 2020, 2, 3788 RSC.
- S. Zhang, H. He, F. Sun, N. Zhao, J. Du, Q. Pan and G. Zhu, A novel adenine-based zinc(II) metal-organic framework featuring the Lewis basic sites for heterogeneous catalysis, Inorg. Chem. Commun., 2017, 79, 55–59 CrossRef CAS.
- H. Cai, Y. X. Wu, Z. Lu, D. Luo, J. X. Sun, G. W. Wu, M. Li, Y. B. Wei, L. M. Zhong and D. Li, Mimicking DNA Periodic Docking Grooves for Adaptive Identification of L-/D-Tryptophan in a Biological Metal-Organic Framework, J. Am. Chem. Soc., 2022, 144, 9559–9563 CrossRef CAS PubMed.
- D. Gao, J. H. Chen, S. Fang, T. Ma, X. H. Qiu, J. G. Ma, Q. Gu and P. Cheng, Simultaneous quantitative recognition of all purines including N6-methyladenine via the host-guest interactions on a Mn-MOF, Matter, 2021, 4, 1001–1016 CrossRef CAS.
- S. Shinkai, K. Araki, T. Matsuda, N. Nishiyama, H. Ikeda, I. Takasu and M. Iwamoto, NMR and crystallographic studies of a p-sulfonatocalix[4]arene-guest complex, J. Am. Chem. Soc., 1990, 112, 9053–9058 CrossRef CAS.
- D. S. Guo and Y. Liu, Supramolecular Chemistry of p-Sulfonatocalix[n]arenes and its Biological Applications, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef CAS PubMed.
- I. Ling, A. N. Sobolev and C. L. Raston, p-Sulfonatocalix[4]arene binding of monovalent, divalent and trivalent metal ions, J. Coord. Chem., 2021, 74, 40–50 CrossRef CAS.
- P. B. Crowley, Protein–Calixarene Complexation: From Recognition to Assembly, Acc. Chem. Res., 2022, 55, 2019–2032 CrossRef CAS PubMed.
- A. Jasat and J. C. Sherman, Carceplexes and Hemicarceplexes, Chem. Rev., 1999, 99, 931–967 CrossRef CAS PubMed.
- S. Mecozzi and J. Rebek Jr., The 55% Solution: A Formula for Molecular Recognition in the Liquid State, Chem. – Eur. J., 1998, 4, 1016–1022 CrossRef CAS.
- G. H. Ning, P. Cui, I. V. Sazanovich, J. T. Pegg, Q. Zhu, Z. Pang, R. J. Wei, M. Towrie, K. E. Jelfs, M. A. Little and A. I. Cooper, Cage inclusion crystals exhibiting guest-enhanced multiphoton harvesting, Chem, 2021, 7, 3135–3170 Search PubMed.
- K. Nakano, K. Sada, Y. Kurozumi and M. Miyata, Importance of Packing Coefficients of Host Cavities in the Isomerization of Open Host Frameworks: Guest-Size-Responsive Isomerization in Cholic Acid Inclusion Crystals with Monosubstituted Benzenes, Chem. – Eur. J., 2001, 7, 209–220 CrossRef CAS PubMed.
- C. G. P. Taylor, S. P. Argent, M. D. Ludden, J. R. Piper, C. Mozaceanu, S. A. Barnett and M. D. Ward, One Guest or Two? A Crystallographic and Solution Study of Guest Binding in a Cubic Coordination Cage, Chem. – Eur. J., 2020, 26, 3054–3064 CrossRef CAS PubMed.
- D. Danielsiek and G. Dyker, An adaptive resorcinarene hemicarcerand, Eur. J. Org. Chem., 2021, 1026–1034 CrossRef CAS.
- A. D. Cardenal and T. R. Ramadhar, Application of Crystalline Matrices for the Structural Determination of Organic Molecules, ACS Cent. Sci., 2021, 7, 406–414 CrossRef CAS PubMed.
- J. Zhou, T. Ke, Y. Song, H. Cai, Z. Wang, Y. Chen, Q. Xu, Z. Zhang, Z. Bao, Q. Ren and Q. Yang, Highly Efficient Separation of C8 Aromatic Isomers by Rationally Designed Nonaromatic Metal–Organic Frameworks, J. Am. Chem. Soc., 2022, 144, 21417–21424 CrossRef CAS PubMed.
- H. Fan, A. Mundstock, A. Feldhoff, A. Knebel, J. Gu, H. Meng and J. Caro, Covalent Organic Framework-Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation, J. Am. Chem. Soc., 2018, 140, 10094–10098 CrossRef CAS PubMed.
- Z. Li and Y. W. Yang, Macrocycle-Based Porous Organic Polymers for Separation, Sensing, and Catalysis, Adv. Mater., 2022, 34, 2107401 CrossRef CAS PubMed.
- J. W. Jordan, A. I. Chernov, G. A. Rance, D. E. Stephen, A. E. Lanterna, F. J. Alves, A. Grüneis, Q. Ramasse, G. N. Newton and A. N. Khlobystov, Host-Guest Chemistry in Boron Nitride Nanotubes: Interactions with Polyoxometalates and Mechanism of Encapsulation, J. Am. Chem. Soc., 2023, 145, 1206–1215 CrossRef CAS PubMed.
- D. E. Braun and U. J. Griesser, Prediction and experimental validation of solid solutions and isopolymorphs of cytosine/5-flucytosine, CrystEngComm, 2017, 19, 3566–3572 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2253871–2253873. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01649h |
| This journal is © the Partner Organisations 2024 |