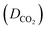Open Access Article
Open Access ArticleAlkylimidazolium-based ionic liquids with tailored anions and cations for CO2 capture†
Abdelbagi Osman *a,
Abobakr K. Ziyada
*a,
Abobakr K. Ziyada b,
Abdul Majeed Khanb and
Fahd Rajaba
b,
Abdul Majeed Khanb and
Fahd Rajaba
aDepartment of Chemical Engineering, College of Engineering, Najran University, P.O. Box 1988, Najran 11001, Saudi Arabia. E-mail: aomustafa@nu.edu.sa; fmrajab@nu.edu.sa
bDepartment of General Studies, Jubail Industrial College, PO Box 10099, Jubail Industrial City 31961, Saudi Arabia. E-mail: taha_a@rcjy.edu.sa; abubakrkhz@yahoo.com; khan_am@rcjy.edu.sa
First published on 29th January 2024
Abstract
A systematic investigation was conducted in the present study to determine how various cations and anions affected the solubility of CO2. To investigate the influence of different cations and anions on the solubility of CO2, twelve ILs were synthesized, characterized, and utilized. These ILs comprised five distinct anions (dioctylsulfosuccinate [DOSS], triflouromethanesulfonate [TFMS], dodecylsulfate [DDS], 3-sulfobezoate [SBA], and benzene sulfonate [BS]), and four distinct cations (1-butyl-3-propanenitrile imidazolium [C2CN Bim], 1-hexyl-3-propanenitrile imidazolium [C2CN Him], 1-octyl-3-propanenitrile imidazolium [C2CN Oim], and 1-decyl-3-propanenitrile imidazolium [C2CN Dim]). The synthesized ILs were characterized using NMR and elemental analysis. Their moisture and halide contents were determined. The gravimetric method (MSB) was employed to determine the solubility of CO2 at various pressures (20, 15, 10, 5, and 1 bar). In addition, the effects of temperature on the solubility of CO2 were investigated. The constant of Henry's law (kH) was also calculated, along with thermodynamic properties including standard enthalpy (H0), entropy (S0), and Gibbs free energy (G0).
1. Introduction
Rapidly increasing industrialization in numerous nations is causing a surge in the demand for carbon dioxide capture and separation.1,2 As a result, for the next few decades, CO2 capture and reduction of the atmospheric CO2 concentration will be critical.3,4 The enhancement of natural carbon sinks, such as forests and oceans, could theoretically mitigate atmospheric CO2 concentrations.5 Nevertheless, the development of novel carbon capture materials is imperative from a commercial and environmental perspective, as existing concepts exhibit long-term promise but are not yet viable.6 Aqueous amine-based solvents are used as CO2-absorbing solvents in many industrial processes nowadays. However, numerous drawbacks exist with these solvents, such as the energy heating requirement to renew the amine. Recently, ionic liquids (ILs) have gained recognition as an alternative material to capture CO2 and for their wide range of engineering applications.ILs typically contain large inorganic and/or organic anions and cations and have melting points below 100 °C.1 Different types of ILs have already been synthesized by combining various anions and cations.1,7 ILs are a class of solvents with unique and versatile properties, including excellent solvency for a wide range of polar and nonpolar compounds, high chemical and thermal stability, negligible vapor pressure, and a broad electrochemical window. These properties make ILs well-suited for a variety of industrial and technological applications, such as biomass processing, electroplating, solar cells, lubricants, and electrolytes.1,8,9 However, these solvents depending on their applications, may not be highly green by type 1 comparisons but may be quite green by type 2 (type 1 comparisons are process- and application-neutral, comparing solvents just on the basis of their mass, whereas type 2 comparisons are tailored to a specific application).10 Moreover, the biodegradability and toxicity of ILs were discussed in details recently.11
ILs are considered as promising CO2 capture solvents, and can physically absorb CO2, which requires less energy to reverse as compared to chemical absorption methods.12,13
The main drawbacks of employing various ILs in the large-scale CO2 are their cost and viscosity that are considered to be higher than that of traditional solvents.14,15 Given that ILs are generally applied in diluted forms with viscosities that are not appreciably higher than water's, the high viscosity issue with ILs can be resolved.11 The low vapor pressure, low volatility, and high stability of ILs in comparison to the solvents already in use are some of the key characteristics that facilitate their usage for CO2 absorption. The efficiency and cost of the CO2 absorption process are significantly affected by the high volatility and high heat degradation of conventional employed solvents such as monoethanolamine, etc. The fact that IL can be regenerated at 1 bar makes it a potentially economical alternative for CO2 uptake from pre-combustion. Moreover, this would reduce the gap in heat transfer between operations and lower the cost of utilities involved. The ability to regenerate the ILs at 1 bar and higher temperatures potentially decrease the cost of the CO2 capture as compared to the high cost of the conventional solvents due to significant equipment expenses connected with vacuum regeneration operations.11,16 Previous research comparing the ILs process's cost and performance indicates that this technology is promising. Furthermore, the potential of ILs to be tailored could be potentially utilized in the development and design of inexpensive, and, thermally stable ILs with high CO2 capture capacity and selectivity.11 Moreover, the high degradation temperatures of ILs prevents equipment against corrosion caused by ILs reacting with impurities.17 Finally, the tuneable nature of ILs allows scientists to design ILs with specific properties tailored to specific applications.1
Computational studies suggest that the anion plays a more significant role than the cation in dissolving CO2 in ILs,18 as the anion is a stronger base and CO2 is a Lewis acid. However, experimental data, reveal that CO2 is more soluble in the [PF6] anion than the [BF4] anion, implying that other factors, such as free volume, also play a role.14 Functionalized ILs, particularly imidazolium-based functionalized ILs, have demonstrated potential for CO2 capture.19 Functionalizing the cation and/or anion with a reactive functional group can significantly enhance CO2 solubility.20 Amine-functionalized ILs absorb CO2 by forming carbamates, while fluoroalkyl groups also exhibit a high affinity for CO2.6,21 Incorporating oxygen-containing functional groups, such as ether and ester groups, into the cation and anion can also increase CO2 solubility by exploiting the ability of the electron-deficient carbon atom to attract electronegative atoms. However, it is crucial to balance the need for high CO2 solubility with the need for easy CO2 desorption from the IL.22,23 Hence, large-scale industrial integration of ILs for CO2 capture requires a comprehensive understanding of their physical and chemical properties. Therefore, experimental techniques and data development are essential for the future of practical, cost-effective, and sustainable CO2 capture using ILs.
The current study meticulously designed IL architectures to evaluate the impact of various structural variations on CO2 solubility. The cations were combined with various alkyl chains and functional groups, while the anions had diverse structures and functional groups. The engineered structures possess features known to enhance the CO2-philicity of a molecule, resulting in augmented CO2 solubility in ILs. Researchers have synthesized and investigated the CO2 solubility in functionalized ILs.24,25 However, no studies have examined the CO2 solubility in imidazolium-based ILs with cations containing nitrile functionality and alkyl chains and anions containing alkyl chains incorporating a sulfonyl group, branched alkyl chain, fluoroalkyl chain, or carbonyl group.
CO2 solubility was investigated by comparing the solubility of CO2 in ILs with varying combinations of cations and anions, as well as ILs at the same pressure and other ILs at varying pressures. To investigate the impact of cations, anions, and pressures on CO2 solubility, ILs containing the cations [C2CN Bim], [C2CN Him], [C2CN Oim], and [C2CN Dim] and incorporating the anions [DDS], [BS], [TFMS], [SBA], and [DOSS] were synthesized, and described.
2. Materials and methods
2.1. Materials
Analytical-grade chemicals were used to produce the ILs without further purification. Their CAS number, source, and grade denote the chemicals. Imidazole (288-32-4, 99%), acrylonitrile (107-13-1, 99%), methanol, anhydrous (67-56-1, 99.8%), 1-bromohexane (111-15-1, 99%), 1-bromodecane (112-29-8, 98%), 1-bromooctane (111-81-1, Aldrich 99%), ethyl acetate, anhydrous (141-78-6, 99.8%), acetone (67-64-1, 99.8%), diethyl ether (60-29-7, 99%), sodium dioctylsulfosuccinate (209-406-4, 98%), sodium triflouromethanesulfonate (2926-30-9, 98%), sodium benzenesulfonate (515-42-4, 97%), sodium dodecylsulfate (205-788-1, 99%), sodium 3-sulfobezoate (17625-03-5, 97%), and dichloromethane (75-09-2, 99.8%), were purchased from Sigma-Aldrich while 1-bromobutane (109-65-9, 98%) was obtained from Merck.2.2. Synthesis
By replacing 1-bromobutane with 1-bromodecane, 1-bromooctcane, and 1-bromohexane, the other nitrile imidazolium-based ILs with decyl (1-decyl-3-propanenitrile imidazolium bromide [C2CN Dim][Br]), octyl (1-octyl-3-propanenitrile imidazolium bromide [C2CN Oim][Br]), and hexyl (1-hexyl-3-propanenitrile imidazolium bromide [C2CN Him][Br]) alkyl chains were synthesized using the same method as described for the synthesis of [C2CN Bim][Br].
Preparation of 1-hexyl-3-propanenitrile imidazolium benzenesulfonate [C2CN Him][BS], 1-hexyl-3-propanenitrile imidazolium sulfobenzoic acid [C2CN Him][SBA], and 1-hexyl-3-propanenitrile imidazolium triflouromethanesulfonate [C2CN Him][TFMS] followed the same protocol as for the synthesis of [C2CN Him][DDS], whereas sodium benzenesulfonate, sodium 3-sulfobezoate and sodium triflouromethanesulfonate were used instead of sodium dodecyl sulfate.
Equimolar amounts of [C2CN Dim][Br] and sodium dioctylsuccinate were dissolved and stirred in acetone for 48 hours to produce 1-decyl-3-propanenitrile imidazolium dioctylsuccinate ([C2CN Dim][DOSS]). The resulting solid product was removed from the mixture, washed with diethyl ether and dried under reduced pressure.
A similar procedure was followed to synthesize 1-octyl-3-propanenitrile imidazolium dioctylsuccinate [C2CN Oim][DOSS], 1-hexyl-3-propanenitrile imidazolium dioctylsuccinate [C2CN Him][DOSS] and 1-butyl-3-propanenitrile imidazolium dioctylsuccinate [C2CN Bim][DOSS] by replacing [C2CN Dim][Br] with [C2CN Oim][Br], [C2CN Him][Br], and [C2CN Bim][Br], respectively.
2.3. Characterization and measurements
The magnetic suspension balance (MSB) is a versatile and precise instrument for measuring the solubility of gases in liquids. It enables real-time monitoring of mass changes, which is essential for detecting equilibrium. This is crucial because equilibrium must be attained before accurate solubility measurements can be made. Additionally, the MSB can be used to confirm the complete degassing of the initial liquid, another prerequisite for accurate results.29
The magnetic suspension balance (MSB) from Rubotherm, Präzisionsmesstechnik GmbH, and Bochum, Germany was used to determine the solubility of the CO2 in the prepared ILs. The instrument employs a magnetic suspension coupling comprising an electromagnet and a suspension magnet, with the electromagnet electronically controlled to maintain the suspension magnet in a frictionless levitation state. The precision and reproducibility of the microbalance were reported to be 0.001 mg and ±0.020 mg, respectively. The pressure and temperature in the measuring cell were controlled to within ±0.05 bar and ±0.2 °C, respectively, as reported.30 To achieve highly accurate weight measurements, all potential environmental disturbances affecting the sample were minimized, controlled, and quantified, except for buoyancy, which was quantified and corrected using MessPro software to control the instrument and record data.
The MSB method for determining the solubility of CO2 in the prepared ILs comprises of four main steps: blank measurement, sample drying, buoyancy correction, and solubility measurement. In the blank measurement, the empty sample container is weighed and its volume is measured. The sample is then dried to remove all water and volatile substances. The volume of the sample inside the sample container is then precisely determined using a buoyancy measurement. The measurement process began at a constant temperature and gradually increased the pressure to the desired value while being continuously monitored. The solubility measurement was considered complete when the MSB showed no further increase in mass. The apparatus, sample preparation, and measurement technique are all described in detail.30–32
3. Results
3.1. Characterization
The synthesized IL structures were confirmed by elemental analysis and NMR spectra. The 1H NMR spectra for the ILs [C2CN Bim][DOSS], [C2CN Him][DOSS], [C2CN Oim][DOSS], [C2CN Dim][DOSS], [C2CN Him][DDS], [C2CN Him][BS], [C2CN Him][TFMS], [C2CN Him][SBA], [C2CN Oim][DDS], [C2CN Oim][BS], [C2CN Oim][TFMS], and [C2CN Oim][SBA] are presented in the ESI† along with the 1H NMR spectra (Fig. SI.1–SI.5†).Elemental analysis, water content, halide content, and purity of all prepared ILs33 are also reported and provided in the ESI.†
3.2. Solubility of CO2 in ILs
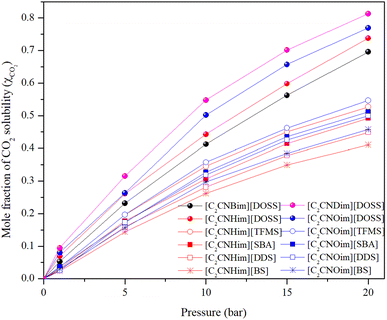
The maximum amount of a gas that can dissolve in a liquid at a given temperature and pressure is its solubility. At equilibrium, the rate of gas molecules entering and leaving the solution is equal. When the pressure increases, more gas molecules collide with the liquid's surface, causing the dissolved gas concentration to increase until a new equilibrium is established.34Table 1 displays the mole fraction versus pressure results for the experimental CO2 solubility measurements taken in the produced ILs at temperatures of 298.15 K and pressures of 1, 5, 10, 15, and 20 bar. When compared to non-functionalized imidazolium-based ILs23,35 and ILs with amine functionality,28,29,36 the time needed for the prepared ILs to reach their maximum solubility capacity is shorter but is still longer than the times reported for [Emim][NTf2] and [Bmim][NTf2].29,37
| xCO2 (bar) | ILs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [C2CN Bim] [DOSS] | [C2CN Him] [DOSS] | [C2CN Him] [TFMS] | [C2CN Him] [SBA] | [C2CN Him] [DDS] | [C2CN Him] [BS] | [C2CN Oim] [DOSS] | [C2CN Oim] [TFMS] | [C2CN Oim] [SBA] | [C2CN Oim] [DDS] | [C2CN Oim] [BS] | [C2CN Dim] [DOSS] | |
| a Standard uncertainties u of temperature, pressure and MSB (mass reading) are 0.05 K, 0.25 kPa and 0.00002 g respectively. The combined standard uncertainties of the CO2 solubility for the ILs [C2CN Bim][DOSS][C2CN Him][DOSS], [C2CN Him][TFMS], [C2CN Him] [SBA], [C2CN Him]DDS], [C2CN Him][BS], [C2CN Oim][DOSS], [C2CN Oim][TFMS], [C2CN Oim][SBA], [C2CN Oim][DDS], [C2CN Oim][BS], and [C2CN Dim][DOSS] were 0.009, 0.014, 0.016, 0.014, 0.010, 0.013, 0.012, 0.013, 0.011, 0.010, 0.013, and 0.014 respectively. | ||||||||||||
| 1 | 0.053 | 0.070 | 0.035 | 0.032 | 0.0267 | 0.023 | 0.081 | 0.041 | 0.037 | 0.025 | 0.030 | 0.095 |
| 5 | 0.232 | 0.261 | 0.197 | 0.177 | 0.1595 | 0.144 | 0.264 | 0.214 | 0.192 | 0.173 | 0.157 | 0.315 |
| 10 | 0.412 | 0.443 | 0.340 | 0.306 | 0.281 | 0.262 | 0.502 | 0.386 | 0.347 | 0.319 | 0.297 | 0.548 |
| 15 | 0.562 | 0.598 | 0.460 | 0.414 | 0.378 | 0.350 | 0.657 | 0.506 | 0.455 | 0.426 | 0.384 | 0.701 |
| 20 | 0.712 | 0.738 | 0.547 | 0.492 | 0.449 | 0.411 | 0.769 | 0.592 | 0.532 | 0.491 | 0.448 | 0.813 |
The solubility data in Table 1 demonstrate that the solubility of CO2 in all of the investigated ILs positively correlates with an increase in pressure. This phenomenon can be attributed to the fact that when the pressure increases, the gas molecules are forced to dissolve into the IL solution to minimize the applied pressure to the greatest extent. Moreover, the solubility of CO2 demonstrated a non-linear pattern with the increase in pressure across all the investigated ILs as shown in Fig. 2.
 | ||
| Fig. 2 CO2 solubility in the [C2CN Him] and [C2CN Oim]-based ILs incorporated with [DDS], [BS], [TFMS], [SBA] and [DOSS] anions at 298.15 K at 20 bar. | ||
Emerging evidences suggest that CO2 solubility in ILs is influenced by more than just interaction strength and free volume.30,38 Seki et al. demonstrated that interactions alone cannot fully explain CO2 sorption in ILs, as was previously believed, and that the strong Lewis acid-base interactions between ILs and dissolved CO2 are not the only factor affecting CO2 solubility.38 Hereafter, we discuss the influence of anions and cations on CO2 solubility.
3.3. Influence of anions on the CO2 solubility
As demonstrated by the CO2 solubility data in Table 1, the anion selection has a substantial effect on CO2 solubility, which is consistent with previous research.32,39,40 Fig. 2 and SI.6 to SI.9 (ESI†) display the isotherms of CO2 solubility in the prepared ILs at 20, 15, 10, 5, and 1 bar. The presented figures illustrate the effect of the anion on the solubility of CO2 in ILs containing [C2CN Him] and [C2CN Oim] cations at 298.15 K.ILs with the [DOSS] anion exhibit a significantly higher affinity for carbon dioxide (CO2) than those with the [DDS], [TFMS], [SBA], and [BS] anions due to their unique properties that enhance CO2 solubility and affinity. These properties include carbonyl and sulfonyl functional groups, which increase the molecule's CO2-philicity and solubility. Additionally, the long and branched alkyl chains on the DOSS anion further enhance CO2 solubility, as demonstrated in previous studies.41 Moreover, the molar free volume of the IL plays a significant role in determining the CO2 solubility capacity. Generally, larger anions result in a larger free volume, which provides more space for CO2 molecules to occupy.22 The molar-free volume of the IL was found to have a significant impact on CO2 solubility, comparable to the anion basicity, which is a well-known key factor affecting CO2 solubility in ILs.6
The fluoroalkyl groups in the [TFMS] anion interact more strongly with CO2, making it easier for CO2 to dissolve in the IL. This is in contrast to ILs based on the [SBA], [DDS], and [BS] anions, which do not form such strong interactions.42,43 Two types of interactions have been identified between anions and carbon dioxide (CO2) in ILs: acid–base interactions and CO2–fluorine interactions. The solubility of CO2 in ILs is positively correlated with the number of CF3 groups in the anion.44,45 The higher CO2 solubility in [SBA]-based ILs than in [DDS] and [BS]-based ILs may be due to the carboxylate ion in the [SBA] anion, which enhances CO2-philicity by increasing van der Waals interactions between CO2 and the anion. The longer alkyl chain of the [DDS] anion increases CO2 solubility in [DDS]-based ILs compared to [BS]-based ILs by strengthening van der Waals interactions between the gas and liquid.
3.4. Influence of cations on the CO2 solubility
The impact of the IL cation on CO2 solubility is examined by comparing the CO2 solubility of synthesized ILs with same anions but different cations. The length of the side chain connecting the CN group to the imidazole ring remains constant, while the attachment of the alkyl chain to the imidazolium cation is altered. These cations were chosen because previous research has shown that adding polar and nonreactive functional groups, such as nitriles, to the cations of ILs can significantly improve CO2/CH4 selectivity while having little to no effect on CO2 solubility compared to non-functionalized ILs.20,46–49From the literature, it is believed that elongating the alkyl chain attached to the imidazolium cation effectively reduces the strength of the strong ion–ion interactions,50 while a shorter side chain connecting the CN group to the IL cation reduces viscosity and melting point, and enhances interactions between the ions and CO2 due to the weaker ionic bonding within the molecule.51
However, because ILs with longer alkyl chains tend to have higher melting points,50 and possibly higher CO2 solubility, butyl to decyl alkyl chains were used in this study. Fig. 3 illustrates the CO2 solubility in [DOSS]-based ILs containing [C2CNBim], [C2CNHim], [C2CNOim], and [C2CNDim] cations at 298.15 K and 5 bar.
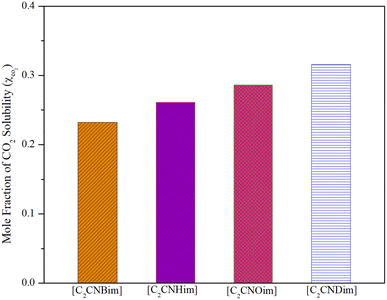 | ||
| Fig. 3 Effect of cations on CO2 solubility in the synthesized ILs at a temperature of 278.15 K and a pressure of 5 bar. | ||
As shown in Fig. 3, an increase in solubility occurred as a result of the cation's alkyl chain lengthening (the CO2 solubility of [C2CNBim] < [C2CNHim] < [C2CNOim] < [C2CNDim]). As predicted, the increased free volume originating from the lengthened alkyl chain of the cation enhances the solubility of CO2 in the ionic liquid (IL).52 At higher pressures, the increase in solubility values with increasing alkyl chain length becomes more noticeable.29
In contrast to conventional ILs, where the increase in solubility is less pronounced for alkyl chains longer than 8 carbon atoms,53 the solubility of CO2 in ILs [C2CNBim][DOSS], [C2CNHim][DOSS], [C2CNOim][DOSS], and [C2CNDim][DOSS] does not exhibit a linear relationship with the length of the cation's alkyl chain. This may be due to the presence of the CN group on the IL side chain and the expansion of the molar volume.24 Solid-state structural analysis of nitrile-functionalized ILs revealed that intermolecular hydrogen bonds form polymeric super-molecular networks.24
Table 1 shows the relationship between pressure and CO2 solubility in ILs. When choosing an IL for CO2 absorption, it is important to consider the process conditions, including pressure and diffusion coefficient, as well as the availability and cost of the material.
The CO2 solubility in [C2CNHim] and [C2CNOim] cations with [TFMS], [SBA], [DDS], and [BS] anions is higher at 15 and 20 bar than in [C2CNOim] with [DOSS] anions at 1, 5 and 10 bar. This demonstrates the importance of considering diffusivity when selecting a material for CO2 absorption. CO2 solubility in ILs increases significantly with pressure, but the rate of increase slows down and eventually plateaus. This is because Henry's sorption in the inter-ion space is responsible for the high rate of solubility at low pressure. As pressure increases, CO2 molecules continuously occupy these spaces, preventing them from settling there.38 To allow for additional CO2 molecules to enter, the inter-ion gaps must be widened, which requires energy. This limits the amount of CO2 that can enter the IL, resulting in the plateauing of solubility. Understanding CO2 diffusivity in ILs is essential for designing and developing ionic liquid-based reaction and separation processes.
CO2 diffusivity was estimated for the prepared ILs using a method developed by Moganty and Baltus,54 as follows:
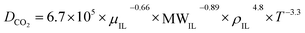 | (1) |
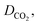 μIL, MWIL and T are the diffusion coefficient (m2 s−1), viscosity of IL (cP), density of IL(g cm−3), molar mass of IL (g mol−1) and temperature (k) respectively. The density and viscosity values used to calculate the diffusivity were published previously.28,36,37 The calculated CO2 diffusivity at temperatures of 298.15 to 348.15 K are reported in Table 2.
μIL, MWIL and T are the diffusion coefficient (m2 s−1), viscosity of IL (cP), density of IL(g cm−3), molar mass of IL (g mol−1) and temperature (k) respectively. The density and viscosity values used to calculate the diffusivity were published previously.28,36,37 The calculated CO2 diffusivity at temperatures of 298.15 to 348.15 K are reported in Table 2.
| Temp-erature, K | ILs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [C2CN Bim] [DOSS] | [C2CN Him] [DOSS] | [C2CN Him] [TFMS] | [C2CN Him] [SBA] | [C2CN Him] [DDS] | [C2CN Him] [BS] | [C2CN Oim] [DOSS] | [C2CN Oim] [TFMS] | [C2CN Oim] [SBA] | [C2CN Oim] [DDS] | [C2CN Oim] [BS] | [C2CN Dim] [DOSS] | |
| 298.15 | 5.18 | 3.24 | 66.64 | 12.49 | 6.35 | 33.85 | — | 25.39 | 8.24 | — | 13.50 | — |
| 308.15 | 9.53 | 5.93 | 111.19 | 21.73 | 11.76 | 56.33 | 3.91 | 42.44 | 14.57 | 6.63 | 25.11 | 2.89 |
| 318.15 | 16.68 | 10.74 | 179.23 | 38.17 | 21.10 | 98.41 | 7.14 | 73.74 | 25.40 | 12.11 | 43.28 | 5.23 |
| 328.15 | 28.16 | 18.17 | 270.87 | 65.92 | 36.82 | 155.78 | 11.91 | 120.71 | 42.20 | 20.38 | 73.24 | 8.73 |
| 338.15 | 48.04 | 29.5 | 418.2 | 112.39 | 59.71 | 229.9 | 18.58 | 189.83 | 71.90 | 31.32 | 121.31 | 13.38 |
| 348.15 | 77.12 | 46.31 | 626.32 | 181.3 | 90.56 | 309.68 | 29.1 | 277.07 | 125.25 | 49.8 | 193.00 | 21.82 |
The diffusion coefficient increases with temperature, as shown in Table 2. The high viscosity of ILs with the [DOSS] anion is likely the main reason for their low diffusion coefficients. ILs with [TFMS] and [BS] anions have higher diffusion coefficients than ILs with other anions, which may be due to their lower viscosity.27,28 Gas diffusion in ILs is partially dependent on viscosity; an increase in viscosity increases diffusion time,29 while the dependence of diffusivity on viscosity decreases with increasing solute molar volume.55 The CO2 diffusivity in ILs with the [DOSS], [SBA], [DDS] and [BS] anions is low compared to that reported for the phosphonium-based IL (diffusion coefficients for [P66614]-based ILs are in the range of 18.4 × 10−8 to 27.1 × 10−8).55 Furthermore, the diffusion coefficients of the current ILs are low compared to that reported for most of the imidazolium-based ILs such as [Hmim][BF4], [Hmim][Tf2N], [Omim][BF4], and [Emim][Tf2N].54 These results are in a good agreement with that reported by Morgan,56 et al., Moganty, et al.,54 and Camper, D., et al.57 Their findings demonstrated that, while the activation energies for self-diffusion and viscosity are similar, they are considerably higher for CO2 diffusion in ILs.54,56,57 This comparison reveals that the molecular motion needed for carbon dioxide diffusion through an IL is significantly similar to that of self-diffusion and viscosity. The larger size of cations and anions in IL compared to CO2 as well as the strong anion–cation coulombic interactions involved are likely the causes of the higher activation energy for self-diffusion and viscosity.54,56,57 Furthermore, solvent viscosity is an essential component of all the formulas used for predicting gas diffusivities in ILs. To focus solely on the relationship between CO2 diffusivity and solvent viscosity, a log–log plot of diffusivity versus solvent viscosity was generated using ILs with viscosities ranging across multiple orders of magnitude. The results showed that CO2 diffusivity is generally dependent on ILs viscosity. In addition, Arshad reported that gas diffusion in an ionic liquid is dependent on its viscosity; that is, the higher the viscosity of the IL, the longer the diffusion time and, consequently, the time needed to attain equilibrium, and vice versa.58 Recently, comprehensive findings on how diffusivity varies based on the type of ILs was reported.11
3.5. Henry's constant
To design and improve separation technologies, it is essential to understand the equilibrium solubility characteristics and thermodynamic factors of any IL. The thermodynamic properties provide insight into the level of orderliness in the liquid/gas mixture and the extent of interaction between the liquid and dissolved gas.59 The Henry's law constant was calculated using experimental data on CO2 solubility in the studied ILs. The Gibbs free energy, enthalpy, and entropy of the solution, among other thermodynamic properties, can be determined using the Henry's law constant for CO2 in the studied ILs.59,60Henry's law constant (kH) is a widely used metric for reporting gas solubility in liquids. It relates the equilibrium mole fraction 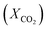 of a solute in the liquid phase to its partial pressure in the gas phase.61 Henry's law can be expressed in a variety of ways, each with its own unit and interpretation. For ILs having a very low or negligible vapor pressure, the gas phase is typically considered to be a pure gas solute.62 Under equilibrium conditions and at infinite dilution, Henry's law constant (kH) can be estimated using the following eqn (2), which assumes a fugacity coefficient of unity:32,62,63
of a solute in the liquid phase to its partial pressure in the gas phase.61 Henry's law can be expressed in a variety of ways, each with its own unit and interpretation. For ILs having a very low or negligible vapor pressure, the gas phase is typically considered to be a pure gas solute.62 Under equilibrium conditions and at infinite dilution, Henry's law constant (kH) can be estimated using the following eqn (2), which assumes a fugacity coefficient of unity:32,62,63
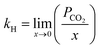 | (2) |
Under increasing pressure, the solubility of CO2 gas in all of the investigated ILs exhibits a nonlinear trend, as shown in Fig. 4. However, when the solubility approaches zero, it becomes possible to determine the limiting slope by employing a second-order polynomial regression analysis on the available data. This approach allows for the determination of Henry's law constants.42 Therefore, eqn (3) has been used to represent the experimental values.6
| kH = ax2 + bx + c | (3) |
Henry's law constant (kH) at infinite dilution has been determined by solving eqn (4) using eqn (3):63
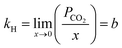 | (4) |
Henry's law constants for each of the ILs under investigation, are presented in Table 3. As mentioned before, the solubility of gases in a solvent is described by Henry's law constant. A decrease in this value represents an increase in the solubility of gases in the solvent.62 Among the ILs under study, [C2CNHim][DOSS] and [C2CNOim][DOSS] displayed the lowest Henry's law constant values for the [C2CNHim] and [C2CNOim] based ILs, respectively, while [C2CNDim][DOSS] showed the lowest Henry's law constant value for the ILs with the [DOSS] anion.
| IL | kH (298.15 K) | IL | kH (298.15 K) |
|---|---|---|---|
| [C2CN Bim][DOSS] | 19.22 | [C2CN Oim][DOSS] | 11.18 |
| [C2CN Him][DOSS] | 15.69 | [C2CN Dim][DOSS] | 07.47 |
| [C2CN Him][TFMS] | 17.02 | [C2CN Oim][TFMS] | 13.04 |
| [C2CN Him][SBA] | 18.99 | [C2CN Oim][SBA] | 14.37 |
| [C2CN Him][DDS] | 20.07 | [C2CN Oim][DDS] | 15.88 |
| [C2CN Him][BS] | 21.16 | [C2CN Oim][BS] | 16.61 |
Moreover, Henry's law constant was used to study the impact of temperature on the CO2 solubility capacity of these ILs. Henry's law constants were determined for the [DOSS] based ILs at pressures of 1, 5, 10, 15, and 20 bar and temperatures of 298.15, 313.15, 328.15, 343.15 and 358.15 K using eqn (4) and presented in Fig. 5.
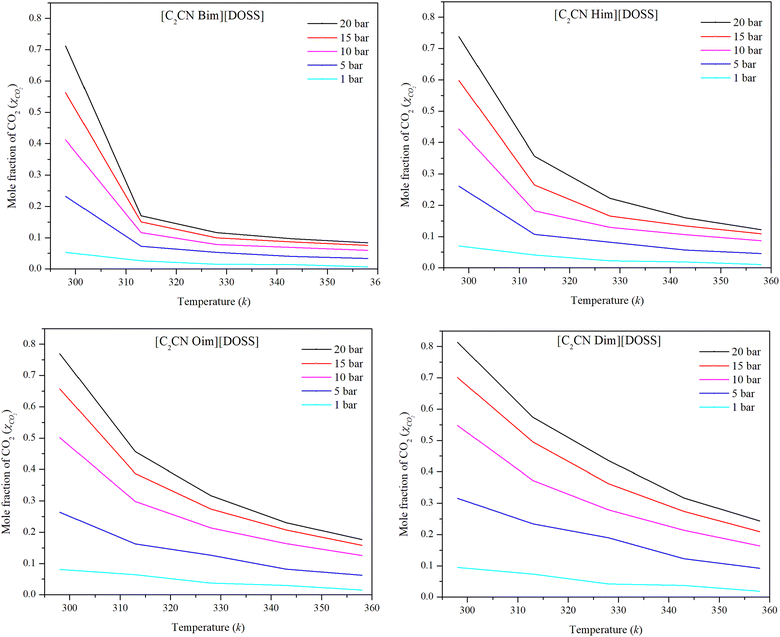 | ||
| Fig. 5 Experimental data on the solubility of CO2 in [DOSS]-based ILs at various temperatures and pressures. | ||
The solubility of CO2 in the IL reduces with increasing temperature, as shown in Fig. 5 is in agreement with the reported results.64 As temperature increases, kinetic energy increases, causing ions to move faster. This reduces the inter-ion gap and may increase the irregular distribution of ions.
3.6. Thermodynamic properties
The thermodynamic parameters such as standard enthalpy (ΔH0), entropy (ΔS0), and Gibbs free energy (ΔG0) influence the solubility of CO2 in ILs. At infinite dilution, the temperature dependence of Henry's law constants can be used to predict their contributions to the gas solvation process in a solvent.32,39,40 The standard enthalpy, entropy, and Gibbs free energy of gas solubility can be derived from Henry's law constants. Eqn (5) can be used to predict the standard Gibbs free energy of a gas solution, given the standard pressure P0 = 1.01325 bar and the Henry's law constant.6The standard entropy of the ionic liquid/gas mixture indicates the degree of order in the mixture, while the standard enthalpy of solvation indicates the strength of the interaction between the liquid and the gas. Gibbs free energy determines whether a gas will dissolve spontaneously in a liquid, in addition to phase stability.32,39,40,65 Additionally, negative ΔG0 values often indicate the formation of one or more chemical complexes.66
The standard enthalpy, entropy, and Gibbs free energy of the gas's solubility were derived from the Henry constants. Having the standard pressure P0 = 1.01325 bar and utilizing Henry's law constant,3 eqn (5) was employed to estimate the standard Gibbs free energy of a gas solution.6
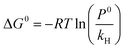 | (5) |
Henry's law constant at infinite dilution is sometimes considered a constant and correlated to the standard enthalpy of gas dissolution. However, in other cases, (ΔH°) is temperature-dependent rather than constant for relatively large temperature ranges.6 By plotting the inverse of absolute temperature against the natural logarithm of Henry's constant (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kH), the molar enthalpy change during gas solubility can be approximated using eqn (6) as follows:6
kH), the molar enthalpy change during gas solubility can be approximated using eqn (6) as follows:6
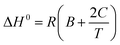 | (6) |
Eqn (7) was utilized to calculate the standard entropy.6
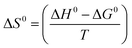 | (7) |
Table 4 presents the Gibbs free energy, standard enthalpy, and entropy of the CO2 solution in the [DOSS]-based ILs at various temperatures. The positive values of ΔG0 that were previously reported indicate the CO2 solubility in the prepared ILs. The negative values of ΔH0 confirm the dissolution of CO2 in the ILs, with a decrease in magnitude from moderately strong acid–base bonds at 298 K to weak acid-base bonds at 343 K, consistent with the previous reports.63
| Temperature (K) | [C2CNDim][DOSS] | [C2CNOim][DOSS] | [C2CNHim][DOSS] | [C2CNBim][DOSS] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG0 | ΔH0 | ΔS0 | ΔG0 | ΔH0 | ΔS0 | ΔG0 | ΔH0 | ΔS0 | ΔG0 | ΔH0 | ΔS0 | |
| 298.15 | 4.95 | −17.91 | −76.68 | 5.95 | −14.38 | −68.20 | 06.79 | −10.31 | −57.37 | 07.30 | −07.55 | −49.79 |
| 313.15 | 6.10 | −17.81 | −76.35 | 6.71 | −14.87 | −68.89 | 07.65 | −11.38 | −60.76 | 08.26 | −08.59 | −53.80 |
| 328.15 | 7.24 | −17.71 | −76.05 | 7.78 | −15.31 | −70.36 | 08.60 | −12.35 | −63.85 | 09.21 | −09.53 | −57.11 |
| 343.15 | 8.38 | −17.63 | −75.79 | 8.86 | −15.71 | −71.60 | 09.61 | −13.23 | −66.56 | 10.18 | −10.39 | −59.95 |
| 358.15 | 9.52 | −17.55 | −75.57 | 9.91 | −16.07 | −72.55 | 10.58 | −14.04 | −68.75 | 11.17 | −11.18 | −62.41 |
The enthalpy of liquids is lower than that of gases, so negative enthalpy changes occur when gases condense. Therefore, the magnitude and sign of the enthalpy of mixing determine the overall partial enthalpy change for solvation. Gas–liquid mixtures with greater solubility typically undergo a negative partial molar enthalpy change due to the dominant enthalpy of condensation.67 A greater negative enthalpy indicates a stronger interaction between the ionic liquid (IL) and CO2.68 In contrast, temperature had a relatively small impact on CO2– [C2CNDim][DOSS] interaction. The negative solvation enthalpies further support the exothermic nature of the solvation process. The correlation between temperature and ΔG0 suggests that more energy is needed for the CO2 solvation process as the temperature rises. The observed solvation entropy values can be explained by the structuring effect, which is caused by the unique interactions between the charged centers of the IL and the solute.63
4. Conclusion
A series of nitrile-based imidazolium ILs incorporating different alkyl chains and sulfonated-based anions were synthesized in order to investigate the effect of the structure, pressure, and temperature on the CO2 solubility. The solubility of CO2 demonstrated a non-linear pattern with the increase in pressure among the investigated ILs. The CO2 solubility in the prepared ILs showed the order; [DOSS] > [TFMS] > [SBA] > [DDS] > [BS] for the anions and [C2CNDim] > [C2CNOim] > [C2CNHim] > [C2CNBim] for the cations. The synthesized [DOSS]-based ILs showed higher CO2 solubility and low diffusivity whereas the [BS]-based ILs showed lower CO2 solubility and higher diffusivity. For Henry's law constant, [C2CN Dim][DOSS] and [C2CN Him][BS] showed the lowest and highest values respectively. The CO2 solubility in the synthesized ILs showed a non-spontaneous process as indicated by the thermodynamic results.Data availability statement
Any additional data will be available upon request.Author contributions
Conceptualization, A. K. Z. and A. O.; methodology, A. K. Z., and A. M. K.; validation, A. K. Z., F. R., and A. M. K.; formal analysis, A. O., and M. M; investigation, F. R. and A. K. Z.; resources, A. K. Z. and A. O.; data curation, A. K. Z., and A. O.; writing—original draft preparation, A. K. Z and A. O.; writing—review and editing, A. K. Z., A. M. K., and F. R.; visualization, A. K. Z. and A. O.; supervision, A. K. Z.; project administration, A. O.; funding acquisition, A. O. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work under the Distinguished Researcher Program grant code (NU/DRP/SERC/12/54).References
- E. Torralba-Calleja, J. Skinner and D. Gutiérrez-Tauste, J. Chem., 2013, 2013, 1–16 CrossRef.
- R. Baker, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef.
- S. D. Kenarsari, D. Yang, G. Jiang, S. Zhang, J. Wang, A. G. Russell, Q. Wei and M. Fan, RSC Adv., 2013, 3, 22739–22773 RSC.
- Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42–55 RSC.
- Y. Liang, MSc, Tsinghua University, 2003.
- F. Karadas, M. Atilhan and S. Aparicio, Energy Fuels, 2010, 24, 5817–5828 CrossRef.
- J. D. Holbrey and K. Seddon, Clean Prod. Process., 1999, 1, 223–236 Search PubMed.
- K. R. Seddon, Nat. Mater., 2003, 2, 363–365 CrossRef PubMed.
- A. E. Jimenez, M. D. Bermudez, F. J. Carrion and G. Martinez-Nicolas, Wear, 2006, 261, 347–359 CrossRef.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- W. F. Elmobarak, F. Almomani, M. Tawalbeh, A. Al-Othman, R. Martis and K. Rasool, Fuel, 2023, 344, 128102 CrossRef.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391–1398 CrossRef.
- K. E. Gutowski, Physical Sciences Reviews, 2018, 3(5), 20170191 Search PubMed.
- M. Ramdin, T. W. de Loos and T. J. Vlugt, Ind. Eng. Chem. Res., 2012, 51, 8149–8177 CrossRef.
- D. Hospital-Benito, J. Lemus, C. Moya, R. Santiago, V. Ferro and J. Palomar, Chem. Eng. J., 2021, 407, 127196 CrossRef.
- F. O. Ochedi, J. Yu, H. Yu, Y. Liu and A. Hussain, Environ. Chem. Lett., 2021, 19, 77–109 CrossRef.
- M. Kosmulski, J. Gustafsson and J. B. Rosenholm, Thermochim. Acta, 2004, 412, 47–53 CrossRef.
- M. Pan, Y. Zhao, X. Zeng and J. Zou, Energy Fuels, 2018, 32, 6130–6135 CrossRef.
- H. S. Schrekker, M. P. Stracke, C. M. L. Schrekker and J. Dupont, Ind. Eng. Chem. Res., 2007, 46, 7389–7392 CrossRef.
- F. Ding, X. He, X. Luo, W. Lin, K. Chen, H. Li and C. Wang, Chem. Commun., 2014, 50, 15041–15044 RSC.
- Y. S. Sistla and V. Sridhar, J. Mol. Liq., 2021, 325, 115162 CrossRef.
- J. Bara, C. Gabriel, T. Carlisle, D. Camper, A. Finotello, D. Gin and R. Noble, Chem. Eng. J., 2009, 147, 43–50 CrossRef.
- X. Zhang, Z. Liu and W. Wang, AIChE J., 2008, 54, 2717–2728 CrossRef.
- D. Zhao, Z. Fei, R. Scopelliti and P. J. Dyson, Inorg. Chem., 2004, 43, 2197–2205 CrossRef PubMed.
- Q. Zhang, Z. Li, J. Zhang, S. Zhang, L. Zhu, J. Yang, X. Zhang and Y. Deng, J. Phys. Chem. B, 2007, 111, 2864–2872 CrossRef PubMed.
- A. K. Ziyada, C. D. Wilfred, M. A. Bustam, Z. Man and T. Murugesan, J. Chem. Eng. Data, 2010, 55, 3886–3890 CrossRef.
- A. K. Ziyada, M. A. Bustam, T. Murugesan and C. D. Wilfred, New J. Chem., 2011, 35, 1111–1116 RSC.
- A. K. Ziyada, M. A. Bustam, C. D. Wilfred and T. Murugesan, J. Chem. Eng. Data, 2011, 56, 2343–2348 CrossRef.
- M. W. Arshad and K. Thomsen, MSc, Technical University of Denmark, 2009.
- A. K. Ziyada and C. D. Wilfred, J. Chem. Eng. Data, 2018, 63, 3672–3683 CrossRef.
- M. S. R. Shahrom, C. D. Wilfred and A. K. Z. Taha, J. Mol. Liq., 2016, 219, 306–312 CrossRef.
- M. Gonzalez-Miquel, J. Bedia, C. Abrusci, J. Palomar and F. Rodriguez, J. Phys. Chem. B, 2013, 117, 3398–3406 CrossRef PubMed.
- M. Shiflett and A. Yokozeki, J. Phys. Chem. B, 2007, 111, 2070–2074 CrossRef PubMed.
- J. C. Kotz, P. Treichel and J. R. Townsend, Chemistry & Chemical Reactivity, Brooks/Cole Pub Co, 2008 Search PubMed.
- J. L. Anthony, J. L. Anderson, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2005, 109, 6366–6374 CrossRef PubMed.
- A. K. Ziyada and C. D. Wilfred, J. Chem. Eng. Data, 2014, 59, 1232–1239 CrossRef.
- A. K. Ziyada and C. D. Wilfred, J. Chem. Eng. Data, 2014, 1385–1390 CrossRef.
- T. Seki, J. D. Grunwaldt and A. Baiker, J. Phys. Chem. B, 2008, 113, 114–122 CrossRef.
- A. M. Tagiuri, Studies of Solubility of CO2 in Ionic Liquids, Kinetics, and Heat of Reactions of CO2 in Promising Cyclic Amines, The University of Regina, Canada, 2019 Search PubMed.
- S. N. V. K. Aki, B. R. Mellein, E. M. Saurer and J. F. Brennecke, J. Phys. Chem. B, 2004, 108, 20355–20365 CrossRef.
- L. A. Blanchard, PhD, University of Notre Dame, 2000.
- G. Yu, S. Zhang, X. Yao, J. Zhang, K. Dong, W. Dai and R. Mori, Ind. Eng. Chem. Res., 2006, 45, 2875–2880 CrossRef.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef PubMed.
- J. Bara, D. Camper, D. Gin and R. Noble, Acc. Chem. Res., 2009, 43, 152–159 CrossRef.
- T. Sarbu, T. J. Styranec and E. J. Beckman, Ind. Eng. Chem. Res., 2000, 39, 4678–4683 CrossRef.
- J. A. Schott, C.-L. Do-Thanh, W. Shan, N. G. Puskar, S. Dai and S. M. Mahurin, Green Chemical Engineering, 2021, 2, 392–401 CrossRef.
- C. Wu, T. P. Senftle and W. F. Schneider, Phys. Chem. Chem. Phys., 2012, 14, 13163–13170 RSC.
- X. Liu, K. E. O'Harra, J. E. Bara and C. H. Turner, J. Phys. Chem. B, 2021, 125, 3665–3676 CrossRef.
- S. D. Hojniak, I. P. Silverwood, A. L. Khan, I. F. Vankelecom, W. Dehaen, S. G. Kazarian and K. Binnemans, J. Phys. Chem. B, 2014, 118, 7440–7449 CrossRef.
- D. Zhao, MSc, Xinan Petroleum University, 2007.
- R. P. Swatloski, PhD, University of Alabama, 2005.
- W. Ren, B. Sensenich and A. Scurto, J. Chem. Thermodyn., 2010, 42, 305–311 CrossRef.
- D. Almantariotis, T. Gefflaut, A. Pa dua, J. Coxam and M. Costa Gomes, J. Phys. Chem. B, 2010, 114, 3608–3617 CrossRef PubMed.
- S. S. Moganty and R. E. Baltus, Ind. Eng. Chem. Res., 2010, 49, 9370–9376 CrossRef.
- B. E. Gurkan, T. R. Gohndrone, M. J. McCready and J. F. Brennecke, Phys. Chem. Chem. Phys., 2013, 15, 7796–7811 RSC.
- D. Morgan, L. Ferguson and P. Scovazzo, Ind. Eng. Chem. Res., 2005, 44, 4815–4823 CrossRef.
- D. Camper, C. Becker, C. Koval and R. Noble, Ind. Eng. Chem. Res., 2006, 45, 445–450 CrossRef.
- M. W. Arshad, Measuring and Thermodynamic Modeling of De-Mixing CO2 Capture Systems, Technical University of Denmark, 2014 Search PubMed.
- J. Huang, A. Riisager, R. Berg and R. Fehrmann, J. Mol. Catal. A: Chem., 2008, 279, 170–176 CrossRef.
- J. Anthony, E. Maginn and J. Brennecke, J. Phys. Chem. B, 2002, 106, 7315–7320 CrossRef.
- A. Henni, P. Tontiwachwuthikul and A. Chakma, Can. J. Chem. Eng., 2005, 83, 358–361 CrossRef.
- L. M. G. Sánchez, PhD, Eindhoven University of Technology, 2008.
- H. W. Pennline, D. R. Luebke, K. L. Jones, C. R. Myers, B. I. Morsi, Y. J. Heintz and J. B. Ilconich, Fuel Process. Technol., 2008, 89, 897–907 CrossRef.
- G. Wang, W. Hou, F. Xiao, J. Geng, Y. Wu and Z. Zhang, J. Chem. Eng. Data, 2011, 56(4), 1125–1133 CrossRef.
- P. Carvalho, V. Álvarez, I. Marrucho, M. Aznar and J. Coutinho, J. Supercrit. Fluids, 2010, 52, 258–265 CrossRef.
- M. Koel, Proc. Est. Acad. Sci., Chem., 2000, 49, 145–155 Search PubMed.
- A. Finotello, J. E. Bara, D. Camper and R. D. Noble, Ind. Eng. Chem. Res., 2008, 47, 3453–3459 CrossRef.
- M. Muldoon, S. Aki, J. Anderson, J. Dixon and J. Brennecke, J. Phys. Chem. B, 2007, 111, 9001–9009 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08335g |
| This journal is © The Royal Society of Chemistry 2024 |