Open Access Article
Open Access ArticleProperties of Ophioglossum vulgatum L. extract Pickering emulsion stabilized by carbon dots and its potential use in cosmetics†
Guomei Xu ab,
Shuyu Chena,
Qiang Shia,
Huayang Wanga,
Lihong Wua,
Pan Pana,
Hanjie Ying
ab,
Shuyu Chena,
Qiang Shia,
Huayang Wanga,
Lihong Wua,
Pan Pana,
Hanjie Ying *b and
Hongxue Xie*a
*b and
Hongxue Xie*a
aKey Laboratory of Biomimetic Sensor and Detecting Technology of Anhui Province, School of Materials and Chemical Engineering, West Anhui University, Lu'an, Anhui 237012, PR China
bState Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing, Jiangsu 211816, PR China
First published on 2nd January 2024
Abstract
Ophioglossum vulgatum L. (O. vulgatum) is a species of fern used in traditional Chinese medicine, however, its application in cosmetics has not yet been studied. This study obtained O. vulgatum extract using 70% ethanol solution and evaporation. Fourier Transform Infrared Spectrometer (FTIR) analysis identified many active components in O. vulgatum extract, such as polyols, amino acids, and flavonoids. A Pickering emulsion of O. vulgatum extract was also prepared, stabilized by a type of carbon dot based on L-arginine (CDs-Arg). The prepared Pickering emulsion was characterized by metallographic microscope and contact angle measurement. The results demonstrated that it was a pH-responsive O/W emulsion. Facial cleanser was then created using the prepared Pickering emulsion as the main component. When squeezed onto hands, the cleanser produced many delicate foams and caused no skin irritation. The prepared Pickering emulsion facilitated the use of O. vulgatum in facial cleanser.
1. Introduction
Ophioglossum vulgatum L. (Ophioglossaceae; O. vulgatum) is a type of fern that has long been used as an important herb. It has many nicknames in folklore, such as Yizhijian, Shexucao, and Maoduncao.1 O. vulgatum has a low toxicity, is bitter and has strong vitality and adaptability. It is commonly located in the northern hemisphere, including both Europe and Asia. In China, O. vulgatum is generally distributed in Yunnan, Sichuan, Guangxi, and Taiwan. It typically grows in shady and wet places in the mountains, riverside areas, and ditches. O. vulgatum plants, including the roots, stems, and leaves, are also used medicinally.2 O. vulgatum contains many chemical components, which are mainly divided into three categories: flavonoids, oils, and proteins,3 including linoleic acid, glycerol trioleate, β-sitosterol, triglycerol n-pentadecanoate, and glycerol mono linoleate. The most well-known components of O. vulgatum are flavonoids and polysaccharides, which are used in the fields of medicine,4 agronomy, and cosmetics. Flavonoids have many pharmacological properties,5 including antioxidant, anti-inflammatory, expectorant, and sedative properties, and have been used to treat asthma.6 O. vulgatum has been shown to lower fevers and detoxify, and has both bactericidal and anti-inflammatory effects. It can also treat some cancers, so it is known as the ‘Medicine King’ in Taiwan, China. In addition, the dried roots of O. vulgatum are ground into powder that is used to treat acute ulcers, wounds, and burns, and the fresh rhizomes of O. vulgatum can be used to cure snake bites. O. vulgatum is therefore valuable in many fields.7 However, its application in cosmetics has not been reported.Cosmetics are fine chemicals applied to the surface of the human body for the purpose of cleaning, maintenance, beauty, or visual modification. Facial cosmetics are one of the fastest growing segments in the cosmetics industry with a long and rich history.8 In primitive society, some tribes smeared animal fat on the skin to make their skin look healthy and shiny, which may be the earliest known skin care routine. From the 5th century BC to the 7th century AD, there were many legends and records about the production and use of cosmetics in various countries: the ancient Egyptians curled their hair with clay; the ancient Egyptian queens painted their eyes with patina, bathing their bodies with donkey's milk; the ancient Chinese also liked to use rouge to smear their cheeks and head oil to moisturise their hair, enhancing the beauty and glamour of their faces. Early cosmetics originated from the chemical industry, as it was difficult to isolate and purify effective ingredients from plants, and the petrochemical synthesis industry was already very developed. As a result, many skincare and cosmetic products come from the chemical industry. Many international and domestic cosmetic brands use the same raw materials that have been used for centuries. In the 1980s, skin experts found that adding a variety of natural ingredients to skin care products had a moisturizing effect on the skin. Technology also advanced, making large-scale natural ingredient extraction and separation possible, leading to natural ingredients being more commonly included in cosmetics. Because natural, plant-based ingredients are greener, safer, and more reliable than chemical synthetic cosmetic components and have significant functionality, cosmetics made from natural ingredients have some significant advantages over chemical cosmetics. This has led the worldwide cosmetic industry to focus research and development on natural herb cosmetics.4 All skincare products contain many ingredients, some of which are water-soluble, and some oil-soluble, which can be evenly mixed using emulsification. Surfactants play an important role in the emulsification process. In an aqueous environment, surfactants form micelles. The oil particles wrapped in the centre of the micelles are equivalent to the oil particles outside the set of a hydrophilic coat, so they can be freely and evenly dispersed in water, forming a stable emulsion.9 When the emulsion is applied to skin, these micelles rupture, releasing the oil particles (usually the active ingredients of cosmetics) to be absorbed by the skin. Traditional surfactants are often chemically synthesized, and can cause serious, irreversible damage to the skin if used in large doses for long periods of time. This study created a novelty surfactant-free O. vulgatum extract Pickering emulsion using CDs-Arg as a stabilizer.
Emulsions stabilized by solid particles are known as “Pickering emulsions.” At the beginning of the last century, Ramsden10 discovered that colloidal particles could play an important role in stabilizing emulsions instead of surfactants. Pickering,11 building on Ramsden's work, systematically studied this type of emulsion, which is why they are now known as Pickering emulsions. Compared with traditional emulsions stabilized by surfactants, Pickering emulsions have unique advantages,12 such as being environmentally friendly, less toxic, cheaper, and having excellent stability. Pickering emulsions thus have significant potential in many fields, including cosmetics, bio-food, medicine, agriculture, and oil.13 Pickering emulsions are mainly stabilized through adsorption of solid particles at the oil-water interface, forming a single or multi-layer film of solid particles,14 which prevents droplets from aggregating, thus stabilizing the emulsion.15 Carbon dots (CDs) are a novel nanomaterial that was used in this study as a stabilizer to form a Pickering emulsion of O. vulgatum extract.16
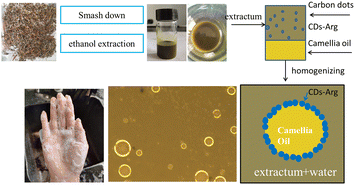
Herein, we propose to develop natural product-based cosmetics from O. vulgatum (Fig. S1†), aiming to prepare natural herb cosmetics and expand the application of natural herbs. The extract of the herb O. vulgatum was used as raw material. To modify the dispersion of O. vulgatum extract in cosmetics and to design it as Pickering emulsion. Carbon dot was used as a stabilizer to prepare Pickering emulsion, for it owns many excellent performances and possess antibacterial properties. A type of carbon dot based on L-arginine (CDs-Arg) was used as the stabilizer for the Pickering emulsion in this study, the extract of O. vulgatum was used as the aqueous phase, and natural mild camellia oil was used as the oil phase. The properties of CDs-Arg have been outlined by previous studies.17 In this study, we have simply investigated the effect of camellia oil, CDs-Arg and pH values on the deformation of Pickering emulsion. The optical micrographs and contact angles of the O. vulgatum extract Pickering emulsion were also studied.
2. Experimental
2.1 Materials
The O. vulgatum samples used in this study were purchased online and washed and dried in a vacuum before using. Rutin was purchased from the National Institute for the Identification of Drug and Biological Products. Camellia oil came from Anhui Yumin Ecological Agricultural Co. and the citric acid, alcohol, sodium hydroxide, potassium bromide, sodium nitrite, and aluminum nitrate were all purchased from the Tianjin Damao Chemical Reagent Factory. All reagents used in this study were used “as is” with no purification. Deionized water created in our lab was used throughout the study and the CDs-Arg used in this study was also created in our lab.2.2 Extraction of O. vulgatum
The fresh O. vulgatum samples were washed with water to remove any silt, dried in an electric oven at 50 °C, and ground into powder with high energy shear milling. The extraction process of O. vulgatum was as follows: 5 g O. vulgatum powder was suspended in 40 mL of 70% alcohol and stirred for 6 h with ultrasonic shaker (Ultrasonic cleaning machine: KH 3200DE, Hechuang, Kunshan, China). The solution was then transferred to a water bath for reflux extraction at 70 °C for 1.5 h. This reflux extraction was performed three times. Then, the extracting solution was gathered and heated under a vacuum to remove the alcohol (IKA Rotary evaporator: RV3, Junhong, Hefei, China). A black greenish-brown solution was finally obtained, then placed them in a vacuum oven (DZF-6000, Shanghai Yiheng Scientific Instrument Co., Ltd) at room temperature for one day, with the final extract being stored in the refrigerator.2.3 Flavonoid determination
The content of all flavonoids in the O. vulgatum extract was estimated using the UV spectrophotometry sodium nitrite-aluminum nitrate–sodium hydroxide color method and rutin as a standard flavonoid. A total of 1 mL O. vulgatum extract was added to a 10 mL volumetric flask and mixed with 0.5 mL 5% sodium nitrite (w/v). After a six-minute interval, 10% 0.5 mL aluminum nitrate (w/v) was added, followed by 4 mol L−1 4 mL sodium hydroxide solution after another six-minute interval. Water was then added until the total volume was 10 mL, and the solution was shaken well and then left for 15 min. Absorbance was tested at 510 nm (Fig. S3†) and water was used as the blank control. The result was expressed in terms of mg rutin per g rutin after a triplicate sample analysis. The flavonoid concentration of the O. vulgatum extract was calculated using the eqn (1), which is the calibration curve using rutin as a standard flavonoid. Rutin standard solution was prepared as above, and the calibration curve was presented is Fig. S4.†| y = 8.03327x + 0.0153, R2 = 0.99927 | (1) |
2.4 Preparation of carbon dot
The type of carbon dot based on L-arginine (CDs-Arg) was made in our lab via hydrothermal method. Put 8 g L-arginine dissolved in 80 mL deionized water, then hydrothermal treated at 200 °C for 4 h. Firstly, the resulting yellow solution was adjusted to neutral with 1% HCl. Then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. Finally, the obtained yellow solution was freeze dried before used. The details and other properties are presented elsewhere.17
000 rpm for 15 min. Finally, the obtained yellow solution was freeze dried before used. The details and other properties are presented elsewhere.17
2.5 Preparation of Pickering emulsion
O. vulgatum extract Pickering emulsion, stabilized by CDs-Arg, was prepared according to the following procedure:18 O. vulgatum extract and water were loaded into a 50 mL flat-bottomed test tube, and CDs-Arg was then added, followed by oil phase camellia oil. The mixture was homogenized with a 1.0 cm head high-speed homogenizer (FJ200-S digital display high-speed homogenizer, Hangzhou Qiwei Instrument Co., Ltd) at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 minutes. The stability of the prepared emulsions was determined by observing the presence of oil and water, separately. Specific formulations of O. vulgatum extract Pickering emulsion are shown in Tables 1–3.
000 rpm for 2 minutes. The stability of the prepared emulsions was determined by observing the presence of oil and water, separately. Specific formulations of O. vulgatum extract Pickering emulsion are shown in Tables 1–3.
| Samples | Water phase | Oil phase | |||
|---|---|---|---|---|---|
| O. vulgatum extract (g) | Water (mL) | Camellia oil (g) | CDs-Arg (g) | pH | |
| 1 | 1.0069 | 19 | 0.0396 | 0.1038 | 5.14 |
| 2 | 1.0066 | 19 | 0.0442 | 0.1038 | 5.15 |
| 3 | 1.0071 | 19 | 0.0481 | 0.1037 | 5.15 |
| 4 | 1.0069 | 19 | 0.0510 | 0.1029 | 5.16 |
| 5 | 1.0070 | 19 | 0.0614 | 0.1032 | 5.15 |
2.6 Measurements
The infrared spectrum of O. vulgatum extract and Pickering emulsion were collected on an IS50 FTIR spectrometer (PerkinElmer Inc), scanning from 500–4000 cm−1 and using KBr as a reference. The O. vulgatum extract and Pickering emulsion were dropped onto a KBr platelet. An XSP-10CC metallographic microscope (Shanghai CAIKON optical instrument Co., Ltd) was used to observe the morphology of O. vulgatum extract Pickering emulsion by applying it onto the surface of a glass slide. The Pickering emulsion type was deduced by testing the size of the contact angle according to the traditional particle-platelet method.19 An M393003 contact angle measuring instrument (Shanghai Zhongping technology Co. Ltd, China) was used to test the three-phase contact angle of the Pickering emulsion at the oil-water interface.3. Results and discussion
3.1 Characteristics of O. vulgatum extract
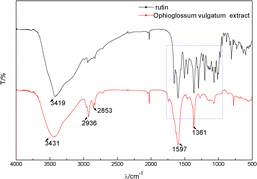
The prepared O. vulgatum extract was a black greenish-brown solution, as shown in Scheme 1.To verify the presence of flavonoids in the extract, rutin was used as a standard flavonoid and its infrared spectrum is also shown in Fig. 1. The FTIR of O. vulgatum extract was similar to that of rutin, except that rutin's had more peaks. O. vulgatum extract showed a narrow and sharp signal at 3431 cm−1, corresponding to the –OH stretches. The adsorption peaks located at 2936 and 2853 cm−1 correspond to the –CH3 and –CH2 groups, respectively. The adsorption band located around 2000 cm−1 might correspond with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl stretching. The adsorption bands appearing at 1597 and 1361 cm−1 could be signals of stretching vibrations of –C
O carbonyl stretching. The adsorption bands appearing at 1597 and 1361 cm−1 could be signals of stretching vibrations of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or might be generated by amide groups.20 Combined with the infrared spectrum of rutin, –COOH, –OH, amide, and other groups were identified in the O. vulgatum extract that were consistent with rutin. The total flavonoid content of O. vulgatum extract was about 2.37% (23.6552 mg g−1), according to the UV spectrophotometry sodium nitrite–aluminum nitrate–sodium hydroxide color method. These data all show O. vulgatum extract's basis for use in cosmetics.
C or might be generated by amide groups.20 Combined with the infrared spectrum of rutin, –COOH, –OH, amide, and other groups were identified in the O. vulgatum extract that were consistent with rutin. The total flavonoid content of O. vulgatum extract was about 2.37% (23.6552 mg g−1), according to the UV spectrophotometry sodium nitrite–aluminum nitrate–sodium hydroxide color method. These data all show O. vulgatum extract's basis for use in cosmetics.
3.2 Formation of O. vulgatum extract Pickering emulsion
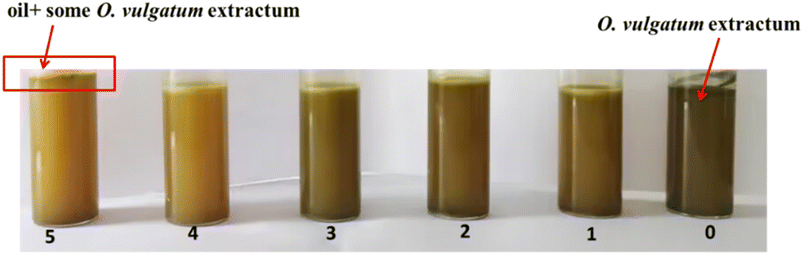
A surfactant-free Pickering emulsion, stabilized by CDs-Arg,21 with O. vulgatum extract as the aqueous phase and natural mild camellia oil as the oil phase, was created in this study. In order to investigate the influence of each of these factors on the formation of the Pickering emulsion, different concentrations were tested, with detailed ingredients listed in Tables 1–3 and Fig. 2–4. Fig. 2 shows the appearance of emulsions at a constant concentration of CDs-Arg with increasing concentration of camellia oil (details in Table 1). Of the six test tubes shown in Fig. 2, the right one was the 0th tube and was muddy and unstratified, containing only the O. vulgatum extract without CDs-Arg or camellia oil. After adding 0.01 g camellia oil, the mixture was homogenized and rested for 24 h. The colour of the solution became lighter with faint stratification observed at the top, as shown in the 1st tube in Fig. 2. The emulsification of the solution increased with the amount of camellia oil added, and when 0.05 g camellia oil was added, the solution changed into a uniform emulsion and the colour changed from brown to yellow (as shown in the 4th tube in Fig. 2). However, as the amount of camellia oil continued to increase, layering occurred, with part of the O. vulgatum extract extracted by the camellia oil (as shown by the brown layer clearly seen in the 6th tube in Fig. 2). Camellia oil is both a hydrophobic substance and an organic solvent. O. vulgatum extract contains some hydrophobic and some hydrophilic components. Based on the principle of polar similarity and compatibility, some hydrophobic substances in O. vulgatum extract were absorbed by camellia oil when the two were mixed. Based on these data, 0.05 g camellia oil was chosen for further studies.Table 2 and Fig. 3 show that carbon dot concentration also significantly impacted the emulsion process. When the concentration of oil phase and water phase remained unchanged, increased CDs-Arg concentration improved the formation and stability of the emulsion,22 but also resulted in turbidity and gelation of the emulsion. The emulsion was clear and translucent at a low carbon dot dosage (0.1 g) but turned to a turbid dispersion when the amount of CDs-Arg increased to 0.3 g, and the color changed from yellow to brown. This could be CDs-Arg contains a lot of hydrophilic groups,23 such as –OH, –COOH, increased CDs-Arg concentration had different results than increased camellia oil concentration. As the content of CDs-Arg increased, the number of hydrophilic groups also could be increased, condensing with the hydrophilic groups in the O. vulgatum extract to obtain hydrogen bonds, leading to an increase in the viscosity of the solution. Based on these results, 0.15 g CDs-Arg was chosen for further studies.
| Samples | Water phase | Oil phase | |||
|---|---|---|---|---|---|
| O. vulgatum extract (g) | Water (mL) | Camellia oil (g) | CDs-Arg (g) | pH | |
| 1 | 1.0012 | 19 | 0.0557 | 0.1026 | 5.31 |
| 2 | 1.0008 | 19 | 0.0554 | 0.1505 | 5.33 |
| 3 | 1.0015 | 19 | 0.0557 | 0.2175 | 5.33 |
| 4 | 1.0009 | 19 | 0.0550 | 0.2554 | 5.37 |
| 5 | 1.0011 | 19 | 0.0549 | 0.3144 | 5.35 |
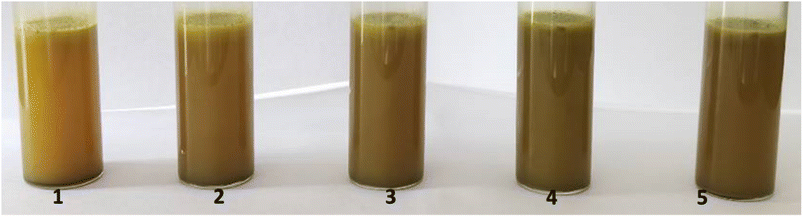
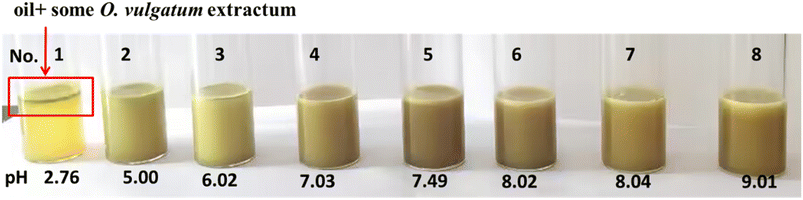
The effect of pH value on the stability of the prepared Pickering emulsion was also investigated.24 The results are shown in Fig. 4, with the components of the emulsion listed in Table 3. As shown in the 2nd tube in Fig. 4, uniform emulsion was obtained by mixing and homogenizing 0.05 g camellia oil, 0.13 g CDs-Arg, 0.5 g O. vulgatum extract, and 10 mL water, resulting in a pH level of 5.00. When a few drops of 1 M citric acid were added to reduce the pH value, the system begun to demulsify, and when the pH value decreased to 2.76, the system completely demulsified.25 Interestingly, the emulsion could be regenerated by adding a few drops of 1 M NaOH after homogenization. The Pickering emulsion became unstable again if more 1 M NaOH was added after the pH increased to 8. These results indicate that when the ingredients of the emulsion were fixed, emulsification/demulsification could be directly impacted by adjusting the pH value.
| Samples | Water phase | Oil phase | |||
|---|---|---|---|---|---|
| O. vulgatum extract (g) | Water (mL) | Camellia oil (g) | CDs-Arg (g) | pH | |
| 1 | 0.5116 | 10 | 0.0554 | 0.1354 | 2.76 |
| 2 | 0.5103 | 10 | 0.0550 | 0.1348 | 5.00 |
| 3 | 0.5111 | 10 | 0.0549 | 0.1352 | 6.02 |
| 4 | 0.5109 | 10 | 0.0548 | 0.1347 | 7.03 |
| 5 | 0.5099 | 10 | 0.0557 | 0.1356 | 7.49 |
| 6 | 0.5113 | 10 | 0.0541 | 0.1351 | 8.02 |
| 7 | 0.5108 | 10 | 0.0551 | 0.1349 | 8.04 |
| 8 | 0.5106 | 10 | 0.0559 | 0.1355 | 9.01 |
3.3 Performance of Pickering emulsion stabilized by CDs-Arg
Previous research26 shows that the formation of Pickering emulsion is closely related to the wettability of solid particles, which can be analysed using the contact angle, so the contact angle θ is an important parameter in the study of Pickering emulsions. Particles with appropriate surface wettability can be maintained between the aqueous and oil phases to stabilize emulsions. If the particles are too hydrophobic or too hydrophilic, they will remain dispersed in the oil or water phase. Contact angle and optical micrographs of the O. vulgatum extract Pickering emulsion were also examined in this study. As shown in Fig. 5, when only O. vulgatum extract was dispersed in water, the contact angle θ = 40.9°, and there were no visible droplets under an optical microscope. After it became a uniform emulsion with the addition of CDs-Arg and camellia oil, the contact angle θ = 51.4°, and with further magnification, it could be clearly seen that these droplets had a distinctive aperture (Fig. S5†). There were small-sized droplets (40–100 nm) visible at current state in TEM images (Fig. S6†). These results demonstrated that CDs-Arg was irreversibly absorbed on the interface of camellia oil and O. vulgatum extract after Pickering emulsion formation, acting as a barrier against aggregation;27 this is also known as the Pickering emulsion formation mechanism. However, when the system was completely demulsified, there were no visible droplets, but rather yellow clumps of substance under the microscope, and the contact angle was reduced to 35.1°. This is likely because citric acid addition produced H+, which reacted with some hydrophilic groups on CDs-Arg, increasing the hydrophobicity of CDs-Arg, resulting in CDs-Arg attaching to the camellia oil, appearing as yellowish clusters under the microscope. The Pickering emulsion could be re-stabilized by adding 1 M NaOH to adjust the pH value. However, when the pH value increased above 8, the Pickering emulsion became unstable again, many non-spherical droplets were observed under an optical microscope, and the contact angle also increased to 44.6°. This is because NaOH has many –OH groups, increasing the hydrophilic content of CDs-Arg when added in large amounts. Because hydrophilic content does not have suitable surface wettability, too much hydrophilic content makes it difficult for the Pickering emulsion to stabilize.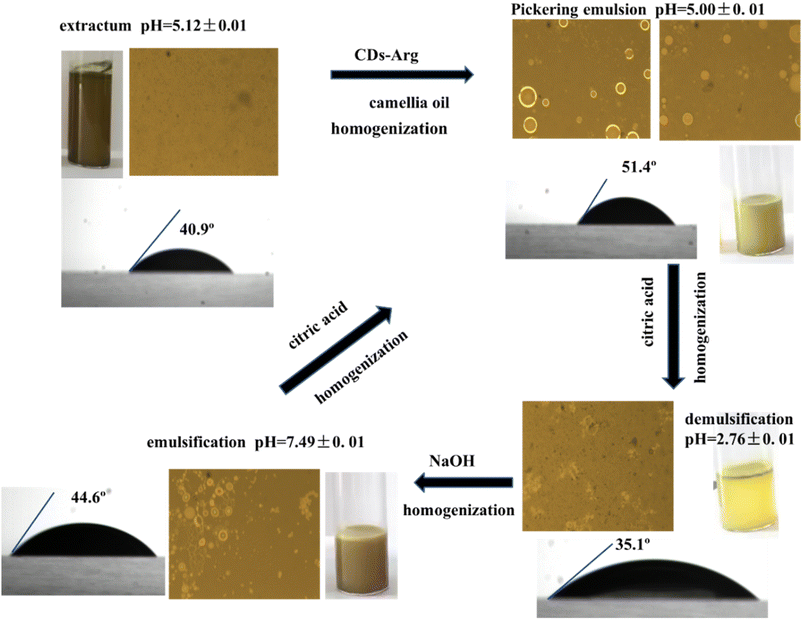 | ||
| Fig. 5 Optical micrographs, contact angle and appearance of the prepared Pickering emulsion varied with pH. | ||
3.4 Application of the O. vulgatum extract Pickering emulsion
A facial cleanser based on the O. vulgatum extract Pickering emulsion was then created. The as-made O. vulgatum extract Pickering emulsion was used as the main component, added with other components according to weight percent. This facial cleanser, when squeezed onto a hand, produced many delicate foams, and caused no skin irritation (Fig. 6). Compared to conventional facial cleansers, Pickering emulsion does not use surfactants for emulsification, thus avoiding skin irritation and sensitization caused by surfactant. These results indicate that Pickering emulsion should be further studied for use in cosmetics. As this is a single, lab-based study, large-scale testing is needed to study the potential of Pickering emulsion in cosmetic products.4. Conclusions
O. vulgatum extract was obtained in this study with a flavonoid content of 23.6552 mg g−1. O. vulgatum extract Pickering emulsion, stabilized by CDs-Arg, and using camellia oil as the oil phase, was then created. Optical micrographs and contact angle of the O. vulgatum extract Pickering emulsion were analysed, only a homogeneous O. vulgatum extract emulsion was formed, many visible droplets could be seen under the optical microscope, the contact angle also increased to above 50°. However, when the system was completely demulsified, no visible droplets were observed under the optical microscope, and the contact angle also decreased to below 50°. More work is undoubtedly needed but the concept shows promise. Facial cleanser was then fabricated from the O. vulgatum extract Pickering emulsion, showing the potential of Pickering emulsion in cosmetic products.Author contributions
Guomei Xu, Hanjie Ying and Hongxue Xie contributed equally during the design, data collection and analysis of the experiment, also the writing of the original work. Shuyu Chen, Qiang Shi, Lihong Wu and Huayang Wang helped and collected data of the experiment. Pan Pan helped with the editing of the manuscript.Conflicts of interest
Authors declare no competing conflict of interests.Acknowledgements
This work was supported by the State Key Laboratory of Materials-Oriented Chemical Engineering (No. SKL-MCE-22B05); Key Project of Natural Science Research in Universities of Anhui Province (No. KJ2021A0941); 2021 High-level Talents Research Initiation Project of West Anhui University (WGKQ2021002); this work was also supported by the undergraduate innovation project (202310376043, s202210376151).References
- H. Azaizeh, B. Saad, K. Khalil and O. Said, J. Evidence-Based Complementary Altern. Med., 2006, 3, 229–235 CrossRef.
- J. Hao, Y. Y. Liang, M. Zhu, J. Y. Ping, P. P. Feng, Y. J. Su and T. Wang, Mitochondrial DNA, Part B: Resources, 2021, 6–9, 2730–2731, DOI:10.1080/23802359.2021.1966333.
- M. O. Nwosu, Econ. Bot., 2002, 56, 255–259 CrossRef.
- F. Y. Luo, J. Z. Pu, Z. Su, S. X. Xing, Q. J. Wang and L. Sun, Pharmacol. Res. - Mod. Chin. Med., 2023, 8, 100282 CrossRef.
- A. B. Carlson, C. A. Mathesius, S. Ballou, M. N. Fallers, T. A. Gunderson, A. Hession, H. Mirsky, B. Stolte, J. Zhang, R. M. Woods, R. A. Herman and J. M. Roper, Food Chem. Toxicol., 2022, 166, 113187 CrossRef CAS.
- B. Křížkovská, L. Hoang, D. Brdová, K. Klementová, N. Szemerédi, A. Loučková, O. Kronusová, G. Spengler, P. Kaštánek, J. Hajšlová, J. Viktorová and J. Lipov, J. Ethnopharmacol., 2023, 312, 116484 CrossRef PubMed.
- L. Zhang and L.-B. Zhang, Mol. Phylogenet. Evol., 2022, 173, 107512 CrossRef.
- A. Krutyansky, S. Halepas and E. M. Ferneini, Oral Maxillofac. Surg. Cases, 2023, 9, 100322 CrossRef.
- C. L. G. Harman, M. A. Patel, S. Guldin and G.-L. Davies, Curr. Opin. Colloid Interface Sci., 2019, 39, 173–189 CrossRef CAS.
- H. Jiang, Y.-F. Sheng and T. Ngai, Curr. Opin. Colloid Interface Sci., 2020, 49, 1–15 CrossRef CAS PubMed.
- F.-N. Huang, Y.-D. Liang and Y.-J. He, Colloids Surf., A, 2019, 580, 123722 CrossRef CAS.
- L.-Y. Zhang, T.-J. Shi, S.-L. Wu and H.-O. Zhou, High Perform. Polym., 2014, 26, 156–165 CrossRef.
- L.-Y. Zhang, T.-J. Shi, D.-X. Tan, H.-O. Zhou and X. Zhou, Fullerenes, Nanotubes Carbon Nanostruct., 2014, 22, 726–737 CrossRef CAS.
- R. Ma, M.-X. Zeng, D.-L. Huang, J. Wang, Z.-D. Cheng and O.-S. Wang, J. Colloid Interface Sci., 2021, 601, 106–113 CrossRef CAS.
- Y. Yang, M.-W. Zhao and L. Lai, Carbon, 2023, 202, 398–413 CrossRef CAS.
- L. Ni, C. Yu, Y.-Y. Xie, Q.-B. Wei, D.-M. Liu, X.-Y. Tan, Y.-W. Ding and J.-S. Qiu, Chem. Commun., 2023, 59, 3261–3264 RSC.
- G.-M. Xu, P. Pan, T. Hu, Zh.-Y. Chen, H.-J. Ying and Y.-F. Cheng, Fullerenes, Nanotubes Carbon Nanostruct., 2023 DOI:10.1080/1536383X.2023.2263107.
- B. V. Farias, D. Brown, A. Hearn, N. Nunn, O. Shenderova and S. A. Khan, J. Colloid Interface Sci., 2020, 580, 180–191 CrossRef CAS PubMed.
- H.-O. Zhou, T.-J. Shi and X. Zhou, J. Biomater. Sci., Polym. Ed., 2014, 25, 641–656 CrossRef CAS.
- R.-Y. Li, Z.-J. Li and J. K. Liu, J. Colloid Interface Sci., 2017, 493, 24–31 CrossRef CAS PubMed.
- R. Cohen, K. A. Mani, M. Pirmatova, G. Jacobi, E. Zelinger, E. Belausov, E. Fallik, E. Banin and G. Mechrez, Colloids Surf., B, 2023, 227, 113355 CrossRef CAS PubMed.
- N. A. Hadi, A. Marefati, M. Matos, B. Wiege and M. Rayner, Carbohydr. Polym., 2020, 240, 116264 CrossRef.
- D. F. Mercado, D. S. Monje, L. M. Ballesteros-Rueda, G. Peñuela Mesa, G. C. Valencia and R. A. Torres-Palma, Adv. Powder Technol., 2023, 34, 104054 CrossRef CAS.
- T.-C. Zhou, Z. Huang and F. Sun, Mater. Today Commun., 2020, 23, 100951 CrossRef CAS.
- B. Hosseinzadeh, N. Nikfarjam and S. H. Kazemi, Colloids Surf., A, 2021, 612, 125978 CrossRef CAS.
- C. Zhang, D.-G. Liao, Y.-N. Wang, P.-Y. Xie, M.-X. Li, L. Zhou, Y.-H. Chen and H.-X. Liu, Ceram. Int., 2023, 49, 8121–8131 CrossRef CAS.
- D. Yu, G.-D. Li, W.-X. Liu, Y.-M. Li, Z.-P. Song, H.-L. Wang, F.-X. Guan and X.-S. Chen, Colloids Surf., A, 2019, 563, 310–317 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06650a |
| This journal is © The Royal Society of Chemistry 2024 |