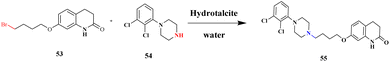Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Clay based heterogeneous catalysts for carbon–nitrogen bond formation: a review
P. Vinoth Kumar and
G. Madhumitha *
*
Chemistry of Heterocycles & Natural Product Research Laboratory, Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore, Tamilnadu, India. E-mail: madhumitha.g@vit.ac.in; dr.g.madhumitha@gmail.com
First published on 5th February 2024
Abstract
Clay and modified clay-based catalysts are widely used in organic transformation. Owing to the interlayer ions and good ion exchange capacity of clay, replacement with another ion and incorporation of different nanomaterials can be done. Due to these significant properties of clay, it can be utilized in the synthesis of various organic compounds. Carbon–nitrogen bonded compounds possess diverse applications in different fields. These compounds are prepared using different solid acid heterogeneous catalysts. This review presents a detailed discussion on clay used for the carbon–nitrogen bond formation reaction, such as the Biginelli reaction and A3 and KA2 coupling reactions. Additionally, other C–N bond formation reactions using various clay-based catalysts such as bentonite, montmorillonite, hydrotalcite and halloysite clay with various metals, metal oxides, Kegging type heteropoly acid and various nanomaterial incorporated clay heterogeneous catalysts are discussed.
1 Introduction
Carbon-heteroatom functionalized compounds have been developed in the past few years; particularly, compounds with C–N bonds are dominant in synthetic chemistry due to their excellent diverse activity in various fields.1 Molecules with carbon–nitrogen bonds are abundantly present, such as amino acids, purine and pyrimidine bases and plant extracted alkaloids.2 These carbon–nitrogen bonded compounds are most sufficiently present in biologically3,4 and pharmacologically active molecules, and they are also present in some natural products, vitamins, drugs, agrochemicals, such as pesticides, herbicides, fungicides,5 synthetic intermediates and polymeric materials.6 Numerous nitrogen containing heterocyclic moieties are present, and they have excellent applications in various fields; some of these compounds play a predominant role in medicinal chemistry and are used against various health problems as they exhibit medicinal properties, such as anti-cancer,7,8 anti-HIV,9 anti-bacterial,10 anti-viral,11 anti-inflammatory,12 anti-diabetic,13 antiulcer, antioxidant,14 and anticonvulsive.15 More reactions are available for the synthesis of various C–N formation analogues, such as Cham-Lam coupling,16–19 Buchwald coupling,20–23 Ullmann coupling,24,25 Biginelli reaction,26,27 A3 coupling,28 KA2 coupling,29 Ugi reaction,30 Aza-Michael addition,31 amidation,32 amino-arylation,33 azide-alkene cycloaddition,34 Fridlander reaction,35 and condensation.36 In the past decades, C–N bond formation reactions have been carried out via diverse catalysts, such as organic catalysts, metal salts, metal complexes, metal oxides and various homogeneous catalysts. The homogeneous and organic catalysts had high selectivity and provided higher yields. However, they had some limitations pertaining to the recovery of the catalyst and requirement of long reaction time, some tedious ligands and reaction conditions for product formation. To avoid the limitations of homogeneous catalysts, heterogeneous catalysts have been introduced. The use of heterogeneous catalysts has been increasing in recent years over homogeneous catalysts, especially in the field of organic synthesis, and they have excellent activity and specific selectivity for the preparation of desired compounds. However, some heterogeneous catalysts have drawbacks owing to their toxic nature, and the recovery of the catalyst from the reaction mixture requires a very tedious procedure. A minute amount of the catalyst in the product affects the nature of the product, and high energy is required to discard the catalyst. Accordingly, green catalysts play a crucial role in chemical transformation, and the major advantages of green catalysts are less energy consumption, low waste formation, eco-friendly, non-hazardous nature, easily recoverable, and high atom economy.37–42 Clay minerals are naturally available inexpensive materials that are heterogeneous in nature, and they do not affect the environment if any chemical reaction is carried out. Various types of clay minerals lead to highly selective products at better yields.43 The clay minerals contain hydrated aluminosilicates with nanostructure layers; they possess both Brønsted and Lewis acidic nature due to which they possess excellent activity against various chemical reactions.44 Owing to the ultrathin interlayer structure of the clay minerals with alternative cationic and anionic species present on the surface that are easily exchangeable, the clays are classified into two types owing to the ion-exchange property: cationic and anionic clay.45 Both positive and negative charges are compensated by the presence of water molecules and ions (Na+, Al3+, Mg+, and Fe3+) in the interlayer.46 Each aluminosilicate sheet in the clay must contain Si–O tetrahedral and Al–O octahedral sheets in an alternative arrangement like a sandwich. Different types of layered structures are present, for example, the (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) type possesses one Si–O tetrahedral sheet and one Al–O octahedral sheet alternatively. Additionally, the kaolin type of clay has (1
1) type possesses one Si–O tetrahedral sheet and one Al–O octahedral sheet alternatively. Additionally, the kaolin type of clay has (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (2
1) and (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) type; the (2
1) type; the (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) layer type has one Al–O octahedral sheet between two Si–O tetrahedral sheets, and the montmorillonite clay has a (2
1) layer type has one Al–O octahedral sheet between two Si–O tetrahedral sheets, and the montmorillonite clay has a (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) type structure in the alternative sandwich model.47 The ions present in the clay strongly hold each other by Van der waals, electrostatic and powerful hydrogen bonding forces. The ions present in the clay sheets can be interchanged (cations and anions) (Fe2+, Al3+, Mg2+, Ca2+, Na+, SO42−, Cl−, and NO3−) without requiring any special process. During the interchanging of ions, the structured nature of nanosheets remains unchanged.48 The amount of water molecules present in clay minerals determines the acidic nature of the peculiar clay. In general, clay exhibits Lewis and Brønsted acidity when heated above 300 °C. By increasing the temperature to 450 °C, it becomes anhydrous. Finally, the powder form of clay possesses an excellent Lewis acidic nature because the Brønsted acidity depends on the hydrogen ions (H+) present in the compounds.49 Clay catalysts are used in various fields, such as semiconductors, dye degradation, supercapacitors, and in the medicinal field.50,51 Owing to the clay mineral's high surface area, high ion-exchange capacity, acidity, and easy exchangeability of ions, clay minerals are the cheapest easily available minerals. In recent years, clay-based materials have been developed.
1) type structure in the alternative sandwich model.47 The ions present in the clay strongly hold each other by Van der waals, electrostatic and powerful hydrogen bonding forces. The ions present in the clay sheets can be interchanged (cations and anions) (Fe2+, Al3+, Mg2+, Ca2+, Na+, SO42−, Cl−, and NO3−) without requiring any special process. During the interchanging of ions, the structured nature of nanosheets remains unchanged.48 The amount of water molecules present in clay minerals determines the acidic nature of the peculiar clay. In general, clay exhibits Lewis and Brønsted acidity when heated above 300 °C. By increasing the temperature to 450 °C, it becomes anhydrous. Finally, the powder form of clay possesses an excellent Lewis acidic nature because the Brønsted acidity depends on the hydrogen ions (H+) present in the compounds.49 Clay catalysts are used in various fields, such as semiconductors, dye degradation, supercapacitors, and in the medicinal field.50,51 Owing to the clay mineral's high surface area, high ion-exchange capacity, acidity, and easy exchangeability of ions, clay minerals are the cheapest easily available minerals. In recent years, clay-based materials have been developed.
Various clay-based materials have been reported, and the metal and metal oxide incorporated clay material, Kegging type heteropoly acid supported clay substance, and different types of nanoparticle-supported clay materials are nowadays used for diverse applications (Fig. 1). All types of clay must have specific activity and play a crucial role in product formation.
 | ||
| Fig. 1 Illustration of the construction of various C–N bond forming compounds using various clay catalysts. | ||
1.1 Montmorillonite
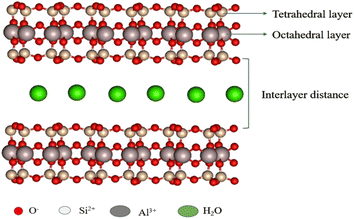
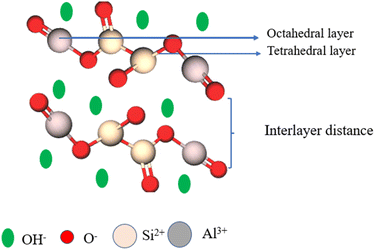
The montmorillonite clay has aluminosilicate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with two tetrahedral sheets (Si–O) in between one octahedral (Al–O) layer (Fig. 2). It comes under the smectite group.52 The molecular formula of montmorillonite is Al2Si4O10 (OH)2. n H2O; it has a very good cation exchange property, and its isomorphic substitution occurs between octahedral and tetrahedral-binded cations. The tetrahedral Si4+ is replaced by trivalent metal ions, such as Al3+; octahedral Al3+ is replaced by divalent metal ions, such as Mg2+; and when Mg2+ ions are introduced in the octahedral layer, the negative charge generated is compensated by Li+, Na+, and Ca2+ ions. This ion-exchange property of montmorillonite clay helps the metal complex, metal nanoparticles, and some organic molecules bound in layers.53,54 The clay has both Brønsted acidity and Lewis acidity nature and excellent ion–exchangeable properties.
1) with two tetrahedral sheets (Si–O) in between one octahedral (Al–O) layer (Fig. 2). It comes under the smectite group.52 The molecular formula of montmorillonite is Al2Si4O10 (OH)2. n H2O; it has a very good cation exchange property, and its isomorphic substitution occurs between octahedral and tetrahedral-binded cations. The tetrahedral Si4+ is replaced by trivalent metal ions, such as Al3+; octahedral Al3+ is replaced by divalent metal ions, such as Mg2+; and when Mg2+ ions are introduced in the octahedral layer, the negative charge generated is compensated by Li+, Na+, and Ca2+ ions. This ion-exchange property of montmorillonite clay helps the metal complex, metal nanoparticles, and some organic molecules bound in layers.53,54 The clay has both Brønsted acidity and Lewis acidity nature and excellent ion–exchangeable properties.
Owing to the ion-exchangeable property of montmorillonite, sodium and calcium montmorillonites are formed naturally. The physical and chemical properties of clay depend on the interlayer cations. Each layer of the montmorillonite possesses a 1 nm thickness and the breadth of the sheets ranges from 100 to 1000 nm range.55,56 The major advantages of montmorillonite are that it is easily available, non-toxic, does not affect the environment, possesses both Lewis and Brønsted acidity nature, has a high surface area, has high pore volume, has high swelling, is expendable in nature, and is easily recoverable and reusable.57,58
1.2 Bentonite
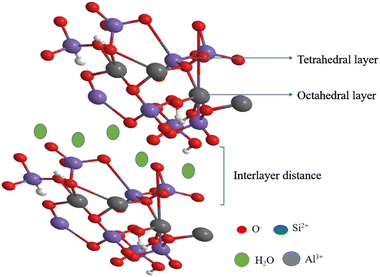
Bentonite is a natural clay that is classified under the smectite family. The molecular formula of the bentonite is Rx(H2O)4 {Al2 x, Mgx)2[(Si, Al)4O10](OH)2}, where a major component of the bentonite contains montmorillonite clay (Fig. 3). It possesses an excellent cation exchange capacity that depends on the interlayer adsorbed cations, such as calcium bentonite and sodium bentonite. Additionally, bentonite has an excellent swelling capacity and low-hydraulic conductivity. The adsorptive capacity of acid-activated bentonite clay possesses excellent catalytic activity owing to its high surface area and porosity. Owing to the hydrophobic nature of clay, it is used in various fields for the purification of water, and bentonite is also a good adsorbent when used as an additive. It is cheap, easily available, has a high surface, and is highly porous, and it can be used as a catalyst in various organic syntheses.59–621.3 Hydrotalcite
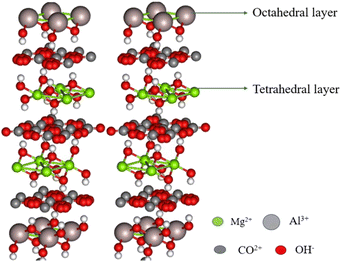
Hydrotalcite is an anionic clay that consists of carboxylate and hydroxyl groups in its interlayer lattice. The molecular formula of hydrotalcite is Mg6Al2(OH)16CO3·4H2O. The hydrotalcite contains a brucite-type positive layer structure of Al3+ or Mg2+ ions, and negatively charged anions are bound between the water molecules (Fig. 4). It has excellent ion-exchange properties. The cations of the hydrotalcite can be exchanged using similar ionic radii metal ions, and the anions of the hydrotalcite can be exchanged using different anionic species and metal complexes (Cl−, Br−, and I−).63–65 The hydrotalcite clay possesses high adsorption capacity, high thermal stability, high surface area and tunable basicity. Owing to this nature, hydrotalcite clay is used in many fields, especially in medicinal applications. The clay exhibits excellent activity when hydrotalcite is activated and supported by metal nanoparticles or Kegging type heteropoly acids.66,671.4 Halloysite
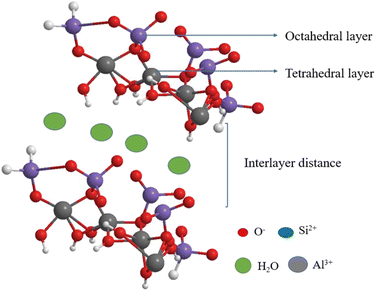
Halloysite nano clay is naturally available hydrated aluminosilicates belonging to the kaolinite group of clay. The molecular formula of halloysite clay is Al2(OH)4Si2O5·2H2O. The structure and reactive nature of Halloysite nanotubes are similar to those of kaolinite clay.68 The water molecule present in the halloysite separates each monolayer; the space between each layer is 10 Å. The SiO4 moiety is located on the outer surface of the HNT clay, and the Al (OH)3 layer is located on the inner surface of the clay.69 Tetrahedral SiO4 and octahedral Al (OH)3 sheets are bound to each other alternatively with water molecules in between separating each layer (Fig. 5). It possesses various morphologies, but the elongated tube type morphology has better activity. It has excellent applications in various fields owing to its high surface area, high thermal and mechanical strength and excellent ion-exchange capacity.70 The HNTs have recently been used in drug delivery, photo-degradation, sewage treatment, and electrical and optical applications.71 The main merits of the HNTs are their biocompatibility, ease of availability, inexpensiveness, eco-friendliness and easy recyclability, and they do not require any tedious procedure.721.5 Kaolin
Kaolin is another naturally and commercially available heterogeneous solid acid; it has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layer of aluminosilicates, and the tetrahedral SiO4 and octahedral AlO2 (OH)4 groups are arranged in a sandwich.73 The Al–O groups are bound between two tetrahedral Si–O moieties and the water molecule is located in the interlayer of each sandwich moiety. The molecular formula of kaolin clay is Al2Si2O5(OH)4; the oxygen atom of tetrahedral Si–O is boned with the octahedral aluminum atom, and one oxygen atom binds with only one Al3+ moiety (Fig. 6). It has a high surface area, strong acidic nature, and excellent thermal stability property. Owing to its diverse properties, kaolin is used in various fields to provide excellent yield.74–76
1 layer of aluminosilicates, and the tetrahedral SiO4 and octahedral AlO2 (OH)4 groups are arranged in a sandwich.73 The Al–O groups are bound between two tetrahedral Si–O moieties and the water molecule is located in the interlayer of each sandwich moiety. The molecular formula of kaolin clay is Al2Si2O5(OH)4; the oxygen atom of tetrahedral Si–O is boned with the octahedral aluminum atom, and one oxygen atom binds with only one Al3+ moiety (Fig. 6). It has a high surface area, strong acidic nature, and excellent thermal stability property. Owing to its diverse properties, kaolin is used in various fields to provide excellent yield.74–76
In 2011, Nagendrappa briefly encapsulated the various clay-based catalysts used for organic synthesis: up to 2010.77 In 2012, Kaur summarized the montmorillonite clay-based catalyst used in various types of organic reactions up to 2012.78 Next, Kumar and his group documented the montmorillonite K10 and different moieties attached MMT-K10 clay catalyst used in organic reactions up to 2014.79 Dutta in 2020 published a metal nano-particle supported montmorillonite clay used in organic transformation, and synthesis of metal nano-particle supported montmorillonite was discussed detailly.80 In 2021, Nagendrappa briefly summarized the clay and clay-based materials used for organic syntheses, such as Biginelli reactions, condensation, addition, oxidation and reduction reactions.81 In our research group, Chellapandi in 2021 detailly discussed the montmorillonite clay-based catalyst used for the synthesis of various N-heterocycles, such as five and six-member heterocycles.82
Organic transformations are mimics of the natural products. It involves the construction of target molecules from small entities. Hetero atom-attached compounds exhibit excellent activity, and C–N bonded compounds are used in medicinal, agricultural and sensor fields. Nowadays, many C–N-coupled organic compounds are available owing to their selective applications in various fields. In Table 1, we discuss the various existing methods of C–N bond formation reactions with their reactivity. Bariwal and his research group in 2013 briefly summarized a C–N bond formation cross-coupling reaction.83 Ghorai et al. in 2017 summarized an iron-based catalyst used in C–N bond formation reactions.84 In 2018, Karkas reported a summarized work of C–N bond formation via electrochemical methods.85 Xia and the research group in the same year reported a summarized work of C–N bond formation using radical-based photo/electro chemistry methods.86 Kaur et al. in 2019 briefly summarized the C–N bond formation reaction using a ruthenium-based catalyst for the synthesis of five-membered N-heterocycles.87 Bharatam and his research group in 2020 summarized the synthesis of drugs and biorelevant N-heterocycle C–N bond formation.88 Schomaker et al. in 2021 reported a briefly summarized work of enantioselective C–N bond formation via nitrene transfer catalyst.89
| S. no | Type of reaction | Catalyst | Product | Yield | Ref. |
|---|---|---|---|---|---|
| 1 | Cham–Lam coupling | Copper iminoarylsulfonate complexes | 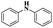 |
99% | 16 |
| [Cu(DMAP)4I]I complex | 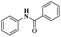 |
90% | 17 | ||
| CuI | 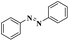 |
92% | 18 | ||
| NiCl2·6H2O | 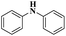 |
85% | 19 | ||
| 2 | Buchwald–Hartwig cross-coupling | [Pd(NHC)(allyl)Cl] | 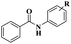 |
88% | 20 |
| N, N-symmetrical benzimidazolium clockPd-PEPPSI complex | 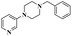 |
93% | 21 | ||
| Cp*Co(III) and Cu(OAc)2 bimetallic catalysis | 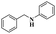 |
91% | 22 | ||
| Pd(dba)2 | 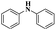 |
94% | 23 | ||
| 3 | Ullmann coupling | Cu/Cu2O | 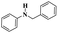 |
77% | 24 |
| CuI | 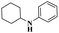 |
98% | 25 | ||
| 4 | Biginelli reaction | Yb (4,6-O-ethylidene-N-(2-hydroxybenzylidene)-β-Dglucopyranosylamine) complex | 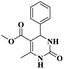 |
95% | 26 |
| Fe3O4-bpy-Ni complex | 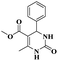 |
90% | 27 | ||
| 5 | A3 coupling | Ag2CO3 | 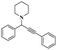 |
90% | 28 |
| 6 | KA2 coupling | Zn(OAc)2 | 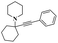 |
91% | 29 |
| 7 | Ugi reaction | Pd(OAc)2 | 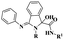 |
82% | 30 |
| 8 | Aza-Michael addition | [Pd(cinnammyl)Cl]2 | 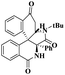 |
95% | 31 |
In this review, we discuss the clay compound-supported catalyst used for the construction of C–N moieties. Clay and modified materials with synthetic methods and properties are discussed in detail in the following protocols (Table 2).
| S. no. | Type of clay | Modified material | Solvent | Synthetic approach | Efficiency | Reference |
|---|---|---|---|---|---|---|
| 1 | Bentonite | Imidazole@Au NP | Ethanol | Conventional heating method | 92% | 90 |
| 2 | Bentonite | B-ZVIN | Solvent free | Greener method | 96% | 91 |
| 3 | Bentonite | TSA | Ethanol | Reflux | 89% | 92 |
| 4 | Bentonite | Fe (III) | Acetonitrile | Conventional heating method | 95% | 93 |
| 5 | Bentonite | Ionic liquid | H2O/EtOH | Greener method | 98% | 94 |
| 6 | Bentonite | TPA | Ethanol | Conventional heating method | 95% | 95 |
| 7 | Halloysite | Amine-F-HPA | Water | Reflux | 96% | 96 |
| 8 | Halloysite | HPA-creation | Water | Reflux | 95% | 97 |
| 9 | Halloysite | Cu@ furfal imine | Water | Ultrasonication | 95% | 98 |
| 10 | Halloysite | Cu-amine-HNT | Ethanol | Ultrasonic method | 93% | 99 |
| 11 | Halloysite | Cu-triazole | Water | Ultrasonic method | 95% | 100 |
| 12 | Halloysite | HNTs | Solvent free | Reflux | 95% | 101 |
| 13 | Hydrotalcite | Mg/Fe | Solvent free | Conventional heating | 90% | 102 |
| 14 | Hydrotalcite | — | Water | Reflux | 94% | 103 |
| 15 | Hydrotalcite | Cu/Fe | TBMP | Conventional heating | 92% | 104 |
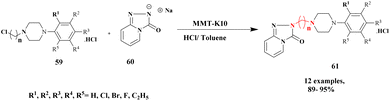
| 16 | MK-10 | — | HCl/Toluene | Greener method | 94% | 105 |
| 17 | MK-10 | — | Solvent free | Greener method | 85% | 106 |
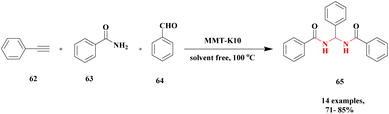
| 18 | MK-10 | — | Solvent free | Greener method | 90% | 107 |
| 19 | MK-10 | NH2 | EtOH | Reflux method | 98% | 108 |
| 20 | MK-10 | — | EtOH | Conventional heating method | 82% | 109 |
| 21 | MK-10 | — | EtOH | Reflux method | 83% | 110 |
| 22 | MK-10 | — | Water | Greener method | 95% | 111 |
| 23 | MK-10 | Metal Schiff base | CH3CN | R.T | 81% | 112 |
| 24 | MMT | Cu/amine | Water/EtOH | Greener method | 95% | 113 |
| 25 | MK-10 | Cu2O/CuO | Water | Green method | 98% | 114 |
| 26 | MK-10 | Cu2O | Water | Green method | 98% | 115 |
| 27 | MMT – KSF | — | Solvent free | Greener method | 87% | 116 |
| 28 | MMT – KSF | GC | Solvent free | Greener method | 96% | 117 |
| 29 | MMT – KSF | HPA | Solvent free | Greener method | 96% | 118 |
| 30 | MMT – KSF | Cu doped | Solvent free | Microwave | 98% | 119 |
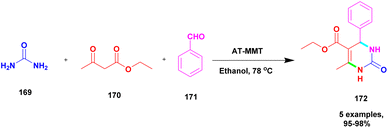
| 31 | HPVAC-MK10 | HPVAC | Solvent free | Greener method | 95% | 120 |
| 32 | HPA-MK10 | HPA | Solvent free | Greener method | 95% | 121 |
| 33 | MMT | CTA-PMo | Solvent free | Greener method | 96% | 122 |
| 34 | MMT | VMWP | Solvent free | Greener method | 92% | 123 |
| 35 | MMT | PVMoK | Solvent free | Greener method | 97% | 124 |
| 36 | MMT-K10 | PVMoK-10 | Solvent free | Greener method | 94% | 125 |
| 37 | Na+-MMT | Cu@imine | Solvent free | Greener method | 96% | 126 |
| 38 | Na+-MMT | [Pmim] HSO4 | Solvent free | Greener method | 90% | 127 |
| 39 | Na+-MMT | Perchloric acid | Solvent free | Greener method | 91% | 128 |
| 40 | Na+- MMT | [Pmim] HSO4 | Solvent free | Greener method | 94% | 129 |
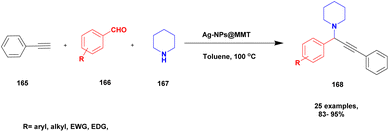
| 41 | MMT | Ag-NP | Toluene | Reflux | 95% | 130 |
| 42 | MMT | Acid activated | Ethanol | Reflux | 98% | 131 |
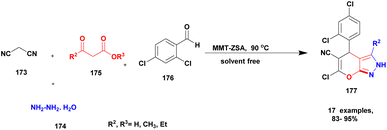
| 43 | Nano clay | Zwitter ionic sulfamic acid | Solvent free | Greener method | 95% | 132 |
| 44 | Natural clay | HPA | Solvent free | Greener method | 93% | 134 |
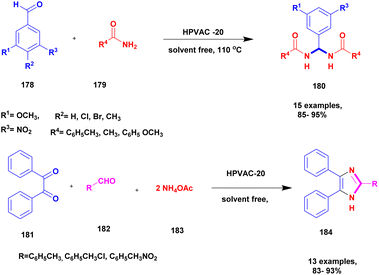
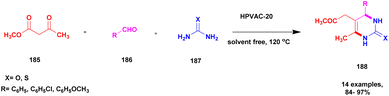
| 45 | Natural clay | HPVAC-20 | Solvent free | Greener method | 92% | 135 |
| 46 | Red brick clay | — | Solvent free | Greener method | 96% | 136 |
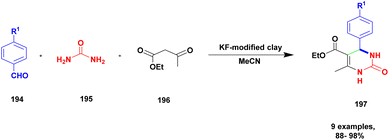
| 47 | KF-clay | — | MeCN | Reflux method | 97% | 137 |
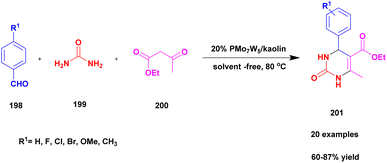
| 48 | Kaoline | PMoW | Solvent free | Greener method | 95% | 138 |
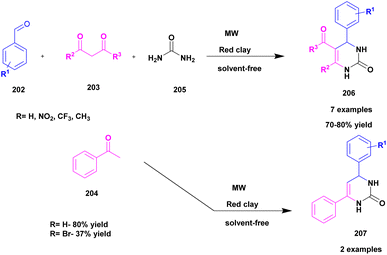
| 49 | Red clay | — | Solvent free | Greener method | 80% | 139 |
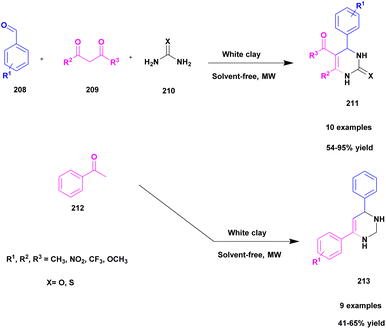
| 50 | White clay | — | Solvent free | Greener method | 95% | 140 |
1.6 Scope of the review
The list of literature discussed in this review was published from 2016 to date. From these studies, the synthesis of compounds with carbon–nitrogen bond in the presence of clay-based catalysts was compiled according to the class of clay along with the published year.2 Discussion
2.1 Bentonite clay-based catalyst using carbon–nitrogen bond formation reactions
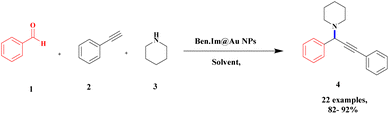
Gholinejad and his group90 addressed the synthesis of propargylamine via A3 coupling in an aqueous solution. The authors introduced gold nanoparticles to assist imidazole-modified bentonite clay as an efficient catalyst for conducting an A3 coupling reaction to prepare propargylamine. Initially, various homogeneous catalysts, including transition metals, were used as catalysts to proceed A3 coupling reaction, but those methods had some drawbacks, such as high cost and difficulty recycling the catalyst. The authors used a natural bentonite clay-modified gold nano-particle catalyst. The gold nanoparticle-assisted imidazole-modified bentonite acts as a better catalyst compared with pure bentonite.The reaction between aromatic aldehyde 1, piperidine 2 and phenylacetylene 3 in the presence of Au-supported imidazole-modified bentonite clay catalyst yielded propargylamine 4 product with up to 90% yield (Scheme 1). The same reaction performed in a different catalyst medium provided a very lower yield compared to the bentonite clay-modified catalyst. Additionally, the catalyst was easily separated from the reaction mixture and reused for four consecutive cycles, which provided a better yield.
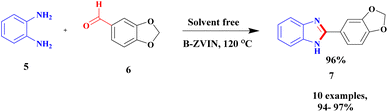
Sravanthi and group91 proposed a facile protocol for the synthesis of benzimidazole via bentonite clay-supported zero-valent iron nanoparticles. The authors synthesized a catalyst, B-ZVIN, using a greener method without any hazardous chemicals. An environmentally friendly Eucalyptus leaf extract was used, and during the synthesis process, no toxic by-product was obtained. The zero-valent iron nanoparticles bound with bentonite clay exhibited excellent catalytic activity and provided a better-coupled product. The reaction did not proceed further without bentonite, and the recoverability of the catalyst was tedious. The reaction between o-phenylenediamine 5 and aromatic aldehyde 6 under solvent-free conditions was carried out in the presence of a bentonite-supported immobilized zerovalent iron nanoparticle catalyst. The expected product fused to benzimidazole 7 was formed at a better yield of up to 95% (Scheme 2).
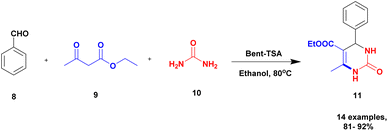
Chopda and his group92 addressed the synthesis of 3, 4-dihydropyrimidine via 12-tungstosilicic acid-supported natural bentonite clay used as a catalyst. The reaction was carried out using a one-pot method under acidic conditions. This reaction had few drawbacks, such as the reaction conditions and the difficulty of reclaiming the catalyst. To avoid these limitations, a solid acid-supported heterogeneous catalyst was utilized. The solid-acid supported catalysts are very costly, so the authors introduce easily available bentonite clay-supported solid-acid catalysts. Tungstosilicic acid-supported bentonite has better catalytic activity compared with pure bentonite. Here, the reaction between benzaldehyde 8, ethyl acetoacetate 9, and diamino-ketone 10 in the presence of a small amount of 30%TSA bentonite catalyst formed the dihydropyrimidine 11 product with up to 89% yield. A simple separation technique and normal filtration were used to easily recover the catalyst and reuse it for another set of reactions, for six cycles without loss of its activity and provided better yield (Scheme 3).
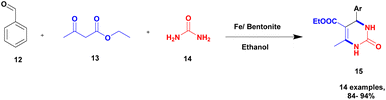
Chopda and his group93 addressed a protocol to carry out the Biginelli reaction via Fe(III)/bentonite clay heterogeneous catalyst. Initially, the Biginelli reaction was performed under different homogeneous catalyst media, and they provided good yields but with some drawbacks. It is very hard to recover the catalyst from the reaction mixture, and some amount of the catalyst remained in the reaction mixture, which affected the formation of the product. To avoid these drawbacks, a heterogeneous catalyst, such as bentonite, was introduced. It is a naturally available clay mineral and possesses a high surface area and an acidic nature. The authors introduced Fe (III) metal ions incorporated into bentonite clay to increase the acidic nature and improve the catalytic activity. The metal ion-incorporated bentonite composite exhibited excellent catalytic activity. Owing to Fe (III) binding with clay, d-space was increased; Fe (III) replaced the other metal ions bonded to the bentonite. When compared with pure and incorporated clay, 30% Fe (III)/bentonite exhibited excellent activity compared with the pure one. The reaction between aromatic aldehyde 12, ethylacatoacetate 13, and diaminoketone 14 in the presence of 30% Fe (III)/bentonite catalyst produced dihydropyrimidine 15 with a better yield of 90% (Scheme 4). The same reaction without iron gave a poor yield.
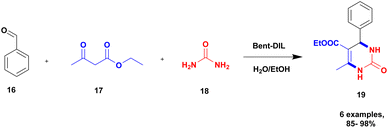
Sadjadi and group94 reported a non-metallic ionic liquid-loaded bentonite catalyst for the synthesis of dihydropyrimidinone using a greener method. Initially, the Biginelli reaction was carried out using different catalysts, but metal-free heterogeneous ionic liquid-loaded bentonite was introduced to provide a better yield. When the ionic liquid reacted with the dendritic moiety, a bent-D-IL catalyst was formed, and it has excellent catalytic activity compared to pure bentonite. The dendritic material has more reactive sites to bind with ionic liquid; this leads to the excellent activity of the composite. The reaction between benzaldehyde 16, ethyl acetoacetate 17, and urea 18 in the presence of the bent-D-IL catalyst provided a dihydropyrimidin 19 product with an excellent yield of up to 98% (Scheme 5). The catalyst helps to achieve higher yields. The dendritic moiety effectively improved the ionic loading, leading to an increased catalytic activity.
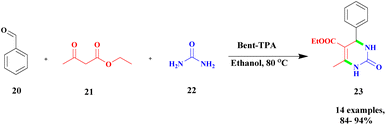
The new protocol for the construction of 3, 4-dihydropyrimidine-2-(1H)-one via heteropoly-12-tungstophosphoric acid-assisted simple bentonite clay composite was used as a heterogeneous catalyst.95 Recently, a heterogeneous catalyst was introduced, and it exhibited excellent catalytic activity. Tungsten-based heteropoly acid was used as a heterogeneous catalyst, which provided a better yield. Here, the authors prepared the tungsten-based heteropoly acid-supported bentonite clay catalyst, which exhibited excellent catalytic activity and provided a very good yield compared with pure HTPA up to 92%. The reaction between the aromatic aldehyde 20, ethylacetoacetate 21, and urea 22 in the presence of bent-TPA catalyst and ethanol solvent provided diaminopyrimidinone 23 up to a 95% yield (Scheme 6). The reaction was performed in three different percentages of TPA-loaded bentonite (10%, 20%, and 30%) and carried out with different solvents as well as using a solvent-free method. The solvent-free condition provided a better yield of up to 91%, while the ethanol medium produced a higher yield of up to 95%. The catalyst was reused for five cycles, and it provided better results.
2.2 Halloysite clay-based catalyst using carbon–nitrogen bond formation reactions
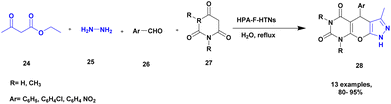
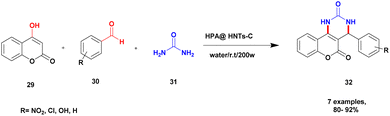
Sadjadi and his group96 reported the synthesis of pyrazolopyranopyrimidines via hetero polyacid-supported amine-functionalized halloysite clay heterogeneous catalysts. The heteropoly acids showed excellent catalysts, with both Brønsted acidity and better redox properties. Owing to this property, it is used as a catalyst in various organic reactions. Halloysite clay nanotubes have been introduced in recent years for various applications, including drug delivery systems. Owing to the large surface area and specific tunability nature of the halloysite, the heteropolyacid-supported halloysite exhibited excellent activity compared with the pristine one. The author performed a four-component domino reaction between ethyl acetoacetic ester 24, hydrazine 25, aromatic aldehyde 26 and barbituric acid 27 in the presence of heretopolyacid over amine-functionalized halloysite nanoparticle. Finally, it provided pyrazolopyranopyrimidines 28 product with a better yield of up to 96% (Scheme 7). When compared with the other earlier methods, the HPA-F-HTNs in water used as solvent provide a better yield. The catalyst in the reaction mixture was easily separated and reused without loss of its activity, even after three cycles with up to 90%. The major advantages of the catalyst were the short reaction time in an aqueous medium and the reusability of the catalyst.A novel heteropoly acid-incorporated creation functionalized halloysite clay heterogeneous catalyst was prepared and used for the synthesis of benzopyranopyrimidine.97 The benzopyranopyrimidine and its derivatives were used for various applications, especially in the medicinal field. The synthesis of this compound requires toxic chemicals, long reaction time, poor yield, and recyclability and reusability of the catalyst were taken into consideration. To avoid this drawback, introducing a heteropoly acid hybrid catalyst would be a better choice. It has a low surface area, an easily soluble nature in organic solvents, and a non-toxic nature. The author introduced the heteropoly acid-supported creation of a functionalized halloysite hybrid catalyst. Owing to halloysite as natural clay, it does not affect the reaction medium, is non-toxic in nature, is inexpensive, and is easily available. The reaction between 4-hydroxycumarin 29 and aromatic aldehyde 30, and diaminoketone 31 presence of an HPA@HNT-C heterogeneous catalyst under ultrasonic irradiation in a water medium provided a benzopyranopyrimidine 32 product with a better yield of up to 95% (Scheme 8). The HPA heterogeneous catalyst provided a better yield at a very short reaction time, and the catalyst was separated easily and reused for another set of reactions. When different substrates were used, better product yields were obtained.
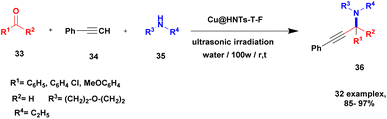
A Cu@furfural imine-supported halloysite clay heterogeneous catalyst was used for three-component A3 and KA2 coupling reactions to synthesize propargylamine derivatives for various applications.98 In the last few years, green chemistry has been prominent owing to the non-toxic and eco-friendly synthesis of compounds to reduce greenhouse gas formation. Halloysite nano clay is an easily available and cheap material that is non-toxic in nature. The surface functionalized halloysite clay was launched recently, and it exhibited excellent activity in various fields for different applications. The author proposed a furfural absorbed copper-supported halloysite nano clay heterogeneous catalyst, and the catalytic performance of the prepared catalyst was studied by performing A3 and KA2 coupling. The reaction between an aldehyde 33, phenylacetylene 34 and secondary amine 35 in the presence of Cu@HNTs-T-F heterogeneous catalyst and water as the solvent using the ultrasonic method yielded the expected product propargylamine 36 with a better yield. The same reaction was carried out using different catalysts, such as CuI-supported HNT and CuCN with better yield, but the reaction took more time compared to the furfural-supported catalyst (Scheme 9). The different substituted substituents bound to various substrates provided better yields in a shorter time. The simple filtration method was used to separate the catalyst, washed with a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio of water and ethanol solution and was reused for another set of reactions. The catalyst was used for four consecutive cycles, which resulted in a better yield without loss in catalytic activity.
1) ratio of water and ethanol solution and was reused for another set of reactions. The catalyst was used for four consecutive cycles, which resulted in a better yield without loss in catalytic activity.
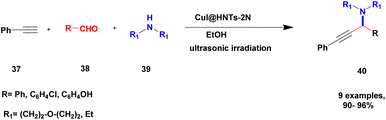
Sadjadi and his group99 documented a visible light-mediated A3 coupling reaction via CuI@amine functionalized modified halloysite clay, and a heterogeneous catalyst was used to upgrade the coupling for the preparation of propargyl amine derivatives. In the past decade, many drawbacks of propargyl amine synthesis have been reported. It requires high temperature, a long reaction time, a specific atmosphere and a specific solvent. To avoid these limitations, the authors introduced inexpensive environmentally friendly halloysite clay nanotubes. The functionalized halloysite nanotubes exhibited excellent activity compared with pure HNTs. Here, CuI-supported amine-functionalized HNTs were introduced owing to their outstanding activity, and the CuI@ HNT-2N composite provided a better yield than CuI@ HNTs-N. The reaction was executed between phenylacetylene 37, aldehyde 38 and secondary amine 39 in the presence of copper-supported halloysite clay catalyst CuI@HNTs-N or CuI@HNTs-2N in ultrasonic irradiation (Scheme 10). The two-nitrogen containing catalyst provided propargyl amine 40 products with a better yield than a single nitrogen CuI@ HNT composite with up to 93%. When the reaction was executed in different solvents, ultrasonic irradiation in the ethanol medium provided an excellent yield of 95% compared with the others. The CuI@ HNT-2N catalyst exhibited the best catalytic activity and reduced the reaction time, and the catalyst was regained easily and reused for five cycles without loss of its activity.
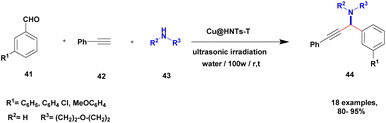
Sadjadi and his group100 documented an extension of the above work. The authors prepared copper incorporated a 1,2,4-triazole-5-methanol functionalized halloysite nano clay catalyst. To execute the one-pot three-component A3 and KA2 coupling reactions for the synthesis of propargylamine derivatives, the reaction was carried out using a conventional method and had some drawbacks. This was avoided by the author by introducing the Cu@HNT-T catalyst. This was driven by the functionalization of HNTs with 1, 2, 4-triazole-5-methanol and then incorporated into copper species. The reaction between carbonyl compounds aldehyde or ketone 41, phenylacetylene 42 and amine 43 in the presence of Cu@HNTs-T catalyst under an ultrasonic medium in a (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio of water
1) ratio of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solvent system yielded propargylamine 44 was formed with up to 95% yield (Scheme 11).
ethanol solvent system yielded propargylamine 44 was formed with up to 95% yield (Scheme 11).
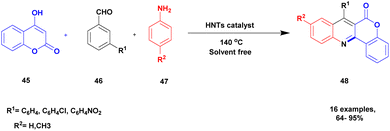
Kachoui and group101 documented a protocol for the multicomponent synthesis of fluorophore chromeno [4, 3-b] quinoline-6-one using a solvent-free greener method and halloysite nano clay as a catalyst. The chromeno-quinoline compounds exhibited very good medicinal properties used against various infections. The earlier method for the synthesis of the compound requires a high temperature and long reaction time, and some of the solvents used are toxic in nature. To avoid these drawbacks, the authors introduced a natural halloysite clay catalyst to perform the reaction. The halloysite clay is easily available, low cost and non-toxic in nature. It has a high surface area and is kinetically and thermally more stable, and it is used in various fields for different application purposes. The reaction between 4-hydroxy coumarin 45, aromatic aldehyde 46, and p-toluidine 47 in the presence of halloysite clay heterogeneous catalyst under the solvent-free condition at 140 °C provided fluorophore chromeno quinoline 48 with a better yield of up to 85% (Scheme 12). When the reaction was carried out in different catalysts and on different substrates, a poor yield was obtained, and when the temperature was decreased or increased above or below 140 °C, a low yield of the product was obtained. The catalyst was reused for five consecutive cycles, which resulted in a better product, and the yield of the product was decreased by 2% after the third cycle, which may be attributed to better catalytic activity.
2.3 Hydrotalcite clay-based catalyst using C–N bond formation reactions
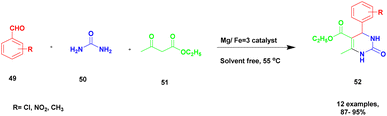
Dabholkar and group102 addressed a protocol for highly efficient Mg/Fe = 3 hydrotalcite catalysts introduced to execute the Biginelli reaction for the construction of dihydropyrimidones using the one-pot solvent-free synthesis method. Over the past decades, the synthesis procedure had some disadvantages and required harsh reaction conditions, high temperatures, expensive reagents, and poor yield. These drawbacks have been overcome by introducing hydrotalcite clay, which has high surface reactivity. When hydrotalcite is calcinated with Mg/Fe at different molar ratios, the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of calcinated Mg/Fe hydrotalcite exhibits excellent catalytic activity. The basicity of the hydrotalcite steadily increases with the Mg/Fe molar ratio and reaching its peak at Mg/Fe = 3. Hence, the reaction between aromatic aldehyde 49 and diaminoketone 50 provided an iminium ion, and it was further reacted with ethylacetoacetate 51 in the presence of Mg/Fe = 3 hydrotalcite calcinated catalyst using a solvent-free one-pot synthesis method (Scheme 13). These methods provide dihydropyrimidone 52 with an excellent yield of up to 90%. The same reaction carried out in different substituent substrates with electron-deficient or electron-rich groups attached with aromatic aldehyde provided a very good yield. Without calcination, a lower yield was obtained. The major advantage of the calcinated clay catalyst is that it can be easily recovered from the reaction mixture without the loss of its catalytic activity and can be reused for another set of reactions.
1 ratio of calcinated Mg/Fe hydrotalcite exhibits excellent catalytic activity. The basicity of the hydrotalcite steadily increases with the Mg/Fe molar ratio and reaching its peak at Mg/Fe = 3. Hence, the reaction between aromatic aldehyde 49 and diaminoketone 50 provided an iminium ion, and it was further reacted with ethylacetoacetate 51 in the presence of Mg/Fe = 3 hydrotalcite calcinated catalyst using a solvent-free one-pot synthesis method (Scheme 13). These methods provide dihydropyrimidone 52 with an excellent yield of up to 90%. The same reaction carried out in different substituent substrates with electron-deficient or electron-rich groups attached with aromatic aldehyde provided a very good yield. Without calcination, a lower yield was obtained. The major advantage of the calcinated clay catalyst is that it can be easily recovered from the reaction mixture without the loss of its catalytic activity and can be reused for another set of reactions.
Soni and group103 documented a protocol to synthesize N-alkylation products using hydrotacite anionic clay, which is an efficient catalyst. This method is a greener approach to reduce the formation of environmentally affected greenhouse gas without any hazardous chemical components to synthesize the expected product. In past decades, a large amount of chemicals has been used to prepare the expected product, and some of it is toxic in nature, providing a large amount of greenhouse gas and affecting the environment. This drawback has been overcome by the author using a greener method to prepare hydrotalcite clay catalysts for N-alkylation. When hydrotalcite was used, a very small amount of carbon dioxide was produced, which was much better than the earlier methods. The reaction between 7-(4-bromo butoxy)-3,4-dihydroquinoline 2H-one 53 with 1-(2,3-dichloro phenyl) piperazine 54 and water in the presence of a lesser amount of hydrotalcite catalyst resulted in the N-alkylation product aripiprazole 55 with an excellent yield of up to 94% (Scheme 14). When similar N-alkylated products were prepared using the hydrotalcite catalyst, a better yield was obtained. When the reaction was completed, the catalyst was regained by filtration and used for another set of reactions. The main advantage of the catalyst was that it reduced the formation of carbon dioxide greenhouse gas and reusability.
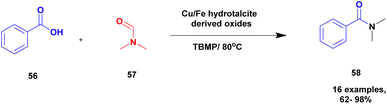
Priya and group104 proposed an oxidative coupling reaction under the calcinated hydrotalcite clay catalyst for the synthesis of N, N-dimethyl substituted amides. Initially, the oxidative amidation reaction was carried out using different catalytic mediums but had drawbacks, such as expansive chemicals, long reaction time, and the reuse of the catalyst. These limitations were overcome using hydrotalcite clay as the catalyst owing to the specific selectivity and specific activity of product formation. The 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of M2+/M3+ transition metal ions was used for calculations with hydrotalcite oxide formation of the heterojunction catalyst. Cu–Fe calcinated hydrotalcite oxides were introduced, and they exhibited excellent activity compared with other calcinated materials. The oxidative coupling between benzoic acid 56 and DMF 57 in the presence of calcinated Cu/Fe = 3
1 ratio of M2+/M3+ transition metal ions was used for calculations with hydrotalcite oxide formation of the heterojunction catalyst. Cu–Fe calcinated hydrotalcite oxides were introduced, and they exhibited excellent activity compared with other calcinated materials. The oxidative coupling between benzoic acid 56 and DMF 57 in the presence of calcinated Cu/Fe = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrotalcite derived oxide catalyst and TBMP occurred by heating the mixture, and the expected N, N-dimethyl amide 58 product was formed with an excellent yield of up to 92% (Scheme 15). When the same reaction was carried out using different transition metals, calcinated hydrotalcite catalysts with very poor yields were obtained. Additionally, the reaction was carried out using different substituted aromatic acids, and products were formed in low yields. Halogens, such as Cl and Br substituted aromatic acids, provided excellent yields with up to 98%. The advantage of the catalyst was that the reaction was completed at a faster rate, non-hazardous, low cost, easily recoverable and reusability of the catalyst for four cycles without loss of its activity.
1 hydrotalcite derived oxide catalyst and TBMP occurred by heating the mixture, and the expected N, N-dimethyl amide 58 product was formed with an excellent yield of up to 92% (Scheme 15). When the same reaction was carried out using different transition metals, calcinated hydrotalcite catalysts with very poor yields were obtained. Additionally, the reaction was carried out using different substituted aromatic acids, and products were formed in low yields. Halogens, such as Cl and Br substituted aromatic acids, provided excellent yields with up to 98%. The advantage of the catalyst was that the reaction was completed at a faster rate, non-hazardous, low cost, easily recoverable and reusability of the catalyst for four cycles without loss of its activity.
2.4 Montmorillonite clay-based catalyst using carbon–nitrogen bond formation
The synthesis of N, N-alkylidene bisamide via one-pot synthesis under a solvent-free situation in the presence of a montmorillonite K10 heterogeneous catalyst was discussed by Lambat and group.106 Initially, many catalysts were introduced to prepare N, N-alkylidene bisamide, but they had few drawbacks, such as sensitivity to harsh medium, reaction time and multiple byproducts. To avoid these drawbacks, the reaction was carried out under solvent-free greener conditions. Here, the authors performed the reaction with a montmorillonite K10 heterogeneous catalyst, which provided a better yield under solvent-free conditions. Compared to the earlier method, this provided a better yield without any by-product formation. The reaction (Scheme 17) between phenylacetylene 62 and benzamide 63 with benzaldehyde 64 in the presence of MK10 heterogeneous catalyst was heated at 100 °C, and the expected alkylidine bisamide 65 product was formed with a better yield of 85%. Through this protocol, the catalyst was separated from the reaction mixture and reused for another set of reactions, which extended to about four cycles.
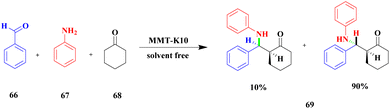
Sanz and group107 reported a mannich reaction for the preparation of β-amino ketones using a solvent free one-pot multicomponent synthesis method. The mannich product has very good activity in various fields mainly in the medical field to prepare synthetic drugs against various diseases. The montmorillonite K10 clay overcame the earlier methods and provided a higher yield. The reaction between aromatic aldehyde 66 and aniline 67 with cyclohexanone 68 in the presence of montmorillonite K10 clay catalyst at 38 °C provided a β-amino ketone 69 of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio of 3-anti/3-syn product 94% at 120 min (Scheme 18). The same reaction was carried out at 5 h with a montmorillonite K10 catalyst, and a selective 3-anti product was formed with 90% yield.
40 ratio of 3-anti/3-syn product 94% at 120 min (Scheme 18). The same reaction was carried out at 5 h with a montmorillonite K10 catalyst, and a selective 3-anti product was formed with 90% yield.
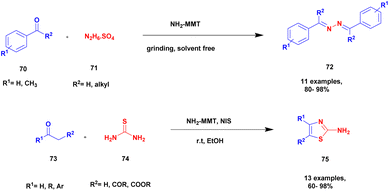
Zarnegar and group108 addressed the synthesis of azines and 2-aminothiazoles using a solvent-free greener method with amine-modified montmorillonite clay as a heterogeneous catalyst. Both azine and 2-aminothiazole derivatives have diverse biological activities and are used in the medicinal field for various applications. However, the earlier methods used in the preparation of the compounds have a few drawbacks, such as prolonged reaction time, poor product formation, hazardous chemicals, many catalysts and reagents needed, and require very high temperatures. To avoid these limitations, a reaction is executed using the solvent-free greener method with a green catalyst. The authors introduced an amine-modified montmorillonite clay (NH2-MMT) heterogeneous catalyst, which does not affect nature and the environment. This amine-modified MMT exhibits both acidic and basic properties. The reaction between aldehyde 70 and hydrazine sulfate 71 in the presence of NH2-MMT catalyst using a solvent-free grinding method provides an excellent coupling product azine 72 with a better yield. Next, methyl carbonyl 73 was reacted with thiourea 74 and N-iodosuccinimide in the presence of ethanol and NH2-MMT catalyst, and a good coupling product 2-aminothiazole 75 was observed with a better yield (Scheme 19). The azines can be prepared using different percentages of NH2-MMT catalyst both in solvent-free grinding method and reflux method, and the solvent-free grinding method provides a very good to a better yield of up to 94% yield. The 2-aminothiazole was prepared using various solvent mediums with different iodine precursors, but the N-iodosuccinimide precursor in the presence of ethanol medium with NH2-MMT catalyst provided an excellent yield of up to 97% yield.
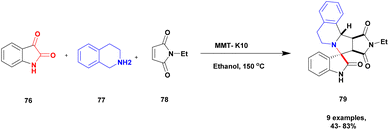
Zhang and his group109 addressed a protocol for the synthesis of spiro oxindole via one-pot synthesis with a montmorillonite K10 clay heterogeneous catalyst to perform the reaction. The spiro oxindole has various biological activities. Hence, the author carried out a [3 + 2] cycloaddition reaction using a greener method in the presence of a montmorillonite K10 clay catalyst. Montmorillonite K10 is easily available, cheap, environmentally friendly and easily recoverable from the reaction mixture and reusable. The reaction between isatin 76 and 1, 2, 3, 4-tetrahydroisoquinoline 77 and N-ethylmalemide 78, in the presence of ethanol solvent and montmorillonite K10 clay heterogeneous catalyst at 150 °C [3 + 2] cycloaddition reaction occurred to provide spirooxindoles 79 better yield with up to 80% (Scheme 20). Initially, the nucleophilic addition of tetrahydroisoquinoline to isatin and then the dehydration process occurred, and an iminium ion was formed. Then, the iminium ion converted into azomethine after deprotonation [3 + 2] cycloaddition occurred with maleimide; finally, the expected product spiro oxindole was formed.
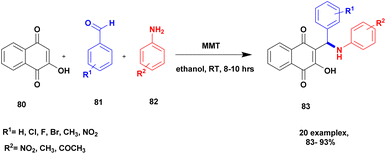
Jayashree and group110 reported a protocol for the synthesis of aminonaphthoquinone derivatives with montmorillonite clay K10 heterogeneous catalysts. The napthoquinoline is mostly used in the medicinal field to cure health problems. In the previous method, the compound was prepared with a transition metal, and a transition metal complex catalyst was used as a catalyst. This method has many drawbacks: it is high cost, toxic in nature, and cannot recycle the catalyst easily. To avoid these limitations, the author introduced a natural montmorillonite K10 clay catalyst, which is easily available, non-toxic, easily separated from the reaction mixture and reusable. The reaction between 2-hydroxy-1,4-napthoquinone 80 and benzaldehyde 81 with aniline 82 in the presence of a montmorillonite K10 catalyst and ethanol solvent provides amino napthaquinoline 83 with a better yield of up to 93% (Scheme 21). When the reactions were performed using various solvents and catalysts, they yielded poor products.
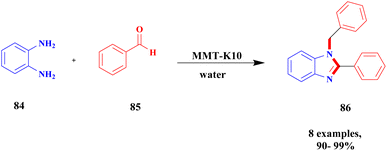
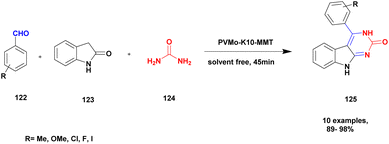
Bonacci et al.111 addressed a procedure for the preparation of benzimidazole derivatives using a montmorillonite K10 heterogeneous catalyst. Benzimidazole has numerous biological applications, and the earlier method of synthesis of the compound has some drawbacks. They needed expensive and toxic solvents and a long reaction time, and recycling of the catalyst was a very tedious process. The heterogeneous catalyst was developed in the last few decades, and it has excellent activity and more applications in various fields because it is more stable and easily recoverable. The authors performed the reaction under microwave irradiation with a natural montmorillonite K10 clay heterogeneous catalyst. Owing to the montmorillonite K-10 features, such as being easily available, non-toxic in nature, and easily recoverable from the reaction mixture, microwave irradiation increases the rate of the reaction and decreases the waste by-product formation. The reaction between ortho-phenylenediamine 84 and aromatic aldehyde 85 in the presence of montmorillonite K10 heterogeneous catalyst with water as a solvent benzimidazole 86 derivative was formed with a better yield (Scheme 22). When performed, the reaction with different substrate selective products was obtained. MK10 helps to reduce waste product formation.
Kannan and group112 reported a montmorillonite K10-supported metal, and a Schiff base complex was used to synthesize pyranoquinoline under aza-Diel's alder reaction. The asymmetric synthesis was an important tool for the construction of novel optically active compounds with very good biological activity. In the past decades, the synthesis of these optically active compounds was carried out using the aza-Diels–Alder reaction in the presence of an excellent asymmetric catalyst. The authors introduced a montmorillonite K10 clay-supported peptide metal complex to prepare optically active compounds. The reaction was carried out without MMT-K10, and it was difficult to remove the Schiff base from the reaction mixture. Mont-K10-supported Cu and Ti metal complexes were used to perform an aza-Diels–alder reaction with a better yield, and MMT-K10 helped to reduce the Schiff-base formation (Scheme 23). The reaction between aromatic aldehyde 87, aniline 88 and 3, 4-dihydro 2H-pyrene 89 in the presence of mont-K10-supported dipeptide Schiff base metal complex provides fused quinoline 90 with a better yield of 84%.
 | ||
| Scheme 23 Synthesis of pyranoquinoline via a Mont-K10 clay-supported DP3 Schiff base metal complex catalyst. | ||
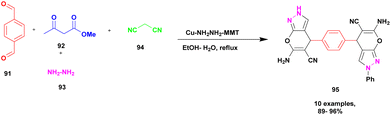
Synthesis of bispyrano [2,3-c] pyrazole was achieved by Ahmadzadeh et al.113 using the one-pot multicomponent method in the presence of copper incorporated amine modified montmorillonite clay catalyst. The four-component coupling occurring between aromatic aldehyde 91, ethyl acetoacetate 92, hydrazine 93, and malononitrile 94 in the presence of Cu (II) anchored amine-modified MMT clay catalyst provided the expected bispyrano [2,3-c] pyrazole 95 compounds of up to 95% within 15 min under water–ethanol solvent medium (Scheme 24). The first condensation reaction occurred between aldehyde and malononitrile, which generated one intermediate; simultaneously, condensation occurred between ethylacetoacetate and hydrazine. Finally, both intermediates are cyclized to form the coupled product. Even for four cycles, the catalyst was reusable, and no catalytic changes were observed. The advantages of this catalyst were its reusability and short reaction time.
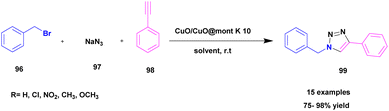
Pathak and his research group114 published a work of azide–alkyne cycloaddition reaction for the synthesis of 1,2,3-triazoles 99 using a Cu2O/CuO@MK-10 heterogeneous catalyst. A simple one-pot click chemistry method was used for synthesizing 1,2,3-triazole. The reaction between sodium azide 97 and phenyl acetylene 98 with benzyl halide 96 in the presence of Cu2O/CuO@MK-10 (10 mg) in water solvent medium at room temperature gave 98% yield in 1 h. The reaction was optimized by changing the catalyst (MK-10, Cu2O, CuO, Cu2O/CuO@MK-10 (5 mg, 10, 20 mg) and solvent (water, DCM, ethanol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol
1 ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water). The optimization in the presence of Cu2O/CuO@MK-10 (10 mg) catalyst in a water solvent medium gave a good yield (Scheme 25). The reaction carried out with different substituent attached derivatives gave a 75–98% yield. When the electron donating group CH3, OCH3 and unsubstituted compound gave 85–98% yield, the electron withdrawing groups Cl and NO2 gave 85–90% yield. The catalyst was separated using a simple filtration technique, then washed with ethanol and reused for another reaction. The catalyst recycled and reused even for five cycles exhibited good catalytic activity.
water). The optimization in the presence of Cu2O/CuO@MK-10 (10 mg) catalyst in a water solvent medium gave a good yield (Scheme 25). The reaction carried out with different substituent attached derivatives gave a 75–98% yield. When the electron donating group CH3, OCH3 and unsubstituted compound gave 85–98% yield, the electron withdrawing groups Cl and NO2 gave 85–90% yield. The catalyst was separated using a simple filtration technique, then washed with ethanol and reused for another reaction. The catalyst recycled and reused even for five cycles exhibited good catalytic activity.
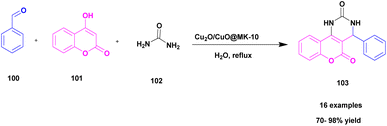
Besharathi and his research group115 developed a protocol for the synthesis of benzopyranopyrimidine derivatives 103 using Cu2O immobilized MK-10 decorated ionic-liquid catalyst (Cu2O@Mont/EAS-1L). The reaction occurred between aromatic aldehyde 100, 4-hydroxy coumarin 101 and urea/thio-urea/guanidine 102 in a water solvent medium in the presence of Cu2O@Mont/EAS-1L catalyst at 60 °C gave a good yield (70–98%) within 15 min. The reaction was optimized under different conditions of catalyst concentration (15, 25, and 35 mg), solvents (water, ethanol, DMF, chloroform, and acetonitrile) and temperature (r.t, 60 °C, and reflux). Finally, the optimization presence of 25 mg Cu2O@Mont/EAS-1L catalyst in water solvent medium at 60 °C showed good activity with 70–98% yield. The reaction was carried out with different substituent attached derivatives, and both electron donating and electron withdrawing groups gave 70–86% yield. When compared with the electron donating & electron withdrawing groups, the unsubstituted derivative gave a good yield of 95–98%. Additionally, compared with earlier reported catalysts, the Cu2O@Mont/EAS-1L showed good catalytic activity with 98% yield. The catalyst was easily recycled from the reaction mixture by applying the normal filtration technique and reused for another set of reactions. After 5 times of recycling, the reactions showed good catalytic activity with 82% yield (Scheme 26).
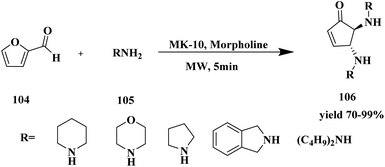
Bonacci et al.116 developed a protocol for the synthesis of bi-functionalized cyclopentanones 106 via solvent free micro-wave medium using montmorillonite K-10 catalyst. The functionalized cyclopentanones were synthesized from furfural, and it was one of the starting materials. Furfural-based compounds are one of the building blacks for the synthesis of various bio active compounds. Nowadays, numerous furfural-based compounds are used as biologically active compounds. From the chiral difunctionalized cyclopentanones, the trans-4,5-disubstituted cyclopentenones exhibited good activity. The reaction carried out between 1 mmol of furfural 104 and 2 mmol of amine 105 in the presence of 20 mol% of MK-10 catalyst at 60 °C for 5 min in microwave showed 99% conversion with 98% yield. The reaction was optimized with different weight percentages of catalyst (10%, 20%, without catalyst), and temperature (r.t, 60 °C, 80 °C, and 100 °C). Additionally, the reaction was carried out with different substituted derivatives (Scheme 27). Finally, the optimization in the presence of MK10–20% at 60 °C in MW showed a 99% yield of trans-4,5-dimorpholinocyclopent-2-enone within 5 min. The catalyst recycled and reused for the further synthesis of bi-functionalized cyclopentanones showed good catalytic activity and high conversion with good yield after the third cycle.
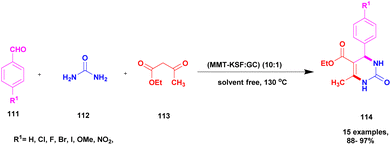
Narayanan and group118 reported a protocol to carry out the Biginelli reaction for the synthesis of dihydropyrimidinone using the solvent-free multicomponent method with montmorillonite-KSF clay – graphene oxide used as a heterogeneous catalyst. The Biginelli type of compound exhibits excellent biological activity and is used against various diseases and infections. Initially, the Biginelli derivatives are prepared using various catalysts, both heterogeneous and homogeneous catalysts. They have some drawbacks: poor product formation, toxic-chemical needed to carry out the reaction with expensive reagents, and catalysts required of the synthetic procedures affect the environment. To overcome these limitations, the reaction was performed using the greener method. The authors were the first to report montmorillonite clay–graphene oxide nanocatalysts using multicomponent reactions for the synthesis of Biginelli products. The graphene oxide exhibits excellent thermal and electrical conductivities, and it possesses various applications in various fields in current research. The different percentages of GO-loaded MMT-KSF clay catalysts were investigated for the synthesis of Biginelli products under solvent-free conditions. The MMT-KSF clay was easily available, did not affect the reaction medium and was non-toxic in nature. The reaction between an aromatic aldehyde 111 and diaminoketone 112 with ethylacetoacetate 113 in the presence of mont-KSF-GO clay at a (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio as the catalyst under solvent-free condition at 130 °C provided 3,4-dihydropyrimidinones 114 in excellent yields of up to 94% (Scheme 29). The reaction carried out in different substrates also provided a better yield, could easily separate the catalyst and be reused even for eight cycles, and has excellent activity with better yield without decreasing its catalytic performance.
1) ratio as the catalyst under solvent-free condition at 130 °C provided 3,4-dihydropyrimidinones 114 in excellent yields of up to 94% (Scheme 29). The reaction carried out in different substrates also provided a better yield, could easily separate the catalyst and be reused even for eight cycles, and has excellent activity with better yield without decreasing its catalytic performance.
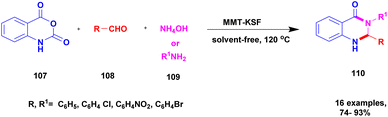
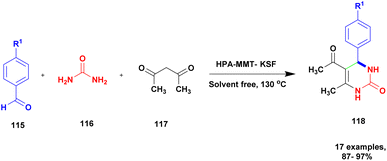
Farooq et al.119 reported a protocol for the construction of dihydropyrimidinones via a solvent free one-pot synthesis using heteropoly acid supported montmorillonite KSF clay as a heterogeneous catalyst. Over the last few decades, the unbelievable growth of multicomponent reactions has been observed in organic synthesis. Selective product formation should not require any separation technique for the removal of intermediate compounds and by-products. In previous procedures, intermediates were produced in each stage and purified for use in the following steps. But MCR uses minimal usage of solvent and avoids multi-step synthesis. Dihydropyrimidinone was prepared using the MCR method with a heteropoly acid-supported montmorillonite KSF catalyst. The reaction between benzaldehyde 115 and urea 116 with ethyl acetoacetate 117 provides a dihydropyrimidinone 118 excellent yield with up to 96% yield (Scheme 30). Whether the reaction was performed without clay or HPA, low yields of 82% and 80%, respectively, were obtained; when both clay and HPA were combined, the yield gradually increased up to 96%. The merits of the catalyst were quick reaction time, excellent yield, ease of separation of the catalyst from the reaction mixture without loss of its catalytic activity and reusability for another set of reactions. Additionally, a non-toxic nature should not affect the nature and environment.
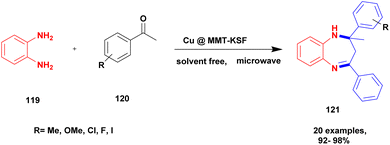
Shaikh et al.120 addressed a protocol for the synthesis of 1, 5-benzodiazepines using a solvent-free microwave method with Cu (II)-doped montmorillonite-KSF nano clay used as a heterogeneous catalyst. The benzodiazepines are used in various fields, such as medicinal, agrochemical, cosmetics and dye and pigment industries. Different types of acidic reagents and iodine molecule-based catalysts as well as some solid phase catalysts have been used in the past decades for the synthesis of benzodiazepine derivatives. They have some disadvantages: poor yield, require high temperature and expensive chemicals to perform the reaction and they affect the environment. To avoid these limitations, the reaction was carried out in an eco-friendly and solvent-free greener method using montmorillonite-KSF clay because it is a naturally available and cheap material. Functionalized montmorillonite clay exhibits excellent activity. Here, the author introduced Cu (II)-doped MMT clay to synthesize benzodiazephines. The reaction between acetophenone 119 and O-phenylene diamine 120 in the presence of Cu (II)-doped MMT-KSF clay using a solvent-free microwave method provided benzodiazepines 121 in excellent yields of up to 98% (Scheme 31). When the same reaction was performed in the presence of different clay catalysts and different solvent mediums, products were formed in poor yields. Simple separation protocols can easily isolate the catalyst from the reaction medium, which can be used again for another set of reactions. Even after five times, the reused catalyst does not show any significant change and provides an excellent yield. The merits of the catalyst were a short reaction time, recyclability, no need for any tedious process, and no by-product formation occurred.
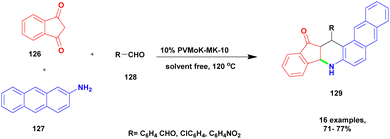
Kumaresan and group122 reported a heteropolyacid-assisted montmorillonite K10 dissimilar catalyst for the preparation of naptho [2, 3-f] quinoline-13 one and naptho [2, 3, -a] acridine 1-2(H) one using the solvent-free greener method. Naturally, the quinoline and acridine compounds have diverse medicinal applications, and some of these derivatives are used against various diseases and infections, such as anti-malarial and anti-hypertensive agents. In earlier methods, synthesis of the quinoline and acridine derivatives has some disadvantages, such as extensive reaction time, poor yield, few reactions needing a toxic solvent, and recovery of the metal catalyst from the reaction mixture requires a very tedious process. To avoid these limitations for carrying out the reaction, a greener solvent-free one-pot synthesis method was used, and provides a better yield without any by-product formation. The authors introduced a heteropoly vanadophophoric acid supported montmorillonite K10 clay heterogeneous catalyst for the reaction between 1, 3-indanedione 126 and 2-amino anthracene 127 with aromatic aldehyde 128 and provided the expected naptho [2, 3-f] quinoline-13 one 129 with a better yield (Scheme 33). Instead of 1, 3-indanedione, 1, 3-cyclohexadiene was used, and it provided naptho [2, 3-a] acridine-1-2(H) product. When compared with the other methods of synthesis, product was formed in low yields. The major advantage of the catalyst was the simple method of synthesising the product, and the non-hazardous greener method was used to carry out the reaction.
Farahani et al.123 reported the synthesis of 2, 4, 5-tri substituted imidazole using the greener method with montmorillonite clay supported heteropoly acid heterogeneous nanocomposite catalyst. The imidazole derivative compounds exhibited excellent activity, especially in the medicinal field. Previously reported synthesis methods have some drawbacks, and to avoid these drawbacks, the reactions were carried out using a greener method. Phosphomolybdic acid was used in various organic reactions. It provided a very good yield, but one of its drawbacks was the difficulty in separating from the reaction mixture. To avoid these limitations, the authors introduced montmorillonite clay; it has a high surface area and is easily available, and it should arrest phosphomolybdic acid mobilization. For the first time, the authors introduced CTA-montmorillonite clay supported HPA for the preparation of substituted imidazole because it provided a better yield in a short time. The reaction between 1, 2-diketone 130 and aldehyde 131 with ammonium acetate 132 in the presence of clay (CTA-montmorillonite clay) supported HPA catalyst provided tri-substituted imidazole 133 product with better yield (Scheme 34). When the reaction was carried out on different substituted substrates, it also provided a better yield. The major advantage of the catalyst was the greener method of synthesis, which can be easily recovered from the catalyst and reused for another set of reactions.
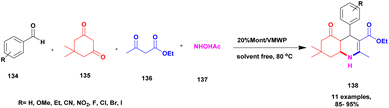
Aher et al.124 reported a Keggin-type phosphoric acid-supported commercial montmorillonite clay heterogeneous catalyst for the preparation of poly-hydro quinoline derivatives. Initially, heteropoly acid was used because it exhibits excellent activity, but it has some demerits, such as poor heat resistance and the least surface area separation from the reaction mixture required a tedious process. To avoid these drawbacks, the authors introduced mixed HPA-keggin type derivatives, such as vanado-molybdotungstophosphoricacid, and subsequently prepared a compound incorporated into natural montmorillonite clay. It has a large surface area, so it helps to increase the catalytic activity of keggin-HPA. The catalytic performance of the prepared catalyst was studied using the preparation of quinoline derivatives. The reaction takes place among benzaldehyde 134, dimedone 135 and acetoacetic ester 136 with ammonium acetate 137, and condensation takes place without solvent at 80 °C, providing polyhydroquinoline 138 at better yield (Scheme 35). When compared with the solvent used method and the presence of any other catalyst, this method at 20% VMWP/Mont provided a better yield with a high percentage. The catalyst was easily recovered by simple filtration and reused for another reaction. Even after four cycles, they provided a better yield without loss of its catalytic activity.
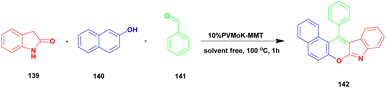
Muthu and group125 published a one-pot multicomponent synthesis of chromeno[2,3-b] indoles under 10%PVMoK (keggin-type of heteropoly-11-molybdo-1-vanadophosphoric acid) supported montmorillonite K-10 clay catalyst. In this reaction, a one-pot three-component condensation reaction occurred between oxindole 139 and β-napthol 140 with aldehyde 141 in the presence of 10% of PVMoK-10 catalyst at 100 °C, providing a coupled chromeno product 142. The authors prepared different % loaded catalysts, among which 10% PVMoK incorporated MMT has better catalytic activity. The mechanistic pathway of the reaction is the first condensation reaction occurring between naphthol and oxindole; subsequently, the condensation product reacts with aldehyde, followed by cyclisation (Scheme 36). Finally, a coupled [2,3-b] indole product was obtained. Easily separable, reusable, and eco-friendly materials are the major advantages of the 10%PVMoK-MMT catalyst.
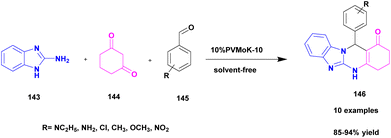
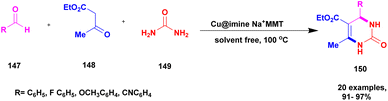
Prasanna and his group126 in 2022 reported a green approach for synthesizing benzoimidazoquinazolinone and indolylxanthenone derivatives using montmorillonite K-10 clay as a catalyst and keggin-type heteropoly-11-molybdo-1-vanadophosphoric acid. A simple one-pot three-component condensation reaction was used in the protocol, with 10% heteropoly-11-molybdo-1-vanadophosphoric acid (H4[PVMo11O40])-loaded montmorillonite K-10 clay material (PVMoK-10) serving as an efficient heterogeneous catalyst. The overall reaction procedure for the synthesis of benzo[4,5]imidazo[2,1b]quinazolin-1(2H)-one derivatives 146 is as follows: 2-aminobenzimidazole 143, 1,3-cyclohexadione 144, substituted aromatic aldehyde 145, and 0.05 g of the catalyst 10% PVMoK-10 were heated for one hour at 100 °C (Scheme 37). The performance of 10% catalysts PVMoK-10 and PV2Mo-K10 is significantly higher than that of raw mont-K10 clay and vanadium-substituted heteropoly acids. The reactions were carried out in various solvent media, including EtOH, MeOH, H2O, MeCN, DCE, DMF, CHCl3, 1,4-dioxane, n-hexane, and toluene. The results demonstrated that the solvent-free reaction setting was the best for the current synthetic transformation, yielding excellent products. Ten benzimidazoquinazolinone derivatives and two indolylxanthenone derivatives were synthesized with a focus on an environmentally friendly method. This methodology has several advantages, including a short reaction time, high yield, reusability of the catalytic material, a straightforward reaction procedure, and solvent-free reaction conditions.
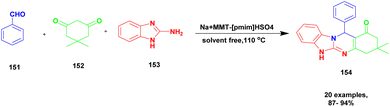
Shirini et al.128 reported a protocol for the synthesis of tetrahydrobenzimidazo [2, 1-b] quinazoline-1(2H)-one via solvent-free conditions using sodium montmorillonite clay incorporated with a Brønsted acidic ionic liquid as the catalyst. The benzimidazo quinazolinones were key compounds for the synthesis of the various biologically active compounds. In recent decades, synthetic methods have had some demerits. For example, they require expensive solvents and catalysts and a hazardous chemical, and some reactions need metal catalysts to proceed. Additionally, a very tedious process occurred that separated the catalyst from the reaction mixture. To avoid this limitation, the authors performed the reaction using the green chemistry method. The three Na+ montorillonote green catalysts, such as sodium montmorillonite, sodium montmorillonite [pmim] Cl, and sodium montmorillonite [pmim] HSO4, were prepared to carry out the reaction. The three Na+MMT[pmim] HSO4 catalysts exhibited excellent activity with 100% conversion of the reactant with better yield (Scheme 39). The reaction between an aromatic aldehyde 151 and dimedone 152 with the 2-aminobenzimidazole 153 in the presence of Na+MMT [pmim] HSO4 Brønsted acidic ionic liquid supported MMT clay heterogeneous catalyst under the without solvent condition at 110 °C provided excellent product tetrahydro benzimidazo quinazoline 154. When the reaction was performed in different catalytic mediums using different solvents, they provided lower yields than the Na+MMT [pmim] HSO4.
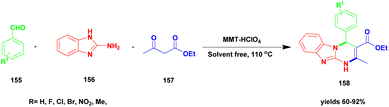
Mashhadinezhad et al.129 documented a Na+ montmorillonite perchloric acid heterogeneous catalyst employed for the synthesis of dihydropyrimidine-attached heterocyclic compounds using a solvent-free green chemistry method. The dihydropyrimidine derivative compounds exhibit excellent biological applications used against various disorders in medicinal fields. The preparation techniques have some drawbacks, and to avoid these drawbacks, the reaction was conducted using a heterogeneous catalyst and a greener method. The authors performed the reaction under natural Na+ – montmorillonite clay incorporated with a perchloric acid catalyst. The reaction between aldehyde 155 and benzimidazole 156 with ethylacetoacetate 157 in the presence of MMT-HClO4 catalyst under a solvent-free method provided the expected dihydrobenzimidazolo pyrimidine 158 derivatives with a better yield of up to 91% (Scheme 40). The same reaction carried out on a substituted substrate with the same MMT-HClO4 catalyst also provided a good yield. When the same reaction was carried out using a different catalyst, a lower yield was observed. The main merits of the prepared catalyst were easy recovery of the catalyst from the reaction mixture and reusability for another set of reactions without the loss of its catalytic activity. Even four cycles provided a better yield, and only 2% of product formation was decreased.
Makhsous et al.130 addressed a protocol for the construction of pyrimido [1, 2-a] benzimidazole moiety and pyrimido [1, 2-a] benzimidazole-3-carboxylate species using a solvent-free greener method with a brønsted acidic ionic liquid supported sodium montmorillonite clay heterogeneous catalyst. The pyrimido benzimidazole compounds have diverse biological activities used against various infections. Previously, executing this reaction under greener conditions had certain drawbacks. Hence, the authors introduced a Brønsted acid ionic liquid-supported sodium montmorillonite nanocomposite. The catalytic activity of the prepared catalyst was investigated by performing a reaction between an aromatic aldehyde 159 and 2-aminobenzimidazole 160 with cyanoacetonitrile 161 in the presence of Na+ MMT [pmim] HSO4 heterogeneous catalyst under solvent-free conditions at 100 °C. The expected pyrimido [1, 2-a] benzimidazole 162 was obtained with a better yield. The reaction was performed using ethylacetoacetate 163 instead of cyanoacetonitrile use, and the product tetrahydrobenzimidazo [1, 2-a] quinazoline-1-2(H)-one 164 derivative was obtained in a very good yield (Scheme 41). The same reaction was carried out in different solvents, and less product was formed. They require a longer time compared with solvent-free Na+MMT [pmim]HSO4 catalyst medium, and it provides a better yield of up to 95%.
Selvakumar et al.135 reported a natural clay-catalyzed heteropolyacid-supported C–N bond formation reaction. One-pot synthesis of compound 3, 4-dihydro pyrimidone and thiones was carried out. Here, the HPVAC-20 catalyst was used with natural clay, and it exhibited excellent catalytic activity compared to the metal catalyst. Initially, the reaction was conducted with various combinations of heteropoly acid supported with natural clay catalysts, and they provided less amount of yield compared to HPVAC-20. The condensation reaction between methyl acetoacetate 185, aldehyde 186, and urea/thiourea 187 occurred under solvent-free condition via a one-pot synthesis method in the presence of HPVAC-20 catalyst and provided 3,4-dihydropyrimidine-one 188 product with excellent yield of 91% (Scheme 46). When the same reaction was executed in the presence of solvent, a lower yield was obtained. The HPVAC-20 is an excellent catalyst, and it easily separates from the reaction medium using simple separation techniques without any change in catalytic activity and is reused for another reaction.
2.4.14.1 Biginelli reaction. In this review, most of the reactions discussed dihydropyrimidinone formation via the Biginelli reaction (Scheme 52). Initially, the heterogeneous clay catalyst activated the carbonyl group formation of the electrophilic carbon centre. The next step was the nucleophilic attack of urea on the electrophilic carbon centre activated with an iminium ion intermediate. Then, the iminium intermediate reacted with ethyl acetoacetate to form a stable intermediate III. The formed intermediate underwent intermolecular cyclization to form intermediate IV; finally, the expected Biginelli derivative product was formed.141
2.4.14.2 A3 coupling reaction. The A3 coupling reaction is one of the major C–N bond formation reactions, and the A3 coupled products have excellent activity in various fields (Scheme 53). The mechanistic pathway of the reaction with an initial condensation reaction takes place between the aldehyde and amine to form an iminium ion intermediate. Next, the formed intermediate reacted with acetylene in the presence of a catalyst, forming a three-component coupled C–N bonded product.142
2.4.14.3 KA2 coupling. The KA2 coupling is the three-component coupling of aldehyde, amine and ketone that reacts to form a C–N bonded product (Scheme 54). The mechanism of KA2 coupling is similar to that of the A3 coupling; instead of aldehyde, the KA2 coupling ketone was used.143
3 Conclusions
In this review, we highlighted the activity of clay-based material for the synthesis of carbon–nitrogen bonded compounds from 2016 to date. Clays are easily available and low-cost catalytic materials; they exhibit excellent catalytic activity and are widely employed for diverse applications in organic synthesis. Over the past few years, clay-based catalysts have attracted synthetic organic chemists. The modified clay catalyst achieves a better yield for different types of organic reactions. The metal, metal oxides, inorganic complexes, and some organic compounds are used to modify clay materials because they have versatile activity to provide highly selective products. Many multicomponent reactions are performed using various clay catalysts, among which the Biginelli reaction and A3 coupling reaction are important for the synthesis of C–N bonded organic products. The metal nanoparticle incorporated and Keggin-type heteropoly acid supported clay catalysts are used for the construction of several carbon–nitrogen bonded heterocyclic compounds. Clay-based catalysts are widely used in organic synthesis owing to their excellent catalytic activity. This kind of catalyst is emerging as an effective one as evident from the literature aforementioned. Though there are some drawbacks to clay as a catalyst, its excellent catalytic activity overshades them, which is of interest to various researchers working in this field. The present review emphasizes various modified clays, such as bentonite, montmorillonite, hydrotalcite, halloysite, red brick, and natural clay supported or catalyzed carbon–nitrogen bond formation reactions to synthesize the selectively coupled product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank VIT management for their support and encouragement.Notes and references
- J. Bariwal and E. Van der Eycken, Chem. Soc. Rev., 2003, 42, 9283–9303 RSC.
- A. F. Pozharskii, A. R. Katritzky and A. T. Soldatenkov, Heterocycles in Life and Society, Wiley, Chichester, 2011 Search PubMed.
- C. Cavedon, P. H. Seeberger and B. Pieber, Eur. J. Org Chem., 2020, 10, 1379–1392 CrossRef.
- M. E. Webb, A. Marquet, R. R. Mendel, F. Rébeillé and A. G. Smith, Nat. prod. Rep., 2007, 24, 988–1008 RSC.
- P. V. Kattamuri, J. Yin, S. Siriwongsup, D. H. Kwon, D. H. Ess, Q. Li and L. Kurti, J. Am. Chem. Soc., 2017, 139, 11184–11196 CrossRef CAS PubMed.
- R. Hili and A. K. Yudin, Nat. Chem. Biol., 2006, 2, 284–287 CrossRef CAS PubMed.
- J. A. Joule, K. Mills and G. F. Smith, Heterocyclic Chemistry, CRC Press, 2020 Search PubMed.
- S. Huang, Y. Han, M. Chen, K. Hu, Y. Qi, P. Sun and X. Zheng, Bioorg. Med. Chem. Lett., 2018, 28, 1143–1148 CrossRef CAS PubMed.
- I. Gallou, Org. Prep. Proced. Int., 2007, 39, 355–383 CrossRef CAS.
- P. G. Do Nascimento, T. L. Lemos, A. M. Bizerra, A. M. Arriaga, D. A. Ferreira, G. M. Santiago and J. G. Costa, Molecules, 2014, 19, 1317–1327 CrossRef PubMed.
- L. Kong, S. Li, Q. Liao, Y. Zhang, R. Sun, X. Zhu and Y. Zhu, Antiviral Res., 2013, 98, 44–53 CrossRef CAS PubMed.
- M. S. Ali, S. A. Ibrahim, S. Jalil and M. I. Choudhary, Phytother. Res., 2007, 21, 558–561 CrossRef CAS PubMed.
- R. M. Pérez Gutiérrez, R. Vargas Solis, E. Garcia Baez and Y. G. Navarro, J. Nat. med., 2009, 63, 393–401 CrossRef PubMed.
- I. E. El Azab, H. S. El-Sheshtawy, R. B. Bakr and N. A. Elkanzi, Molecules, 2021, 26, 708 CrossRef CAS PubMed.
- J. Regan, S. Breitfelder, P. Cirillo, T. Gilmore, A. G. Graham, E. Hickey and C. Torcellini, J. Med. Chem., 2002, 45, 2994–3008 CrossRef CAS PubMed.
- D. V. Hardouin, G. L. Bano and F. Schaper, ACS Catal., 2018, 8, 7308–7325 CrossRef.
- S. Roy, M. J. Sarma, B. Kashyap and P. Phukan, Chem. Commun., 2016, 52, 1170–1173 RSC.
- Y. Wang, R. Xie, L. Huang, Y. N. Tian, S. Lv, X. Kong and S. Li, Org. Chem. Front., 2021, 8, 5962–5967 RSC.
- D. S. Raghuvanshi, A. K. Gupta and K. N. Singh, Org. Lett., 2012, 14, 4326–4329 CrossRef CAS PubMed.
- G. Li, T. Zhou, A. Poater, L. Cavallo, S. P. Nolan and M. Szostak, Catal. Sci. Technol., 2020, 10, 710–716 RSC.
- M. V. Reddy, G. Anusha and P. V. Reddy, New J. Chem., 2020, 44, 11694–11703 RSC.
- A. K. Srivastava, C. Sharma and R. K. Joshi, Green Chem., 2020, 22, 8248–8253 RSC.
- N. Kathewad, M. C. Anagha, N. Parvin, S. Parambath, P. Parameswaran and S. Khan, Dalton Trans., 2019, 48, 2730–2734 RSC.
- M. Li, X. Xing, Z. Ma, J. Lv, P. Fu and Z. Li, ACS Sustainable Chem. Eng., 2018, 6, 5495–5503 CrossRef CAS.
- A. F. Quivelli, P. Vitale, F. M. Perna and V. Capriati, Front. Chem., 2019, 7, 723 CrossRef CAS PubMed.
- V. K. Madduluri, S. K. Mishra and A. K. Sah, Inorg. Chem. Commun., 2020, 120, 108165 CrossRef CAS.
- D. Girija, H. B. Naik, B. V. Kumar, C. N. Sudhamani and K. N. Harish, Arabian J. Chem., 2019, 12, 420–428 CrossRef CAS.
- N. Li, S. Xu, X. Wang, L. Xu, J. Qiao, Z. Liang and X. Xu, Chin. Chem. Lett., 2021, 32, 3993–3997 CrossRef CAS.
- N. V. Tzouras, S. P. Neofotistos and G. C. Vougioukalakis, ACS omega, 2019, 4, 10279–10292 CrossRef CAS PubMed.
- Z. L. Ren, P. He, W. T. Lu, M. Sun and M. W. Ding, Org. Biomol. Chem., 2018, 16, 6322–6331 RSC.
- C. Liu, L. Song, L. Van Meervelt, V. A. Peshkov, Z. Li and E. V. Van der Eycken, Org. Lett., 2021, 23, 5065–5070 CrossRef CAS PubMed.
- C. L. Allen and J. M. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC.
- H. Yin, M. Jin, W. Chen, C. Chen, L. Zheng, P. Wei and S. Han, Tetrahedron Lett., 2012, 53, 1265–1270 CrossRef CAS.
- J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 RSC.
- J. Xu, Q. Chen, Z. Luo, X. Tang and J. Zhao, RSC adv., 2019, 9, 28764–28767 RSC.
- R. F. Gomes, N. R. Esteves, J. A. Coelho and C. A. Afonso, J. Org. Chem., 2018, 83, 7509–7513 CrossRef CAS PubMed.
- B. S. Kumar, A. Dhakshinamoorthy and K. Pitchumani, Catal. Sci. Technol., 2014, 4, 2378–2396 RSC.
- X. Pei, Y. Li, L. Lu, H. Jiao, W. Gong and L. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 44418–44426 CrossRef CAS PubMed.
- F. A. Etzkorn, Green Chemistry: Principles and Case Studies, Royal Society of Chemistry, 2019 Search PubMed.
- P. T. Anastas, L. B. Bartlett, M. M. Kirchhoff and T. C. Williamson, Catal. Today, 2000, 55, 11–22 CrossRef CAS.
- M. Lancaster, Green Chemistry: an Introductory Text, Royal society of chemistry, 2020 Search PubMed.
- J. H. Clark and D. J. Macquarrie, Handbook of Green Chemistry and Technology, John Wiley & Sons, 2008 Search PubMed.
- M. Kurian and S. Kavitha, IOSR J. Appl. Chem., 2016, 1, 47–54 CAS.
- G. Brown, Crystal structures of clay minerals and their X-ray identification, The Mineralogical Society of Great Britain and Ireland, 1982, vol. 5 Search PubMed.
- F. Bergaya and G. Lagaly, Handbook of Clay Science Part A: Fundamentals, 2nd edn, 2013 Search PubMed.
- A. Vaccari, Appl. Clay Sci., 1999, 14, 161–198 CrossRef CAS.
- M. I. Carretero and M. Pozo, Appl. Clay Sci., 2009, 46, 73–80 CrossRef CAS.
- C. I. Sainz-Diaz, V. Timon, V. Botella and A. Hernandez-Laguna, Am min, 2000, 85, 1038–1045 CrossRef CAS.
- G. Nagendrappa, Resonance, 2002, 7, 64–77 Search PubMed.
- E. M. Araújo, T. J. A. Melo, L. N. L. Santana, G. A. Neves, H. C. Ferreira, H. L. Lira and I. S. Araújo, Mater. Sci. Eng. B, 2004, 112, 175–178 CrossRef.
- T. Chellapandi and G. Madhumitha, Appl. Nanosci., 2021, 11, 1379–1391 CrossRef CAS.
- F. Bergaya and G. Lagaly, Dev. clay sci., 2006, 1, 1–18 CAS.
- M. Mashhadinezhad, Iran. J. Catal., 2013, 3, 119–121 CAS.
- M. Hechelski, A. Ghinet, B. Louvel, P. Dufrénoy, B. Rigo, A. Daïch and C. Waterlot, ChemSusChem, 2018, 11, 1249–1277 CrossRef CAS PubMed.
- M. Dessalegne, F. Zewge, W. Mammo, G. Woldetinsae and I. Diaz, Bull. Chem. Soc. Ethiop., 2018, 32, 199–211 CrossRef CAS.
- A. M. Youssef, H. E. Nasr, A. M. Ramadan and W. S. Mohamed, Drug. Egypt J. Chem., 2012, 55, 143–159 CrossRef.
- L. Krstić, S. Sukdolak and S. Solujić-Sukdolak, J. Serb. Chem. Soc., 2002, 67, 325–329 CrossRef.
- B. K. G. Theng, The Chemistry of Clay-Organic Reactions, The Chemistry of Clay-Organic Reactions, 1974 Search PubMed.
- J. H. Park, H. J. Shin, M. H. Kim, J. S. Kim, N. Kang, J. Y. Lee and D. D. Kim, Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems, J. Pharm. Invest., 2016, 46, 363–375 CrossRef CAS PubMed.
- J. Nones, H. G. Riella, A. G. Trentin and J. Nones, Appl. Clay Sci., 2015, 105, 225–230 CrossRef.
- N. Wahab, M. Saeed, M. Ibrahim, A. Munir, M. Saleem, M. Zahra and A. Waseem, Front. Chem., 2019, 7, 654 CrossRef CAS PubMed.
- B. Bananezhad, M. R. Islami, E. Ghonchepour, H. Mostafavi, A. M. Tikdari and H. R. Rafiei, Polyhedron, 2019, 162, 192–200 CrossRef CAS.
- K. Kaneda and T. Mizugaki, Green Chem., 2019, 21, 1361–1389 RSC.
- B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127–14136 CrossRef CAS PubMed.
- X. Duan, J. Lu and D. G. Evans, Assembly Chemistry of Anion-Intercalated Layered Materials, in Modern Inorganic Synthetic Chemistry, Elsevier, 2011, p. 375 Search PubMed.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173–301 CrossRef CAS.
- U. Costantino, V. Ambrogi, M. Nocchetti and L. Perioli, Microporous Mesoporous Mater., 2008, 107(1–2), 149–160 CrossRef CAS.
- P. Yuan, A. Thill and F. Bergaya, Nanosized Tubular Clay Minerals: Halloysite and Imogolite, Elsevier, 2016 Search PubMed.
- S. Barrientos-Ramírez, E. V. Ramos-Fernández, J. Silvestre-Albero, A. Sepúlveda-Escribano, M. M. Pastor-Blas and A. González-Montiel, Microporous Mesoporous Mater., 2009, 120, 132–140 CrossRef.
- D. Grabka, M. Raczyńska-Żak, K. Czech, P. M. Słomkiewicz and M. A. Jóźwiak, Appl. Clay Sci., 2015, 114, 321–329 CrossRef CAS.
- Y. Zhang, A. Tang, H. Yang and J. Ouyang, Appl. Clay Sci., 2016, 119, 8–17 CrossRef CAS.
- M. Massaro, V. Schembri, V. Campisciano, G. Cavallaro, G. Lazzara, S. Milioto and S. Riela, RSC adv., 2016, 6, 55312–55318 RSC.
- M. O. Eyankware, C. Ogwah and J. C. Ike, Res. Ecol., 2021, 3(2), 32–45 CrossRef CAS.
- S. Attique, M. Batool, M. I. Jalees, K. Shehzad, U. Farooq, Z. Khan and A. T. Shah, Turk. J. Chem., 2018, 42, 684–693 CAS.
- N. Nami, M. Tajbakhsh and M. Vafakhah, QJICC, 2019, 7, 93–101 CAS.
- J. P. Nguetnkam, R. Kamga, F. Villiéras, G. E. Ekodeck, A. Razafitianamaharavo and J. Yvon, J. Colloid Interface Sci., 2005, 289, 104–115 CrossRef CAS PubMed.
- G. Nagendrappa, Appl. Clay Sci., 2011, 53, 106–138 CrossRef CAS.
- N. Kaur and D. Kishore, J. Chem. Pharm. Res., 2012, 4, 991–1015 CAS.
- B. S. Kumar, A. Dhakshinamoorthy and K. Pitchumani, Catal. Sci. Technol., 2014, 4, 2378–2396 RSC.
- D. K. Dutta, J. Mater. NanoSci., 2019, 6, 19–31 CAS.
- G. Nagendrappa and R. R. Chowreddy, Catal. Surv. Asia., 2021, 25, 231–278 CrossRef CAS.
- T. Chellapandi and G. Madhumitha, Mol. Diversity, 2021, 1–29 Search PubMed.
- J. Bariwal and E. Van der Eycken, Chem. Soc. Rev., 2013, 42, 9283–9303 RSC.
- S. K. Ghorai, V. G. Gopalsamuthiram, A. M. Jawalekar, R. E. Patre and S. Pal, Tetrahedron, 2017, 73, 1769–1794 CrossRef CAS.
- M. D. Kärkäs, Chem. Soc. Rev., 2018, 47, 5786–5865 RSC.
- Y. Zhao and W. Xia, Chem. Soc. Rev., 2018, 47, 2591–2608 RSC.
- N. Kaur, N. Ahlawat, Y. Verma, P. Grewal and P. A. Bhardwaj, Curr. Org. Chem., 2019, 23, 1901–1944 CrossRef CAS.
- F. A. Sofi and P. V. Bharatam, Curr. Org. Chem., 2020, 24, 2293–2340 CrossRef CAS.
- M. Ju and J. M. Schomaker, Nat. Rev. Chem, 2021, 5, 580–594 CrossRef CAS PubMed.
- M. Gholinejad, R. Bonyasi, C. Najera, F. Saadati, M. Bahrami and N. Dasvarz, ChemPlusChem, 2018, 83, 431–438 CrossRef CAS PubMed.
- K. Sravanthi, D. Ayodhya and P. Y. Swamy, Mater. Sci. Technol., 2019, 2, 298–307 Search PubMed.
- L. V. Chopda and P. N. Dave, ChemistrySelect, 2020, 5, 2395–2400 CrossRef CAS.
- L. V. Chopda and P. N. Dave, ChemistrySelect, 2020, 5, 14161–14167 CrossRef CAS.
- S. Sadjadi and F. Koohestani, J. Mol. Liq., 2020, 319, 114393 CrossRef CAS.
- L. V. Chopda and P. N. Dave, Arabian J. Chem., 2020, 13, 5911–5921 CrossRef CAS.
- S. Sadjadi, M. M. Heravi and M. Daraie, Res. Chem. Intermed., 2017, 43, 2201–2214 CrossRef CAS.
- S. Sadjadi, M. M. Heravi and M. Malmir, Res. Chem. Intermed., 2017, 43, 6701–6717 CrossRef CAS.
- S. Sadjadi, T. Hosseinnejad, M. Malmir and M. M. Heravi, New J. Chem., 2017, 41, 13935–13951 RSC.
- S. Sadjadi and N. Bahri-Laleh, J. Porous Mater., 2018, 25, 821–833 CrossRef CAS.
- S. Sadjadi, M. M. Heravi, M. Malmir and F. Noritajer, Mater. Chem. Phys., 2019, 223, 380–390 CrossRef CAS.
- T. Ataee-Kachouei, M. Nasr-Esfahani, I. M. Baltork, V. Mirkhani, M. Moghadam, S. Tangestaninejad and R. Kia, ChemistrySelect, 2019, 4, 2301–2306 CrossRef CAS.
- V. Dabholka, K. Badhe and S. Kurade, Curr. Chem. Lett., 2017, 6, 77–90 CrossRef.
- M. Soni, N. Joshi and J. Vardia, Chem. Pharm., 2017, 6, 14–20 Search PubMed.
- P. S. Samudrala, A. V. Nakhate, S. S. R. Gupta, K. B. Rasal, G. P. Deshmukh, C. R. Gadipelly and L. K. Mannepalli, Appl. Catal., B, 2019, 240, 348–357 CrossRef CAS.
- T. L. Lambat and S. S. Deo, Synth. Catal., 2016, 1, 1 Search PubMed.
- T. L. Lambat, S. S. Deo, F. S. Inam, T. B. Deshmukh and A. R. Bhat, Karbala. Int. J. Mod. Sci., 2016, 2, 63–68 CrossRef.
- F. Gómez-Sanz, M. V. Morales-Vargas, B. González-Rodríguez and M. L. Rojas-Cervantes, Appl. Clay Sci., 2017, 143, 250–257 CrossRef.
- Z. Zarnegar, R. Alizadeh and M. Ahmadzadeh, J. Mol. Struct., 2017, 1144, 58–65 CrossRef CAS.
- X. Zhang, M. Liu, W. Qiu, J. Evans, M. Kaur, J. P. Jasinski and W. Zhang, ACS Sustain. Chem. Eng., 2018, 6, 5574–5579 CrossRef CAS.
- S. Jayashree and K. Shivashankar, Synth. Commun., 2018, 48, 1805–1815 CrossRef CAS.
- S. Bonacci, G. Iriti, S. Mancuso, P. Novelli, R. Paonessa, S. Tallarico and M. Nardi, Catalysts, 2020, 10, 845 CrossRef CAS.
- V. Kannan and K. Sreekumar, J. Heterocycl. Chem., 2021, 58, 153–160 CrossRef CAS.
- M. Ahmadzadeh, M. Sadeghi and J. Safari, J. Chem., 2021, 1–11 Search PubMed.
- C. Pathak and G. Borah, Chem. Pap., 2022, 76, 4749–4761 CrossRef CAS.
- Z. Besharati, M. Malmir and M. M. Heravi, Inorg. Chem. Commun., 2022, 143, 109813 CrossRef CAS.
- S. Bonacci, M. Nardi, P. Costanzo, A. De Nino, M. L. Di Gioia, M. Oliverio and A. Procopio, Catalysts, 2019, 9, 301 CrossRef.
- S. U. Tekale, S. B. Munde, S. S. Kauthale and R. P. Pawar, Org. Prep. Proced. Int., 2018, 50, 314–322 CrossRef CAS.
- D. P. Narayanan, A. Gopalakrishnan, Z. Yaakob, S. Sugunan and B. N. Narayanan, Arabian J. Chem., 2020, 13, 318–334 CrossRef CAS.
- S. Farooq, F. A. Alharthi, A. Alsalme, A. Hussain, B. A. Dar, A. Hamid and S. Koul, RSC adv., 2020, 10, 42221–42234 RSC.
- I. N. Shaikh, M. A. Baseer, D. B. Ahmed, S. F. Adil, M. Khan and A. Alwarthan, J. King Saud Univ., Sci., 2020, 32, 979–985 CrossRef.
- M. Kumaresan, K. Selvakumar and P. Sami, Mater. Today: Proc., 2017, 4, 12437–12447 Search PubMed.
- M. Kumaresan, V. Karthika, K. Selvakumar and P. Sami, Synth. Commun., 2019, 49, 2856–2868 CAS.
- M. Masteri-Farahani, A. Ezabadi, R. Mazarei, P. Ataeinia, S. Shahsavarifar and F. Mousavi, Appl. Organomet. Chem., 2020, 34, e5727 CrossRef CAS.
- D. S. Aher, K. R. Khillare, L. D. Chavan and S. G. Shankarwar, ChemistrySelect, 2020, 5, 7320–7331 CrossRef CAS.
- P. Antony Muthu, K. Murugan, S. Meenakshisundaram and S. Ponnusamy, Res. Chem. Intermed., 2021, 47, 3583–3595 CrossRef CAS.
- A. M. Prasanna, M. Kumaresan, K. Selvakumar, M. Swaminathan and P. Sami, Res. Chem. Intermed., 2022, 48, 5029–5044 CrossRef CAS.
- A. Khorshidi, K. Tabatabaeian, H. Azizi, M. Aghaei-Hashjin and E. Abbaspour-Gilandeh, RSC adv., 2017, 7, 17732–17740 RSC.
- F. Shirini, M. Mazloumi and M. Seddighi, J. Nanosci. Nanotechnol., 2018, 18, 1194–1198 CrossRef CAS PubMed.
- M. Mashhadinezhad, F. Shirini, M. Mamaghani and M. Rassa, Polycyclic Aromat. Compd., 2019, 40, 1417–1433 CrossRef.
- M. Makhsous, F. Shirini, M. Seddighi and M. Mazloumi, Polycyclic Aromat. Compd., 2020, 40, 494–501 CrossRef CAS.
- S. J. Borah and D. K. Das, Catal. Lett., 2016, 146, 656–665 CrossRef CAS.
- A. Phukan, S. J. Borah, P. Bordoloi, K. Sharma, B. J. Borah, P. P. Sarmah and D. K. Dutta, Adv. Powder Technol., 2017, 28, 1585–1592 CrossRef CAS.
- J. Safari and M. Ahmadzadeh, J. Taiwan Inst. Chem. Eng., 2017, 74, 14–24 CrossRef CAS.
- K. Selvakumar, T. Shanmugaprabha, M. Kumaresan and P. Sami, Synth. Commun., 2017, 47, 2115–2126 CrossRef CAS.
- K. Selvakumar, T. Shanmugaprabha, M. Kumaresan and P. Sami, Synth. Commun., 2018, 48, 223–232 CrossRef CAS.
- N. Kerru, L. Gummidi, S. V. Bhaskaruni, S. N. Maddila and S. B. Jonnalagadda, Mol. Diversity, 2020, 24, 889–901 CrossRef CAS PubMed.
- S. Bentahar, M. A. Taleb, A. Sabour, A. Dbik, M. El Khomri, N. El Messaoudi and R. Mamouni, Russ. J. Org. Chem., 2019, 55, 1423–1431 CrossRef CAS.
- D. S. Aher, K. R. Khillare, L. D. Chavan and S. G. Shankarwar, RSC adv., 2021, 11, 2783–2792 RSC.
- B. Anjaneyulu and D. Rao, Int. J. Eng. Res. Sci. Technol., 2015, 3, 26–37 Search PubMed.
- J. Farhi, I. N. Lykakis and G. E. Kostakis, Catalysts, 2022, 12, 660 CrossRef CAS.
- N. V. Tzouras, S. P. Neofotistos and G. C. Vougioukalakis, ACS omega, 2019, 4, 10279–10292 CrossRef CAS PubMed.
- S. P. Babar, Int. res. j. pharm. med. sci., 2022, 5, 9–12 Search PubMed.
- S. P. Babar and V. M. Kamble, Int. j. res. appl. sci. eng. technol., 2021, 9, 467–472 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |