Open Access Article
Open Access ArticleFacile preparation of a CoNiS/CF electrode by SILAR for a high sensitivity non-enzymatic glucose sensor
Shi Wang†
 ,
Ruirui Zhang†,
Saiwen Ding,
Jialin Ao and
Ting Shu
,
Ruirui Zhang†,
Saiwen Ding,
Jialin Ao and
Ting Shu *
*
Hubei University of Science and Technology, Xianning, Hubei, China. E-mail: stzjj@sina.com
First published on 4th April 2024
Abstract
The nanomaterials for non-enzymatic electrochemical sensors are usually pre-synthesized and coated onto electrodes by ex situ methods. In this work, amorphous cobalt-nickel sulfide (CoNiS) nanoparticles were facilely prepared on copper foam (CF) by the in situ successive ionic layer adsorption and reaction (SILAR) method, and as-prepared CoNiS/CF was studied as an electrode for non-enzymatic glucose sensing. It was analyzed by field emission scanning electron microscopy (FESEM), energy dispersive X-ray analysis (EDAX) and X-ray photoelectron spectroscopy (XPS). The electrochemical performance was investigated by cyclic voltammetry (CV) and chronoamperometry (CA). This binary sulfide electrode showed better performance toward glucose oxidation compared to the corresponding single sulfide and showed a wide linear range of 0.005 to 3.47 mM, a high sensitivity of 2298.7 μA mM−1 cm−2 and a low detection limit of 2.0 μM. The sensor exhibited high sensitivity and good repeatability and stability and was able to detect glucose in an actual sample. This work provides a simple and fast in situ electrode preparation method for a high-sensitivity glucose sensor.
1. Introduction
Glucose directly participates in the metabolism of the human body and is the primary energy source for the human body. However, a high glucose level will damage the health of patients with diabetes. Several methods have been developed to detect glucose, including chromatography,1 colorimetry,2 fluorescence,3 and electrochemical techniques.4 Among them, electrochemical techniques show advantages of easy access to instruments, simple operation, and easy miniaturization. Electrochemical technology includes various methods, such as cyclic voltammetry (CV),5 differential pulse voltammetry (DPV),6 electrochemical impedance spectroscopy (EIS),7 and chronoamperometry (CA), which can be flexibly applied. Enzyme electrochemical glucose sensors have high selectivity, but their sensitivity to environmental factors such as temperature, humidity, and pH affect their accuracy.8 Therefore, enzyme-free electrochemical glucose sensors have received widespread attention. Various non-enzymatic materials, mainly nanomaterials, have been used for glucose sensors, which are known as nanoenzymes. For example, noble metals,9,10 carbon-based nanomaterials,11,12 and transition metal nanomaterials (mainly copper, cobalt, nickel, including their metals,13,14 oxides,15,16 hydroxides,17,18 sulfides19–23) have been reported as electrode materials for electrochemical glucose sensing.Transition metal sulfides have applications in the fields of photocatalysis,24 supercapacitors,25 sensors26 and solar cells27 due to their excellent physical and chemical properties, such as redox reversibility, capacitance and conductivity. Metal sulfides like CuS,28 CoS29 and NiS30 were used as nanozymes for non-enzymatic glucose sensors. Li et al. prepared amorphous CoS on a reduced graphene oxide-poly(3,4-ethylenedioxythiophene) composite by electrodeposition, and the glucose sensor exhibited a linear range of 0.0002–1.38 mM, and sensitivity of 113.46 μA mM−1 cm−2 with a detection limit of 0.079 μM.29 Lin et al. electrodeposited an α-NiS nanosphere film on ITO for glucose detection, and the sensor showed a linear range of 1–35 μM and a sensitivity of 8.4 μA μM−1 cm−2.30
However, monometallic materials have few active sites and limited electrochemical activity, which limit their application in non-enzymatic glucose sensors. Enhanced electrochemical performance can be observed on electrodes based on multimetallic material due to their synergistic effect.31,32 Binary metal sulfides have large redox reaction sites and high electrical conductivity compared to monometallic sulfides.33 Vilian et al. prepared Ni2CoS4 nanopetals on carbon nanofibers (Ni2CoS4–CNF) by an electrospinning-assisted hydrothermal method. The Ni2CoS4–CNF-based glucose sensor exhibited an extremely low detection limit (0.25 nM) and a wide linear range (5–70 nM).34 Cao et al. electrodeposited a nickel cobalt sulfide nanosheet film on a titanium mesh (Ni–Co–S/TM), which showed a wide linear response range of 0.001–3.0 mM, a sensitivity of 3291.5 μA mM−1 cm−2 and a low limit of detection of 0.12 μM for glucose sensing.35
In addition to the type of electrode material, the electrode preparation method and morphology of the material have significant impacts on the performance of sensors. Generally, in situ preparation of materials on the electrode shows better sensing performance compared to an ex situ method due to good contact and direct electron transfer. For instance, Li et al. reported that electrodeposited CoS had better electrocatalytic activity than drop casting CoS for glucose sensing.36 The in situ methods include electrospinning, electrodeposition, atomic vapor deposition, and the successive ionic layer adsorption and reaction (SILAR) method. The SILAR method has the advantages of low cost, fast speed, and easy operation. For instance, CuS,37 CuO,38 and Au nanoparticles39 have been prepared on different substrates by SILAR to be used as glucose sensor electrodes. As for the morphology of the materials, nanomaterials with a small particle size and a larger specific surface area generally have more catalytic sites, better contact with electrolyte solution, and better electrocatalytic activity. These nanomaterials are usually pre-synthesized by a hydrothermal method and are later coated on the substrate by an ex situ method to prepare sensor electrodes. However, sensors prepared by an ex situ method generally have low sensitivity due to the poor contact between the material and the substrate.
In this work, monolayer cobalt nickel sulfide nanoparticles were prepared by SILAR on copper foam for non-enzymatic glucose detection. Copper foam has a larger surface area and better adsorption than ordinary smooth substrates (such as conductive glass and glassy carbon electrodes), so it is easier to use a SILAR method to prepare materials in situ. This method avoids the use of non-conductive connectors, allowing good contact between the material and substrate. Metal sulfide nanoparticles with a size smaller than 50 nm were grown on copper foam, which increased the catalytic performance of the electrode. Compared with single cobalt sulfide or nickel sulfide, the synergistic effect of bimetallic cobalt nickel sulfide significantly improved the performance of the sensor. The sensor exhibited high sensitivity and good repeatability and stability and was used to detect glucose in an actual sample. Most importantly, compared to other in situ methods, this method was simple and fast.
2. Experimental section
2.1. Materials
Glucose (Glu), NaOH, Na2S·9H2O, CoSO4·7H2O, and NiSO4·6H2O came from Kelong Reagent Co. Ltd (Chengdu, China). Uric acid (UA), dopamine, ascorbic acid (AA), and cysteine were bought from Wokai Biotechnology Co. Ltd (Shanghai, China). Methanol was bought from Sinopharm Chemical Reagent Co. Ltd. All of the above reagents were of analytical grade and used directly without further treatment. The serum sample was purchased from Shanghai Acmec Biochemical Co. Ltd.2.2. Fabrication of CoNiS/CF
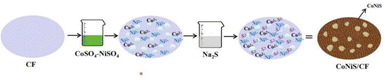
The optimized preparation process of the CoNiS/CF electrode was as follows: copper foam with an area of 1.5 × 0.8 cm2 was ultrasonically cleaned with acetone, RO water and absolute ethanol in sequence for 10 min. As CoNiS has a large current response, to obtain electrodes with a low detection limit, the concentration of the precursor solution needs to be low, and the dipping time in the precursor solution need to be short. The naturally dried copper foam was immersed in a mixed solution of 0.05 M CoSO4–NiSO4 (Co/Ni molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 10 s, which was then washed with deionized water to remove the residual solution and immersed in a solution of 0.05 M Na2S for another 10 s. After that, it was washed with deionized water and dried at 50 °C for 3 h to obtain the CoNiS/CF electrode. A schematic diagram of electrode preparation using SILAR is shown in Fig. 1. To enhance the hydrophilicity of the copper foam, the solvent for both solutions was a mixed solvent of water and methanol (Vwater/Vmethanol = 4
1) for 10 s, which was then washed with deionized water to remove the residual solution and immersed in a solution of 0.05 M Na2S for another 10 s. After that, it was washed with deionized water and dried at 50 °C for 3 h to obtain the CoNiS/CF electrode. A schematic diagram of electrode preparation using SILAR is shown in Fig. 1. To enhance the hydrophilicity of the copper foam, the solvent for both solutions was a mixed solvent of water and methanol (Vwater/Vmethanol = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For comparison, CoS/CF and NiS/CF electrodes were prepared by the same procedure, except that the mixed solution was replaced with 0.05 M NiSO4 or 0.05 M CoSO4 solution.
1). For comparison, CoS/CF and NiS/CF electrodes were prepared by the same procedure, except that the mixed solution was replaced with 0.05 M NiSO4 or 0.05 M CoSO4 solution.
2.3. Characteristics and electrochemical measurement
Scanning electron microscope (SEM) images were obtained from a Hitachi model SU 8020 UHR field emission scanning electron microscope (FESEM) (Japan), and the compositional analysis was performed by energy-dispersive X-ray spectroscopy (EDS). X-ray photo electron spectroscopy (XPS) analysis was tested with a Themo Escalab 250 (Thermo Fisher Scientific, USA) XPS system with a monochromatized Al Kα line source (1486.7 eV).Cyclic voltammetry (CV) and chronoamperometry (CA) were performed with a three-electrode system on a CHI 760E electrochemical workstation (Chenhua Inc., China). The working electrode was the as-prepared electrode with an active area of 0.8 cm2; the counter electrode was a Pt wire; and the reference electrode was an Ag/AgCl electrode. NaOH (0.1 M) was used as the electrolyte. CVs were performed in the potential window of 0–0.8 V at a scan rate of 20 mV s −1.
3. Results and discussion
3.1. Characterization of CoNiS/CF
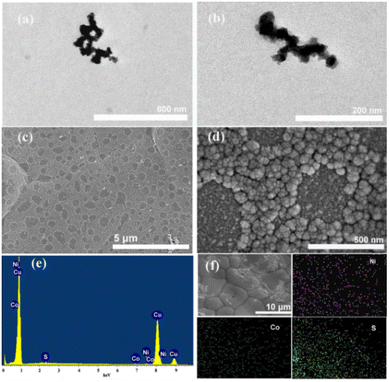
Fig. 2 shows the TEM and SEM images and EDS results of the CoNiS/CF electrode. Fig. 2(a and b) are the TEM images of CoNiS, which show that CoNiS nanoparticles were interconnected, and the size was less than 50 nm. As can be seen from Fig. 2(c), the copper foam surface was covered with a monolayer of rough aggregates. There were some relatively smooth areas which were irregular or nearly circular. These smooth areas were not connected but were independently and uniformly distributed on the copper foam. Fig. 2(d) shows clearly that the rough area was a layer of aggregates that showed a cauliflower-like morphology, which is a typical structure of a sulfide. These aggregates had sizes of about 50–100 nm and were composed of smaller nanoparticles. During the SILAR process, the copper foam first adsorbed Co2+–Ni2+ ions on its surface, and then S2− ions were adsorbed; when S2− ions met Co2+–Ni2+ ions, they reacted immediately to form the CoNiS nanoparticles on the surface of the copper foam, forming the rough cauliflower-like morphology, which increased the specific surface area and added active sites for the electrocatalysis of glucose. It can be inferred from Fig. 1 that the rough area was the place where Co2+–Ni2+ ions were adsorbed. In the Co2+–Ni2+ ion adsorption process, the liquid film containing Co2+–Ni2+ ions did not densely spread over the foam copper, but covered most of it, leaving some irregular or near circular smooth areas, which were the surface of foamed copper. Fig. 2(e) displays the EDS spectrum, indicating that Co, Ni, and S elements existed on the electrode. The map images in Fig. 2(f) demonstrate that Co, Ni, and S elements were distributed uniformly on the copper foam. The atomic ratio of Co to Ni was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
The elemental composition of the CoNiS/CF electrode was analyzed by XPS. Fig. 3(a) is a full XPS spectrum, showing the presence of Co, Ni, and S elements in the material, where the presence of C and O is usually attributed to contamination caused by the use of carbon as a corrective material and oxygen adsorbed onto the material in the testing process, respectively. As Fig. 3(b) shows, peaks at binding energies of 856.5 eV and 874.1 eV correspond to Ni2p3/2 and Ni2p1/2 of Ni3+ with minor Ni2+, respectively, and peaks at 862.1 eV and 880.1 eV are satellite peaks.40,41 In Fig. 3(c), peaks at binding energies of 781.8 eV and 797.2 eV are ascribed to Co2p3/2 and Co2p1/2 of Co2+ with minor Co3+, respectively, and satellite peaks are located at 786.7 eV and 803.2 eV.40,41 Fig. 3(d) shows two peak at 162.4 eV and 163.2 eV, which are attributed to S 2p.42 The above results indicate that the CoNiS nanoparticles had been successfully grown on CF.
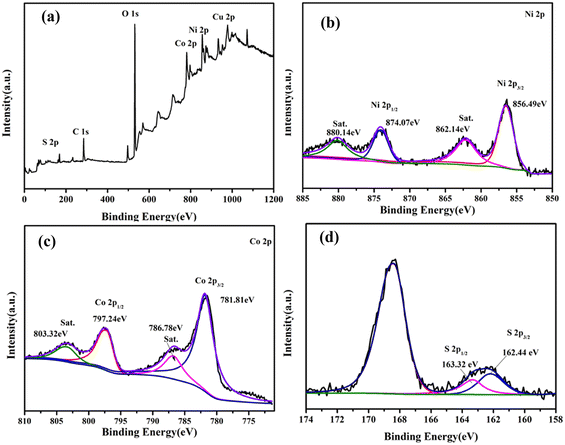 | ||
| Fig. 3 XPS spectra of the CoNiS/CF electrode: (a) survey spectrum, (b) Ni 2p, (c) Co 2p and (d) S 2p. | ||
3.2. Electrochemical properties of CoNiS/CF
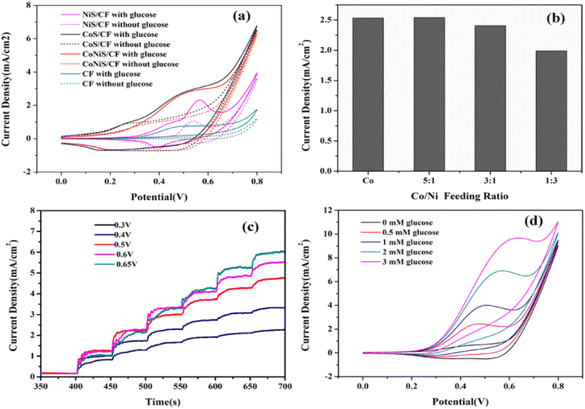
To study the electrochemical properties of the sensors, they were investigated by CV and CA. The CV curves of various electrodes with and without 0.5 mM glucose are presented in Fig. 4(a), which shows that the electrodes had oxidation peaks towards glucose at about 0.4–0.6 V. The oxidation peak currents increased significantly compared with those without glucose, indicating they all had an electrocatalytic effect on glucose. The bare CF had the lowest current responses, while the CoNiS/CF electrode exhibited current responses higher than those of CF or NiS/CF and close to those of CoS/CF. However, the current change before and after glucose addition for the CoNiS/CF electrode was larger than that of CoS/CF, indicating its better electrocatalytic activity for glucose. The feeding ratio of Co/Ni was optimized to achieve a better synergistic effect. From Fig. 4(b), a low oxidation peak current was obtained with a Co/Ni feeding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; when the ratios were 3
3; when the ratios were 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the oxidation peak currents became higher, both nearing that of CoS/CF. Thus, the Co/Ni feeding ratio of 3
1, the oxidation peak currents became higher, both nearing that of CoS/CF. Thus, the Co/Ni feeding ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was ultimately used for the CoNiS/CF electrode. To improve the sensitivity of the CoNiS/CF electrode for glucose detection, its amperometric response was tested by adding 0.5 mM glucose to 0.1 M NaOH at different potentials (0.4, 0.5, 0.6, and 0.65 V). Fig. 4(c) shows that the current responses increased with the increase in potential; when the potential was greater than 0.6 V, the current responses no longer increased. Thus, 0.6 V was selected as the final detection potential. The CV curves of CoNiS/CF with different concentrations of glucose are shown in Fig. 4(d); the oxidation/reduction peak near 0.6 V increased gradually and linearly with an increase in glucose from 0.0 to 3.0 mM, indicating that the electrode was sensitive to glucose in this range.
1 was ultimately used for the CoNiS/CF electrode. To improve the sensitivity of the CoNiS/CF electrode for glucose detection, its amperometric response was tested by adding 0.5 mM glucose to 0.1 M NaOH at different potentials (0.4, 0.5, 0.6, and 0.65 V). Fig. 4(c) shows that the current responses increased with the increase in potential; when the potential was greater than 0.6 V, the current responses no longer increased. Thus, 0.6 V was selected as the final detection potential. The CV curves of CoNiS/CF with different concentrations of glucose are shown in Fig. 4(d); the oxidation/reduction peak near 0.6 V increased gradually and linearly with an increase in glucose from 0.0 to 3.0 mM, indicating that the electrode was sensitive to glucose in this range.
Under alkaline conditions, Co2+ and Ni2+ change into Co3+ and Ni3+ at around 0.4–0.6 V; then Co3+ and Ni3+ react with glucose by electro-oxidation near 0.6 V to generate glucolactone. Therefore, in summary, the mechanism of electrocatalytic reactions between CoNiS/CF and glucose may include following processes:
| CoS + OH− → CoSOH + e− | (1) |
| NiS + OH− → NiSOH + e− | (2) |
| Co3+ + glucose → Co2+ + gluconolactone | (3) |
| Ni3+ + glucose → Ni2+ + gluconolactone | (4) |
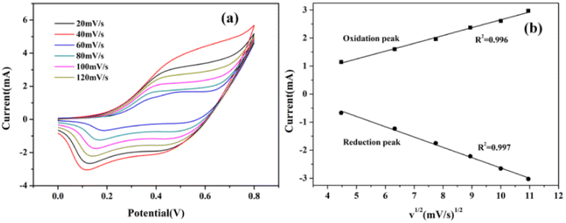
The CV curves of the CoNiS/CF electrode towards glucose (0.3 mM in 0.1 M NaOH) with different scan rates are shown in Fig. 5(a). When scan rate went from 20 to 120 mV s−1, the peak current intensity increased with the increase in scan rate. As shown in Fig. 5(b), the peak current of the redox peak of glucose was proportional to the square root of the scan rate, indicating that the redox reaction of glucose on this electrode was controlled by a diffusion process.
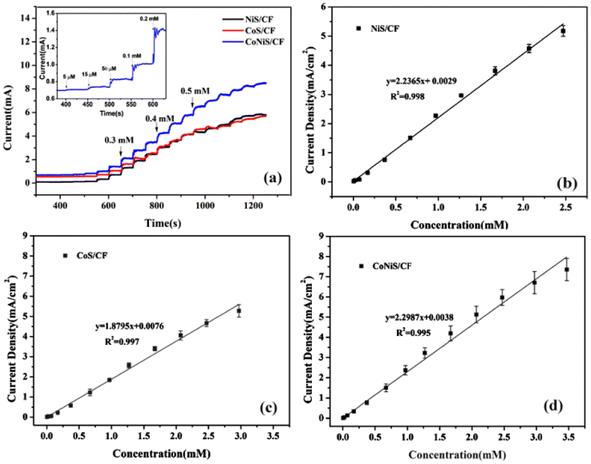
The current responses of the NiS/CF, CoS/CF, and CoNiS/CF electrodes were tested by CA with the addition of different concentrations of glucose to 0.1 M NaOH solution at a voltage of 0.6 V. The amperometric responses of the electrodes are shown in Fig. 6(a), and their linear calibration plots are shown in Fig. 6(b–d). As shown in Fig. 6(b), the linear equation of NiS/CF was j (mA cm−2) = 2.2365 C (mM) + 0.0029 with R2 of 0.998, which showed a linear relationship with the glucose concentration in the range of 0.005–2.47 mM with a sensitivity of 2.2365 mA mM−1 cm−2 and a detection limit of 2.1 μM. Fig. 6(c) shows that Co/CF had a linear relationship in the range of 0.005–2.97 mM (j (mA cm−2) = 1.8795 C (mM) + 0.0076, R2 = 0.997), a sensitivity of 1.8795 mA mM−1 cm−2, and a detection limit of 4.9 μM. Fig. 6(d) shows that CoNiS/CF had a linear relationship in the range of 0.005–3.47 mM (j (mA cm−2) = 2.2987 C (mM) + 0.0038, R2 = 0.995), a sensitivity of 2.2987 mA mM−1 cm−2, and a detection limit of 2.0 μM. It can be seen that the electrochemical catalytic performance of CoNiS/CF was better than those of NiS/CF and CoS/CF, consistent with the results of CV measurement. Table 1 is a comparison of the performance of the as-prepared CoNiS/CF sensor with reported Co or Ni sulfide based glucose sensors. It can be seen from the table that our sensor had higher sensitivity and wider linearity than most of the listed sensors, indicating that the CoNiS/CF sensor prepared by this simple SILAR method showed good performance and might be used for the non-enzymatic detection of glucose.
| Electrode | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | Detection limit (μM) | Ref. |
|---|---|---|---|---|
| NiCoS/Ti mesh | 3290 | 0.001–3.0 | 0.12 | 34 |
| CoS-ED | 330 | 0.08–1.0 | — | 36 |
| CoS@C/GCE | 697 | 0.01–0.96 | 2.0 | 43 |
| CoS-PPy-CP | 1110 | 0.0005–0.4665 | 0.14 | 44 |
| CoS/Co-MOF | 4600 | 0.005–1.17 | 0.11 | 45 |
| NiS | 5.78 | 0.005–0.06 | 0.052 | 46 |
| NiS | 54.6 | 0.02–5.0 | 0.0083 | 47 |
| NiS/S-g-C3N4 | 80 | 0.001–2.1 | 1.5 | 48 |
| NiCo2S4/EGF-7 | 7431.96 | 0.0005–3.571 | 0.167 | 49 |
| P-NiCo2S4/ITO | 250 | 0.001–5.2 | 0.46 | 50 |
| NiCo2S4/GCE | 858.57 | 0.005–0.1 | 2.0 | 51 |
| NiCo2S4/FTO | 1890 | 0.2–2.4 | 2.226 | 52 |
| NiCo2S4/Ni/CFP | 283 | 0.0005–6.0 | 0.0005 | 53 |
| CoNiS/CF | 2298.7 | 0.005–3.47 | 2.0 | This work |
3.3. Selectivity, reproducibility and stability
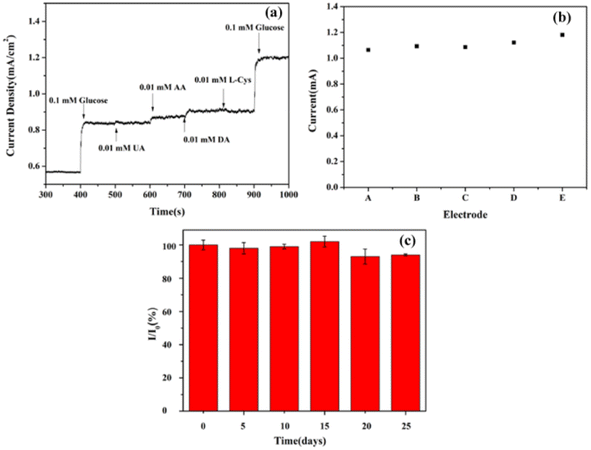
In actual glucose testing, common interfering substances in serum include ascorbic acid (AA), dopamine (DA), uric acid (UA), and cysteine, whose concentrations are approximately 10% of glucose concentration. Therefore, selective testing is essential. The procedure was as follows: 0.1 mM of glucose, 0.01 mM of AA, DA, UA, cysteine and 0.1 mM of glucose were added to 0.1 M NaOH solution; their amperometric responses are recorded in Fig. 7(a). No interferents produced an evident current response, whereas the additions of 0.1 M glucose before and after the interferents produced significant and almost identical current responses, respectively, indicating that the CoNiS/CF electrode showed good selectivity to glucose.Reproducibility and stability are also important factors for evaluating sensors. Five independent CoNiS/CF electrodes were used to measure 0.6 M glucose under same conditions. The relative standard deviation (RSD) of the five amperometric current responses was 4.0% (Fig. 7(b)). The long-term stability of CoNiS/CF electrodes was tested by recording the first current response value I0 toward 0.5 mM glucose in 0.1 M NaOH. They were then stored in a 2–4 °C refrigerator, and the current response intensity I was tested every five days under same conditions. As shown in Fig. 7(c), after 25 days, the current intensity ratio I/I0 of the CoNiS/CF electrode remained above 93%, indicating that the electrodes showed good stability and could withstand long-term continuous glucose testing.
To examine the practicality of the CoNiS/CF electrode, commercial serum was added to 0.1 M NaOH containing standard glucose solution under constant stirring (the added serum was diluted 1000 times), the current response was recorded by CA, and the glucose content was calculated according to the linear equation. The recoveries of the three samples were 103.3–105.0%, and the RSD of all test results was less than 5.0% (Table 2), indicating that the sensor was feasible for the detection of actual samples.
| Sample | Standard concentration (μM) | Measured concentration (μM) | Recovery (%) | RSD (%) n = 5 |
|---|---|---|---|---|
| 1 | 450 | 465 | 103.3 | 4.50 |
| 2 | 500 | 525 | 105.0 | 3.95 |
| 3 | 550 | 569 | 103.4 | 4.34 |
4. Conclusions
In summary, a novel CoNiS/CF electrode was fabricated by the SILAR method for an enzyme-free glucose sensor. The optimized electrode enhanced the electrochemical response and increased the sensing performance through the synergistic effect of cobalt and nickel. The sensor was found to have good electrocatalytic activity for glucose oxidation in 0.1 M NaOH solution, which exhibited a linear range of 0.005–3.47 mM, a sensitivity of 2298.7 μA mM−1 cm−2, and a detection limit of 2.0 μM. In addition, the sensor showed good selectivity, reproducibility and stability. It also achieved satisfactory recoveries in real serum sample measurement, indicating the application feasibility of a CoNiS/CF electrode. This study provides a simple and fast method for the in situ preparation of electrodes for high-sensitivity glucose sensors.Author contributions
Shi Wang: conceptualization, formal analysis, writing – original draft. Ruirui Zhang: conceptualization, methodology. Ning Li: validation, visualization, formal analysis. Jialin Ao: validation, visualization. Saiwen Ding: investigation, formal analysis. Ting Shu: conceptualization, methodology, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Educational Commission of Hubei Province of China (D20222802).Notes and references
- Y. Zhou, J. P. Chen, L. Gan, W. Xu, Y. Liu, Y. G. Zhao and Y. Zhu, J. Chromatogr., 2022, 1685, 463564 CrossRef CAS PubMed.
- M. Srivastava, S. K. Srivastava, R. P. Ojha and R. Prakash, Microchem. J., 2022, 182, 107850 CrossRef CAS.
- S. K. Vaishanav, J. Korram, R. Nagwanshi, K. K. Ghosh and M. L. Satnami, Sens. Actuators, B, 2017, 245, 196–204 CrossRef CAS.
- H. Kim, H. Choi, C. S. Park, H. S. Yim, D. Kim, S. Lee and Y. Lee, Biosensors, 2023, 13, 248 CrossRef PubMed.
- N. Sheibani, M. Kazemipour, S. Jahani and M. M. Foroughi, Microchem. J., 2019, 149, 103980 CrossRef CAS.
- T. Iranmanesh, S. Jahani, M. M. Foroughi, M. S. Zandi and H. H. Nadiki, Anal. Methods, 2020, 12, 4319–4326 RSC.
- M. M. Foroughi, S. Jahani, S. Rashidi, O. Tayari and M. Moradalizadeh, Mater. Chem. Phys., 2024, 315, 128893 CrossRef CAS.
- N. Lu, C. L. Shao, X. H. Li, F. J. Miao, K. X. Wang and Y. C. Liu, Ceram. Int., 2016, 42, 11285–11293 CrossRef CAS.
- K. Shim, W. C. Lee, M. S. Park, M. Shahabuddin, Y. Yamauchi, M. S. A. Hossain, Y. B. Shim and J. H. Kim, Sens. Actuators, B, 2019, 278, 88–96 CrossRef CAS.
- F. Li, X. Chen, H. Wang, M. Liu and H. L. Peng, ACS Appl. Mater. Interfaces, 2023, 15, 13290–13298 CrossRef PubMed.
- C. Tiwari, S. S. Jha, R. Kumar, M. Chhabra, B. D. Malhotra and A. Dixit, Mater. Sci. Eng., B, 2022, 285, 115931 CrossRef CAS.
- J. Mohapatra, B. Ananthoju, V. Nair, A. Mitra, D. Bahadur, N. V. Medhekar and M. Aslam, Appl. Surf. Sci., 2018, 442, 332–341 CrossRef CAS.
- M. Pak, A. Moshaii, H. Siampour, S. Abbasian and M. Nikkhah, Microchim. Acta, 2020, 187, 276 CrossRef CAS PubMed.
- G. Mo, X. Zheng, N. Ye and Z. Ruan, Talanta, 2021, 225, 121954 CrossRef CAS PubMed.
- S. Sedaghat, C. R. Piepenburg, A. Zareei, Z. Qi, S. Peana, H. Wang and R. Rahimi, ACS Appl. Nano Mater., 2020, 3, 5260–5270 CrossRef CAS.
- W. Liu, G. Chai, X. Zhao, Y. Dai and Y. Qi, Sens. Actuators, B, 2020, 321, 128485 CrossRef CAS.
- N. Shi, S. Sun, B. Zhang, Q. Du, Y. Liao, X. Liao, G. Yin, Z. Huang, X. Pu and X. Chen, Nanotechnology, 2020, 31, 325502 CrossRef CAS PubMed.
- J. Tashkhourian, S. F. Nami-Ana and M. Shamsipur, Anal. Chim. Acta, 2018, 1034, 63–73 CrossRef CAS PubMed.
- B. Kim, S. H. Lee, M. Cho and Y. Lee, Sens. Actuators, B, 2017, 249, 161–167 CrossRef.
- M. Keerthi, B. Mutharani, S. M. Chen and P. Ranganathan, Microchim. Acta, 2019, 186, 807 CrossRef CAS PubMed.
- J. Zhua, X. Penga, W. Nie, Y. Wang, J. Gao, W. Wen, J. N. Selvaraj, X. Zhang and S. Wang, Biosens. Bioelectron., 2019, 141, 111450 CrossRef PubMed.
- W. Wu, B. Yu, H. Wu, S. Wang, Q. Xia and Y. Ding, Mater. Sci. Eng., C, 2017, 70, 430–437 CrossRef CAS PubMed.
- P. K. Kannan and C. S. Rout, Chem.–Eur. J., 2015, 21, 9355–9359 CrossRef CAS PubMed.
- S. Khan, H. Choi, D. Kim, S. Y. Lee, Q. Zhu, J. Zhang, S. Kim and S. H. Cho, Chem. Eng. J., 2020, 395, 125092 CrossRef CAS.
- R. Barik and P. P. Ingole, Curr. Opin. Electrochem., 2020, 21, 327–334 CrossRef CAS.
- B. Chatterjee and A. Bandyopadhyay, Mater. Sci. Eng., B, 2023, 297, 116781 CrossRef CAS.
- L. H. Kharboot, N. A. Fadil, T. A. A. Bakar, A. S. M. Najib, N. H. Nordin and H. Ghazali, Materials, 2023, 16, 2881 CrossRef CAS PubMed.
- K. P. Sharma, M. Shin, G. P. Awasthi, M. B. Poudel, H. J. Kim and C. Yu, Int. J. Biol. Macromol., 2022, 206, 708–717 CrossRef CAS PubMed.
- A. Meng, L. Sheng, K. Zhao and Z. Li, J. Mater. Chem. B, 2017, 5, 8934–8943 RSC.
- S. Lin, J. B. Shi, C. M. Peng, B. C. Zheng, F. C. Cheng, M. W. Lee, H. W. Lee, P. F. Wu and Y. J. Liu, Nanoscale Res. Lett., 2018, 13, 109 CrossRef PubMed.
- W. Li, S. Lv, Y. Wang, L. Zhang and X. Cui, Sens. Actuators, B, 2019, 281, 652–658 CrossRef CAS.
- J. Ding, L. Zhong, X. Wang, L. Chai, Y. Wang, M. Jiang, T. T. Li, Y. Hua, J. Qian and S. Huang, Sens. Actuators, B, 2020, 306, 127551 CrossRef CAS.
- P. Kulkarni, S. K. Nataraj, R. G. Balakrishna, D. H. Nagaraju and M. V. Reddy, J. Mater. Chem. A, 2017, 5, 22040–22094 RSC.
- E. Vilian, S. K. Hwang, K. S. Ranjith, Y. Cho, Y. S. Huh and Y. K. Han, Microchim. Acta, 2021, 188, 106 CrossRef PubMed.
- X. Cao, K. Wang, G. Du, A. M. Asiri, Y. Ma, Q. Lu and X. Sun, J. Mater. Chem. B, 2016, 4, 7540–7544 RSC.
- X. Li, S. Sharma, D. W. M. Arrigan and D. S. Silvester, J. Electrochem. Soc., 2022, 169, 056505 CrossRef CAS.
- C. Wei, X. Zou, Q. Liu, S. Li, C. Kang and W. Xiang, Electrochim. Acta, 2020, 334, 135630 CrossRef CAS.
- A. S. Patil, R. T. Patil, G. M. Lohar and V. J. Fulari, Appl. Phys. A, 2021, 127, 101 CrossRef CAS.
- J. Li, L. Yan, H. Wang, H. Wang, X. Chen, L. Wang and M. Wu, J. Mater. Sci.: Mater. Electron., 2017, 8, 3067–3074 CrossRef.
- J. Yang, M. Cho and Y. Lee, Biosens. Bioelectron., 2016, 75, 15–22 CrossRef CAS PubMed.
- W. Li, S. Lv, Y. Wang, L. Zhang and X. Cui, Sens. Actuators, B, 2019, 281, 652–658 CrossRef CAS.
- X. Liu, L. Ai and J. Jiang, Powder Technol., 2015, 283, 539–548 CrossRef CAS.
- P. Qu, Z. Gong, H. Cheng, W. Xiong, X. Wu, P. Pei, R. Zhao, Y. Zeng and Z. Zhu, RSC Adv., 2015, 5, 106661–106667 RSC.
- Y. Qi, Y. Hu, X. Wu, W. Wu, J. Bao, H. Yang, J. Zhao, C. Hou and D. Huo, J. Electrochem. Soc., 2021, 168, 107507 CrossRef CAS.
- S. Ramesh, A. T. A. Ahmed, Y. Haldorai, V. Kakani, C. Bathula and H. S. Kim, J. Alloys Compd., 2023, 967, 171760 CrossRef CAS.
- R. Singh and M. M. Ayyub, ACS Appl. Electron. Mater., 2021, 3, 1912–1919 CrossRef CAS.
- M. Arivazhagan, Y. M. Santhosh and G. Maduraiveeran, Micromachines, 2021, 12, 403 CrossRef PubMed.
- S. Vinot, P. M. Rajaitha, A. Venkadesh, K. S. S. Devi, S. Radhakrishnan and A. Pandikumar, Nanoscale Adv., 2020, 2, 4242–4250 RSC.
- Q. Guo, T. Wu, L. Liu, Y. He, D. Liu and T. You, J. Alloys Compd., 2020, 819, 153376 CrossRef CAS.
- X. Lang, D. Chu, Y. Wang, D. Ge and X. Chen, Biosensors, 2022, 12, 823 CrossRef CAS PubMed.
- D. Chen, H. Wang and M. Yang, Anal. Methods, 2017, 9, 4718–4725 RSC.
- H. Yuan, C. Ma, Z. Gao and L. Zhang, Appl. Phys. A, 2019, 125, 61 CrossRef.
- K. J. Babu, T. R. Kumar, D. J. Yoo, S. M. Phang and G. Gnana Kumar, ACS Sustainable Chem. Eng., 2018, 6, 16982–16989 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |