Open Access Article
Open Access ArticleIntermediate diradical character and thermal cis–trans isomerization of near-infrared absorbing thionated squaraine dyes†‡
Taishi
Oka
a,
Takeshi
Maeda
 *a,
Daisuke
Sakamaki
*a,
Daisuke
Sakamaki
 b,
Naoya
Suzuki
b,
Naoya
Suzuki
 a,
Shigeyuki
Yagi
a,
Shigeyuki
Yagi
 a,
Shintaro
Kodama
a,
Shintaro
Kodama
 a and
Hideki
Fujiwara
a and
Hideki
Fujiwara
 *b
*b
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka Metropolitan University, Naka-ku, Sakai 599-8531, Japan. E-mail: tmaeda@omu.ac.jp
bDepartment of Chemistry, Graduate School of Science, Osaka Metropolitan University, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: hfuji@omu.ac.jp
First published on 23rd October 2024
Abstract
We synthesized squaraine dyes incorporating 4-membered thionated oxocarbon and chalcogenopyrylium moieties, which displayed intermediate diradical character as confirmed by bond-length analysis and temperature-dependent behaviors observed in 1H NMR and ESR spectroscopy. The 1H NMR signals of the cyclobutenedithione dyes disappeared at lower temperatures compared to the cyclobutenedione analogues, indicating a lower thermal transition to the excited triplet state and thus greater diradical character. Moreover, we identified cis–trans isomerization in the thionated dyes. Notably, an increase in diradical character lowered the activation energy of this isomerization, facilitating the structural transition between cis and trans isomers. These dyes exhibit strong near-infrared absorption, with slight temperature-dependent shifts likely arising from the closely matched electronic transition energies of the isomers.
Introduction
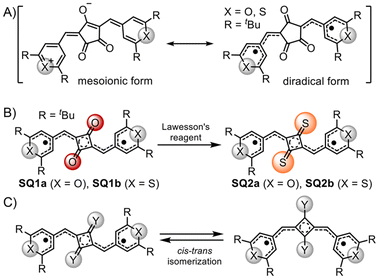
Singlet diradicaloids are molecules characterized by intermediate diradical character, which is illustrated by resonance structures that include both closed-shell and open-shell forms.1–3 In these molecules, two electrons with antiparallel spins are only partially coupled in the ground state. This partial coupling causes the electrons to occupy separate spatial regions to minimize electron repulsion, thereby reducing the covalent character of the π bond and stabilizing the diradical resonance forms. As a result, singlet diradicaloids can facilitate thermal excitation from singlet to triplet states.4–7 Over the past two decades, various stable singlet diradicaloids have been demonstrated, including π-extended quinodimethanes like Chichibabin's hydrocarbon,8–10 quinoidal oligothiophenes,11–13 and graphene fragments.14,15 These compounds have attracted significant interest due to their unique physical properties stemming from diradical forms, which offer substantial potential for applications in nonlinear optics, singlet fission, and semiconductor materials.16–19The diradical character not only influences the physical properties of these molecules but also affects their structural conversions. For instance, some singlet diradicaloids induced structural transformations through diradical configurations (Fig. 1A).20 Kozaki, Okada, et al. reported the thermal cis–trans isomerization of a dithienoquinoid analogue of Chichibabin's hydrocarbon (Fig. 1B).21 More recently, Wu et al. reported that oxindolyl-based Chichibabin's hydrocarbons induced cis–trans isomerization due to the contribution of the diradical forms (Fig. 1C).22 These cases collectively highlight the pivotal role of intermediate diradical character in driving dynamic structural changes.
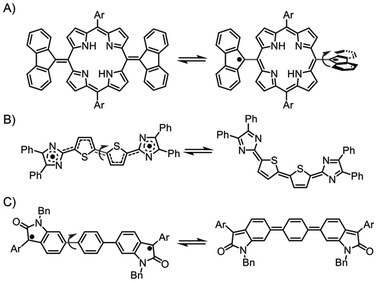 | ||
| Fig. 1 An example of a structural change in a diradical form (A). Thermal cis–trans isomerization of Chichibabin's hydrocarbon analogues (B and C). | ||
Until recently, the range of compounds classified as singlet diradicals was relatively narrow. However, it is now clear that a variety of compounds with extended π-electron systems exhibit sustained singlet diradical character. In fact, even molecules traditionally considered as closed-shell, such as certain diketopyrrolopyrrole derivatives and donor–acceptor conjugated polymers with quinoid repeat units, can exhibit intermediate diradical character.23–28 We also demonstrated that croconaine dyes, typically classified as closed-shell species, actually exhibited intermediate diradical character.29 These dyes consisting of chalcogenopyrylium and five-membered oxocarbons (see Fig. 2A), displayed notable temperature-dependent magnetic properties due to their thermally accessible triplet states.
In addition to the croconaine dyes, chalcogenopyrylium-based squaraine dyes, that were four-membered oxocarbon analogues for the variable applications,30–33 also exhibited intermediate diradical character.34 Although we previously confirmed that the chalcogenopyrylium moiety influences the contribution of the diradical structure, the impact of the central oxyallyl structure on the diradical character remains unclear. Thioxyallyl derivatives, where sulphur atom replaces the oxygen atom in the oxyallyl structure, are known to exhibit diradical character, unlike oxyallyl derivatives.35 Therefore, investigating the effect of sulphur-substitutions in the central skeleton on diradical character of squaraine dyes is of particular interest.
In this study, we synthesized novel chalcogenopyrylium-based squaraine dyes featuring a cyclobutenedithione central skeleton (SQ2a: X = O, SQ2b: X = S) to evaluate their intermediate diradical character (Fig. 2B). These dyes exist as cis–trans isomers, where their intermediate diradical character is likely to influence the isomerization behavior. To investigate this further, we examined the cis–trans isomerization of the obtained dyes and compared their behaviour with existing squaraine dyes containing cyclobutenediones (SQ1a–b).
Results and discussion
SQ2a and SQ2b were synthesized by thionation of precursor dye SQ1a and SQ1b with cyclobutenedione, respectively, using Lawesson's reagent in moderate yields (77% for SQ2a and 74% for SQ2b, see ESI‡).36 They are stable in the solid state even at high temperature and in solution under dark conditions (Fig. S1‡), allowing for reliable measurements of 1H NMR, ESR, and electronic absorption spectra.Fig. 3 displays the X-ray structures of SQ2a and SQ2b, with detailed information available in the ESI.‡ Both dyes were analysed as planar trans isomers. In the crystal structure of SQ2a, two crystallographically independent molecules are present. We investigated the degree of diradical character by examining four major resonance forms (α to δ) chosen from 16 possible resonance forms (Fig. 3C, and Fig. S3‡). To evaluate their contribution, we analysed the difference in average bond lengths along the thio-oxyallyl part, denoted as ΔD = ave(g, p) − ave(h, q), where ave(a, b) represents the average of bond lengths a and b. For closed-shell mesoionic structures (α and β), ΔD = 0 due to identical bond lengths in the double and single bond pairs (g, p) and (h, q). In contrast, for the diradical δ form, ΔD > 0 because the outer parts (g and p) are single bonds while the inner parts (h and q) are double bonds. The γ form shows the reverse trend with ΔD < 0. Importantly, the ΔD values from the X-ray structures of SQ2a and SQ2b are positive (ΔD > 0), suggesting that the large contribution of diradical δ form, with unpaired electrons on the chalcogenopyrylium components (Table 1).
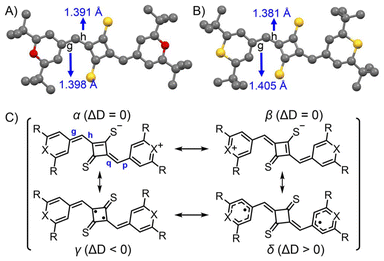 | ||
| Fig. 3 Single crystal X-ray structures of SQ2a (A) and SQ2b (B) at 100 K. Bond lengths of the methine part (g and p) are shown in the Table 1. Resonance contributors of mesoinonic structures (α and β), thio-oxyallyl diradical (γ), and chalcogenopyranyl radical (δ) (C). ΔD is defined as ΔD = ave(g, p) − ave(h, q). | ||
The singlet diradical nature of SQ2a–b was also confirmed by DFT calculations (see ESI, Table S3‡).37–40 The ΔD value obtained from the crystal structure was intermediate between the values for the singlet and triplet states from DFT-optimized structures at the UB3PW91/cc-pVDZ level (Table 1). This suggests that SQ2a–b is in a state that lies between a pure singlet and triplet state.
The diradical index y0, where y0 = 0 and y0 = 1 correspond to a perfect closed-shell and an open-shell electronic structure, respectively, is commonly used to express the degree of contribution of the diradical form in singlet diradicaloids (see ESI‡).41 We estimated y0 values for trans isomers of SQ2a and SQ2b using the spin-projected unrestricted Hartree–Fock (PUHF) theory using the 6-31G(d,p) basis set, obtaining values of 0.24 and 0.37, respectively (Table 2). These results suggest that the thiopyrylium skeleton enhances the contribution of the diradical form.
| Dye | Signal-disappearance temperature in 1H NMR a | Vibrational frequency (cm−1) b |
λ
max (nm) (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε)c ε)c |
ΔG‡d (kcal mol−1) |
y
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
|---|---|---|---|---|---|
| a The temperatures at which the signals assigned to the protons of the chalcogenopyrylium skeleton disappear in DMSO-d6. b Frequencies of the stretching bands around methine groups in CHCl3. Details are shown in ESI.‡ c Electronic absorption maxima in DMSO. The values in parentheses indicate logarithm of molar absorptivity. d The Gibbs free energies of activation (ΔG‡) for the cis–trans isomerization calculated by dynamic NMR method. Details are shown in ESI.‡ e The diradical index calculated by the spin-projected unrestricted Hartree–Fock (PUHF) theory using the 6-31G(d,p) basis set. Details are shown in ESI.‡ | |||||
| SQ2a | 333 | 1480 | 754 (5.22) | 13.7 | 0.24 |
| SQ2b | >303 | 1470 | 849 (5.28) | 13.4 | 0.37 |
| SQ1a | <423 | 1487 | 721 (5.37) | — | 0.37 |
| SQ1b | 403 | 1478 | 822 (5.44) | 14.5 | 0.47 |
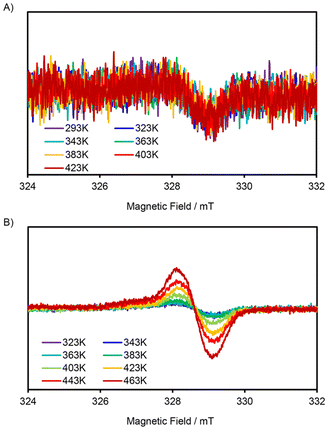
In addition to the bond length analyses, ESR spectra of these dyes displayed a signature of singlet diradicaloids. While the signal intensity of SQ2a was very low, both SQ2a and SQ2b exhibited signals with g values of 2.006, indicating the presence of carbon-centred radicals (Fig. 4). While SQ2a displayed negligible temperature-dependent behaviour, SQ2b showed increased signal intensity with rising temperature, indicating a thermal transition from singlet to triplet states that reflects its singlet diradical nature. This observation suggests that the thiopyrylium skeleton likely enhances the diradical contribution.
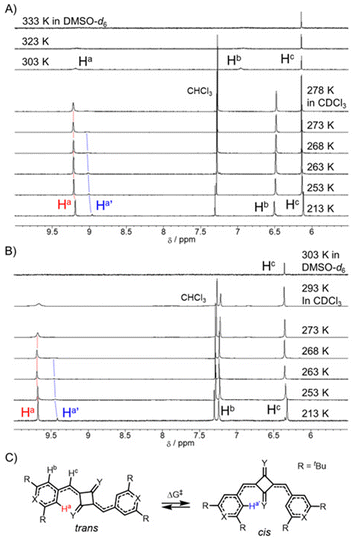
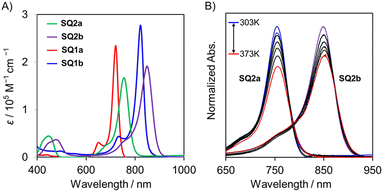
Unlike metal-free organic dyes with closed-shell structures, SQ2a–b exhibit characteristic temperature-dependent behavior in their 1H NMR spectra in DMSO-d6 due to their diradical character (Fig. 5A for SQ2a, Fig. 5B for SQ2b). At 303 K, SQ2a showed signals attributed to the proton on the pyrylium component. As the temperature increased, the signal broadened and completely disappeared at 333 K (Table 2). In SQ2b, no signal corresponding to the proton on the thiopyrylium components was observed, even at 303 K, where DMSO-d6 barely freezes. These results indicate an increase in thermally excited triplet species with increasing temperature, confirming the intermediate diradical nature of SQ2a–b. Thus, 1H NMR studies, along with ESR data, clearly show that the intermediate diradical character of SQ2b is higher than that of SQ2a.
We previously reported that SQ1a–b, with cyclobutenedione skeletons (precursors to SQ2a–b), also exhibits intermediate diradical character. For SQ1a–b, the proton signals disappear above 400 K, whereas for SQ2a–b, the proton signals vanish at a lower temperature, around 300–330 K (Table 2). This is because SQ2a–b reach their thermally excited triplet state at a lower temperature, indicating a smaller energy gap between the singlet and triplet states and a significant contribution from the diradical forms. Thus, thionation of the central skeleton enhances the contribution of the diradical structure, even though the diradical index y0 values for SQ1a–b are estimated to be larger than those for SQ2a–b due to limitations in computational accuracy.
The single crystal of SQ1b contains both cis and trans isomers, confirming that cis–trans isomerization occurs in solution.42 Similar to SQ1b, SQ2a–b also exhibited cis and trans isomers at lower temperatures in CDCl3 (Fig. 5A for SQ2a, Fig. 5B for SQ2b and Fig. S9–10‡ for SQ1a–b). Signals corresponding to the protons on the chalcogenopyrylium component coalesced at 278 K for SQ2a and at 273 K for SQ2b, suggesting that the isomerization is promoted at higher temperature condition and the signals for isomers is no longer observed in the NMR timescale. The Gibbs free energies of activation (ΔG‡) for the cis–trans isomerization of SQ2a and SQ2b were estimated using the dynamic NMR method, resulting in values of 13.7 and 13.4 kcal mol−1, respectively (Fig. 5C, and Table 2).43 Although some error was introduced by the inability to observe peak shifts at temperatures below 213 K due to the freezing of CDCl3 used in the measurements, SQ2b, with a cyclobutenedithione skeleton, exhibits a lower ΔG‡ than SQ1b, which has a cyclobutenedione skeleton (ΔG‡ = 14.5 kcal mol−1).44 The higher diradical contribution of SQ2b compared to SQ1b may lower the activation barrier and promote isomerization.
To measure the strength of the C–C double bond involved in isomerization, FT-IR spectra of these dyes in CHCl3 were recorded. The IR absorption corresponding to the stretching vibration around the methine group in SQ2b was observed at a lower wavenumber compared to SQ2a, indicating that the bond in the methine group of SQ2b is relatively weaker (Table 2, Fig. S11 and 12‡). Thus, increased diradical character appears to diminish the double bond character of the methine group in the ground state, thereby promoting cis–trans isomerization.
The electronic absorption spectra for the dyes SQ2a and SQ2b, compared with those of SQ1a and SQ1b, are illustrated in Fig. 6 and summarized in Table 2. SQ2a and SQ2b each exhibit strong and sharp absorption peaks at 754 nm and 849 nm, respectively. The dyes with the cyclobutenedithione skeleton (SQ2a, SQ2b) exhibited absorption bands in a longer wavelength region compared to those with corresponding cyclobutenedione analogues (SQ1a, SQ1b). Notably, SQ2b, which has a high contribution from the diradical structure, demonstrated a lower transition energy. This is consistent with our previous results that a greater diradical character results in reduced transition energy according to the perturbation theory proposed by Fabian et al.34,45 We also examined the temperature-dependence in absorption spectra of these dyes (Fig. 6B, and Fig. S15‡). With increasing temperature, the absorption bands of SQ2a and SQ2b were red-shifted by 2 nm and 5 nm, respectively. This change in absorption band with temperature might be attributed to cis–trans isomerization. DFT calculations estimate that the transition energy of the cis isomer is slightly lower than that of the trans isomer, suggesting that the equilibrium shifts towards the trans isomer at low temperatures due to cis–trans isomerization (Table S3 and S4‡).
Conclusions
In summary, we synthesized cyclobutenedithione-based dyes SQ2a–b and analysed their intermediate diradical character using 1H NMR, ESR, and theoretical calculations. Our results reveal that dyes incorporating a thiopyrylium component exhibit large degree of diradical character compared to those with a pyrylium component. Furthermore, SQ2a–b demonstrate a higher degree of diradical character than their cyclobutenedione counterparts (SQ1a–b). This enhanced diradical character promotes cis–trans isomerization, which is attributed to the weakening of the C–C double bonds involved. Additionally, SQ2a–b show subtle temperature-dependent changes in their electronic absorption spectra within the near-infrared region. Our study exemplifies the impact of diradical character on the physical and chemical properties of squaraine dyes, offering valuable insights for the design of advanced near-infrared absorbing dyes.Data availability
The data supporting this article have been included as part of the ESI.‡ Crystallographic data for SQ2a, SQ2b,SQ1a, and SQ1b are available in the CCDC under deposit numbers 2382744, 2382745, 2194625 and 2194626,‡ respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 24K01573, ENEOS Tonengeneral Research/Development Encouragement & Scholarship Foundation, Iketani Science and Technology Foundation, and JST, the Establishment of University Fellowships Towards the Creation of Science Technology Innovation, Grant Number JPMJFS2138.References
- M. Abe, Diradicals, Chem. Rev., 2013, 113, 7011–7088 CrossRef CAS PubMed.
- T. Kubo, Recent Progress in Quinoidal Singlet Biradical Molecules, Chem. Lett., 2015, 44, 111–122 CrossRef.
- T. Y. Gopalakrishna, W. Zeng, X. Lu and J. Wu, From open-shell singlet diradicaloids to polyradicaloids, Chem. Commun., 2018, 54, 2186–2199 RSC.
- H. Hayashi, J. E. Barker, A. Cárdenas Valdivia, R. Kishi, S. N. MacMillan, C. J. Gómez-García, H. Miyauchi, Y. Nakamura, M. Nakano, S. Kato, M. M. Haley and J. Casado, Monoradicals and Diradicals of Dibenzofluoreno[3,2-b]fluorene Isomers: Mechanisms of Electronic Delocalization, J. Am. Chem. Soc., 2020, 142, 20444–20455 CrossRef CAS.
- Q. Jing, T. Tao, H. Phan, Y. Han, T. Y. Gopalakrishna, T. S. Herng, G. Li, L. Yuan, J. Ding and C. Chi, Diazuleno-s-indacene Diradicaloids: Syntheses, Properties, and Local (anti)Aromaticity Shift from Neutral to Dicationic State, Angew. Chem., Int. Ed., 2018, 57, 16737–16741 CrossRef.
- J. J. Dressler, M. Teraoka, G. L. Espejo, R. Kishi, S. Takamuku, C. J. Gomez-Garcia, L. N. Zakharov, M. Nakano, J. Casado and M. M. Haley, Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets, Nat. Chem., 2018, 10, 1134–1140 CrossRef CAS PubMed.
- W. Zeng, T. Y. Gopalakrishna, H. Phan, T. Tanaka, T. S. Herng, J. Ding, A. Osuka and J. Wu, Superoctazethrene: An Open-Shell Graphene-like Molecule Possessing Large Diradical Character but Still with Reasonable Stability, J. Am. Chem. Soc., 2018, 140, 14054–14058 CrossRef CAS PubMed.
- V. G. Jiménez, P. Mayorga-Burrezo, V. Blanco, V. Lloveras, C. J. Gómez-García, T. Šolomek, J. M. Cuerva, J. Veciana and A. G. Campaña, Dibenzocycloheptatriene as end-group of Thiele and tetrabenzo-Chichibabin hydrocarbons, Chem. Commun., 2020, 56, 12813–12816 RSC.
- T. Nishiuchi, S. Aibara, H. Sato and T. Kubo, Synthesis of π-Extended Thiele's and Chichibabin's Hydrocarbons and Effect of the π-Congestion on Conformations and Electronic States, J. Am. Chem. Soc., 2022, 144, 7479–7488 CrossRef CAS PubMed.
- Y. Hayashi, S. Suzuki, T. Suzuki and Y. Ishigaki, Dibenzotropylium-Capped Orthogonal Geometry Enabling Isolation and Examination of a Series of Hydrocarbons with Multiple 14π-Aromatic Units, J. Am. Chem. Soc., 2023, 145, 2596–2608 CrossRef CAS.
- T. Takahashi, K. Matsuoka, K. Takimiya, T. Otsubo and Y. Aso, Extensive quinoidal oligothiophenes with dicyanomethylene groups at terminal positions as highly amphoteric redox molecules, J. Am. Chem. Soc., 2005, 127, 8928–8929 CrossRef CAS PubMed.
- R. P. Ortiz, J. Casado, V. Hernandez, J. T. López Navarrete, P. M. Viruela, E. Orti, K. Takimiya and T. Otsubo, On the Biradicaloid Nature of Long Quinoidal Oligothiophenes : New Experimental Evidences Guided by Theoretical Studies, Angew. Chem., Int. Ed., 2007, 46, 9057–9061 CrossRef CAS.
- J. Casado and J. T. López Navarrete, The longest quinoidal oligothiophene: a Raman story, Chem. Rec., 2011, 11, 45–53 CrossRef CAS.
- Q. Jiang, H. Wei, X. Hou and C. Chi, Circumpentacene with Open-Shell Singlet Diradical Character, Angew. Chem., Int. Ed., 2023, 62, e202306938 CrossRef CAS PubMed.
- Y. Yano, N. Mitoma, H. Ito and K. Itami, Correction to A Quest for Structurally Uniform Graphene Nanoribbons: Synthesis, Properties, and Applications, J. Org. Chem., 2021, 86(5), 4372–4373 CrossRef CAS PubMed.
- K. Kamada, K. Ohta, T. Kubo, A. Shimizu, Y. Morita, K. Nakasuji, R. Kishi, S. Ohta, S.-I. Furukawa, H. Takahashi and M. Nakano, Strong two-photon absorption of singlet diradical hydrocarbons, Angew. Chem., Int. Ed., 2007, 46, 3544–3546 CrossRef CAS PubMed.
- K. Kamada, S. Fuku-en, S. Minamide, K. Ohta, R. Kishi, M. Nakano, H. Matsuzaki, H. Okamoto, H. Higashikawa, K. Inoue, S. Kojima and Y. Yamamoto, Impact of Diradical Character on Two-Photon Absorption: Bis(acridine) Dimers Synthesized from an Allenic Precursor, J. Am. Chem. Soc., 2013, 135, 232–241 CrossRef CAS PubMed.
- K. S. Mayer, D. J. Adams, N. Eedugurala, M. M. Lockart, P. Mahalingavelar, L. Huang, L. A. Galuska, E. R. King, X. Gu, M. K. Bowman and J. D. Azoulay, Topology and ground state control in openshell donor-acceptor conjugated polymers, Cell Rep. Phys. Sci., 2021, 2, 100467 CrossRef CAS.
- W. Chao, H. Hua and K. Tajima, Essential Role of Triplet Diradical Character for Large Magnetoresistance in Quinoidal Organic Semiconductor with High Electron Mobility, Adv. Sci., 2022, 9, 2201045 CrossRef.
- H. Zhang, H. Phan, T. S. Herng, T. Y. Gopalakrishna, W. Zeng, J. Ding and J. Wu, Conformationally Flexible Bis(9-fluorenylidene)porphyrin Diradicaloids, Angew. Chem., Int. Ed., 2017, 56, 13484–13488 CrossRef CAS.
- M. Kozaki, A. Isoyama and K. Okada, Detection of a diradical intermediate in the cis–trans isomerization of 5,5′-bis(4,5-diphenyl-2H-imidazol-2-ylidene)-5,5′-dihydro-Δ2,2′-bithiophene, Tetrahedron Lett., 2006, 47, 5375–5378 CrossRef CAS.
- J. Wang, X. Xu, H. Phan, T. S. Herng, T. Y. Gopalakrishna, G. Li, J. Ding and J. Wu, Stable Oxindolyl-Based Analogues of Chichibabin's and Müller's Hydrocarbons, Angew. Chem., Int. Ed., 2017, 56, 14154–14158 CrossRef CAS PubMed.
- W. Wang, L. Ge, G. Xue, F. Miao, P. Chen, H. Chen, Y. Lin, Y. Ni, J. Xiong, Y. Hu, J. Wu and Y. Zheng, Fine-tuning the diradical character of molecular systems via the heteroatom effect, Chem. Commun., 2020, 56, 1405–1408 RSC.
- Z. Chen, W. Li, M. A. Sabuj, Y. Li, W. Zhu, M. Zeng, C. S. Sarap, M. M. Huda, X. Qiao, X. Peng, D. Ma, Y. Ma, N. Rai and F. Huang, Evolution of the electronic structure in open-shell donor-acceptor organic semiconductors, Nat. Commun., 2021, 12, 5889 CrossRef CAS.
- Z. Chen, W. Li, Y. Zhang, Z. Wang, W. Zhu, M. Zeng and Y. Li, Aggregation-Induced Radical of Donor–Acceptor Organic Semiconductors, J. Phys. Chem. Lett., 2021, 12, 9783–9790 CrossRef CAS PubMed.
- T. L. D. Tam, G. Wu, S. W. Chien, S. F. V. Lim, S.-W. Yang and J. Xu, High Spin Pro-Quinoid Benzo[1,2-c;4,5-c′]bisthiadiazole Conjugated Polymers for High-Performance Solution-Processable Polymer Thermoelectrics, ACS Mater. Lett., 2020, 2, 147–152 CrossRef CAS.
- L. Huang, N. Eedugurala, A. Benasco, S. Zhang, K. S. Mayer, D. J. Adams, B. Fowler, M. M. Lockart, M. Saghayezhian, H. Tahir, E. R. King, S. Morgan, M. K. Bowman, X. Gu and J. D. Azoulay, Open-Shell Donor–Acceptor Conjugated Polymers with High Electrical Conductivity, Adv. Funct. Mater., 2020, 30, 1909805 CrossRef CAS.
- H. Cai, H. Tang, T. Wang, C. Xu, J. Xie, M. Fu, X. Luo, Z. Hu, Y. Zhang, Y. Deng, G. Li, C. Liu, F. Huang and Y. Cao, An n-Type Open-Shell Conjugated Polymer with High-Spin Ground-State and High Intrinsic Electrical Conductivity, Angew. Chem., Int. Ed., 2024, 63, e202402375 CrossRef CAS PubMed.
- A. Rajca, Organic Diradicals and Polyradicals: From Spin Coupling to Magnetism?, Chem. Rev., 1994, 94, 871–893 CrossRef CAS.
- P. Sun, Q. Wu, X. Sun, H. Miao, W. Deng, W. Zhang, Q. Fan and W. Huang, J-Aggregate squaraine nanoparticles with bright NIR-II fluorescence for imaging guided photothermal therapy, Chem. Commun., 2018, 54(95), 13395–13398 RSC.
- S. Maryala, D. Sato, S. S. Pandey, S. Hayase and T. Kato, Efficient near infrared fluorescence detection of elastase enzyme using peptide-bound unsymmetrical squaraine dye, Bioorg. Med. Chem. Lett., 2017, 27(17), 4024–4029 CrossRef PubMed.
- C.-A. Shen, D. Bialas, M. Hecht, V. Stepanenko, K. Sugiyasu and F. Würthner, Polymorphism in Squaraine Dye Aggregates by Self-Assembly Pathway Differentiation: Panchromatic Tubular Dye Nanorods versus J-Aggregate Nanosheets, Angew. Chem., Int. Ed., 2021, 60, 11949–11958 CrossRef CAS PubMed.
- C.-L. Sun, Q. Liao, T. Li, J. Li, J.-Q. Jiang, Z.-Z. Xu, X.-D. Wang, R. Shen, D.-C. Bai, Q. Wang, S.-X. Zhang, H.-B. Fu and H.-L. Zhang, Rational design of small indolic squaraine dyes with large two-photon absorption cross section, Chem. Sci., 2015, 6, 761–769 RSC.
- T. Maeda, T. Oka, D. Sakamaki, H. Fujiwara, N. Suzuki, S. Yagi, T. Konishi and K. Kamada, Unveiling a new aspect of oxocarbons: open-shell character of 4- and 5-membered oxocarbon derivatives showing near-infrared absorption, Chem. Sci., 2023, 14, 1978 RSC.
- W. Ando, Y. Hanyu, T. Furuhata and T. Takata, Thioxyallyl ion from allene episulfide with acid, J. Am. Chem. Soc., 1983, 105, 6151–6152 CrossRef CAS.
- T. Ozturk, E. Ertas and O. Mert, Use of Lawesson's Reagent in Organic Syntheses, Chem. Rev., 2007, 107, 5210–5278 CrossRef CAS PubMed.
- D. López-Carballeira, D. Casanova and F. Ruipérez, Potential Use of Squarates and Croconates as Singlet Fission Sensitizers, ChemPhysChem, 2018, 19, 2224–2233 CrossRef.
- S. Pradhan, Y. Kurokawa and S. S. Pandey, Design and Synthesis of Novel NIR-Sensitive Unsymmetrical Squaraine Dyes for Molecular Photovoltaics, Phys. Status Solidi A, 2023, 220, 2300145 CrossRef CAS.
- V. V. Kurdyukov, A. I. Tolmachev, M. L. Dekhtyar, Y. G. Vlasenko and A. N. Chernega, A.N. Synthesis, spatial structure and spectral properties of pyrylo-4 (thio) squaraines variously substituted in cyclobutene moiety (2015) Journal of Physical Organic Chemistry, J. Phys. Org. Chem., 2015, 28, 452–459 CrossRef CAS.
- S. Ito, T. Nagami and M. Nakano, Diradical Character Based Design for Singlet Fission of Condensed-Ring Systems with 4nπ Electrons, J. Phys. Chem. Lett., 2016, 7, 3925–3930 CrossRef CAS PubMed.
- K. Yamaguchi, T. Fueno and H. Fukutome, A molecular-orbital theoretical classification of reactions of singlet ground-state molecule, Chem. Phys. Lett., 1973, 22(3), 461–465 CrossRef CAS.
- The crystal structures of SQ1a and SQ1b are available in the CCDC under deposit numbers 2194625 and 2194626,‡ respectively.
- H. Friebolin, Basic One- and Two-Dimensional NMR Spectroscopy, Willey-VCH, 1991, p. 309 Search PubMed.
- Unfortunately, the activation free energy of SQ1a could not be calculated because the signal for Ha in cis-SQ1a could not be identified.
- J. Fabian and R. Zahradník, The Search for Highly Colored Organic Compounds, Angew. Chem., Int. Ed. Engl., 1989, 28, 677–694 CrossRef.
Footnotes |
| † Dedicated to Prof. Dr Frank Würthner on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2194625 and 2194626. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01722f |
| This journal is © the Partner Organisations 2025 |