Silver-mediated synthesis of 1,4-dihydropyridine sulfones via [4 + 2] cyclization of N-allenylsulfonamides and enaminones with a 1,3-sulfonyl shift†
Shuguang
Zhou
a,
Shuang-Feng
Dong
a,
Xin
Zhang
a,
Shen-Yuan
Zhang
c,
Teck-Peng
Loh
 *bd and
Jie-Sheng
Tian
*bd and
Jie-Sheng
Tian
 *a
*a
aSchool of Chemistry and Chemical Engineering, Northwestern Polytechnical University (NPU), Xi'an 710072, China. E-mail: tjs@nwpu.edu.cn
bCollege of Advanced Interdisciplinary Science and Technology, Henan University of Technology, Zhengzhou, 450001, China
cSchool of Chemistry and Molecular Engineering, Nanjing Tech University (NanjingTech), Nanjing 211816, China
dSchool of Chemistry, Chemical Engineering and Biotechnology (CCEB), Nanyang Technological University, Singapore 637371, Singapore. E-mail: teckpeng@ntu.edu.sg
First published on 14th November 2023
Abstract
This study outlines the development of an innovative tandem reaction involving [4 + 2] cyclization with 1,3-sulfonyl migration from N-allenylsulfonamides and enaminones, expanding the scope of synthetic methodologies for multifunctional 1,4-dihydropyridine derivatives. The outlined approach furnishes a broad spectrum of sulfonyl-substituted 1,4-dihydropyridine derivatives with good to excellent yields, which can be subsequently converted into diverse sulfonyl-substituted compounds such as pyridine, tetrahydropyridine, and piperidine derivatives through simplified operation. The methodological advancements achieved in this research not only demonstrate the versatility and applicability of the synthesized derivatives across varied substrates and conditions but also present promising potential for the development and diversification of unsymmetrical 1,4-dihydropyridine sulfones.
Introduction
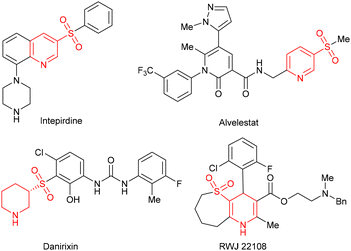
(Hydro)pyridine sulfone derivatives are important structural frameworks found in many biologically active molecules.1 Some representative examples include the 5-HT6 receptor antagonist intepirdine,2 neutrophil elastase inhibitor alvelestat,3 CXCR2 antagonist danirixin,4 and active structure RWJ 221085 (Fig. 1). Among these N-heterocycles, the synthesis of 1,4-dihydropyridines (1,4-DHPs) has attracted wide attention in view of their feasibility for further functional modification.6 For their preparation, the Hantzsch condensation proves to be an effective method leading to symmetrical 1,4-DHPs.7 In order to enrich the structure of 1,4-DHPs and better regulate their activities, recent efforts have focused on the synthesis of unsymmetrical structures tethered by a functional group,8 such as β-sulfonylation of piperidine9 and [4 + 2] annulation of N-tosylated α,β-imides and aldehydes (Scheme 1a).10 Despite advancements, constructing unsymmetrical 1,4-DHPs with active groups remains a high-priority objective.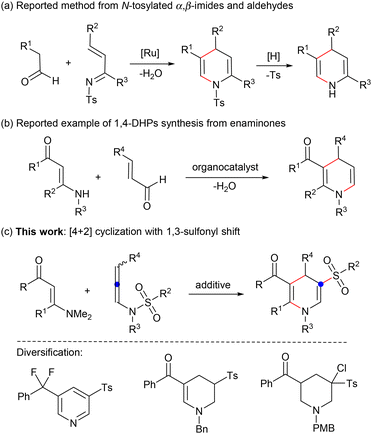 | ||
| Scheme 1 Design of [4 + 2] cyclization of N-allenylsulfonamides and enaminones with a 1,3-sulfonyl shift. | ||
Concurrently, enaminones bearing the active carbonyl and amino groups show unique reactivities in organic transformations,11 especially for the installation of N-heterocycles through C–H activation.12 Nevertheless, reports on the synthesis of 1,4-DHPs from enaminones are still scarce (Scheme 1b).13 Hence, it is still of great significance to construct functionalized 1,4-DHPs with enaminones. Inspired by the interesting N- to C-sulfonyl migration,14 we envisage that [4 + 2] cascade cyclization of N-allenylsulfonamides15 and enaminones with a 1,3-sulfonyl shift may be achieved for the synthesis of 1,4-dihydropyridine sulfones (Scheme 1c). If successful, a diversity of functionalized pyridine, tetrahydropyridine, and piperidine derivatives containing a sulfone group can be obtained through simple conversion.
Herein, we describe an Ag-promoted tandem reaction involving 1,3-sulfonyl migration/[4 + 2] cycloaddition to synthesize 1,4-dihydropyridine sulfones. This approach employs the easily prepared N-allenylsulfonamides and enaminones as the raw materials, showcasing the advantages of wide substrate scope and easy derivatization to pyridine, tetrahydropyridine and piperidine substituted with a sulfonyl group.
Results and discussion
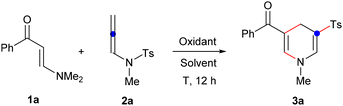
To validate this hypothesis, we initiated our synthetic exploration by investigating the reaction between enaminone 1a and N-allenylsulfonamide 2a (Table 1). Employing [Cp*RhCl2]2 as a catalyst and AgOAc as the additive in DCE at 100 °C for 12 hours resulted in the formation of the desired 1,4-dihydropyridine sulfone 3a with an 86% yield16 (entry 1). Interestingly, we observed that the reaction could also proceed without [Cp*RhCl2]2 (entry 2), but no product formation occurred without AgOAc (entry 3). Subsequently, we tested various additives including copper acetate, silver carbonate, and silver trifluoroacetate, but these led to either no product or products in low yields (entries 4–6). Reducing the quantity of silver acetate to 1.0 equivalent yielded only 65% of the product (entry 7). Temperature proved crucial and lowering it to 80 °C resulted in only a trace amount of 3a (entry 8). Attempts with different solvents such as acetonitrile and 1,4-dioxane could not improve the yield (entries 9 and 10). Thus, the optimized conditions are identified as follows: enaminones reacting with 1.5 equivalents of N-allenylsulfonamides in DCE at 100 °C for 12 hours, facilitated by 2.0 equivalents of silver acetate.| Entry | Additive (equiv.) | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: enaminone 1a (0.4 mmol, 1.0 equiv.), N-allenylsulfonamide 2a (0.6 mmol, 1.5 equiv.), additive, and solvent (1.5 mL) at a specified temperature for 12 h. b Isolated yields based on 1a. c [Cp*RhCl2]2 (2.0 mol%). | ||||
| 1c | AgOAc (2.0) | DCE | 100 | 86 |
| 2 | AgOAc (2.0) | DCE | 100 | 88 |
| 3 | — | DCE | 100 | 0 |
| 4 | Cu(OAc)2 (2.0) | DCE | 100 | Trace |
| 5 | Ag2CO3 (2.0) | DCE | 100 | 30 |
| 6 | AgTFA (2.0) | DCE | 100 | 28 |
| 7 | AgOAc (1.0) | DCE | 100 | 65 |
| 8 | AgOAc (2.0) | DCE | 80 | Trace |
| 9 | AgOAc (2.0) | MeCN | 100 | 80 |
| 10 | AgOAc (2.0) | 1,4-Dioxane | 100 | 21 |
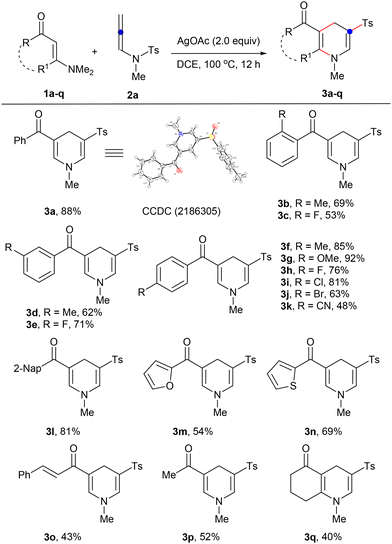
This reaction exhibited remarkable versatility. Both aromatic and alkyl-substituted, linear and cyclic enaminones led to a diverse array of 1,4-dihydropyridine sulfones 3a–q, with yields ranging from 40–92% (Scheme 2). X-ray crystallography validated the structure of 3a. For phenyl-substituted enaminones, every isomer, ortho-, meta-, and para-substituted, responded well, with the para-substituted forms outperforming due to presumed lower steric hindrance (1fvs.1b, 1d, 1hvs.1c, 1e). Electron-donating groups such as methyl in the ortho position (1b) performed better than those in the meta position (1d), whereas electron-withdrawing groups such as fluorine showed an inverse relationship. Various aromatic substituents and alkyl groups also demonstrated compatibility, delivering products 3l–p in 43–81% yields. Notably, cyclic enaminone 1q proved to be an effective substrate, achieving the target tetrahydroquinolin-5(1H)-one 3q in 40% yield. The versatility and compatibility of the substrates underscored the broad applicability of this methodology.
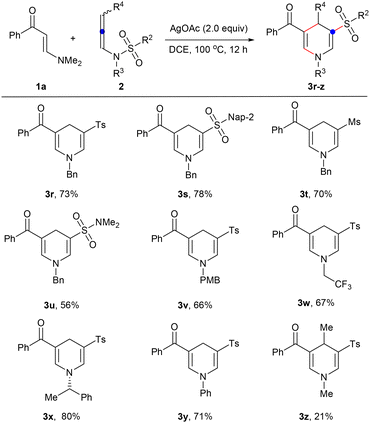
Next, the scope of N-allenylsulfonamides was expanded under the optimized reaction conditions (Scheme 3). First, different N-sulfonyl groups were investigated, and it was found that simple phenyl 2b, fused naphthyl 2c, alkyl 2d, and dimethylamino 2e substituted sulfonyl groups could successfully migrate to produce various 1,4-dihydropyridine sulfones 3r–u in 56–78% yields. Then, a variety of N-substituents such as methyl 2a, easily removable benzyl 2f and p-methoxybenzyl 2g, functionalized trifluoroethyl 2h, chiral secondary carbon chain 2i, and aromatic ring 2j have also been studied and can be well converted into the desired products 3a, 3s, 3v–y. Finally, internal N-allenylsulfonamide 2k has also been attempted, and it is gratifying that 4-substituted 1,4-dihydropyridine 3z has also been isolated in a yield of 21%.
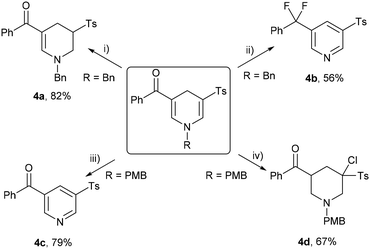
Given the multifunctional nature of unsymmetrical 1,4-dihydropyridine sulfones, characterized by unsaturated structures, diverse double bonds, and carbonyl groups, we delved deeper into exploring the potential diversification of these compounds. Initially, a 5 mmol scale reaction yielded N-benzyl-protected 3r in 81% yield. Subsequent conversions of 3r and 3v are outlined in Scheme 4. Triethylsilane selectively reduced the sulfonyl group-bearing double bond in trifluoroacetic acid (TFA)17 to yield tetrahydropyridine sulfone 4a in 82% yield (Scheme 4(i)). Remarkably, treatment of 3r with N,N-diethylaminosulfur trifluoride (DAST) in air at room temperature18 resulted in deoxofluorinated, debenzylated, and dehydrocyclized pyridine 4b (Scheme 4(ii)). Replacing the N-substituent of 1,4-dihydropyridine sulfone with p-methoxybenzyl facilitated the synthesis of pyridine 4c, achieving 79% yield in TFA in air at room temperature19 (Scheme 4(iii)). Finally, the reaction of 3v with N-chlorosuccinimide (NCS), followed by the addition of borane, resulted in chlorinated piperidine 4d in 67% yield9 (Scheme 4(iv)).
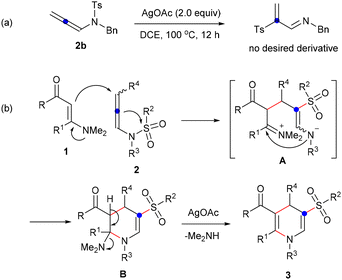
To investigate whether a 1,3-sulfonyl shift occurred first, enaminone 1 was omitted from the reaction system, revealing no detection of any 2-tosylprop-2-en-1-imine derivative (Scheme 5a). Consequently, based on the observed results and relevant reports,20 a proposed reaction process for the formation of 1,4-dihydropyridine sulfones is illustrated in Scheme 5b. With the assistance of AgOAc, the addition of enaminone 1 to N-allenylsulfonamide 2, accompanied by a 1,3-sulfonyl shift, produced zwitterionic species A. This was followed by intramolecular nucleophilic cyclization yielding tetrahydropyridine B containing an N,N-dimethyl group. Ultimately, 1,4-dihydropyridine sulfone 3 was formed through the elimination of dimethylamine, facilitated by the assistance of silver acetate.
Conclusions
In conclusion, we have successfully developed an efficient and practical tandem reaction strategy involving [4 + 2] cyclization with 1,3-sulfonyl migration from N-allenylsulfonamides and enaminones, contributing significantly to the synthesis of multifunctional compounds. This approach has enabled the synthesis of a diverse array of sulfonyl-substituted 1,4-dihydropyridine derivatives in good to excellent yields. These derivatives present vast possibilities for further conversion into structurally diverse compounds such as sulfonyl-substituted pyridine, tetrahydropyridine, and piperidine derivatives through simple operation. The systematic synthetic exploration carried out in this study has demonstrated substantial versatility and applicability across varying substrates and conditions, underlining the potential for further exploration and diversification of unsymmetrical 1,4-dihydropyridine sulfones. The continuation of constructing functionalized 1,4-dihydropyridines through multi-component cascade reactions and their applicability in drug synthesis are the current focal points of ongoing investigations in our laboratory.Author contributions
S. Zhou, S.-F. Dong, X. Zhang, and S.-Y. Zhang performed the experiments. S. Zhou analyzed the experimental data. J.-S. Tian conceived the project and drafted the paper. J.-S. Tian and T.-P Loh supervised the project and reviewed the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Fundamental Research Funds for the Central Universities (G2020KY0501, D5000210701), the Natural Science Basic Research Plan of Shaanxi Province (2023-JC-YB-109), and the grant of MOE-Tier 1 (M4012045.110 RG12/18-(S)) for the generous financial support.References
- (a) Y. Ozols, B. Vigante and G. Duburs, 1,4-Dihydro-pyridines Containing Sulfur, Chem. Heterocycl. Compd., 1995, 30, 1386–1400 CrossRef; (b) K. Miyashita, M. Nishimoto, T. Ishino, H. Murafuji, S. Obika, O. Muraoka and T. Imanishi, Studies on Novel and Chiral 1,4-Dihydropyridines. V, Hantzsch-type 1,4-Dihydropyridines Having a Chiral Sulfinyl Group: Syntheses, Structures, and Biological Activity as a Calcium Channel Antagonist, Tetrahedron, 1997, 53, 4279–4290 CrossRef CAS; (c) O. Elso, G. G. Linares and V. Sulsen, Current Status on 1,4-Dihydropyridine Derivatives against Human Pathogenic Parasites, Curr. Med. Chem., 2023, 30, 1689–1711 CrossRef CAS PubMed; (d) R. Davis, J. R. Kern, L. J. Kurz and J. R. Pfister, Enantioselective Synthesis of Dihydropyridine Sulfones, J. Am. Chem. Soc., 1988, 110, 7873–7874 CrossRef CAS; (e) V. D. Nguyen, V. T. Nguyen, G. C. Haug, H. T. Dang, H. D. Arman, W. C. Ermler and O. V. Larionov, Rapid and Chemodivergent Synthesis of N-Heterocyclic Sulfones and Sulfides: Mechanistic and Computational Details of the Persulfate-Initiated Catalysis, ACS Catal., 2019, 9, 4015–4024 CrossRef CAS; (f) S. R. Wisniewski, J. M. Stevens, M. Yu, K. J. Fraunhoffer, E. O. Romero and S. A. Savage, Utilizing Native Directing Groups: Synthesis of a Selective IKur Inhibitor, BMS-919373, via a Regioselective C-H Arylation, J. Org. Chem., 2019, 84, 4704–4714 CrossRef CAS PubMed.
- (a) J. Rychtyk, A. Partyka, J. Gdula-Argasińska, K. Mysłowska, N. Wilczyṅska, M. Jastrzębska-Więsek and A. Wesołowska, 5-HT6 Receptor Agonist and Antagonist Improve Memory Impairments and Hippocampal BDNF Signaling Alterations Induced by MK-801, Brain Res., 2019, 146375 CrossRef CAS PubMed; (b) C. K. Callaghan, V. Hok, A. Della-Chiesa, D. J. Virley, N. Upton and S. MōMara, Age-related Declines in Delayed Non-match-to-sample Performance (DNMS) are Reversed by the Novel 5HT6 Receptor Antagonist SB742457, Neuropharmacology, 2012, 63, 890–897 CrossRef CAS PubMed; (c) X. Codony, J. M. Vela and M. J. Ramírez, 5-HT6 Receptor and Cognition, Curr. Opin. Pharmacol., 2011, 11, 94–100 CrossRef CAS PubMed.
- T. Stevens, K. Ekholm, M. Gränse, M. Lindahl, V. Kozma, C. Jungar, T. Ottosson, H. Falk-Håkansson, A. Churg, J. L. Wright, H. Lal and A. Sanfridson, AZD9668: Pharmacological Characterization of a Novel Oral Inhibitor of Neutrophil Elastase, J. Pharmacol. Exp. Ther., 2011, 339, 313–320 CrossRef CAS PubMed.
- B. E. Miller, S. Mistry, K. Smart, P. Connolly, D. C. Carpenter, H. Cooray, J. C. Bloomer, R. Tal-Singer and A. L. Lazaar, The Pharmacokinetics and Pharmacodynamics of Danirixin (GSK1325756) - A Selective CXCR2 Antagonist - in Healthy Adult Subjects, BMC Pharmacol. Toxicol., 2015, 16, 18–30 CrossRef PubMed.
- J. H. Dodd, C. F. Schwender, J. B. J. Moore, D. M. Ritchie, Y. Gray-Nunez, D. Loughney, T. Kirchner, W. C. Miller and S. Mockoviak, Design and Discovery of RWJ 22108 - A Novel Bronchoselective Calcium Channel Blocker, Drug Des. Discovery, 1998, 15, 135–148 CAS.
- For selected reviews, see: (a) R. Lavilla, Recent Developments in the Chemistry of Dihydropyridines, J. Chem. Soc., Perkin Trans. 1, 2002, 1141–1156 RSC; (b) J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines, Chem. Rev., 2012, 112, 2642–2713 CrossRef CAS PubMed.
- P. Galatsis, in Name Reactions in Heterocyclic Chemistry, ed. J. J. Li, Wiley, Hoboken, NJ, 2005, pp. 304–307 Search PubMed.
- (a) P. M. Parthiban, 1,4-Dihydropyridine: Synthetic Advances, Medicinal and Insecticidal Properties, RSC Adv., 2022, 12, 29253–29290 RSC; (b) V. K. Sharma and S. K. Singh, Synthesis, Utility and Medicinal Importance of 1,2- & 1,4-Dihydropyridines, RSC Adv., 2017, 7, 2682–2732 RSC; (c) B. Borah, M. Patat, S. Swain and L. R. Chowhan, Recent Advances and Prospects in the Transition-Metal-Free Synthesis of 1,4-Dihydropyridines, ChemistrySelect, 2022, 7, e202202484 CrossRef CAS; (d) J. Jiang, J. Yu, X.-X. Sun, Q.-Q. Rao and L.-Z. Gong, Organocatalytic Asymmetric Three-Component Cyclization of Cinnamaldehydes and Primary Amines with 1,3-Dicarbonyl Compounds: Straightforward Access to Enantiomerically Enriched Dihydropyridines, Angew. Chem., Int. Ed., 2008, 47, 2458–2462 CrossRef CAS PubMed; (e) K. Islam, D. K. Das and A. T. Khan, Hydrated Ferric Sulfate Catalyzed Synthesis of 5,6-Unsubstituted 1,4-Dihydropyridines Using Three-component Reaction, Tetrahedron Lett., 2014, 55, 5613–5617 CrossRef CAS; (f) X. Hu, L. Chen, H. Li, Q. Xu, X. Liu and X. Feng, Enantioselective Catalytic Hantzsch Dihydropyridine Synthesis, ACS Catal., 2023, 13, 6675–6682 CrossRef CAS.
- R. J. Griffiths, W. C. Kong, S. A. Richards, G. A. Burley, M. C. Willis and E. P. A. Talbot, Oxidative β-C-H Sulfonylation of Cyclic Amines, Chem. Sci., 2018, 9, 2295–2300 RSC.
- K. Maeda, T. Terada, T. Iwamoto, T. Kurahashi and S. Matsubara, Ruthenium-Porphyrin-Catalyzed [4+2] Cycloaddition of α,β-Unsaturated Imines and Aldehydes, Org. Lett., 2015, 17, 5284–5287 CrossRef CAS PubMed.
- For selected reviews, see: (a) S. Branko and S. Jurij, Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones, Chem. Rev., 2004, 104, 2433–2480 CrossRef PubMed; (b) P. Lue and J. V. Greenhill, Enaminones in Heterocyclic Synthesis, Adv. Heterocycl. Chem., 1997, 67, 207–343 CrossRef; (c) A.-Z. A. Elassar and A. A. El-Khair, Recent Developments in the Chemistry of Enaminones, Tetrahedron, 2003, 59, 8463–8480 CrossRef CAS; (d) J. P. Michael, K. De, B. Charles, D. Gravestock, G. D. Hosken, A. S. Howard, C. M. Jungmann, R. W. M. Krause, A. S. Parsons, S. C. Pelly and T. V. Stanbury, Enaminones: Versatile Intermediates for Natural Product Synthesis, Pure Appl. Chem., 1999, 71, 979–988 CrossRef CAS.
- M.-Z. Lu, J. Goh, M. Maraswami, Z. Jia, J.-S. Tian and T.-P. Loh, Recent Advances in Alkenyl sp2 C-H and C-F Bond Functionalizations: Scope, Mechanism, and Applications, Chem. Rev., 2022, 122, 17479–17646 CrossRef CAS PubMed.
- (a) A. Noole, M. Borissova, M. Lopp and T. Kanger, Enantioselective Organocatalytic Aza-Ene-Type Domino Reaction Leading to 1,4-Dihydropyridines, J. Org. Chem., 2011, 76, 1538–1545 CrossRef CAS PubMed; (b) J.-P. Wan, S.-F. Gan, G.-L. Sun and Y.-J. Pan, Novel Regioselectivity: Three-Component Cascade Synthesis of Unsymmetrical 1,4- and 1,2-Dihydropyridines, J. Org. Chem., 2009, 74, 2862–2865 CrossRef CAS PubMed.
- For selected reviews, see: A. J. Flynn, A. Ford and A. R. Maguire, Synthetic and Mechanistic Aspects of Sulfonyl Migrations, Org. Biomol. Chem., 2020, 18, 2549–2610 RSC.
- (a) T. Lu, Z. Lu, Z.-X. Ma, Y. Zhang and R. P. Hsung, Allenamides: A Powerful and Versatile Building Block in Organic Synthesis, Chem. Rev., 2013, 113, 4862–4904 CrossRef CAS PubMed; (b) L.-L. Wei, H. Xiong and R. P. Hsung, The Emergence of Allenamides in Organic Synthesis, Acc. Chem. Res., 2003, 36, 773–782 CrossRef CAS PubMed; (c) J.-Y. Wang, W.-J. Hao, S.-J. Tu and B. Jiang, Engaging Yne-Allenes in Cycloaddition Reactions: Recent Developments, Chin. J. Chem., 2022, 40, 1224–1242 CrossRef CAS.
- S. Zhou, D.-Y. Liu, S. Wang, J.-S. Tian and T.-P. Loh, An Efficient Method for the Synthesis of 2-Pyridones via C-H Bond Functionalization, Chem. Commun., 2020, 56, 15020–15023 RSC.
- G. S. Lal, G. P. Pez, R. J. Pesaresi, F. M. Prozonic and H. Cheng, Bis(2-methoxyethyl)aminosulfur Trifluoride: A New Broad-Spectrum Deoxofluorinating Agent with Enhanced Thermal Stability, J. Org. Chem., 1999, 64, 7048–7054 CrossRef CAS.
- (a) T. Umemoto, R. P. Singh, Y. Xu and N. Saito, Discovery of 4-tert-Butyl-2,6-dimethylphenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation with High Stereoselectivity, J. Am. Chem. Soc., 2010, 132, 18199–18205 CrossRef CAS PubMed; (b) D. Behera, S. Thiyagarajan, P. K. Anjalikrishna, C. H. Suresh and C. Gunanathan, Ruthenium(II)-Catalyzed Regioselective 1,2-Hydrosilylation of N-Heteroarenes and Tetrel Bonding Mechanism, ACS Catal., 2021, 11, 5885–5893 CrossRef CAS.
- M. M. Hinman, T. A. Rosenberg, D. Balli, C. Black-Schaefer, L. E. Chovan, D. Kalvin, P. J. Merta, A. M. Nilius, S. D. Pratt, N. B. Soni, F. L. Wagenaar, M. Weitzberg, R. Wagner and B. A. Beutel, Novel Antibacterial Class: A Series of Tetracyclic Derivatives, J. Med. Chem., 2006, 49, 4842–4856 CrossRef CAS PubMed.
- (a) M. Kimura, Y. Wakamiya, Y. Horino and Y. Tamaru, Efficient Synthesis of 4-Ethenylidene-2-oxazolidinones via Palladium-Catalyzed Aminocyclization of 2-Butyn-1,4-diol Biscarbamates, Tetrahedron Lett., 1997, 38, 3963–3966 CrossRef CAS; (b) Y. Horino, M. Kimura, Y. Wakamiya, T. Okajima and Y. Tamaru, Efficient Entry to Tetrahydropyridines: Addition of Enol Ethers to Allenesulfonamides Involving a Novel 1,3-Sulfonyl Shift, Angew. Chem., Int. Ed., 1999, 38, 121–124 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization of the synthesized compounds, NMR spectra, and X-ray crystallographic data of 3a. CCDC 2186305. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01681a |
| This journal is © the Partner Organisations 2024 |