Open Access Article
Open Access ArticlePerformance and mechanism of a bioelectrochemical system for reduction of heavy metal cadmium ions†
XiaXia Wang,
Yu Zhao *,
Li'E. Jin* and
Bin Liu
*,
Li'E. Jin* and
Bin Liu
Institute of Clean Chemical Engineering, College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China. E-mail: zhaoyu@tyut.edu.cn; lejin2003@163.com
First published on 12th February 2024
Abstract
This study explores the removal of Cd(II) from wastewater using a microbial electrolysis cell (MEC) to investigate the electrochemical performance and removal kinetics of an anodic polarity reversal biocathode and the mechanism of action of electrochemically active bacteria. Comparative electrochemical methods showed that using an anodic polarity reversal biocathode resulted in greater than 90% removal of different concentrations of Cd(II) within three days, which may be related to the catalytic effect of anodic electrochemically active bacteria. However, due to the ability of bacteria to regulate, up to nearly 2 mg L−1 of Cd(II) ions will remain in solution. As shown by the linear fitting relationship between scanning speed and peak current, the removal process was dominated by adsorption control for 20–80 mg L−1 Cd(II) and diffusion control for 100 mg L−1 Cd(II). The analysis of raw sludge and sludge containing Cd(II) showed that Arcobacter and Pseudomonas were the primary cadmium-tolerant bacteria, and that the ability to remove Cd(II) was the result of a synergistic collaboration between autotrophic and heterotrophic Gram-negative bacteria.
1. Introduction
Pollution caused by Cd(II) metal is a severe threat to ecological stability and human health. Cd is commonly used in industries such as electroplating and mining, and it often accumulates in industrial wastewater in various forms, including ions and compounds. From wastewater, Cd accumulates in the soil and eventually throughout the food chain, which results in organisms becoming the ultimate bearers of heavy metals. Cd(II) is not an essential element in the human body, and it can cause chronic poisoning of the kidneys and chondromalacia.1 However, because the half-life of Cd(II) is 10–30 years, Cd will continue to be hazardous to life and health even if its enrichment is stopped.2Bioelectrochemical systems (BESs) utilize anodic microorganisms to degrade organic matter and release the resulting electrons to the surface of a solid anode, where the electrons are transported through an external circuit and are received at the cathode for reduction reaction.3 The BES consists of a microbial fuel cell (MFC) that generates electrical energy and a microbial electrolysis cell (MEC) that produces hydrogen energy. Based on the MFC, the MEC has been developed for applications such as hydrogen and methane production, heavy metal ion reduction, and coupling to other devices. In addition, the performance of the cathode material in the MEC affects the overall power reduction efficiency.
Biocathodes are currently a new technology whereby pollution treatment and energy saving are combined. Electroactive microorganisms (membranes) on low-cost electrode carriers are a cathodic reaction catalyst, with living microbial cells (electrical nutrients) as the basis to reduce overpotential with accompanying high-performance operation. There are various advantages to using microorganisms as catalysts, such as self-regeneration, adaptability, mild operating conditions, and low cost.4–6 Although early research on the biocathode MEC focused on hydrogen production, one of the most promising applications of the MEC is to combine hydrogen production with the removal of heavy metal ions from wastewater by simultaneously acquiring electrons and generating a green and clean energy source, which is hydrogen.7
Yiran1 et al. discovered that an increase in voltage led to an increase in Cd(II) removal for biocathodes domesticated at different voltages. Aradhana31 et al. explored the role of using biocathodes for the removal of mixed metal ions in an MFC facility, and showed that the removal of nickel(II) and cadmium(II) was high at low concentrations versus high concentrations because the toxic environment at high concentrations exceeded the tolerance of the microbial cells and caused damage to the biocathodes. Therefore, adding an appropriate concentration of metal ions to the solution is crucial for the survival of microorganisms and the proper functioning of the biocathode. The application of MEC devices in metal removal or recovery has become a promising technology, such as the use of NaHCO3 as a carbon source to recover various heavy metal ions on a biocathode in a double-chamber electrochemical system.8 Additionally, there were greater advantages with single-chamber bioelectrochemistry in the treatment of a combination of organics and heavy metal wastewater.9
Electroactive microorganisms are indispensable components of BESs, and the species composition varies from system to system. However, most microorganisms originate from the same phylum, such as Proteobacteria, Acidobacteria, and Bacteroidetes. According to previous studies, the Proteobacteria phylum contains several types of electroactive microorganisms, most of which are metal-reducing organisms that can be effectively used in wastewater treatment.10,11 Alternatively, there are two pathways for electron transfer: intracellular and extracellular, where intracellular transfer refers to the simultaneous transfer of electrons by electrochemically active bacteria utilizing their specific metabolic pathways (i.e., the release of extracellular polymeric substances). Extracellular transfer refers to redox reactions within the MEC system relying on the conductance of electrons over long distances (several centimeters) by the microorganism's flagella and cytochromes, which enables the diffusion of electrons from the cell to the solution.12,13
The polarity-reversal domestication of a band into a biocathode has also been employed. Wu5 compared in situ and ex situ domestication methods for biocathodes. The ex situ domestication method accelerated the domestication time of the biocathode and enhanced the treatment capacity of the biocathode. The biocathode was domesticated in individually acclimatized Cd(II) in a double-chamber MEC to achieve nearly complete removal of the metal, and the performance of the reaction system was evaluated based on metal removal and coulombic efficiency. An investigation of Cd(II) removal kinetics and high-throughput 16S rDNA gene sequencing were conducted to characterize the microbial communities of the original and Cd(II)-containing sludge for comparing the metal removal rates of the biocathodes at different times and at various initial Cd(II) concentrations.
2. Materials and methods
2.1 MEC configuration and start-up
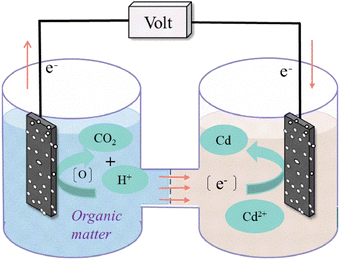
The double-chamber MEC reactor was constructed from Plexiglas using a cation exchange membrane (CMI-7000S Hangzhou Hua Membrane Technology Co., Ltd) to separate the anode and cathode chambers. The effective volume of the cathode and anode chambers was 80 mL (diameter: 5 cm, height: 8 cm, Tianjin Gosh Rui Co., Ltd), and the cathode and anode electrode material consisted of carbon felt (2.5 cm × 5.0 cm, 0.8 cm thick, Tianjin Haitian Muzi Graphite Products Factory). A saturated Ag/AgCl reference electrode (0.241 V vs. SHE) was placed in the cathode chamber of the reactor to collect cathode potential data.2.2 Inoculation and handling
The MEC (Fig. 1) reactor was started using the same inoculum in all experiments, which was collected from the Yang Jia Bao Wastewater Treatment Plant, Taiyuan, China. The reactor electrolyte consisted of mixed bacteria (20 mL) and buffer solution (60 mL), where the mixed bacteria consisted of aerobic and anaerobic bacteria in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and the buffer solution contained the following components: NaH2PO4·2H2O (3.32 g L−1), Na2HPO4·12H2O (10.32 g L−1), NH4Cl (0.31 g L−1), KCL (0.13 g L−1), CH3COONa (1 g L−1), and trace minerals (10 mL L−1). In addition, the initial pH in the electrolyte varied from 6.8 to 7.2. The circuit current was monitored by applying an external voltage of 1.0 V and subsequently connecting a multimeter (UT52 Multimeter) and recording the current every hour until it exceeded 1 mA and stabilized at a maximum value for three consecutive cycles. This indicated that the removal process in the anodic domestication of anhydrous sodium acetate biofilm carbon felts was well established. All experiments were performed using the same inoculum for the reactor inoculation start-up process.
1 ratio, and the buffer solution contained the following components: NaH2PO4·2H2O (3.32 g L−1), Na2HPO4·12H2O (10.32 g L−1), NH4Cl (0.31 g L−1), KCL (0.13 g L−1), CH3COONa (1 g L−1), and trace minerals (10 mL L−1). In addition, the initial pH in the electrolyte varied from 6.8 to 7.2. The circuit current was monitored by applying an external voltage of 1.0 V and subsequently connecting a multimeter (UT52 Multimeter) and recording the current every hour until it exceeded 1 mA and stabilized at a maximum value for three consecutive cycles. This indicated that the removal process in the anodic domestication of anhydrous sodium acetate biofilm carbon felts was well established. All experiments were performed using the same inoculum for the reactor inoculation start-up process.
A double-chamber MEC was constructed to domesticate the biocathode by reversing the polarity of the abovementioned biological anode, and the electrolyte simulating cadmium-containing wastewater as a biocathode inoculum was based on the same inoculum as described above for the anode with the addition of Cd(II). CH3COONa was used as the only carbon source for the cathode microorganisms. The initial Cd(II) concentration in the solution was increased in gradients of 5, 10, and 20 mg L−1, and the length of each cycle was four days. Biofilm cathodes are tolerant of the toxicity of a single heavy metal, and were obtained when the circuit current stabilized. All experimental MECs were domesticated in a constant temperature shaker at 35 °C.
2.3 Methods of testing and analysis
Cd(II) was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Thermo Fisher CAPPRO) in all experiments. The removal rate of Cd(II) (mg L−1), coulometric efficiency (CEca, Cd), energy efficiency per unit (ηE, Cd), and energy consumption per unit (kW h kg−1) were calculated for the cathode of the MEC device, as shown in the ESI.†The electrochemical behavior of the biocathode and abiotic cathode was determined by cyclic voltammetry (CV) using a three-electrode configuration with an electrochemical workstation (CS2350M, CORRTEST, Wuhan, China) at scanned potentials of −1.2 V to 0.2 V (vs. SHE) and a scan rate of 1.0 mV s−1. CV is an electrochemical method used to characterize redox reactions on the surface of a reactive electrode, and it was used to analyze the response of the cathodic electrode to the presence of Cd(II), with the cathode as the working electrode, Ag/AgCl as the reference electrode, and the anode as the counter electrode. The Tafel slope is an essential parameter for determining the catalytic activity of the cathodic hydrogen precipitation reaction, and we used a scan rate of 1–10 mV s−1 to study the cathodic reaction kinetics. The kinetics of the electrode process were analyzed by electrochemical impedance spectroscopy (EIS). The change in impedance with sinusoidal frequency was measured to study the mechanism of the electrode material, and used an equivalent circuit fitted with the electrochemical workstation.
In sampling the original sludge and the Cd(II)-containing sludge for microbial identification, 16S rDNA can be used as a characteristic nucleic acid sequence for revealing biological species.13–15 First, the genomic DNA of the samples was extracted by the sodium dodecyl sulfate (SDS) method and then tested for purity and concentration. Second, PCR amplification of selected V3–V4 variable regions, which are highly variable regions of bacterial genes, was performed using specific primers containing the barcode and Phusion® High-Fidelity PCR polymerase. Then, PCR products were detected by 2% agarose gel electrophoresis, and cut gel recovery was then performed using an AxyPrepDNA Gel Extraction Recovery Kit (Axygen). The PCR-amplified recovered products were detected and quantified using a QuantiFluor™-ST Blue Fluorescence Quantification System (Promega).
Next, library construction was performed using the NEB Next®Ultra™ DNA Library Prep Kit for Illumina (New England Biolabs, USA), and the quality of the built libraries was confirmed by an Agilent Bioanalyzer 2100 system and Qubit. Lastly, based on the characteristics of the amplified 16S region, a small fragment library was constructed for paired-end sequencing using the Illumina MiSeq sequencing platform. Through the clustering of operational taxonomic units (OTUs), species annotation and abundance analysis can reveal the species composition of the samples. It can also explore the differences between the samples to identify the differential bacterial communities.
3. Results and discussion
3.1 Performance of the abiotic and biotic cathodes
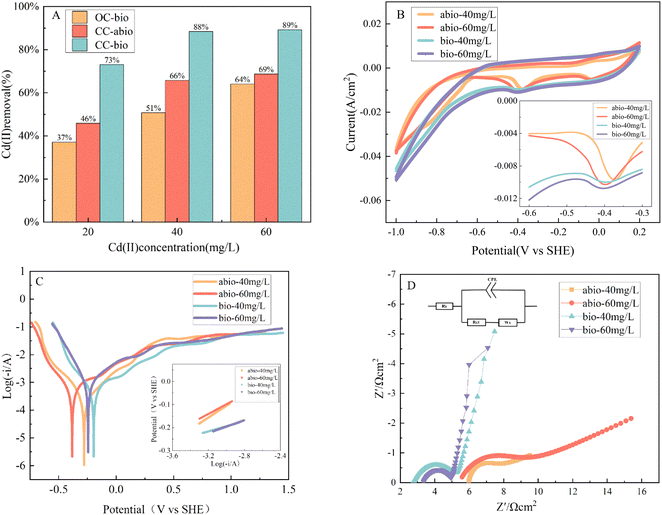
Abiotic and polarity-reversed biological cathodes were compared under open-circuit (OC) and closed-circuit (CC) conditions for three initial concentrations of Cd(II) ranging from 20 mg L−1 to 60 mg L−1 after 24 h of treatment in the MEC. The Cd(II) removal rate was significantly higher in the CC-biotic than in the OC-biotic under CC-abiotic conditions at all three concentrations (Fig. 2A). Therefore, this indicates that electrophilic microorganisms and circuit currents play a significant role in Cd(II) removal.Under OC-biotic conditions, Cd(II) removal can be related to the physical adsorption of carbon felt and the presence of bacteria, such as biosorption and bioreduction of Cd(II). The addition of sodium acetate as a carbon source for microorganisms, ammonium ions in the cathodic electrolyte medium,16 and metal-containing trace elements may also serve as electron donors for trophic bacteria (chemo-living bacteria).17
In this experiment, the high efficiency of Cd(II) removal by OC organisms may be attributed to the fact that the biocathode used has been subjected to extensive experiments to convert toxicity-tolerant microorganisms. Moreover, the removal of Cd(II) from the simulated wastewater by acetate was unchanged, which excluded the effect of Cd(II) and acetate complexation on the removal process, and acetate was found to facilitate the growth of heterotrophic microorganisms on the biocathode of the MEC.17
This study compared the electrochemical properties of abiotic and polarity-reversed abiotic cathodes under CC conditions. The CV (Fig. 2B, S1 and S2†) analyses of the abiotic and biotic cathodes showed a Cd(II) reduction peak potential of approximately −0.4 V. In contrast, the reduction peak potentials and reduction peak currents in a 20 mg L−1 Cd(II) solution of abiotic and biotic cathodes did not indicate a significant difference. Therefore, this study utilized Cd(II) concentrations of 40 and 60 mg L−1 as an example for analysis.
The reduction peak potential of the biocathode was negatively shifted compared with that of the abiotic cathode (Fig. 2B), which indicates that more negative peak potentials of the biocathode were more favorable for the reduction reaction and reduced the total free energy required for electron transfer during electrochemical processes. The biocathode also exhibited a more positive reduction onset potential than the abiotic cathode [biotic/abiotic: 0.124 V/0.100 V at 40 mg L−1; biotic/abiotic: 0.128 V/0.091 V at 60 mg L−1] (Fig. S1†) and a small reduction peak current. These differences can be attributed to electrochemically active bacteria on the electrode surface, increased mass transfer resistance, and decreased peak current.18 However, the electrochemically active bacteria shifted the peak and reduction onset potential to the left, confirming the catalytic activity of polar inversion biofilms for metal reduction.
The kinetic parameters were determined using Tafel polarization curves, as shown in Fig. 2C. The Tafel curves for different concentrations and electrodes exhibited similarities, and the intercept and slope of the Tafel curves were obtained by linear fitting, with the slope = (1 − α)F/2.3RT.18 The transfer coefficient α is 0.98 for the abiotic cathode and 0.99 for the biotic cathode. The lower Tafel slope [biotic/abiotic: 14.25 mV dec−1/24.89 mV dec−1 at 40 mg L−1; biotic/abiotic: 13.94 mV dec−1/21.29 mV dec−1 at 60 mg L−1] (Fig. 2C) indicated a favorable electrochemical performance, which suggested that the biocathode exhibited superior electrochemical performance compared to the abiotic cathode. Based on the intercepts of the fitted curves, the exchange current density (j0) was higher at the abiotic cathode than at the biotic cathode. This observation was in accordance with the results of the CV analysis, and emphasized that the catalytic role of electrochemically active bacteria and the kinetic reaction rate of electrodeposition are related to the speed of electron transfer.
The equivalent circuit of the cathode resistance consists of an ohmic resistor Rs, a double-layer charge transfer resistor Rct, a capacitor Cd, and diffusion impedance Zw.19 From the Nyquist plots of the different cathodes and the corresponding equivalent circuit diagrams, the EIS curves for both electrodes are characterized by semicircular arcs and diagonal lines, with the charge transfer impedance (Rct) derived from the diameter of the semicircle, which represents the resistance to electron transfer between the electrode surface and the adsorbed material on the electrode. Diffusion impedance Zw is the concentration impedance of reactants diffusing from the solution to the electrode reaction interface, and the combination with Rct is helpful for understanding the kinetics of the electrochemical reactions with the redox reactions on the electrode.
Based on the data in Fig. 2D and Table 1, it is evident that there was lower ohmic resistance, charge transfer resistance, and diffusive impedance for the biocathodes with polarity reversal as compared to the non-biocathodes. The biological cathodes exhibited a lower ohmic resistance, resulting in increased conductivity and more rapid electron transfer. As noted by Ha et al.,20 the charge transfer resistance of the electrode is linked to the activation energy of the cathode, and serves as an indicator of the kinetics of the cathodic reaction. Moreover, electrodes with low charge transfer resistance were more conducive to reduction reactions, and the magnitude of the diffusive impedance is proportional to the slope of the oblique line.
| Cd(II) mg L−1 | Rs/Ω | Rct/Ω | W/Ω | Slope | Intercept | |
|---|---|---|---|---|---|---|
| Abiotic | 40 | 5.9471 | 4.6473 | 9.607 | 0.24893 | 0.63613 |
| 60 | 5.4264 | 3.98 | 9.976 | 0.21289 | 0.35052 | |
| Biotic | 40 | 2.7346 | 2.6872 | 26.255 | 0.14250 | 0.31638 |
| 60 | 3.3384 | 1.7643 | 20.544 | 0.13941 | 0.22284 |
The polarity of the reversed bioelectric polarographic line indicated an angle greater than 45° from the real axis, signifying rapid ion diffusion toward the electrode. In contrast, the non-biological polarographic line fell below 45° from the real axis, which may have occurred due to the electrode surface roughness and the variability of the solution state, indicating that the biocathode surface absorbed more electroactive bacteria and reduced the impedance for solution diffusion to the electrode surface. However, the charge transfer resistance for both electrodes was higher in the 40 mg L−1 solution as compared to the 60 mg L−1 solution, and this difference was mainly due to the increased concentration of metal ions in the cathode electrolyte, which led to facilitated electron movement, lower resistance, and decreased charge transfer resistance. These phenomena indicate a greater driving force for kinetics, more rapid charge transfer, and stronger reducing capabilities at the electrode.21,22
3.2 Effect of Cd(II) concentration
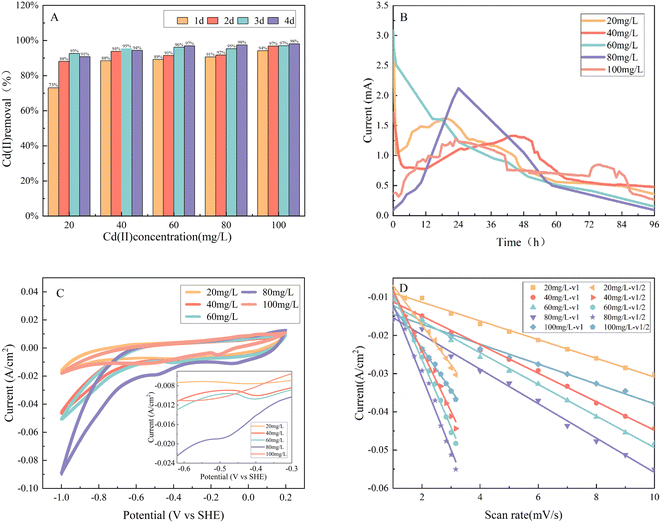
We investigated the influence of Cd(II) concentration on system performance and circuit current magnitude across a concentration range of 20–100 mg L−1. The data showed that the removal of five initial Cd(II) concentrations from biofilms at the same applied voltage of 0.5 V ranged from 90.80% to 98.08% after four days (Fig. 3A), suggesting that heterotrophic microorganisms within the biofilm gradually adapted to the Cd(II)-tolerant bacteria in the replicated experiments at progressively higher concentrations. These Cd(II) removal rates were higher than those of 69.2 ± 1.5% in the non-ex situ domestication of biocathodes at the same applied voltage.1 The Cd(II) removal rates were also higher than 70% for the previous biocathodes under the same carbon source.31 However, different regularities were observed at different times in the removal rates for Cd(II) at low (≤40 mg L−1) and high (≥60 mg L−1) concentrations in wastewater.For instance, the removal rate in the cathodic solution with an initial concentration of 20 mg L−1 Cd(II) gradually increased from 73.03% to 92.57% within a day and reached the highest removal rate on the third day. By contrast, a decrease in the removal rate (90.80%) was observed on the fourth day of the treatment process (Fig. 3A). This trend was also observed for Cd(II) wastewater with an initial concentration of 40 mg L−1. On the contrary, the rate of removal of high-concentration Cd(II) (60 mg L−1) from wastewater increased from 89.19% to 96.93% within four days (Fig. 3A).
This trend is also observed from Fig. S3 (ESI),† where the standard deviation of the removal rate on the third day was smaller. In addition, combined with the standard deviation graph of different Cd(II) concentrations, the relative error at higher concentrations was small, illustrating that an enhanced Cd(II) removal rate by the electrochemical bacteria and the biological cathode achieved optimal removal capacity under this repeated selective pressure with elevated Cd(II) levels and prolonged acclimation time.
The decreased removal rate at low concentrations may be due to accumulated or adsorbed Cd(II) within the microorganisms being re-released in solution, which occurred because microorganisms can regulate and maintain the internal balance of the system. The residual Cd(II) ions in high- and low-concentration wastewater solutions are approximately 2.00 ± 0.2 mg L−1, which indicates that the removal of Cd(II) by the biocathode is unlikely to reach 100%. Nevertheless, following extensive domestication, the cathode exhibited a breakthrough capacity for applicability beyond environments characterized by low concentrations and low toxicity.
The circuit currents at different concentrations were observed for four days of operation at 0.5 V (Fig. 3B). There were two patterns exhibited by the circuit currents of the biocathode: one was a curve that rapidly decreased from a higher point at the beginning and then gradually increased and subsequently decreased, and the other was a curve that gradually increased from the lowest point to the highest point and then decreased. The initial rise to the highest point represents the oxidation and reduction of organic material by microorganisms on the electrodes. The subsequent gradual increase signifies the progressive decomposition of the organic material due to substrate depletion.
In the study by Colantonio and Kim,23 the magnitude of the current generated conferred no direct effect on the removal rate of cadmium from wastewater during the operation of the MEC unit. There were trends in output currents caused by different concentrations of Cd(II), as shown in Fig. 3B, and the removal rates on the third day were all 90% (Fig. 3A). This finding excludes a direct impact of the circuit current and the removal rate for different concentrations of Cd(II).
Table S2† shows the comparison of cathodic electrons (CEca, Cd) and energy efficiency (ηE, Cd) consumed for the cathodic reduction of Cd(II) and cathodic energy consumption per unit (kW h kg−1) for different initial Cd(II) concentrations, as described in detail in the ESI and Table S2.† As a result, the cathodic CEan, Cd and ηE, Cd of the MEC device tended to decrease with the increase in removal time for the five Cd(II) concentrations, while the power consumption gradually rose [CEan, Cd: 1.60% (1D)–0.79% (4D); ηE, Cd: 1.29% (1D)–0.63% (4D)].
The example in Table S2† [energy consumption: 14.91 (1D)–30.37 (4D) kW h kg−1; applied voltage: 0.5 V 20 mg L−1 Cd(II)] also reflects the progressive decrease of Cd(II) in solution with the increase in removal time. Conversely, the initial Cd(II) concentration increased from 20 mg L−1 to 100 mg L−1; the cathodic CEan, Cd rose from 0.79% to 4.83%; and ηE, Cd improved from 0.63% to 3.89% in a gradual manner. In contrast, the electrical energy consumption gradually decreased from 30.37 kW h kg−1 to 4.93 kW h kg−1 (Table S2†) on the fourth day of treatment conditions in the MEC unit. Accordingly, the electron utilization and energy utilization efficiencies for the reduction in Cd(II) ions increased with the increase in concentration, and the electrical energy consumption decreased with the decrease in concentration, which indicates that higher concentrations are favorable for the rapid reduction in Cd(II) ions.
Fig. 3C illustrates the CV analysis conducted across a spectrum of initial Cd(II) concentrations, ranging from 20 mg L−1 to 100 mg L−1. A reduction peak appeared at the biocathode with acetate as the carbon source, and the reduction peak on the cathode progressively shifted to the left as the concentration increased. This finding indicates that the presence of the Cd(II) electron acceptor significantly favored the electron transfer of the cathode, although the reason for this favorable effect was unclear because bacterial cells catalyze the reduction in Cd(II) and the production of acetate and hydrogen through several possible mechanisms.24,25
For example, respiratory processes in bacterial cells may be a physiological behavior of Cd(II)-tolerant EABs in the limited environment of the MEC system or may be a significant channel for them to transfer cathodic electrons to the cathodic solution for facilitating the reduction in metal ions.26 From Fig. 3D and Table 2, peak currents were systematically examined at varying scan rates, spanning from 1–10 mV s−1, across different initial Cd(II) concentrations to elucidate the rate-limiting step governing the behavior of the biocathode. As the scan rate increased, it led to incremental peak current, accompanied by shifts in positive and negative potential, signifying that the biocathode biocathode was reversible.
| 20 mg L−1 | 40 mg L−1 | 60 mg L−1 | 80 mg L−1 | 100 mg L−1 | |
|---|---|---|---|---|---|
| Rv12 | 0.99386 | 0.99520 | 0.99526 | 0.99444 | 0.98871 |
| Rv1/22 | 0.98444 | 0.98418 | 0.98933 | 0.97228 | 0.99684 |
The peak current displays a linear relationship with the scan rate (ν), which indicates adsorption control, and the phenomenon is known as ‘thin-film behavior’; when the peak current is linearly related to the square root of the scan rate (ν1/2), it indicates diffusion control.11,27 The fitting analysis showed that the linear relationship between the peak current and scan rate (ν) was superior to that of the square root of the scan rate (ν1/2) for initial Cd(II) concentrations from 20 mg L−1 to 80 mg L−1, and the removal of Cd(II) by the biocathode for this concentration range was controlled by adsorption. When the initial concentration of Cd(II) was 100 mg L−1, the linear relationship between the peak current and the scan rate (ν1/2) was inferior when compared to the square root relationship for diffusion control (Table 2).
The voltage loss during electrodeposition at a standstill is presumed to be due to the charge transfer resistance of the electrode.19 However, in the current study, the diffusion impedance of the biocathode and abiocathode electrodes was higher than the charge transfer resistance in both Cd(II) solutions (Fig. 2D and Table 1); the diffusion impedance was also higher than the charge transfer in solutions with different concentrations of Cd(II) (Fig. S4B and Table S1†).
The analysis suggested that diffusive resistance may be the primary resistance to the electrodeposition process of metals due to the difference between the supply and demand for metal ion reduction and diffusion control, and that this phenomenon is more pronounced when there is a reduction in the amount of metal ions. Equivalent circuit fitting showed that the diffusion impedance of the system gradually decreased when the Cd(II) ion concentration increased from 20 mg L−1 to 80 mg L−1. In comparison, it tended to increase at a high Cd(II) ion concentration of 100 mg L−1. This result was consistent with the analyses of the plots of the relationship between the peak currents and the sweeping speeds at the primary and one-half power, so that the electrochemical kinetics of the system for Cd(II) removal up to 100 mg L−1 was controlled by adsorption. In contrast, the removal process at a concentration of 100 mg L−1 Cd(II) was likely to shift towards diffusion control, as it becomes challenging for microorganisms to eliminate Cd(II) from the water. Thus, an appropriate increase in the concentration of the cathodic electrolyte may accelerate the motion of metal ions toward the cathodic electrode, and a decrease in the concentration of metal ions in the solution may lead to an increase in the diffusive resistance.19
3.3 Cd(II) removal and kinetic properties of the MEC
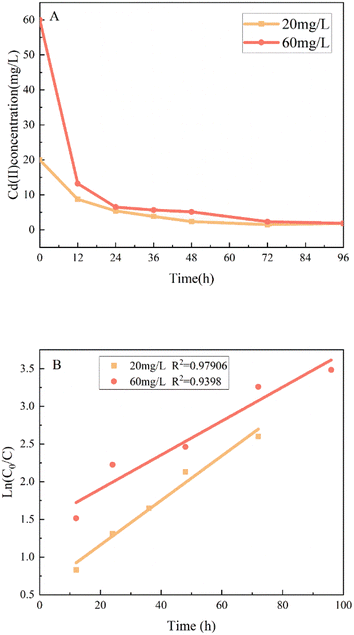
The Cd(II) reduction reaction in the MEC described above occurs by removal kinetics.Pseudo first-order kinetics:
In![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/C = Kobst C0/C = Kobst
| (1) |
3.4 Microbial community analysis
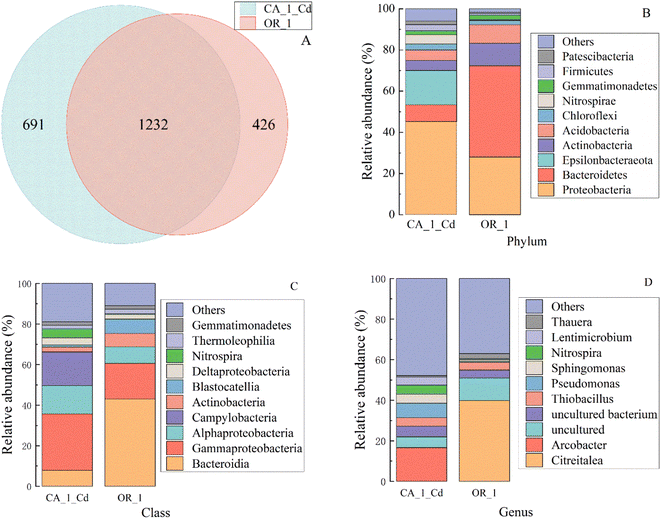
In this experiment, microbial community analyses of the original sludge and the Cd(II)-containing sludge were conducted to further explore the effect of Cd(II) on the biological communities. The results of the OTU number (CA_1_Cd:1923, OR_1:1658) (Table S3†) and Shannon's index (CA_1_Cd:7.59, OR_1:5.75) (Table S3†) for the two sludges indicated that Cd(II) was selective for the survival of microorganisms. The similarities and differences of microorganisms in the two silts are shown using a Venn diagram (Fig. 5A),29 which showed 691 and 426 different species. However, most of these strains are identical because the same slime had been inoculated, and a few changed under the pressure of Cd(II) and adapted to the toxic living environment. In addition, the species distributions of 16S rRNA gene sequences were analyzed at the phylum, class, and genus level in conjunction with single-sample multilevel species composition maps (Fig. S5 and S6†) and community structure component plots (Fig. 5B–D).Remarkable differences in the structure of microbial communities were observed between different samples. According to the results of 16S rRNA gene analysis, Proteobacteria, Bacteroidetes, Acidobacteria, and Actinobacteria were the predominant phyla in the two sludge communities at the phylum level (Fig. 5B).30 However, their abundance distributions were quite different, with an increase in the relative abundance of Proteobacteria from 28.00% in the original sludge to 45.22% in the Cd(II) solution. Conversely, the relative abundance of Bacteroidetes decreased from 44.28% in the original sludge to 8.00% in the Cd(II) solution. The relative abundance of new Epsilonbacteraeata was analyzed from the graph, and it increased from 0% in the original sludge to 16.66% in the Cd(II)-containing sludge.
This increase indicated that Proteobacteria and Epsilonbacteraeata were the phyla that were tolerant of heavy metals, and were also the main electron-producing phyla.31 At the class level (Fig. 5C), compared with the original sludge, the reactor with Cd(II)-containing sludge increased the abundance of Campylobacteria and Gammaproteobacteria while decreasing the number of Lactobacillus, as compared to the original sludge. As reported, Campylobacteria and Proteobacteria promoted the reduction of Cd(II) and electricity generation using nanoconductors (flagella) or conductivity-related cytochrome in electron generation and transport.32,33 These results showed that Gammaproteobacteria, Alphaproteobacteria, and Campylobacteria were more characteristic of cadmium tolerance. However, most of the Bacteroidia gradually disappeared under prolonged operational time, which suggests that they were not involved in the reduction reaction of the heavy metal Cd(II) ions or the absence of anti-cadmium properties.
Further study at the genus level (Fig. 5D) provided additional detailed information on the microbial community, and it was determined that the biodiversity of the sludge containing Cd(II) was more complex than that of the original sludge. After domestication by prolonged addition of Cd(II), the dominant bacteria Citreitalea in the original sludge gradually disappeared, and two absent species of bacteria, Arcobacter and Lentimicrobium, were simultaneously added under Cd(II) stress. In addition, the relative abundances of the species Pseudomonas, Sphingomonas, Nitrospira, and Thiobacillus increased from 0.62%, 0.80%, 0.34%, and 3.77% in the original sludge to 7.14%, 4.45%, 4.50%, and 4.23%, respectively, in the Cd(II)-containing sludge.
As reported, Arcobacter, Pseudomonas, and Sphingomonas are Gram-negative bacteria that can survive in microaerobic and anaerobic environments. Pseudomonas can consume various carbon sources and participate in metal reduction and electron production.34 Sphingosphinomonas is an endophytic bacterium with efficient metabolic regulation and can repair the toxicity damage in plants caused by Cd.35,36 Lentibacterium is a fermenting Gram-negative bacterium that may metabolize organic matter by apoptosis in an anaerobic environment. Nitrospira oxidizes nitrite to nitrate, and Thiobacillus oxidizes thiosulfate and elemental sulfur to sulfate,37 and both are autotrophic Gram-negative bacteria. Gradually disappearing Citreitalea are strictly aerobic Gram-negative bacteria, indicating that aerobic bacteria were not involved in Cd(II) removal.
The community analysis showed that the original bacterial community was transformed into an organic biological community with higher power generation efficiency and anaerobic fermentation by drug-resistant bacteria, which were numerically dominant during the acclimatization to the heavy metal ion Cd(II). Gram-negative bacteria possess an outer lipid membrane that protects them from toxic and harmful environments, while the flagellum facilitates bacterial motility and electron transport. In Cd(II)-containing sludge, Gram-negative bacteria are classified as autotrophic and heterotrophic bacteria, where autotrophic bacteria derive their metabolic energy from the oxidation of electrons, and heterotrophic bacteria are responsible for transferring electrons along the electron transport chain to the cell membrane.38
These results confirmed that the removal of Cd(II) ions in the MEC system is the result of a synergistic mechanism of multiple parthenogenetic anaerobic bacteria in anaerobic and microaerobic environments,39 and that the diversity of microbial communities contributes to maintaining the balance within the biological system. Arcobacter and Lentimicrobium were the dominant Cd-tolerant bacteria in the MEC system.
4. Conclusion
In this investigation, we examined the influence of biocathodes and abiotic cathodes on the performance of microbial electrolysis cells (MECs) operating under open-circuit (OC) and closed-circuit (CC) conditions. Our findings revealed that the polarity-reversing biocathode is more favorable for Cd(II) removal in MEC devices. Then, the removal rate and reaction mechanism for cadmium(II) were investigated for different concentrations of cadmium(II) at an applied voltage of 0.5 V under CC conditions. Remarkably, the removal rate exceeded 90% within three days, with the removal reaction in the MEC reactor adhering to the pseudo-first-order kinetic equation. The microbial population analysis showed a gradual decrease in the numbers of Bacteroidetes and Epsilon in the raw sludge after the addition of Cd(II), with changes in Alphaproteobacteria and Gammaproteobacteria in the Proteobacteria. These findings highlight the close relationship between Cd(II) removal and the composition of cathodic bacterial communities.Author contributions
Author 1 (first author): conceptualization, data curation, formal analysis, methodology, software, investigation, writing – original draft; author 2 (corresponding author): conceptualization, funding acquisition, resources, supervision, writing – review and editing, visualization, investigation; author 3 (corresponding author): resources, supervision; author 4: writing – review and editing, data curation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We received support from Special Funds for Guiding Local Scientific and Technological Development by the Central Government (YDZJSX2021A015) and Research Project Supported by the Shanxi Scholarship Council of China, 2021-054.References
- C. Yiran, Effects of Applied Voltage and Carbon Source on Cd(II) Removal Electrolysis cells[D], Dalian University of Technology, 2015 Search PubMed.
- C. Renlian, Study on the Transport and Transformation Process of Cd, Fe, and Mn in Soil Bioelectrochemical System and Their Microbial Mechanism Transport and Transformation Process of Cd, Fe, and Mn in Soil Bioelectrochemical System and Their Microbial Mechanism[D], Guangdong University of Technology, 2021 Search PubMed.
- Z. Xin, Removal of Cu(II) and Ni(II) from Wastewater by SMFC-SMEC Coupled System [D]. Harbin Institute of Technology, 2015 Search PubMed.
- Q. Wei, B. Li and X. Zhao, et al., Enhanced removal performance and microbial community diversity analysis of a microbial electrolytic cell with a double chamber for the treatment of wastewater containing p-bromoaniline, Biochem. Eng. J., 2022, 185, 108539 CrossRef CAS.
- X. Wu, X. Zhu and T. Song, et al., Effect of acclimatization on hexavalent chromium reduction in a biocathode microbial fuel cell, Bioresour. Technol., 2015, 180, 185–191 CrossRef CAS PubMed.
- Z. He and T. L. Angenent, Application of Bacterial Biocathodes in Microbial Fuel Cells, Electroanalysis, 2006, 18, 2009–2015 CrossRef CAS.
- Y. Li, Y. Wu and B. Liu, et al., Self-sustained reduction of multiple metals in a microbial fuel cell–microbial electrolysis cell hybrid system, Bioresour. Technol., 2015, 192, 238–246 CrossRef CAS PubMed.
- L. Huang, H. Xue and Q. Zhou, et al., Imaging and distribution of Cd(II) ions in lactotrophs and its response to current and electron transfer inhibitor in microbial electrolysis cells, Sens. Actuators B Chem., 2018, 255, 244–254 CrossRef CAS.
- O. Modin, X. Wang and X. Wu, et al., Bioelectrochemical recovery of Cu, Pb, Cd, and Zn from dilute solutions, J. Hazard. Mater., 2012, 235–236 Search PubMed.
- Y. Zhang, Z. Jian and Y. Liu, Application of Electrochemically Active Bacteria as Anodic Biocatalyst in Microbial Fuel Cells, Chin. J. Anal. Chem., 2015, 43, 155–163 CAS.
- B. Yu, J. Tian and L. Feng, Remediation of PAH polluted soils using a soil microbial fuel cell: Influence of electrode interval and role of microbial community, J. Hazard. Mater., 2017, 336, 110–118 CrossRef CAS PubMed.
- M. C. Martinez and H. L. Alvarez, Application of redox mediators in bioelectrochemical systems, Biotechnol. Adv., 2018, 36, 1412–1423 CrossRef PubMed.
- D. R. Lovely, Happy together: microbial communities that hook up to swap electrons, ISME J., 2017, 11, 327–336 CrossRef PubMed.
- P. Christian, L. Steffen and S. Jie, et al., Filamentous bacteria transport electrons over centimeter distances, Nature, 2012, 49, 218–221 Search PubMed.
- W. Zhi, Z. Ge and Z. He, et al., Methods for understanding microbial community structures and functions in microbial fuel cells: A review, Bioresour. Technol., 2014, 171, 461–468 CrossRef CAS PubMed.
- C. Shaoan, X. Defeng and D. F. Call, et al., Direct biological conversion of electrical current into methane by electromethanogenesis, Environ. Sci. Technol., 2009, 43, 3953–3958 CrossRef PubMed.
- Y. Chen, J. Shen and L. Huang, et al., Enhanced Cd(II) removal with simultaneous hydrogen production in biocathode microbial electrolysis cells in the presence of acetate or NaHCO3, Int. J. Hydrogen Energy, 2016, 41, 13368–13379 CrossRef CAS.
- L. Zhang, Z. Xu and Z. He, Selective recovery of lead and zinc through controlling cathodic potential in a bioelectrochemical-assisted electrodeposition system, J. Hazard. Mater., 2020, 38, 121941 CrossRef PubMed.
- W. Yuhang, W. Yehua and T. Jing, et al., Aqueous Li-ion cells with superior cycling performance using multi-channeled polyaniline/Fe2O3 nanotube anodes, J. Mater. Chem. A, 2014, 2, 20177–20181 RSC.
- T. P. Ha, H. Moon and H. B. Kim, et al., Determination of charge transfer resistance and capacitance of microbial fuel cell through a transient response analysis of cell voltage, Biosens. Bioelectron., 2010, 25, 1629–1634 CrossRef PubMed.
- T. M. Noori, C. Mukherjee and M. Ghangrekar, Enhancing performance of microbial fuel cell by using graphene supported V2O5-nanorod catalytic cathode, Electrochim. Acta, 2017, 228, 513–521 CrossRef.
- L. Meng, Study of Pb(II) Removal of Microbial Electrolysis Cells Derived by Cr(VI)-reduced Microbial Fuel Cells[D], South China University of Technology, 2019 Search PubMed.
- N. Colantonio and Y. Kim, Cadmium (II) removal mechanisms in microbial electrolysis cells, J. Hazard. Mater., 2016, 311, 134–141 CrossRef CAS PubMed.
- R. A. Rowe, P. Rajeev and A. Jain, et al., Tracking Electron Uptake from a Cathode into Shewanella Cells: Implications for Energy Acquisition from Solid-Substrate Electron Donors, mBio, 2018, 9, e02203–e02217 CrossRef PubMed.
- C. He, Z. Mu and H. Yang, et al., Electron acceptors for energy generation in microbial fuel cells fed with wastewaters: A mini-review, Chemosphere, 2015, 140, 12–17 CrossRef CAS PubMed.
- X. Hou, L. Huang and P. Zhou, et al., Electrosynthesis of acetate from inorganic carbon (HCO3−) with simultaneous hydrogen production and Cd(II) removal in multifunctional microbial electrosynthesis systems (MES), J. Hazard. Mater., 2019, 371, 463–473 CrossRef CAS PubMed.
- Y. Haihang, H. Liping and Z. Guoquan, et al., Physiological metabolism of electrochemically active bacteria directed by combined acetate and Cd(II) in single-chamber microbial electrolysis cells, J. Hazard. Mater., 2022, 42, 127538 Search PubMed.
- T. Song, Y. Jin and J. Bao, et al., Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell, J. Hazard. Mater., 2016, 317, 73–80 CrossRef CAS PubMed.
- Z. Xin, W. Yi and W. Ling, et al., Removal of Ni (II) and microbial dynamics in single-chamber microbial electrolysis cell, J. Microbiol., 2016, 56, 1794–1801 Search PubMed.
- A. W. Jeremiasse, H. V. M. Hamelers, E. Croese and C. J. N. Buisman, et al., Acetate enhances the startup of a H2-producing microbial biocathode, Biotechnol. Bioeng., 2012, 109, 657–664 CrossRef CAS PubMed.
- S. Aradhana and K. Anubha, Removal of Cd and Ni with enhanced energy generation using biocathode microbial fuel cell: Insights from molecular characterization of biofilm communities, J. Clean. Prod., 2021, 315, 127940 CrossRef.
- W. Liu, A. Wang and D. Sun, et al., Characterization of microbial communities during anode biofilm reformation in a two-chambered microbial electrolysis cell (MEC), J. Biotechnol., 2012, 157(4), 628–632 CrossRef CAS PubMed.
- I. Shun'ichi, W. Kazuya and Y. Soichi, et al., Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell, Appl. Environ. Microbiol., 2008, 74(23), 7348–7355 CrossRef PubMed.
- T. P. Hai, B. Nico and A. Peter, et al., Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer, Appl. Microbiol. Biotechnol., 2008, 77(5), 1119–1129 CrossRef PubMed.
- S. Z. Li, B. Zhao and M. Jin, et al., A comprehensive survey on the horizontal and vertical distribution of heavy metals and microorganisms in soils of a Pb/Zn smelter, J. Hazard. Mater., 2020, 400, 123255 CrossRef CAS PubMed.
- P. Zeng, Z. H. Guo and X. Y. Xiao, et al., Effects of tree-herb co-planting on the bacterial community composition and the relationship between specific microorganisms and enzymatic activities in metal(loid)-contaminated soil, Chemosphere, 2019, 220, 237–248 CrossRef CAS PubMed.
- C. Wu, L. Shi and S. Xue, et al., Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils, Sci. Total Environ., 2019, 647, 1158–1168 CrossRef CAS PubMed.
- H. Liping, W. Qiang and J. Linjie, et al., Adaptively Evolving Bacterial Communities for Complete and Selective Reduction of Cr(VI), Cu(II), and Cd(II) in Biocathode Bioelectrochemical Systems, Environ. Sci. Technol., 2015, 49(16), 9914–9924 CrossRef PubMed.
- Y. Zhang, Q. He and L. Xia, et al., Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode, Biochem. Eng. J., 2018, 138, 179–187 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07771c |
| This journal is © The Royal Society of Chemistry 2024 |