Open Access Article
Open Access ArticleSynthesis, biological evaluation and molecular docking of novel nereistoxin derivatives containing phosphonates as insecticidal/AChE inhibitory agents†
Qiaoli Yan,
Xiaogang Lu ,
Jin Wang,
Zixuan Zhang
,
Jin Wang,
Zixuan Zhang ,
Runli Gao,
Chengxin Pei and
Hongmei Wang
,
Runli Gao,
Chengxin Pei and
Hongmei Wang *
*
State Key Laboratory of NBC Protection for Civilian, Beijing 102205, China. E-mail: hongmei_ricd@yeah.net
First published on 29th January 2024
Abstract
In continuation of our program aimed at the discovery and development of natural product-based insecticidal agents, a series of novel nereistoxin derivatives containing phosphonate were synthesized and characterized by 31P, 1H, 13C NMR and HRMS. The bioactivities of the derivatives were evaluated for the acetylcholinesterase (AChE) inhibition potency and insecticidal activity. The AChE inhibitory effects of the derivatives were investigated using the in vitro Ellman method. Half of the compounds exhibited excellent inhibition of AChE. All the compounds were assessed for insecticidal activities against Mythimna separate (Walker) and Rhopalosiphum padi in vivo. Some derivatives displayed promising insecticidal activity against Rhopalosiphum padi. Compounds 5b and 6a displayed the highest activity against R. padi, showing LC50 values of 17.14 and 18.28 μg mL−1, respectively, close to that of commercial insecticide flunicotamid (LC50 = 17.13 μg mL−1). Compound 9g also showed notable insecticidal activity, with an LC50 value of 23.98 μg mL−1. Additionally, the binding modes of the active compounds 5b, 6a and 9g with AChE were analyzed in-depth though molecular docking and the intrinsic reasons for the differences in the strength of the compound's activities were elucidated. In summary, our findings demonstrate the potential of these nereistoxin derivatives as promising candidates for the development of novel pesticides.
1 Introduction
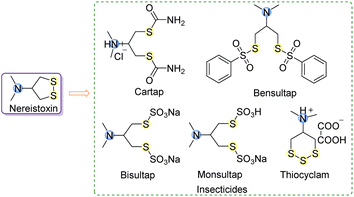
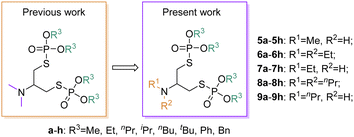
Nowadays, pesticides continue to be extensively employed to enhance crop yields and protect crops from pests.1 The increasing application of synthetic agrochemicals is progressively leading to the emergence of resistance in various pest populations, posing significant challenges in pest management and crop protection.2–4 In view of the increasing pesticide resistance and the difficulties in rapidly producing new pesticides,5,6 it is a prominent way to search for original insecticidal compounds from natural products and their secondary metabolites, as well as utilize these compounds as lead structures for further modification. Nereistoxin (NTX, Fig. 1), a natural neurotoxin with insecticidal activity, was initially isolated from Lumbriconereis heteropoda, an organism inhabiting marine sediments,7 which marked a significant milestone as the first successful development and application of an animal-derived insecticide in the history of pesticide development. Currently, only five commercially available insecticides are synthesized with NTX as the primary compound, as depicted in Fig. 1. These NTX analogs have demonstrated effective control over lepidopteran, hemipteran, and coleopteran pests.8,9 Furthermore, they are considered safe for humans and domestic animals since they can be broken down and excreted. However, the long-term, repeated, and extensive use of these insecticides has inevitably led to the development of pesticide resistance.10,11 Therefore, there is a pressing need to synthesize new compounds to enrich and advance the development of nereistoxin-derived insecticides.Organophosphorus insecticides are known for their broad-spectrum insecticides, rapid action and significant effects.12 Despite their numerous benefits, they cause serious reverberations on humans and animals, including organ failure and damage to the nervous system.13,14 Consequently, researchers are actively exploring innovative strategies to mitigate its major adverse effect on humans. The modification of organophosphorus insecticides aims to retain their efficient insecticidal activity while reducing the environmental impact. This endeavor has led to the development of new pesticides that are relatively safe for mammals and environmentally friendly. By combining organophosphorus components with the natural product nereistoxin, our objective is to explore new compounds that strike a balance between excellent insecticidal activity and environmentally sound characteristics. Our prior research focused on synthesizing N,N-dimethyl nereistoxin derivatives containing phosphonates and their bioactivities. We have screened compounds with insecticidal potential.15 To enhance the activities of the new S–P bond nereistoxin derivatives associated with phosphonates, we conducted a detailed study of derivatives with varied substituents on N atom (Fig. 2, the alkyl substituents on the N atom are changed) and the synthesis, characterization and biological activities (acetylcholinesterase inhibitory activity and insecticidal activity against Mythimna separata Walker and Rhopalosiphum padi) were fully studied. Our findings revealed that the introduction of various alkyl substituents on the N atom resulted in differing degrees of acetylcholinesterase inhibition and insecticidal activity. These results highlight the potential of these S–P bond nereistoxin derivatives as insecticidal agents and offer valuable insights for further development in the field of insect pest control. Moreover, molecular docking was conducted to illustrate the binding modes of active compounds with AChE, aiming to provide insights into the variations in their activity strengths.
2 Results and discussion
2.1 Chemistry
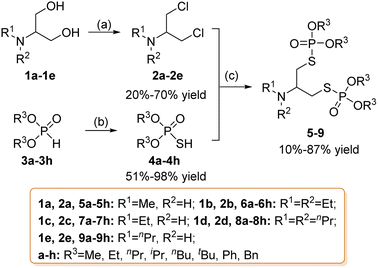
The general synthetic route of compounds 5–9 is shown in Scheme 1. The key intermediate 2a–2e was conveniently synthesized from 1a–1e. The hydroxyl of 1a–1e was chlorinated with SOCl2 to obtain 2a–2e. Other crucial intermediates, such as S–hydrogen phosphorothioates 4a–4h, were obtained from diphosphite 3a–3h. Some of them were commercially available and others were obtained via reaction of the appropriate alcohol and PCl3, with pyridine as an acid-binding agent. Diphosphite 3a–3h was reacted with S8 and triethylamine to obtain 4a–4h. The end-products of 5–9 were synthesized via a substitution reaction between intermediates 2a–2e and various substituted S–hydrogen phosphorothioates 4a–4h. The target compounds 5–9 were characterized via 1H NMR, 13C NMR, 31P NMR and HRMS.2.2 The stability of the products
The polarity of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and the formation of p–dπ conjugation between the O/S atoms connected to it, the electron cloud density on the C connected to O in alkoxy groups and P is lower, making P or C atoms more susceptible to be attacked by nucleophilic reagent attack and then hydrolysis. For –OR, as the electron-donating ability of alkyl groups follows the order CH3 < C2H5< C3H7, the electron-withdrawing ability is OCH3 > OC2H5 > OC3H7. Therefore, the electron cloud density of the P atom connected to OCH3 is lower than that of the P atom connected to other alkoxy groups, making it more susceptible to hydrolysis through nucleophilic substitution reaction. That is, methyl phosphate is less stable than other alkyl phosphates. The N atoms of compounds 5a, 7a, and 9a are a secondary amine substituted by two alkyl groups, making 5a, 7a, and 9a excellent nucleophilic reagents themselves. In terms of electron-donating ability, H < CH3 < C2H5, so the electron cloud density on the C atom attached to O in –OR, CH3 < C2H5 < C3H7, so compared with other alkyl S–hydrogen phosphorothioates, C atoms attached to O in dimethyl phosphorothioate are easier to be attacked by nucleophilic reagents. During the synthesis of 5a, 7a, and 9a, there is an excess of dimethyl S–hydrogen phosphorothioates, and the compound is prone to react with the excess dimethyl thiophosphate during purification to converted into products with one more methyl substitution on the N atom, and we have monitored the corresponding products by LC-MS during purification. Therefore, 5a, 7a, and 9a are unstable during the purification process (see ESI Fig. S140–S160†).
O bond and the formation of p–dπ conjugation between the O/S atoms connected to it, the electron cloud density on the C connected to O in alkoxy groups and P is lower, making P or C atoms more susceptible to be attacked by nucleophilic reagent attack and then hydrolysis. For –OR, as the electron-donating ability of alkyl groups follows the order CH3 < C2H5< C3H7, the electron-withdrawing ability is OCH3 > OC2H5 > OC3H7. Therefore, the electron cloud density of the P atom connected to OCH3 is lower than that of the P atom connected to other alkoxy groups, making it more susceptible to hydrolysis through nucleophilic substitution reaction. That is, methyl phosphate is less stable than other alkyl phosphates. The N atoms of compounds 5a, 7a, and 9a are a secondary amine substituted by two alkyl groups, making 5a, 7a, and 9a excellent nucleophilic reagents themselves. In terms of electron-donating ability, H < CH3 < C2H5, so the electron cloud density on the C atom attached to O in –OR, CH3 < C2H5 < C3H7, so compared with other alkyl S–hydrogen phosphorothioates, C atoms attached to O in dimethyl phosphorothioate are easier to be attacked by nucleophilic reagents. During the synthesis of 5a, 7a, and 9a, there is an excess of dimethyl S–hydrogen phosphorothioates, and the compound is prone to react with the excess dimethyl thiophosphate during purification to converted into products with one more methyl substitution on the N atom, and we have monitored the corresponding products by LC-MS during purification. Therefore, 5a, 7a, and 9a are unstable during the purification process (see ESI Fig. S140–S160†).
Compounds containing tert-butyl groups (5f, 6f, 7f, 8f, and 9f) are unstable due to the high steric hindrance of tert-butyl, making them more prone to hydrolyzing –OC(CH3)3 into –OH.
Since the benzyl group forms a carbonium ion and enters displacement reactions much more readily than aliphatic groups do, the benzyl group are theoretically more likely to hydrolyze than aliphatic phosphates.16 Therefore, 5h, 7h, and 9h are unstable and more likely to hydrolyze.
2.3 Biological activities
In this study, in accordance with the guidelines for antipesticide laboratory biological activity tests, the insecticidal activities of all synthesized derivatives 5a–5h, 6a–6h, 7a–7h, 8a–8h and 9a–9h against common agricultural pests (Mythimna separate Walker and Rhopalosiphum padi) were evaluated in the laboratory, and the representative insecticides chlorpyrifos and flunicotamid were used as the positive controls, respectively. In addition, the target compounds 5a–5h, 6a–6h, 7a–7h, 8a–8h and 9a–9h were evaluated for acetylcholinesterase inhibitory activities in vitro.| Comp. | R1 | R2 | R3 | IC50 (μM) | Ki (mM−1 min−1) |
|---|---|---|---|---|---|
| a Compounds are unstable in purification.b Hydrolyzed easily.c Poor solubility. | |||||
| 5a | Me | H | Me | —a | — |
| 5b | Me | H | Et | 0.5243 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338.64 ± 6948.86 338.64 ± 6948.86 |
| 5c | Me | H | nPr | 0.3506 | 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215.90 ± 6501.13 215.90 ± 6501.13 |
| 5d | Me | H | iPr | 23.43 | 2254.33 ± 321.25 |
| 5e | Me | H | nBu | 0.4041 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 859.10 ± 3840.74 859.10 ± 3840.74 |
| 5f | Me | H | tBu | —b | — |
| 5g | Me | H | Ph | —a | — |
| 5h | Me | H | Bn | —a | — |
| 6a | Et | Et | Me | 0.6813 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 184.77 ± 13 184.77 ± 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 731.42 731.42 |
| 6b | Et | Et | Et | 3.133 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341.50 ± 1855.40 341.50 ± 1855.40 |
| 6c | Et | Et | nPr | 8.494 | 6503.80 ± 486.92 |
| 6d | Et | Et | iPr | 99.77 | 792.54 ± 46.93 |
| 6e | Et | Et | nBu | 17.25 | 4203.51 ± 878.03 |
| 6f | Et | Et | tBu | —b | — |
| 6g | Et | Et | Ph | —c | — |
| 6h | Et | Et | Bn | —c | — |
| 7a | Et | H | Me | —a | — |
| 7b | Et | H | Et | 1.383 | 202.01 ± 59.01 |
| 7c | Et | H | nPr | 19.33 | 7.01 ± 5.12 |
| 7d | Et | H | iPr | 28.77 | 9.60 ± 0.44 |
| 7e | Et | H | nBu | 36.73 | 2.26 ± 0.12 |
| 7f | Et | H | tBu | —a | — |
| 7g | Et | H | Ph | —a | — |
| 7h | Et | H | Bn | —a | — |
| 8a | nPr | nPr | Me | 7.039 | 6531.33 ± 210.66 |
| 8b | nPr | nPr | Et | 12.86 | 2723.50 ± 183.68 |
| 8c | nPr | nPr | nPr | 45.03 | 1066.84 ± 98.27 |
| 8d | nPr | nPr | iPr | 871.00 | 80.87 ± 1.00 |
| 8e | nPr | nPr | nBu | 57.98 | 792.57 ± 8.06 |
| 8f | nPr | nPr | tBu | —a | — |
| 8g | nPr | nPr | Ph | —a | — |
| 8h | nPr | nPr | Bn | —a | — |
| 9a | nPr | H | Me | —a | — |
| 9b | nPr | H | Et | 8.641 | 21.62 ± 4.64 |
| 9c | nPr | H | nPr | 0.9667 | 79.47 ± 18.53 |
| 9d | nPr | H | iPr | 269.7 | 0.67 ± 0.07 |
| 9e | nPr | H | nBu | 5.42 | 26.75 ± 0.59 |
| 9f | nPr | H | tBu | —a | — |
| 9g | nPr | H | Ph | —a | — |
| 9h | nPr | H | Bn | —a | — |
To assess the mode of action of the nereistoxin derivatives, the activity of human AChE was assayed for each compound. All compounds were evaluated for their inhibitory activity on AChE using Ellman's method, except for certain compounds characterized that are easy hydrolysis, poor solubility and instability. It is difficult to calculate the IC50 value of nereistoxin because it is such a weak AChE inhibitor.
The compounds exhibited varying levels of inhibition on human AChE with IC50 values ranging from 0.3506 μmol L−1 to 871 μmol L−1 (Table 1). The results demonstrated that these derivatives, in general, exhibited exceptional inhibitory potency against human AChE compared with the similar phosphorothioates reported previously.19 Notably, compounds 5c, 5e, 5b, 6a, and 9c showed significant AChE inhibitory activity, with IC50 values less than 1 μmol L−1. Among them, compound 5c displayed the most potent inhibition with the lowest IC50 value of 0.3506 μmol L−1. It's worth noting that the N-methyl-substituted compounds showed higher inhibitory activity against AChE compared to other N-alkyl-substituted compounds with the same phosphate ester substituents. Additionally, for N,N-dialkyl compounds, the AChE inhibition decreases as the number of C atoms increases when the substituents of the phosphate ester are straight-chain alkyl groups, such as the inhibitory activity of 6a < 6b < 6c < 6e, 8a < 8b < 8c < 8e.
Based on the preliminary results (Table 2), compounds with excellent bioactivity were selected for further bioassays against M. separate at different concentrations (the different concentrations of 10a, 12b, 12d and 12e were 125, 250, 500, 1000, 2000 μg mL−1). The results of these bioassays are expressed in Table 3 as half of the lethal concentration (LC50, μg mL−1).
| Comp. | R1 | R2 | R3 | Corrected mortality rate ±SD (%) | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| a Compounds are unstable in purification.b Hydrolyzed easily. | |||||
| 5a | Me | H | Me | —a | — |
| 5b | Me | H | Et | 8.33 ± 2.35 | 25 ± 2.37 |
| 5c | Me | H | nPr | 8.33 ± 4.11 | 25 ± 0 |
| 5d | Me | H | iPr | 0 | 8.33 ± 1.21 |
| 5e | Me | H | nBu | 0 | 16.67 ± 2.74 |
| 5f | Me | H | tBu | —b | — |
| 5g | Me | H | Ph | 0 | 25 ± 3.50 |
| 5h | Me | H | Bn | —a | — |
| 6a | Et | Et | Me | 58.33 ± 1.98 | 66.67 ± 2.26 |
| 6b | Et | Et | Et | 8.33 ± 0.91 | 25 ± 3.51 |
| 6c | Et | Et | nPr | 8.33 ± 1.63 | 8.33 ± 1.47 |
| 6d | Et | Et | iPr | 0 | 25 ± 2.28 |
| 6e | Et | Et | nBu | 0 | 8.33 ± 0.69 |
| 6f | Et | Et | tBu | —b | — |
| 6g | Et | Et | Ph | 0 | 33.33 ± 0.62 |
| 6h | Et | Et | Bn | 16.67 ± 1.59 | 16.67 ± 1.94 |
| 7a | Et | H | Me | —a | — |
| 7b | Et | H | Et | 0 | 0 |
| 7c | Et | H | nPr | 0 | 0 |
| 7d | Et | H | iPr | 0 | 0 |
| 7e | Et | H | nBu | 20 ± 1.49 | 0 |
| 7f | Et | H | tBu | —b | — |
| 7g | Et | H | Ph | 8.33 ± 1.57 | 33.33 ± 1.82 |
| 7h | Et | H | Bn | —a | — |
| 8a | nPr | nPr | Me | 8.33 ± 1.38 | 16.67 ± 0.75 |
| 8b | nPr | nPr | Et | 16.67 ± 1.24 | 58.33 ± 2.17 |
| 8c | nPr | nPr | nPr | 16.67 ± 1.38 | 16.67 ± 0.99 |
| 8d | nPr | nPr | iPr | 8.33 ± 2.21 | 50 ± 2.88 |
| 8e | nPr | nPr | nBu | 16.67 ± 2.29 | 58.33 ± 2.28 |
| 8f | nPr | nPr | tBu | —b | — |
| 8g | nPr | nPr | Ph | 0 | 16.67 ± 1.73 |
| 8h | nPr | nPr | Bn | 0 | 8.33 ± 1.27 |
| 9a | nPr | H | Me | —a | — |
| 9b | nPr | H | Et | 0 | 0 |
| 9c | nPr | H | nPr | 0 | 0 |
| 9d | nPr | H | iPr | 0 | 0 |
| 9e | nPr | H | nBu | 0 | 0 |
| 9f | nPr | H | tBu | —b | — |
| 9g | nPr | H | Ph | 8.33 ± 1.96 | 16.67 ± 3.25 |
| 9h | nPr | H | Bn | —a | — |
| Nereistoxin | — | — | — | 100 ± 0 | 100 ± 0 |
| Chlorpyrifos | — | — | — | 100 ± 0 | 100 ± 0 |
| Comp. | y = ax + b | LC50 (μg mL−1) | R2 | CI (95%) |
|---|---|---|---|---|
| 6a | y = 1.5794x + 0.5582 | 649 | 0.9433 | 383.76–1097.72 |
| 8b | y = 1.1530x + 1.6038 | 882 | 0.9903 | 404.33–1924.88 |
| 8d | y = 1.2229x + 1.4573 | 789 | 0.9880 | 391.17–1591.31 |
| 8e | y = 1.2229x + 1.4132 | 857 | 0.9705 | 413.00–1779.19 |
| Nereistoxin | y = 1.8644x + 0.9391 | 150.71 | 0.9784 | 92.25–246.21 |
| Chlorpyrifos | y = 1.6690x + 2.9764 | 16.31 | 0.9829 | 9.44–28.20 |
Table 3 reveals that among the 29 new compounds, only 6a, 8b, 8d and 8e attained the criteria for further assays (48 h corrected mortality >50%). Notably, compounds 8b, 8d and 8e belong to the N-propyl substituted class, suggesting that these compounds possess more significant insecticidal potential than other classes in the experimental group. However, the LC50 of the synthesized compounds ranged from 649 μg mL−1 to 882 μg mL−1, which is much higher than the LC50 of the nereistoxin (150.71 μg mL−1). Correspondingly, the LC50 of the positive control chlorpyrifos is only 16.31 μg mL−1, which means that the insecticidal activity of the synthesized compounds against M. separate is much weaker compared to that of chlorpyrifos. Compared with the previous research,15 the LC50 values of N,N-dimethyl compounds against M. separate ranged from 136.86 to 836.34 μg mL−1, that is, compared to analogues, the lead compound nereistoxin displayed more potent effect on the third instar larvae of M. separata. The structure–activity relationship (SAR) analysis revealed that the insecticidal activity against M. separate was greatly influenced by the type of the substituents on N and P atoms. The mortality rates of N,N-dialkyl compounds were generally higher than those of N-alkyl compounds, such as the mortality rates of N,N-dimethyl substituents > N-methyl substituents, N,N-diethyl substituents (6) > N-ethyl substituents (7), N,N-dipropyl substituents (8) > N-propyl substituents (9), indicating that dialkyl substituent on N atom has higher insecticidal activity against M. separate. Moreover, for N,N-dialkyl compounds, the mortality rate of branched substituents on the P atom exceeded that of straight-chain substituents, e.g. mortality rate 6d > 6c, 8d > 8c, indicating that the branched alkane substituent has higher activity than that of straight-chain alkane substituents.
| Comp. | R1 | R2 | R3 | Corrected mortality rate ±SD (%) | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| a Compounds are unstable in purification.b Hydrolyzed easily. | |||||
| 5a | Me | H | Me | —a | — |
| 5b | Me | H | Et | 12.44 ± 1.62 | 87.74 ± 2.08 |
| 5c | Me | H | nPr | 5.19 ± 0.91 | 60.61 ± 3.01 |
| 5d | Me | H | iPr | 6.76 ± 1.91 | 59.28 ± 4.11 |
| 5e | Me | H | nBu | 7.09 ± 2.70 | 47.96 ± 3.65 |
| 5f | Me | H | tBu | —b | — |
| 5g | Me | H | Ph | 4.62 ± 3.90 | 44.74 ± 1.05 |
| 5h | Me | H | Bn | —a | — |
| 6a | Et | Et | Me | 6.65 ± 5.32 | 84.21 ± 4.68 |
| 6b | Et | Et | Et | 14.22 ± 2.87 | 49.45 ± 1.48 |
| 6c | Et | Et | nPr | 5.65 ± 2.18 | 21.92 ± 5.70 |
| 6d | Et | Et | iPr | 10.42 ± 2.34 | 16.78 ± 3.12 |
| 6e | Et | Et | nBu | 1.59 ± 2.24 | 33.56 ± 3.26 |
| 6f | Et | Et | tBu | —b | — |
| 6g | Et | Et | Ph | 10.17 ± 4.91 | 12.88 ± 3.73 |
| 6h | Et | Et | Bn | 1.23 ± 1.74 | 46.32 ± 2.42 |
| 7a | Et | H | Me | —a | — |
| 7b | Et | H | Et | 10.92 ± 4.91 | 21.82 ± 1.19 |
| 7c | Et | H | nPr | 29.28 ± 3.43 | 85.08 ± 1.69 |
| 7d | Et | H | iPr | 9.04 ± 4.17 | 43.20 ± 4.86 |
| 7e | Et | H | nBu | 9.48 ± 4.28 | 23.80 ± 5.52 |
| 7f | Et | H | tBu | —b | — |
| 7g | Et | H | Ph | 2.62 ± 1.86 | 82.50 ± 3.92 |
| 7h | Et | H | Bn | —a | — |
| 8a | nPr | nPr | Me | 8.35 ± 3.95 | 26.32 ± 2.22 |
| 8b | nPr | nPr | Et | 10.35 ± 2.55 | 89.07 ± 2.66 |
| 8c | nPr | nPr | nPr | 11.64 ± 1.98 | 15.55 ± 2.13 |
| 8d | nPr | nPr | iPr | 10.10 ± 0.71 | 45.37 ± 3.89 |
| 8e | nPr | nPr | nBu | 4.46 ± 1.99 | 24.82 ± 3.38 |
| 8f | nPr | nPr | tBu | —b | — |
| 8g | nPr | nPr | Ph | 6.58 ± 1.83 | 17.36 ± 4.56 |
| 8h | nPr | nPr | Bn | 1.08 ± 1.52 | 22.95 ± 0.58 |
| 9a | nPr | H | Me | —a | — |
| 9b | nPr | H | Et | 36.98 ± 1.79 | 90.21 ± 0.80 |
| 9c | nPr | H | nPr | 49.87 ± 1.13 | 84.00 ± 1.28 |
| 9d | nPr | H | iPr | 31.32 ± 0.91 | 31.43 ± 0.83 |
| 9e | nPr | H | nBu | 23.51 ± 3.83 | 39.06 ± 2.25 |
| 9f | nPr | H | tBu | —b | — |
| 9g | nPr | H | Ph | 1.23 ± 1.75 | 84.08 ± 2.99 |
| 9h | nPr | H | Bn | —a | — |
| Nereistoxin | — | — | — | 12.71 ± 1.61 | 40.22 ± 0.12 |
| Flunicotamid | — | — | — | 75.11 ± 1.96 | 84.00 ± 2.78 |
The corrected mortality rates of all compounds at 24 h were lower than that of the positive control, flunicotamid (75.11%). Half of the compounds had a mortality rate of less than 10% at 24 h, while the mortality rate of nereistoxin was 12.71%. Only six compounds had a higher corrected mortality rate than nereistoxin at 24 h. With the time extension to 48 h, the corrected mortality rates of 5b, 6a, 7c, 7g, 8b, 9b, 9c and 9g increased from 1.23–9.87% to 80–90%. The corrected mortality rate of the positive control, flunicotamid, at 48 h was 84%, indicating that the compounds (0.2 mg L−1) could achieve the same insecticidal effect as the positive control (0.04 mg L−1) after 48 h.
It can also be seen from Table 4 that the corrected mortality rate of nereistoxin at 48 h increased only from 12.71% at 12 h to 40.22%. In comparison, 9b had the highest corrected mortality rate of 90.21%. The corrected mortality rate for 9g increased from 1.23% at 24 h to 84.08% at 48 h, a 68-fold enhancement. This indicates that such compounds can exert efficient insecticidal activity in a short period of time, which may be similar to the mode of action of organophosphorus insecticides.
Based on these preliminary results (Table 4), some of the compounds with corrected mortality rates more than 80% at 48 h were chosen for further bioassays against R. padi at different concentrations (12.5, 25, 50, 100, and 200 μg mL−1); the results of these bioassays are presented as half of the lethal concentration (LC50; μg mL−1) in Table 5.
| Comp. | y = ax + b | LC50 (μg mL−1) | R2 | CI (95%) |
|---|---|---|---|---|
| 5b | y = 1.2105x + 3.5061 | 17.14 | 0.9637 | 11.53–25.48 |
| 6a | y = 1.2805x + 0.9847 | 18.28 | 0.9695 | 12.72–26.26 |
| 7c | y = 1.0586x + 2.9346 | 89.35 | 0.9899 | 70.59–113.09 |
| 7g | y = 2.3099x + 1.5743 | 30.41 | 0.9579 | 25.39–36.43 |
| 8b | y = 1.2102x + 3.1332 | 34.88 | 0.9727 | 26.47–45.96 |
| 9b | y = 1.2496x + 2.6866 | 71.02 | 0.9779 | 58.22–86.62 |
| 9c | y = 1.5811x + 2.1970 | 59.27 | 0.9832 | 51.44–68.29 |
| 9g | y = 1.3763x + 3.1009 | 23.98 | 0.9701 | 17.65–32.58 |
| Flunicotamid | y = 1.0967x + 3.6471 | 17.13 | 0.9720 | 11.25–26.07 |
The tested compounds, including 5b, 6a, 7c, 7g, 8b, 9b, 9c and 9g, displayed excellent biological activity against R. padi in the laboratory, with LC50 values range from 17.14 μg mL−1 to 89.35 μg mL−1 (Table 5), all of which were less than 90 μg mL−1. These results indicated that the compound exhibited outstanding insecticidal activity against R. padi. It is worth mentioning that R. padi is one of the most destructive wheat pests. They feed on the sap of flowers, trees and crops, thus the affected leaves roll longitudinally to the back of the leaves, and then wither and fall off. The honeydew it secretes affects the photosynthesis of wheat leaves,22 reducing the yield and quality of wheat. Additionally, this aphid is also a transmission vector of wheat yellows virus.23,24 Chemical control remains the primary method for managing wheat aphids. However, due to their short life cycle, large and rapid reproduction, frequent control measures, and high drug dosages, drug resistance develops rapidly.25–27 Thus, it is imperative to search for novel, alternative, effective pesticides.
We therefore concluded that compounds 5b (LC50 = 17.13 μg mL−1) and 6a (LC50 = 18.28 μg mL−1) showed comparable bioactivities to the positive control flunicotamid (LC50 = 17.13 μg mL−1).
Similarly, the structure–activity relationship (SAR) analysis revealed that the insecticidal activity against R. padi was influenced by the substituents on the N and P atoms. For N-alkyl-substituted compounds with identical substituents on the N atom, the LC50 values showed that 7g < 7c and 9g < 9c, indicating that compounds with aryl substituents on phosphate esters exhibited higher activity than those with straight-chain substituents. For N-alkyl-substituted compounds with the same substituents on phosphate ester, it was observed that the shorter the carbon chain of the substituent on the N atom, the lower the activity of the compound. Comparing LC50 and mortality rates, the insecticidal activity shows that 9c > 7c > 5c, and 9g > 7g > 5g. For N,N-dialkyl substitution, the mortality rates of alkyl-substituted compounds on phosphate esters exceeded those of aryl-substituted compounds. For example, the mortality rate of 6a is greater than that of other N,N-dialkyl substituted compounds, and the mortality rate of 8b surpassed that of other N,N-dipropyl substituted compounds, indicating that alkyl substituted compounds on the phosphate ester have high activity among N,N-dialkyl substituted compounds. In addition, the activity of the compounds against R. padi is correlated with their AChE inhibition activity. In general, compounds with strong insecticidal activity also have potent AChE inhibition ability.
2.4 Molecular docking
Docking simulation was performed to illustrate the differences in binding sites and binding modes between different active compounds with AChE (PDB: 6WVO). Compounds 5b, 6a, and 9g were singled out as the most promising active compounds, based on a comprehensive evaluation of AChE inhibition, anti-aphid activity and compound substituents. The results of the docking simulations are presented in Table 6, and the binding sites and binding modes of these compounds to AChE are visually depicted in Fig. 3–5.| Comp. | Binding energy (kcal mol−1) | H-bond residues | No. of H-bond | π–π/cation–π/anion–π stacking residues |
|---|---|---|---|---|
| 5b | −7.0 | Ser125 | 1 | — |
| 6a | −6.5 | Tyr124 | 1 | — |
| 9g | −8.5 | Tyr510, His381, Arg525, Ala528 | 4 | HIS381, Arg525, Asp400 |
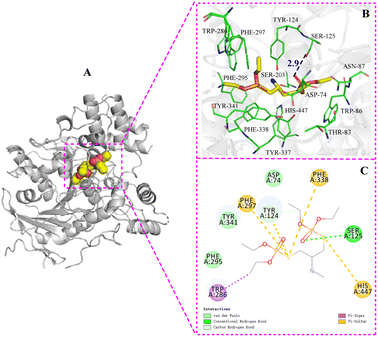 | ||
| Fig. 3 The binding mode of AChE with 5b. (A) The 3D structure of the complex, (B) the hydrogen bond donor receptor network of the complex, (C) the 2D binding mode of the complex. | ||
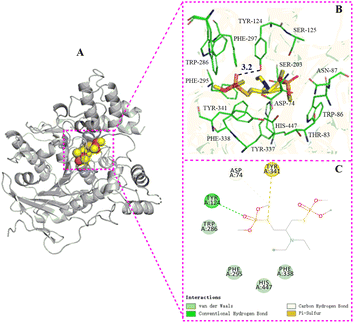 | ||
| Fig. 4 The binding mode of AChE with 6a. (A) The 3D structure of the complex, (B) the hydrogen bond donor receptor network of the complex, (C) the 2D binding mode of the complex. | ||
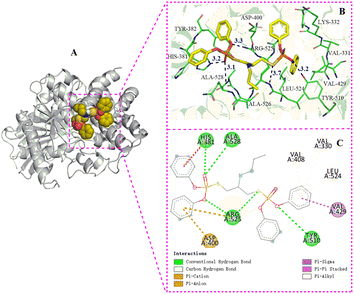 | ||
| Fig. 5 The binding mode of AChE with 9g. (A) The 3D structure of the complex, (B) the hydrogen bond donor receptor network of the complex, (C) the 2D binding mode of the complex. | ||
The information extracted from the docking simulation indicates that compound 5b and 6a bind to the same active pocket of AChE but adopt distinct binding modes. Each of them forms a hydrogen bond with Ser125 and Tyr124 respectively within the AChE active pocket through the oxygen atom of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group on the phosphate moiety. The length of hydrogen bond between 5b and Ser125 is predicted to be shorter than that of 6a and Tyr124 (2.9 Å vs. 3.2 Å). Furthermore, in comparison to 6a, 5b interacts with a greater number of amino acid residues in the AChE active pocket, which caused that the binding energy of 5b with AChE is slightly lower (−7.0 kcal mol−1) than that of 6a with AChE (−6.5 kcal mol−1), indicating that 5b has a stronger affinity with AChE and exhibits more potent AChE inhibitory activity than 6a. Experimental AChE inhibitory activity assays corroborate these findings by revealing that the IC50 value of 5b (0.5243 μM) is slightly lower than that of 6a (0.6813 μM), thus affirming the superior AChE inhibitory activity of 5b. Additionally, regarding anti-aphid activity, 5b has an LC50 of 17.14 μg mL−1, slightly lower than that of 6a (LC50 = 18.28 μg mL−1). This suggests a close relationship between AChE inhibitory activity and insecticidal effectiveness, where a stronger AChE inhibitory effect corresponds to more potent insecticidal activity.
O group on the phosphate moiety. The length of hydrogen bond between 5b and Ser125 is predicted to be shorter than that of 6a and Tyr124 (2.9 Å vs. 3.2 Å). Furthermore, in comparison to 6a, 5b interacts with a greater number of amino acid residues in the AChE active pocket, which caused that the binding energy of 5b with AChE is slightly lower (−7.0 kcal mol−1) than that of 6a with AChE (−6.5 kcal mol−1), indicating that 5b has a stronger affinity with AChE and exhibits more potent AChE inhibitory activity than 6a. Experimental AChE inhibitory activity assays corroborate these findings by revealing that the IC50 value of 5b (0.5243 μM) is slightly lower than that of 6a (0.6813 μM), thus affirming the superior AChE inhibitory activity of 5b. Additionally, regarding anti-aphid activity, 5b has an LC50 of 17.14 μg mL−1, slightly lower than that of 6a (LC50 = 18.28 μg mL−1). This suggests a close relationship between AChE inhibitory activity and insecticidal effectiveness, where a stronger AChE inhibitory effect corresponds to more potent insecticidal activity.
Compound 9g is a phosphate with four phenyl substituents, significantly differing in structure from compounds 5b and 6a. Molecular docking results revealed that in comparison to 5b and 6a, 9g binds to a distinct active pocket of AChE, and forms different types of interactions with amino acid residues at the active site. The oxygen atoms of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O on the phosphate in 9g form hydrogen bonds with Tyr510, His381, Arg525 and Ala528. Additionally, the presence of phenyl substituents on the phosphate ester of 9g leads to π–π stacking, cation–π, and anion–π interactions with HIS381, Arg525 and Asp400. Table 6 shows that the binding energy of 9g with AChE is −8.5 kcal mol−1, which is lower than that of 5b and 6a. This indicates that, compared to 5b and 6a, 9g exhibits stronger binding affinity with AChE. Theoretically speaking, 9g should have stronger AChE inhibitory activity and, consequently, more potent anti-aphid activity. However, because the AChE inhibitory activity experiment was conducted in a PBS buffer, the hydrophobicity of the benzene ring in 9g limits the solubility in this solution, making it challenging to determine an exact IC50 value. As a result, it was not possible to accurately compare the IC50 values of 5b, 6a and 9g, thereby hindering a direct comparison of their AChE inhibitory activities. Regarding the anti-aphid activity, the LC50 value of 9g anti-aphid was 23.98 μg mL−1, slightly higher than the LC50 values for 5b and 6a. This difference may be attributed to the presence of four phenyl substituents in compound 9g, which reduces its solubility in the physiological environment of aphids and may hinder metabolism, resulting in lower insecticidal activity.
O and P–O on the phosphate in 9g form hydrogen bonds with Tyr510, His381, Arg525 and Ala528. Additionally, the presence of phenyl substituents on the phosphate ester of 9g leads to π–π stacking, cation–π, and anion–π interactions with HIS381, Arg525 and Asp400. Table 6 shows that the binding energy of 9g with AChE is −8.5 kcal mol−1, which is lower than that of 5b and 6a. This indicates that, compared to 5b and 6a, 9g exhibits stronger binding affinity with AChE. Theoretically speaking, 9g should have stronger AChE inhibitory activity and, consequently, more potent anti-aphid activity. However, because the AChE inhibitory activity experiment was conducted in a PBS buffer, the hydrophobicity of the benzene ring in 9g limits the solubility in this solution, making it challenging to determine an exact IC50 value. As a result, it was not possible to accurately compare the IC50 values of 5b, 6a and 9g, thereby hindering a direct comparison of their AChE inhibitory activities. Regarding the anti-aphid activity, the LC50 value of 9g anti-aphid was 23.98 μg mL−1, slightly higher than the LC50 values for 5b and 6a. This difference may be attributed to the presence of four phenyl substituents in compound 9g, which reduces its solubility in the physiological environment of aphids and may hinder metabolism, resulting in lower insecticidal activity.
Molecular docking analysis reveals that the phosphate moiety of the compound significantly influences AChE inhibitory activity. The binding of compounds to AChE primarily relies on the formation of hydrogen bonds between phosphate esters (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O bonds) and amino acid residues of AChE. Different phosphate ester substituents resulted in varied interactions with amino acid residues. Alkyl groups, being flexible, share the same active pocket probably with different binding modes to AChE. In contrast, aryl (phenyl)-substituted phosphate esters have a higher molecular weight and greater spatial hindrance, causing them to bind to different active sites in comparison to alkyl-substituted compounds. Moreover, the presence of the aryl group allows for the formation of π–π stacking, cation–π, and anion–π interactions between the compounds and AChE amino acid residues. Consequently, aryl-substituted compounds are expected to exert a stronger AChE inhibitory effect.
O and P–O bonds) and amino acid residues of AChE. Different phosphate ester substituents resulted in varied interactions with amino acid residues. Alkyl groups, being flexible, share the same active pocket probably with different binding modes to AChE. In contrast, aryl (phenyl)-substituted phosphate esters have a higher molecular weight and greater spatial hindrance, causing them to bind to different active sites in comparison to alkyl-substituted compounds. Moreover, the presence of the aryl group allows for the formation of π–π stacking, cation–π, and anion–π interactions between the compounds and AChE amino acid residues. Consequently, aryl-substituted compounds are expected to exert a stronger AChE inhibitory effect.
3 Experimental section
3.1 Chemistry
3.1.2.1 Synthesis of N-alkyl-1,3-dichloropropane and N,N-dialkyl-1,3-dichloropropane (2a–2e). Compounds 2a–2e were synthesized by refluxing 1 mmol (1 eq.) N-alkyl-1,3-propanediol or N,N-dialkyl-1,3-propanediol with 6 mmol (6 eq.) of SOCl2 for 0.5 h–1 h.
3.1.2.2 Synthesis of S–hydrogen phosphorothioates (4a–4h). S–hydrogen phosphorothioates 4a–4h were prepared by the reaction of 1.1 mmol (1.1 eq.) of S8 and 1 mmol (1 eq.) of corresponding diphosphate 3a–3h in 30 mL of dry diethyl ether, then 1.1 mmol (1.1 eq.) Et3N was added dropwise and stirred overnight at room temperature.
3.1.2.3 Synthesis of phosphorothioic acid, S,S′-[alkyl amino-1,3-propanediyl]O,O,O′O′-tetra ester (5–9). Novel synthesized compounds 5–9 were prepared by the reaction of 3 mmol (3 eq.) S–hydrogen phosphorothioates 4a–4h, NaH 3 mmol (3 eq.) and 1 mmol (1 eq.) N-alkyl-1,3-dichloropropane and N,N-dialkyl-1,3-dichloropropane 2a–2e in 20 mL of dry acetonitrile at 50 °C. Then the precipitate was filtered, and the filtrate was concentrated and extracted with dichloromethane. The organic phase was dried with anhydrous MgSO4 and then concentrated, and finally purified using silica gel column. All the synthesized compounds were characterized by 1H, 13C, 31P NMR and HRMS. The purity of all compounds is over 95% (see ESI†).
3.2 Biological assays
All of the bioassays were performed on representative test organisms reared in the laboratory. The bioassay was repeated in triplicate. Insecticidal assessments were made on a dead/alive basis, and mortality rates were corrected using Abbott's formula.The formulas involved in the experiment are as follows:
| Inhibitory mortality rate (%) = (B412 − T412) × 100/B412 |
| Ki = ln[v0/vt]/[IN]t |
| Corrected mortality rate (%) = (T − C) × 100/(1 − C) |
3.3 Molecular docking
The potential binding modes and the main interactions of compounds 5b, 6a and 9g with the AChE were determined using AutoDock Vina (version 1.1.2). The crystal structure of AChE (PDB ID: 6WVO) was obtained from Protein Data Bank (https://www.rcsb.org/). After water and other irrelevant small molecules were removed by Pymol 2.1 software, the AChE structure was pretreated and converted into pdbqt format using AutoDockTools v.1.5.6. The size of the grid box was set to 60 × 70 × 80 Å with a grid spacing 0.375 Å, and the grid was centered at −17.222, −38.784, and 28.591 (in x, y, and z dimensions, respectively). The optimized 5b, 6a and 9g at the DFT/B3LYP/6-31G(d) level was also pretreated and converted into pdbqt format using AutoDockTools v.1.5.6.4 Conclusions
In this study, a series of novel nereistoxin derivatives were designed, synthesized and evaluated for their biological activity, specifically involving acetylcholinesterase inhibitory activity and insecticidal activity. The AChE assay demonstrated that the studied compounds effectively inhibited AChE activity. However, the insecticidal activity of these compounds against M. separate indicated that the in vivo insecticidal activity was not only related to the AChE inhibition. This suggests that AChE may not be the unique active site for the compounds to exert insecticidal effect against M. separate in vivo. Whether it will be related to the active site AChR, where nereistoxin derivatives act, etc., remains to be further confirmed. In addition, the preliminary bioassay showed that 8 target compounds had significant insecticidal activity against R. padi compared to the positive control. Among them, compound 5b exhibited the highest activity against R. padi, with an LC50 of 17.14 μg mL−1. Simultaneously, 5b also showed strong AChE inhibitory activity, suggesting that compounds may exert their insecticidal activity against R. padi as an acetylcholinesterase inhibitor. The SAR analysis provided some valuable insights into the discovery and development of new insecticidal agents against M. separate and R. padi. In addition, molecular docking revealed significant differences in the binding sites and binding modes of the more active compounds 5b, 6a, and 9g with AChE. These differences were attributed to the larger steric hindrance of the aryl group and the fact that compared to alkyl substituents, increasing in π–π stacking/cation–π/anion–π interactions of aryl substituents with the active site of AChE could easily cause more inhibition potent in AChE. Thus, the aryl-substituted compounds theoretically have higher AChE inhibitory activity and more potent anti-aphid activity. However, their lower water solubility prevented us from obtaining the IC50 values. The docking results inspired us that incorporating hydrophilic groups into aryl-substituted compounds may offer potential avenues for exploring novel acetylcholinesterase inhibitors and nereistoxin pesticides. This work advances the development and deployment of nereistoxin-based insecticides and organophosphorus pesticides while also identifying viable candidate insecticides to solve current pesticide resistance challenges.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thanked Dr Rongxiu Zhu from the Institute of Theoretical Chemistry, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, People's Republic of China for assistance with the molecular docking simulation, and we also gratefully acknowledged the College of Plant Protection and Key Laboratory of Botanical Pesticide R&D in Shaanxi Province, Northwest A&F University, Yangling, Shaanxi 712100, China for the insecticidal activity assays.Notes and references
- T. Pirzada, B. V. de Farias, R. Mathew, R. H. Guenther, M. V. Byrd, T. L. Sit, L. Pal, C. H. Opperman and S. A. Khan, Curr. Opin. Colloid Interface Sci., 2020, 48, 121 CrossRef PubMed.
- C. Bass and C. M. Jones, Curr. Opin. Colloid Interface Sci., 2018, 27, iv Search PubMed.
- B. Borel, Nature, 2017, 543, 302 CrossRef PubMed.
- N. Liu, Annu. Rev. Entomol., 2015, 60, 537 CrossRef PubMed.
- R. Consortium, Evol. Appl., 2010, 3, 375 CrossRef PubMed.
- J. Sun, P. Liang and X. Gao, Pest Manage. Sci., 2012, 68, 285 CrossRef PubMed.
- S. Nitta, Yakugaku Zasshi, 1934, 54, 648 CrossRef PubMed.
- K. Konishi, Agric. Biol. Chem., 1970, 34, 926 Search PubMed.
- H. Mitsudera, T. Kamdcado, H. Uneme and Y. Kono, Agric. Biol. Chem., 1990, 54, 1719 Search PubMed.
- L. Cheng, G. Yu, Z. Chen and Z. Li, Agric. Sci. China, 2008, 7, 847 CrossRef.
- X. Jiang, K. Wang and M. Yi, Chin. J. Pestic. Sci., 2000, 2, 44 CAS.
- B. K. Singh, Nat. Rev. Microbiol., 2009, 7, 156 CrossRef CAS PubMed.
- H. A. Mekkawy, A. A. Massoud, E. M. Hammouda and M. A. Hassan, Toxicol. Lett., 1998, 95, 144 CrossRef.
- L. Gorecki, O. Soukup and J. Korabecny, Trends Pharmacol. Sci., 2022, 43, 593 CrossRef CAS PubMed.
- Q. Yan, X. Lu, Z. Zhang, Q. Jin, R. Gao, L. Li and H. Wang, Molecules, 2023, 28, 4846 CrossRef CAS PubMed.
- E. Dyguda-Kazimierowicz, S. Roszak and W. A. Sokalski, J. Phys. Chem. B, 2014, 118, 7277 CrossRef CAS PubMed.
- M. Jokanović, Toxicology, 2018, 410, 125 CrossRef.
- O. Politeo, I. Carev and A. Veljaca, Croat. Chem. Acta, 2019, 92, 11 CrossRef.
- B. Kaboudin, S. Emadi and A. Hadizadeh, Bioorg. Chem., 2009, 37, 101 CrossRef CAS.
- S. Sanada-Morimura and M. Matsumura, Appl. Entomol. Zool., 2011, 46, 443 CrossRef CAS.
- Z. Che, X. Yu, X. Zhi, L. Fan, X. Yao and H. Xu, J. Agric. Food Chem., 2013, 61, 8148 CrossRef CAS PubMed.
- C.-A. Dedryver, A. Le Ralec and F. Fabre, C. R. Biol., 2010, 333, 539 CrossRef PubMed.
- L. R. Nault, Ann. Entomol. Soc. Am., 1997, 90, 521 CrossRef.
- R. Hossain, W. Menzel, C. Lachmann and M. Varrelmann, Plant Pathol., 2021, 70, 584 CrossRef.
- S. Yang, E. Fitches, P. Pyati and J. A. Gatehouse, Pest Manage. Sci., 2015, 71, 951 CrossRef.
- C. C. Voudouris, A. N. Kati, E. Sadikoglou, M. Williamson, P. J. Skouras, O. Dimotsiou, S. Georgiou, B. Fenton, G. Skavdis and J. T. Margaritopoulos, Pest Manage. Sci., 2016, 72, 671 CrossRef.
- Q. Tang, K. Ma, Y. Hou and X. Gao, Pestic. Biochem. Physiol., 2017, 143, 39 CrossRef.
- F. Worek, H. Thiermann, L. Szinicz and P. Eyer, Biochem. Pharmacol., 2004, 68, 2237 CrossRef PubMed.
- S. Sanada-Morimura and M. Matsumura, Appl. Entomol. Zool., 2011, 46, 443 CrossRef.
- Z. Che, X. Yu, X. Zhi, L. Fan, X. Yao and H. Xu, J. Agric. Food Chem., 2013, 61, 8148 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08004h |
| This journal is © The Royal Society of Chemistry 2024 |