Open Access Article
Open Access ArticleDevelopments in conducting polymer-, metal oxide-, and carbon nanotube-based composite electrode materials for supercapacitors: a review
Aarti Tundwal
a,
Harish Kumar
 *a,
Bibin J. Binoj
a,
Rahul Sharma
*a,
Bibin J. Binoj
a,
Rahul Sharma
 a,
Gaman Kumar
a,
Rajni Kumari
a,
Ankit Dhayal
a,
Abhiruchi Yadav
a,
Devender Singh
a,
Gaman Kumar
a,
Rajni Kumari
a,
Ankit Dhayal
a,
Abhiruchi Yadav
a,
Devender Singh
 b and
Parvin Kumar
c
b and
Parvin Kumar
c
aDept of Chemistry, Central University of Haryana, Mahendergarh-123031, India. E-mail: harishkumar@cuh.ac.in
bDept of Chemistry, MDU, Rohtak-124001, India
cDept of Chemistry, Kurukshetra University, Kurukshetra, India
First published on 20th March 2024
Abstract
Supercapacitors are the latest development in the field of energy storage devices (ESDs). A lot of research has been done in the last few decades to increase the performance of supercapacitors. The electrodes of supercapacitors are modified by composite materials based on conducting polymers, metal oxide nanoparticles, metal–organic frameworks, covalent organic frameworks, MXenes, chalcogenides, carbon nanotubes (CNTs), etc. In comparison to rechargeable batteries, supercapacitors have advantages such as quick charging and high power density. This review is focused on the progress in the development of electrode materials for supercapacitors using composite materials based on conducting polymers, graphene, metal oxide nanoparticles/nanofibres, and CNTs. Moreover, we investigated different types of ESDs as well as their electrochemical energy storage mechanisms and kinetic aspects. We have also discussed the classification of different types of SCs; advantages and drawbacks of SCs and other ESDs; and the use of nanofibres, carbon, CNTs, graphene, metal oxide–nanofibres, and conducting polymers as electrode materials for SCs. Furthermore, modifications in the development of different types of SCs such as pseudo-capacitors, hybrid capacitors, and electrical double-layer capacitors are discussed in detail; both electrolyte-based and electrolyte-free supercapacitors are taken into consideration. This review will help in designing and fabricating high-performance supercapacitors with high energy density and power output, which will act as an alternative to Li-ion batteries in the future.
1. Introduction
In recent decades, rapid globalization has increased the consumption of energy. At the global level, the human population is increasing at an alarming rate, raising energy requirements. The combined effect of these two factors leads to strain on the existing power infrastructure. Because of the rising usage of environmentally friendly and renewable energy systems in recent years, the consumption of hydrocarbon-based energy resources has decreased.1 Automobiles, portable and non-portable electronic gadgets, fuel cells, etc. use electrochemical systems such as battery packs, supercapacitors (SCs), and bio-fuel cells to convert chemical energy into electrical energy or for energy storage.2 A straightforward Ragone plot explains how batteries have moderated power and energy sectors, whereas SCs are thought of as elevated-power systems and fuel cells as high-energy systems (Fig. 1). The latest research is focused on the fabrication of SCs because of their unique properties, which has led to the testing of different composites as electrode materials such as carbon nanotubes (CNTs), conductive polymers (CPs), and metal oxide (MO) nanoparticles (NPs). The variety of uses of elastic and blendable SCs that need matching electrodes and components has led to the improvement of specific electrode materials.3 It leads to environment-friendly and minimum-cost energy storage systems with emerging ecology and modern pollution free society.4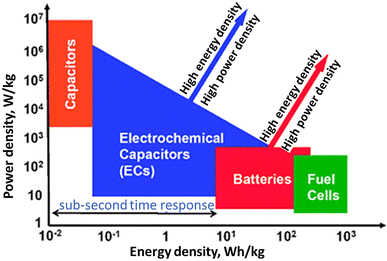 | ||
| Fig. 1 Ragone graph of power density vs. energy density (ED) for different ESDs [Reproduced with permission from ref. 2. A copyright of RSC, 2008]. | ||
SCs are devices in which electrical energy can be stored via double-layer (DL) charging, faradaic reactions, or a combination of both. They are employed via thin dielectric layers and large surface area electrodes, which give high power density, outstanding periodic life, and a rapid charging/discharging rate, which in turn leads to high electrochemical performance.5,6 The Leyden jar created in 1746, Netherlands, where electrical charges could be held on the plates of a so-called condenser, today known as a capacitor, was the first double-layer SC. The first patent, however, was only issued in 1957 and detailed a large surface area carbon-based capacitor.7 SCs, their components, and their implementations are covered in a broader sense.8–16 The rise in ED at the system level is mentioned in many articles published and has been emphasized about widely applicable outcomes.17 The benefits of asymmetric and hybrid SC techniques have been explored along with the subsequent aspects.18,19
SCs play a major role in ESDs due to their unique characteristics and advantages. A few advantages of SCs over conventional batteries are as follows: (i) high power density: SCs can deliver and absorb electrical energy at a much higher rate compared to batteries. Their high power density allows for quick charging and discharging, making them suitable for applications that require quick bursts of energy, (ii) long cycle life: SCs have an exceptionally long cycle life, with the ability to undergo hundreds to thousands or even millions of charge–discharge cycles,9 (iii) efficient energy transfer: SCs show low internal resistance, and hence efficient energy transfer between the storage device and the load. This characteristic minimizes energy losses following charge and discharge cycles, leading to higher overall system efficiency,10 (iv) wide temperature range: SCs can operate across a broad temperature range, from extreme cold to high heat, without significant degradation in performance.12 This versatility makes them suitable for various environments and applications, including automotive, aerospace, and industrial sectors, (v) safety and environmental friendliness: SCs are generally considered safe due to their stable chemistry and lack of toxic materials. Unlike some battery chemistries, they do not pose significant risks of overheating, leakage, or explosion,16 and (vi) complementary to rechargeable batteries: SCs can be used in conjunction with batteries to create hybrid energy storage systems that leverage the strengths of both technologies.17,18 By combining the high EDs and power densities of batteries and the rapid response of SCs, these hybrid systems can enhance the overall performance, extending battery life and improving energy management.
Hence, SCs have high power density, long cycle life, efficient energy transfer, wide temperature range, safety, and environmental friendliness. These qualities make them indispensable in ESDs, supporting different applications where quick energy delivery, longevity, and reliability are essential requirements.
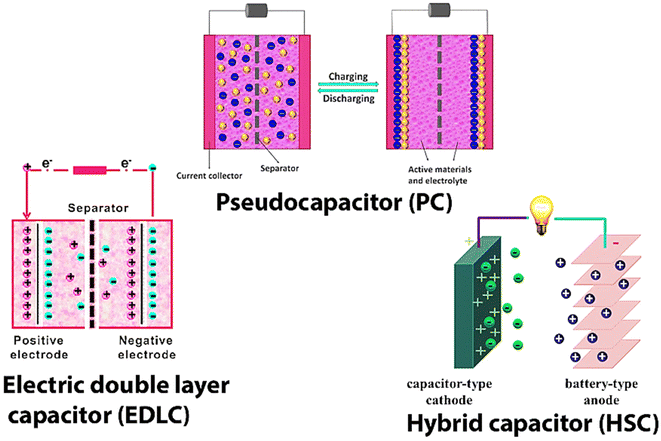
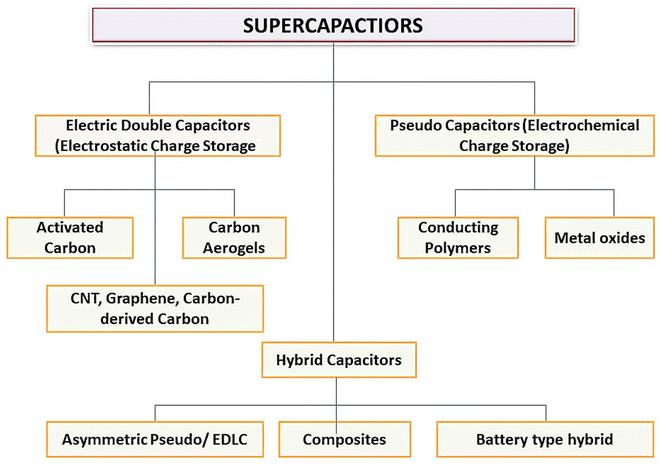
SCs may be divided into three types: Pseudo Capacitors (PCs), Electro-chemical Double Layer Capacitors (EDLC), and a hybrid kind produced by combining EDLCs and PCs (Fig. 2). Fig. 2 shows the working and internal structure of different types of SCs.
For the first time, we are introducing composite materials based on conducting polymers like polyaniline (PANI), polypyrrole (PPy), and polythiophene (PTh), CNTs [Single Walled Carbon Nanotubes (SWCNTs), and Multi-Walled Carbon Nanotubes (MWCNTs)] and transition MO NPs used in the fabrication of PCs, EDLCs, and SCs. Progress made towards development and modification done in increasing the charge storing capacity and ED of SCs by modifying the electrode material and electrolyte are discussed in detail. Kinetics and structural changes taking place at the surface of the electrode during the charging and discharging processes are discussed at full length. A comparison between rechargeable batteries and SCs is also made at relevant places. This review is mainly focused on the charge storage principle of SCs and the properties of modified electrode materials used in the latest SCs. This review is structured as SCs and their types, strategies of nanofiber (NF) fabrication, and different kinds of electrode materials used, including C material (graphitic carbon, graphene oxide, and CNTs), transition MO-based NF, and CP-based electrode materials used in SCs. The use of different MO/CPs-based composite as electrode materials (binary composite) in SCs is investigated in detail. NC materials are made of carbon along with MO NPs and CPs (tertiary composite) as the anode and cathode material. Lastly, we discussed the future scope and conclusions. This review article will certainly help researchers working in the field of ESDs to increase their performance and power output.
2. SCs as ESDs
SCs, also known as ultra-capacitors or electrochemical capacitors, are a type of ESD that stores electrical energy through the electrostatic separation of charges at two plates. They are different from traditional rechargeable batteries and have different advantages over batteries like rapid charge and discharge capabilities and longer cycle life. SCs are classified based on their unique characteristics and applications as EDLCs, PCs, hybrid SCs, symmetric and asymmetric SCs, flexible and printable SCs, etc.EDLCs store energy by forming an electric DL at the electrode–electrolyte interface. They have high power density, quick charge/discharge rates, and long cycle life. EDLCs are used for short-term energy storage, such as in regenerative braking systems, and peak power shaving in renewable energy systems. PCs store energy through fast and reversible faradaic reactions at the electrode–electrolyte interface. They offer higher EDs as compared to EDLCs but may have lower power density. Common electrode materials used in EDLCs are transition MOs (e.g., RuO) and CPs.
Hybrid SCs combine the features of both EDLCs and PCs to provide a balance between energy and power density. These devices typically consist of an EDLC electrode and a PC electrode. Symmetric SCs have identical electrodes and are used for high-power applications.
Asymmetric SCs have different electrodes with varying properties to achieve a balance between energy and power density. Flexible and printable SCs are designed to be flexible, lightweight, and even printable on various substrates. They are used in wearable electronics, smart textiles, and other applications where traditional rigid SCs would be impractical. The choice of SC type depends on the specific requirements of the application, such as ED, power density, voltage range, and environmental conditions. Here, we will discuss a few important SCs used in ESDs.
2.1. EDLCs as ESDs
An electrolyte, a separator, and two electrodes made of C-based materials make EDLCs. EDLCs can conserve charge electrostatically when utilizing a non-faradaic technique that prohibits the charge from moving from the electrode to the electrolyte.20–22 Electrochemical double layers are the basis for EDLCs' energy storage system. Ions in the medium migrate over the divider and the porous electrodes. When voltage is applied, as a consequence of the affinity between opposing charges generated by the potential difference. Ion recombination is prevented by an extra layer of charge which is built up at the electrodes. Electrodes composed of NCs possess a large Specific Surface Area (SSA), which provides a large surface for electrochemical reactions, ion recombination, charge storage capacity, etc. and hence, EDLCs show improved EDs.23,24Additionally, the efficient storage mechanism of EDLCs enables much faster energy absorption, distribution, and increased production of power. Chemical reactions are prevented via the non-faradaic technique. It eliminates the swelling that occurs when rechargeable batteries, especially Li-ion batteries (LIBs), are charged and drained due to the active substance present as an electrolyte.
There are a few key distinctions between EDLCs and rechargeable batteries, that is, (i) batteries can only survive a few thousand cycles at best, but EDLCs can have large cycle life, and (ii) in EDLCs, the electrolytic solvent does not contribute significantly to the charge storage process; instead, it increases ionic mobility and minimizes resistance between the two electrodes. However, Solid-State Electrolytes (SSEs) have some contribution to increase the charge storage capacity of EDLCs.25–30 EDLC devices only have small EDs due to the electrochemical surface charging mechanism. Because of this, the majority of recent research on EDLCs has focused on improving energy efficiency and extending the temperature range in which batteries cannot work. The effectiveness of EDLCs can be influenced by the type of electrolyte used.
2.2. Pseudocapacitors (PCs)
PCs are a type of ESD that combines the principles of traditional capacitors and rechargeable batteries. They are designed to provide high EDs and rapid charge/discharge capabilities, making them ideal for a large number of applications. In the 1960s, the pseudo-capacitance concept was introduced to understand surface faradaic processes like hydrogen evolution and under-potential deposition (UPD). UPD is defined as the electrodeposition of metal monolayers onto a different metal substrate at less negative potential than the electrodeposition of the same metal as the substrate.21 The CV shows significant and highly reversible peaks due to the formation of different structures of the electrodeposited monolayers on the surface of the electrode.Compared to electrostatically charged EDLCs, PCs use the faradaic principle to store charge, which includes the exchange of charges between the two electrodes.24 The cycling stability of SCs was found to be intermediate between LIBs and EDLCs. If a voltage is supplied in a PC, the electrode material undergoes reduction and oxidation. A faradaic current flows via the SC electrodes as a result of the transfer of charge via the DL. Due to the involvement of the faradaic principle to store the charge, PCs can reach higher specific capacity and EDs than EDLCs. MO NPs and CPs are two examples that are used in PCs to increase charge-storage capacity.25 This raises the interest of researchers in these materials, but they also possess cyclical instability and low power density due to their faradaic nature, which necessitates a reduction–oxidation process comparable to that of batteries.31–33
Fig. 3 shows different types of electrochemical energy harnessing methods which depend on capacity which influences the degree of electrode surface modification (structural changes), over-potential, and types of electrode kinetics. The main advantage of PCs is their short cycle life ranging from 10 seconds to 10 min.
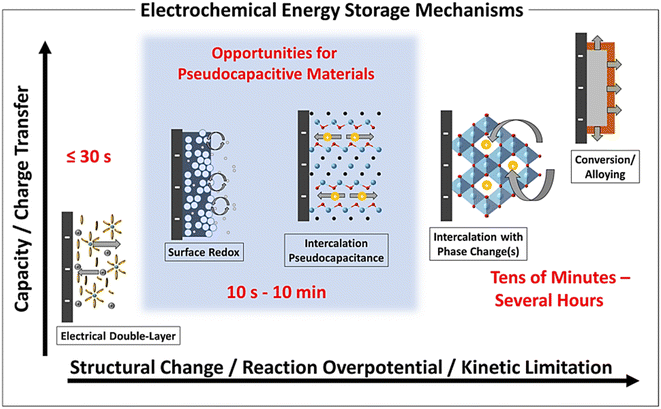 | ||
| Fig. 3 Different electrochemical energy harnessing mechanisms of PCs as a function of characteristic capacity, degree of structural modifications in the electrodes, reaction over-potential, and limits of the chemical kinetics [Reproduced with permission from ref. 30. A copyright of ACS, 2020]. | ||
The kinetic behavior of a battery involves chemical reactions that occur during the charge and discharge processes. These reactions typically involve the insertion and extraction of ions or molecules into/from the electrode materials. PCs exhibit faster kinetics than batteries because their charge and discharge processes involve rapid surface redox reactions, which do not require the diffusion of ions deep into the electrode material. Batteries have slower charge and discharge rates compared to PCs due to the relatively sluggish nature of the chemical reactions involved. This slower kinetics is a limiting factor in applications that require rapid energy exchange. However, PCs can deliver and store electrical energy quickly, making them suitable for applications requiring rapid energy cycling, such as regenerative braking in vehicles.
Batteries often undergo significant structural changes during charge and discharge cycles. For example, in LIBs, the anode and cathode materials can expand and contract as Li ions intercalate and de-intercalate, leading to mechanical stress and eventual electrode degradation.
The structural changes during the charging and discharging of batteries can limit their cycle life, as repeated expansion and contraction lead to electrode cracking and capacity fading with time. PCs typically undergo minimal structural changes during charge and discharge processes because their energy storage primarily relies on surface redox reactions. There is less volume change in the electrode materials compared to batteries. PCs often have longer cycle lives compared to batteries due to the reduced mechanical stress on the electrode materials. This makes them more durable and capable of withstanding a large number of charge–discharge cycles.
PCs are mainly composed of two components: electrodes and an electrolyte. The electrodes in PCs are made of highly conductive materials, such as Activated Carbon (AC), graphene, CNT, CP, and MO-based composites, which provide a large surface area for electrochemical reactions.34–37 The quick charge transfer reactions due to the adsorbed species on the electrode surface depend upon UPD, intermediates adsorbed on ions present in the electrolyte, and electrode composition.28 The electrolyte is a liquid or gel-like substance containing ions that facilitate the charge transfer between the electrodes. The working principle of PCs is based on two electrochemical processes that are double-layer capacitance (Cdl) and redox reactions. Cdl is produced in PCs when a voltage is applied across the electrodes which results in the formation of an electrical DL at the electrode–electrolyte (e–i) interface. The DL comprises two regions: the Helmholtz–Perrin and diffuse-charge layers. The Helmholtz–Perrin layer regularly contains tightly bound ions (in a plane), while the diffuse charge layer contains loosely bound randomly arranged ions. The separation of charges at the e–i interface creates a DL capacitance (Cdl), which allows for the rapid storage of charge and instantaneous release of electrical energy. In addition to DL capacitance, PCs also exhibit pseudo-capacitance resulting from reversible faradaic redox reactions.37 This involves the transfer of electrons between the two electrodes. The electrode material undergoes oxidation and reduction reactions during the charging and discharging of PCs. This redox reaction chemistry contributes to the overall capacitance of the PC and hence increases its total energy storage capacity. Fig. 4 shows different types of pseudocapacitance mechanisms that is adsorption, electrochemical, and intercalation. The shape and area of the CV region demonstrate the differences in the electrochemical performance of SCs.
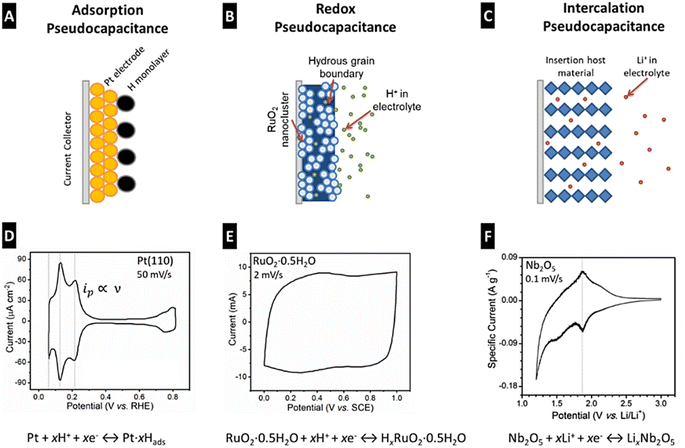 | ||
| Fig. 4 Different types of pseudo-capacitance mechanisms: adsorption (A), electrochemical (B), and intercalation (C). CV of the Pt (110) electrode in HClO4 solution at 50 mV s−1 versus SHE (D). CV of RuO2·H2O in H2SO4 at 2 mV s−1 w.r.t. SCE (E). CV of the Nb2O5 electrode in non-aqueous Li-ion electrolyte at 0.1 mV s−1 w.r.t. SCE (F). The area in the CV cover was the maximum for electrochemical type SCs [Reproduced with permission from ref. 30. A copyright of ACS, 2020]. | ||
The combination of Cdl and pseudo-capacitance enables PCs to achieve higher energy storage densities than traditional capacitors while maintaining fast charge/discharge cycles. The working principle of PCs involves the process of charging and discharging. During charging, when a voltage is applied, ions from the electrolyte are attracted to the electrode surfaces, creating a DL. Simultaneous redox reactions take place, leading to the reversible storage of charges in the form of ions at the e–i interface. This results in the accumulation of electrical energy in the PCs. During discharging, the stored charges are released, and the ions return to the electrolyte, generating a flow of electrons through an external circuit. This discharge process can occur rapidly, enabling high-power delivery when needed.
PCs offer many advantages as compared to other ESDs, which include the following: (i) high power density: PCs can deliver and store energy rapidly due to the Cdl and redox reaction chemistry, (ii) long cycle life: PCs possess excellent cycle life with minimal degradation, making them suitable for long-lasting applications, (iii) high operating temperature range: PCs can operate in a wide range of temperatures, increasing their applicability in different environments, and (iv) high EDs: PCs have higher energy storage capacities than traditional capacitors, although they generally have lower EDs as compared to rechargeable batteries.
The first RuO2 transition metal oxide-based PCs were reported by Transatti et al. in 1971.13 The high cost of RuO2 limits its large-scale production. Later, in 1999, amorphous MnO2·nH2O in neutral aqueous solution-based PCs showed better performance.26 The observed specific capacitance was 200 F g−1 in KCl solution. In the late 1980s, CP-based PCs were developed.27 The theoretical capacity of PANI-based PCs was 146 mA h g−1.31 Carbon/CP based NC electrode materials were also used in PCs to increase the theoretical capacity of PCs.38,39 In the 1980s, Li-ion intercalation was tested using organic electrolytes.40 The discovery of carbon nitrides that is Ti3C2Tx (MXenes), a new electrode material for PCs, led to an increase in the electrochemical performance of PCs.15 This choice was driven by the remarkable properties exhibited by MXenes, including exceptional electrical conductivity,41 electrochemical activity, hydrophilicity, environmentally benign nature, layered shape, large surface area, and thermal stability.42,43
PCs find applications in many fields, like energy storage systems, hybrid vehicles, renewable energy systems, portable electronics, and grid-level energy storage. They are particularly useful in situations where high power delivery and rapid charging/discharging are essential requirements.
2.3. Hybrid capacitors (HCs)
In PCs, EDLCs offer higher specific capacity while also having great periodic stability and power performance. In HCs, the hybrid system delivers a combination of both blending a power source that is an electrode that functions like a capacitor and a source of energy that is an electrode that functions like a battery in the same cell.35,36 HCs, also known as asymmetric capacitors, are a type of ESD that combines the features of both capacitors and rechargeable batteries. They are designed to provide a balance between high power density (like capacitors) and high EDs like LIBs, making them suitable for applications that require both rapid charge/discharge capabilities and long-term energy storage.By raising the cell voltage with the appropriate electrode composition, the ED and power density of HCs may be improved. In the past, many combinations of aqueous and inorganic electrolytes with positive and negative electrodes have been investigated. The faradaic electrode frequently boosts the ED at the price of cycle stability. This is the main limitation of hybrid devices contrasted to EDLCs.37
HCs consist of two main components: an EDLC electrode and a battery electrode. The EDLC electrode is typically made of a high conductivity material with a large SSA, like AC, graphene, or CNTs. The battery electrode is composed of a high-capacity material, such as an MO or a CP. A separator separates both electrodes and is exposed to an electrolyte. The working principle of HCs is based on the combination of an electrochemical DL capacitance and battery-like faradaic reactions. Similar to traditional SCs, HCs utilize the principle of Cdl. When a voltage is applied, an electrical DL forms at the e–i interface between the EDLC electrode and the electrolyte. The charge separation at this interface creates a DL capacitance, allowing for rapid charge and discharge of electrical energy. In addition to Cdl, HCs also exhibit faradaic reactions at the battery electrode. The battery electrode undergoes reversible redox reactions during the charging and discharging processes. These reactions involve the transfer of charge between the two electrodes and the electrolyte, leading to the charge storage and release of electrical energy.38 The faradaic reactions contribute to the overall energy storage capacity of the HCs. The combination of Cdl and faradaic reactions allows HCs to achieve a balance between high power density and high ED.39–41 The operation principle of HCs involves charging and discharging reactions. During charging, a voltage is applied for a fixed duration, and opposite charges are attracted to the EDLC electrodes, leading to the formation of an electrified interface. Simultaneously, faradaic reactions occur at the battery electrode, enabling the reversible storage of charges at the electrode surface. This results in the accumulation of electrical energy in the HCs. During discharging, the stored charges are released. The discharge process involves reciprocal redox reactions at the battery electrode and the release of charges from the electrified interface at the EDLC electrode. This discharge process can occur rapidly, providing high-power delivery when needed.42
HCs have many advantages as compared to traditional capacitors and rechargeable batteries, which are as follows: (i) high power density: HCs can deliver and store energy rapidly due to the combination of Cdl and faradaic reactions, (ii) high EDs: HCs provide a higher energy storage capacity compared to traditional capacitors, although typically lower than rechargeable batteries, and (iii) long cycle life: HCs exhibit excellent cycle life, with minimal degradation over multiple charge/discharge cycles. HCs find applications in many fields, including electric vehicles, renewable energy systems, portable electronics, and power backup systems. They are particularly useful in applications that need great power and energy storage for longer duration, striking a balance between the capabilities of capacitors and batteries. The present research focuses on the three primary types of hybrid SCs—composite, asymmetric, and battery-type—which may be distinguished by their electrode topologies.
2.4. Composite hybrid SCs
To generate a single electrode that can collect charges both physically and chemically, electrodes are composed of AC and MO or CP-based materials. The highly porous structure of AC imparts a Cdl of high charge and SSA to enhance the interfacial interaction between pseudo-capacitive materials and the electrolyte. By using the faradaic process, pseudo-capacitive materials increase the capacitance of the composite electrode. Two-fold and three-fold composite materials are commonly used in HCs. Whereas ternary composites utilize three distinct electrode materials, a single electrode and binary composite electrode employ two independent electrode materials. Every composite falls into a variety of size categories, such as nanometers, micrometers, sub-micrometers, etc.38Composite hybrid SCs combine the advantages of both traditional electrochemical SCs and rechargeable batteries, offering high ED and power density. Some key areas of progress in the development of composite hybrid SCs include the following: (i) material development: researchers are constantly exploring different advanced materials to improve the performance of SCs. For example, graphene and other carbon-based materials have been widely investigated due to their excellent electrical conductivity and large surface area, which will increase energy storage capacity, (ii) nanostructured electrode materials: the use of nanostructured electrode materials has shown promise in enhancing the energy storage capacity of SCs. By employing nanomaterials, such as CNTs or MO NPs, CPs, MXenes, etc., researchers have increased the surface area available for charge storage, leading to improved capacitance,39 (iii) hybridization with rechargeable batteries: combining SCs with rechargeable batteries in a hybrid configuration has gained attention as a means to achieve high EDs and power densities. This approach combines the high energy storage capacity of rechargeable batteries with the high power density and rapid charge/discharge capabilities of SCs, (iv) advanced electrolytes: electrolytes play a crucial role in the performance of hybrid SCs. Researchers have been exploring the use of novel electrolytes, such as ionic liquids or gel electrolytes, to improve the ED and stability of composite hybrid SCs, and (v) device architecture: optimizing the device architecture is another avenue of progress done in hybrid SCs. Researchers are developing innovative designs, such as asymmetric SCs or interdigitated structures, to enhance overall performance by maximizing energy and power densities. It's worth noting that the field of energy storage is actively evolving, and a lot of progress has been made in increasing the ED and the power density of composite hybrid SCs.
2.5. Asymmetric hybrid capacitors
Asymmetric HCs merge faradaic and non-faradaic reactions by coupling EDLCs with an electrode for PCs. The positive electrode is made up of CPs or MOs and the negative electrode is composed of C-based materials.412.6. Battery-type hybrid capacitors
Battery-type HCs, like asymmetric hybrids, combine two distinct electrodes. In this type of hybrid capacitor, the electrodes from the battery and the SCs are joined. The advantages of batteries and SCs were combined in one capacitor using the design shown in Fig. 2.The energy storage and release mechanism of the EDLCs occurs at the electrified interface, which is created electrochemically. The lack of chemical oxidation–reduction (redox) processes renders the non-faradaic charge storage mechanism. EDLCs possess increased cycle lifetimes since only physical charge transfer occurs during the process. PCs, on the other hand, are built using high-energy electrode materials that are composed of CPs, metal-doped C, or metal oxides. In faradaic redox reactions, these CP and C-based materials are used.39–42 These electrode materials enable the development of SCs with higher EDs. When compared to EDLCs, PCs frequently have higher EDs but also have shorter cycle lives and slower speeds. As mentioned, both EDLC and PC processes are present in the hybrid SCs.
The basic building block of an SC includes the two electrodes segregated from one another by a selectively permeable membrane acting as a separator (Fig. 2). The electrodes and separator are impregnated with an electrolyte solution, which permits ionic current to flow between them while obstructing electronic current from the cell. Current collectors receive current from the electrodes. The same equations that govern regular capacitors also apply to SCs, and the capacitance may be computed from the conventional capacitance, which would be calculated using eqn (1).
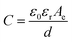 | (1) |
The SCs may be made from diverse materials depending on the type of energy storage required for the task and the necessary capacitance ranges. The major commercial material for SCs is carbon, which is widely used and has a wide range of potential transformations. There is currently a huge variety of materials suitable for SCs. MO like Ni, Co, Mn, and Ru are commonly used. In recent years, the usage of CNTs and CPs in SCs has increased (Fig. 5). SCs are significantly more advantageous than rechargeable batteries because of the novel materials used in the fabrication of electrode materials (Table 1).43,44 Table 1 shows the advantages and disadvantages of SCs and other ESDs.
| Types | Benefits | Drawbacks | References |
|---|---|---|---|
| SCs | (i) High power density | (i) Lesser EDs | 39–42 |
| (ii) Extended cycle life | |||
| (iii) Quick charging speed | (ii) Spontaneous discharge | ||
| (iv) Effective performance at low-temperature | |||
| (v) Efficient charging and discharging circuit | |||
| LIBs | (i) Enlarged-working platform | (i) The cost of Li metal is high due to the relatively lower abundance | 45 and 46 |
| (ii) High EDs | |||
| (iii) Extended periodic stability | (ii) Overcharging and over-discharging line management are required | ||
| (iv) Efficient charging and discharging | |||
| (v) Good safety at room temperature, zero carbon emission | |||
| (vi) Can perform at a wide temperature range | |||
| Lithium sulfur batteries (LSBs) | (i) Higher EDs | (i) Low-performance rate | 47 and 48 |
| (ii) High theoretical capacity | |||
| (iii) Less toxic and environmental preservation | (ii) Poor Coulomb efficiency poorly stable cycles | ||
| (iv) Good charge–discharge efficiency | |||
| (v) Log cycle life | |||
| Na-ion batteries (SIBs) | (i) EDs comparable to LIBs | (i) Compared to LIBs, SIBs have a slow charge–discharge rate | 172 |
| (ii) Better stability | (ii) Large size and weight compared to LIBs | ||
| (iii) Better safety records | (iii) Low EDs compared to LIBs | ||
| (iv) Extended service life | |||
| (v) Easy access to raw materials | (iv) SIBs require different anode material as compared to carbon in LIBs | ||
| (vi) The ability of SIBs to discharge to 0 V due to lack of over-discharge characteristics |
Nonetheless, SCs provide the required instant current and minimize the battery current in the event of transitory power interruption. The SCs may act as a complement to the present battery technology. To compensate for transitory and momentary disturbances, large battery units can be used parallel to electrochemical SCs. This would considerably lessen the unnecessary strain placed on batteries from frequent interruptions.49 Recent SC research has exclusively focused on figuring out how to preserve high power density, quick charge/discharge, and cycle stability while increasing EDs.50,51
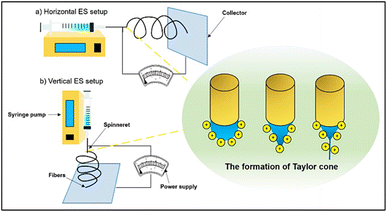 | ||
| Fig. 6 Setup of the ES system: (a) parallel (horizontal) setup and (b) perpendicular (vertical) setup [Reproduced with permission from ref. 55. A copyright of Elsevier, 2019]. | ||
The primary factors that affect the morphology of the ES fibers are the tip-collector distance, applied voltage, flow velocity, and the polymeric solution's viscosity.56 Additionally, the assembly of the fibers may be accomplished by utilizing a variety of collector types. For instance, static collectors are used to construct fibers with a web-like structural morphology, whereas cylindrical spinning drum collectors are used to create non-woven fibers.57,58
CNFs can be created mainly within two phases, starting with stabilization and ending with carbonization. To stabilize the ES NFs, they are first put in a tube furnace and heated between 250 and 300 °C. The polymer is now transformed into a compound with a double-stranded polymer with the connectivity of a ladder, which aids in maintaining the fibrous structural morphology at elevated temperatures. The stabilized fibers are next turned into CNFs that resemble graphite by carbonization in an inactive environment at a temperature exceeding 505 °C, as seen in Fig. 7. Fig. 7 shows the variation in structural morphology of poly-acrylonitrile (PAN) to CNFs. Additionally, the majority of non-carbon components (H2O, NH3, HCN, CO2, N2, and CO) are removed along this procedure,71 which reduces the diameter dimensions of CNFs and increases accessible surface area and hence conductivity also gets increased. Furthermore, the creation of a porous structural morphology in CNFs, i.e., microporous (<2 nm in size), mesoporous (2–50 nm), and macroporous (>50 nm) has been extensively researched for flexible SCs (FSCs). Feng and his team observed that micro-porous CNFs (2.0 nm) can boost charge transfer resistance because they partially stop electrolytes from entering the microporous electrode, even during charging/discharging.72 As they have higher electrochemical performance, mesoporous (2.0–50 nm) CNFs are favorable to alleviate this ionic diffusion.73,74 Intriguingly, micro/mesoporous CNFs produced from PAN-based fibers, a silica pattern, and potassium hydroxide treatment have SSAs up to 1796 m2 g−1, which promotes the quick passage and diffusion of electroactive species while displaying a large electrical capacity of 198 F g−1.75 Although their capacitance has grown, a study has been done to control the void size within porous CNFs for better electrochemical performance in SCs.76
3. Electrode materials
SCs have two properties, specific capacitance, and charge-storage density, which are affected by the composition of electrode materials used.3.1. Carbon materials
All types of carbon compounds are used as electrode materials while making SCs. Large surface area, low price, accessibility, and well-established electrode production technology are the few reasons for using C-based materials in SCs.78 C-based materials use an electrochemical DL storage system, which forms at the electrolyte/electrode interface. Thus, the capacitance mostly depends on the area of the surface where electrolyte ions come into contact with the electrode. Electrochemical performance is significantly influenced by SSA, pore structure, shape and size distribution of pores, functional groups at the surface, and electrical properties.79–81 In particular, when it comes to carbon materials, they have large SSA, which enhances the capacity for the charge to build up at the electrode–electrolyte contact. When boosting specific capacitance for carbon materials, surface functionalization must take into consideration the pore size and large SSA. A few examples of carbon compounds used in fabricating the electrode materials of SCs include charcoal filters, carbon aerogels, CNTs, graphene, and other forms of carbon.Different studies have assessed this connection, and there appears to be an imbalance between specific capacitance and a particular surface area of AC. A comparatively low capacitance was achieved with a high SSA of around 3000 m2 g−1.86 This demonstrates that not all pores are productive while charges are accumulating. Therefore, even if the SSA in EDLC is a key performance parameter, other factors are also taken into account in carbon materials, such as pore size distribution, which has a significant impact on electrochemical performance.87 Moreover, over-activation results in a high pore volume, which has limitations, i.e., small conductivity and density, which causes low EDs and a decrease in power capacity.
An attempt was made to ascertain how different electrolytes affect the functionality of AC in affecting the capacitance of SCs. It was found that aqueous electrolytes present between AC-based electrodes have a higher capacitance (ranging from 100 F g−1 to 300 F g−1) than organic electrolytes (150 F g−1).88 Cooked chicken bone waste along with KOH electrolyte was used as an electrode material for SCs. The chicken bone-based SCs show a specific energy of 11.3 W h kg−1 and a specific power of 8.50 kW kg−1.89
As opposed to AC, CNTs often have a tiny SSA (500 m2 g−1 or less), which results in lower EDs. To increase CNT's specific capacitance, KOH can be chemically used as an activator. The aforementioned technique can significantly enhance CNTs' surface area (by a two- to three-fold factor) while preserving their nano-tubular architecture.83
CNTs have been extensively investigated as electrode materials for SCs due to their unique properties, including high surface area, excellent electrical conductivity, and mechanical strength. To increase the performance of CNT-based SCs, researchers have developed NCs by incorporating CNTs with other materials. Here are some examples of NCs used as electrode materials in CNT-based SCs: (i) CNTs/MO NCs: MOs such as MnO2, RuO2, and NiO have been combined with CNTs to form NC-based electrode materials of SCs.88–94 These materials provide additional pseudocapacitance, which significantly increases the energy storage capacity of SCs. The combination of CNTs with MO can be achieved through various methods, including electrodeposition, CVD, or hydrothermal synthesis, (ii) CNTs/CPs NCs: CPs, such as PPy, PANI, and poly(3,4-ethylene dioxythiophene), have been integrated with CNTs to create NC based electrode materials of SCs. The CPs contribute to the overall capacitance of the SCs, while CNTs provide enhanced electrical conductivity and mechanical stability. These NCs can be synthesized through methods such as in situ polymerization or electrochemical deposition, (iii) CNTs/graphene NCs: graphene, another carbon-based material, has been combined with CNTs to form NC-based electrode materials of SCs. The combination of these two-dimensional carbon materials offers a large surface area, high electrical conductivity, and excellent mechanical properties. The NC structure facilitates charge transfer and improves the overall performance of the SCs, and (iv) CNTs/metal NCs: metal NPs, such as Au, Ag, and Pt, have been incorporated into CNTs to fabricate NCs. The presence of metal NPs enhances the electrochemical activity of the electrode, leading to increased energy storage capacity. The synthesis of CNTs/metal NCs can be achieved through methods such as chemical reduction or electrodeposition. The specific choice of NC-based electrode materials in SCs depends on the desired properties and performance requirements of the SCs.
CVD, arc discharge, micro-mechanical exfoliation,104 unfolding CNTs, chemical and electrochemical processes, and intercalation of carbon–graphite are just a few of the procedures that may be used to produce different types of graphene.105–107 The high intrinsic surface capacitance and SSA of single-layer graphene were utilized in the fabrication of graphene-based electrode materials for SCs. Efforts were made to prevent graphene sheets from stacking on one another during the synthesis of graphene along with electrode production. Blended graphene sheets were developed to achieve high efficiency and prevent face-to-face stacking. An ED of 85.6 W h kg−1 was achieved at standard temperature and 136 W h kg−1 at 80 °C with the current at 1.0 A g−1. The reported EDs are similar to that of the Ni–H battery.97 Aqueous and organic electrolytes were investigated with chemically modified graphene (CMG), and electrical capacities up to 135 and 99 F g−1 were produced.4 The results are better than carbon-based SC electrodes. For example, when the potential is 1.0 volt and an aqueous electrolyte is present, the graphene material used in SC electrodes produces a better capacitance of 205 F g−1 with an ED of 28.5 W h kg−1. A single-layer graphene oxide (GO) sheet was depleted in the experiment using gas-based hydrazine at ambient temperature. The graphene produced by this method is less agglomerated than graphene manufactured using an aqueous solution-based synthesis of chemically altered graphene.108 Three distinct approaches were investigated to discover better capacitance by employing graphene as the electrode material for SCs. The first approach used thermally induced graphitic oxide exfoliation, the second involved heating nano-diamonds to a temperature of 1650 °C in a helium environment to produce graphene, and the third involved decomposing camphor over nickel NPs. The graphitic oxide was thermally exfoliated to provide an excellent specific capacity of 118 F g−1 and an ED of 32 W h kg−1.109 It is essential to create SCs with significant capacitance and fast charging at high current densities since high-power applications are the main application of SCs.
A modified Hummers technique and tip sonication method were used to synthesize graphene. Graphene is chemically exfoliated at high temperatures. A novel approach was studied which uses C-precursors to exfoliate graphene owing to unique chemistry taking place at the surface revealed by a low-temperature exfoliation method. The resulting capacitance is greater than that of high-temperature exfoliated graphene.110,111 The problem of agglomeration and restacking that graphene faces can be minimized in different ways. Graphite oxide was thermally reduced at a high temperature, and then quickly cooled with liquid nitrogen to create sheets of graphene with a high degree of exfoliation. A better specific capacitance of 349 F g−1 was achieved. Problems caused by graphene-based electrodes can be improved by using graphene oxide-based NCs.112
To conclude, constant efforts are being made to improve the conductivity of SC electrodes by incorporating graphene and its derivatives. Graphene's high electrical conductivity helps in achieving better charge–discharge rates. Attempts were also made towards the development of hybrid materials for hybrid structure electrodes by combining graphene with other materials, such as MO or CPs, which have been explored to optimize energy storage and increase specific capacitance. CNTs are often used in composite electrode materials when used with other materials to increase SC performance. CNT–CP composites, CNT–MO composites, and CNT–graphene composites have been tested for improving the conductivity and capacitance of SCs.
Techniques for aligning CNTs in the electrode structure were developed to maximize the utilization of CNTs' unique properties, such as their high aspect ratio. Porous structures of AC were also utilized towards the development of hybrid electrode materials for SCs. Researchers are focusing on tailoring the pore structure of AC to optimize ion transport and increase surface area. Hierarchically structured AC with micro, meso, and macro-pores has been thoroughly investigated.
New methods of chemical activation have been explored to create AC with improved performance characteristics, such as higher specific capacitance and better charge–discharge rates. Additional manufacturing techniques, such as 3D printing, have been applied to create complex electrode structures with improved performance and design flexibility. Advances in nanomaterial synthesis and integration techniques have enabled the development of electrodes with precisely controlled nanoscale features, leading to enhanced performance.
The use of ionic liquids as advanced electrolytes has gained attention for improving the ED and stability of SCs. Researchers have also explored the use of pseudo-capacitive electrolytes to increase the overall capacitance and ED of SCs. Researchers continue to work on increasing the ED of SCs to bridge the gap between traditional SCs and batteries. Efforts are also being made to enhance the durability and long-term stability of SCs for various applications.
3.2. MO NF-based SCs
MO-NFs have received great attention for usage as PC electrodes in the development of novel high-performance ESDs due to their better specific energy when compared to EDLCs.113,114 Certain MO-NFs, such as Co3O4, RuO2, V2O5, MnO2, and NiO, are frequently employed in SCs due to their high specific capacitance.115–120 Few important MO-NF-based SCs include RuO NFs, MnO NFs, NiO NFs, CoO NFs, CoO NFs, and VO NFs.MO NPs are often employed as electrode materials in SCs due to their unique properties, which make them suitable for energy storage applications. The electrochemical reactions in SCs involve the storage of electrical energy through the reversible adsorption and desorption of ions at the electrode–electrolyte interface.113–115 These MO NPs participate in the electrode reactions in different ways. (i) MO NPs have a high surface area and are characterized by a large number of active sites. The high surface area is crucial for enhancing the electrode–electrolyte interface, promoting more significant ion adsorption, and thereby increasing the capacitance of the SCs.116 (ii) During the charging process, MO NPs undergo oxidation, and metal ions within the oxide matrix release electrons. Simultaneously, cations from the electrolyte, typically in the form of an electrolyte solution, are adsorbed onto the surface of the MO NPs. This process is reversible during the discharge cycle. (iii) SCs operate based on two main mechanisms: double-layer capacitance and pseudocapacitance. MO NPs contribute to both. (iv) Double-layer capacitance can occur at the interface between the electrode and the electrolyte. The high surface area of MO NPs facilitates the formation of an electric double layer, where charged ions are attracted to the charged surface of the NPs.117 (v) Some MO, especially transition MO like RuO or MnO, exhibit pseudocapacitance. This involves fast and reversible faradaic redox reactions at the surface of the metal oxide nanoparticles. The MO NPs store charge through the reversible electrochemical reaction of the metal ions with the electrolyte.118 (vi) The stability of MO NPs during repeated charge/discharge cycles is essential for the longevity of the SCs. Some metal oxides may undergo structural changes during cycling, affecting their performance over time. Researchers often focus on designing MO NP structures that can withstand numerous cycles without significant degradation.119
MO NPs in SC electrodes participate in electrochemical reactions by providing a high surface area for ion adsorption, promoting double-layer capacitance, and, in some cases, contributing to pseudocapacitance through reversible faradaic redox reactions. The specific properties of the MO and the electrode design play a crucial role in determining the performance characteristics of the SCs.
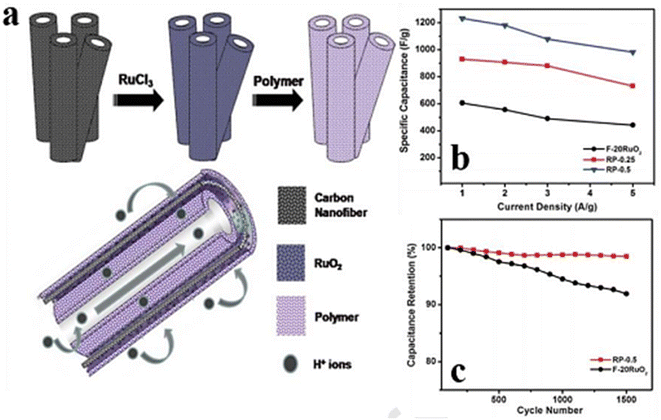 | ||
| Fig. 8 (a) Steps for the production of double-walled CNFs modified with ruthenium dioxide and poly(benzimidazole). (b) Specific capacitance of F-20 RuO2, RP-0.25, and RP-0.5 at different current densities from 1.0 to 5.0 A g−1, and (c) electrochemical action of the materials RP-(0.5) and F-(20) RuO2 in terms of stability for 1500 cycles when the amount of current is 1.0 A g−1.125 [Reproduced with permission from ref. 125. A copyright of RSC, 2013]. | ||
Two redox peaks in the Cyclic Voltammetry (CV) of RuOx/GNF were observed as a result of the faradaic behavior of RuOx, showing appreciable capacitance. By adding a sacrificial material that will degrade throughout the carbonization process, one can make nano-structured cavities in the carbon polymeric network. Kim et al.125 described the ES of two copolymers, poly methyl methacrylate (PMMA), and polyacrylonitrile (PAN), accompanied by a chemical activation method, to produce RuO2 and high-mesopore-activated CNFs that is RuPM-ACNFs. The optimum PAN@PMMA mix ratio (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) produced the largest energy output of 24 W h kg−1 at a power rate of 410 W kg−1 with a small semi-circle (Rct = 1.07), which was mostly due to the presence of mesoporous RuPM that may operate as binding sites for rapid ion transportation. A lot of interest has also arisen in ternary hybrid composites based on RuO2 for application in high-performance SCs.
30) produced the largest energy output of 24 W h kg−1 at a power rate of 410 W kg−1 with a small semi-circle (Rct = 1.07), which was mostly due to the presence of mesoporous RuPM that may operate as binding sites for rapid ion transportation. A lot of interest has also arisen in ternary hybrid composites based on RuO2 for application in high-performance SCs.
Balan and his coworkers126 used double-walled CNFs of RuO2 poly-benzimidazole (PBI), abbreviated as CNFs-RuO2-PBI (RP) composite, to achieve a better maximum specific capacitance of 1060 F g−1 when the scan rate was 20 mV s−1 over a voltage of 1.3 V for RP-1 (Fig. 9). Fig. 9 shows the design for the preparation of the MnO2/PCNFs composite, and (b) SEM of the MnO2/PCNFs composite. By applying a modified polyol technique to embed RuO2 NPs on functionalized porous CNFs (F-CNFs), this ternary hybrid electrode was developed. In the next step, phosphoric-acid-doped PBI was tested. The RuO2 NPs were evenly covered with a very thin layer of H3PO4 doped PBI without aggregation, as seen in the TEM pictures (Fig. 10). As a result, the resistance caused by charge transport at the electrode–electrolyte interface was decreased. However, creating a ternary composite electrode material requires a lot of time and effort, thus being inappropriate for mass production. As a result, Kim et al.125 suggested ES and activation as a quick and easy way to prepare RuO2/ACNF. It's interesting to note that the electrode that was already constructed (20% RuO2 concentration) had a better electrical conductivity (4.8 S cm−1) and a big SSA (678 m2 g−1), which produced an excellent electrical capacity of 535 F g−1 corresponding to 22 mV s−1 scans per seconds.
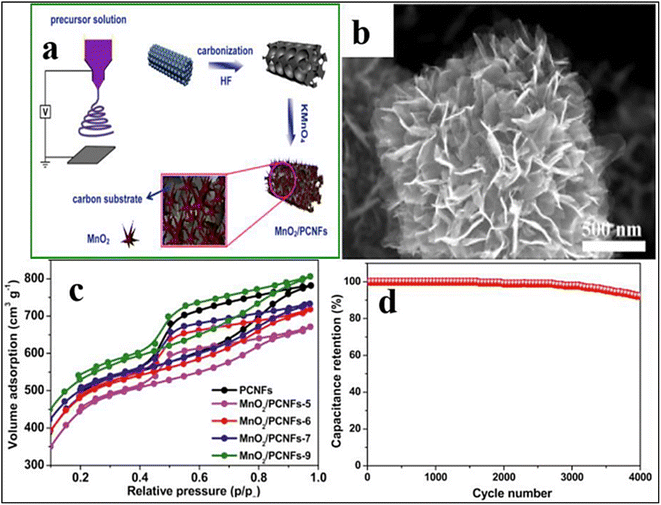 | ||
| Fig. 9 (A) Design for the preparation of the MnO2/PCNF composite, (b) SEM of the MnO2/PCNF composite, (c) isotherm for nitrogen adsorption and desorption of PCNFs and MnO2/PCNF composite, and (d) cycling stability of MnO2/PCNFs as an electrode over 4.0 × 103 cycles at a current of 0.5 A g−1 [Reproduced with permission from ref. 55. A copyright of Elsevier, 2019]. | ||
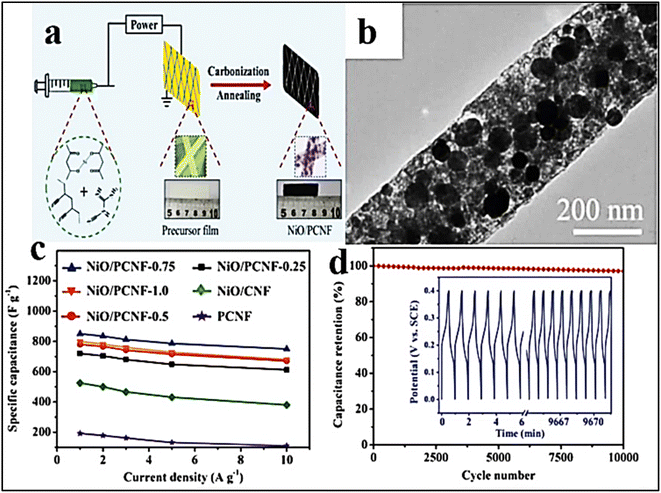 | ||
Fig. 10 (a) Fabrication process of the composite made of NiO and PCNF, (b) TEM surface morphology of NiO/PCNF-(0.75), (c) electrical capacity of NiO/PCNF at a composition of 0.25, 0.5, 0.75, and 1.0 against current density (A g−1), and (d) NiO/PCNF-(0.75) cycling stability at a 10 A g−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles [Reproduced with permission from ref. 157. A copyright of Wiley, 2018]. 000 cycles [Reproduced with permission from ref. 157. A copyright of Wiley, 2018]. | ||
Since the electrolyte ion may permeate through the MnO2 shell in a short (10 nm) distance, mesoporous MnO2 core–shells covered on the perpendicular alignment of CNFs (MnO2/CNFs) may offer a larger capacitive performance of 473 F g−1.135 Furthermore, Klankowski et al.136 and Wen et al.137 have researched the creation of CNFs using degradable, inexpensive bamboo chopsticks. The created carbon-fiber sheets (CFSs) were electrodeposited with MnO2 NPs (20–50 nm), which resulted in materials with impressive constant capacitance (376 F g−1 at 1.0 A g−1), amazing EDs (11.0 W h kg−1 at 530 W h g−1), and better long-term periodic stability (99.4% performance was preserved even after 5000 cycles). The synergistic effects of the faradaic charge storage mechanism of EDLC, the enlarged electro-active sites, and the migration of the electrolyte ion contribute to the outstanding electrochemical activities of CFs/MnO2. Core–shell fibers have been thoroughly studied to achieve a high electrochemical activity in hybrid SCs.137 For example, PAN solution and PAN–Mn(NO3)2–PAN mixture were employed as the core and shell solutions, respectively, when C/MnO2 NC fibres (C/MnOx) were synthesized utilizing the core–shell ES technique by Pech and Maensiri.138 Different MnO2-based nanostructured materials possess different applications, especially in ESDs. The carbon/MnOx-based composite SCs show the highest specific capacitance of 213.6 F g−1 at 0.5 A g−1 current density, energy density of 30 mW h g−1, and power density of 249 mW g−1.138
The C/MnOx hybrid was able to acquire a high SSA, which in turn increased the electrical capacity to 214 F g−1 in 6.0 M KOH by using EDLC and pseudo-capacitance of carbon and MnOx. Zhou et al.139 used a one-step ES process followed by in situ deposition to produce the MnO2 nanoflakes/porous CNFs (MnO2/PCNFs) as depicted in Fig. 8. SEM imaging shows that manganese oxide nanoflakes grow vertically, forming a sizable lateral area between manganese oxide and the electrolyte ions on the outermost layer of PCNFs. The MnO2/PCNF nanoflakes were highly mesoporous and had a high SSA (1815 m2 g−1), which allowed them to retain their initial capacitance even after 4000 cycles. According to the electrochemical impedance spectroscopy (EIS) experiments,140 the greater charge resistance of MnO2/PCNFs compared to PCNFs was caused by the electrode's increased induction of high electronic conductivity. To rationally create hierarchical hollow MnO2 NFs, Xu et al.141 used the hydrothermal technique. The hollow MnO2 NFs have an SSA of 195 m2 g−1 and an average mesopore size that is similar to 4.0 nm, according to Brunauer–Emmett–Teller (BET) measurements, offering a sizable number of active sites for electron transport. Also, they show adequate retention; the electrical capacity of MnO2 NFs could be maintained between 290 and 214 F g−1 when the amount of current per gram was in the range of 1.0 and 10.0 A g−1.
For optimal capacitive nature and reversibility, galvanostatic charge–discharge arcs are fabricated in triangular and uniform shapes. Lately, it has been investigated how dopants like boron, which have a pseudo-capacitive effect, might enhance carbon materials' electrochemical performance. Yang and Kim142 used a one-step ES and carbonization process to hybridize MnO2 with boron as a dopant to PAN@pitch based-CNFs (PPBMn). It demonstrates how a huge amount of electroactive spots for redox activities are made possible by the low-impedance electron transport channels found in boron and MnO2. The PPBMn shows a high ED of 25.67 W h kg−1 and a better electrical capacity of 209 F g−1. Chi et al. used boron as a dopant in MnO2 crystallites with a possible bonding with hydrogen, producing a high specific capacity of 365 F g−1 at a scan rate of 2 mV s−1, which is much greater than that of electrodes that are not doped (306 F g−1).143
Furthermore, the interior structure of boron-doped MnO2 contains Mn2+, which results in low cycling stability (80.5% after 9000 cycles). The combined usage of materials in EDLC with NFs based on MnO2 overcomes the drawbacks of pseudo-capacitive materials. Encouraging high electrical conductivity, it boosts the number of electroactive locations for immediate ion diffusion. Lee and Kim used ES and thermal processing to generate SCs utilizing MnO2/HPCNFs/G (MnO2/hierarchical porous CNFs/graphene).144 As evidenced by the exceptional electrochemical properties, the HPCNFs/consistent graphitic MnO2 pattern, which might lower the charge transport barrier, is strongly supported in this category by the strong rate capability attributes and high specific power of graphene. Even at high scan speeds (10–100 mV s−1), MnO2/HPCNFs/G retained the quasi-rectangular CV shape, resulting in an improved consistent capacitance of 212 F g−1 and strong electrochemical reversibility. Lee et al. have fabricated MnO2/porpus carbon fibre and graphene based composite electrode material for SCs.144 The SCs electrode with a concentration of 5 wt. percent graphene gives a better specific capacitance (210 F g−1), good rate capability, and high energy density (24 W h kg−1) in an electrolyte of 6.0 M KOH solution.
According to Śliwak and Grygleweicz, the counter electrode should be a high voltage non-symmetric SC based on AC, and the positive electrode should be MnO2/oxidized CNFs.145 They demonstrated high-efficiency and stable performance (94%) after 5000 cycles when the electrical power was at 100 W kg−1 with an ED of 25 W h kg−1 in the potential range 0–2.2 V. After 1.0 × 103 charge/discharge cycles at 2.0 V, the efficiency is still above 97% when the positive electrode is formed of reduced graphene oxide (rGO) CNFs and Mn2CO3, and the negative electrode is likewise comprised of rGO in 1.0 M Na2SO4. δ-MnO2 NFs and SWCNTs were produced by Wang et al. utilizing vacuum filtering.146 The real capacitance rose to 965 mF cm−2, which is greater than that of SWCNTs and δ-MnO2 films when used with PVA and KOH as the electrolytes. The findings explain why SWCNTs are superior to other materials. They also help to explain why δ-MnO2/SWCNT hybrid electrodes have enlarged surface area and an improved electrical conductivity of up to 81.2 S cm−1 (Fig. 9).
Yang et al. proposed the direct thermal decomposition of Metal–Organic Frameworks (MOFs) consisting of Ni and Zn for the in situ production of diluted NiO–CNFs.147 The covalent bonding of organic molecules and metal ions in MOFs improves the electrochemical properties and the final product's porosity.155 Only 28% of the specific capacitance was depleted from its starting capacitance even at high scan rates, because of the mesoporous structure of NiO/CNFs MOFs. This results in a good rate capability of SCs. Moreover, a significant quantity of NiO was distributed across the surface of CNFs, increasing the energy output of SCs to 33.5 W h kg−1 with outstanding rate capability and minimal internal resistance. The potential drop observed was small throughout the system.
Zhang et al. used NiO sheets to make hollow tube NFs using the ES process using citric acid.148 Because of their larger surface area and potential electrocapacitive properties, the NCs synthesized from NiO/citric acid exhibit high electrical capacity, i.e., 338 F g−1 compared with NiO alone. Furthermore, due to the short electron diffusion paths produced by the development of a hollow structure, a minimal charge transfer resistance was observed, i.e., 0.25 Ω. NiO has a very low intrinsic conductivity, which is considered a drawback that is rectified by synthesizing a porous NiO/citric acid structure to enhance the reactive surface area. Wang et al. have reported metal organic framework based 3D nanostructures for hybrid supercapacitors.154
Zang et al. have synthesized NiO NFs using a hydrothermal method followed by calcination. Because of their unique surface morphology and quick electron transport during the faradaic process, they showed a capacitive performance of 885 F g−1 with 12% capacitance fading even after 1000 cycles.155 Kundu and Liu156 developed an easy method to speed up the formation of porous NiO NFs on a nickel foam by the ES process. This simple process yields a 738 F g−1 specific capacitance and exceptional stability performance even after 8000 cycles. With the help of active surface sites, the Ni foam was successfully bonded to the porous structure of NiO-NFs, which increased the rate of ion diffusion and enhanced capacitive performance.
Li et al. synthesized porous Ni-doped CNFs (PNCNFs) with NiO NPs scattered throughout the framework, which had a better synergistic impact over the NiO NPs and PNCNFs and significantly increased specific capacity to 852 F g−1 in 6.0 M KOH electrolyte.157 Due to the breakdown of dicyandiamide (DCDA), which aids in the uniform dispersion of NiO NPs across the whole framework, different gases (CO, CO2, H2O, and NH3) evolved throughout the entire nano framework. The NiO NPs do not naturally agglomerate. As a result of the creation of pores on the PNCNF surfaces and the presence of non-aggregated NiO, non-breakable charge transport channels may be induced, which improves stability performance by reducing ion diffusion resistance. Additionally, this approach demonstrates that as the Ni concentration on the surface of PCNFs grows, rate capacity improves, leading to an improvement in cycle stability of 97% after 1.0 × 103 cycles (Fig. 10). Fig. 10 shows the fabrication method of NiO and PCNF based electrode materials for SCs. TEM images of electrode materials were shown (Fig. 10b). The variation of specific capacity (F g−1) was observed against current (A g−1) for different types of composite electrodes (Fig. 10c). The specific capacity was found to be higher for the NiO/PCNF-0.75 composite-based electrode material in all current density measurements. The capacity retention power of the NiO/PCNF-0.75 composite-based electrode material was observed after different cycle numbers (Fig. 10d). It was observed that the capacity retention of the NiO/PCNF-0.75 composite-based electrode material was more than 80% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
Utilizing the thermal breakdown of MO and the annealing of vapour-grown CNFs (VGCNFs), Kim et al. created Mn/Co binary oxide NFs.164 VGCNFs have multi-dimensional pathways for electron transfer throughout the system and further minimize the separation between the CoMnO2 electrode and bulky electrolyte. This system produces specific capacitance up to 630 F g−1 at a scan rate of 5 mV s−1. When compared to other materials, Ni froth and CoMnO2/VGCNFs demonstrate greater capacitance efficiency, keeping 95% of their original capacitance even after 1.0 × 103 cycles. 1D spinel CoMnO2 porous NFs were also produced at a low cost for solid-state SCs using the one-step ES approach.165 Additionally, certain holes or gaps that were created in between spherical CoMnO2 particles might serve as an ion buffering resource. This special characteristic of CoMnO2 particles results in a reduced diffusion resistance of up to 6.11 ohm and a good specific energy density of 75 W h kg−1 with a power density of 2000 W kg−1.165
Utilizing GO with a larger surface area improves capacitance and conductivity, which solves the issue of capacity fading at higher cycles. V2O5/CNFs were synthesized by Kim et al.169 utilizing the carbonization and ES techniques. The V2O5 utilized in this procedure was used in weight percentages of 5, 10, and 20%. They investigated several V2O5 weight percentages to determine which weight % was best for the CNFC microporous structure. Finally, they concluded that V2O5-20 is the material that works well in the microporous structure of CNF–Cs and produces great results, such as specific capacitance up to 152 F g−1 and impressive ED of 19 W h kg−1 (because of the enlarged area of the region between CNFCs and V2O5 (20%)). Multichannel CNFs with amorphous V2O5 doped into their microporous structure have been found by Huang et al. to provide a higher specific capacity of 745 F g−1 with a current density of 0.5 A g−1.170 An improved relationship between V2O5 high capacitance and enlarged surface area can provide SCs with outstanding electrochemical characteristics.
The correlation between V2O5 in the multichannel CNF microporous structure increases the region where the electrode and electrolyte interact, which lowers the charge transfer resistance by up to 0.25 Ω. Additionally, due to their numerous oxidation states in the same framework, researchers are currently concentrating on generating mixed transition MO NF composites (binary spinel) to improve specific capacitance and achieve higher efficacy in the stability performance of SCs.170 For instance, the NCs made from V2O5 and CuxO (CuxO/V2O5) by varying the weight fraction of the vanadium and copper precursors exhibit much higher performance and specific capacitance than either CuxO or V2O5 alone.171 Large interfacial area in the NCs created from V2O5 and CuxO precursors reduces charge transfer resistance by 0.2 Ω. Additionally, it increases the charge capacity of bimetallic oxides.171
To conclude, different transition MO, including RuO2, MnO2, and Co3O4, have been extensively studied for their high specific capacitance and electrochemical stability of SCs. Researchers have designed MO NPs with controlled sizes, shapes, and surface structures to optimize their electrochemical properties. NPs with high SSA and porosity are desirable for improving capacitance. Hybrid electrode materials that combine MO NPs with carbonaceous materials, such as graphene, CNTs, or AC, were developed to increase the capacitance and electrical conductivity of SCs. Doping MO NPs with other elements or ions can improve their electrochemical performance. For example, nitrogen-doped MOs can exhibit enhanced capacitance due to the improved electronic conductivity and altered surface chemistry of the composite electrode material of SCs. Atomic layer deposition has been employed to deposit MO NPs with precise control over thickness and uniformity. This technique enables the creation of tailored electrode materials with enhanced electrochemical properties.
Researchers have been working on strategies to increase the ED of SCs using MO NPs. This includes optimizing the design of asymmetric SCs and exploring novel materials for higher voltage operation. Continuous efforts have been made to improve the long-term stability and cycle life of MO-based SCs by mitigating issues like particle aggregation, dissolution, and structural degradation during charge–discharge cycles.
Exploration of alternative MO NPs beyond the commonly studied ones, such as V2O5, WO3, and NiO, for their potential in SC applications is essentially required. Sustainable and environmentally friendly synthesis methods for MO NPs have been a focus of research to reduce the environmental impact of SC uses and production. MO-based SCs have been integrated into various electronic and ESDs, such as wearable electronics, energy harvesters, and hybrid energy storage systems.
3.3. CP-based SCs
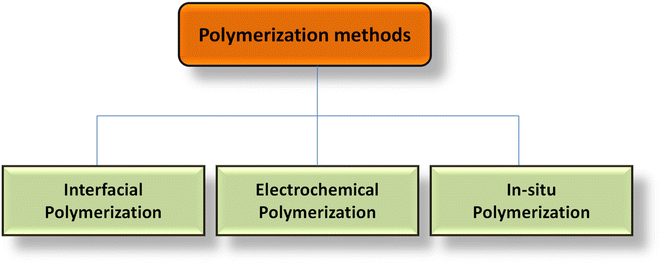
Particularly in the case of PCs, CPs are regarded as an outstanding electrode material for SCs. At the surface and across the electrode, they exhibit reversible redox behavior that allows them to retain charges. Conjugate double bonds with free electrons that are delocalized throughout the whole structure are necessary for CPs.172,173 This gives CPs the ability to conduct heat and electricity. p-Doping or n-doping can increase the conductivity of the polymers. Doping decreases the distance between the conduction band (CB) and the valence band (VB), allowing electrons to travel more freely inside the system in a larger sense; however, this also includes redox polymers containing redox centers, polymeric electrolytes, or polyelectrolytes. Redox polymers and CPs possess different methods for transferring electrons despite having different structural configurations.174For intrinsic CPs, sometimes referred to as ICPs, the conduction process is carried out by the movement of the electronic charge from end-to-end conjugation.175,176 The hopping process may occur between both the chains, and any flaws if present retard the conduction of electrons.177 PANI, PPy, polyacetylene (PA), PTh, etc. are a few examples of CPs. These CPs are used to make SC electrodes, which have several benefits including strong conductivity, flexibility, affordability, and ease of synthesis. Furthermore, several investigators have observed the electrochemical properties of CPs and their NCs as electrodes and have explored numerous techniques to enhance their characteristics.178–186 Table 2 shows different types of CPs, their structures, energy gap, and electrical conductivity. CPs are synthesized by the chemical oxidation technique, electrochemical polarization technique, vapor phase polymerization techniques, template polymerization, solid state polymerization, microwave-assisted polymerization, in situ polymerization, and many more. Fig. 11 shows different polarization methods for fabricating CP-based electrode materials.
| Types of CPs | Structures | Energy gap (eV) | Conductivity (S cm−1) | References |
|---|---|---|---|---|
| Polyaniline | 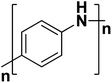 |
3.2 | 300–200 | 180–186 |
| Polypyrrole | 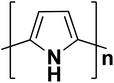 |
3.1 | 103–1.7 × 105 | 102–105 |
| Polyacetylene | 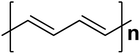 |
1.5 | 102–1.7 × 103 | 172–174 |
| Polythiophene | 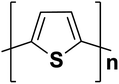 |
2.0 | 10 × 103 | 219–223 |
| Poly(p-phenylene vinylene) | 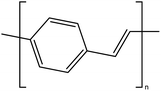 |
2.5 | 3.5 × 103 | 175 and 176 |
| P3HT | 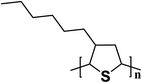 |
2.3 | 6.675 × 10−5–0.34 | 177 |
| Polyphenylene | 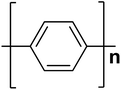 |
3.0 | 102 × 103 | 17 |
CPs are generally used as electrode materials in SCs due to their unique electrical and electrochemical properties. The diffusion and absorption properties of CPs play a significant role in determining the efficiency of SCs.180–182 These adsorption and diffusion properties of CPs influence the application of SCs in one or another way. CPs have a porous structure that allows ions from the electrolyte to diffuse and penetrate the bulk of the CPs during the charging and discharging processes. This high ion diffusion capability is crucial for achieving high capacitance and fast charge/discharge rates.183 CP electrodes usually have a large specific surface area, which enhances the electrode–electrolyte interface and promotes efficient ion diffusion. The higher the surface area, the more ions can be stored, contributing to the overall capacitance of the SCs. CPs can absorb and hold a significant number of electrolytes within their structure. This absorption capacity contributes to the increase in the overall charge storage capacity of the SCs. Some CPs exhibit swelling/shrinking behavior during the redox processes associated with charging and discharging. This reversible swelling allows the CPs to accommodate the volume changes associated with ion absorption and desorption, contributing to the longevity and stability of the SCs.184,185
The efficient diffusion and absorption properties of CPs contribute to high capacitance values. This means that the SCs can store a large amount of electrical energy per unit mass or volume, making them more efficient in terms of energy storage. The ability of CPs to facilitate rapid ion diffusion allows for fast charge and discharge cycles. This property is crucial for applications where quick bursts of energy are required. The swelling and contraction behavior, combined with the overall structural stability of CPs, influences the cycling stability of the SCs. A stable and reversible redox process, along with minimal structural degradation over numerous cycles, is essential for long-lasting and efficient SCs.186
The diffusion and absorption properties of CPs have a significant impact on the efficiency of SCs by influencing key performance factors such as capacitance, charge/discharge rates, and cycling stability. The ability of CPs to effectively accommodate ions and maintain structural stability during repeated cycling contributes to the overall effectiveness and reliability of the SCs.187–190
Different synthetic approaches were reported for synthesizing CPs in the current scenario and also had several applications for electrochemical storage devices.92,179 We will focus on three major CP (PANI, PPy, and PTh) based NCs, their electrochemical properties, and their impact as electrode materials in SCs.
The electrochemical performance of SCs using the CPs–C composite as the electrode material exhibits a notable increase in efficiency and stability performance of SCs in comparison to individual CP components and CP–carbon material composite. Examples include the PANI/CNTs composite and PANI/CNFs composite, both exhibiting maximum specific capacitance in comparison to the separate components.191–195 PANI/graphene nanoplate (GN) composites were created by Zhang et al.196 using the in situ polymerization process under acidic conditions. SCs having a maximum electrical capacity of 482 F g−1 when the amount of current is 0.1 A g−1 were produced via the associated mechanism. They have great cycling stability efficiency and retain their original capacitance after many cycles. Chemical precipitation was used by Gómez et al. to create a PANI/GN composite.202 The composite made up of PANI and NPs emphasized high-end features and applications.197 It shows a maximum electrical capacity of 500 F g−1 at a current density of 0.1 A g−1. Both examples demonstrate that using a composite CPs–carbon material as an electrode in SCs may increase the specific capacitance of SCs. Furthermore, the NCs made by any CPs, especially PANI and GO, show increased electrochemical activity and greater specific capacity because of the synergetic effect between them.198–200 The reason why NCs retain their original capacitance to such a great extent is that they offer a far bigger interior surface area than individual components. This characteristic of NCs guarantees increased performance stability and increased efficiency of PANI composite-based electrodes and hence electrochemical characteristics. Additionally, there is little room for improvement in the specific capacitance of carbon materials. Current research thus focuses on modifications in the composite electrode material created by CPs–MO-based SCs.
Researchers recently created a CPs–MO composite, which offered benefits including good cycle stability, excellent conductivity, and a large surface area.201,202 Gemeay et al. discovered that the amount of crystallinity of the NCs PANI/MnO2 in an acidic medium is influenced by the acidic anions present in the media.203 Zhang and coworkers created PANI@intercalated multilayer MnO2 in N-methyl-2-pyrrolidone solvent via an exchange reaction method.204 The corresponding process mechanism demonstrates that the composite created had improved conductivity. When the current was 1.0 A g−1, the composite had a maximum electrical capacity of 350 F g−1. Even after 1000 cycles, the composite still had 95% of its initial capacitance. A PANI nanorod-MnO2 NPs composite film was created by Chen et al.205 When compared to earlier instances and individual components, the NC film created has a significant amount of surface area. As a result, they successfully created electrode materials using the PANI-based NCs, which offer minimum expensive performance which is more effective, good charge storage capability, and are eco-friendly.
 | ||
| Fig. 12 Surface morphology of PPy films produced using interfacial polymerization technique. (a) PPy fabricated without a surfactant at 25 °C, (b and c) PPy fabricated using Tween 80 and (d and e) PPy fabricated using Span 80 as surfactants at 25 °C and 0 °C [Reproduced with permission from ref. 206. A copyright of Wiley, 2014]. | ||
Li and Yang207 used methyl orange/FeCl3 as a reactive, self-destructing template to create a pure PPy film using a chemical oxidation approach. They used 0.5 M FeCl3, and the resulting film was composed of nanotubes of 5 to 6 μm with diameters of 50 to 60 nm. The polymer resulted in significantly better electrochemical performance because of the resulting tube-like structure, which reached a maximum electrical capacity of 578 F g−1 at 0.2 A g−1. Even after 1000 cycles in an electrolyte containing 1.0 M KCl, it maintained a capacitance of 80%. Using in situ polymerization, Xu et al. prepared conductive cotton textiles that were covered with PPy nanorods. The FeCl3-methyl orange complex was used as a template.208
Although the fabrics produced by the aforementioned technique have a good specific capacity of 328 F g−1 and ED of 25 W h kg−1 at a current of 0.7 mA cm−2, they cannot be employed as electrodes for SCs. Although, they can maintain a capacitance of 64% after 500 cycles, their periodical stability is quite low. Rajesh et al.209 created PPy films using phytic acid, a dopant, using the electron polymerization process. As a result, the SC conductivity rises, reaching a maximum electrical capacity of 345 F g−1 at a scan rate of 5.0 mV s−1. The preservation of initial capacitance of 90% at 10 A g−1 even after 4 × 103 cycles shows that the PPy-based electrode has high specific capacitance.
In general, the synthetic approach, dopant types, template used, and other variables had an impact on the microporous structure and reversible redox characteristics of PPy-based composite electrodes. Even if these parameters are adjusted, some of the qualities of PPy-based composite electrodes can be improved, but other features may deteriorate, making PPy-based electrodes still unable to fulfil the needs of practical applications. Due to the potential for these hybrid composites to improve the energy storage capacity of PPy composites, researchers have recently concentrated on the development of PPy–carbon material composites or PPy–MO NCs.209–211 Because their reversible redox characteristics have been improved throughout the system, the composites exhibit higher electrochemical performance and better mechanical properties when compared to pure PPy.
Tang et al. used the electron deposition approach to create the CNT/PPy film. The CNT/PPy served as the anode in CNTs/MnO2/KCl@CH2@CH–SiO2/poly-acrylamide/CNTs–PPy SCs.210 When the electron deposition was 2000 s, the specific capacity of these SCs reached a high of 638 F g−1 when the amount of current was 1.0 mA cm−2. Additionally, the ED reached a maximum value of roughly 40 W h kg−1 having a power of 520 kW kg−1 and a strain of 100%, demonstrating greatly increased stability. The composite showed better efficiency in specific capacitance compared with individual components. By using an in situ chemical oxidation approach, Yang et al. created unique PPy/CNTs composites that are connected in their structure.211 The conductivity and thermal stability of the associated composites were enhanced when PPy was evenly coated on the surface of air plasma-activated CNTs. Overall, the PPy/plasma-activated CNTs composite's electrochemical characteristics are improved and are higher than those of the PPy/CNTs composite. Additionally, the conjugated structure created at the active sites made available by the nitrogen groups on PPy strengthens the interaction between CNTs. The development of composites with better electrochemical characteristics is facilitated by PPy. By making adjustments to the elements that are essential for the creation of the composite, the mechanical characteristics of the PPy/CNTs composite may also be enhanced. Chen et al. synthesized a durable and flexible CNT-based electrode material for SCs.212 In this combination, a vacuum-filtered CNT film was covered with PPy that was electronically deposited.
The mechanical qualities of the electrode material are improved by this method, which also increases electrochemical performance. SCs using the aforementioned electrode (PPy/CNT) produced long-term stability and exceptional flexibility, retaining ∼95% of their initial capacity even after 1.0 × 104 cycles. However, despite significant improvements in electrochemical efficiency for PPy/CNT composite's cycling stability, these NC electrode materials still have lower energy and power densities than LIBs due to their limited capacity for charge build-up.212 Due to these factors, SCs using the PPy/CNT composite as their electrode material are unable to satisfy the demands of real-world applications. As the demand for improved electrode materials grows, researchers concentrate on creating composite materials using PPy and metal oxides. High theoretical capacitances are present in MO-based composites.213 For instance, a PPy/MnO2 nanowire composite was created by Shayeh et al. using an electrochemical oxidation method.215 Increased specific capacitance due to this process can reach 210 F g−1, almost twice as much as the value of a single PPy (104 F g−1).
Metal oxides are regarded as the optimum active material for PCs, including Co3O4,213 MnO2,214 NiO,216 CuO,217 WO3,218 VOx,219 and V2O5.220 Their hypothetical capacitance was more than that of pure PPy. The theoretical capacitance of Co3O4 is greater than 3000 F g−1; however, in fact, it was discovered that the experimental capacitance of the corresponding MO is lower than the theoretical value.213 It is because standard dense electrode films have a restriction on ion diffusion, and low conductivity results in worse electron transport.221,222 Additionally, they also encounter volume changes as a result of the unstable charge/discharge transfer. Better capacitance is produced by adjusting the charge/discharge process and enhancing conductivity.
Researchers concentrate on creating NCs out of various electroactive materials including CPs and MO since they offer so many benefits. They have good electrical, mechanical, and electrochemical capabilities, resulting in the development of ESDs.220 3D NCs made of a PPy@CoO hybrid nanowire array on nickel foam were created by Zhou et al. and exhibited good pseudo-capacitive performance.223 Since it has great rate capability, its specific capacitance reaches a maximum of up to 2225 F g−1 at a current of 1.0 mA cm−2, which leads to the retention of an initial specific capacity of 99.7% after 2000 cycles at a scan rate of 20 mA cm−2. It served as a positive electrode in an aqueous asymmetric SC with a hybrid array that can provide EDs of up to 34 W h kg−1 as its maximum. It also has a higher power density, which was measured at 5500 W kg−1. The perfect synergetic effect between the PPy and the CoO nanowires is the reason why this SC exhibits exceptional stability for 2000 cycles.
Ji et al.216 created a PPy nanotube/MnO2 composite using a simple method that exhibits outstanding cyclic stability and specific capacitance of a maximum of 405 F g−1 at a current of 1.0 A g−1. Even after 800 cycles, it still retains up to 89% of its original capacitance. The efficiency results from a superior synergistic interaction between PPy and MnO2, which increases the capacitance. Coated MnO2 also prevents structural changes in PPy during long-term cycles. Sun et al.221 used a two-step process to synthesize the resulting PPy/V2O5, which exhibits greater electrochemical performance efficiency than either component alone. Furthermore, using a direct electrochemical co-deposition process on an interdigital-like electrode, Qian et al.218 created a core/shell CuO/PPy nanosheet array for distinctive on-chip SCs. An exceptional electrical capacity of 1276 F cm−3 and an energy per volume of 29 W h cm−3 are produced by this process. The device's ability to maintain its specific capacitance up to 100% at a current density of 2.6 A cm−3 even after 3000 cycles is its main benefit. The device's strong electrochemical characteristics demonstrate its potential for use in practical SC electrode applications.
In general, electrode materials for SC composites consisting of PPy and MO frequently demonstrate good electrochemical performance, including power density, high EDs, exceptional, superior cycle stability, and amazing specific capacity, due to the synergistic effect of the two composites.222
Gnanakan et al. used the dilute polymerization process to create perfect PTh-based NPs and PTh/tartaric acid (TA) NPs in which TA functions as a dopant.231 These two NPs had a specific capacity of 135 and 157 F g−1, respectively. Nejati used an oxidative chemical vapor deposition approach to make a PTh film without any replacement, which is covered with an ultrathin layer over a variety of substrates.232 According to the experimental findings, the specific capacity of an activated carbon electrode wrapped using the PTh film rose to 50% when compared to an active carbon electrode that was not coated, and up to 90% of its initial capacity might still be present even after 5.0 × 103 cycles. By using a sequential ionic layer deposition and reaction approach with FeCl3 as the oxidizing agent, Patil et al. created an amorphous form of PTh.233 In 0.1 M LiClO4 metal salt solution, this mechanism produces a capacitance of 255 F g−1.
In general, pure PTh-based electrode materials for SCs are often impacted by a variety of variables, which has an impact on their electrochemical performance. These elements include the surface structure of PTh, the methods used for production, the creation of the substrate, and more. Due to the adjustment of the aforementioned parameters in the recent scenario, the electrochemical performance was significantly enhanced. However, it is still unable to satisfy the demands of practical applications because its inherent limitations, such as rapid loss of power density and low specific capacitance, make it inferior to PPy and PANI.233
Nowadays, researchers are focussing on the fabrication of PTh-based NC materials to address the drawbacks of pure PTh-based electrode materials. To create PTh NCs, there are primarily two types of methods: one involves creating composites of PTh and carbon nanomaterials, and the other involves PTh and MO. A PTh/MWCNTs composite, which may be used as a representative example for the first category, was created by Fu et al.235 The aforementioned electrochemical polymerization process was used to create the PTh NCs. This PTh/MWCNT composite outperforms individual MWCNT and pure PTh in terms of electrochemical performance. At a scan speed of 60 mV s−1, it had a maximum electrical capacity of 110 F g−1. Even after 1000 continuous cycles, the SCs employing the aforementioned PTh/MWCNT composites as the electrodes still retain up to 90% of their initial capacitance. Through the use of an electrochemical polymerization process, Zhang et al. created a PTh/MWCNT composite of thickness 2–3 nm.236 When the amount of current was 1.0 A g−1, this composite then displayed an increasing specific capacity of 218 F g−1 because of the insignificant internal resistance, which promoted strong electron transport between the two electrodes of SCs.
Ambade et al. gave an easy method to fabricate graphite/thiophene/benzylidene (GTB) and GO/thiophene/benzylidene (GO-TB) using in situ polymerization of conjugated polymer PTh (PTCPs).239 By creating and manufacturing GTB and GO-TB, the PTCP film was non-covalently bound on both the graphene and GO sheets in the NCs, which improves the synergistic impact of the microstructures and also delivers improved doping in graphene. The electrochemical experiments show that the GO-TB composite exhibited better electrochemical performance, resulting in lower serial resistance, excellent specific capacity, and EDs of 298 F g−1 and 150 W h kg−1, respectively. It also showed high cycling stability and increased ionic conductivity. Because of the synergistic impact, PTh/carbon material composites demonstrated greater capacitance and cycle stability when compared to single components. Different factors affect the performance of these SCs like the type of PTh, the shape and dimensions of the electrode, production techniques, and efficiency of accumulation of PTh on carbon materials. As previously described for PANI and PPy-based MO composites, PTh-based composites are also appropriate for the production of electrode materials for SCs. Because the specific capacity of SCs with PTh electrodes is substantially less than that of PPy and PANI-based SCs, progress in the development of PTh/MO composites is slower than that of PANI/MO and PPy/MO composites. There is no effective way to enhance the electrochemical performance of CP-based SCs.
Currently, there has been some research performed on PTh/MO composite-based electrodes of SCs.237,238 To improve the electrochemical performance efficiency, more research is required. The composites formed between the above-mentioned CPs and other materials like activated carbon, CNTs, etc. also had merits and demerits.
All three CP and NC-based SCs have merits and demerits. Merits of PANI CPs include a high range of specific capacitance, ease of synthesis, and ease of control of the conductivity. It has high flexibility. Doping and de-doping are simple in the PANI. PANI also shows high theoretical capacitance. The demerit of PANI is that the specific capacitance depends upon the fabrication method. It also shows poor cycling stability. PANI-based electrodes are applied in proton-type electrolytes only.198–204
Merits of PPy are high specific capacitance per volume, being easy to synthesize, and have greater cycling stability than PANI. It also shows better flexibility than PANI. It is mainly used to neutralize electrolytes. Demerits of PPy are as follows: doping and de-doping is difficult, it has low specific capacitance per unit gram, and it can be used only as a material for the cathode.219–222
PTh has advantages, i.e., it is also flexible, the synthesis technique is much easier, and stability in the environment is very high. The cycling stability of PTh is favorable. Demerits of PTh are that its conductivity, and specific capacitance are very poor.223–238
While all three, i.e. PANI, PPy, and PTh, CPs-based electrode materials used separately in SCs offer advantages for energy storage applications, there are some differences in their properties and performance. PANI exhibits pseudocapacitive behavior, which means that the charge storage mechanism involves redox reactions at the electrode–electrolyte interface. PANI-based electrodes generally have high specific capacitance and good cycling stability. However, the low intrinsic conductivity of PANI limits its overall energy density and power density compared to other materials. PPy-based electrodes exhibit pseudocapacitive behavior, providing high specific capacitance. PPy has a relatively higher intrinsic conductivity compared to PANI, allowing for better charge transfer and higher power density. However, PPy electrodes may suffer from decreased cycling stability over long-term use due to structural degradation and potential leaching of dopant ions. PTh-based electrodes in SCs exhibit a combination of both EDLC and pseudocapacitance, providing a balanced energy storage mechanism. PTh offers good cycling stability and long-term electrochemical performance. However, compared to PANI and PPy, PTh typically has a lower specific capacitance, limiting its overall energy storage capacity. Overall, the choice of CPs for SCs electrodes depends on the specific requirements of the application. Researchers often explore composite materials and hybrid systems to combine the advantages of different CPs and optimize the overall performance of SCs.
4. Ternary NC, i.e., carbon material/CP/MO-based SCs
In the past, researchers were focused on creating binary NC-based SCs since they attracted more attention due to their superior electrochemical and mechanical capabilities compared to their separate components.239 In the current scenario, researchers worked hard to develop carbon material-based ternary NCs because the composites show better resultant electrochemical and mechanical properties than CP-based binary NCs.240 Different factors affect the electrochemical performance of carbon-based ternary NCs, including synergistic effect, conductivity, surface area, internal resistance, specific capacitance, cycling stability, etc. This section discusses the most recent advancements in various carbon-based ternary NCs used as SC electrode materials.Due to the potential physical and chemical relevance of carbon materials, CPs, and MO, different researchers have attempted to synthesize carbon/CP/MO-based composites.241,242 A dynamic electrode for SCs was fabricated by Chen et al.242 using a nanowire network of freestanding NiMoO4 that is equally dispersed in all directions. This standalone NiMoO4 nanowire array, which was coated with the CP PANI and a conductive carbon cloth, is recognized as an effective electrode layer for PCs. The final composite showed better electrochemical performance and may be employed as an outstanding electrode in SCs. This indicates improved cycle durability and rate capabilities. A CP/MO electrode material based ternary composite with carbon cloth was prepared through hydrothermal methods and also had application in the field of ESDs.243 Fig. 13 shows the fabrication process of carbon cloth/PANI/NiMoO4-based flexible electrode materials used in SCs.
 | ||
| Fig. 13 (a) Representation of a fabrication process for carbon cloth/PANI/NiMoO4, (b) digital photo of pristine carbon cloth, and (c) digital view of carbon cloth/PANI/NiMoO4 and (d) final product of carbon cloth/PANI/NiMoO4 [Reproduced with permission from ref. 242. A copyright of Elsevier, 2015]. | ||
In comparison to individual components, the aforementioned NCs have an exceptional specific capacitance of 1340 F g−1 at 1.0 mA cm−2, and they preserve their original capacitance of 97% even after 2000 cycles. Dynamic graphene oxide (GO), PPy, and manganese oxide (GO/PPy/MnOx) NCs were fabricated by Ng et al.245 and used as a working electrode in SCs. They showed strong cycling stability and better specific capacitance even after 1000 cycles and were able to maintain an initial capacitance of 96.5%. Using GO and PPy, MOs like TiO2 (ref. 246) and ZnO247 different ternary electrode materials for SCs were also tested. Using GO, ternary composites demonstrated good electrochemical and mechanical characteristics.
Sk's team developed the MWCNT/MnO2/PANI ternary NCs using in situ polymerization. They reported a unique MWCNT-based composite electrode material with pseudo capacitance.243 The specific capacitance of these NCs can reach a maximum of 530 F g−1 and EDs can reach a maximum of 74 W h kg−1, while the π–π interactions, and n–π interactions between constituents improve the electrochemical performance. The power density reached as high as 11.35 kW kg−1. A pseudo-capacitive shell was produced after evenly coating CNT with MnO2. The resulting NC working electrode produces increased capacitive performance up to a maximum value of 529.5 F g−1 at 0.1 A g−1 current density and also maintains its original capacitance up to 98.5% after 1.0 × 103 cycles at a 5 A g−1 current. Having a power density of 100 W kg−1, it offers a greater energy content of up to 38.5 W h kg−1 while retaining 60% of its initial capacitance. By coating PPy on MnO2 NPs that were placed on CNT cloth, Yun et al.248 attempted to increase the energy output and cycle dependability. The SCs with these ternary NCs also showed remarkable flexible capacity. These ternary composite-based electrode materials demonstrated significant use in SCs.249 Table 3 shows the development made towards improving the electrochemical performance of SCs.
| Type of electrode material used | Specific capacitance achieved (F g−1) | Current density | Specific surface area (m2 g−1) | Reference |
|---|---|---|---|---|
| AC | 300 | 0.5 A g−1 | 3000 | 88 |
| Graphene | 550 | 0.1 A g−1 | 2630 | 97 |
| GO | 349 | — | — | 111 |
| RuO NFs | 2000 | — | — | 121 |
| CNFs/RuO/PBI | 1060 | 0.5 A g−1 | 678 | 125 |
| MnO2 | 1375 | — | — | 127 |
| MnO2/PCNFs | 290 | — | 1815 | 140 |
| MnO2/CNTs | 965 | — | — | 146 |
| NiO/citric acid | 338 | 0.5 A g−1 | — | 148 |
| CNFs/NiO | 852 | — | — | 158 |
| Co3O4 | 3560 | — | 485 | 160 |
| CoMnO2/VG CNFs | 630 | 0.1 A g−1 | — | 165 |
| GO/V2O5 | 455 | — | — | 167 |
| V2O5/CNFs | 745 | 0.5 A g−1 | — | 168 |
| PANI/GN | 482 | 0.1 A g−1 | — | 199 |
| PANI/MnO2 | 350 | 1.0 A g−1 | 872 | 203 |
| PPy/FeCl3 | 578 | 0.2 A g−1 | — | 208 |
| CNT/PPy | 638 | 1.0 mA cm−2 | — | 209 |
| PPy/CoO | 2225 | 1.0 mA cm−2 | 612 | 221 |
| PPy/MnO2 | 405 | 1.0 A g−1 | — | 215 |
| PPy/CuO | 1276 | 2.6 A cm−3 | 478 | 217 |
| PTh/TiO2 | 1533 | — | — | 228 |
| PTh/LiClO4 | 255 | 232 | ||
| Graphite/PTh/benzylidine | 298 | 236 | ||
| Carbon/PANI/NiMnO4 | 1340 | 1 mA cm−2 | 244 | |
| PANI/MWCNT/MnO2 | 530 | 0.1 A g−1 | 248 |
CP-based electrodes used in SCs and their modification were discussed by Wustoni et al.250 In another research, intrinsic CPs combined with MO NP-based electrode materials were designed and fabricated to improve the performance of SCs.251 The probable reason for electrode material degradation with an increase in cycle life was also investigated. The reason for electrode surface rupture and degradation in the mechanical stability of electrode materials with cycle life was poor linking between metal NPs and CPs. The MO NPs start rupturing from the electrode surface with an increase in the charge/discharge cycle.
In another research, flexible SCs based on CNT-based electrodes were reported.252 The fiber fabrics (FF) were used in CNT-based electrodes to increase the flexibility of SCs. The functionalization of electrode materials was done with MnO2 NPs. Ogihara et al. reported an intercalated MOF-based active electrode material for Li-ion-based ESDs.253 They used 0.8 V potential for intercalation of Li-ions in the MOF matrix. This hybrid ESD shows an 85% retention capacity at 400 mA g−1. The performance of this hybrid ESD was compared with that of an AC-based positive electrode, which shows 91% capacity retention even after 1000 discharge/charge cycles at a current of 0.15 A cm−2. Brunet Cabré et al. have reported Ti/C/T/MXene electrode-based PSCs.254 The Ti metal was used instead of Li metal for de-intercalation/intercalation reactions. The acidic medium was used as an electrolyte. Deprotonation/protonation of the oxygen functional group on the surface of the MXene-based electrode was followed. The capacity retention of MXene in the presence of Ti ions was found to be higher than that of CNT and CP-based ESDs.
Zhang et al. have reported integrated electrochemical SCs for portable ESDs used in drones and E-vehicles.255 They have fabricated heavy load-bearing integrated ESDs comprised of electrochemical capacitors based on polymeric solid-state electrolytes. They observed an ED of 0.131 W h m−2, power density of 0.13 W m−2, and specific capacitance of 32.5 F cm−2. Li et al. have reported 2D and 3D porous material MXene hydrogel-based PSCs for high-performance ESDs.256 The different MXene used in PSCs are Mo/Ti/C/Tx, Ti/C/Tx, and Nb/C/Tx. 3D MXene-based porous materials show large SSA, good electrical conductivity, and mechanical properties. PSCs show a high specific capacitance of 230 F g−1 at 10 V s−1 or 3.32 F cm−2 at 10 mV s−1, high ED of 9.3 × 10−6 W h cm−2, and power density of 7.0 mW cm−2.
Liang et al. have reported novel semiconductor-based electrode materials for advanced SCs.257 The performance of semiconductor-based electrode materials was compared with that of AC, CNT, and CP-based electrode materials. The performance of Si and MO-based semiconductor electrode materials was found to be almost comparable to that of AC-based electrode materials but was lesser than that of CP, CNT, and MXene-based electrode materials of SCs. Panwar et al. have reported a MoS2/graphene-based electrode material for ultrathin SCs.258 They used the field effect transistor (FET) concept in their SCs. They prepared a few atomic thick layers of MoS2/graphene in FET-based SCs. A gel electrolyte was used between the two electrodes of FETs of SCs. They observed that on combining MoS2/graphene, the capacitance increases to 3000% in contrast to MoS2 in the absence of graphene, which shows an 18% increase in capacitance.
Biswas et al. have reported anthraquinone/polydiacetylene-based asymmetric SCs.259 They observed an increase in electrochemical performance in terms of high capacity retention (>90%), specific capacitance (340 F g−1) and ED, and power density. Panwar et al. have fabricated FET-based SCs comprised of MoS2/graphene.258 They compared the electrochemical performance of ultrathin SCs with that of MoS2 and graphene electrode material-based SCs. They observed that electrochemical performance increases almost 1000 times when combining MoS2/graphene.
Ma et al. investigated the aging behavior of SCs with an increase in charging/discharging cycles.260 This aging behavior (deterioration) of the electrode surface with an increase in the discharge/charge cycle of SCs was based on the nature and composition of electrode materials used in SCs. The performance of single-material-based electrodes was found to be better with an increase in cycle life as compared to composite electrode material-based SCs.
In general, the ternary NCs improved specific capacitance, ED, power density, cycle stability, and other properties, especially using different CNTs. Several parameters, including electrical and thermal conductivity, internal resistance, and the kind of CNTs, CPs, and metal oxides, were utilized, which have an impact on the electrochemical and mechanical characteristics of ternary composites. Researchers were able to produce more effective ternary NC electrode materials for SCs by adjusting the different features. The effect of AC on the performance of SCs was comparable to MnO2/PCNF-based electrodes used in SCs. A high theoretical capacity of 3000 F g−1 was not attained experimentally in the case of PPy/CoO-based SCs. The maximum specific capacitance observed was 2225 F g−1 in PPy/CoO NC-based SCs. The Co3O4 when used alone shows a very high specific capacitance of NiO 710 F g−1 with a specific area of 485 m2 g−1. Out of the three investigated CPs that is PANI, PPy, and PTh, the PPy-based composite material shows good electrochemical performance in comparison to the two other CPs. The performance of SWCNTs and MWCNTs is almost comparable with some better results in the case of SWCNTs. The influence of graphene and GO on the performance of SCs was not up to mark. But when the carbon-based cloth was used with PANI/NiMoO4 its performance increased appreciably.
By combining MO, CPs, and CNTs into ternary nanocomposites, the resulting electrode materials of SCs can achieve a synergistic effect, maximizing their specific capacitance, electrical conductivity, cycling stability, and power density. The precise composition, morphology, and fabrication methods of these NCs can be tailored to optimize the desired properties for specific SC applications.
The use of nanomaterials like CNTs, graphene, MO NPs, and CPs in electrode materials for SCs increases their stability by different mechanisms.261–263 A few important are as follows: (i) nanomaterials typically have a large surface area-to-volume ratio. This increases the number of active sites for electrochemical reactions, thus facilitating better charge storage and faster ion diffusion, (ii) nanomaterials show good electrical conductivity compared to their bulk material. This property reduces the internal resistance of the SCs, leading to increased charge and discharge rates and overall improved stability, (iii) the unique structural properties of nanomaterials contribute to increased mechanical strength. This is crucial for maintaining the structural integrity of the electrode material during repeated charge–discharge cycles, leading to a longer lifespan of SCs, (iv) nanomaterials offer better electrochemical performance, i.e., higher capacitance and energy density. This results in more efficient and stable SCs, (v) nanomaterials are often more resistant to volume changes during the charge–discharge process. This minimizes electrode swelling and shrinking, reducing the likelihood of mechanical degradation and maintaining stability with time of operation, (vi) the nanoscale features of nanomaterials facilitate better accessibility for ions, improving ion diffusion and reducing the chances of concentration polarization. This leads to more consistent and stable performance of the SCs, and (vii) nanomaterials can be easily modified or functionalized to tailor their surface properties. This allows for better control over interactions with electrolytes and ions, optimizing the electrode–electrolyte interface and enhancing stability.
In a nutshell, the incorporation of nanomaterials in SC electrodes addresses different challenges, including increased surface area, improved conductivity, enhanced mechanical strength, and optimized electrochemical performance. These factors collectively contribute to the stability and longevity of SCs (Table 4).
| Nanomaterial | Type of electrolyte used | Impact of electrolyte on the performance of SCs | References |
|---|---|---|---|
| Carbon and CNTs | Aqueous (e.g., H2SO4) | (i) High surface area for increased capacitance | 90–94 and 262–267 |
| (ii) Good electrical conductivity for fast charge/discharge | |||
| (iii) Enhanced stability due to mechanical strength | |||
| Transition metal dichalcogenides (e.g., MoS2) | Ionic liquids | (i) High specific capacitance and energy density | 268–270 |
| (ii) Improved stability in non-aqueous environments | |||
| (iii) Slower ion diffusion may affect charge/discharge rates | |||
| MXene nanosheets | Aqueous (e.g., Na2SO4) | (i) High conductivity and large surface area | 15, 39 and 271–274 |
| (ii) Good stability and mechanical strength | |||
| (iii) Suitable for environmentally friendly aqueous systems | |||
| (iv) Promising for high-performance supercapacitors |
5. Future perspective
SCs have an encouraging future in ESDs. In future SCs will improve to be very essential and beneficial in terms of the following: (i) high power density: SCs to be developed in future will deliver and store energy at a much higher rate as compared to rechargeable batteries (LIBs), making them suitable for applications in rapid energy requiring equipment/machinery, (ii) rapid charging: futuristic SCs will be charged and discharged quickly, enabling shorter charging times for ESDs and electric vehicles (EVs), (iii) long cycle life: new generation SCs will have a longer lifespan than traditional rechargeable batteries, with the ability to undergo 100 s to 1000 s of charge–discharge cycles without significant degradation, (iv) increased efficiency: future research should focus on to improve the ED of SCs, allowing them to store more energy per unit volume or weight, which would enhance their overall efficiency, (v) hybrid energy storage systems: future of SCs lies in combining SCs with other energy storage technologies, such as rechargeable batteries, which can create hybrid systems that take advantage of both technologies, providing higher EDs and power output, (vi) grid energy storage: in future, SCs can play a role in developing grid-scale energy storage systems, providing fast-response power to stabilize the grid during peak demand or intermittent renewable energy generation, (vii) portable electronics: future of SCs also lies in increasing the performance of portable electronic devices by providing quick bursts of power during high-demand activities, improving user experience and reducing reliance on batteries, (viii) SC materials: future research of SCs will be focused on developing advanced materials with higher capacitance and EDs, such as graphene and CNT-based electrodes, which could lead to significant advancements in SC technology, (ix) sustainable energy storage: futuristic SCs will also provide environmental benefits as they do not rely on rare earth materials or heavy metals typically used in rechargeable batteries. Their longer lifespan and recyclability contribute to a more sustainable energy storage solution, and (x) integration with renewable energy: futuristic SCs can also help in addressing the intermittent nature of renewable sources of energy like wind, and solar by storing energy during low-demand periods and releasing it at peak demand, facilitating a more reliable and stable renewable energy grid.In a nutshell, SCs have great potential as ESDs, offering high power density, rapid charging, long cycle life, and the ability to integrate with other technologies, making them suitable for numerous applications in the future.
6. Conclusions
SCs or ultracapacitors are ESDs that bridge the gap between traditional capacitors and rechargeable batteries, especially LIBs. They offer higher power density and faster charge–discharge rates compared to rechargeable batteries while providing higher EDs than conventional capacitors. The specific capacitance is a crucial parameter that determines the energy storage capacity of SCs. Researchers have made significant progress in improving the specific capacitance of SCs. By utilizing advanced materials, such as C-based nanomaterials (such as graphene, CPs, and CNTs) and transition MO, a high specific capacitance was achieved in the range of 10 to a few 100 F g−1. The cut-off power density in the range of 100 to a few 1000 W kg−1 has been reported for SCs. These values demonstrate their potential use in large power appliances, like regenerative braking in electric vehicles, peak power leveling in renewable energy systems, and various other power-intensive applications.Out of the three investigated CPs that is PANI, PPy, and PTh, the PPy-based composite material shows good electrochemical performance in comparison to PANI and PTh CPs. The performance of SWCNTs and MWCNTs is almost comparable with some better results in the case of SWCNTs. The influence of graphene and GO on the performance of SCs was not up to mark. But when the carbon-based cloth was used with PANI/NiMoO4 its performance increased appreciably.
Advancements in electrode design, including nano-structuring and hierarchical architectures, have contributed to improved power density in SCs. The development of new electrolytes, SWCNTs, MWCNTs, and CPs has led to increased ionic mobility and faster charge–discharge rates of SCs. Latest developments in electrode materials for SCs include the use of (i) C-based materials, such as AC, CNTs, and graphene (G, rGO, and GO), which have a high SSA, excellent electrical conductivity, and good chemical stability, (ii) transition MO, such as RuO2, MnO2, and NiO, which have shown high specific capacitance, (iii) CPs, including PANI, PPy, PTh, etc., which have been reported as electrode materials for SCs. These CP-based electrode materials exhibit high electrical conductivity and redox activity, (iv) MOF-based electrode materials in SCs. MOFs possess high surface areas and tuneable structures, offering potential for SC applications, and (v) 2D materials: in addition to graphene, other 2D materials, like TMDs and MXenes, have emerged as promising candidates for SC electrodes. These materials offer high surface areas, excellent electrical conductivity, and unique electrochemical properties, which can enhance the energy storage performance of SCs.
Abbreviations
| CNTs | Carbon Nanotubes |
| SCs | Supercapacitors |
| EDs | Energy Densities |
| EDLCs | Electrical Double Layer Capacitors |
| ESDs | Energy Storage Devices |
| MO | Metal Oxide |
| NPs | Nanoparticles |
| NCs | Nanocomposites |
| PCs | Pseudo Capacitors |
| HCs | Hybrid Capacitors |
| ES | Electrospinning |
| BET | Brunauer–Emmett–Teller |
| Rct | Charge Transfer Resistance |
| SSA | Specific Surface Area |
| ESR | Equivalent Series Resistance |
| PVP | Polyvinyl Pyrrolidone |
| PEO | Polyethylene Oxide |
| PVA | Polyvinyl Alcohol |
| PAN | Polyacrylo Nitrile |
| PVDF | Polyvinylidene Fluoride |
| AC | Activated Carbon |
| CMG | Chemically Modified Graphene |
| GO | Graphene Oxide |
| NFs | Nanofibers |
| TMDs | Transition Metal Dichalcogenides |
| MOFs | Metal–Organic Frameworks |
| DL | Double Layer |
| CPs | Conducting Polymers |
| PANI | Polyaniline |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| SWCNTs | Single-Walled Carbon Nanotubes |
| MWCNTs | Multiwalled Carbon Nanotubes |
| LIBs | Lithium-Ion batteries |
| Cdl | Double Layer Capacitance |
| CVD | Chemical Vapour Deposition |
| CNFs | Carbon Nano Fibres |
| PVAC | Polyvinyl Acetate |
| FSCs | Flexible Supercapacitors |
| GNFs | Graphene Nano Fibres |
| CV | Cyclic Voltammetry |
| PMMA | Polymethyl Methacrylate |
| CFSs | Carbon Fibre Sheets |
| PCNFs | Porous Carbon Nano Fibres |
| EIS | Electrochemical Impedance Spectroscopy |
| rGO | Reduced Graphene Oxide |
| DCDAP | Dicyanodiamide |
| VGCNFs | Vapour Grown Carbon Nano Fibres |
| CB | Conduction Band |
| VB | Valence Band |
| ICPs | Intrinsic Conducting Polymers |
| GNs | Graphene Nanoplates |
| UPD | Underpotential Deposition |
Author contributions
Aarti Tundwal: validation; and writing – original draft. Harish Kumar: conceptualization; supervision; formal analysis; data curation; validation; investigation; resources; experimental design; and writing – review and editing. Bibin J. Binoj: writing; formal analysis; and validation. Rahul Sharma: formal analysis; and validation. Gaman Kumar: formal analysis; and validation. Rajni Kumari: formal analysis; and validation. Ankit Dhayal: formal analysis; and validation. Abhiruchi Yadav: data curation; and validation. Devender Singh: data curation; and validation. Parvin Kumar: formal analysis; and validation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
It is not possible to complete this manuscript for us without acknowledging the specific research support provided to us by CUH authorities. We duly acknowledge all types of research support provided by CUH authorities for writing this review article.References
- R. Tawn and J. Browell, Renewable Sustainable Energy Rev., 2022, 153, 111758 CrossRef.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourga and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226–252 RSC.
- Z. Zhao, K. Xia, Y. Hou, Q. Zhang, Z. Ye and J. Lu, Chem. Soc. Rev., 2021, 50, 12702–12743 RSC.
- A. S. Aricò, P. Bruce, B. Scrosati, J. M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef PubMed.
- W. Wu, L. Yang, S. Chen, Y. Shao, L. Jing, G. Zhao and H. Wei, RSC Adv., 2015, 5, 91645–91653 RSC.
- Leyden Jar Electrical Instrument, [online], available: https://www.britannica.com/technology/Leyden-jar Search PubMed.
- R. Sharma, H. Kumar, G. Kumar, S. Sharma, R. Aneja, A. K. Sharma, R. Kumar and P. Kumar, Chem. Eng. J., 2023, 468, 143706, DOI:10.1016/j.cej.2023.143706.
- E. Dhandapani, T. Sadhasivam, K. P. Ramesh, K. P. Ramesh, R. Vasudevan and N. Duraisamy, J. Energy Storage, 2022, 52, 104937 CrossRef.
- K. K. Patel, T. Singhal, V. Pandey, T. P. Sumangala and M. S. Sreekanth, J. Energy Storage, 2021, 44, 103366 CrossRef.
- M. I. A. Abdel Maksoud, R. A. Fahim, A. E. Shalan, M. A. Elkodous, S. O. Olojede, A. I. Osman, C. Farrell, A. H. Al-Muhtaseb, A. S. Awed, A. H. Ashour and D. W. Rooney, Environ. Chem. Lett., 2020, 19, 375–439 CrossRef.
- M. Bigdeloo, E. Kowsari, A. Ehsani, A. Chinnappan, S. Ramakrishna and R. A. Akbari, J. Energy Storage, 2021, 37, 102474 CrossRef.
- P. Forouzandeh, V. Kumaravel and S. C. Pillai, Catalysts, 2020, 10, 969 CrossRef CAS.
- S. Trasatti and G. Buzzanca, J. Electroanal. Chem. Interfacial Electrochem., 1971, 29, A1–A5 CrossRef.
- S. Suriyakumar, P. Bhardwaj, A. N. Grace and A. M. Stephan, Batteries Supercaps, 2021, 4, 571–584 CrossRef CAS.
- M. R. Lukatskaya, S. Kota, Z. Lin, M. Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Nat. Energy, 2017, 2, 17105 CrossRef CAS.
- N. S. Shaikh, S. B. Ubale, V. J. Mane, J. S. Shaikh, V. C. Lokhande, S. Praserthdam, C. D. Lokhande and P. Kanjanaboos, J. Alloys Compd., 2022, 893, 161998 CrossRef CAS.
- O. Vasile, AIP Conf. Proc., 2014, 1597, 98–120 Search PubMed.
- A. Afif, S. M. Rahman, A. T. Azad, J. Zaini, M. A. Islan and A. K. Azad, J. Energy Storage, 2019, 25, 100852 CrossRef.
- Y. Kumar, A. Gupta, A. K. Thakur, S. J. Uke, V. Khatri, A. Kumar, M. Gupta and Y. Kumar, J. Nanopart. Res., 2021, 23, 119 CrossRef CAS.
- M. V. Kiamahalleh, S. H. S. Zein, G. Najafpour, S. A. Sata and S. Buniran, Nano, 2012, 7, 1230002 CrossRef.
- E. Herrero, L. J. Buller and H. D. Abruña, Chem. Rev., 2001, 101, 1897–1930 CrossRef CAS PubMed.
- M. Jayalakshmi and K. Balasubramanian, Int. J. Electrochem. Sci., 2008, 3, 1196–1217 CrossRef CAS.
- M. S. Halper and J. C. Ellenbogen, Supercapacitors: A Brief overview, The MITRE Corporation, McLean, Virginia, USA, 2006 Search PubMed.
- H. Choi and H. Yoon, Nanomaterials, 2015, 5, 906–936 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- H. Y. Lee and J. B. Goodenough, J. Solid State Chem., 1999, 144, 220–223 CrossRef CAS.
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc. Chem. Commun., 1977, 578 Search PubMed.
- M. Gholamian, J. Sundaram and A. Q. Contractor, Langmuir, 1987, 3, 741–744 CrossRef CAS.
- S. M. Ata, Adv. Mat. Fabricat. Methods Electrochem. Supercapacitors, Dissertion, McMaster University, Canada, 2012.
- S. Fleischmann, J. B. Mitchell, R. Wang, C. Zhan, D. Jiang, V. Presser and V. Augustyn, Chem. Rev., 2020, 120, 6738–6782 CrossRef CAS.
- A. M. Bryan, L. M. Santino, Y. Lu, S. Acharya and J. d'Arcy, Chem. Mater., 2016, 28, 5989–5998 CrossRef CAS.
- S. Mohapatra, A. Acharya and G. Roy, Lat.-Am. J. Phys. Educ., 2012, 6, 380–384 Search PubMed.
- S. M. Chen, R. Ramachandran, V. Mani and R. Saraswathi, Int. J. Electrochem. Sci., 2014, 9, 4072–4085 CrossRef.
- A. Taleb, M. Afaq, H. A. Albalwi, F. Ismail, A. Irshad and M. M. Ibrahim, Mater. Sci. Eng. B, 2024, 299, 116916, DOI:10.1016/j.mseb.2023.116916.
- R. Jerome and A. K. Sundramoorthy, Anal. Chim. Acta, 2020, 1132, 110–120, DOI:10.1016/j.aca.2020.07.060.
- H. Shehzad, J. Chen, M. T. Shuang, Z. Liu, Z. H. Farooqi, A. Sharif, E. Ahmed, L. Zhou, A. Irfan, R. Begum, F. Iqbal and J. Ouyang, Colloids Surf., A, 2024, 680, 132637, DOI:10.1016/j.colsurfa.2023.132637.
- B. Lee, S. Cho, B. J. Jeong, S. H. Lee, D. S. Kim, S. H. Kim, J.-H. Park, H. K. Yu and J. Choi, Sens. Actuators, B, 2024, 401, 134913, DOI:10.1016/j.snb.2023.134913.
- K. Naoi and P. Simon, Electrochem. Soc. Interface, 2008, 17, 34–37 CrossRef CAS.
- H. Lv, Q. Pan, Y. Song, X.-X. Liu and T. Liu, Nano-Micro Lett., 2020, 12, 118 CrossRef CAS PubMed.
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539 CrossRef CAS.
- Z. Hadi, J. Y. Khademzadeh, Y. Zare, M. T. Munir and K. Y. Rhee, J. Mater. Res. Technol., 2024, 28, 4229–4238, DOI:10.1016/j.jmrt.2024.01.014.
- S. P. Nagalingam and A. N. Grace, Mater. Today Chem., 2022, 26, 101113, DOI:10.1016/j.mtchem.2022.101113.
- F. Guo, Y. Wang, K. Xue, L. Liu, J. Li and Y. Huang, Compos. Sci. Technol., 2024, 110425, DOI:10.1016/j.compscitech.2023.110425.
- Y. Lv, S. Huang and Y. Zhao, Sci. Adv. Mater., 2019, 11, 418–424 CrossRef CAS.
- Z. Zhang, S. Mu, B. Zhang, L. Tao, S. Huang, Y. Huang, F. Gao and Y. Zhao, J. Mater. Chem. A, 2016, 4, 2137–2146 RSC.
- J. He, T. Tao, F. Yang and Z. Sun, ChemSusChem, 2022, 15, e202200817 CrossRef CAS PubMed.
- J. Li, J. Fleetwood, W. B. Hawley and W. Kays, Chem. Rev., 2021, 122, 903–956 CrossRef PubMed.
- A. Cresce and K. Xu, Carbon Energy, 2021, 3, 721–751 CrossRef.
- Y. Chen, T. Wang, H. Tian, D. Su, Q. Zhang and G. Wang, Adv. Mater., 2021, 33, 2003666 CrossRef CAS PubMed.
- M. N. Rantho, M. J. Madito, K. O. Oyedotun, D. J. Tarimo and N. Manyala, AIP Adv., 2020, 10, 065113, DOI:10.1063/5.0011862.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- N. Lingappan, S. Lim, G. H. Lee, H. V. Tung, V. H. Luan and W. Lee, Funct. Compos. Struct., 2022, 4, 012001 CrossRef.
- J. V. Patil, S. S. Mali, A. S. Kamble, C. S. Hong, J. S. Kim and P. S. Patil, Appl. Surf. Sci., 2017, 423, 641–674 CrossRef CAS.
- D. Tian, X. Lu, W. Li, Y. Li and C. Wang, Acta Phys.-Chim. Sin., 2020, 36, 1904056 Search PubMed.
- M. A. A. M. Abdah, N. H. N. Azman and S. Kulandaivalu, Mater. Des., 2020, 186, 108199 CrossRef.
- X. Li, Y. Chen, H. Huang, Y. W. Mai and L. Zhou, Energy Storage Mater., 2016, 5, 58–92 CrossRef.
- S. Peng, L. Li, J. K. Y. Lee, L. Tian, M. Srinivasan, S. Adams and S. Ramakrishna, Nano Energy, 2016, 22, 361–395 CrossRef CAS.
- L. Xiang Ye, B. Tian-Jiao, W. Xin, Z. Bing, Y. Zhen-Zhen and H. Tie-Shi, Prog. Chem., 2020, 33, 1159–1174 Search PubMed.
- S. Bhoyate, P. K. Kahol, B. Sapkota, S. Mishra, F. Perez and R. K. Gupta, Surf. Coat. Technol., 2018, 345, 113–122 CrossRef CAS.
- A. A. M. A. Abdah, N. M. Rahman and Y. Sulaiman, Ceram. Int., 2019, 45, 8433–8439 CrossRef.
- M. M. Modawe, S. Kulandaivalu, N. M. Rahman and Y. Sulaiman, Int. J. Hydrogen Energy, 2018, 43, 17328–17337 CrossRef.
- C. Ma, R. Wang, Z. Xie, H. Zhang, Z. Li and J. Shi, J. Porous Mater., 2017, 24, 1437–1445 CrossRef CAS.
- Q. Liu, J. Zhu, L. Zhang and Y. Qiu, Renewable Sustainable Energy Rev., 2018, 81, 1825–1858 CrossRef CAS.
- M. A. A. Mohd Abdah, N. A. Zubair, N. H. N. Azman and Y. Sulaiman, Mater. Chem. Phys., 2017, 192, 161–169 CrossRef CAS.
- S. Nur, S. Mamat, S. A. Rasyid, Z. Zainal and Y. Sulaiman, Electrochim. Acta, 2018, 261, 548–556 CrossRef.
- S. Nur, S. Mamat, S. A. Rasyid, Z. Zainal and Y. Sulaiman, J. Polym. Sci., Part A: Polym. Chem., 2017, 56, 50–58 Search PubMed.
- M. A. A. Mohd Abdah, N. A. Rahman and Y. Sulaiman, Electrochim. Acta, 2018, 259, 466–473 CrossRef CAS.
- J. Yan, J. H. Choi and Y. U. Jeong, Mater. Des., 2018, 139, 72–80 CrossRef CAS.
- J. K. Gan, Y. S. Lim, A. Pandikumar, N. M. Huang and H. N. Lim, RSC Adv., 2015, 5, 12692–12699 RSC.
- S. K. Nataraj, K. S. Yang and T. M. Aminabhavi, Prog. Polym. Sci., 2012, 37, 487–513 CrossRef CAS.
- S. Peng, L. Li, J. Kong Yoong Lee, L. Tian, M. Srinivasan, S. Adams and S. Ramakrishna, Nano Energy, 2016, 22, 361–395 CrossRef CAS.
- H. Feng, H. Hu, H. Dong, Y. Xiao, Y. Cai, B. Lei, Y. Liu and M. Zheng, J. Power Sources, 2016, 302, 164–173 CrossRef CAS.
- L. Qie, W. Chen, H. Xu, X. Xiong, Y. Jiang, F. Zou, X. Hu, Y. Xin, Z. Zhang and Y. Huang, Energy Environ. Sci., 2013, 6, 2497 RSC.
- C. Huang, C. T. Hsieh, P. C. Kuo and H. Teng, J. Mater. Chem., 2012, 22, 7314 RSC.
- D. Lee, J. Y. Jung, M. J. Jung and Y. S. Lee, Chem. Eng. J., 2015, 263, 62–70 CrossRef CAS.
- M. S. A. Rahaman, A. F. Ismail and A. Mustafa, Polym. Degrad. Stab., 2007, 92, 1421–1432 CrossRef CAS.
- D. Zhu, C. Xu, N. Nakura and M. Matsuo, Carbon, 2002, 40, 363–373 CrossRef CAS.
- Z. Zhai, L. Zhang, T. Du, B. Ren, Y. Xu, S. Wang, J. Miao and Z. Liu, Mater. Des., 2022, 221, 111017 CrossRef CAS.
- H. Yang, S. Kannappan, A. S. Pandian, J. H. Jang, Y. S. Lee and W. Lu, Nanotechnology, 2017, 28, 445401 CrossRef.
- Z. Bo, Z. Wen, H. Kim, G. Lu, K. Yu and J. Chen, Carbon, 2012, 50, 4379–4387 CrossRef CAS.
- A. Singh, N. Tiwari, P. B. Karandikar, and A. Dubey, Internat. Conf. on Industrial Instrumentation and Control (ICIC), 2015, DOI:10.1109/iic.2015.7150826.
- N. Syarif, Int. Trans. J. Eng., Manage., Appl. Sci. Technol., 2012, 3, 21–34 CAS.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS.
- A. Khamkeaw, T. Asavamongkolkul, T. Perngyai, B. Jongsomjit and M. Phisalaphong, Molecules, 2020, 25, 4063 CrossRef CAS PubMed.
- Y. Yang, K. Ge, S. Ur Rehman and H. Bi, Rare Met., 2022, 41, 3957–3975 CrossRef CAS.
- K. Lota, A. Sierczyńska, I. Acznik and G. Lota, Chemik, 2013, 67, 1138–1145 CAS.
- F. Lufrano and P. Staiti, Int. J. Electrochem. Sci., 2010, 5, 903–916 CrossRef CAS.
- Y. Cai, Z. Qin and L. Chen, Prog. Nat. Sci.: Mater. Int., 2011, 21, 460–466 CrossRef.
- D. J. Tarimo, K. O. Oyedotun, N. F. Sylla, A. A. Mirghni, N. M. Ndiaye and N. Manyala, J. Energy Storage, 2022, 51, 104378, DOI:10.1016/j.est.2022.104378.
- P. Tamilarasan, A. Kumar Mishra, and S. Ramaprabhu, 2011 Int. Conf. on Nanoscience, Technology and Societal Implications, 2011, pp. 1–5 Search PubMed.
- C. Du and N. Pan, Nanotechnol. Law Bus., 2007, 4, 3 Search PubMed , http://ningpan.net/publications/101-150/125.pdf.
- M. G. Sumdani, M. R. Islam, A. N. A. Yahaya and S. I. Safie, Polym. Eng. Sci., 2021, 62, 269–303 CrossRef.
- M. S. Halper and J. C. Ellenbogen, Supercapacitors: A brief overview, The MITRE Corporation, McLean, Virginia, USA, 2006 Search PubMed.
- J. Li, X. Cheng, A. Shashurin and M. Keidar, Graphene, 2012, 1, 1–13 CrossRef CAS.
- C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef PubMed.
- I. Ahmad, R. Pawara, R. Girase, A. Pathan, V. Jagatap, N. C. Desai, Y. O. Ayipo, S. J. Surana and H. Patel, ACS Omega, 2022, 7, 21820–21844 CrossRef CAS PubMed.
- C. H. Wu, J. Zhu, B. Zhang, H. Shi, H. Zhang, S. Yuán, Y. Yu, G. Chen and C. Chen, J. Colloid Interface Sci., 2023, 650, 1871–1880 CrossRef CAS.
- M. Fu, W. Chen, Y. Lei, H. Yu, Y. Lin and M. Terrones, Adv. Mater., 2023, 35, 2300940 CrossRef CAS.
- J. Rajendran, A. K. Sundramoorthy, D. Ganapathy, R. Atchudan, M. A. Habila and D. Nallaswamy, J. Hazard. Mater., 2022, 440, 129705 CrossRef CAS PubMed.
- P. Karthika, N. Rajalakshmi and K. S. Dhathathreyan, Soft Nanosci. Lett., 2012, 2, 59–66 CrossRef CAS.
- T. Kim, G. J. Jung, S. M. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed.
- T. Y. Kim, H. W. Lee, M. Stoller, D. R. Dreyer, C. W. Bielawski, R. S. Ruoff and K. S. Suh, ACS Nano, 2010, 5, 436–442 CrossRef PubMed.
- K. C. Moon, Z. B. Li, Y. D. Yao, Z. Lin, Q. Liang, J. C. Agar, M. Song, M. Liu, and C. P. Wong, Proceedings 60th Electronic Components and Technology Conference (ECTC), 2010, DOI:10.1109/ectc.2010.5490644.
- D. J. Tarimo, K. O. Oyedotun, A. A. Mirghni and N. I. Manyala, Int. J. Hydrogen Energy, 2020, 45, 13189–13201, DOI:10.1016/j.ijhydene.2020.03.059.
- R. Ramachandran, M. Saranya, V. Velmurugan, B. P. C. Raghupathy, S. K. Jeong and A. N. Grace, Appl. Energy, 2015, 153, 22–31 CrossRef CAS.
- F. Zhang, K. Yang, G. Liu, Y. Chen, M. Wang, S. Li and R. Li, Composites, Part A, 2022, 160, 107051 CrossRef CAS.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS.
- M. A. Pope, S. Korkut, C. Punckt and I. A. Aksay, J. Electrochem. Soc., 2013, 160, A1653–A1660 CrossRef CAS.
- Z. S. Iro, Int. J. Electrochem. Sci., 2016, 11, 10628–10643 CrossRef CAS.
- W. Lv, D. M. Tang, Y. B. He, C. H. You, Z. Q. Shi, X. C. Chen, C. M. Chen, P.-X. Hou, C. Liu and Q. H. Yang, ACS Nano, 2009, 3, 3730–3736 CrossRef CAS PubMed.
- J. Yan, J. Liu, Z. Fan, T. Wei and L. Zhang, Carbon, 2012, 50, 2179–2188 CrossRef CAS.
- R. Lakra, R. Kumar, P. K. Sahoo, D. Thatoi and A. Soam, Inorg. Chem. Commun., 2021, 133, 108929 CrossRef CAS.
- R. Liang, Y. Du, P. Xiao, J. Cheng, S. Yuan, Y. Chen, J. Yuan and J. Chen, Nanoscale Adv., 2021, 3, 6294–6309 RSC.
- T. E. Balaji, H. Tanaya Das and T. Maiyalagan, ChemElectroChem, 2021, 8, 1723–1746 CrossRef CAS.
- J. Zheng, R. Zhang, P. S. Yu and X. F. Wang, J. Alloys Compd., 2019, 772, 359–365 CrossRef CAS.
- Z. Zhang, Z. Xu, Z. Yao, Y.-Q. Meng, Q. Xia and D. Li, J. Alloys Compd., 2019, 805, 396–403 CrossRef CAS.
- S. Mothkuri, S. Chakrabarti, H. Gupta, B. Padya, T. N. Rao and P. K. Jain, Mater. Today: Proc., 2020, 26, 142–147 CAS.
- T. F. Yi, J. Mei, B. Guan, P. Cui, S. Luo, Y. Xie and Y. Liu, Ceram. Int., 2020, 46, 421–429 CrossRef CAS.
- J. Lin, Y. Yan, H. Wang, X. Zheng, Z. Jiang, Y. Wang, S. Ding, J. Cao, W. Fei and J. Feng, J. Alloys Compd., 2019, 794, 255–260 CrossRef CAS.
- C. C. Hu, K. H. Chang, M. C. Lin and Y. T. Wu, Nano Lett., 2006, 6, 2690, DOI:10.1021/nl061576a.
- L. Q. Chen, Y. Hou, J. H. Kang, A. Hirata, T. Fujita and M. Chen, Adv. Energy Mater., 2013, 3, 851–856, DOI:10.1002/aenm.201300024.
- Y. Xu, J. Wei, L. Tan, J. Yu and Y. Chen, J. Mater. Chem. A, 2015, 3, 7121–7131 RSC.
- J. J. Jhao, C. H. Lin, T. K. Yeh, H. C. Wu, M. C. Tsai and C. K. Hsieh, Surf. Coat. Technol., 2017, 320, 263–269 CrossRef CAS.
- B. H. Kim, C. D. Kim and G. S. Lee, J. Electroanal. Chem., 2016, 760, 64–70 CrossRef CAS.
- B. K. Balan, H. D. Chaudhari, U. K. Kharul and S. Kurungot, RSC Adv., 2013, 3, 2428, 10.1039/C2RA22776B.
- K. S. Yang, Y. W. Joo, K. T. Chan, J. H. Kim, and W. J. Lee, in Proc. 10th WSEAS Int. Conf. on Computers, 2006, pp. 888–892 Search PubMed.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2004, 16, 3184–3190, DOI:10.1021/cm049649j.
- Y. Liu, M. Liu, P. Zheng, D. Ge and L. Yang, Mater. Des., 2019, 182, 108022, DOI:10.1016/j.matdes.2019.108022.
- A. V. Radhamani, M. Krishna Surendra and M. S. Ramachandra Rao, Mater. Des., 2018, 139, 162–171 CrossRef CAS.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- J. Shin, J. K. Seo, R. Yaylian, A. Huang and Y. S. Meng, Int. Mater. Rev., 2019, 65, 356–387 CrossRef.
- N. Yu, H. Yin, W. Zhang, Y. Liu, Z. Tang and M.-Q. Zhu, Adv. Energy Mater., 2015, 6, 1501458 CrossRef.
- X. Song, H. Wang, Z. Li, C. Du and R. Guo, Chem. Rec., 2022, 22, e202200118 CrossRef CAS PubMed.
- T. Yue, B. Shen and P. Gao, Renewable Sustainable Energy Rev., 2022, 158, 112131 CrossRef CAS.
- S. A. Klankowski, G. P. Pandey, G. Malek, C. R. Thomas, S. L. Bernasek, J. Wu and J. Li, Nanoscale, 2015, 7, 8485–8494 RSC.
- Y. Wen, T. Qin, Z. Wang, X. Jiang, S. Peng, J. Zhang, J. Hou, F. Huang, D. He and G. Cao, J. Alloys Compd., 2017, 699, 126–135 CrossRef CAS.
- O. Pech and S. Maensiri, J. Alloys Compd., 2019, 781, 541–552, DOI:10.1016/j.jallcom.2018.12.088.
- D. Zhou, H. Lin, F. Zhang, H. Niu, L. Cui, Q. Wang and F. Qu, Electrochim. Acta, 2015, 161, 427–435 CrossRef CAS.
- N. H. Kwon, K.-G. Lee, H. K. Kim and S. J. Hwang, Mater. Chem. Front., 2021, 5, 3549–3575 RSC.
- H. Xu, S. Li, J. Yang and J. Hu, J. Colloid Interface Sci., 2018, 513, 448–454 CrossRef PubMed.
- C. M. Yang and B. H. Kim, J. Alloys Compd., 2019, 780, 428–434 CrossRef CAS.
- H. Z. Chi, H. Zhu and L. Gao, J. Alloys Compd., 2015, 645, 199–205 CrossRef CAS.
- D. G. Lee and B.-H. Kim, Synth. Met., 2016, 219, 115–123 CrossRef CAS.
- A. Śliwak and G. Gryglewicz, Energy Technol., 2014, 2, 819–824 CrossRef.
- L. Wang, M. M. Huang, S. Chen, L. P. Kang, X. He, Z. Lei, F. Shi, H. Xu and Z.-H. Liu, J. Mater. Chem. A, 2017, 5, 19107–19115 RSC.
- Y. Yang, F. Yang, H. Hu, S. Lee, Y. Wang, H. Zhao, D. Zeng, B. Zhou and S. Hao, Chem. Eng. J., 2017, 307, 583–592 CrossRef CAS.
- S. Zhang, Y. Pang, Y. Wang, B. Dong, S. Lu, M. Li and S. Ding, J. Alloys Compd., 2018, 735, 1722–1729 CrossRef CAS.
- B.-S. Yin, Q. Wang, S.-W. Zhang, C. Liu, Q. Ren and K. Ke, ACS Appl. Mater. Interfaces, 2016, 8, 26019–26029 CrossRef CAS.
- G. Cheng, W. Yang, C. Dong, T. Kou, Q. Bai, H. Wang and Z. Zhang, J. Mater. Chem. A, 2015, 3, 17469–17478 RSC.
- Y. Wang, S. Huang, Y. Lu, S. Cui, W. Chen and L. Mi, RSC Adv., 2017, 7, 3752–3759 RSC.
- K. S. Hui, K. S. Hui, K. S. Hui and K. S. Kim, J. Mater. Chem. A, 2016, 4, 9113–9123 RSC.
- G. Zhu, J. Chen, Z. Zhang, Q. Kang, X. Feng, Y. Li, Z.-D. Huang, L. Wang and Y. Ma, Chem. Commun., 2016, 52, 2721–2724 RSC.
- R. Wang, D. Jin, Y. Zhang, S. Wang, J. Lang, X. Yan and L. Zhang, J. Mater. Chem. A, 2017, 5, 292–302 RSC.
- L. Zang, J. Zhu and Y. Xia, J. Mater. Eng. Perform., 2013, 23, 679–683 CrossRef.
- M. Kundu and L. Liu, Mater. Lett., 2015, 144, 114–118 CrossRef CAS.
- Q. Li, J. Guo, D. Xu, J. Guo, X. Ou, Y. Hu, H. Qi and F. Yan, Small, 2018, 14, 1704203, DOI:10.1002/smll.201800426.
- S. Aloqayli, C. K. Ranaweera, Z. Wang, K. Siam, P. K. Kahol, P. Tripathi, O. N. Srivastava, B. K. Gupta, S. R. Mishra, F. Perez, X. Shen and R. K. Gupta, Energy Storage Mater., 2017, 8, 68–76 CrossRef.
- S. J. Mammadyarova, Azerb. Chem. J., 2021, 2, 80–93 CrossRef.
- S. Ameen, M. S. Akhtar, H.-K. Seo, Y. S. Kim and H. S. Shin, Chem. Eng. J., 2012, 187, 351–356 CrossRef CAS.
- X. Wang, A. Hu, C. Meng, C. Y. Wu, S. Yang and X. Hong, Molecules, 2020, 25, 269 CrossRef CAS PubMed.
- D. Majumdar and S. Ghosh, J. Energy Storage, 2020, 34, 101995 CrossRef.
- M. Yaseen, M. A. K. Khattak, M. Humayun, M. Usman, S. S. Shah, S. Bibi, B. S. U. Hasnain, S. M. Ahmad, A. Khan, N. Shah, A. A. Tahir and H. Ullah, Energies, 2021, 14, 7779 CrossRef CAS.
- J. E. Kim, Y. G. Ko, S. Y. Lee, D. Y. Lee, S. W. Kim, Y. Kim and C.-C. Yang, Int. J. Electrochem. Sci., 2022, 46, 23564–23577 CAS.
- J. Bhagwan, V. Sivasankaran, K. L. Yadav and Y. Sharma, J. Power Sources, 2016, 327, 29–37, DOI:10.1016/j.jpowsour.2016.07.040.
- Y. Ju, X. Liu, X. Ye, M. Dai, B. Fang, X. Shen and L. Liu, ACS Appl. Nano Mater., 2023, 6, 13792–13823 CrossRef CAS.
- N. M. Ndiaye, B. D. Ngom, N. F. Sylla, T. M. Masikhwa, M. J. Madito, D. Momodu, T. Ntsoane and N. Manyala, J. Colloid Interface Sci., 2018, 532, 395–406 CrossRef CAS PubMed.
- R. Thangappan, S. Kalaiselvam, A. Elayaperumal and R. Jayavel, Solid State Ionics, 2014, 268, 321–325 CrossRef CAS.
- B. H. Kim, C. H. Kim, K. S. Yang, A. Rahy and D. J. Yang, Electrochim. Acta, 2012, 83, 335–340 CrossRef CAS.
- G. Huang, C. Li, J. Bai, X. Sun and H. Liang, Int. J. Hydrogen Energy, 2016, 41, 22144–22154 CrossRef CAS.
- T. Prasankumar, V. S. Irthaza Aazem, P. Raghavan, K. Prem Ananth, S. Biradar, R. Ilangovan and S. P. Jose, J. Alloys Compd., 2017, 695, 2835–2843 CrossRef CAS.
- S. J. Uke, S. P. Mardikar, A. Kumar, Y. Kumar, M. Gupta and Y. Kumar, R. Soc. Open Sci., 2021, 8, 210567, DOI:10.1098/rsos.210567.
- M. G. Tadesse, E. Kasaw, B. Fentahun, E. Loghin and J. F. Lübben, Energies, 2022, 15, 2471 CrossRef CAS.
- H. Mu, J. Bai, C. Li and W. Sun, J. Alloys Compd., 2019, 775, 872–882 CrossRef CAS.
- R. Holze, Polymers, 2020, 12, 1835 CrossRef CAS PubMed.
- V. V. Kondratiev and R. Holze, Chem. Pap., 2021, 75, 4981–5007 CrossRef CAS.
- S. Banerjee, K. Kar and K. Das, Recent Pat. Mater. Sci., 2014, 7, 173–203 CrossRef CAS.
- M. Samtham, D. Singh, K. Hareesh and R. S. Devan, J. Energy Storage, 2022, 51, 104418 CrossRef.
- R. Holze, Molecules, 2022, 27, 546 CrossRef CAS PubMed.
- S. Banerjee and K. Kar, Recent Pat. Mater. Sci., 2014, 7, 131–150 CrossRef CAS.
- Z. Li and L. Gong, Materials, 2020, 13, 548 CrossRef CAS.
- D. Li, J. Huang and R. B. Kaner, Acc. Chem. Res., 2008, 42, 135–145 CrossRef PubMed.
- S. R. Sivakumar, W. J. Kim, J. A. Choi, D. R. MacFarlane, M. Forsyth and D. W. Kim, J. Power Sources, 2007, 171, 1062–1068 CrossRef.
- H. Li, J. Wang, Q. Chu, Z. Wang, F. Zhang and S. Wang, J. Power Sources, 2009, 190, 578–586, DOI:10.1016/j.jpowsour.2009.01.052.
- J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. Wei, ACS Nano, 2010, 4, 5019–5026 CrossRef CAS.
- C. Bavatharani, E. Muthusankar, S. M. Wabaidur, Z. A. Alothman, K. M. Alsheetan, M. Mana AL-Anazy and D. Ragupathy, Synth. Met., 2021, 271, 116609 CrossRef CAS.
- S. P. Nagalingam and A. N. Grace, Mater. Today Chem., 2022, 26, 101113 CrossRef.
- H. Wang, Y. Diao, Y. Lu, H. Yang, Q. Zhou, K. Chrulski and J. M. D’Arcy, Nat. Commun., 2020, 11, 3882 CrossRef CAS.
- Z. Zhao, G. F. Richardson, Q. Meng, S. Zhu, H.-C. Kuan and J. Ma, Nanotechnology, 2015, 27, 042001 CrossRef.
- M. N. Gueye, A. Carella, J. Faure-Vincent, R. Demadrille and J.-P. Simonato, Prog. Mater. Sci., 2020, 108, 100616 CrossRef CAS.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- A. Imani and G. Farzi, J. Mater. Sci.: Mater. Electron., 2015, 26, 7438–7444 CrossRef CAS.
- Z. Niu, P. Luan, Q. Shao, H. Dong, J. Li, J. Chen, D. Zhao, L. Cai, W. Zhou, X. Chen and S. Xie, Energy Environ. Sci., 2012, 5, 8726 RSC.
- H. Mi, X. Zhang, S. An, X. Ye and S. Yang, Electrochem. Commun., 2007, 9, 2859–2862 CrossRef CAS.
- C. Tran, R. Singhal, D. Lawrence and V. Kalra, J. Power Sources, 2015, 293, 373–379 CrossRef CAS.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392–1401 CrossRef CAS.
- A. Kausar, Mater. Res. Innovations, 2021, 26, 1–13 Search PubMed.
- O. Okhay and A. Tkach, Nanomaterials, 2022, 12, 2531 CrossRef CAS PubMed.
- A. M. Díez-Pascual, Polymers, 2021, 13, 2978 CrossRef.
- X. Cai, K. Sun, Y. Qiu and X. Jiao, Crystals, 2021, 11, 947 CrossRef CAS.
- R. Vinodh, R. S. Babu, S. Sambasivam, C. V. V. M. Gopi, S. Alzahmi, H.-J. Kim, A. L. F. de Barros and I. M. Obaidat, Nanomaterials, 2022, 12, 1511 CrossRef CAS PubMed.
- H. Gómez, M. K. Ram, F. Alvi, P. Villalba, E. Stefanakos and A. Kumar, J. Power Sources, 2011, 196, 4102–4108 CrossRef.
- A. H. Gemeay, I. A. Mansour, R. G. El-Sharkawy and A. B. Zaki, Eur. Polym. J., 2005, 41, 2575–2583 CrossRef CAS.
- X. Zhang, L. Ji, S. Zhang and W. Yang, J. Power Sources, 2007, 173, 1017–1023 CrossRef CAS.
- L. Chen, L.-J. Sun, F. Luan, Y. Liang, Y. Li and X.-X. Liu, J. Power Sources, 2010, 195, 3742–3747 CrossRef CAS.
- Q. Yang, Z. Hou and T. Huang, J. Appl. Polym. Sci., 2014, 132, 41615, DOI:10.1002/app.41615.
- M. Li and L. Yang, J. Mater. Sci.: Mater. Electron., 2015, 26, 4875–4879 CrossRef CAS.
- J. Xu, D. Wang, L. Fan, Y. Yuan, W. Wei, R. Liu, S. Gu and W. Xu, Org. Electron., 2015, 26, 292–299 CrossRef CAS.
- M. Rajesh, C. Justin Raj, B.-G. Kim, B.-B. Cho and J. M. Ko, Electrochim. Acta, 2016, 220, 373–383 CrossRef CAS.
- Q. Tang, M. Chen, C. Yang, W. Wang, H. Bao and G. Wang, Chem. Mater., 2015, 7, 15303–15313 CAS.
- L. Yang, Z. Shi and W. Yang, Electrochim. Acta, 2015, 153, 76–82 CrossRef CAS.
- Y. Chen, L. Du, P. Yang, P. Sun, X. Yu and W. Mai, J. Power Sources, 2015, 287, 68–74 CrossRef CAS.
- H. Wang, Y. Zhang, H. Ang, Y. Zhang, H. Tan, Y. Zhang, Y. Guo, J. C. Franklin, X.-L. Wu, S. Madhavi, H. J. Fan and Q. Yan, Adv. Funct. Mater., 2016, 26, 3082–3093 CrossRef CAS.
- L. Cao, F. Xu, Y. Y. Liang and H.-L. Li, Adv. Mater., 2004, 16, 1853–1857 CrossRef CAS.
- J. S. Shayeh, S. O. R. Siadat, M. Sadeghnia, K. Niknam, M. Rezaei and N. Aghamohammadi, J. Mol. Liq., 2016, 220, 489–494 CrossRef CAS.
- J. Ji, X. Zhang, J. Liu, L. Peng, C. Chen, Z. Huang, L. Li, X. Yu and S. Shang, Mater. Sci. Eng., B, 2015, 198, 51–56 CrossRef CAS.
- W. Ji, Y. Zhang, X. Cui, J. Chen, D. Liu, H. Deng and Q. Fu, Chem. Commun., 2015, 51, 7669–7672 RSC.
- T. Qian, J. Zhou, N. Xu, T. Yang, X. Shen, X. Liu, S. Wu and C. Yan, Nanotechnology, 2015, 26, 425402 CrossRef.
- F. Wang, X. Zhan, Z. Cheng, Z. Wang, Q. Wang, K. Xu, M. Safdar and J. He, Small, 2014, 11, 749–755 CrossRef PubMed.
- M. Yu, Y. Zeng, Y. Han, X. Cheng, W. Zhao, C. Liang, Y. Tong, H. Tang and X. Lu, Adv. Funct. Mater., 2015, 25, 3534–3540 CrossRef CAS.
- X. Sun, Q. Li and Y. Mao, Electrochim. Acta, 2015, 174, 563–573 CrossRef CAS.
- R. J. Choudhary, S. Ansari and B. Purty, J. Energy Storage, 2020, 29, 101302 CrossRef.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085, DOI:10.1021/nl400378j.
- S. Patil, N. R. Chodankar, Y. S. Huh and D. W. Lee, J. Power Sources, 2020, 453, 227766 CrossRef CAS.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- L. Nyholm, G. Nyström, A. Mihranyan and M. Strømme, Adv. Mater., 2011, 23, 3751–3769 CrossRef CAS PubMed.
- B. Senthilkumar, P. Thenamirtham and R. Kalai Selvan, Appl. Surf. Sci., 2011, 257, 9063–9067 CrossRef CAS.
- A. Laforgue, P. Simon, C. Sarrazin and J.-F. Fauvarque, J. Power Sources, 1999, 80, 142–148 CrossRef CAS.
- R. B. Ambade, S. B. Ambade, R. R. Salunkhe, V. Malgras, S. H. Jin, Y. Yamauchi and S.-H. Lee, J. Mater. Chem. A, 2016, 4, 7406–7415 RSC.
- R. Prabhu, M. Rajasekhar and A. Subramanian, Int. J. Electrochem. Sci., 2009, 4, 1289–1301 CrossRef.
- S. R. P. Gnanakan, N. Murugananthem and K. Dai, Polym. Adv. Technol., 2009, 22, 788–793 CrossRef.
- S. Nejati, T. E. Minford, Y. Y. Smolin and K. K. S. Lau, ACS Nano, 2014, 8, 5413–5422 CrossRef CAS PubMed.
- B. H. Patil, A. D. Jagadale and C. D. Lokhande, Synth. Met., 2012, 162, 1400–1405 CrossRef CAS.
- A. Alabadi, S. Razzaque, Z. Dong, W. Wang and B. Tan, J. Power Sources, 2016, 306, 241–247 CrossRef CAS.
- C. Fu, H. Zhou, R. Liu, Z. Huang, J. Chen and Y. Kuang, Mater. Chem. Phys., 2012, 132, 596–600 CrossRef CAS.
- H. Q. Zhang, Z. Hu, M. Li, L.-W. Hu and S. Jiao, Mater. Chem. Phys., 2014, 2, 17024–17030 CAS.
- J. Xiao, H. Li, H. Zhang, S. He, Q. Zhang, K. Liu, S. Jiang, A. Greiner and K. Zhang, J. Bioresour. Bioprod., 2022, 7, 245–269 CrossRef CAS.
- Q. Lu and Y. Zhou, J. Power Sources, 2011, 196, 4088–4094 CrossRef CAS.
- R. B. Ambade, S. B. Ambade, N. K. Shrestha, Y. C. Nah, S.-H. Han, W. Lee and S. H. Lee, Chem. Commun., 2013, 49, 2308 RSC.
- A. R. Akbar, W. Tian, M. I. Qadir, Z. Khaliq, Z. Liu, M. Tahir, Y. Hu, C. Xiong and Q. Yang, Colloids Surf., A, 2021, 610, 125644 CrossRef CAS.
- Y. Li, C. Zhu, T. Lu, Z. Guo, D. Zhang, J. Ma and S. Zhu, Carbon, 2013, 52, 565–573 CrossRef CAS.
- Y. Chen, B. Liu, Q. Liu, J. Wang, J. Liu, H. Zhang, S. Hu and X. Jing, Electrochim. Acta, 2015, 178, 429–438 CrossRef CAS.
- M. M. Sk, C. Y. Yue and R. K. Jena, Synth. Met., 2015, 208, 2–12 CrossRef CAS.
- X. Wang, P. Xu, P. Zhang and S. Ma, Materials, 2021, 14, 7148 CrossRef CAS PubMed.
- C. H. Ng, H. N. Lim, Y. S. Lim, W. K. Chee and N. M. Huang, Int. J. Energy Res., 2014, 39, 344–355 CrossRef.
- L. Jiang, X. Lu, C. Xie, G. Wan, H. Zhang and T. Youhong, J. Phys. Chem. C, 2015, 119, 3903–3910 CrossRef CAS.
- W. K. Chee, H. San Lim, I. Harrison, M. Ali, Z. Zainal, C. R. Ng and N. Huang, Electrochim. Acta, 2015, 157, 88–94 CrossRef CAS.
- T. G. Yun, B. Il Hwang, D. Kim, S. Hyun and S. M. Han, ACS Appl. Mater. Interfaces, 2015, 7, 9228–9234 CrossRef CAS.
- D. Majumdar, ChemElectroChem, 2020, 8, 291–336 CrossRef.
- S. Wustoni, D. Ohayon, A. Hermawan, A. Nuruddin, S. Inal, Y. S. Indartono and B. Yuliarto, Polym. Rev., 2023, 1–59 Search PubMed.
- M. Ikram and R. Holze, Polymers, 2023, 15, 730 CrossRef PubMed.
- Y. Han, H. Ha, C. Choi, H. Yoon, P. Matteini, J. Cheong and S. Hwang, Appl. Sci., 2023, 13, 5290 CrossRef.
- N. Ogihara, M. Hasegawa, H. Kumagai, R. Mikita and N. Nagasako, Nat. Commun., 2023, 14, 1472 CrossRef CAS PubMed.
- M. Brunet Cabré, D. Spurling, P. Martinuz, M. Longhi, C. Schröder, H. Nolan, V. Nicolosi, P. E. Colavita and K. McKelvey, Nat. Commun., 2023, 14, 374 CrossRef PubMed.
- J. Zhang, J. Yan, Y. Zhao, Q. Zhou, Y. Ma, Y. Zi, A. Zhou, S. Lin, L. Liao, X. Hu and H. Bai, Nat. Commun., 2023, 14, 64 CrossRef CAS PubMed.
- K. Li, J. Zhao, A. Zhussupbekova, C. E. Shuck, L. Hughes, Y. Dong, S. Barwich, S. Vaesen, I. V. Shvets, M. Möbius, W. Schmitt, Y. Gogotsi and V. Nicolosi, Nat. Commun., 2022, 13, 6884 CrossRef CAS.
- C. Liang, S. Wang, S. Sha, S. Lv, G. Wang, B. Wang, Q. Li, J. Yu, X. Xu and L. Zhang, J. Mater. Chem. C, 2023, 11, 4288–4317 RSC.
- V. Panwar, P. S. Chauhan, S. Kumar, R. Tripathi and A. Misra, ACS Energy Lett., 2023, 8, 1510–1519 CrossRef CAS.
- S. Biswas, N. Shauloff, R. Bisht and R. Jelinek, Adv. Sustainable Syst., 2023, 7, 2370018 CrossRef.
- N. Ma, D. Yang, S. Riaz, L. Wang and K. Wang, Technologies, 2023, 11, 38 CrossRef.
- W. K. Chee, H. N. Lim, Z. Zainal, N. M. Huang, I. Harrison and Y. Andou, J. Phys. Chem. C, 2016, 120, 4153–4172 CrossRef CAS.
- J. Rajendran, A. N. Reshetilov and A. K. Sundramoorthy, RSC Adv., 2021, 11, 3445–3451 RSC.
- Y. Han, Y. Ge, Y. Chao, C. Wang and G. G. Wallace, J. Energy Chem., 2018, 27, 57–72 CrossRef.
- Z. S. Iro, C. Subramani, J. Rajendran and A. K. Sundramoorthy, Carbon Lett., 2021, 31, 237 CrossRef.
- M. Halim, G. Liu, R. E. A. Ardhi, C. Hudaya, O. Wijaya, S. H. Lee, A. Y. Kim and J. K. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 20566–20576 CrossRef CAS.
- M. X. Tran, A. Kim and J. K Lee, Appl. Surf. Sci., 2018, 461, 161–170 CrossRef CAS.
- A.-Y. Kim, R. E. A. Ardhi, G. Liu, J. Y. Kim, H. J. Shin, D. Byun and J. K. Lee, Carbon, 2019, 153, 62–72 CrossRef CAS.
- L. Li, Z. Wei, J. Liang, J. Ma and S. Huang, Results Chem., 2021, 3, 100205 CrossRef CAS.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- L. Khandare, D. J. Late and N. B. Chaure, Front. Chem., 2023, 11 DOI:10.3389/fchem.2023.1166544.
- S. A. Thomas, A. Patra, B. M. Al-Shehri, M. Selvaraj, A. Aravind and C. S. Rout, J. Energy Storage, 2022, 55, 105765 CrossRef.
- S. Kumar, M. A. Rehman, S. Lee, M. Kim, H. Hong, J. Y. Park and Y. Seo, Sci. Rep., 2021, 11, 649 CrossRef CAS PubMed.
- R. Ma, Z. Chen, D. Zhao, X. Zhang, J. Zhuo, Y. Yin, X. Wang, G. Yang and F. Yi, J. Mater. Chem. A, 2021, 9, 11501–11529 RSC.
- Y. Ying, Q. Fan, Z. Li and P. Fu, Mater. Today Sustain., 2023, 24, 100551 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |