Open Access Article
Open Access ArticleChemically heparinized PEEK via a green method to immobilize bone morphogenetic protein-2 (BMP-2) for enhanced osteogenic activity†
MeeiChyn Goha,
Kiyoon Min a,
Young Ha Kimb and
Giyoong Tae
a,
Young Ha Kimb and
Giyoong Tae *a
*a
aSchool of Materials Science and Engineering, Gwangju Institute of Science and Technology (GIST), Gwangju 61005, Republic of Korea. E-mail: gytae@gist.ac.kr
bKorea Institute of Science and Technology, Hwarang-ro 14-gil 5, Seongbuk-gu, Seoul 02792, Republic of Korea
First published on 8th January 2024
Abstract
Osseointegration remains one of the major challenges in the success of bone-related implants. Recently, polyetheretherketone (PEEK) has emerged as an alternative material in orthopedic and dental applications due to its bone-mimicking mechanical properties. However, its bioinertness resulting in poor osseointegration has limited its potential application. So, the surface modification of PEEK with bone morphogenetic protein-2 (BMP-2) can be a potential approach for improving osseointegration. In this study, we proposed the chemical modification of heparin onto PEEK through an environmentally benign method to exploit the BMP-2 binding affinity of heparin. The heparin was successfully functionalized on the PEEK surface via a combination of ozone and UV treatment without using organic solvents or chemicals. Furthermore, BMP-2 was efficiently immobilized on PEEK and exhibited a sustained release of BMP-2 compared to the pristine PEEK with enhancement of bioactivity in terms of proliferation as well as osteogenic differentiation of MG-63. The significant synergistic effect of BMP-2 and heparin grafting on osteogenic differentiation of MG-63 was observed. Overall, we demonstrated a relatively safe method where no harsh chemical reagent or organic solvent was involved in the process of heparin grafting onto PEEK. The BMP-2 loaded, heparin-grafted PEEK could serve as a potential platform for osseointegration improvement of PEEK-based bone implants.
1. Introduction
Polyetheretherketone (PEEK), a synthetic semi-crystalline linear polyaromatic thermoplastic, has attracted great attention as a promising alternative biomedical implant to titanium and its alloys, especially in orthopedic and dental devices.1,2 PEEK possesses numerous superior characteristics over metallic implants, including the absence of a metal allergy, good biocompatibility, satisfactory mechanical properties, radiolucency, good chemical stability, and sterilization resistance.3,4 One of the most important characteristics of PEEK is its closely matched elastic modulus with that of natural human bone, which can alleviate the impact of stress shielding.5,6 However, PEEK has a low surface energy owing to its relatively hydrophobic surface, which limits cellular adhesion.7,8 This inherent bio-inert nature of PEEK results in inferior osseointegration, thus its clinical applications as orthopedic and dental implants are severely hampered. It is known that surface characteristics, including both physical and chemical properties, are crucial factors in cell responses.9,10 By altering the surface characteristics of PEEK, its osseointegration capability may be improved. Over the past few years, numerous strategies have been applied to alter the surface characteristics of PEEK to enhance its osteogenic activity and bone-implant integration. By deposition, metals,11–13 ceramics,14,15 peptides,16,17 or proteins18,19 have been coated on the surface of PEEK to enhance cell adhesion, osteogenesis, and bone-implant integration of stem cells and osteoblast cells.Delivery of an osteoinductive agent, such as bone morphogenetic protein-2 (BMP-2), is one of the promising approaches for improving the osseointegration of implants through osteogenic differentiation of osteoblast progenitors or stem cells,20 due to its high osteoinductivity.21 A high initial dose and repeated administration of high-cost BMP-2 are necessary to achieve its biological activity and maintain its effective concentration because of its short half-life, rapid degradation, and fast clearance in vivo.22 However, the most concerning issue in BMP-2 therapy is the adverse effects of BMP-2 at a supra-physiological concentration, such as bone overgrowth and adverse immune responses.23,24 Therefore, strategies to provide controlled release of BMP-2 for effective delivery have been pursued.
Heparin-grafting has been widely applied for this purpose because heparin has high binding affinities to various growth factors, such as basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF),25 as well as a protective effect against degradation and fast clearance in vivo.26 There are a lot of studies showing the improvement of osteogenic activity and bone-implant integration by the controlled release of BMP-2 through heparin-grafted or immobilized materials.22,27–29 However, there has been no heparin grafting or immobilization on PEEK for delivering BMP-2 and improving the osteogenic properties of PEEK. Several attempts have been made to immobilize BMP-2 on the surface of PEEK to enhance implant osseointegration and induce osteogenic differentiation of osteoblasts. However, prior studies that introduced BMP-2 on PEEK surfaces were limited to physical adsorption due to the challenging chemical modification of PEEK.30 Furthermore, PEEK is inherently hydrophobic, necessitating surface modification for BMP-2 immobilization. Some studies coated PEEK implants with collagen or polyelectrolyte multilayers to create hydrophilic surfaces.18,19 In the case of chemical modification, sulfonated PEEK and TiO2-deposited PEEK needed harsh chemicals during the surface modification despite enhanced BMP-2 adsorption resultantly.31,32 On the other hand, heparin modification on PEEK has been reported for other purposes. For instance, the surface of PEEK was physically immobilized using a cationic 2-methacryloyloxyethyl trimethylammonium chloride to prolong blood coagulation33 or chemically engrafted with heparin via EDC/NHS chemistry after amine functionalization for characterizing heparinized PEEK, but without loading BMP-2 or conducting osteogenic studies.34
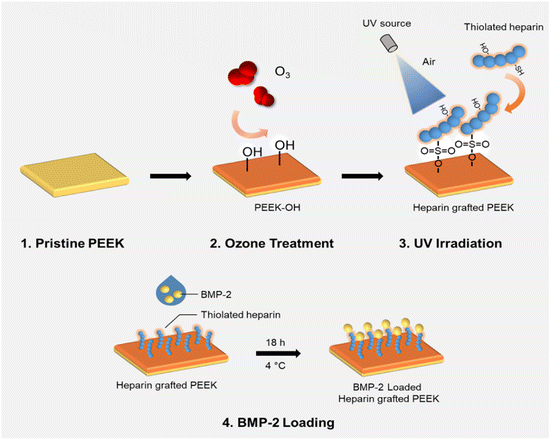
In our previous studies, we developed bioactive thiolated heparin-based hydrogel systems that showed controlled release of growth factors, such as BMP-2, to regenerate fibrocartilage,35 epidermal growth factor (EGF) to accelerate wound healing,36 as well as multiple growth factors (bFGF, EGF, and HGF) to promote the hepatic differentiation of human-derived adipose stem cells.25 Thus, we aimed to graft thiolated heparin onto the PEEK surface to provide a hydrophilic surface following BMP-2 immobilization to improve the osteogenic activity of pre-osteoblasts. The surface modification through thiol-ol reaction using UV and ozonation and following characteristic changes were analyzed. Then, the immobilization and controlled release of BMP-2 on heparinized PEEK were investigated. The bioactivity of loaded BMP-2 on heparinized PEEK was evaluated using osteogenesis-induced pre-osteoblasts.
2. Experimental
2.1. Materials and instrumentation
Thiolated heparin synthesized using a modification of our previous report37 (in ESI†) was grafted onto the ozonized PEEK surface (in ESI†) with the assistance of UV light irradiation. Briefly, the PEEK sheet after ozone treatment for 60 min was immediately immersed in a 1 wt% aqueous Hep-cySH solution for 15 min and taken out for drying at RT for ∼20 min, followed by gently blowing the surface with compressor air. Then, the sheet was irradiated with 365 nm UV light using an OmniCure series 1000 light source (EXFO, Vanier, Quebec, Canada) for 10 min in the presence of air, with a 12 cm distance between the sample and the UV probe. Following UV irradiation, the sheet was washed thoroughly with surfactant-containing distilled water, followed by distilled water for 24 h. After the washing process, the PEEK sheet was dried using compressed air and stored in an argon gas-purged container for further experiments. The amount of grafted heparin on the PEEK sheet was determined by the TBO assay (in ESI†).382.2. In vitro BMP-2 loading and release study
Recombinant Human/Murine/Rat BMP-2 (BMP-2) was loaded on the PEEK sheet (0.5 cm × 0.5 cm) by physical adsorption at 4 °C. Briefly, 50 ng/20 μL BMP-2 solution was dropped on PEEK and hPEEK sheets and incubated for 18 h at 4 °C. After incubation, the concentration of BMP-2 in the drop solution was measured by Human/Murine/Rat BMP-2 Standard ABTS ELISA Development Kit. The amount of immobilized BMP-2 on the PEEK sheet was calculated as a difference in the amounts of BMP-2 in the original solution and the final solution. To investigate the release kinetics of BMP-2, the sheets were incubated at 37 °C in PBS containing 0.1% BSA and 0.01% sodium azide for predetermined time points. The released amount of BMP-2 was measured using ELISA.2.3. In vitro studies
MG-63 cells from Korea Cell Line Bank (Seoul, Korea) were used to confirm the biocompatibility of pristine PEEK and surface-modified PEEK. With BMP-2 loading, cell proliferation, alkaline phosphatase activity, and mineralization of MG-63 cells on them were also characterized. Cells were cultured in Dulbecco's modified eagle's medium (DMEM) supplement with 10% FBS and 1% antibiotics-antimycotics before detaching for experiments. For all in vitro cellular response experiments, PEEK sheets were sterilized with 70% ethanol and rinsed with sterilized deionized water before use. The dimension of the PEEK sheet was 0.5 cm × 0.5 cm.2.4. Statistical analysis
Data were expressed as mean ± standard deviation. Data were statistically analyzed by using Student's t-test. The differences were considered significant when p < 0.05.3. Results and discussion
3.1. Grafting Hep-cySH to PEEK for preparing hPEEK
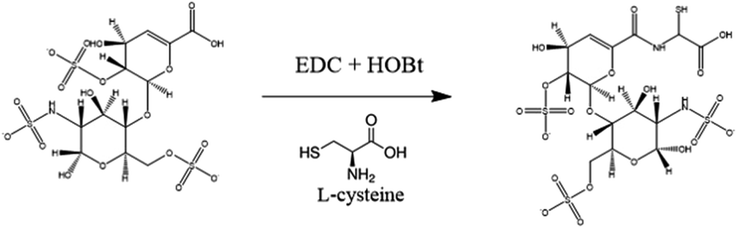
In this study, thiolated heparin was prepared using L-cysteine, a natural amino acid, instead of cysteamine that we previously reported (Fig. 1). So, thiolated heparin was composed of only natural materials present in human. L-Cysteine has been used in pharmaceuticals, medicines, and nutraceuticals,39 and heparin has been used as an anticoagulant drug. Thus, the degraded products of thiolated heparin in vivo are not likely to cause biocompatibility issues. The replacement of cysteamine with L-cysteine did not change significantly the thiolation reaction of heparin. The degree of thiolation could be controlled from ∼10 to ∼40% by the amount of EDC/HOBt relative to the carboxyl group of heparin. The 1H-NMR peak of thiol groups appeared at ∼1.2 ppm (Fig. S1†), demonstrating the successful conjugation of cysteine, in addition to the calculation of the degree of substitution using an Ellman's assay. Previously, it was reported that ∼40% thiolation did not affect significantly the binding affinity of heparin to heparin-binding molecules in contrast to the significant loss in anticoagulant activity of heparin.40 Considering the purpose of heparin grafting to PEEK and the obvious advantage of a higher thiol density for better grafting reaction, Hep-cySH with ∼40% thiolation was used for grafting to PEEK.As proper delivery of BMP-2 is effective for improving osteogenic activity as well as osseointegration of pre-osteoblast cells, we aimed to develop an easy and efficient method for industrial application to graft Hep-cySH onto PEEK surface by combining ozone treatment and UV irradiation (Scheme 1). First, the PEEK sheet was treated with ozone to introduce peroxides onto the surface.41 Since the ozonation time is one of the variable parameters, the effect of ozone treatment time on peroxide generation on the PEEK surface was analyzed spectrophotometrically by using an iodide method. The concentration of peroxides on PEEK increased linearly with ozonation time and reached a plateau after 60 min (Fig. S2†). So, 60 min was chosen as the ozonation time for further experiments. The peroxide generation on the PEEK surface was further proven by the XPS O 1s spectra as shown in Fig. S3.† Then, by immersing the peroxide-functionalized PEEK sheet into 1 wt% aqueous Hep-cySH solution at room temperature, the adsorption of Hep-cySH onto the oxidized PEEK sheet was induced. Next, the Hep-cySH adsorbed PEEK sheet was taken out from the solution, and it was UV-irradiated for 10 min for reacting with the thiol or disulfide group of Hep-cySH bound on the PEEK sheet via thiol-ol reaction to anchor the Hep-cySH in the presence of air, mediated by the similar reaction mechanisms of previous reports.42,43 The heparin grafted amount was 31 ± 3.0 μg cm−2 as quantified by TBO assay.
To demonstrate the potential of practical applications of the present heparin grafting method, a commercial lumbar bone cage made of PEEK was grafted with heparin through the same process as the PEEK sheets. As shown in Fig. S4,† the uniform and even staining of TBO on a heparin-grafted PEEK cage was observed in contrast to no TBO staining on of a pristine PEEK cage. Thus, not only a flat two-dimensional PEEK sheet but also a complex three-dimensional shape of a bone cage was also successfully grafted by heparin with the developed method.
In previous studies, heparin was either covalently or non-covalently immobilized onto the PEEK surface.33,34 However, a series of complicated chemical treatment steps were involved to introduce the functional group for covalently immobilizing heparin onto the PEEK surface. This method also might have limitations for biomedical applications due to the side effect of the residuals from chemical treatment. On the other hand, the non-covalently binding of heparin on the PEEK surface through ionic interaction may raise the stability issue because the non-covalent bonding can cause heparin desorption and gradually release into the bloodstream.3,33 In this study, by adopting the thiol-ol chemistry between thiol and hydroxyl groups assisted by UV irradiation in the presence of oxygen, we demonstrated the covalent grafting of thiolated heparin onto the PEEK surface by combining ozone treatment and UV irradiation. Both of these methods are considered to be low cost, easy-to-handle, and industrial friendly, hence, it has the potential to scale up for industrial application.44
3.2. Characterization of heparin-grafted PEEK surface
To confirm the grafting of thiolated heparin on the PEEK surface, PEEK, oPEEK, and hPEEK were analyzed by XPS. As shown in Fig. 2A, the characteristic peaks of S 2p and N 1s were detected only from hPEEK in comparison with PEEK or oPEEK, supporting the presence of heparin. The high-resolution S 2p spectrum of hPEEK (the inset) showed two peak components with binding energies of ∼162 and ∼168 eV. The higher binding energy component, assigned to sulfur atoms bonded to two or three oxygen atoms such as sulfone, sulfonate, or sulfonic acid, was attributed to the sulfonate group (–SO3) generated from the conjugation of thiolated heparin with the hydroxyl group of PEEK via thiol-ol reaction or the sulfonate unit from thiolated heparin itself.42,45 On the other hand, the lower binding energy component was attributed to the remaining unreacted thiol group (–SH) of thiolated heparin. Compared to thiolated heparin, the ratio of –SO3 peak to –SH peak of hPEEK was higher (Fig. S5†), also supporting the covalent conjugation of heparin on PEEK. Thus, the results of XPS spectra validated that heparin was successfully grafted to the PEEK surface. The water contact angles of PEEK, oPEEK, and hPEEK were measured to analyze the change in hydrophobicity of the PEEK surfaces (Fig. 2B). PEEK itself is very hydrophobic, so the water contact of PEEK was 85 ± 2°, while the water contact angle decreased to 35 ± 2° for oPEEK which underwent only ozone treatment and UV irradiation. This result revealed that the surface became more hydrophilic after ozone treatment and UV irradiation. By grafting heparin, additional hydrophilicity from heparin led to the further decrease of the water contact angle to 28 ± 2°. Since the proper hydrophilicity of implants is one of the important factors for cellular responses, the enhancement in biological activities of PEEK was expected as the hydrophobicity of the PEEK surface was reduced by heparin grafting.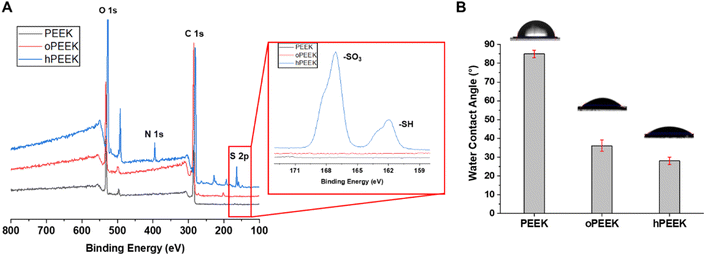 | ||
| Fig. 2 (A) XPS wide-scan spectra of PEEK, oPEEK, and hPEEK. The inset: the high resolution S 2p spectrum. (B) Water contact angle of PEEK, oPEEK, and hPEEK. *p < 0.05 and ***p < 0.0001. | ||
3.3. In vitro loading and release of BMP-2
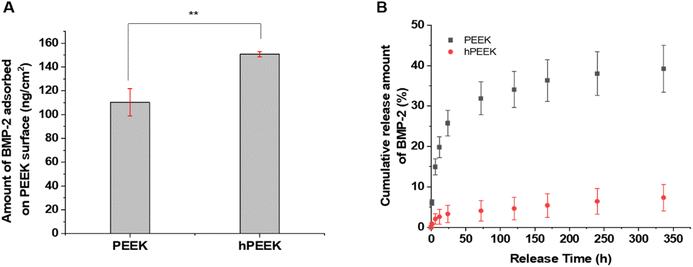
Grafted heparin on the PEEK surface would provide BMP-2 loading via heparin-binding affinity to BMP-2. However, pristine PEEK could also load BMP-2 via non-specific physisorption. The difference in BMP-2 loading amount between PEEK and hPEEK was significant and large, but less than 50% (Fig. 3A). Thus, simply in terms of the loading amount of BMP-2, the benefit of heparin grafting might be regarded to be not dramatic. However, the release of loaded BMP-2 from the PEEK surfaces showed a clear difference (Fig. 3B). Very slow release of BMP-2 without initial burst was observed from hPEEK. Over 90% of loaded BMP-2 remained on the hPEEK surface. In contrast, a much faster release (∼40% release in 2 weeks) with some initial burst was observed from PEEK. Thus, even without considering the biological activity of BMP-2, heparin grafting could provide a dramatic benefit for immobilizing BMP-2 on the PEEK surface mediated by the specific heparin-binding affinity of BMP-2, which involves hydrophobic association, hydrogen bonding, and electrostatic interactions.22,46The microstructures of the films before and after BMP-2 loading were observed using scanning electron microscopy (SEM) (Fig. S6†). Compared to PEEK, some changes in morphology were observed on hPEEK and PEEK/BMP2 and more on hPEEK/BMP2, representing surface grafting of heparin and BMP-2 loading.
3.4. In vitro cellular responses
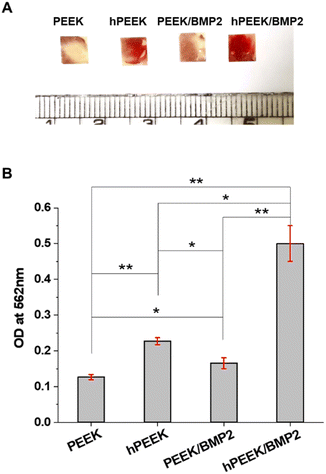
Mineralization induced by osteoblast cells occurs during the late stage of osteoblastic differentiation where cells started to deposit mineral matrix, leading to calcium deposition.49,55 The cells on PEEK samples after 14 days of culture were stained with Alizarin red S to evaluate the calcium deposition qualitatively and quantitatively. Overall, the calcium deposition results were similar to the ALP activity results. As shown in Fig. 6A, strong positive stains (bright red colors) were developed on hPEEK and hPEEK/BMP2 with more intense staining for hPEEK/BMP2. The semi-quantitative analysis of calcium deposition by extracting Alizarin red S (Fig. 6B) confirmed the positive effect of heparin itself and the importance of heparin grafting for further enhancement by BMP-2 loading in contrast to a minimal effect of BMP-2 loading without heparin grafting. This observation indicated that the osteogenic activity by heparin grafting and BMP-2 loading could successfully lead to the mineralization (calcium deposition) by osteoblast cells on the PEEK surface, which is necessary for osseointegration with the surrounding bone for in vivo applications. Thus, both results of ALP activity and calcium deposition revealed a synergistic effect of heparin and BMP-2 on enhancing osteogenic differentiation. Different from previous studies on heparin immobilization on PEEK,33,34 the present study demonstrated the successful enhancement of the osteogenic activity of PEEK, which is the most important characteristic for applying orthopedic or dental implants.
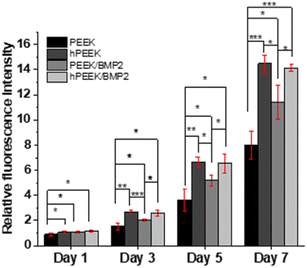
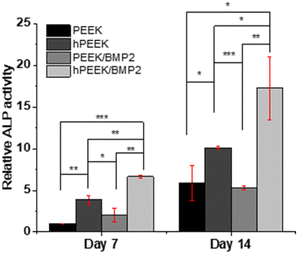
We observed each distinct role of heparin, BMP-2, and their synergistic roles. In terms of cell proliferation, no additional benefit of BMP-2 loading was observed for heparinized PEEK (hPEEK vs. hPEEK/BMP2 in Fig. 4), while BMP-2 incorporation on the PEEK film (PEEK/BMP2) also enhanced cell proliferation. We supposed that the hydrophilic and bioactive heparin itself already provided a sufficient effect on cell adhesion and proliferation on the hydrophobic PEEK.56 Thus, the additional effect of BMP-2 on proliferation was minor in this case. As the PEEK film is very hydrophobic (Fig. 2B), cells face challenges in adhering and proliferating on the pristine PEEK film. Thus, the surface coating of PEEK directly with BMP-2 (PEEK/BMP2) improved the initial adhesion of cells by making the surface more hydrophilic and possibly by facilitating the adsorption of serum proteins in the cell culture media. In summary, compared to the pristine PEEK, the heparin engraftment elevated 1.75-fold increase in cell proliferation (both hPEEK and hPEEK/BMP2), and the direct adsorption of BMP-2 (PEEK/BMP2) enhanced 1.4-fold increase in cell proliferation based on the metabolic activity on day 7.
On the other hand, the immobilization of BMP-2 on heparinized PEEK film effectively enhanced the osteogenic activity of MG-63. It has been reported that BMP-2 promotes osteogenic differentiation of MG-63 through specific signaling pathways (such as BMP-2/RUNX-2 MAPKs, and Akt signaling pathways).57,58 Since BMP-2 showed a low efficiency of immobilization on the PEEK without heparin engraftment, the difference between PEEK and PEEK/BMP2 in terms of osteogenic activities was not remarkable. However, after BMP-2 immobilization on heparinized PEEK, significant enhancement was observed both in terms of ALP activity and mineralization of MG-63 (Fig. 5 and 6), while heparin itself also enhanced the osteogenic activity of MG-63. Heparin engraftment on PEEK (hPEEK) showed a 1.7-fold and 1.8-fold increase in ALP activity and alizarin red S staining results, while BMP-2 immobilization on hPEEK (hPEEK/BMP2) showed 2.9- and 4.0-fold increase in ALP activity and alizarin red S staining results, respectively. Therefore, although heparin engraftment itself improved the osteogenic differentiation of MG-63 significantly, the synergistic effect of heparin and BMP-2 incorporation was clearly observed. The synergistic index for osteogenic differentiation of MG-63, calculated as (‘hPEEK/BMP2’)/(‘hPEEK’ + ‘PEEK/BMP2’), was 1.12 for ALP activity and 1.27 mineralization on day 14, while that for MG-63 proliferation was 0.55. Therefore, the synergistic effect of heparin and BMP-2 was evident in the osteogenic activities but not in the cell proliferation.
In addition to surface modification, PEEK has been developed to enhance osseointegration and osteogenic activities by fabricating PEEK composites with other materials. Carbon fiber-reinforced PEEK composites have been shown to enhance mechanical properties and bioactivities for osseointegration in vitro and in vivo.59,60 Hydroxyapatite has also been blended with PEEK powder for bone tissue repair.61 However, the approach of composite materials requires the process of new materials development by adjusting the mechanical, physical, and chemical properties of PEEK. Additionally, it is challenging to create homogenous and reproducible materials, and the surface exposure of bioactive materials would be limited compared to surface modification techniques. In contrast, the surface modification approach can be applied to existing products. We also demonstrated that our strategy can modify the commercial PEEK cage conformally (Fig. S5†).
4. Conclusions
A relatively green method to graft heparin onto PEEK via the thiol-ol reaction was developed by combining ozone and UV treatment without using any chemical reagent or organic solvent, but by using biocompatible cysteine, an amino acid, for thiolation on heparin. The method is low-cost and easy to handle and can be applied to complex shapes like the commercial PEEK implant. Successful heparin grafting was confirmed by XPS and water contact angle analysis. Heparin grafting on PEEK provided the efficient immobilization of BMP-2 compared to a fast release of loaded BMP-2 from pristine PEEK. Heparin grafting itself enhanced the bioactivity of PEEK in terms of the proliferation and the osteogenic differentiation of MG-63 compared to pristine PEEK. The effect of BMP-2 loading onto PEEK on more specific osteogenic activities including ALP activity and calcium deposition of MG-63 could be achieved only after heparin grafting. The novel system is expected to be applied for orthopedic and dental regeneration, overcoming the limitations of conventional metal/metal alloy-based materials. Further in vivo studies of this BMP-2 loaded, heparin-grafted PEEK will be necessary to provide more convincing results of osteogenic activity enhancement as well as osseointegration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Special thanks to Professor Yunho Lee from the School of Earth Science and Environmental Engineering for providing the ozone generator and Woongbae Lee for instructing the ozone generator usage. Financial support by the National Research Foundation of Korea (NRF) funded by MSIT of Korea (2021R1A2C2004722) and Gwangju Institute of Science and Technology (GIST) Research Institute (GRI) grant funded by the GIST in 2020.References
- J. Knaus, D. Schaffarczyk and H. Cölfen, Macromol. Biosci., 2020, 20, 1900239 Search PubMed.
- S. Verma, N. Sharma, S. Kango and S. Sharma, Eur. Polym. J., 2021, 147, 110295 Search PubMed.
- E. Buck, H. Li and M. Cerruti, Macromol. Biosci., 2020, 20, 1900271 Search PubMed.
- D. Almasi, N. Iqbal, M. Sadeghi, I. Sudin, M. R. Abdul Kadir and T. Kamarul, Int. J. Biomater., 2016, 2016, 1–12 Search PubMed.
- L. Liu, Y. Zheng, Q. Zhang, L. Yu, Z. Hu and Y. Liu, RSC Adv., 2020, 10, 16971 Search PubMed.
- Y. Zheng, L. Liu, L. Xiao, Q. Zhang and Y. Liu, Colloids Surf., B, 2019, 173, 591–598 Search PubMed.
- S. Mishra and R. Chowdhary, Clin. Implant. Dent. Relat. Res., 2019, 21, 208–222 Search PubMed.
- G. Primc, Polymers, 2022, 14, 5319 Search PubMed.
- H. Amani, H. Arzaghi, M. Bayandori, A. S. Dezfuli, H. Pazoki-Toroudi, A. Shafiee and L. Moradi, Adv. Mater. Interfaces, 2019, 6, 1900572 Search PubMed.
- B. J. Papenburg, E. D. Rodrigues, M. Wessling and D. Stamatialis, Soft Matter, 2010, 6, 4377 Search PubMed.
- B. C. Cheng, S. Koduri, C. A. Wing, N. Woolery, D. J. Cook and R. C. Spiro, Med. Devices: Evidence Res., 2018, 11, 391–402 Search PubMed.
- D. J. Hickey, B. Lorman and I. L. Fedder, Colloids Surf., B, 2019, 175, 509–516 Search PubMed.
- Z. Pang, Z. Pan, M. Ma, Z. Xu, S. Mei, Z. Jiang and F. Yin, PLoS One, 2021, 16, 725–740 Search PubMed.
- A. Oyane, M. Nakamura, I. Sakamaki, Y. Shimizu, S. Miyata and H. Miyaji, PLoS One, 2018, 13, e0206524 Search PubMed.
- J. W. Durham, S. A. Montelongo, J. L. Ong, T. Guda, M. J. Allen and A. Rabiei, Mater. Sci. Eng. C, 2016, 68, 723–731 Search PubMed.
- X. Meng, J. Zhang, J. Chen, B. Nie, B. Yue, W. Zhang, Z. Lyu, T. Long and Y. Wang, J. Mater. Chem. B, 2020, 8, 10190–10204 Search PubMed.
- M. Yakufu, Z. Wang, Y. Wang, Z. Jiao, M. Guo, J. Liu and P. Zhang, RSC Adv., 2020, 10, 9777–9785 Search PubMed.
- R. Guillot, I. Pignot-Paintrand, J. Lavaud, A. Decambron, E. Bourgeois, V. Josserand, D. Logeart-Avramoglou, E. Viguier and C. Picart, Acta Biomater., 2016, 36, 310–322 Search PubMed.
- Y.-W. Du, L.-N. Zhang, X. Ye, H.-M. Nie, Z.-T. Hou, T.-H. Zeng, G.-P. Yan and P. Shang, Front. Mater. Sci., 2015, 9, 38–50 Search PubMed.
- D. Lee, M. Wufuer, I. Kim, T. H. Choi, B. J. Kim, H. G. Jung, B. Jeon, G. Lee, O. H. Jeon, H. Chang and D. S. Yoon, Sci. Rep., 2021, 11, 746 Search PubMed.
- J. Yang, P. Shi, M. Tu, Y. Wang, M. Liu, F. Fan and M. Du, Food Sci. Hum. Wellness, 2014, 3, 127–135 Search PubMed.
- M. H. Hettiaratchi, L. Krishnan, T. Rouse, C. Chou, T. C. McDevitt and R. E. Guldberg, Sci. Adv., 2020, 6, eaay1240 Search PubMed.
- S.-Y. Park, K.-H. Kim, S. Kim, Y.-M. Lee and Y.-J. Seol, Pharmaceutics, 2019, 11, 393 Search PubMed.
- I. El Bialy, W. Jiskoot and M. Reza Nejadnik, Pharm. Res., 2017, 34, 1152–1170 Search PubMed.
- Y. Hwang, M. Goh, M. Kim and G. Tae, Biomaterials, 2018, 165, 94–104 Search PubMed.
- Y. Liang and K. L. Kiick, Acta Biomater., 2014, 10, 1588–1600 Search PubMed.
- H. S. Yang, W.-G. La, Y.-M. Cho, W. Shin, G.-D. Yeo and B.-S. Kim, Exp. Mol. Med., 2012, 44, 350 Search PubMed.
- S. E. Kim, Y.-P. Yun, K.-S. Shim, K. Park, S.-W. Choi, D. H. Shin and D. H. Suh, Colloids Surf., B, 2015, 134, 453–460 Search PubMed.
- S. E. Kim, S.-H. Song, Y. P. Yun, B.-J. Choi, I. K. Kwon, M. S. Bae, H.-J. Moon and Y.-D. Kwon, Biomaterials, 2011, 32, 366–373 Search PubMed.
- W. Liu, H. Wang, C. Liu, J. Wang, X. Cheng, C. Liu, L. Qiao, S. Zhang and X. Jian, Colloids Surf., B, 2020, 194, 111173 Search PubMed.
- Z. Sun, L. Ouyang, X. Ma, Y. Qiao and X. Liu, Colloids Surf., B, 2018, 171, 668–674 Search PubMed.
- C. Han, T. Jang, H. Kim and Y. Koh, J. Biomed. Mater. Res., 2014, 102, 793–800 Search PubMed.
- K. Ishihara, S. Yanokuchi, Y. Teramura and K. Fukazawa, Colloids Surf., B, 2020, 192, 111021 Search PubMed.
- H. Sun, R. Chen, S. Liu and G. Xu, Chem. Res. Chin. Univ., 2012, 28, 542–545 Search PubMed.
- J. Lee, W. I. Choi, G. Tae, Y. H. Kim, S. S. Kang, S. E. Kim, S.-H. Kim, Y. Jung and S. H. Kim, Acta Biomater., 2011, 7, 244–257 Search PubMed.
- M. Goh, Y. Hwang and G. Tae, Carbohydr. Polym., 2016, 147, 251–260 Search PubMed.
- N. Clemente Plaza, M. Reig García-Galbis and R. Martínez-Espinosa, Molecules, 2018, 23, 575 Search PubMed.
- G. Tae, Y.-J. Kim, W.-I. Choi, M. Kim, P. S. Stayton and A. S. Hoffman, Biomacromolecules, 2007, 8, 1979–1986 Search PubMed.
- Z. Lu, R. Yin, J. Yao and C. K. Y. Leung, Composites, Part B, 2019, 177, 107446 Search PubMed.
- M. Li, D. Mitra, E.-T. Kang, T. Lau, E. Chiong and K. G. Neoh, ACS Appl. Mater. Interfaces, 2017, 9, 1847–1857 Search PubMed.
- L. Li, J. Li, X. Du, A. Welle, M. Grunze, O. Trapp and P. A. Levkin, Angew. Chem., Int. Ed., 2014, 53, 3835–3839 Search PubMed.
- M. Flejszar and P. Chmielarz, Materials, 2020, 13, 999 Search PubMed.
- Y. Zheng, L. Liu, Y. Ma, L. Xiao and Y. Liu, Ind. Eng. Chem. Res., 2018, 57, 10403–10410 Search PubMed.
- K. Webb, V. Hlady and P. A. Tresco, J. Biomed. Mater. Res., 1998, 41, 422–430 Search PubMed.
- J. Wu, L. Li, C. Fu, F. Yang, Z. Jiao, X. Shi, Y. Ito, Z. Wang, Q. Liu and P. Zhang, Colloids Surf., B, 2018, 169, 233–241 Search PubMed.
- B. A. Springer, M. W. Pantoliano, F. A. Barbera, P. L. Gunyuzlu, L. D. Thompson, W. F. Herblin, S. A. Rosenfeld and G. W. Book, J. Biol. Chem., 1994, 269, 26879–26884 Search PubMed.
- Y.-P. Yun, J. Y. Lee, W. J. Jeong, K. Park, H.-J. Kim, J.-J. Song, S. E. Kim and H.-R. Song, BioMed Res. Int., 2015, 2015, 1–10 Search PubMed.
- P.-L. Kuo, Y.-T. Huang, C.-H. Chang and J.-K. Chang, Biol. Pharm. Bull., 2006, 29, 119–124 Search PubMed.
- G. Hélary, F. Noirclère, J. Mayingi, B. Bacroix and V. Migonney, J. Mater. Sci.: Mater. Med., 2010, 21, 655–663 Search PubMed.
- A. Alcheikh, G. Pavon-Djavid, G. Helary, H. Petite, V. Migonney and F. Anagnostou, J. Mater. Sci.: Mater. Med., 2013, 24, 1745–1754 Search PubMed.
- Y. Niu, L. Guo, F. Hu, L. Ren, Q. Zhou, J. Ru and J. Wei, PLoS One, 2020, 15, 2403–2417 Search PubMed.
- T. Ma, J. Zhang, S. Sun, W. Meng, Y. Zhang and J. Wu, Eur. Polym. J., 2023, 183, 111757 Search PubMed.
- S. E. Kim, Y.-P. Yun, J. Y. Lee, K. Park and D. H. Suh, Colloids Surf., B, 2014, 123, 191–198 Search PubMed.
- K. Gwon, E. Kim and G. Tae, Acta Biomater., 2017, 49, 284–295 Search PubMed.
- J.-O. Jeong, S. I. Jeong, J.-S. Park, H.-J. Gwon, S.-J. Ahn, H. Shin, J. Y. Lee and Y.-M. Lim, RSC Adv., 2017, 7, 8963–8972 Search PubMed.
- L. V. Dorofeyeva, Acta Virol., 1975, 19, 497 Search PubMed.
- Y. Pang, L. Liu, H. Mu and V. Priya Veeraraghavan, Saudi J. Biol. Sci., 2021, 28, 4916–4920 Search PubMed.
- Y. Li, W. Hu, G. Han, W. Lu, D. Jia, M. Hu and D. Wang, Chem.-Biol. Interact., 2018, 283, 51–58 Search PubMed.
- J. Wang, W. Yu, R. Shi, S. Yang, J. Zhang, X. Han, Z. Zhou, W. Gao, Y. Li and J. Zhao, J. Biomed. Mater. Res., 2023, 111, 505–512 Search PubMed.
- W. Yu, H. Zhang, L. A, S. Yang, J. Zhang, H. Wang, Z. Zhou, Y. Zhou, J. Zhao and Z. Jiang, Colloids Surf., B, 2020, 193, 111098 Search PubMed.
- J. Zheng, H. Zhao, Z. Ouyang, X. Zhou, J. Kang, C. Yang, C. Sun, M. Xiong, M. Fu, D. Jin, L. Wang, D. Li and Q. Li, Composites, Part B, 2022, 232, 109508 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07660a |
| This journal is © The Royal Society of Chemistry 2024 |