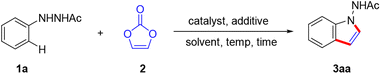Open Access Article
Open Access ArticleRhodium-catalyzed annulation of hydrazines with vinylene carbonate to synthesize unsubstituted 1-aminoindole derivatives†
Yichun Chen‡
,
Ziqi Lu‡,
Wenfen He,
Huanyi Zhu,
Weilong Lu,
Junjun Shi ,
Jie Sheng and
Wucheng Xie
,
Jie Sheng and
Wucheng Xie *
*
School of Environment and Chemical Engineering, Foshan University, Foshan 528000, China. E-mail: wuchengxie@fosu.edu.cn
First published on 6th February 2024
Abstract
Herein, we describe rhodium-catalysed C–H bond activation for [3 + 2] annulation using hydrazide and vinylene carbonate, providing an efficient method for synthesising unsubstituted 1-aminoindole compounds. Characterised by high yields, mild reaction conditions, and no need for external oxidants, this transformation demonstrates excellent regioselectivity and a wide tolerance for various functional groups.
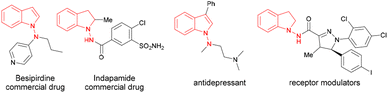
Indole scaffolds,1 among the most important N-heterocycles, have a wide range of applications, from pharmaceuticals and agrochemicals to renewable energy.2 Particularly, 1-aminoindoles3 serve as core components of numerous bioactive molecules, such as commercial drugs like besipirdine4b and indapamide,4c as well as antidepressant and receptor modulators (Fig. 1). Traditional synthetic methods5 for these moieties are often complex, involving most synthetic routes requiring multiple steps and electrophilic N-amination. The necessity for strong bases, less secure N-aminating reagents, and harsh reaction conditions have limited their synthetic utility and practicality. Consequently, developing more efficient and economically viable synthetic strategies for the 1-aminoindole moiety has attracted significant attention.
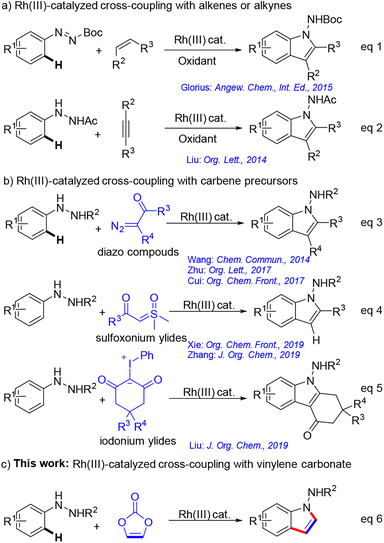
Over the past two decades, transition metal-catalysed C–H bond activation6 has emerged as a valuable approach for constructing complex molecular scaffolds. Rh(III) complexes,7 in particular, have been instrumental in catalysing annulation reactions.8 However, only a few studies have been conducted on the synthesis of 1-aminoindole skeletons via directed C–H bond activation. Glorius et al.8a developed an Rh(III)-catalysed oxidative annulation method using aryl-substituted diazene carboxylates and alkenes, producing C2, C3-disubstituted 1-aminoindoles (Scheme 1a, eqn (1)). Liu et al.8b achieved the oxidative annulation of hydrazines with alkynes using 1,3-dinitrobenzene as an oxidant (Scheme 1a, eqn (2)). However, these methods require stoichiometric oxidants.
The coupling of arenes with carbene precursors9 in a redox-neutral manner has introduced important alternatives to oxidative annulation reactions. Wang's group10a realized Rh(III)-catalyzed tandem annulation of arylhydrazines with diazo compounds as carbene precursors to yield 1-aminoindole products (Scheme 1b, eqn (3)). Zhu et al.10b successfully used this strategy to synthesise N-aminoindoles via N-Boc cleavage (Scheme 1b, eqn (3)). Cui's group10c he synthesis of 1-aminoindoles through a three-component cyclisation using in situ-formed hydrazones as guiding groups (Scheme 1b, eqn (3)). Owing to their relatively higher safety and stability, sulfoxonium ylides11 have been demonstrated as carbene surrogates for diazo compounds. Xie12a and Zhang12b independently described a rhodium-catalyzed redox-neutral reaction involving arylhydrazines and sulfoxonium ylides to create C2-substituted 1-aminoindole derivatives (Scheme 1b, eqn (4)). Recently, Li and his colleagues13 reported an Rh(III)-catalyzed C–H bond activation reaction of arylhydrazines using iodonium ylides as a carbene precursor (Scheme 1b, eqn (5)). Despite the considerable progress with respect to the synthesis of 1-aminoindole derivatives, the synthesis of C2, C3-unsubstituted 1-aminoindoles through directed C–H bond activation remains underexplored (Scheme 1c, eqn (6)).
Vinylene carbonate14 is a stable and readily available substance that can be produced on an industrial scale. In 2019, Miura's group15 pioneered the use of vinylene carbonate as an acetylene surrogate strategy in C–H functionalization and cascade annulations. Building upon this concept, the facile synthesis of various heterocycles, such as isoquinolines, isocoumarins, quinazolines, quinoline quinolone, pyrazolidinones, cinnolines, and indoles using vinylene carbonate have been realized.16 In line with our ongoing interest in heterocycle synthesis, we introduce a novel method for synthesising crucial C2, C3-unsubstituted 1-aminoindoles derivatives. This approach involves Rh(III)-catalyzed C–H bond activation and cascade annulation from arylhydrazines and vinylene carbonate (Scheme 1c).
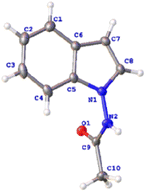
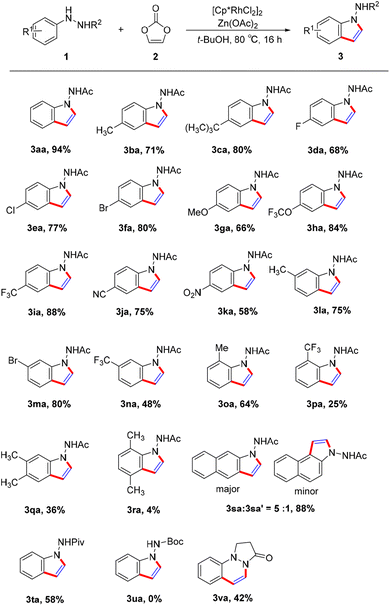
To initiate our investigation of this reaction, we selected 1N′-phenylacetohydrazide (1a) and vinylene carbonate (2a) as the model substrates for the coupling reaction (Table 1). We were pleased to discover that the desired 1-aminoindole product 3aa was obtained in 66% yield in the presence of a catalytic combination of [Cp*RhCl2]2 and Zn(OAc)2 in DMF (Table 1, entry 1). NMR spectroscopy and high-resolution mass spectrometry confirmed the structure of 3aa, which was further validated via X-ray crystallographic analysis17 (Fig. 2). A control experiment demonstrated the necessity of the Rh(III) catalyst; alternative catalysts such as Cp*Co(CO)I2, [Cp*IrCl2]2 and [p-cymeneRuCl2]2 did not facilitate the reaction (Table 1, entries 3–5). The use of the cationic catalyst [Cp*Rh(MeCN)3](SbF6)2 resulted in a decreased yield (Table 1, entry 2). Various additives were screened, but it was disappointing that no reaction occurred when NaOAc or KOAc was used (Table 1, entries 7 and 8). A range of solvents was tested, including t-BuOH, MeCN, DME, DCE, dioxane, TAA, toluene and CH3OH, with t-BuOH proving to be the optimal solvent, achieving a 94% yield of the target product 3aa (Table 1; entry 9). When CH3OH was used as the solvent, the reaction did not proceed (Table 1, entry 16). The reaction efficiency was negatively affected by both increments and decrements in reaction temperature (Table 1, entries 17 and 18). Altering the reaction duration to 12 or 20 h resulted in a reduced yield (Table 1, entries 19 and 20).
| Entry | Catalyst | Additive | Solvents | Yieldb (%) |
|---|---|---|---|---|
| a 1a (0.20 mmol), 2a (0.40 mmol), catalyst (5 mol%), additive (20 mol%), solvent (2.0 mL), N2, 80 °C, 16 h.b Isolated yield.c 60 °C.d 100 °C.e For 12 h.f For 20 h. | ||||
| 1 | [Cp*RhCl2]2 | Zn(OAc)2 | DMF | 66 |
| 2 | [Cp*Rh(MeCN)3][SbF6]2 | Zn(OAc)2 | DMF | 41 |
| 3 | Cp*Co(CO)I2 | Zn(OAc)2 | DMF | nd |
| 4 | [Cp*IrCl2]2 | Zn(OAc)2 | DMF | nd |
| 5 | [p-CymeneRuCl2]2 | Zn(OAc)2 | DMF | nd |
| 6 | None | Zn(OAc)2 | DMF | nd |
| 7 | [Cp*RhCl2]2 | NaOAc | DMF | nd |
| 8 | [Cp*RhCl2]2 | KOAc | DMF | nd |
| 9 | [Cp*RhCl2]2 | Zn(OAc)2 | t-BuOH | 94 |
| 10 | [Cp*RhCl2]2 | Zn(OAc)2 | MeCN | 91 |
| 11 | [Cp*RhCl2]2 | Zn(OAc)2 | DME | 86 |
| 12 | [Cp*RhCl2]2 | Zn(OAc)2 | DCE | 85 |
| 13 | [Cp*RhCl2]2 | Zn(OAc)2 | Dioxane | 80 |
| 14c | [Cp*RhCl2]2 | Zn(OAc)2 | TAA | 76 |
| 15 | [Cp*RhCl2]2 | Zn(OAc)2 | Toluene | 70 |
| 16 | [Cp*RhCl2]2 | Zn(OAc)2 | CH3OH | nd |
| 17c | [Cp*RhCl2]2 | Zn(OAc)2 | t-BuOH | 66 |
| 18d | [Cp*RhCl2]2 | Zn(OAc)2 | t-BuOH | 80 |
| 19e | [Cp*RhCl2]2 | Zn(OAc)2 | t-BuOH | 90 |
| 20f | [Cp*RhCl2]2 | Zn(OAc)2 | t-BuOH | 90 |
With the optimized conditions established, we explored the substrate scope of 1-acetyl-2-phenylhydrazine (Scheme 2). The transformation exhibited a broad substrate scope, accommodating 2-acetyl-1-arylhydrazines with various substituents. 2-Acetyl-1-arylhydrazines, whether bearing electron-donating group (e.g., Me, OMe, and OCF3) or electron-withdrawing groups (e.g., CF3, CN, NO2) at the para position, all reacted effectively with vinylene carbonate, yielding the corresponding products (3ba–3ka) in moderate to good yields. This functional group compatibility proves highly beneficial for synthesizing complex molecules. Furthermore, substrates with halogen substituents (e.g. F, Cl and Br) at different positions were well-tolerated, enabling further derivatisation through coupling reactions. This transformation demonstrated excellent regioselectivity, with coupling occurring at the less hindered position for meta-substituted substrates (3la–3na). The reaction seems to be sensitive to steric hindrance, as ortho-substituted phenylacetohydrazines (1m–1o) exhibited limited reactivity (3oa–3ra). When disubstituted derivatives were used in the reaction with vinylene carbonate, the product yield was reduced (3qa, 3ra). Moreover, when N′-(naphthalen-2-yl)acetohydrazide (1s) was used, the C–H bond at the 3-position, with less steric hindrance was preferred to be functionalized (3sa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3sa′ = 5
3sa′ = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 88%).
1, 88%).
Our investigation also included hydrazines with different substitutions at the R2 position. A pivaloyl (Piv) group led to the formation of 1-aminoindole derivatives (3ta) in 58% yield, while an N-Boc substituent failed to produce the desired product (3ua). Additionally, disubstituted hydrazine 1v was also explored and the corresponding product (3va) could be isolated in 42% yield.
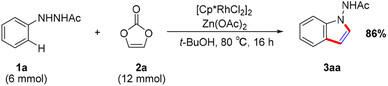
To demonstrate the synthesis utility of our catalytic system, we performed a scaled-up reaction with 6 mmol of hydrazine (1a), obtaining product 3aa in 86% yield (Scheme 3).
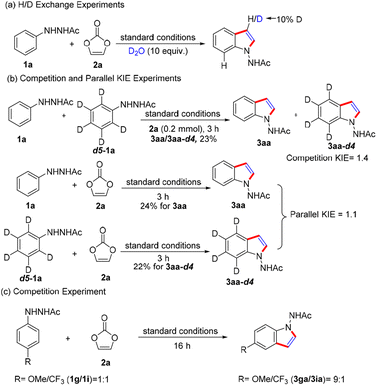
A series of experiments was conducted to investigate the reaction mechanism (Scheme 4). Initially, an H/D exchange study was carried out under standard conditions in the presence of D2O. Only 10% deuterium was incorporated at the C3 position of 3aa but not at other positions (Scheme 4a). The results indicate a protonation process was possibly involved in the transformation. Furthermore, a kinetic isotope effect (KIE) experiment was investigated. An intermolecular competitive KIE experiment using equivalent substrates 1a and 1a-d5 was implemented, revealing a KIE value of 1.4 (Scheme 4b). Moreover, a KIE value of 1.1 was detected based on parallel experiments (Scheme 4b). These results suggested that the C–H bond cleavage might not be involved in the rate-determining step of the overall reaction. Additionally, an intermolecular competition reaction between 1g and 1i was carried out in a one-pot fashion under the optimized reaction conditions, and the products were isolated in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (3ga/3ia), demonstrating higher reactivity for the electron-rich substrate (Scheme 4c).
1 ratio (3ga/3ia), demonstrating higher reactivity for the electron-rich substrate (Scheme 4c).
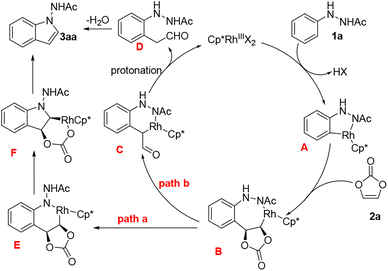
Based on the aforementioned experiments and previous reports,18 we propose two possible catalytic cycle pathways (Scheme 5, path a and path b). The cycle begins with the activation of a C–H bond, catalysed by rhodium(III), leading to the formation of a five-membered rhodacycle intermediate A. Subsequently, intermediate A coordinates with vinylene carbonate and undergoes migratory insertion of vinylene carbonate into the Rh–C bond, resulting in the formation of a seven-membered metallacycle complex B. Rhodacycle B may further undergo intramolecular amide attack towards Rh, yielding the six-membered E (path a). This is followed by C–N reductive elimination, which is succeeded by the formation of a bond into the adjacent C–O bond, giving rise to intermediate F. Formal β-oxygen elimination is affected to liberate the desired product (3aa) along with the regenerated catalyst, and the extrusion of CO2 (path a).
An alternative possibility is that 1,2-Rh–C bond migration and decarboxylation may occur from intermediate B, resulting in the formation of intermediate C (path b). Subsequently, a protonolysis process generates the corresponding aldehyde intermediate D, along with the regeneration of the Cp*Rh(III). After eliminating water through intramolecular condensation, the final product 3aa is formed.
Conclusions
In summary, we developed a new method for the step-economical synthesis of C2, C3-unsubstituted 1-aminoindole derivatives. This method involves rhodium-catalysed annulation of hydrazines with vinylene carbonate and is scalable to a gram-scale level without substantial yield loss. This protocol showcases a broad substrate scope and exceptional tolerance for various functional groups.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (22001036), Guangdong Basic and Applied Basic Research Foundation (2022A1515111147) for financial support.Notes and references
- (a) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (b) J. A. Homer and J. Sperry, J. Nat. Prod., 2017, 80, 2178 CrossRef CAS PubMed; (c) Y. C. Zhang, F. Jiang and F. Shi, Acc. Chem. Res., 2020, 53, 425 CrossRef CAS PubMed; (d) Y. Meng, B. Yu, H. Huang, Y. Peng, E. Li, Y. Yao, C. Song, W. Yu, K. Zhu, K. Wang, D. Yi, J. Du and J. Chang, J. Med. Chem., 2021, 64, 925 CrossRef CAS PubMed; (e) B. Prabagar, Y. Yang and Z. Shi, Chem. Soc. Rev., 2021, 50, 11249 RSC; (f) A. Dhiman, R. Sharma and R. K. Singh, Acta Pharm. Sin. B, 2022, 12, 3006 CrossRef CAS PubMed.
- (a) G. Zhou, D. Wu, B. Snyder, R. G. Ptak, H. Kaur and M. Gochin, J. Med. Chem., 2011, 54, 7220 CrossRef CAS; (b) C. W. Lee and J. Y. Lee, Adv. Mater., 2013, 25, 5450 CrossRef CAS PubMed; (c) H. Mizoguchi, H. Oikawa and H. Oguri, Nat. Chem., 2014, 6, 57 CrossRef CAS PubMed; (d) I. Cho, S. K. Park, B. Kang, J. W. Chung, J. H. Kim, K. Cho and S. Y. Park, Adv. Funct. Mater., 2016, 26, 2966 CrossRef CAS; (e) N. Chadha and O. Silakari, Eur. J. Med. Chem., 2017, 134, 159 CrossRef CAS PubMed; (f) J. Hwang, J. Park, Y. J. Kim, Y. H. Ha, C. E. Park, D. S. Chung, S.-K. Kwon and Y.-H. Kim, Chem. Mater., 2017, 29, 2135 CrossRef CAS; (g) X. Feng, D. Liao, D. Liu, A. Ping, Z. Li and J. Bian, J. Med. Chem., 2020, 63, 15115 CrossRef CAS PubMed; (h) C. Qian, Z. Ma, X. Fu, X. Zhang, Z. Li, H. Jin, M. Chen, H. Jiang, X. Jia and Z. Ma, Adv. Mater., 2022, 34, 2200544 CrossRef CAS PubMed; (i) X. Fu, X. Zhang, C. Qian, Z. Ma, Z. Li, H. Jiang and Z. Ma, Chem. Mater., 2022, 35, 347 CrossRef.
- (a) E. J. Glamkowski and P. A. Eieitano, J. Med. Chem., 1979, 22, 106 CrossRef CAS PubMed; (b) G. Cignarella and P. Sanna, J. Med. Chem., 1981, 24, 1003 CrossRef CAS PubMed; (c) B. A. Frontana-Uribe, C. Moinet and L. Toupet, Eur. J. Org Chem., 1999, 1999, 419 CrossRef; (d) J. Chi, C. Hang, Y. Zhu and H. Katayama, Synth. Commun., 2010, 40, 1123 CrossRef CAS; (e) S. S. Chaudhaery, K. K. Roy, N. Shakya, G. Saxena, S. R. Sammi, A. Nazir, C. Nath and A. K. Saxena, J. Med. Chem., 2010, 53, 6490 CrossRef CAS PubMed.
- (a) K. Andersen, J. Perregaard, J. Arn, J. B. Nielsen and M. Begtrup, J. Med. Chem., 1992, 35, 4823 CrossRef CAS; (b) J. T. Klein, L. Davis, G. E. Olsen, G. S. Wong, F. P. Huger, C. P. Smith, W. W. Petko, M. Cornfeldt, J. C. Wilker and R. Blitzer, J. Med. Chem., 1996, 39, 570 CrossRef CAS; (c) F. Nie, J. Lu and W. Niu, Anal. Chim. Acta, 2005, 545, 129 CrossRef CAS; (d) H. Johansson, M. W. Boesgaard, L. Norskov-Lauritsen, I. Larsen, S. Kuhne, D. E. Gloriam, H. Brauner Osborne and D. Sejer Pedersen, J. Med. Chem., 2015, 58, 8938 CrossRef CAS PubMed; (e) C.-C. Tseng, G. Baillie, G. Donvito, M. A. Mustafa, S. E. Juola, C. Zanato, C. Massarenti, S. Dall'Angelo, W. T. A. Harrison, A. H. Lichtman, R. A. Ross, M. Zanda and I. R. Greig, J. Med. Chem., 2019, 62, 5049 CrossRef CAS PubMed.
- (a) F. Melkonyan, A. Topolyan, M. Yurovskaya and A. Karchava, Eur. J. Org Chem., 2008, 2008, 5952 CrossRef; (b) A. Kaga, X. Peng, H. Hirao and S. Chiba, Chem.–Eur. J., 2015, 21, 19112 CrossRef CAS PubMed; (c) T. T. H. Luong, J.-D. Brion, M. Alami and S. Messaoudi, J. Org. Chem., 2015, 80, 751 CrossRef CAS PubMed; (d) U. Huynh, M. Uddin, S. E. Wengryniuk, S. L. McDonald and D. M. Coltart, Tetrahedron Lett., 2016, 57, 4799 CrossRef CAS; (e) H. Wang, H. Jung, F. Song, S. Zhu, Z. Bai, D. Chen, G. He, S. Chang and G. Chen, Nat. Chem., 2021, 13, 378 CrossRef CAS; (f) P. Y. Vemuri and F. W. Patureau, Org. Lett., 2021, 23, 3902 CrossRef CAS PubMed; (g) G. Li, L. Yang, J.-J. Liu, W. Zhang, R. Cao, C. Wang, Z. Zhang, J. Xiao and D. Xue, Angew. Chem., Int. Ed., 2021, 60, 5230 CrossRef CAS PubMed.
- For a recent review on C–H functionalization, see: (a) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC; (b) J. R. Hummel, J. A. Boerth and J. A. Ellman, Chem. Rev., 2017, 117, 9163 CrossRef CAS PubMed; (c) Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247 CrossRef CAS PubMed; (d) Y. Wei, P. Hu, M. Zhang and W. Su, Chem. Rev., 2017, 117, 8864 CrossRef CAS PubMed; (e) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787 CrossRef CAS; (f) S. Prakash, R. Kuppusamy and C.-H. Cheng, ChemCatChem, 2018, 10, 683 CrossRef CAS; (g) Y. Hu, B. Zhou and C. Wang, Acc. Chem. Res., 2018, 51, 816 CrossRef CAS PubMed; (h) T. Gensch, M. J. James, T. Dalton and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 2296 CrossRef CAS PubMed; (i) P. Gandeepan and L. Ackermann, Chem, 2018, 4, 199 CrossRef CAS; (j) G. Duarah, P. P. Kaishap, T. Begum and S. Gogoi, Adv. Synth. Catal., 2019, 361, 654 CrossRef CAS; (k) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192 CrossRef CAS PubMed; (l) P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192 CrossRef CAS PubMed; (m) S. V. Kumar, S. Banerjeea and T. Punniyamurthy, Org. Chem. Front., 2020, 7, 1527 RSC; (n) W. Ma, Ni. Kaplaneris, X. Fang, L. Gu, R. Mei and L. Ackermann, Org. Chem. Front., 2020, 7, 1022 RSC; (o) S. K. Sinha, S. Guin, S. Maiti, J. P. Biswas, S. Porey and D. Maiti, Chem. Rev., 2021, 122, 5682 CrossRef PubMed; (p) T. Rogge, N. Kaplaneris, N. Chatani, J. Kim, S. Chang, B. Punji, L. L. Schafer, D. G. Musaev, J. Wencel-Delord, C. A. Roberts, R. Sarpong, Z. E. Wilson, M. A. Brimble, M. J. Johansson and L. Ackermann, Nat. Rev. Methods Primers, 2021, 1, 43 CrossRef CAS; (q) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed; (r) L. Guillemard, N. Kaplaneris, L. Ackermann and M. J. Johansson, Nat. Rev. Chem., 2021, 5, 522 CrossRef CAS PubMed; (s) T. Dalton, T. Faber and F. Glorius, ACS Cent. Sci., 2021, 7, 245 CrossRef CAS PubMed; (t) S. K. Sinha, S. Guin, S. Maiti, J. P. Biswas, S. Porey and D. Maiti, Chem. Rev., 2021, 122, 5682 CrossRef PubMed; (u) T. Dalton, T. Faber and F. Glorius, ACS Cent. Sci., 2021, 7, 245 CrossRef CAS PubMed; (v) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed; (w) T. Rogge, N. Kaplaneris, N. Chatani, J. Kim, S. Chang, B. Punji, L. L. Schafer, D. G. Musaev, J. Wencel-Delord, C. A. Roberts, R. Sarpong, Z. E. Wilson, M. A. Brimble, M. J. Johansson and L. Ackermann, Nat. Rev. Methods Primers, 2021, 1, 43 CrossRef CAS; (x) R. Logeswaran and M. Jeganmohan, Adv. Synth. Catal., 2022, 364, 2113 CrossRef CAS; (y) R. Maayuri and P. Gandeepan, Org. Biomol. Chem., 2023, 21, 441 RSC.
- (a) T. Satoh and M. Miura, Chem.–Eur. J., 2010, 16, 11212 CrossRef CAS PubMed; (b) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814 CrossRef CAS PubMed; (c) N. Kuhl, N. Schroder and F. Glorius, Adv. Synth. Catal., 2014, 356, 1443 CrossRef CAS; (d) G. Song and X. Li, Acc. Chem. Res., 2015, 48, 1007 CrossRef CAS PubMed; (e) B. Ye and N. Cramer, Acc. Chem. Res., 2015, 48, 1308 CrossRef CAS PubMed; (f) K. Shin, H. Kim and S. Chang, Acc. Chem. Res., 2015, 48, 1040 CrossRef CAS PubMed; (g) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC.
- (a) D. Zhao, S. Vásquez-Céspedes and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 1657 CrossRef CAS PubMed; (b) D. Y. Li, H. J. Chen and P. N. Liu, Org. Lett., 2014, 16, 6176 CrossRef CAS PubMed.
- For selected recent reviews on C–H/carbene precursors, see: (a) F. Hu, Y. Xia, C. Ma, Y. Zhang and J. Wang, Chem. Commun., 2015, 51, 7986 RSC; (b) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810 CrossRef CAS PubMed; (c) S. Nunewar, S. Kumar, S. Talakola, S. Nanduri and V. Kanchupalli, Chem.–Asian J., 2021, 16, 443 CrossRef CAS PubMed; (d) S. Kumar, V. Borkar, M. Mujahid, S. Nunewar and V. Kanchupalli, Org. Biomol. Chem., 2022, 21, 24 RSC.
- (a) Y. Liang, K. Yu, B. Li, S. Xu, H. Song and B. Wang, Chem. Commun., 2014, 50, 6130 RSC; (b) P. Shi, L. Wang, S. Guo, K. Chen, J. Wang and J. Zhu, Org. Lett., 2017, 19, 4359 CrossRef CAS PubMed; (c) Z. Yang, X. Lin, L. Wang and X. Cui, Org. Chem. Front., 2017, 4, 2179 RSC.
- (a) M. Barday, C. Janot, N. R. Halcovitch, J. Muir and C. Aïssa, Angew. Chem., Int. Ed., 2017, 56, 13117 CrossRef CAS PubMed; (b) Y. Xu, X. Zhou, G. Zheng and X. Li, Org. Lett., 2017, 19, 5256 CrossRef CAS PubMed; (c) C. You, C. Pi, Y. Wu and X. Cui, Adv. Synth. Catal., 2018, 360, 4068 CrossRef CAS; (d) S. Ji, K. Yan, B. Li and B. Wang, Org. Lett., 2018, 20, 5981 CrossRef CAS PubMed; (e) C. Hong, X. Jiang, S. Yu, Z. Lu and Y. Zhang, Chin. J. Org. Chem., 2021, 41, 888 CrossRef CAS; (f) A. Singh, S. Kumar and C. M. R. Volla, Org. Biomol. Chem., 2022, 21, 879 RSC.
- (a) W. Xie, X. Chen, J. Shi, J. Li and R. Liu, Org. Chem. Front., 2019, 6, 2662 RSC; (b) N. Lv, Z. Chen, Z. Liu and Y. Zhang, J. Org. Chem., 2019, 84, 13013 CrossRef CAS PubMed.
- H. Li, H. Gu, Y. Lu, N. Xu, N. Han, J. Li, J. Liu and J. Liu, J. Org. Chem., 2019, 84, 13013 CrossRef PubMed.
- (a) X. Gao, R. Zhai, X. Chen and S. Wang, Chin. J. Org. Chem., 2023, 43, 3119 CrossRef; (b) Y. Ge, Q. Yan and J. Nan, Org. Chem. Front., 2023, 96, 5717 RSC.
- K. Ghosh, Y. Nishii and M. Miura, ACS Catal., 2019, 9, 11455 CrossRef CAS.
- (a) X. Li, T. Huang, Y. Song, Y. Qi, L. Li, Y. Li, Q. Xiao and Y. Zhang, Org. Lett., 2020, 22, 5925 CrossRef CAS PubMed; (b) G. Mihara, K. Ghosh, Y. Nishii and M. Miura, Org. Lett., 2020, 22, 5706 CrossRef CAS PubMed; (c) K. Ghosh, Y. Nishii and M. Miura, Org. Lett., 2020, 22, 3547 CrossRef CAS PubMed; (d) L. Wang, K.-C. Jiang, N. Zhang and Z.-H. Zhang, Asian J. Org. Chem., 2021, 10, 1671 CrossRef CAS; (e) C. Wang, X. Fan, F. Chen, P.-C. Qian and J. Cheng, Chem. Commun., 2021, 57, 3929 RSC; (f) J. Nan, Q. Ma, J. Yin, C. Liang, L. Tian and Y. Ma, Org. Chem. Front., 2021, 8, 1764 RSC; (g) X. Huang, Y. Xu, J. Li, R. Lai, Y. Luo, Q. Wang, Z. Yang and Y. Wu, Chin. Chem. Lett., 2021, 32, 3518 CrossRef CAS; (h) J. Singh, S. Sharma and A. Sharma, J. Org. Chem., 2021, 86, 24 CrossRef CAS PubMed; (i) J. Nan, J. Yin, X. Gong, Y. Hu and Y. Ma, Org. Lett., 2021, 23, 8910 CrossRef CAS PubMed; (j) M. S. Park, K. Moon, H. Oh, J. Y. Lee, P. Ghosh, J. Y. Kang, J. S. Park, N. K. Mishra and I. S. Kim, Org. Lett., 2021, 23, 5518 CrossRef CAS PubMed; (k) Y. Hu, J. Nan, J. Yin, G. Huang, X. Ren and Y. Ma, Org. Lett., 2021, 23, 8527 CrossRef CAS PubMed; (l) F.-P. Hu, X.-G. Zhang, M. Wang, H.-S. Wang and G.-S. Huang, Chem. Commun., 2021, 57, 11980 RSC; (m) N. Li, X. Zhang and X. Fan, Tetrahedron Lett., 2022, 103, 153984 CrossRef CAS; (n) P. Kumar and M. Kapur, Chem. Commun., 2022, 58, 4476 RSC; (o) S. Kim, H. K. Park, J. Y. Kang, N. K. Mishra and I. S. Kim, Synthesis, 2022, 54, 4461 CrossRef CAS; (p) Y. Yu, Y. Wang, B. Li, Y. Tan, H. Zhao, Z. Li, C. Zhang and W. Ma, Adv. Synth. Catal., 2022, 364, 838 CrossRef CAS; (q) G. Huang, J. T. Yu and C. Pan, Eur. J. Org Chem., 2022, e202200279 CrossRef CAS; (r) J. L. Song, L. Xiao, S. Y. Chen, Y. C. Zheng, Y. Z. Liu, S. S. Zhang and B. Shu, Adv. Synth. Catal., 2023, 365, 1457 CrossRef CAS; (s) S. Jothi Murugan and M. Jeganmohan, J. Org. Chem., 2023, 88, 1578 CrossRef CAS PubMed; (t) S. Zhang, S. Hu, K. Wang, Z. Yang, H. Liu and J. Wang, Adv. Synth. Catal., 2023, 365, 238 CrossRef CAS; (u) V. Kumar and P. Gandeepan, Eur. J. Org Chem., 2023, e202300914 CrossRef CAS.
- CCDC 2302860 for compounds 3aa.
- (a) B. Shen, S. Liu, L. Zhu, K. Zhong, F. Liu, H. Chen, R. Bai and Y. Lan, Organometallics, 2020, 39, 2813 CrossRef CAS; (b) J. Kitano, Y. Nishii and M. Miura, Org. Lett., 2022, 24, 5679 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data. CCDC 2302860. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra07466h |
| ‡ Y. Chen and Z. Lu contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2024 |