Visible-light-driven C(sp3)–H alkylation of heterobenzylic amines via electron donor–acceptor complexes†
Yumei
Huo
a,
Beibei
Chen
a,
Zhaoqing
Xu
 *ab,
Xiaoyu
Ren
*a,
Rupeng
Qi
a and
Chao
Wang
*ab,
Xiaoyu
Ren
*a,
Rupeng
Qi
a and
Chao
Wang
 *ab
*ab
aKey Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, China. E-mail: zqxu@lzu.edu.cn; renxy@lzu.edu.cn; wangchao@lzu.edu.cn
bResearch Unit of Peptide Science, Chinese Academy of Medical Sciences, 2019RU066, Lanzhou 730000, China
First published on 17th November 2023
Abstract
Heterobenzylic amines such as α-alkyl- substituted oxadiazole methylamine skeletons present as essential motifs in pharmaceuticals and pesticides, due to their unique biological activities. Photoredox reactions mediated by electron donor–acceptor (EDA) complexes are relatively environmentally friendly, and have attracted much attention in current organic synthesis research. Herein, we report a C–H alkylation of heterobenzylic amines under visible light irradiation, involving an EDA complex formed upon association of alkyl iodide and organophosphines in catalytic amounts.
Introduction
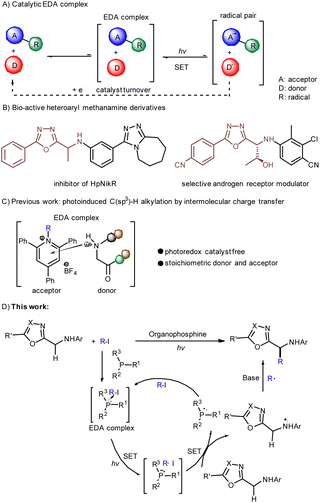
Primary amines, particularly heterobenzylic amines (HBAs), have been intensively investigated due to their widespread presence in many pharmaceuticals, agrochemicals,1 dyes, and materials. Consequently, synthetic organic chemists have a long-standing interest in general methods for the synthesis of HBAs. Although many strategies have been looked into, including the reduction of imines,2 enamines3 and N-heteroarenes, hydroamination of alkenes, addition of amines to a Michael acceptor, allylic amination, insertion of nitrenes into C–H bonds, etc., they are mostly indirect methods for obtaining HBAs and require metal catalysis,4,5 which limits their future applications and development.In recent years, photoredox-promoted radical coupling reactions have been extensively studied due to their mild reaction conditions and excellent functional group tolerance.6 In this area, photoredox reactions mediated by electron donor–acceptor (EDA) complexes show great potential to unlock reaction manifolds that are unavailable using conventional thermal methods.7 In general, exogenous photosensitizers are required for the electron transfer (ET) processes in photosensitizer-catalyzed redox reactions. Note in particular that the photoreactions promoted by EDA complexes rely on a new molecular aggregate formed by an electron-rich donor substrate (D) and an electron-deficient acceptor molecule (A), with this aggregate contributing to the intermolecular charge transfer event under photoirradiation without the assistance of an extra photoredox catalyst.8 The classical EDA photochemical approaches are based on the intermediates involved in EDA complex formation with stoichiometric amounts of donor and acceptor, where both components end up in the product structure.9 According to further research, the donor or acceptor can also act as a catalyst to promote the radical coupling between an electron-deficient or electron-rich substrate and another substrate, decoupling the complexation/photoactivation steps from the substrate functionalization10,11 (Scheme 1A).
1,3,4-Oxadiazole and its derivatives are widely used in pesticides, pharmaceuticals and materials due to their unique biological and optical activities. Notably, we further found that a specific 5-aryl-1,3,4-oxadiazole-2-methylamine skeleton plays a particularly important role in many pharmacologically active molecules, including Helicobacter pylori nickel response regulator (NikR),12 the selective androgen receptor modulator (SARM),13 and others.14,15 (Scheme 1B). In 2021, Huo and co-workers described a photocatalytic α-C(sp3)–H alkylation of 5-aryl-1,3,4-oxadiazole-2-methylamines, which required the addition of copper as a photoredox catalyst to reduce the redox-active esters via single electron transfer (SET).16 Despite this progress, the transition-metal-free strategy for this transformation, while highly desirable, has still not been realized.
Very recently, we disclosed a visible-light-promoted deaminative C(sp3)–H alkylation of glycine by EDA-complex-mediated radical–radical cross-coupling, with this process using glycine as a donor and alkyl pyridinium salts as an acceptor to achieve the preparation of unnatural α-amino acids and modification of peptides17 (Scheme 1C). Inspired by this part of our work, we attempted to construct a modified EDA-mediated system consisting of an electron-deficient acceptor in association with a catalytic donor through electron transfer to generate radicals involved in the subsequent coupling reactions, for a proper design and synthesis of 5-aryl-1,3,4-oxadiazole-2-methylamines.
Compared with the frequently used alkyl radical precursors (redox-active esters18,19 and Katritzyl salts17), alkyl halides constitute a class of alkylation reagents with higher atom economy, and are abundant industrial feedstocks with annual global production on the order of tons.20–22 However, alkyl halides are rarely involved in EDA complex reactions as electron acceptors.23 Organophosphines are widely used as ligands due to their electron-donating ability in transition-metal-catalyzed reactions. Recently, several groups have presented the visible-light-promoted transformation of triarylphosphine as an SET medium.9,24 Inspired by these pioneering works, we proposed SETs of electron-deficient alkyl iodides with catalytic amounts of organophosphine donors, with 5-aryl-1,3,4-oxadiazole-2-methylamines (HBAs) each undergoing a one-electron reduction to regenerate the corresponding donor in a turnover event, and in the meantime with HBAs of interest trapping the resulting alkyl radical (Scheme 1D). This protocol readily facilitated visible-light-promoted formation of α-C–H-functionalized HBAs without the assistance of an extra photoredox catalyst.
Results and discussion
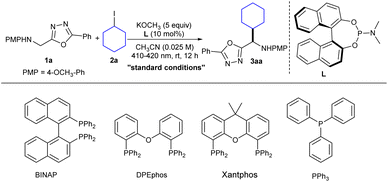
On the basis of our hypothesis, we studied the feasibility of using organophosphines as catalytic donors in visible-light-mediated EDA complexes to C(sp3)–H alkylation of heterobenzylic amines. To our delight, the desired product was obtained in 85% yield when using 5-phenyl-1,3,4-oxadiazole-2-methylamine (1a) and iodocyclohexane (2a, 4 equiv.) as the substrates, (11bS)-N,N-dimethyldinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-amine (L, 10 mol%) (which is less expensive than the racemic material) as the catalytic donor, KOCH3 (5 equiv.) as an additive providing an alkaline environment, dry CH3CN as solvent, and visible light irradiation (24 W, 410–420 nm) at room temperature for 12 hours (Table 1, entry 1, see the ESI† for details). First, the control experiment showed that organophosphine, KOCH3 and photoirradiation were indispensable for this reaction (entries 2–5). Replacing L with another tertiary amine electron donor, namely DIPEA, led to a significantly lower yield11,25 (entry 6), which indicated that organophosphines were more suitable electron donors than were tertiary amines. Substitution of L with BINAP, DPEphos, Xantphos or triphenyl phosphorus, frequently used electron donors, resulted in dramatic decreases in yield (entries 7–10). Other alkyl halides, namely chlorocyclohexane and bromocyclohexane, were tested, and the results revealed that iodocyclohexane was optimal (entries 11 and 12). The high BDE values of C–Cl and C–Br bonds in the alkyl substrates were apparently responsible for their low reactivities.26| Entry | Change from the “standard conditions” | Yieldb (%) |
|---|---|---|
| a Conditions 1a (0.05 mmol), 2a (0.2 mmol), L (10 mol%), base (0.25 mmol), solvent (2 mL), Ar, 12 h, r.t., and under visible light (410–420 nm). b Yield was determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. | ||
| 1 | Standard conditions | 85 |
| 2 | Without L | Trace |
| 3 | Without KOCH3 | N.D. |
| 4 | Without L and KOCH3 | N.D. |
| 5 | Without hv | N.D. |
| 6 | DIPEA instead of L | 15 |
| 7 | BINAP instead of L | 30 |
| 8 | DPEphos instead of L | 46 |
| 9 | Xantphos instead of L | 53 |
| 10 | PPh3 instead of L | 13 |
| 11 | Chlorocyclohexane instead of iodocyclohexane | N.D. |
| 12 | Bromocyclohexane of instead iodocyclohexane | 32 |
With the optimal reaction conditions established, the substrate scopes of the reaction were investigated (Scheme 2). We first examined the heteroaryl methylamine compounds, with various substituents including electron-donating and electron-withdrawing groups at the phenyl p- or m-position; they provided products with good to excellent yields under standard conditions (3ba–ia, 70–85%). Note that the desired product was obtained with the yield of 85% when the 5-benzene para position was substituted with a strongly electron-withdrawing group (3ha). In addition, using other aromatics, namely naphthalene, furan, or thiophene, instead of the 5-phenyl worked well under the standard conditions (3ja–la, 80–83%). Notably, the oxazoles with sulfur-containing aliphatic groups (3ma, 77% and 3na, 63%) and a glucose derivative (3oa, 71%) were successfully obtained with acceptable yields. Various heterobenzylic amines are widely present in natural products and drug molecules due to their biological activity. Therefore, the modification of different types of heterobenzylic amines has become particularly important. And fortunately, reactions of many other heterocycles, namely oxazole, thiazole, pyridine and benzoxazole compounds, all proceeded smoothly with uniformly satisfactory yields (3pa–sa, 65–80%).
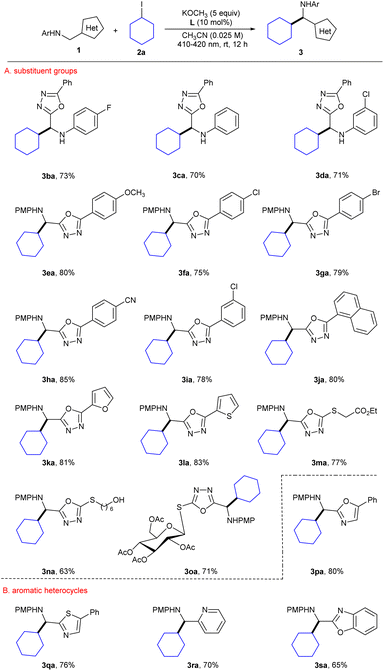 | ||
| Scheme 2 Substrate scope with respect to heteroaryl methanamines. All reactions were conducted on a scale of 0.2 mmol. Yields refer to isolated yields. | ||
We next focused our investigation of substrate scope on alkyl iodides. The reactions were successfully applied to various unactivated alkyl iodides (Scheme 3). Primary alkylation (3ab–ad, 51–70%), secondary alkylation (3aa, 3ae–3ag, 82–85%), and tertiary alkylation (3ah, 80% and 3ai, 66%) were all applicable with satisfactory results. Importantly, alkyl iodides bearing halogen (3ad) and heterocycle (3af and 3ag) functional groups all reacted smoothly. In addition, iodomethane was also investigated as an alkyl source, but no methylated product was obtained. Furthermore, the reaction could be conducted on scales of 1.5 and 3.5 mmol to provide the product in acceptable yields (3aa).
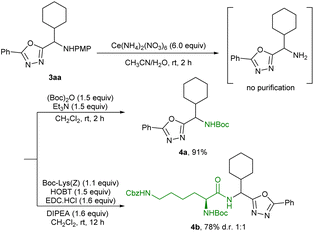
Synthetic applications of α-alkyl HBAs were demonstrated (Scheme 4). The PMP group of 3aa was easily removed by subjecting it to oxidation with excess ammonium cerium nitrate and the remaining material was applied in subsequent transformations without further purification. For example, the PMP protecting group in 3aa was converted to the frequently-used protecting groups –Boc (4a). Notably, in the presence of 1-hydroxybenzotriazole (HOBT), 1-(3-dimethylaminopr-opyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) and N,N-diisopropylethylamine (DIPEA), 3aa was directly applied for the preparation of the unnatural α-amino acid 4b, which provided more possibilities for producing peptide drugs containing unnatural α-amino acid moieties.
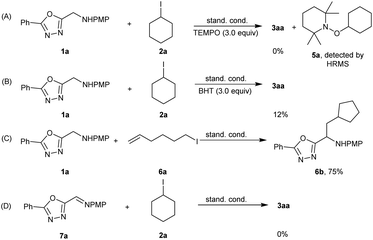
To gain further insight into the reaction mechanism, a series of mechanistic studies were conducted (see the ESI† for details). First, radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO, 3.0 equiv.) was added to the reaction solution under the standard conditions; this addition significantly suppressed the transformation, and the radical trapping intermediate 5a was detected using HRMS (Scheme 5A), which revealed the involvement of a radical intermediate during the reaction process. Addition of butylated hydroxytoluene (BHT, 3.0 equiv.) led to a dramatic decrease of the yield (Scheme 5B, 12%). To confirm that the mechanism involved a radical pathway, we also proceeded to carry out a radical clock experiment using 6-iodohex-1-ene (6a) as the reaction partner. Here, the ring-closing product 6b was obtained under standard conditions (Scheme 5C). When (E)-4-methoxy-N-((5-phenyl-1,3,4-oxadiazol-2-yl)methylene)aniline (7a) was used instead of 4-methoxy-N-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)aniline (1a), no 3aa was detected, which indicated that 7a was not formed as the intermediate for this transformation (Scheme 5D).
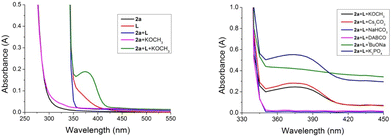
To clarify the mechanism of this photo-induced intermolecular charge transfer, experiments involving the use of UV/Vis absorption spectroscopy to identify the presence of an intermolecular EDA complex were first conducted (see the ESI† for details). As shown in Scheme 6 (left), after mixing of iodocyclohexane (2a), organophosphine (L) and KOCH3 in CH3CN, the maximum absorption in the optical absorption spectrum underwent a bathochromic shift to the visible region. This mixture was visibly yellow, confirming the formation of a charge-transfer molecular aggregate.8,27 Surprisingly, we found significantly different absorptions with the addition of different bases, and note that the participation of some bases such as NaHCO3 and DABCO did not obviously change the absorption and did not promote the reaction either, which indicated the crucial roles of KOCH3 (Scheme 6, right). Based on these experimental results, we hypothesized that the base might play two roles in the reactions: (1) promoting the formation of an EDA complex between iodoalkane and organophosphine; and (2) providing a basic condition to facilitate the deprotonation of heterobenzylic amine.28
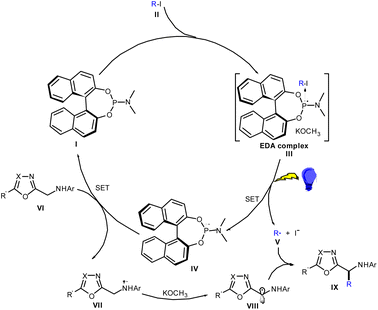
On the basis of the above-described investigation, a mechanism was derived, and is depicted in Scheme 7. According to this mechanism, first a colored EDA complex was formed in the presence of I and II (with a ternary EDA complex perhaps forming in the presence of KOCH3). Under violet LED (410–420 nm) irradiation, an SET created a radical pair of alkyl radical V and radical cation IV. Then, the reduced heterobenzylic VI and IV underwent single-electron transfer to obtain intermediate VII, while the formation of organophosphine I completed the catalytic cycle. The radical cation VII was deprotonated under basic conditions to provide the stable a-carbon radical VIII. Finally, according to the proposed mechanism, radical–radical cross-coupling between V and VIII provided the alkylation product IX.
Conclusions
In conclusion, we have disclosed a visible-light-promoted C(sp3)–H alkylation of HBAs occurring via ground-state EDA associations between organophosphines and iodoalkane. The reactions were made effective for the introduction of all kinds of alkyl groups by directly using the alkyl iodides, without the assistance of an extra photoredox catalyst, a feature providing more possibilities for the modification of heterobenzylic amines and laying a foundation for the subsequent research and development of new drugs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the NSFC (no. 21971098, 22001105, 22201112, 22271126 and 22301114), Innovation Project of Medicine and Health Science and Technology of Chinese Academy of Medical Sciences (no. 2019-I2M-5-074), Natural Science Foundation of Gansu Province (no. 23JRRA1091 and 23JRRA1147), China Postdoctoral Science Foundation (no. 2023TQ0143) and Key R&D Project of Gansu Province (no. 22YF7WA010).References
- Top 200 Small Molecule Drugs by Sales in 2018, a poster made by J. T. Njardarson, group. https://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster.
- T. Irrgang and R. Kempe, Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen, Chem. Rev., 2020, 120, 9583–9674 CrossRef CAS PubMed.
- T. C. Nugent and M. El-Shazly, Chiral Amine Synthesis-Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction, Adv. Synth. Catal., 2010, 352, 753–819 CrossRef CAS.
- M. T. Pirnot, Y. M. Wang and S. L. Buchwald, Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes, Angew. Chem., Int. Ed., 2016, 55, 48–57 CrossRef CAS PubMed.
- A. Trowbridge, S. M. Walton and M. J. Gaunt, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines, Chem. Rev., 2020, 120, 2613–2692 CrossRef CAS PubMed.
- S. P. Pitre and L. E. Overman, Strategic Use of Visible-Light Photoredox Catalysis in Natural Product Synthesis, Chem. Rev., 2022, 122, 1717–1751 CrossRef CAS PubMed.
- G. S. Lima, T. de M. Lima, M. Duarte, I. D. Jurberg and M. W. Paixão, Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications, ACS Catal., 2016, 6, 1389–1407 CrossRef.
- Q. Guo, M. Wang, H. Liu, R. Wang and Z. Xu, Visible–Light–Promoted Dearomative Fluoroalkylation of β–Naphthols through Intermolecular Charge Transfer, Angew. Chem., Int. Ed., 2018, 57, 4747–4751 CrossRef CAS PubMed.
- G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS PubMed.
- M. C. Fu, R. Shang, B. Zhao, B. Wang and Y. Fu, Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide, Science, 2019, 363, 1429–1434 CrossRef CAS PubMed.
- H. Xu, X. Li, Y. Dong, S. Ji, J. Zuo, J. Lv and D. Yang, Thianthrenium-Enabled Phosphorylation of Aryl C–H Bonds via Electron Donor–Acceptor Complex Photoactivation, Org. Lett., 2023, 25, 3784–3789 CrossRef CAS PubMed.
- A. Segura-Cabrera, X. Guo, A. Rojo-Dominguez and M. A. Rodriguez-Perez, Integrative Computational Protocol for the Discovery of Inhibitors of the Helicobacter Pylori Nickel Response Regulator (NikR), J. Mol. Model., 2011, 17, 3075–3084 CrossRef CAS PubMed.
- P. Miller, M. Shomali, C. R. Lyttle, L. S. O'Dea, H. Herendeen, K. Gallacher, D. Paquin, D. R. Compton, B. Sahoo, S. A. Kerrigan, M. S. Burge, M. Nickels, J. L. Green, J. A. Katzenellenbogen, A. Tchesnokov and G. Hattersley, Design, Synthesis, and Preclinical Characterization of the Selective Androgen Receptor Modulator (SARM) RAD140, ACS Med. Chem. Lett., 2011, 2, 124–129 CrossRef PubMed.
- V. Padmavathi, G. D. Reddy, S. N. Reddy and K. Mahesh, Synthesis and Biological Activity of 2-(bis((1,3,4-oxadiazolyl/1,3,4thiadiazolyl)methylthio)methylene)malononitriles, Eur. J. Med. Chem., 2011, 46, 1367–1373 CrossRef CAS PubMed.
- G. D. Reddy, S. J. Park, H. M. Cho, T. J. Kim and M. E. Lee, Antiallergic Activity Profile in Vitro RBL-2H3 and in Vivo Passive Cutaneous Anaphylaxis Mouse Model of New Sila-Substituted 1,3,4-Oxadiazoles, J. Med. Chem., 2012, 55, 6438–6444 CrossRef PubMed.
- P. Niu, J. Yang, Y. Yuan, Y. Zhang, C. Zhou, X. Bao and C. Huo, Photocatalyzed Redox-Neutral Decarboxylative Alkylation of Heteroaryl Methanamines, Green Chem., 2021, 23, 774–779 RSC.
- C. Wang, R. Qi, H. Xue, Y. Shen, M. Chang, Y. Chen, R. Wang and Z. Xu, Visible-Light-Promoted C(sp3)–H Alkylation by Intermolecular Charge Transfer: Preparation of Unnatural α-Amino Acids and Late-Stage Modification of Peptides, Angew. Chem., Int. Ed., 2020, 59, 7461–7466 CrossRef CAS PubMed.
- R. Qi, C. Wang, Y. Huo, H. Chai, H. Wang, Z. Ma, L. Liu, R. Wang and Z. Xu, Visible Light Induced Cu-Catalyzed Asymmetric C(sp3)-H Alkylation, J. Am. Chem. Soc., 2021, 143, 12777–12783 CrossRef CAS PubMed.
- C. Wang, M. Guo, R. Qi, Q. Shang, Q. Liu, S. Wang, L. Zhao, R. Wang and Z. Xu, Visible-Light-Driven, Copper-Catalyzed Decarboxylative C(sp3)-H Alkylation of Glycine and Peptides, Angew. Chem., Int. Ed., 2018, 57, 15841–15846 CrossRef CAS PubMed.
- K. Weissermel and H.-J. Arpe, Industrial organic chemistry, Wiley Online Library, New York, 2003 Search PubMed.
- W. Liu, L. Li and C. J. Li, Empowering a Transition-Metal-Free Coupling between Alkyne and Alkyl Iodide with Light in Water, Nat. Commun., 2015, 6, 6526 CrossRef CAS PubMed.
- C. Wang, Y. Lei, M. Guo, Q. Shang, H. Liu, Z. Xu and R. Wang, Photoinduced, Copper-Promoted Regio- and Stereoselective Decarboxylative Alkylation of α,β-Unsaturated Acids with Alkyl Iodides, Org. Lett., 2017, 19, 6412–6415 CrossRef CAS PubMed.
- L.-J. Cheng and N. P. Mankad, C–C and C–X Coupling Reactions of Unactivated Alkyl Electrophiles using Copper Catalysis, Chem. Soc. Rev., 2020, 49, 8036–8064 RSC.
- H. Lu, D.-Y. Wang and A. Zhang, Visible Light-Promoted Phosphine-Catalyzed Difluoroalkylation of Arenes and Heterocycles, J. Org. Chem., 2020, 85, 942–951 CrossRef CAS PubMed.
- A. Dewanji, L. van Dalsen, J. A. Rossi-Ashton, E. Gasson, G. E. M. Crisenza and D. J. Procter, A General Arene C–H Functionalization Strategy via Electron Donor–Acceptor Complex Photoactivation, Nat. Chem., 2023, 15, 43–52 CrossRef CAS PubMed.
- X. Y. Wang, Y. Q. He, Y. Zhou, L. Lu, X. R. Song, Z. Z. Zhou, W. F. Tian and Q. Xiao, Photoalkylation/-arylation of ortho-Diketones with Unactivated Organic Halides, Org. Lett., 2023, 25, 3847–3852 CrossRef CAS PubMed.
- C. Liu, N. Shen and R. Shang, Photocatalytic Decarboxylative Alkylation of Silyl Enol Ether and Enamide with N-(acyloxy)phthalimide using Ammonium Iodide, Org. Chem. Front., 2021, 8, 4166–4170 RSC.
- J. Xie, J. Yu, M. Rudolph, F. Rominger and A. S. K. Hashmi, Monofluoroalkenylation of Dimethylamino Compounds through Radical–Radical Cross–Coupling, Angew. Chem., Int. Ed., 2016, 55, 9416–9421 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, optimization details, experimental procedures, characterization data of all compounds, and mechanistic studies. See DOI: https://doi.org/10.1039/d3qo01765f |
| This journal is © the Partner Organisations 2024 |

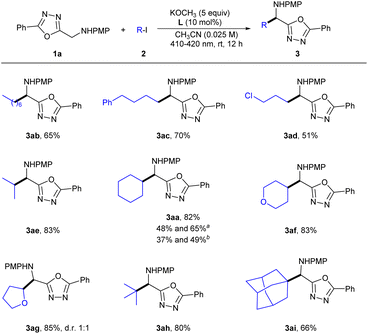
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 mmol after 12 and 36 h, respectively.
1.5 mmol after 12 and 36 h, respectively.