Open Access Article
Open Access ArticleStreptomyces monashensis MSK03-mediated synthesis of gold nanoparticles: characterization and antibacterial activity
Supavadee Kerdtooba,
Panjamaphon Chanthasenab,
A'liyatur Rosyidahc,
Wanwisa Limphiratd,
Watsana Penkhruea,
Phongsakorn Gantaa,
Wissarut Srisakvarangkoola,
Montri Yasawongef and
Nawarat Nantapong *a
*a
aSchool of Preclinical Sciences, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima 30000, Thailand. E-mail: nawarat@sut.ac.th
bDepartment of Medical Technology, Faculty of Allied Health Sciences, Nakhonratchasima College, Nakhon Ratchasima 30000, Thailand
cResearch Center for Vaccine and Drug, National Research and Innovation Agency (BRIN), Bogor, West Java, Indonesia
dSynchrotron Light Research Institute, 111 University Avenue, Nakhon Ratchasima, Thailand
eProgramme on Environmental Toxicology, Chulabhorn Graduate Institute, Bangkok 10210, Thailand
fCenter of Excellence on Environmental Health and Toxicology (EHT), OPS, MHESI, Bangkok 10400, Thailand
First published on 5th February 2024
Abstract
Nanotechnology is a cutting-edge field with diverse applications, particularly in the utilization of gold nanoparticles (AuNPs) due to their stability and biocompatibility. AuNPs serve as pivotal components in medical applications, with a specific emphasis on their significant antibacterial efficacy. This study focuses on synthesizing AuNPs using the cell-free supernatant of Streptomyces monashensis MSK03, isolated from terrestrial soil in Thailand. The biosynthesis process involved utilizing the cell-free supernatant of S. monashensis MSK03 and hydrogen tetrachloroauric acid (HAuCl4) under controlled conditions of 37 °C and 200 rpm agitation. Characterization studies revealed spherical AuNPs with sizes ranging from 7.1 to 40.0 nm (average size: 23.2 ± 10.7 nm), as confirmed by TEM. UV-Vis spectroscopy indicated a localized surface plasmon resonance (LSPR) band at 545 nm, while XRD analysis confirmed a crystalline structure with characteristics of cubic lattice surfaces. The capping molecules on the surface of AuNPs carry a negative charge, indicated by a Zeta potential of −26.35 mV, and FTIR analysis identified functional groups involved in reduction and stabilization. XANES spectra further confirmed the successful reduction of Au3+ to Au0. Moreover, the synthesized AuNPs demonstrated antibacterial activity against drug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii. Interestingly, the AuNPs showed non-toxicity to Vero cell lines. These significant antibacterial properties of the produced nanoparticles mean they hold great promise as new antimicrobial treatments for tackling the increasing issue of antibiotic resistance.
Introduction
In recent years, there has been a surge of interest in nanotechnology for the production of metal nanoparticles (NPs).1,2 This field involves manipulating nanosized materials (1–100 nm) to generate novel features such as chemical, biological, electrical, and catalytic qualities that bulk materials cannot attain.3,4 Metal nanoparticles find wide-ranging applications in medicine and materials engineering.2,3,5–8 For instance, gold nanoparticles synthesized using a combination of five cow-derived products (urine, dung, milk, curd, ghee) showed antimicrobial activity against human pathogens, Klebsiella pneumoniae and Bacillus subtilis. Copper iodide nanoparticles were used for monitoring of dopamine, aiding in the early diagnosis of neurodegenerative disorders.9 Additionally, the determination of vitamin B12 (VB12) was achieved by developing an electrochemical sensor prepared through the electrophoretic deposition of tin dioxide nanoparticles.6 Furthermore, titanium carbide–MXene nanoparticles were produced using plastic and applied in a self-charging device.7Among NPs, gold nanoparticles (AuNPs) have been studied extensively by many researchers due to their unique properties, such as antimicrobial activity, low toxicity, ease of production, and precision targeting.2,10,11 Recently, there has been a lot of research focused on antibacterial properties of AuNPs making them a promising candidate for antibiotic supplementation. The antibacterial activity of AuNPs is determined by several factors, including size, dose, and the type of bacteria.2,3 Previous studies by Shamaila and colleagues demonstrated that chemically synthesized AuNPs exhibit antibacterial action against Staphylococcus aureus, Escherichia coli, K. pneumoniae, and B. subtilis, with the activity being size and dose-dependent.2 Furthermore, different types of bacteria showed distinct responses to AuNPs. For example, the antibacterial activity of biologically synthesized PG-AuNPs by panchagavya (PG) was found to be strong against Gram-negative bacteria and moderate against Gram-positive bacteria.3
The synthesis of AuNPs can be achieved through chemical, physical, and biological methods.1 However, chemical and physical approaches, which often involve the use of toxic agents, raise concerns for both the environment and human health. In contrast, biological synthesis is considered a safe, non-toxic, and environmentally friendly alternative.1,3,12,13 This approach entails the utilization of bacteria, fungi, plants, and other natural raw materials for the synthesis of nanoparticles.3 Microorganisms, especially Streptomyces species, are effective in producing AuNPs due to their ability to grow in a low-cost medium and facilitate controlled reduction of metal ions.14 Streptomycetes, Gram-positive filamentous bacteria that serve as a valuable source of antibiotics, have proven the potential to synthesize AuNPs.15 Previous reports highlight various Streptomyces species, such as Streptomyces cyaneus Alex-SK121, Streptomyces sp. NH21, Streptomyces hygroscopicus BDUS49, Streptomyces griseus M8, Streptomyces misionensis PYA9, Streptomyces sp. U30, and Streptomyces viridogens HM10, in the synthesis of AuNPs with antimicrobial activity against various bacteria.16–22
In this study, we synthesized AuNPs using the cell-free supernatant of Streptomyces monashensis MSK03, isolated from terrestrial soil in Thailand. The biosynthesis of AuNPs was characterized using various techniques, including UV–visible spectroscopy, X-ray diffraction (XRD) spectroscopy, energy-dispersive X-ray (EDX) spectroscopy, transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy, and X-ray absorption near edge structure (XANES) spectroscopy. Additionally, we explored the antibacterial potential of these nanoparticles against clinical drug-resistant pathogens and assessed their cytotoxicity profiles. To our knowledge, this is the first report on the utilization of S. monashensis for synthesizing AuNPs. Our findings significantly contribute to understanding the potential of Streptomyces species in the field of nanoparticle biosynthesis, paving the way for future research and applications.
Experimental
Microorganisms
Streptomyces sp. MSK03 was isolated from terrestrial soil at Sakaerat Environmental Research Station (SERS), Nakhon Ratchasima, Thailand. To assess the antimicrobial activity, drug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii were used. These pathogenic bacteria were obtained from clinical samples at Suranaree University of Technology Hospital (SUTH), Thailand.Isolation of Streptomyces sp. MSK03
Soil samples were randomly collected randomly from the upper surface of the soil at a depth of 10–15 cm using a sterile technique. A total of 1 g of soil was suspended in 99 mL of sterile water and incubated at room temperature with shaking at 200 rpm for 30 minutes. After incubation, the soil suspension was allowed to settle, and then it was subjected to 10-fold serial dilution up to 10−6. The Streptomyces strain was isolated using the actinomycete isolation agar (AIA, Himedia, India) medium through a serial dilution procedure. The agar plates were incubated at 37 °C for a duration of 7 days or until the colonies appeared. The streptomycetes colonies were further purified on International Streptomyces Project 2 (ISP2) agar medium (4 g L−1 of yeast extract, 10 g L−1 of malt extract, 4 g L−1 of glucose, 15 g L−1 of agar, and pH 7.2).Identification of Streptomyces sp. MSK03
For molecular identification of Streptomyces sp. strain MSK03, genomic DNA was extracted using the modified method for fungal DNA extraction.23 The 16S ribosomal RNA (rRNA) gene was amplified using polymerase chain reaction (PCR) with universal 16S rRNA primers, 27F 5′ AGAGTTTGATCCTGGCTCAG 3′ and 1525R 5′ AAGGAGGTGWTCCARCC 3′.24 The PCR product was then purified using the Gel/PCR Purification Mini Kit (Favorgen™, Taiwan), and the purified PCR products were sent for sequencing at Macrogen, Korea. The sequence of the 16S rRNA gene was compared to the EzBioCloud 16S rRNA database (http://www.ezbiocloud.net) for identification purposes.Preparation of cell-free supernatant
Streptomyces sp. MSK03 was cultured in a 500 mL Erlenmeyer flask with 100 mL of Starch Casein Broth (SCB) (10 g L−1 of soluble starch, 0.3 g L−1 of casein, 2 g L−1 of KNO3, 2 g L−1 of NaCl, 2 g L−1 of K2HPO4, 0.05 g L−1 of MgSO4, 0.02 g L−1 of CaCO3, 0.01 g L−1 of FeSO4, and pH 7.2) and incubated at 37 °C under shaking conditions at 200 rpm for 5 days. After the incubation period, the culture was centrifuged at 4 °C and 8000 rpm for 5 minutes. The resulting cell-free supernatant was carefully collected and utilized for the biosynthesis of AuNPs.Biosynthesis of AuNPs
Biosynthesis of AuNPs was carried out by following the methods outlined by Khadivi et al.,12 Soltani et al.,25 and Balagurunathan et al.22 with minor modification. In brief, 1 mM Hydrogen tetra chloroauric acid (HAuCl4·3H2O; Sigma-Aldrich, USA.) was combined with the cell-free supernatant of Streptomyces sp. MSK03 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume-to-volume ratio. The reaction mixture underwent an incubation period at 37 °C with continuous shaking at 200 rpm for 72 hours, and the pH was adjusted to 7. As a control experiment, the cell-free supernatant and HAuCl4 solution was used. After the incubation period, the synthesis of AuNPs was visually monitored as the mixture transitioned from a pale yellow hue to a pale pink or purple color. This distinctive color change served as a visible marker for the successful reduction of Au3+ to Au0, a key milestone in the biosynthesis process. The entire mixture was centrifuged at 4 °C, 8000 rpm for 5 minutes, and the AuNPs were washed with sterile DI water, then collected for further studies.
1 volume-to-volume ratio. The reaction mixture underwent an incubation period at 37 °C with continuous shaking at 200 rpm for 72 hours, and the pH was adjusted to 7. As a control experiment, the cell-free supernatant and HAuCl4 solution was used. After the incubation period, the synthesis of AuNPs was visually monitored as the mixture transitioned from a pale yellow hue to a pale pink or purple color. This distinctive color change served as a visible marker for the successful reduction of Au3+ to Au0, a key milestone in the biosynthesis process. The entire mixture was centrifuged at 4 °C, 8000 rpm for 5 minutes, and the AuNPs were washed with sterile DI water, then collected for further studies.
Characterization of MSK03-AuNPs
Zeta potential and hydrodynamic size distribution analysis. Zeta potential measurement and hydrodynamic particle size distribution analysis of the MSK03-AuNPs were conducted using a Zeta-sizer instrument (Malvern Instrument Ltd, USA). The zeta potential analyzer was utilized to measure the zeta potential, indicating the surface charge of the AuNPs.27,28 On the other hand, the dynamic light scattering (DLS) technique was employed to measure the average particle size distribution of the biosynthesized AuNPs.16,28,29
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 eV. The obtained XANES data was averaged and normalized using the Demeter package, version 8.9.26.32. This XANES analysis was carried out at the XANES beamline (beamline 8) at the Synchrotron Light Research Institute (SLRI), Thailand.
919 eV. The obtained XANES data was averaged and normalized using the Demeter package, version 8.9.26.32. This XANES analysis was carried out at the XANES beamline (beamline 8) at the Synchrotron Light Research Institute (SLRI), Thailand.Determination of the antimicrobial activity of AuNPs
The antimicrobial activity of MSK03-AuNPs against drug-resistant pathogens P. aeruginosa and A. baumannii was assessed using the agar well diffusion method. The Mueller–Hinton agar (Himedia™, India) was inoculated with mid-log phase (5 × 105 CFU mL−1) test pathogens using the spread plate technique. Subsequently, a 6 mm diameter hole was aseptically punched in the agar using a sterile cork borer. One hundred microliters of the MSK03-AuNPs (56.55 μg) were then added into the well and allowed to diffuse into the agar at room temperature for 1 hour. The culture broth of MSK03 and HAuCl4 were used as control substances. Following this, all plates were incubated at 37 °C for 24 hours. The formation of a clear zone around the well was observed and measured to determine the antimicrobial activity of MSK03-AuNPs against the drug-resistant pathogens.Cytotoxicity assay
The MTT assay was performed to analyze the inhibitory concentration (IC50) of synthesized AuNPs.36–38 The Vero cell line (kidney tissue of an African green monkey) was cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS). A total of 104 cells per well were seeded in 96-well plates and incubated at 37 °C with 5% CO2 for 24 hours. After 24 hours, the cells were treated with different concentrations of MSK03-AuNPs (ranging from 32 to 512 μg mL−1) in serum-free media and incubated for an additional 24 hours. Subsequently, the cells were treated with MTT (0.5 mg mL−1) and incubated for 4 hours to allow the formation of formazan crystals. The formed formazan crystals were solubilized by removing the MTT solution and adding 100 μL of 100% DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10% SDS (9
10% SDS (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to each well. The absorbance of the purple-blue formazan dye was then measured at 570 nm using a spectrophotometer. The percentage cytotoxicity of each concentration was calculated and utilized for IC50 determination using the GraphPad Prism 5.0 program.
1) to each well. The absorbance of the purple-blue formazan dye was then measured at 570 nm using a spectrophotometer. The percentage cytotoxicity of each concentration was calculated and utilized for IC50 determination using the GraphPad Prism 5.0 program.
Results and discussion
Isolation and identification of Streptomyces sp. MSK03
Streptomyces is the genus of the actinomycetales order with more than 500 members, including those industrially significant for their unrivaled ability to produce a wide range of bioactive secondary metabolites with interesting biological activities.15 The present study attempted to eco-friendly synthesizes AuNPs using the soil bacterium Streptomyces sp. MSK03 and investigated their biological properties, particularly antibacterial and cytotoxic effects. The strain MSK03 was isolated from forest soil at SERS, Nakhon Ratchasima province, Thailand. The identification of Streptomyces sp. MSK03 was based on its physiological and morphological properties, as depicted in Fig. 1. The strain exhibited leathery growth with white aerial mycelium and dark yellow substrate mycelium on ISP2 medium, and its colony released light yellow soluble pigments visible as exudates after incubation at 37 °C for 10 days. | ||
| Fig. 1 Colony morphology of soil-isolated S. monashensis MSK03 on ISP2 agar after 10 days of incubation. | ||
Molecular techniques based on 16S rRNA gene sequences were employed to identify the species level of bacterial strain.39 The 16S rRNA gene sequence of strain MSK03 was amplified using 27F and 1525R primers.24 The 16S rRNA gene sequence of MSK03 was submitted to GenBank with the accession number ON159854. The 16S rRNA of MSK03 showed a high similarity of 99.79% to Streptomyces monashensis MUSC 1JT (KP998432). Organisms are typically identified at the species level when their 16S rRNA gene sequences exhibit more than 99% identity.39 Thus, we propose that strain MSK03 belongs to the S. monashensis species, which, to our knowledge, has not been previously employed in metal nanoparticle synthesis.
Biosynthesis of MSK03-AuNPs
The use of streptomycetes in gold nanoparticle (AuNP) biosynthesis offers a versatile and advantageous approach.14,15,40 With inherent biocompatibility and low toxicity, streptomycetes are well-suited for biomedical applications.14,15 Their enzymatic activity serves as eco-friendly reducing and stabilizing agents, while their metal-tolerant nature facilitates efficient nanoparticle reduction.14,41 The controlled synthesis allows for fine-tuning of AuNPs size and shape.42–44 Streptomycetes' abundance and diversity in natural environments provide a rich resource for strain optimization, ensuring economic viability and environmental friendliness in large-scale production.14,15 Furthermore, the potential for nanoparticle functionalization and antibacterial properties further underscores the diverse benefits of employing streptomycetes in AuNP biosynthesis.42,43,45 In this study, AuNPs were synthesized using the extracellular cell-free supernatant of S. monashensis MSK03, which acted as reducing and stabilizing agents. The synthesis process involved mixing the cell-free supernatant of MSK03 with 1 mM HAuCl4 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, followed by incubation at 37 °C for 72 hours. The initial light yellow mixture changed noticeably during incubation into a purple color, demonstrating the formation of surface plasmon resonance (SPR), characteristic of AuNPs (Fig. 2). The color of the solution changed from light yellow to purple upon the formation of AuNPs, indicating that Au3+ was reduced to Au0.22,29,46–50 Thus, S. monashensis MSK03 demonstrated successful biosynthesis of AuNPs, a capability not shared by all Streptomyces strains. This study leads the recognition of S. monashensis as a novel candidate for the eco-friendly synthesis of AuNPs, marking an unexplored avenue in nanoparticle biosynthesis research.
1 ratio, followed by incubation at 37 °C for 72 hours. The initial light yellow mixture changed noticeably during incubation into a purple color, demonstrating the formation of surface plasmon resonance (SPR), characteristic of AuNPs (Fig. 2). The color of the solution changed from light yellow to purple upon the formation of AuNPs, indicating that Au3+ was reduced to Au0.22,29,46–50 Thus, S. monashensis MSK03 demonstrated successful biosynthesis of AuNPs, a capability not shared by all Streptomyces strains. This study leads the recognition of S. monashensis as a novel candidate for the eco-friendly synthesis of AuNPs, marking an unexplored avenue in nanoparticle biosynthesis research.
Characterization of gold nanoparticles
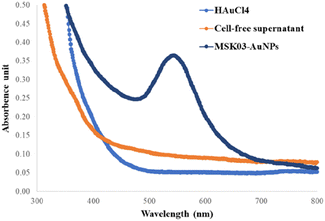
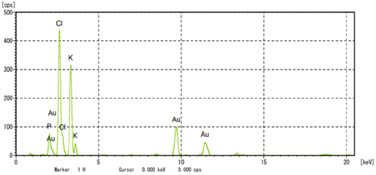
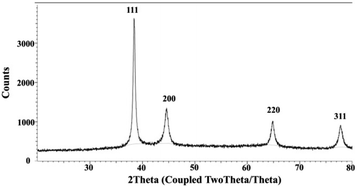
To confirm the formation of AuNPs, UV-Vis spectrophotometry techniques were employed to determine the position of the localized surface plasmon resonance (LSPR) bands of the synthesized AuNPs.22,29,48,51 The LSPR band of MSK03-AuNPs exhibited a wavelength of maximum absorbance at 545 nm, falling within the absorption range typically observed for spherical AuNPs (500–580 nm).43,52,53 In contrast, no absorption spectrum corresponding to AuNPs was observed in the cell-free supernatants or HAuCl4 solutions, as depicted in Fig. 3. This confirmed that the characteristic absorption peak at 545 nm was indeed due to the presence of the synthesized AuNPs, confirming the existence of spherical AuNPs produced by S. monashensis MSK03.In this study, multiple analyses were performed to demonstrate the formation of AuNPs, including EDXRF, XRD, and XANES.25,33,54 The elemental mapping obtained from TEM-EDXRF and XRD techniques confirmed the presence of Au elements in the sample. The EDXRF spectra indicated strong optical absorption peaks corresponding to metallic Au at 2.2, 9.7, and 11.5 keV, with the Au element detected at 8.08% in the MSK03-AuNPs (Fig. 4). The crystal structure of MSK03-AuNPs was elucidated through XRD analysis, as shown in Fig. 5. The XRD pattern exhibited four distinct diffraction peaks at 2θ values of 38.3°, 44.5°, 64.9°, and 77.8°, corresponding to the (111), (200), (220), and (311) crystallographic planes, respectively, of the face-centered cubic (fcc) structure of metallic gold. This unequivocally confirmed that the crystal structure of MSK03-AuNPs exhibited the characteristic features of a cubic lattice surface.
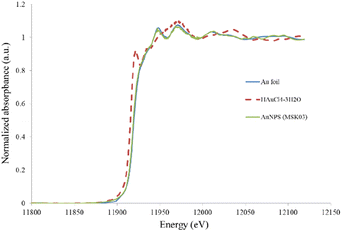
Additionally, XANES analysis was employed to investigate the local geometry and structure of gold in the synthesized AuNPs. Fig. 6 illustrates a comparison between the XANES spectra profiles of MSK03-AuNPs and a standard material. The spectra of MSK03-AuNPs exhibited a similar energy profile to that of Au metal foil, indicating a partial reduction of Au3+ to Au0 during the biosynthesis process.
Based on the EDXRF, XRD, and XANES analyses, it can be concluded that S. monashensis MSK03 cell-free supernatants can effectively reduce Au3+ into Au0. The detailed mechanism of the reduction process of Au3+ to Au0 remains unclear. Several researchers have proposed the reducing agents for biosynthesized AuNPs, including lipids, carbohydrates, nucleic acids, or proteins.26 For the biosynthesized AuNPs using Streptomyces spp., cell wall reductive enzymes and enzymes on the cytoplasmic membrane have been suggested as potential reducing agents.28,55
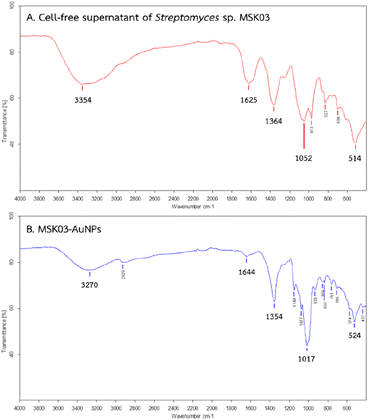
To identify the possible organic functional groups acting as capping, stabilizing, and reducing agents in the synthesized AuNPs, FTIR analysis was employed. The functional groups of biomolecules attached to the surface of AuNPs could play a role in the reduction of Au3+ to Au0, making FTIR analysis crucial in identifying these biomolecules.56,57 Fig. 7 presents the FTIR spectra of the cell-free supernatant of S. monashensis MSK03 and MSK03-AuNPs, each showing five major peaks. A shift in peak positions was observed from 3354, 1625, 1364, 1052, and 514 cm−1 in the cell-free supernatant of MSK03 to 3270, 1644, 1354, 1017, and 524 cm−1 in the MSK03-AuNPs, corresponding to N–H or O–H stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching or N–H bending, C–N stretching or N–H bending, C–H stretching, and C–H bending or metal–ligand stretching, respectively. The absorption peaks at 3270, 1644, and 1354 cm−1 are indicative of functional groups associated with carbohydrates, amines, and amides, respectively, while the remaining peaks at 1017 and 524 cm−1 correspond to the amine functional groups of the protein. Changes in peak intensity or shifts in wavenumbers of functional groups from the cell-free supernatant to AuNPs can provide insights into the specific functional groups involved in the interaction with AuNPs.58 For example, Kumar and Rao (2016) reported that proteins containing functional groups such as amide, carboxyl, and hydroxyl from Streptomyces coelicoflavus SRBVIT13 were attached to the surface of AuNPs, acting as stabilizing agents.59 In our study, the FTIR results of the biosynthesized AuNPs using the extracellular cell-free supernatant of S. monashensis MSK03 indicated that proteins with functional groups like carboxyl, hydroxyl, amine, and amide groups from S. monashensis MSK03 are bound to AuNPs, serving as capping, reducing, and stabilizing agents.
O stretching or N–H bending, C–N stretching or N–H bending, C–H stretching, and C–H bending or metal–ligand stretching, respectively. The absorption peaks at 3270, 1644, and 1354 cm−1 are indicative of functional groups associated with carbohydrates, amines, and amides, respectively, while the remaining peaks at 1017 and 524 cm−1 correspond to the amine functional groups of the protein. Changes in peak intensity or shifts in wavenumbers of functional groups from the cell-free supernatant to AuNPs can provide insights into the specific functional groups involved in the interaction with AuNPs.58 For example, Kumar and Rao (2016) reported that proteins containing functional groups such as amide, carboxyl, and hydroxyl from Streptomyces coelicoflavus SRBVIT13 were attached to the surface of AuNPs, acting as stabilizing agents.59 In our study, the FTIR results of the biosynthesized AuNPs using the extracellular cell-free supernatant of S. monashensis MSK03 indicated that proteins with functional groups like carboxyl, hydroxyl, amine, and amide groups from S. monashensis MSK03 are bound to AuNPs, serving as capping, reducing, and stabilizing agents.
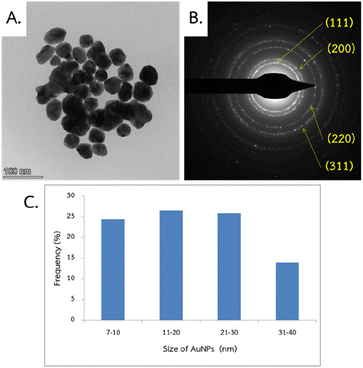
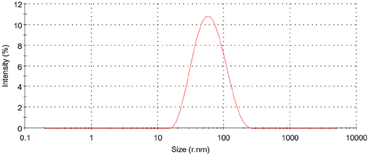
TEM and DLS analyses were employed to evaluate the morphological properties (size and shape) of nanoparticles. The TEM technique typically focuses solely on the size of the core particle, excluding capping agents or other linked molecules.60,61 The obtained images revealed that MSK03-AuNPs exhibited spherical forms (Fig. 8A). To confirm AuNPs formation and crystalline structure, SAED analysis revealed distinct diffraction rings, confirming the face-centered cubic (fcc) structure of gold (JCPDS: 04-0784), as indicated by (111), (200), (220), and (311) lattice planes (Fig. 8B).30,62 The TEM/SAED results were consistent with the XRD results. The average particle size of MSK03-AuNPs was measured to be 23.2 ± 10.7 nm, with a size range varying from 7.1 to 40.0 nm (Fig. 8C). On the other hand, DLS measures the hydrodynamic size, which includes the core, capping agents, and other absorbed molecules on the nanoparticle's surface.63,64 The analysis revealed that the MSK03-AuNPs exhibited an average size of 46.34 nm, with a polydispersity index (PDI) of 0.268 (Fig. 9). Previous reports have indicated that the particle size observed in TEM images is generally smaller than that measured by the DLS analyzer.29,64–67 In our study, TEM analysis revealed the spherical shape of gold nanoparticles synthesized by MSK03, with an average particle size of 23.2 nm, which was smaller than the hydrodynamic size (46.34 nm) of the particles. The size of the nanomaterial is important because it affects its physical properties, cell penetration, and interactions with living cell molecules. Smaller particles have a larger surface area than larger particles for the same amount of material, and surface activity of AuNPs is also higher.68
Zeta potential analysis has been instrumental in understanding the stability of the synthesized nanostructures. This analytical technique is widely employed to describe the surface charge, stability, and aggregation conditions of colloidal particles, including AuNPs.63,64,69 Large positive or negative zeta potential values indicate strong physical stability due to electrostatic repulsion among individual particles. Conversely, zeta potential values below −25 mV or above +25 mV typically indicate high stability.70–75 Furthermore, studies have suggested that zeta potential values closer to −30 mV indicate higher stability for metal nanoparticles.76,77 In our study, the zeta potential value of MSK03-AuNPs was determined to be −26.35 (±1.29) mV, indicating the presence of negative charge and potential repulsion among the AuNPs. This negative charge on the MSK03-AuNPs is attributed to the presence of biological molecules in the cell-free supernatant, which act as colloidal stabilizers for the MSK03-AuNPs. Consequently, our findings validate the high level of stability exhibited by MSK03-AuNPs.
Antimicrobial activity of MSK03-AuNPs
Due to the rise of microbial infections and increased drug-resistant rates, novel antimicrobials are being investigated. The World Health Organization (WHO) has prioritized pathogens under the acronym ESKAPE (Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter species) due to their profound threat to human health.78 These antibiotic-resistant microorganisms can lead to severe and often fatal infectious diseases, such as bloodstream infections and pneumonia.79 In this context, biosynthesized nanoparticles (NPs) have emerged as promising agents for addressing antibiotic-resistant infections, offering potential alternatives to conventional antibiotics.80Despite these advancements, there remains a dearth of studies on antibacterial gold nanoparticles (AuNPs) synthesized from actinomycetes.27 This study addresses this gap by evaluating the antibacterial activity of MSK03-AuNPs against drug-resistant Gram-negative bacteria, specifically P. aeruginosa and A. baumannii, both recognized by the WHO as significant global threats. Multidrug-resistant strains of these bacteria present formidable challenges in treatment due to their extensive antimicrobial resistance, potential for outbreaks, and association with adverse outcomes such as increased mortality rates and treatment failure.81–85 A systematic review identified that A. baumannii and P. aeruginosa infections had mortality rates of 47% and 23%, respectively.83,85
The antibacterial activity of MSK03-AuNPs, with an average particle size of 23.2 (±10.7) nm, was assessed using the agar well diffusion method. The inhibition zones for P. aeruginosa and A. baumannii were measured at 11.20 (±0.67) and 9.44 (±0.80) mm, respectively. Importantly, control substances, including HAuCl4 and cell-free supernatant, exhibited no inhibitory effect (Table 1). Similarly, El-Batal and Tamie (2015) observed the sensitivity of the pathogen P. aeruginosa to AuNPs synthesized from the marine bacterium Streptomyces cyaneus Alex-SK121.16 The AuNPs synthesized by strain Alex-SK121, with an average particle size of 12.63 nm, exhibited an inhibition zone of 26 ± 2 mm against P. aeruginosa when tested using the agar well diffusion method. Based on the information above, it has been demonstrated that smaller nanoparticles exhibit higher activity against pathogens compared to larger ones.
| Tested pathogens | Inhibition zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| HAuCl4 | S. monashensis MSK03 | Streptomyces cyaneus Alex-SK121 (ref. 16) | Streptomyces sp. NH21 (ref. 17) | Streptomyces griseus M8 (ref. 19) | Streptomyces sp. U30 (ref. 21) | ||
| Supernatant | AuNPs | ||||||
| a Each value represents the mean ± SD of three independent experiments; ND = not detect. | |||||||
| P. aeruginosa | 0 | 0 | 11.20 ± 0.67 | 26 ± 2 | 0 | 20 | 23 |
| A. baumanii | 0 | 0 | 9.44 ± 0.80 | ND | ND | ND | ND |
The mechanisms underlying the antimicrobial activity of AuNPs have been reported.86 These nanoparticles operate in two stages: firstly, they alter the membrane potential and decrease adenosine triphosphate (ATP) synthase activity, thereby slowing down the metabolic process; secondly, they inhibit the ribosome's subunit for tRNA binding, effectively disrupting its biological process.86,87 Remarkably, these actions are achieved while demonstrating lower toxicity to mammalian cells. In addition, the certain mechanisms of antimicrobial activity are required for further research.86
The antimicrobial activity of AuNPs synthesized by Streptomyces spp. against several Gram-positive and Gram-negative bacteria has been reported.16–22 However, there has no report of antibacterial activity of AuNPs on A. baumannii (Table 1). Hence, our study marks the first investigation of the inhibitory effects of AuNPs, synthesized by Streptomyces, on the growth of A. baumannii. The findings from this study might be led the way for the development of new strategies to solving the problems caused by infections that are resistant to antibiotics. Future studies could explore the mechanistic details of MSK03-AuNPs' antimicrobial action, assess their possible clinical utility.
Cytotoxicity of MSK03-AuNPs
Cytotoxicity testing is a crucial approach to determining the safety of nanomaterials for potential biomedical use. Gold nanoparticles are commonly favored for biomedical applications due to their remarkable compatibility with human cells.38 In this study, we evaluated the cytotoxicity of MSK03-AuNPs on Vero cell line using the MTT assay. MSK03-AuNPs at different concentration ranging from 32 to 512 μg mL−1 were tested for cytotoxicity. The IC50 of MSK03-AuNPs was greater than 512 μg mL−1, indicating that the nanoparticles exhibited non-cytotoxicity on the Vero cell line. The viability of the Vero cell was greater than 92.5% after 24 hours of exposure to MSK03-AuNPs at a concentration of 512 μg mL−1. It has been demonstrated that when cell viability remains above 80% following exposure to a test substance, the substance is considered non-toxic.88,89 AuNPs synthesized from various natural sources, including plants and fungi, were evaluated for cytotoxicity using Vero cells, revealing no toxicity.90–94 However, to the best of our knowledge, there have been no previous reports on the cytotoxicity of AuNPs synthesized by Streptomyces in Vero cells. Therefore, our study represents the first report of the cytotoxic effects of Streptomyces-synthesized AuNPs on Vero cells.Our findings hold significance, as they indicate that AuNPs synthesized by S. monashensis MSK03 have the potential to act as effective antimicrobial agents against drug-resistant pathogens. The potential of AuNPs to interact with bacterial cells makes it feasible to conjugate antibiotics onto them. Thus, the AuNPs not only facilitate antibiotic penetration into bacteria but also have a synergistic effect.95 Green synthesis AuNPs have been reported to have a synergistic effect with antibiotics. For instance, Garcinia mangostana extract was employed in one study to bio-synthesize AuNPs, which were then conjugated with streptomycin and azithromycin. The conjugated AuNPs had higher bactericidal efficiency against Staphylococcus spp. compared to free streptomycin and azithromycin.96 Therefore, this synergistic effect could be used as an alternative to treat multidrug-resistant bacterial infections. Overall, the biocompatibility of AuNPs with human cells, as demonstrated by their lack of cytotoxicity, makes them promising candidates for various biomedical applications. This is especially significant in the context of therapeutic medical treatments and drug delivery systems.
Conclusions
The findings of this study underscore the potential of actinomycetes as a valuable biological source for synthesizing gold nanoparticles. Our results demonstrate that a mixture of the cell-free supernatant from the actinomycetes strain S. monashensis MSK03 and 1 mM HAuCl4 is favorable for the formation of AuNPs. The AuNPs, biosynthesized through the mediation of actinobacteria, were characterized using standard procedures, including UV-vis spectroscopy, XRD patterns, and XANES spectra, confirming the successful synthesis of AuNPs. FTIR results revealed that biological molecules, such as carboxyl, hydroxyl, amine, and amide groups, contributed to the reduction and stabilization of MSK03-AuNPs. The synthesized nanoparticles were spherical, with an average particle size of 23.2 nm and a surface charge of −26.35 mV. Gold nanoparticles derived from S. monashensis MSK03 exhibited activity against drug-resistant pathogens (A. baumannii and P. aeruginosa) while being non-toxic to the Vero cell line. Consequently, this study not only introduces a novel avenue for the biosynthesis of AuNPs but also highlights the significant potential of MSK03-derived gold nanoparticles as effective agents for future medical therapies.Author contributions
SK: methodology, investigation, formal analysis, data curation, visualization, writing – original draft; PC: methodology, data curation, visualization, writing – review & editing; AR: methodology, data curation, visualization, writing – review & editing; WL: resources, formal analysis (FTIR, XRD, XANES), data curation, writing – review & editing; WP: data curation, writing – review & editing; PG: data curation, writing – review & editing; WS: data curation, writing – review & editing; MY: methodology, data curation, writing – review & editing; NN: conceptualization, methodology, project administration, supervision, validation, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Suranaree University of Technology, Thailand Science Research and Innovation (TSRI), and the National Science, Research, and Innovation Fund (NSRF) (FRB660044/0240 project code 180874) as well as the Center of Excellence on Environmental Health and Toxicology (EHT), OPS, Ministry of Higher Education, Science, Research and Innovation. Supavadee Kerdtoob extends gratitude to the External Grants and Scholarships for Graduate Students (One Research One Graduate: OROG), Suranaree University of Technology for providing tuition funding. We are deeply grateful to Assoc. Prof. Dr Nuannoi Chudapongse for her invaluable suggestions and contribution of HAuCl4, which greatly enhanced the quality of this work.References
- M. Bhagat, S. Rajput, S. Arya, S. Khan and P. Lehana, Bull. Mater. Sci., 2015, 38, 1253–1258 CrossRef CAS.
- S. Shamaila, N. Zafar, S. Riaz, R. Sharif, J. Nazir and S. Naseem, Nanomaterials, 2016, 6, 71 CrossRef PubMed.
- S. Sathiyaraj, G. Suriyakala, A. Dhanesh Gandhi, R. Babujanarthanam, K. S. Almaary, T.-W. Chen and K. Kaviyarasu, J. Infect. Public Health, 2021, 14, 1842–1847 CrossRef PubMed.
- K. A. Altammar, Front. Microbiol., 2023, 14, 1155622 CrossRef PubMed.
- X. Li, S. M. Robinson, A. Gupta, K. Saha, Z. Jiang, D. F. Moyano, A. Sahar, M. A. Riley and V. M. Rotello, ACS Nano, 2014, 8, 10682–10686 CrossRef CAS PubMed.
- A. Sharma, S. Arya, D. Chauhan, P. R. Solanki, S. Khajuria and A. Khosla, J. Mater. Res. Technol., 2020, 9, 14321–14337 CrossRef CAS.
- B. Padha, S. Verma, Prerna, A. Ahmed, S. P. Patole and S. Arya, Appl. Energy, 2024, 356, 122402 CrossRef CAS.
- S. B. Rana, R. P. P. Singh and S. Arya, J. Mater. Sci.: Mater. Electron., 2017, 28, 2660–2672 CrossRef CAS.
- K. R. B. Singh, P. Singh, S. Mallick, J. Singh and S. S. Pandey, Int. J. Biol. Macromol., 2023, 253, 127587 CrossRef CAS PubMed.
- P. Boomi, R. Ganesan, G. Prabu Poorani, S. Jegatheeswaran, C. Balakumar, H. Gurumallesh Prabu, K. Anand, N. Marimuthu Prabhu, J. Jeyakanthan and M. Saravanan, Int. J. Nanomed., 2020, 7553–7568 CrossRef CAS PubMed.
- V. Nayak, K. R. B. Singh, R. Verma, M. D. Pandey, J. Singh and R. Pratap Singh, Mater. Lett., 2022, 313, 131769 CrossRef CAS.
- F. Khadivi Derakhshan, A. Dehnad and M. Salouti, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2012, 42, 868–871 CrossRef CAS.
- M. Bhagat, R. Anand, R. Datt, V. Gupta and S. Arya, J. Inorg. Organomet. Polym. Mater., 2019, 29, 1039–1047 CrossRef CAS.
- K. S. Kumar, G. Kumar, E. Prokhorov, G. Luna-Bárcenas, G. Buitron, V. Khanna and I. Sanchez, Colloids Surf. A Physicochem. Eng. Asp., 2014, 462, 264–270 CrossRef.
- P. Manivasagan, J. Venkatesan, K. Sivakumar and S.-K. Kim, Microbiol. Res., 2014, 169, 262–278 CrossRef CAS PubMed.
- A. El-Batal and M. Al Tamie, J. Chem. Pharm. Res., 2015, 7, 1020–1036 CAS.
- M. Składanowski, M. Wypij, D. Laskowski, P. Golińska, H. Dahm and M. Rai, J. Cluster Sci., 2017, 28, 59–79 CrossRef.
- S. Sadhasivam, P. Shanmugam, M. Veerapandian, R. Subbiah and K. Yun, BioMetals, 2012, 25, 351–360 CrossRef CAS PubMed.
- M. M Hamed and L. S. Abdelftah, Egypt. J. Aquat. Biol. Fish., 2019, 23, 173–184 CrossRef.
- B. Abirami, V. Akshata, M. Radhakrishnan, R. Namitha, K. Govindaraju, V. Gopikrishnan and K. Manigundan, Int. J. Agric. Technol., 2023, 19, 323–338 CAS.
- R. Sayed and H. Saad, Egypt. J. Chem., 2021, 64, 7213–7222 Search PubMed.
- R. Balagurunathan, M. Radhakrishnan, R. B. Rajendran and D. Velmurugan, Indian J. Biochem. Biophys., 2011, 48, 331–335 CAS.
- T. H. Al-Samarrai and J. Schmid, Lett. Appl. Microbiol., 2000, 30, 53–56 CrossRef CAS PubMed.
- D. Lane, 16S/23S rRNA Sequencing, in Nucleic Acid Techniques in Bacterial Systematic, ed. Stackebrandt E. and Goodfellow M., John Wiley and Sons, New York, 1991, pp. 115–175 Search PubMed.
- N. M. Soltani, B. G. Shahidi and N. Khaleghi, Nanomedicine, 2015, 2, 153–159 Search PubMed.
- M. Shah, V. Badwaik, Y. Kherde, H. K. Waghwani, T. Modi, Z. P. Aguilar, H. Rodgers, W. Hamilton, T. Marutharaj and C. Webb, Front. Biosci., 2014, 19, 1320–1344 CrossRef PubMed.
- M. Skladanowski, M. Wypij, D. Laskowski, P. Golinska, H. Dahm and M. Rai, J. Cluster Sci., 2017, 28, 59–79 CrossRef CAS.
- P. Manivasagan, J. Venkatesan, K. Sivakumar and S. K. Kim, Crit. Rev. Microbiol., 2016, 42, 209–221 CrossRef CAS PubMed.
- V. R. Ranjitha and V. R. Rai, 3 Biotech, 2017, 7, 299 CrossRef CAS PubMed.
- N. E.-A. El-Naggar, N. H. Rabei, M. F. Elmansy, O. T. Elmessiry, M. K. El-Sherbeny, M. E. El-Saidy, M. T. Sarhan and M. G. Helal, Sci. Rep., 2023, 13, 12686 CrossRef CAS PubMed.
- S. Shaikh, J. Fatima, S. Shakil, S. M. Rizvi and M. A. Kamal, Saudi J. Biol. Sci., 2015, 22, 90–101 CrossRef CAS PubMed.
- S. R. Sathish Kumar and K. V. Bhaskara Rao, IET Nanobiotechnol., 2016, 10, 308–314 CrossRef PubMed.
- J. W. Jeffery, Methods in X-ray Crystallography, Academic Press, London, 1971 Search PubMed.
- G. S. Henderson, F. M. De Groot and B. J. Moulton, Rev. Mineral. Geochem., 2014, 78, 75–138 CrossRef CAS.
- Y. Konishi, T. Tsukiyama, N. Saitoh, T. Nomura, S. Nagamine, Y. Takahashi and T. Uruga, J. Biosci. Bioeng., 2007, 103, 568–571 CrossRef CAS PubMed.
- S. Vijayakumar and S. Ganesan, J. Nanomater., 2012, 2012, 14 Search PubMed.
- V. Pivodová, J. Franková, A. Galandáková and J. Ulrichová, Nanobiomedicine, 2015, 2, 7 CrossRef PubMed.
- P. C. Chen, S. C. Mwakwari and A. K. Oyelere, Nanotechnol. Sci. Appl., 2008, 45–65 Search PubMed.
- M. Drancourt, P. Berger and D. Raoult, J. Clin. Microbiol., 2004, 42, 2197–2202 CrossRef CAS PubMed.
- N. Pantidos and L. E. Horsfall, J. Nanomed. Nanotechnol., 2014, 5, 1 Search PubMed.
- S. B. Zotchev, J. Biotechnol., 2012, 158, 168–175 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
- X. Hu, Y. Zhang, T. Ding, J. Liu and H. Zhao, Front. Bioeng. Biotechnol., 2020, 8, 990 CrossRef PubMed.
- M. Shah, V. D. Badwaik and R. Dakshinamurthy, J. Nanosci. Nanotechnol., 2014, 14, 344–362 CrossRef CAS PubMed.
- Y. N. Slavin, J. Asnis, U. O. Häfeli and H. Bach, J. Nanobiotechnol., 2017, 15, 1–20 CrossRef PubMed.
- M. Hassanisaadi, G. H. S. Bonjar, A. Rahdar, S. Pandey, A. Hosseinipour and R. Abdolshahi, Nanomaterials, 2021, 11, 2033 CrossRef CAS PubMed.
- T. Bennur, Z. Khan, R. Kshirsagar, V. Javdekar and S. Zinjarde, Sensor. Actuator. B Chem., 2016, 233, 684–690 CrossRef CAS.
- D. Prakash, V. Mahale, A. Bankar, N. Nawani, V. Mahale and D. Prakash, Indian J. Exp. Biol., 2013, 51, 969–972 CAS.
- N. F. Zonooz, M. Salouti, R. Shapouri and J. Nasseryan, J. Cluster Sci., 2012, 23, 375–382 CrossRef CAS.
- M. Sapkal and A. Deshmukh, Res. J. Biotechnol., 2008, 3, 36–39 CAS.
- A. Sharma, S. Sharma, K. Sharma, S. P. Chetri, A. Vashishtha, P. Singh, R. Kumar, B. Rathi and V. Agrawal, J. Appl. Phycol., 2016, 28, 1759–1774 CrossRef CAS.
- G. E. J. Poinern, A Laboratory Course in Nanoscience and Nanotechnology, CRC Press, 2014 Search PubMed.
- H.-S. Kim, Y. Seo, K. Kim, J. W. Han, Y. Park and S. Cho, Nanoscale Res. Lett., 2016, 11, 230 CrossRef PubMed.
- K. Ricketts, C. Guazzoni, A. Castoldi and G. Royle, Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip., 2016, 816, 25–32 CrossRef CAS.
- A. Ahmad, S. Senapati, M. I. Khan, R. Kumar, R. Ramani, V. Srinivas and M. Sastry, Nanotechnology, 2003, 14, 824 CrossRef CAS.
- T. Elavazhagan and K. D. Arunachalam, Int. J. Nanomed., 2011, 6, 1265 CrossRef CAS PubMed.
- S. A. Dahoumane, E. K. Wujcik and C. Jeffryes, Enzyme Microb. Technol., 2016, 95, 13–27 CrossRef CAS PubMed.
- W. K. A. Wan Mat Khalir, K. Shameli, S. D. Jazayeri, N. A. Othman, N. W. Che Jusoh and N. M. Hassan, Front. Chem., 2020, 8, 620 CrossRef PubMed.
- S. R. Sathish Kumar and K. V. Bhaskara Rao, IET Nanobiotechnol., 2016, 10, 308–314 CrossRef PubMed.
- B. Schaffer, U. Hohenester, A. Trügler and F. Hofer, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 041401 CrossRef.
- M. Montes, A. Mayoral, F. Deepak, J. Parsons, M. Jose-Yacamán, J. Peralta-Videa and J. Gardea-Torresdey, J. Nanopart. Res., 2011, 13, 3113–3121 CrossRef CAS.
- S. Akhtar, S. M. Asiri, F. A. Khan, S. T. Gunday, A. Iqbal, N. Alrushaid, O. A. Labib, G. R. Deen and F. Z. Henari, Arab. J. Chem., 2022, 15, 103594 CrossRef CAS.
- a. k. Mittal, A. Kaler, A. Mulay and U. Banerjee, J. Nanoparticles, 2013, 2013 Search PubMed.
- A. Muthuvel, K. Adavallan, K. Balamurugan and d. n. k. Narendran, Biomed. Prev. Nutr., 2014, 4, 325–332 CrossRef.
- C. H. N. de Barros, G. C. F. Cruz, W. Mayrink and L. Tasic, Nanotechnol. Sci. Appl., 2018, 1–14 CAS.
- R. I. MacCuspie, K. Rogers, M. Patra, Z. Suo, A. J. Allen, M. N. Martin and V. A. Hackley, J. Environ. Monit., 2011, 13, 1212–1226 RSC.
- T. G. Souza, V. S. Ciminelli and N. D. S. Mohallem, J. Phys.: Conf. Ser., 2016, 773, 012039 CrossRef.
- C. Liang, J. Y. Cheong, G. Sitaru, S. Rosenfeldt, A. S. Schenk, S. Gekle, I.-D. Kim and A. Greiner, Adv. Mater. Interfaces, 2022, 9, 2100867 CrossRef CAS.
- F. Graily-Moradi, A. Maadani Mallak and M. Ghorbanpour, in Biogenic Nano-Particles and Their Use in Agro-Ecosystems, Springer, 2020, pp. 187–204 Search PubMed.
- S. L. Brock, Nanostructures and Nanomaterials, Imperial College Press, London, 2004 Search PubMed.
- J. D. Clogston and A. K. Patri, Methods Mol. Biol., 2011, 697, 63–70 CrossRef CAS PubMed.
- J. D. Clogston and A. K. Patri, in Characterization of Nanoparticles Intended for Drug Delivery, Springer, 2011, pp. 63–70 Search PubMed.
- M. Horie and K. Fujita, in Advances in molecular toxicology, Elsevier, 2011, vol. 5, pp. 145–178 Search PubMed.
- K. E. Sapsford, K. M. Tyner, B. J. Dair, J. R. Deschamps and I. L. Medintz, Anal. Chem., 2011, 83, 4453–4488 CrossRef CAS PubMed.
- A. J. Shnoudeh, I. Hamad, R. W. Abdo, L. Qadumii, A. Y. Jaber, H. S. Surchi and S. Z. Alkelany, in Biomaterials and Bionanotechnology, ed. R. K. Tekade, Academic Press, 2019, pp. 527–612 Search PubMed.
- A. Gade, A. Ingle, C. Whiteley and M. Rai, Biotechnol. Lett., 2010, 32, 593–600 CrossRef CAS PubMed.
- M. Rai, A. Ingle, S. Gaikwad, I. Gupta, A. Gade and S. Silvério da Silva, J. Appl. Microbiol., 2016, 120, 527–542 CrossRef CAS PubMed.
- G. Mancuso, A. Midiri, E. Gerace and C. Biondo, Pathogens, 2021, 10, 1310 CrossRef CAS PubMed.
- Z. Breijyeh, B. Jubeh and R. Karaman, Molecules, 2020, 25, 1340 CrossRef CAS PubMed.
- N. Gürsoy, B. Y. Öztürk and İ. Dağ, Turk. J. Biol., 2021, 45, 196–213 CrossRef PubMed.
- H. Giamarellou and I. Karaiskos, Antibiotics, 2022, 11, 1009 CrossRef CAS PubMed.
- D. Alrahmany, A. Omar, A. Alreesi, G. Harb and I. Ghazi, Antibiotics, 2022, 11, 1086 CrossRef CAS PubMed.
- A. Vivo, M. A. Fitzpatrick, K. J. Suda, M. M. Jones, E. N. Perencevich, M. A. Rubin, S. Ramanathan, G. M. Wilson, M. E. Evans and C. T. Evans, BMC Infect. Dis., 2022, 22, 491 CrossRef CAS PubMed.
- C. J. Kim, K. H. Song, N. K. Choi, J. Ahn, J. Y. Bae, H. J. Choi, Y. Jung, S. S. Lee, J. H. Bang, E. S. Kim, S. M. Moon, J. E. Song, Y. G. Kwak, S. H. Chun, Y. S. Kim, K. H. Park, Y. M. Kang, P. G. Choe, S. Lee and H. B. Kim, Sci. Rep., 2022, 12, 13934 CrossRef CAS PubMed.
- D. Alrahmany, A. F. Omar, A. Alreesi, G. Harb and I. M. Ghazi, Antibiotics, 2022, 11, 1086 CrossRef CAS PubMed.
- Y. Cui, Y. Zhao, Y. Tian, W. Zhang, X. Lu and X. Jiang, Biomaterials, 2012, 33, 2327–2333 CrossRef CAS PubMed.
- T. Al Hagbani, S. M. D. Rizvi, T. Hussain, K. Mehmood, Z. Rafi, A. Moin, A. S. Abu Lila, F. Alshammari, E.-S. Khafagy, M. Rahamathulla and M. H. Abdallah, Polymers, 2022, 14, 771 CrossRef CAS PubMed.
- R. Chen, K. Qiu, G. Han, B. K. Kundu, G. Ding, Y. Sun and J. Diao, Chem. Sci., 2023, 14, 10236 RSC.
- J. López-García, M. Lehocký, P. Humpolíček and P. Sáha, J. Funct. Biomater., 2014, 5, 43–57 CrossRef PubMed.
- P. Priya Tharishini, N. Saraswathy, K. Smila, D. Yuvaraj, M. Chandran and P. Vivek, Int. J. ChemTech Res., 2014, 6, 4241–4250 CAS.
- M. A. Meléndez-Villanueva, K. Morán-Santibañez, J. J. Martínez-Sanmiguel, R. Rangel-López, M. A. Garza-Navarro, C. Rodríguez-Padilla, D. G. Zarate-Triviño and L. M. Trejo-Ávila, Viruses, 2019, 11, 1111 CrossRef PubMed.
- K. Priya and P. R. Iyer, Egypt. Liver J., 2020, 10, 15 CrossRef.
- M. K. Soliman, S. S. Salem, M. Abu-Elghait and M. S. Azab, Appl. Biochem. Biotechnol., 2023, 195, 1158–1183 CrossRef CAS PubMed.
- V. Nachiyar, S. Sunkar and P. Prakash, Der Pharma Chem., 2015, 7, 31–38 CAS.
- S. M. D. Rizvi, A. S. A. Lila, A. Moin, T. Hussain, M. A. Kamal, H. Sonbol and E.-S. Khafagy, Pharmaceutics, 2023, 15, 430 CrossRef CAS PubMed.
- R. Nishanthi, P. Palani, 16th International Conference of Nanotechnology, Japan, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |