Open Access Article
Open Access ArticleNi–Fe bimetallic catalysts with high dispersion supported by SBA-15 evaluated for the selective oxidation of benzyl alcohol to benzaldehyde
V. L. Mangesha,
Murali Govindarajana,
Rama Bhadri Raju Chekurib,
Tamizhdurai Perumal *c,
Kumaran Rajendranc,
Kavitha Chandrasekaranc,
Nadavala Siva Kumar
*c,
Kumaran Rajendranc,
Kavitha Chandrasekaranc,
Nadavala Siva Kumar d,
Praveen Kumar Basivi
d,
Praveen Kumar Basivi e,
Salwa B. Alreshaidanf and
Ahmed S. Al-Fateshd
e,
Salwa B. Alreshaidanf and
Ahmed S. Al-Fateshd
aDepartment of Mechanical Engineering, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, Andhra Pradesh 522502, India
bSagi Rama Krishnam Raju Engineering College, Bhimavaram-534204, Andhra Pradesh, India
cDepartment of Chemistry, Dwaraka Doss Goverdhan Doss Vaishnav College (Autonomous) (Affiliated to the University of Madras, Chennai), 833, Gokul Bagh, EVR Periyar Road, Arumbakkam, Chennai 600 106, Tamil Nadu, India. E-mail: tamizhvkt2010@gmail.com; Tel: +91-9677146579
dDepartment of Chemical Engineering, King Saud University, P.O. Box 800, Riyadh 11421, Saudi Arabia. E-mail: snadavala@ksu.edu.sa; Tel: +966-537228108
ePukyong National University Industry-University Cooperation Foundation, Pukyong National University, Busan 48513, Republic of Korea
fDepartment of Chemistry, Faculty of Science, King Saud University, P.O. Box 800, Riyadh 11451, Saudi Arabia
First published on 11th January 2024
Abstract
A wetness impregnation method was used to impregnate the substrate with a substantial quantity of oleic acid together with a metal precursor, leading to significantly dispersed Ni–Fe bimetallic catalysts based on mesoporous SBA-15. Using a wide variety of characterization methods, such as XRD, BET, and TEM Analysis, the physiochemical properties of the catalyst were determined. The addition of the metal does not have any effect on the structural characteristics of the SBA-15 catalyst, as validated by transmission electron microscopy (TEM), which shows that the prepared SBA-15 supported catalyst has a hexagonal mesoporous structure. The catalytic capabilities of the Ni–Fe-SBA-15 catalysts were evaluated in the conversion of BzOH using tert-butyl hydroperoxide (TBHP) as an oxidant and acetonitrile as a solvent. The Ni/Fe-SBA-15 (NFS-15) catalytic composition is the best of the developed catalysts, with a maximum conversion of 98% and a selectivity of 99%. In-depth investigations were conducted into the molar ratio of TBHP to BzOH, the dosage of the catalyst, the reaction rate, temperature, and solvent. The recycling investigations indicate that the synthesized Ni/Fe-SBA-15 (NFS-15) catalyst seems to be more durable up to seven successive cycles.
1. Introduction
Industries use selective oxidation techniques to convert alcohols into aldehydes and ketones.1–4 Finding high-purity aldehydes at a reasonable cost is still a top objective for researchers.5,6 Traditionally, we generated the hazardous organic waste, such as benzaldehyde, via toluene, benzyl chloride, and hydrolysis, respectively.7 This conversion is more expensive overall9–12 despite its limited selectivity, loss of conversion, toxic by-products, and substrate specificity.8 Researchers employed liquid-phase selective oxidation with a noble metal catalyst and a variety of oxidants to get around these constraints.13–16 Hydrogen peroxide (H2O2) is a powerful oxidizing agent that is also good for the environment and contains more active oxygen.17–20 The inability to reuse the catalyst and the challenge of separating it from the catalytic system are two drawbacks of homogeneous catalysis.21As a result, researchers are becoming more interested in the synthesis of affordable and environmentally acceptable solid acid catalysts.22 The activity of the catalysts can be further increased by supporting materials.23 Mahmoud Nasrollahzadeh and other researchers thoroughly evaluated the preferential oxidation of alcohols enabled by polymers.24 Because of its significant surface area and well-defined high porosity, ordered mesoporous silica is thought to have better catalyst support.25 In many heterogeneous catalyzes, metal catalysts, including Ru,26 Co,27 Au,28 and Pd,29 are used. Additionally, benzyl alcohol was catalytically converted using an SBA-15-assisted Au–Pd catalyst.30 The expense of oxidation procedures is raised by the noble metal's high price and agglomeration on the support surface.31 However, oxidation catalysts made of nickel are thought to be superior to those made of iron. This is attributed to stable oxide (nickel oxide) formation, which results in the catalytic abilities of semiconductor metal oxide rather than metal catalytic properties.32
Because of its good chemical stability and possible catalytic uses, Ni/Fe is a great option.33 It has been extensively used in the photocatalytic reduction of CO2, photocatalytic splitting of water, and photocatalytic synthesis of hydrogen.34 Due to its exceptional photocatalytic activity, this comes from a quick and effective charge change between the oxidation states and has attracted a lot of attention.35,36 The electrical configuration of the metal may be rapidly and drastically changed to perfectly accommodate its immediate surroundings.37 Recently, Ni/Fe has been employed in the selective oxidation of benzyl alcohol as a substrate for Au–Pd nanoparticles and as a catalyst or photocatalyst. A photocatalyst made of Ni/Fe was also employed with SBA-15 as a support.38,39 Researchers were synthesised the series of mesoporous NixCoy/SBA-15 catalysts with different Ni/Co ratios for the selective oxidation of alcohol using oxygen as the sole oxidant and it displayed the best catalytic performance with a benzyl alcohol conversion of 96.2%.40 However, selective oxidation is used for the majority of the aforementioned processes. CeO2, MnO2, and Ni–Fe composites oxidation catalytic agents were created by Xiaodong Zhang et al. and investigated in the process of oxidizing CO and toluene.41
Oleic acid inhibits nanoparticles against agglomeration by providing steric stability against van der Waals along with magnetic attractive interactions.42 Gong et al. investigated the influence of only one dosage of oleic acid (4 g in 100 ml) on the structural features of Fe3O4 nanoparticles. They observed that oleic acid, particularly present in the form of a bilayer, inhibit Ostwald ripening and promotes the formation of monodispersed magnetic nanospheres.43 In the instance of Co nanoparticles, an oleic acid/cobalt ratio of 0.15 resulted in erratically formed black precipitates. Conversely, as the oleic acid molar ratio reached to 0.6, the capping agent developed an extremely dense monolayer on the outermost layer of the nanoparticles, which restored them against agglomeration and also prohibited the Co from oxidizing in the air.44 However, as reported earlier, owing to the efficiency of oleic acid involved in the synthesis of the catalysts, likely as a result of the synthesis of metal oleate species, which stops the particles from clumping together during calcination. Additionally, the calcination process causes the metallic oxides to self-assemble, which results in significant dispersion and core–shell metal particles.
Investigations on the selective oxidation of benzyl alcohol utilizing Ni/Fe and SBA-15 in the liquid phase are still needed. The particle size, the kind of support, and the interaction between the support and the Ni/Fe catalyst are some of the factors that affect the Ni/Fe catalyst's ability to catalyze. This study involved the development of Ni/Fe-SBA-15 catalysts for the selective oxidation of benzyl alcohol (BzOH). In-depth research was done on the factors, such as catalyst quantity, metal loading, the substrate to oxidant ratio, and reaction temperature, that affect the efficiency of the oxidation from a catalytic perspective. The regeneration powers of the catalyst were also investigated in this work.
2. Experimental information
2.1. Preparation of catalysts and supports
Previous studies have shown that acidic conditions can be exploited to produce Ni–Fe bimetallic supported SBA-15 catalysts.45 The Ni–Fe loaded SBA-15 was prepared in the manner described below. Add 4 grams of P123, a triblock co-polymer (Mol. Wt 5800) to 20 ml of HCl and 130 ml of water. TEOS (14 ml) is added, and the mixture was subjected to thorough stirring. Nickel nitrate and iron nitrate were added to the mixture, and stirred for 7.5 h at 45 °C. Afterward, the mixture was allowed to age for 15.5 hours at 80 °C and cooled down. The cooled precipitate was dried, filtered, and washed in 25 ml water. Post-synthesis, the product was calcined for 6 hours at a temperature of 550 °C with a heating rate of 1 °C min−1 and progressively cooled.46Two sets of Ni–Fe bimetallic catalysts, each with a 7% metal loading (5 wt% Ni-2 wt% Fe/SBA-15 and 4 wt% Ni-3 wt% Fe/SBA-15), have been made, and compared with a mono metal catalyst comprising 7% Ni/SBA-15. Iron(III) nitrate nonahydrate and nickel(VI) nitrate hexahydrate, respectively, served as the precursors for the nickel and iron. Our previously reported core–shell precursor with in situ self-assembly technique was used to make the catalysts. Using an incipient wetness impregnation technique, a small amount of oleic acid (OA), nOA/n metal is, controlled at a molar ratio of 0.30, was utilised in this approach. The dissolved iron(III) nitrate nonahydrate and nickel hydrate hexahydrate were added, thoroughly mixed, and then combined with the SBA-15 support. The samples were aged for 12 h and then dried for around 6 h at 60 °C while being agitated occasionally. The catalyst material was then heated for 12 h at 100 °C before being calcined for 4 h at 700 °C in an electric furnace.47
2.2. Catalyst characterization study
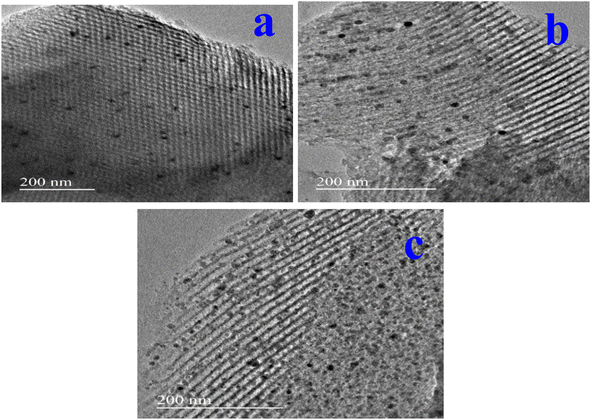
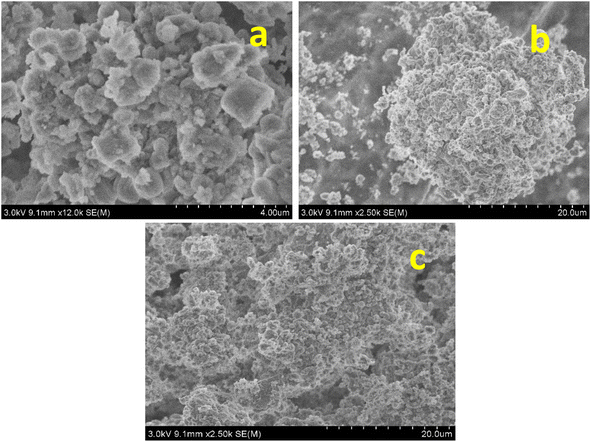
Crystallographic tests were carried out using a Cu Ka radiation source and an X-ray diffractometer (Rigaku Miniflex II). The catalyst's functional group was identified using Fourier transform infrared spectra (FTIR; PerkinElmer). The Micromeritics ASAP2020 equipment was employed to measure the synthesized catalyst's surface exposed area and pore volume. High-resolution scanning electron microscopy was utilized to take pictures of the materials on a Hitachi S-4800 device (HR-SEM). High-resolution transmission electron microscopy (HR-TEM) pictures of the samples were captured using JEOL equipment (JEM 2010). In order to capture thermogravimetric data from the samples, we used a thermogravimetric analyser made by PerkinElmer with model number TGA 7. At room temperature, a PerkinElmer Fourier transform infrared (FTIR) spectrometer was utilized to record the as-synthesized samples by using the KBR pellet approach.2.3. Catalytic activity in the selective oxidation of benzyl alcohol
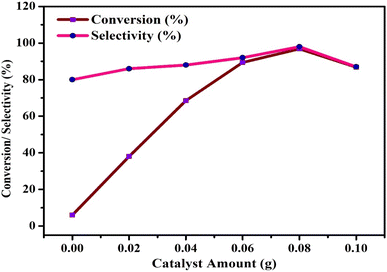
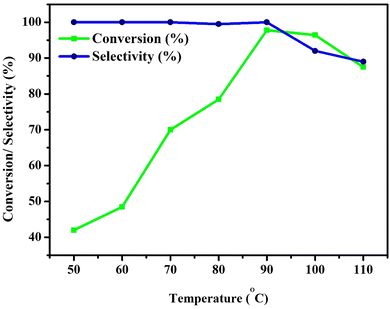
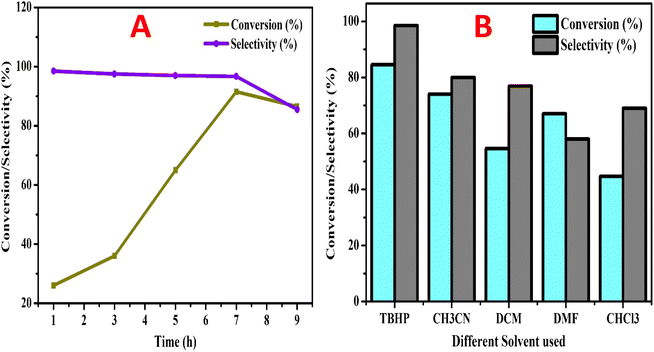
Benzyl alcohol (BzOH – 20 mmol) was oxidized over 0.1 g of various 7% Ni and Fe catalysts (NFS-15) in a batch reactor (round bottom flask with Liebig condenser and temperature controller) utilizing tert-butyl hydroperoxide (TBHP – 25 mmol) as the oxidant and acetonitrile (10 ml) as the solvent. The aforementioned combination was thereafter heated at 100 °C for 7 hours. The products were identified using a gas chromatograph with a flame ionization detector and a capillary column composed of 70 percent cyanopropyl polysphenylene siloxane, known as SGEBPX70, with the following specifications: 0.54 mm (length), 30 m (internal diameter), and 0.60 m (film thickness). The impacts of altering experimental parameters, such as concentration (7wt%Ni/SBA-15, 5wt%Ni-2wt%Fe/SBA-15, 4wt%Ni-3wt%Fe/SBA-15), catalyst loading (0.00–0.15 g), process temperature (50 to 110 °C), and TBHP/BzOH ratio (0.25–2.00), were assessed using the most active sample, Ni/Fe-SBA-15. Optimizing the process parameters maximized the BzOH conversion and benzaldehyde (BzH) selectivity. The possibility of recycling the sample was also checked.3. Results and discussion
3.1. Characterization using spectroscopy and physicochemical
| Catalyst | SBETa (m2 g−1) | Pore volumeb (cm3 g−1) | Dpc (nm) | d100d (nm) |
|---|---|---|---|---|
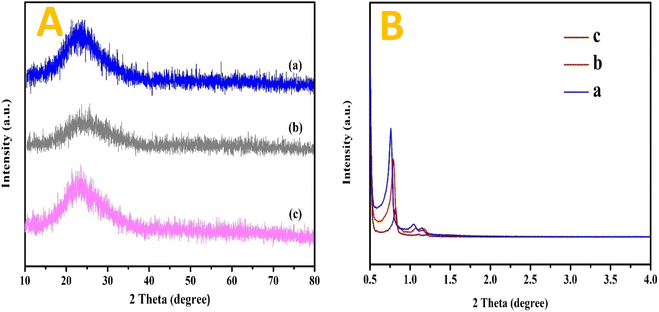
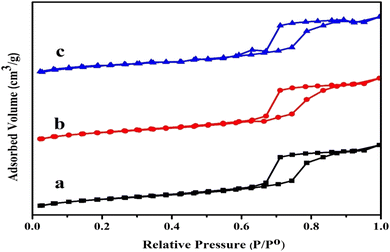
| a Specific surface area by BET analysis.b Total pore volume estimated at P/P0 = 0.99.c Average pore diameter calculated by Barrett–Joyner–Halenda (BJH) method.d From low angle XRD pattern (Fig. 1B). | ||||
| 7Ni/SBA-15 | 698.12 | 0.720 | 7.14 | 0.80 |
| 5Ni–2Fe/SBA-15 | 652.80 | 0.692 | 7.05 | 0.84 |
| 4Ni–3Fe/SBA-15 | 642.38 | 0.681 | 7.00 | 1.08 |
Table 1 also provides a summary of the catalyst's physical textural characteristics. Due to the mesoporous SBA-15 support's intrinsic properties, as well as every sample of the catalyst examined possessing a sizable specified surface area. In fact, compared to the Ni/SBA-15 monometallic catalyst, the surface area would have been around 698.12 m2 gcat−1, and the surface area decreased to approximately 642.38 m2 gcat−1 when the Ni metal was substituted with merely 2 wt% Fe. However, the subsequent substitution of Ni metal up to 3 wt% Fe did not exhibit a material surface area that differed noticeably from the substitute of 2 weight percent Fe. The pore volume, meanwhile, gradually reduced from 0.72 with an upsurge in the Fe loading.53 Furthermore, the pore capacity marginally decreased from 0.72 to 0.68 cm3 gcat−1 with an increase in Fe loading. Reduction in the specific surface area from 698.12 m2 g−1 of SBA-15 to 642.38 m2 g−1 after metal loading could be attributed to the surface coverage by metal species, while no clear fluctuation in pore volume and average pore diameter indicated metal species might be mainly loaded on the exterior surface of SBA-15 by impregnation, without blocking the pore channels. As a whole, the mass transfer resistance of something like the catalyst's pore network containing the hydrocarbon feed may be decreased by the existence of bigger pores. However, the pore diameter with Fe was slightly smaller, at about 7.00 nm, compared to the unsubstituted Ni/SBA-15 catalyst's 7.14 nm. These findings suggest that combining with Fe atoms causes the metals to migrate further deeper inside the host matrix of mesoporous silica, which results in a bit of constriction among the walls. However, the difference in pore size is only noticeably significant since the Fe atomic radius (126 pm) is not appreciably more important than that of Ni (124 pm).54
4. Selective catalytic oxidation study
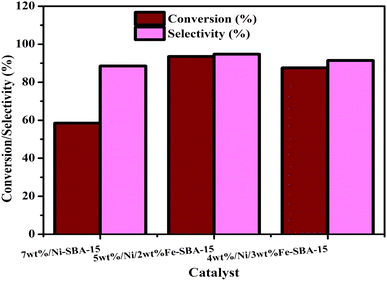
4.1. Catalytic activity of Ni/Fe-SBA-15 catalysts on the oxidation of benzyl alcohol
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
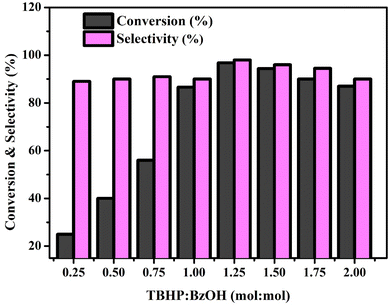
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzOH ratio was examined. Fig. 9 shows how the TBHP/BzOH molar ratio affects the oxidation of benzyl alcohol. The proportion of conversion rises from 27% to 93.5% with a molar ratio increase from 0.25 to 1.25. According to the data, increasing the TBHP level significantly improves benzyl alcohol conversion.62 To improve conversion, the TBHP
BzOH ratio was examined. Fig. 9 shows how the TBHP/BzOH molar ratio affects the oxidation of benzyl alcohol. The proportion of conversion rises from 27% to 93.5% with a molar ratio increase from 0.25 to 1.25. According to the data, increasing the TBHP level significantly improves benzyl alcohol conversion.62 To improve conversion, the TBHP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzOH ratio must be greater than 1.25. The conversion rate of benzyl alcohol, even with a TBHP
BzOH ratio must be greater than 1.25. The conversion rate of benzyl alcohol, even with a TBHP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzOH ratio of 1.0, is lower than expected, and the same can be attributed to the fact that TBHP's ability to be oxidized declines after 88.5 percent conversion. At further increase beyond 1.25 and up to 2.00, the conversion of benzyl alcohol reduces to 87%, and selectivity drops to 88.6%. This may be because TBHP, a chemical that readily transforms benzaldehyde into benzoic acid, decomposes under conditions of high oxygen concentration. Lower concentrations of TBHP result in restricted conversion, while higher concentrations of TBHP result in further oxidation. According to the experimental results, 1.25 is the perfect TBHP
BzOH ratio of 1.0, is lower than expected, and the same can be attributed to the fact that TBHP's ability to be oxidized declines after 88.5 percent conversion. At further increase beyond 1.25 and up to 2.00, the conversion of benzyl alcohol reduces to 87%, and selectivity drops to 88.6%. This may be because TBHP, a chemical that readily transforms benzaldehyde into benzoic acid, decomposes under conditions of high oxygen concentration. Lower concentrations of TBHP result in restricted conversion, while higher concentrations of TBHP result in further oxidation. According to the experimental results, 1.25 is the perfect TBHP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzOH ratio for the best BzOH transformation and BzH selectivity.63
BzOH ratio for the best BzOH transformation and BzH selectivity.63
| Catalyst | Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| NFS-15 | Conversion | 90 | 88.5 | 87.0 | 86.4 | 85.6 | 85.0 | 84.8 |
| Selectivity | 97.5 | 96.4 | 98.8 | 98.4 | 97.6 | 94.6 | 93.6 |
| Catalyst entry | Catalyst dose (g) | Time (h) | Temperature (°C) | Conversion (%) | Selectivity (%) to aldehyde | References |
|---|---|---|---|---|---|---|
| TiMCM-41-20Ph-2(55)a | 0.1 | 3 | 50 | 72 | 86 | 64 |
| Anion resin H3PW4O24 | 0.350 | 6 | 70 | 92.4 | 98.1 | 65 |
| Ti(acac)-SBA-15 | 0.01 | 2 | 60 | 19.9 | 19.3 | 66 |
| Ti(acac)-MCM-41 cal | 0.01 | 2 | 60 | 8.1 | 7.5 | 67 |
| 10%Me-TiMCM-41NP | 0.03 | 0.5 | 60 | 8.4 | 13.4 | 68 |
| TiMCM-41 LP | 0.03 | 2 | 70 | 5.7 | 10.3 | 69 |
| Cu(II)-cyclamSBA-16 | 0.05 | 4 | 80 | 47.3 | 3.6 | 70 |
| 10 MeTiM-1 | 0.05 | 7 | 80 | 45.8 | 74.76 | 71 |
| NFS-15 | 0.08 | 7 | 90 | 93.5 | 97.5 | This work |
5. Conclusion
Throughout this research, the efficacy of monometallic Ni supported on SBA-15 catalysts was compared to bimetallic Ni–Fe supported on SBA-15 catalysts for the selective oxidation of benzyl alcohol to benzaldehyde. A highly distributed Ni/FeSBA-15 catalyst based on SBA-15 was synthesized using the oleic acid aided incipient wetness impregnation approach. The NFS-15 catalyst achieved the highest rate of conversion and product selectivity. This could be as a result of the fact that NFS-15 exhibits a more synergistic interaction between Fe and Ni than the other catalysts produced in this study. The NFS-15 catalyst demonstrated 98% BzOH conversion and 99% BzH selectivity under optimised conditions (TBH P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BzOH ratio – 1.25; catalyst amount – 0.08 g; reaction temperature – 90 °C, reaction time 4 h). The NFS-15 catalyst's recycling effectiveness was evaluated up to 7 cycles, and it was discovered that the catalytic activity was sustained up to 7 cycles. This Ni and Fe loaded SBA-15 catalyst is affordable and efficient from an industrial perspective when compared to more costly metal catalysts. In terms of conversion, selectivity, and reaction temperature, the NFS-15 is not only better than other Ni-based catalysts mentioned in the literature, but it also outperforms them.
BzOH ratio – 1.25; catalyst amount – 0.08 g; reaction temperature – 90 °C, reaction time 4 h). The NFS-15 catalyst's recycling effectiveness was evaluated up to 7 cycles, and it was discovered that the catalytic activity was sustained up to 7 cycles. This Ni and Fe loaded SBA-15 catalyst is affordable and efficient from an industrial perspective when compared to more costly metal catalysts. In terms of conversion, selectivity, and reaction temperature, the NFS-15 is not only better than other Ni-based catalysts mentioned in the literature, but it also outperforms them.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to sincerely thank the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia, for funding this research work through the Project number (IFKSUOR3-544-2).Notes and references
- Z. Luo, A. S. Poyraz, C.-H. Kuo, R. Miao, Y. Meng, S.-Y. Chen, T. Jiang, C. Wenos and S. L. Suib, Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity, Chem. Mater., 2015, 27, 6–17 CrossRef CAS.
- M. Fukui, W. Koshidaa, A. Tanakaa, K. Hashimotoa and H. Kominamia, Photocatalytic hydrogenation of nitrobenzenes to anilines over noble metal-free TiO2 utilizing methylamine as ahydrogen donor, Appl. Catal., B, 2020, 268, 118446 CrossRef CAS.
- Z. Hu, H. Quan, Z. Chen, Y. Shao and D. Li, New insight into efficient visible-light-driven photocatalytic organic transformation over CdS/TiO2 photocatalysts, Photochem. Photobiol. Sci., 2018, 17, 51–59 CrossRef CAS PubMed.
- X. Li, J. Wang, Y. Men and Z. Bian, TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions, Appl. Catal., B, 2016, 187, 115–121 CrossRef CAS.
- N. Qin, Y. Liu, W. Wu, L. Shen, X. Chen, Z. Li and L. Wu, One Dimensional CdS/TiO2 Nanofiber Composites as Efficient Visible Light-Driven Photocatalysts for Selective Organic Transformation: Synthesis, Characterization, and Performance, Langmuir, 2015, 31, 1203–1209 CrossRef CAS PubMed.
- M. Zhang, Q. Wang, C. Chen, L. Zang, W. Ma and J. Zhao, Oxygen Atom Transfer in the Photocatalytic Oxidation of Alcohols by TiO2: Oxygen Isotope Studies, Angew. Chem., Int. Ed., 2009, 48, 6081–6084 CrossRef CAS PubMed.
- K. Yaemsunthorn, M. Kobielusz and W. Macyk, TiO2 with Tunable Anatase-to-Rutile Nanoparticles Ratios: How Does the Photoactivity Depend on the Phase Composition and the Nature of Photocatalytic Reaction?, ACS Appl. Nano Mater., 2021, 4, 633–643 CrossRef CAS.
- J. Zhu, S. Zhu, X. Kong, Y. Liang, Z. Li, S. Wu, S. Luo, C. Chang and Z. Cui, Rutile-Coated B-Phase TiO2 Heterojunction Nanobelts for Photocatalytic H2 Evolution, ACS Appl. Nano Mater., 2020, 3, 10349–10359 CrossRef CAS.
- A. J. Gardecka, C. Bishop, D. Lee, S. Corby, I. P. Parkin, A. Kafizas and S. Krumdieck, High efficiency water splitting photoanodes composed of nano-structured anatase-rutile TiO2 heterojunctions by pulsed-pressure MOCVD, Appl. Catal., B, 2018, 224, 904–911 CrossRef CAS.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO2 Photocatalysis: Mechanisms and Materials, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlope, J. W. J. Hamiltone, J. A. Byrnee, K. O'Sheaf, M. H. Entezarig and D. D. Dionysioua, A Review on The Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- J. Harris, R. Silk, M. Smith, Y. Dong, W.-T. Chen and G. I. N. Waterhouse, Hierarchical TiO2 Nanoflower Photocatalysts with Remarkable Activity for Aqueous Methylene Blue PhotoOxidation, ACS Omega, 2020, 5, 18919–18934 CrossRef CAS PubMed.
- M. Toyoda, Y. Nanbu, Y. Nakazawa, M. Hirano and M. Inagaki, Effect of crystallinity of anatase on photoactivity for methylene blue decomposition in water, Appl. Catal., B, 2004, 49, 227–232 CrossRef CAS.
- V. Etacheri, M. K. Seery, S. J. Hinder and S. C. Pillai, Highly Visible Light Active TiO2-xNx Heterojunction Photocatalysts, Chem. Mater., 2010, 22, 3843–3853 CrossRef CAS.
- V. Kumaravel, S. Mathew, J. Bartlett and S. C. Pillai, Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances, Appl. Catal., B, 2019, 244, 1021–1064 CrossRef CAS.
- N. Serpone, Is the Band Gap of Pristine TiO2 Narrowed by Anion and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts?, J. Phys. Chem. B, 2006, 110, 24287–24293 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, VisibleLight Induced Photocatalysis in Nitrogen doped Titanium Dioxides, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- X. Pan, M.-Q. Yang, X. Fu, N. Zhang and Y.-J. Xu, Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications, Nanoscale, 2013, 5, 3601–3614 RSC.
- X. Zhang, Y. Wang, B. Liu, Y. Sang and H. Liu, Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photo catalysts, Appl. Catal., B, 2017, 202, 620–641 CrossRef CAS.
- R. I. Bickley, T. G. Carreno, J. S. Lee, L. Palmisano and R. J. D. Tilley, A Structural Investigation of Titanium Dioxide Photocatalysts, J. Solid State Chem., 1991, 92, 178–190 CrossRef CAS.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. A. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Band Alignment of Rutile and Anatase TiO2, Nat. Mater., 2013, 12, 798–801 CrossRef CAS PubMed.
- X. Zhou, E. Wierzbicka, N. Liu and P. Schmuki, Black and white anatase, rutile and mixed forms: band-edges and photocatalytic activity, Chem. Commun., 2019, 55, 533–536 RSC.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR, J. Phys. Chem. B, 2003, 107, 4545–4549 CrossRef CAS.
- M. Nasrollahzadeh, M. Sajjadi, M. Shokouhimehr and R. S. Varma, Recent developments in palladium (nano)catalysts supported on polymers for selective and sustainable oxidation processes, Coord. Chem. Rev., 2019, 397, 54–75 CrossRef CAS.
- S. Sato, R. Nakamura and S. Abe, Visible-light sensitization of TiO2 photocatalysts by wet-method N doping, Appl. Catal., A, 2005, 284, 131–137 CrossRef CAS.
- Z. Liu, X. Zhang, S. Nishimoto, M. Jin, D. A. Tryk, T. Murakami and A. Fujishima, Anatase TiO2 Nanoparticles on Rutile TiO2Nanorods: A Heterogeneous Nanostructure via Layer-by-Layer Assembly, Langmuir, 2007, 23, 10916–10919 CrossRef CAS PubMed.
- Z. Xing, J. Zhang, J. Cui, J. Yin, T. Zhao, J. Kuang, Z. Xiu, N. Wan and W. Zhou, Recent advances in floating TiO2-basedphotocatalysts for environmental application, Appl. Catal., B, 2018, 225, 452–467 CrossRef CAS.
- B. R. Venugopal, E. P. Samuel, C. Shivakumara and M. Rajamathi, Macroporous metal oxide foams through self-sustained combustion reactions, J. Porous Mater., 2009, 16, 205–208 CrossRef CAS.
- B. R. Venugopal, S. Naik, M. Antony, G. Ramalingam, M. Rajamathi and S. Raghavan, Amorphous, Monoclinic, and Tetragonal Porous Zirconia Through a Controlled Self-Sustained Combustion Route, J. Am. Ceram. Soc., 2011, 94, 1747–1755 CrossRef.
- K. Nagaveni, M. S. Hegde, N. Ravishankar, G. N. Subbanna and G. Madras, Synthesis and Structure of Nanocrystalline TiO2 with Lower Band Gap Showing High Photocatalytic Activity, Langmuir, 2004, 20, 2900–2907 CrossRef CAS PubMed.
- S. Sakthivel and H. Kisch, Daylight Photocatalysis by CarbonModified Titanium Dioxide, Angew. Chem., Int. Ed., 2003, 42, 4908–4911 CrossRef CAS PubMed.
- S. U. M. Khan, M. Al-Shahry and W. B. Ingler Jr, Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2, Science, 2002, 297, 2243–2245 CrossRef CAS PubMed.
- K. Nagaveni, G. Sivalingam, M. S. Hegde and M. Giridhar, Solar photocatalytic degradation of dyes: high activity of combustion synthesized nano TiO2, Appl. Catal., B, 2004, 48, 83–93 CrossRef CAS.
- D. A. H. Hanaor and C. C. Sorrell, Review of the anatase to rutile phase transformation, J. Mater. Sci., 2011, 46, 855–874 CrossRef CAS.
- S. Yurdakal, G. Palmisano, V. Loddo, V. Augugliaro and L. Palmisano, Nanostructured Rutile TiO2 for Selective Photocatalytic Oxidation of Aromatic Alcohols to Aldehydes in Water, J. Am. Chem. Soc., 2008, 130, 1568–1569 CrossRef CAS PubMed.
- G.-J. T. Brink, I. W. C. E. Arends and R. A. Sheldon, Green, Catalytic Oxidation of Alcohols in Water, Science, 2000, 287, 1636–1639 CrossRef PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Environmental Applications of Semiconductor Photocatalysis, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- T. Mallat and A. Baiker, Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts, Chem. Rev., 2004, 104, 3037–3058 CrossRef CAS PubMed.
- Y. Shiraishi, N. Saito and T. Hirai, Titanosilicate Molecular Sieve for Size-Screening Photocatalytic Conversion, J. Am. Chem. Soc., 2005, 127, 8304–8306 CrossRef CAS PubMed.
- R. Li, Y. Zhang, B. Xing, M. Huang, T. Wang, X. Hong, B. Zhou, B. Li, D. Jia and S. Qi, Modulating the electronic structure of Co-Ni bimetal oxides on mesoporous silica for promoting selective oxidation of alcohol, Microporous Mesoporous Mater., 2023, 350, 112407 CrossRef CAS.
- X. Zhang, F. Bi, Z. Zhu, Y. Yang, S. Zhao, J. Chen, X. Lv, Y. Wang, J. Xu and N. Liu, The promoting effect of H2O on rod-like MnCeOx derived from MOFs for toluene oxidation: A combined experimental and theoretical investigation, Appl. Catal., B, 2021, 297, 120393 CrossRef CAS.
- R. Tadmor, R. E. Rosensweig, J. Frey and J. Klein, Resolving the Puzzle of Ferrofluid Dispersants, Langmuir, 2000, 16, 9117–9120 CrossRef CAS.
- T. Gong, D. Yang, J. Hu, W. Yang, C. Wang and J. Q. Lu, Preparation of monodispersed hybrid nanospheres with high magnetite content from uniform Fe3O4 clusters, Colloids Surf., A, 2009, 339, 232–239 CrossRef CAS.
- Y. Lu, X. Lu, T. Brian, T. H. Mayers and Y. Xia, Synthesis and characterization of magnetic Co nanoparticles: A comparison study of three different capping surfactants, J. Solid State Chem., 2008, 181, 1530–1538 CrossRef CAS.
- W.-T. Chen, A. G. Dosado, A. Chan, D. S. Waterhouse and G. I. N. Waterhouse, Highly Reactive Anatase Nanorod Photocatalysts Synthesized by Calcination of Hydrogen Titanate Nanotubes: Effect of Calcination Conditions on Photocatalytic Performance for Aqueous Dye Degradation and H2 Production in Alcohol-Water Mixtures, Appl. Catal., A, 2018, 565, 98–118 CrossRef CAS.
- H. Xu, G. Li, G. Zhu, K. Zhu and S. Jin, Enhanced Photocatalytic Degradation of Rutile/Anatase TiO2 Heterojunction Nano flowers, Catal. Commun., 2015, 62, 52–56 CrossRef CAS.
- G. Lui, J.-Y. Liao, A. Duan, Z. Zhang, M. Fowler and A. Yu, Graphene-Wrapped Hierarchical TiO2 Nano flower Composites with Enhanced Photocatalytic Performance, J. Mater. Chem. A, 2013, 1, 12255–12262 RSC.
- H. Choi, A. C. Sofranko and D. D. Dionysiou, Nanocrystalline TiO2 Photocatalytic Membranes with a Hierarchical Mesoporous Multilayer Structure: Synthesis, Characterization, and Multifunction, Adv. Funct. Mater., 2006, 16, 1067–1074 CrossRef CAS.
- H. Maleki and V. Bertola, TiO2 Nanofilms on Polymeric Substrates for the Photocatalytic Degradation of Methylene Blue, ACS Appl. Nano Mater., 2019, 2, 7237–7244 CrossRef CAS.
- M.-C. Wu, K.-C. Hsiao, Y.-H. Chang and K. Kordás, Core−Shell Heterostructures of Rutile and Anatase TiO2 Nanofibers for Photocatalytic Solar Energy Conversion, ACS Appl. Nano Mater., 2019, 2, 1970–1979 CrossRef CAS.
- J. Huo, C. Yuan and Y. Wang, Nanocomposites of ThreeDimensionally Ordered Porous TiO2 Decorated with Pt and Reduced Graphene Oxide for the Visible-Light Photocatalytic Degradation of Waterborne Pollutants, ACS Appl. Nano Mater., 2019, 2, 2713–2724 CrossRef CAS.
- Z. He, Q. Cai, H. Fang, G. Situ, J. Qiu, S. Song and J. Chen, Photocatalytic Activity of TiO2 Containing Anatase Nanoparticles and Rutile Nanoflower Structure Consisting of Nanorods, J. Environ. Sci., 2013, 25, 2460–2468 CrossRef CAS PubMed.
- L. Yu, Y. Lin and D. Li, Visible-light-induced aerobic oxidation of alcohols in a green catalytic system of carbonate-like species doped TiO2, Appl. Catal., B, 2017, 216, 88–94 CrossRef CAS.
- D. I. Enache, D. Barker, J. K. Edwards, S. H. Taylor, D. W. Knight, A. F. Carley and G. J. Hutchings, Solvent-free oxidation of benzyl alcohol using titania-supported gold–palladium catalysts: effect of Au–Pd ratio on catalytic performance, Catal. Today, 2007, 122(3–4), 407–411 CrossRef CAS.
- G. Gao, R. Rong, Z. Zhang, B. Pan, X. Sun, Q. Zhang, G. Zheng, K. Xu and L. GaoIn, Selective oxidation of benzyl alcohol to benzaldehyde over CoFe2O4 nanocatalyst, Catal. Commun., 2023, 183, 106757 CrossRef CAS.
- J. Tsuji, Synthetic Applications of the Palladium-Catalyzed Oxidation of Olefins to Ketones, Synthesis, 1984, 1984, 369–384 CrossRef.
- M. A. Fouad, H. Abdel-Hamid and M. S. Ayoup, Two decades of recent advances of Ugi reactions: synthetic and pharmaceutical applications, RSC Adv., 2020, 10, 42644–42681 RSC.
- S. Semwal and J. Choudhury, Switch in Catalyst State: Single Bifunctional Bi-state Catalyst for Two Different Reactions, Angew. Chem., Int. Ed., 2017, 56, 5556–5560 CrossRef CAS PubMed.
- L. Liu, S. Zhang, X. Fu and C.-H. Yan, Metal-free aerobic oxidative coupling of amines to imines, Chem. Commun., 2011, 47, 10148–10150 RSC.
- F. Bottaro, A. Takallou, A. Chehaiber and R. Madsen, CobaltCatalyzed Dehydrogenative Coupling of Amines into Imines, Eur. J. Org Chem., 2019, 2019, 7164–7168 CrossRef CAS.
- T. Xiao, S. Xiong, Y. Xie, X. Dong and L. Zhou, Coppercatalyzed synthesis of benzazoles via aerobic oxidative condensation of o-amino/mercaptan/hydroxyanilines with benzylamines, RSC Adv., 2013, 3, 15592–15595 RSC.
- K. Singh, A. Kaur, V. S. Mithu and S. Sharma, Metal-Free Organocatalytic Oxidative Ugi Reaction Promoted by Hypervalent Iodine, J. Org. Chem., 2017, 82, 5285–5293 CrossRef CAS PubMed.
- K. Kaizuka, H. Miyamura and S. Kobayashi, Remarkable effect of bimetallic nanocluster catalysts for aerobic oxidation of alcohols: combining metals changes the activities and the reaction pathways to aldehydes/carboxylic acids or esters, J. Am. Chem. Soc., 2010, 132, 15096–15098 CrossRef CAS PubMed.
- M. Shibasaki and Y. Yamamoto, Multimetallic Catalysts in Organic Synthesis, Wiley, Weinheim, Germany, 2006 Search PubMed.
- I. Bratko and M. Gomez, Polymetallic complexes linked to a single-frame ligand: cooperative effects in catalysis, Dalton Trans., 2013, 42, 10664–10681 RSC.
- M. Shibasaki, M. Kanai, S. Matsunaga and N. Kumagai, Recent progress in asymmetric bifunctional catalysis using multimetallic systems, Acc. Chem. Res., 2009, 42, 1117–1127 CrossRef CAS PubMed.
- M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kiely and G. J. Hutchings, Designing bimetallic catalysts for a green and sustainable future, Chem. Soc. Rev., 2012, 41, 8099–8139 RSC.
- C. Kaub, S. Lebedkin, A. Li, S. V. Kruppa, P. H. Strebert, M. M. Kappes, C. Riehn and P. W. Roesky, Bimetallic d10-metal complexes of a bipyridine substituted N-heterocyclic carbene, Chem.–Eur. J., 2018, 24, 6094–6104 CrossRef CAS PubMed.
- T. Dang-Bao, D. Pla, I. Favier and M. Gomez, Bimetallic nanoparticles in alternative solvents for catalytic purposes, Catalysts, 2017, 7, 207 CrossRef.
- C. Parmeggiani and F. Cardona, Transition metal based catalysts in the aerobic oxidation of alcohols, Green Chem., 2012, 14, 547–564 RSC.
- T. Punniyamurthy, S. Velusamy and J. Iqbal, Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen, Chem. Rev., 2005, 105, 2329–2364 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |