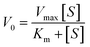Open Access Article
Open Access ArticleSynthesis of Os@ZIF-8 nanocomposites with enhanced peroxidase-like activity for detection of Hg2+†
Zijie Wei,
Cuifeng Jiang *,
Jinshan Wang
*,
Jinshan Wang and
Yue Chen
and
Yue Chen
School of Materials Science and Engineering, Yancheng Institute of Technology, Yancheng, 224051, China. E-mail: cuifengj123@163.com
First published on 26th March 2024
Abstract
Metal organic framework (MOF)-derived nanostructures display remarkable characteristics and have broad application potential. Os@ZIF-8 nanocomposites were prepared by a depositional method. The Os nanoparticles distributed on the surface of ZIF-8. The nanocomposites displayed enhanced peroxidase-like activity with smaller Km for both 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2 compared to Os NPs due to the confinement effect and large surface area that ZIF-8 provided. From the average reaction rate constants obtained from three different temperatures, the activation energy values were determined. The kinetic data indicated that the Os@ZIF-8 NCs are catalytically more active than Os NPs. In addition, quantitative measurement of Hg2+ was performed based on the formation of Os–Hg alloy. Os@ZIF-8 NCs had a wide detection range between 0 μM and 71.43 μM for Hg2+ with a limit of detection (LOD) of 2.29 μM. Using a MOF with a large surface area to load Os nanoparticles to achieve enhanced nanozyme activity is the novelty of this work.
1. Introduction
As a heavy metal, mercury is a common environmental pollutant due to its low degradation rate and high toxicity.1,2 Ingested mercury can generate highly toxic methylmercury through methylation and accumulate in the brain through the blood–brain barrier.3–5 Eventually, it results in neurological diseases such as spasms and epilepsy.4,5 Thus, mercury poisoning is a big concern in our life. It is of great importance to detect mercury in water. So far, many analytical methods have been exploited to detect mercury, including atomic emission spectrometry (AES),6 atomic absorption spectroscopy,7 colorimetric methods,8,9 fluorescence methods10 and electrochemical methods.2 Especially, colorimetric methods based on nanozymes have attracted lots of interest due to simplicity.11Nanozymes are a class of nanomaterials with enzyme-like catalytic activity that have been applied in diverse fields, such as chemical,12 biological sensing,13 medical diagnosis14 and environmental protection.15 Especially, noble metal nanoparticles have attracted lots of interest due to the unique advantages of high catalytic activity, simple synthesis and good stability.16 However, rarely has attention been paid to osmium nanoparticles. Osmium is a precious metal with low compressibility, high melting point and high bulk modulus.17,18 Recent studies have revealed that Os nanozymes exhibited remarkable higher peroxidase-like activity and negligible oxidase-like activity in comparison with other noble metal-based nanozymes (e.g. Pt and Au).19 This enabled Os-based NPs to be peroxidase-like specific nanozymes with high enzymatic activity. However, for practical applications, an issue still needs to be addressed. That is the easy aggregation of individual Os NPs, which may result in the decrease of the activity. Nanoparticles supported on metal–organic frameworks (MOFs) are a good choice to resolve this problem.20 Especially, due to the advantages of large surface area, small pore size and easy preparation, zeolite imidazolate framework-8 (ZIF-8) has become a good candidate to support nanoparticles.20–23 Inspired by this enlightenment, Os@ZIF-8 nanocomposites were designed and applied for detection of mercury.
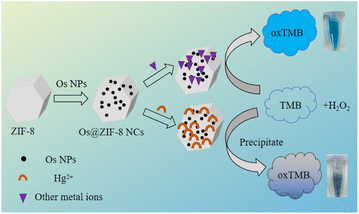
Here, a Os@ZIF-8 NCs composite was prepared (Scheme 1). This nanocomposite has a large surface area and porous structure, which resulted in enhanced peroxidase-like activity. The steady-state kinetic and catalytic reaction kinetics were investigated. The high peroxidase-like activity was believed to originate from the strong binding between the nanocomposite and the substrate. The lower activation energy further explained the excellent catalytic ability. Using Os@ZIF-8 NCs as a probe, Hg2+ were detected with lower detection limit and wider linear range.
2. Experimental
2.1 Chemicals
K2OsCl6, NaBH4 and terephthalic acid (TA) were purchased from Aladdin Industrial Corporation. 2-Methylimidazole, zinc acetate, hydrogen peroxide (98%), CaCl2 and sodium acetate were purchased from Jiangsu Tongsheng Chemical Reagent Company. Acetate buffer was prepared by mixing glacial acetic acid and sodium acetate in proportion. 3,3′,5,5′-Tetramethylbenzidine (TMB), CrCl3 were obtained from Sangon Biotech (Shanghai) Co., Ltd. Hg was purchased from National Testing Center for Nonferrous Metals and Electronic Materials. KCl, NaCl, MgCl2·6H2O, ZnCl2, AlCl3, CuCl2·2H2O, FeCl3, MnCl2 and glacial acetic were obtained from Sinopharm Chemical Reagent Co., Ltd. PbCl2 was obtained from Shanghai Shisi Hewei Chemical Co., Ltd.2.2 Preparation of Os@ZIF-8 NCs
0.015 mL K2OsCl6 (0.1 M) was added into 1 mL H2O in a round bottom flask and stirred vigorously for 30 minutes at room temperature. Then, 0.1 mL NaBH4 (0.2 M) prepared with ice water was slowly added into the mixture and stirred in dark for 90 minutes to obtain Os nanoparticles.2 mL 2-methylimidazole (0.56 M) and 2 mL zinc acetate (70 mM) (prepared in methanol) were added into a round bottom flask and stirred for 24 hours at room temperature. Then, the obtained ZIF-8 was centrifuged and washed with methanol. The washed ZIF-8 was dissolved in methanol solution 5 mL (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). After that, 1 mL Os NPs was added into the above solution and stirred for 45 minutes. The mixture is allowed to stand for 24 hours to obtain Os@ZIF-8 NCs.
4). After that, 1 mL Os NPs was added into the above solution and stirred for 45 minutes. The mixture is allowed to stand for 24 hours to obtain Os@ZIF-8 NCs.
2.3 Characterization
The morphology and microstructure was observed and analyzed by transmission electron microscope (TEM, JEM-2100F) and an energy dispersive X-ray spectrometer (EDS, X-MaxN 80T IE250). X-ray photoelectron spectroscopy (XPS) measurement of the products was carried out using a ESCALAB 250Xi spectrometer. Fourier-transform infrared spectroscopy (FTIR) was recorded by using NEXUS-670. The surface area, pore volume, and pore size distribution of the catalysts were determined from N2 adsorption–desorption isotherms using Beckman Coulter SA3100. XRD measurement was obtained by X-ray diffractometer (X'Pert3Powder). Size distribution of nanoparticles was obtained from the Malvern Zetasizer (Zetasizer Nano). Other characterization methods refer to our previous report.242.4 Enzyme-mimetic activities of Os@ZIF-8 NCs
The enzyme-mimic activities of Os@ZIF-8 NCs were evaluated by using TMB in the presence or absence of H2O2. Typically, in the absence or presence of 100 μL H2O2 (50 mM), 50 μL Os@ZIF-8 NCs, 100 μL TMB (8 mM) and 100 μL acetic acid buffer (50 mM, pH = 4.6) were mixed and spectra were measured after 10 min incubation at room temperature. After the enzyme-mimetic experiment, the mixture was centrifuged or under natural precipitation (by standing for 1.5 hours). Then, the recycling experiment was conducted by using the precipitate.The steady-state kinetics of TMB oxidation were measured at room temperature with changes in the concentration of TMB (0, 0.1, 0.2, 0.5, 1, 2, 3, 4, 5, 6, 8 mM) with H2O2 fixed at 50 mM or H2O2 (0, 0.1, 0.5, 1, 5, 10, 20, 30, 40, 50 mM) with TMB fixed at 8 mM. The calculation is according to our previous literature.24
In the case of TA oxidation, 0.025 M TA was prepared in 0.1 M NaOH solution. Then, 20 μL TA (25 mM), 200 μL H2O2 (50 mM), 200 μL acetic acid buffer (pH = 4.6) and 100 μL Os@ZIF-8 NCs were incubated in 700 μL H2O in the dark for 1 h. Finally, the fluorescence spectrum of the reaction mixture was collected.
2.5 Detection of Hg2+
50 μL Hg with different concentrations were mixed with 50 μL Os@ZIF-8 NCs. Then, 100 μL TMB (8 mM), 100 μL H2O2 (50 mM), 100 μL acetate buffer (50 mM, pH = 4.6) and 300 μL deionized water were added and incubated for 5 min. Then, the obtained solution was measured for UV-vis absorption. For Hg2+ detection, the absorption value at 652 nm (characteristic peak for oxTMB) was used as the signal. Different concentration of Hg2+ gave different absorbance value. The curve of absorbance versus concentration could be fitted linearly, obtaining the calibration function. For selective experiments, 71.43 μM of other metal ions were added.3. Results and discussion
3.1 Characterization of Os@ZIF-8 NCs
The detailed structural characteristics of Os@ZIF-8 NCs were investigated. Firstly, the morphology and microstructure of prepared nanomaterials was characterized by transmission electron microscopy (TEM). As showed in Fig. 1a, the original ZIF-8 exhibited hexagonal morphology. After deposition of Os NPs (Fig. 1b), it can be seen that Os NPs dispersed on the surface of ZIF-8. To further make clear the element distribution of Os@ZIF-8, EDS layered image and element mapping were employed. Fig. 1c indicates that the C, N and Zn elements from ZIF-8 were uniformly dispersed on the whole nanocomposite, while the Os element mainly distributed on the surface of ZIF-8. The EDS results in Fig. S1a† showed the presence of elemental C, N, Zn and Os, in accordance with the element mapping results. The size of the nanocomposite is mainly in the range of 450–600 nm as showed in Fig. S1b†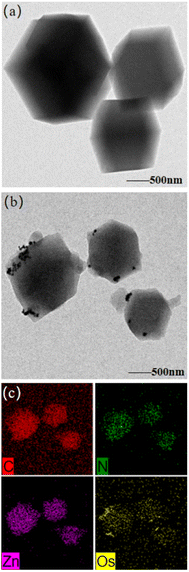 | ||
| Fig. 1 Typical TEM images of (a) pristine ZIF-8, (b) Os@ZIF-8 NCs, (c) element mapping of Os@ZIF-8 NCs. | ||
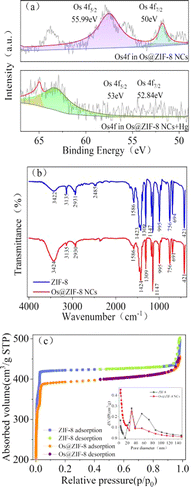
X-ray photoelectron spectroscopy (XPS) analysis was performed to determine the oxidation state of Os in the composite. The binding energy of Os 4f7/2 and Os 4f5/2 appeared at 50 eV and 55.99 eV, which indicated the presence of Os0 (Fig. 2a).17,25 In order to analyze the structure of ZIF-8, FTIR spectra were measured. As showed in Fig. 2b, the characteristic peak stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in the imidazole ring at 421 cm−1 and 1586 cm−1, the planar bending of the imidazole ring near 1423 cm−1, all kept the same for ZIF-8 and Os@ZIF-8 NCs. The results showed that Os NPs had no effect on the structure and functional groups of ZIF-8.
N bond in the imidazole ring at 421 cm−1 and 1586 cm−1, the planar bending of the imidazole ring near 1423 cm−1, all kept the same for ZIF-8 and Os@ZIF-8 NCs. The results showed that Os NPs had no effect on the structure and functional groups of ZIF-8.
The permanent porosity of ZIF-8 was confirmed by reversible N2 sorption experiments. Both ZIF-8 and Os@ZIF-8 NCs exhibited type-I adsorption isotherm behavior (Fig. 2c), with surface areas of 1425.94 m2 g−1 and 1337.87 m2 g−1 respectively. The BET surface areas value of ZIF-8 is similar to former reported value.26 The pore volume are calculated to be 0.76 and 0.69 mL g−1 for ZIF-8 and Os@ZIF-8 NCs. Both the smaller surface area and pore volume of Os@ZIF-8 NCs further illustrate the distribution of Os NPs over the surface of ZIF-8. XRD measurement in Os nanoparticles, ZIF-8, Os@ZIF-8 NCs have been conducted. However, distinct characteristic peaks of Os were not observed neither in Os NPs nor in Os@ZIF-8 NCs. There was no significant change between ZIF-8 and Os@ZIF-8 NCs. The relative data has been added in Fig. S1c.†
3.2 Mimetics property of Os@ZIF-8 NCs
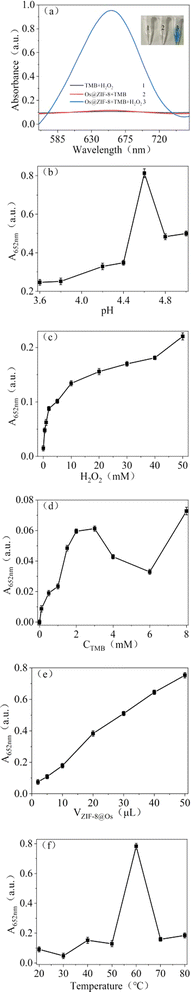
Combination of the nanozyme property of Os NPs and porous structure of ZIF-8, the prepared nanocomposites were expected to have broad application in nanocatalysis. The mimetic property of Os@ZIF-8 NCs was evaluated by employing chromogenic substrate TMB, which would turn to blue color in the appropriate catalyst. The results were showed in Fig. 3a, Os@ZIF-8 NCs + TMB + H2O2 system exhibited blue color and significant absorption at 652 nm, indicating the peroxidase-like activity of Os@ZIF-8 NCs. However, Os@ZIF-8 NCs + TMB displayed almost colorless. It indicates the Os@ZIF-8 NCs doesn't possess oxidase-like activity. In order to obtain the high catalytic activity, the experimental conditions were optimized. As shown in Fig. 3b–e, the optimal conditions were pH 4.6, H2O2 50 mM. TMB 8 mM, and 50 μL Os@ZIF-8 NCs. It is interesting that the optimal temperature for Os@ZIF-8 NCs was 60 °C (Fig. 3f), which was different from the previous reports that Os NPs exhibited very low peroxidase-like activity when the temperature was higher than 55 °C. It is possibly due to the stronger stability of Os under ZIF-8 loading.27,28To make clear the exact catalytic mechanism of Os@ZIF-8 nanozyme, TA was chosen to conduct the related experiments because it could detect ˙OH free radicals.29–31 As shown in Fig. S2,† in the TA oxidation experiment, fluorescence emission was observed after co-incubation of Os@ZIF-8 and H2O2, indicating the generation of ˙OH free radicals. The fluorescence intensity increased compared to TA + H2O2. However, the intensity of TA + H2O2 + Os@ZIF-8 NCs decreased compared to the TA + Os@ZIF-8 NCs. It is proposed that the quenching effect appeared in the presence of both Os@ZIF-8 NCs and H2O2.32,33
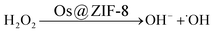 | (1) |
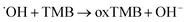 | (2) |
Two steps were involved in the catalytic process as shown in eqn (1) and (2). Firstly, ˙OH free radicals was generated from H2O2 in the presence of Os@ZIF-8. In this step, Os@ZIF-8 played the role of catalyst. Secondly, ˙OH free radicals oxidized TMB to oxTMB, accompanying the color change from colorless to blue. Totally, the peroxidase-like activity required both Os@ZIF-8 and H2O2, which can also be verified from Fig. 3a, where Os@ZIF-8 + TMB almost displayed colorless.
3.3 Kinetic analysis
The Michaelis–Menten equation describes the relationship of Km, V0 (initial velocity) and Vmax (maximum velocity):Lineweaver–Burk double reciprocal was obtained from Michaelis–Menten equation. The Km and Vmax could be calculated from Lineweaver–Burk double reciprocal. Smaller Km indicates stronger binding, and vice versa.34 As showed in Fig. 4a and b, the steady state kinetic of Os@ZIF-8 NCs followed Michaelis–Menten trend. The Km and Vmax were obtained from the Lineweaver–Burk double reciprocal (Fig. 4c and d). To further analyze the mimicking property of Os@ZIF-8 NCs, the steady-state kinetic analysis of pure Os NCs was conducted and presented in Fig. S3.†
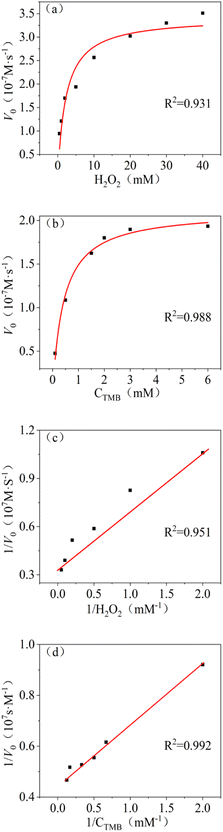 | ||
| Fig. 4 Steady-state kinetic analysis with Os@ZIF-8 NCs, Michaelis–Menten trend of (a) H2O2 and (b) TMB, Lineweaver–Burk plot of (c) H2O2 and (d) TMB. | ||
As listed in Table 1, Os@ZIF-8 NCs displayed much smaller Km for TMB and H2O2 compared to pure Os NPs. Zeta potential of Os and Os@ZIF-8 were measured to be −26 mV and 14 mV. Considering the positive property of TMB, Os NPs was supposed to have stronger binding to TMB. However, the result was different. This phenomenon indicates other factors showed intense effect on the catalytic activity. It is speculated that two reasons are responsible for the result. On one hand, the porous structure of ZIF-8 could exhibit confinement effect for contact of Os NPs and substrate. This hypothesis could be verified from Fig. 2d. The average pore size of Os@ZIF-8 NCs is smaller than ZIF-8 due to a mount of Os distributed on the pore. On the other hand, the large surface area of Os@ZIF-8 NCs may be more easily to absorb substrate molecules. In addition, compared with other nanozymes, such as GSH-AgNPs,35 Fe3O4 NPs,36 Cu@Au/Pt24 and Cu(OH)2 SCs,37 Os@ZIF-8 NCs also displayed smaller Km for TMB. All these data indicated the excellent peroxidase-like activity of prepared nanocomposites.
| Catalyst | Substrate | Km (mM) | Vmax (10−7 M s−1) | Ref. |
|---|---|---|---|---|
| GSH-AgNPs | H2O2 | 58.6 | 2.15 | 35 |
| TMB | 4.17 | 24.7 | ||
| Fe3O4 NPs | H2O2 | 0.16 | 0.18 | 36 |
| TMB | 1.372 | 0.419 | ||
| Cu@Au/Pt | H2O2 | 8.54 | 2.43 | 24 |
| TMB | 6.64 | 6.14 | ||
| Cu(OH)2 SCs | H2O2 | 0.199 | 4.251 | 37 |
| TMB | 2.488 | 4.483 | ||
| Os NPs | H2O2 | 27.342 | 43.197 | This work |
| TMB | 1.53 | 6.585 | ||
| Os@ZIF-8 NCs | H2O2 | 0.516 | 2.21 | This work |
| TMB | 0.926 | 2.5776 |
In the reaction from TMB to oxTMB, Os@ZIF-8 NCs played the role of catalyst. Therefore, the activation energy is another important parameter to understand the catalytic reaction. Fig. S4† displays the experimental results obtained from three different temperature (20 °C, 40 °C, 50 °C). It can be seen, the absorbance at 652 nm increased with time for both Os NPs and Os@ZIF-8 NCs at each temperature. After that, it reached a plateau, indicating the accomplishment of the reaction from TMB to oxTMB. The first-order kinetics for the oxidation of TMB could be applied to our system. As shown in Fig. 5a–c, the ln(A/A0) (the initial absorption value at 652 nm was A0, the absorption value at t time was A) is linearly to time. According to the linear relationship, the average reaction rate constant (k) for both catalyst at three temperature were calculated and listed in Table S1.† Moreover, based on the Arrhenius equation ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln
k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − Ea/RT and the linear fitting of ln
A − Ea/RT and the linear fitting of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k versus 1/T (Fig. 5d), the apparent activation energy (Ea) were obtained. The activation energy of Os@ZIF-8 NCs (57.58 kJ mol−1) is lower than that of Os NPs (391.67 kJ mol−1), implying a better catalytic activity for Os@ZIF-8 NCs. It is probably due to the confinement effect of ZIF-8 for contact of Os NPs and substrates.25 “Confinement effects” mainly related to three aspects. One is containment of catalyst (Os@ZIF-8) and substrate (TMB) in the same space. The other is the inhibition of catalyst aggregation. The third is protecting the reaction between Os@ZIF-8 and TMB from exposure to potentially deleterious substances.38
k versus 1/T (Fig. 5d), the apparent activation energy (Ea) were obtained. The activation energy of Os@ZIF-8 NCs (57.58 kJ mol−1) is lower than that of Os NPs (391.67 kJ mol−1), implying a better catalytic activity for Os@ZIF-8 NCs. It is probably due to the confinement effect of ZIF-8 for contact of Os NPs and substrates.25 “Confinement effects” mainly related to three aspects. One is containment of catalyst (Os@ZIF-8) and substrate (TMB) in the same space. The other is the inhibition of catalyst aggregation. The third is protecting the reaction between Os@ZIF-8 and TMB from exposure to potentially deleterious substances.38
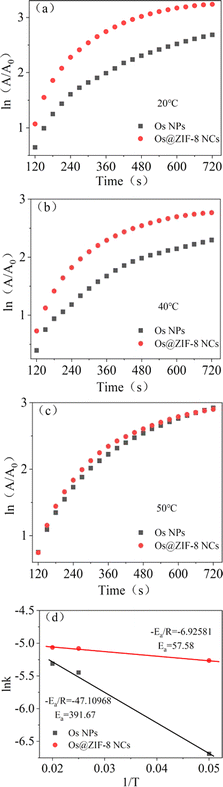 | ||
| Fig. 5 (a)–(c)The Arrhenius plots for reactions catalyzed by Os NPs and Os@ZIF-8 NCs, (d) the activation energies (Ea) can be calculated from the slope of the linear fitting. | ||
3.4 Recycling experiment
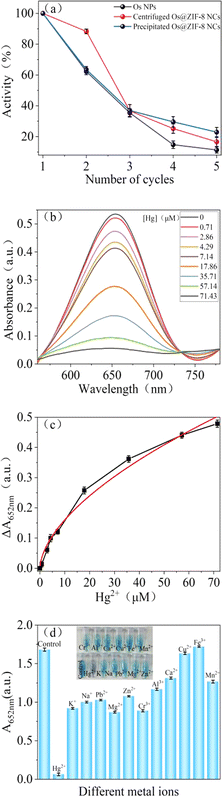
Recyclability is another important factor for application of nanozyme. Therefore, recycling experiments were conducted on the prepared pure Os NPs and Os@ZIF-8 NCs. Natural precipitation and centrifugation were used to evaluate the recycle effect. The results in Fig. 6a showed that the activity of pure Os NPs and precipitated Os@ZIF-8 NCs deceased to 60% after twice recycle. However, the activity of centrifuged Os@ZIF-8 NCs remained 90%. It was possible that during natural precipitation, some samples may not precipitate thoroughly enough, resulting in some losses. After 4 times recycle, the activity of precipitated and centrifuged Os@ZIF-8 NCs are 22.93% and 19.57%, respectively. However, the activity of pure Os NPs is only 8.44%. It is proposed that the centrifugation of Os NPs can't separate all the nanoparticles due to their small size. The same trend was observed for 5 times recycle. In a word, Os@ZIF-8 NCs could maintain higher activity compared to pure Os NPs after recycling experiments.3.5 Detection of Hg2+
To explore the application of Os@ZIF-8 NCs, quantitatively detection of Hg2+ was performed under optimized condition. Fig. 6b demonstrates the effect of Hg2+ concentration on the peroxidase-like activity of Os@ZIF-8 NCs. It can be seen that as the concentration of Hg2+ increases, the absorption value at 652 nm decreases. Fig. 6c shows that ΔA at 652 nm versus concentration of Hg2+ (ΔA = A0 − A, where A0 and A are absorption intensities at 652 nm in the absence and presence of Hg2+, respectively). It can be seen that the absorbance exhibits a nonlinear variation between 0 and 71.43 μM. However, 0–17.86 μM and 17.86–71.43 μM showed linear relationship, respectively. The limit of detection (LOD) was calculated to be 2.29 μM according to the rule of 3σ/S, σ represents the standard deviation of the sample, and S represents the slope of the sample. Table 2 lists the detection limits and detection ranges of other materials for detecting Hg2+. It can be seen that Os@ZIF-8 NCs has a wide range and low detection limit compared with other materials. It is worth to mention, the black flocculent generated upon addition of Hg2+were collected for XPS analysis. As showed in Fig. 2a, the two peaks for Os 4f7/2 (50 eV) and Os 4f5/2 (55.99 eV) disappeared, while a new peak at 63 eV appeared. It was reported binding energy of Hg0 at 100.7 and 104.7 eV.39 The new peak located between Os0 and Hg0, which indicated the formation of Os–Hg alloy.Then, the influence of nanocomposite on the detection of Hg2+ was evaluated. Firstly, Os@ZIF-8 NCs have long-term storage stability and can be ultrasonically dispersed upon reuse. The activity of Os@ZIF-8 NCs with storage days was added in Fig. S5a.† It can be seen, the activity almost unchanged even after one day storage. However, the activity decreased sharply after one day. Therefore, Os@ZIF-8 NCs could be used in one day for Hg2+ detection to get good sensitivity. Moreover, the response time of the nanocomposite for Hg2+ detection was showed in Fig. S5b.† The absorbance tended to stabilize at 300 seconds. Therefore, 5 minutes was chosen as the response time for testing. In addition, we also investigated the influence of temperature on the detection of Hg2+. As shown in Fig. S6,† the trend was similar under the three temperatures (20 °C, 35 °C, 50 °C), so the influence of temperature on the detection of Hg2+ was negligible.
In addition, to evaluate whether other metal ions will affect the detection, selective experiments were conduct. 71.43 μM of K+, Na+, Mg2+, Pb2+, Zn2+, Cr3+, Al3+, Cu2+, Ca2+, Fe3+, Mn2+ were used to perform selectivity experiment. As shown in Fig. 6d, the solution color with Hg was lighter than with other metal ions. Meanwhile, there is almost no absorption peak upon addition of Hg2+. The absorption value at 652 nm for all the metal ions was summarized in Fig. 6d. It is clear that the value is much lower for Hg2+ than other metal ions. Fig. 6d shows that the nanocomposite is not specific for Hg2+ detection. However it is more sensitive to Hg2+. Nitrogen of ZIF-8 has lone pairs of electrons, which could form coordination bond with metal ions. The coordination compound perhaps blocked the active site of Os NPs, resulting decrease of catalytic ability. However, the binding force between Hg2+ and ZIF-8 is much stronger compared to other metal ions. Therefore, it is more sensitive to Hg2+.
4. Conclusions
In summary, we have successfully deposited Os NPs on the surface of ZIF-8. Enhanced peroxidase-like activity verified as stronger binding to TMB and H2O2 was achieved thanks to the confinement effect and large surface area that ZIF-8 provided. Importantly, Os@ZIF-8 NCs had smaller activation energy compared to Os NPs, further verifying the better catalytic ability. Moreover, Os@ZIF-8 NCs exhibited better recoverability. In addition, the nanocomposite provided a sensitive approach for selective detection of Hg2+. It is supposed to be widely applied in water pollution.Conflicts of interest
The authors declared that they have no conflicts of interest to this work.Acknowledgements
This work was supported by Natural Science Foundation of China (21605128).Notes and references
- A. Hu, G. Chen, A. Huang, Z. Cai, T. Yang, C. Ma, L. Li, H. Gao, J. Gu, C. Zhu, Y. Wu, X. Qiu, J. Xu, J. Shen and L. Zhong, J. Fluoresc., 2023, 34, 905–913 CrossRef PubMed.
- L. Huang, Y. Cao and D. Diao, Sens. Actuators, B, 2020, 305, 127495 CrossRef CAS.
- K. Abbas, H. Znad and M. R. Awual, Chem. Eng. J., 2018, 334, 432–443 CrossRef CAS.
- T. A. Saleh, G. Fadillah, E. Ciptawati and M. Khaled, TrAC, Trends Anal. Chem., 2020, 132, 116016 CrossRef CAS.
- N. Liang, X. Ge, Y. Zhao, L. Xia, Z.-L. Song, R.-M. Kong and F. Qu, J. Hazard. Mater., 2023, 454, 131455 CrossRef CAS PubMed.
- R. Wang and W.-X. Wang, Environ. Pollut., 2018, 234, 288–296 CrossRef CAS PubMed.
- V. A. Lemos and L. O. dos Santos, Food Chem., 2014, 149, 203–207 CrossRef CAS PubMed.
- D. Zhang, S. Yang, Q. Ma, J. Sun, H. Cheng, Y. Wang and J. Liu, Food Chem., 2020, 313, 126119 CrossRef PubMed.
- S. Zhang, H. Li, Z. Wang, J. Liu, H. Zhang, B. Wang and Z. Yang, Nanoscale, 2015, 7, 8495–8502 RSC.
- H. Wang, X. Wang, M. Liang, G. Chen, R.-M. Kong, L. Xia and F. Qu, Anal. Chem., 2020, 92, 3366–3372 CrossRef CAS PubMed.
- J. Dai, L. Wang, F. Xu and G. Ma, Ionics, 2022, 28, 5251–5255 CrossRef CAS.
- Q. Yang, Y.-Y. Mao, Q. Liu and W.-W. He, Rare Met., 2023, 42, 2928–2948 CrossRef CAS.
- M. Feng, X. Li, X. Zhang and Y. Huang, TrAC, Trends Anal. Chem., 2023, 166, 117220 CrossRef CAS.
- X. Zhang, X. Chen and Y. Zhao, Nano-Micro Lett., 2022, 14, 95 CrossRef CAS PubMed.
- J. Venkatesan, P. K. Gupta, S. E. Son, W. Hur and G. H. Seong, J. Cluster Sci., 2022, 34, 23–43 CrossRef.
- W. Shou, S.-T. Yang, Y.-L. Wang and L.-H. Guo, Chin. J. Anal. Chem., 2021, 49, 676–685 CAS.
- S. B. He, M. T. Lin, L. Yang, H. A. A. Noreldeen, H. P. Peng, H. H. Deng and W. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 44541–44548 CrossRef CAS PubMed.
- S. B. He, Q. Q. Zhuang, L. Yang, M. Y. Lin, Y. Kuang, H. P. Peng, H. H. Deng, X. H. Xia and W. Chen, Anal. Chem., 2020, 92, 1635–1642 CrossRef CAS PubMed.
- M. Gerami, H. Farrokhpour and N. Orangi, J. Phys. Chem. A, 2023, 127, 3991–4004 CrossRef CAS PubMed.
- X. Wang, R. Xu, Y. Tian, X. Gao, W. Zhang, Z. Sun, Y. Mou, X. Sun, Y. Guo and F. Li, Sens. Actuators, B, 2023, 388, 133856 CrossRef CAS.
- F. K. Arabbani, D. Vasu, S. Sakthinathan, T. W. Chiu and M. C. Liu, Electroanalysis, 2023, 35, e202200284 CrossRef CAS.
- Y. Xie, X. Dong, N. Cai, F. Yang, W. Yao and L. Huang, Foods, 2023, 12, 813 CrossRef CAS PubMed.
- Y. Guo, J. Zhang, J. Liu, N. Wang and X. Su, Anal. Chim. Acta, 2023, 1276, 341649 CrossRef CAS PubMed.
- C. Jiang, X. Wei, S. Bao, H. Tu and W. Wang, RSC Adv., 2019, 9, 41561–41568 RSC.
- R. L. Papporello, E. E. Miró and J. M. Zamaro, Microporous Mesoporous Mater., 2015, 211, 64–72 CrossRef CAS.
- Y. Wang, F. Wang and J. Zhang, Cryst. Growth Des., 2019, 19, 3430–3434 CrossRef CAS.
- S. He, L. Yang, P. Balasubramanian, S. Li, H. Peng, Y. Kuang, H. Deng and W. Chen, J. Mater. Chem. A, 2020, 8, 25226–25234 RSC.
- X. Cui, J. Zhang, Y. Liu, W. Shao, Y. Tang and H. Sun, ChemNanoMat, 2023, 9 DOI:10.1002/cnma.202300310.
- Z. Fu, W. Zeng, S. Cai, H. Li, J. Ding, C. Wang, Y. Chen, N. Han and R. Yang, J. Colloid Interface Sci., 2021, 604, 113–121 CrossRef CAS PubMed.
- X. Jiang, P. Gray, M. Patel, J. Zheng and J. J. Yin, J. Mater. Chem. B, 2020, 8, 1191–1201 RSC.
- B. Han, H. Guan, B. Peng, Y. Zhang and Y. Liu, Anal. Methods, 2022, 14, 4832–4841 RSC.
- X. Liu and F. Liu, Carbohydr. Polym., 2023, 310, 120726 CrossRef CAS PubMed.
- H. M. Abdul Hakkeem, A. Babu, N. Shilpa, A. A. Venugopal, A. P. Mohamed, S. Kurungot and S. Pillai, Carbohydr. Polym., 2022, 292, 119723 CrossRef CAS PubMed.
- L. Ou, T. L. Herzog, C. M. Wilmot and C. B. Whitley, Mol. Genet. Metab., 2014, 111, 113–115 CrossRef CAS PubMed.
- C. Jiang, Z. Bai, F. Yuan, Z. Ruan and W. Wang, Spectrochim. Acta, Part A, 2022, 265, 120348 CrossRef CAS PubMed.
- N. Bagheri, A. Khataee, J. Hassanzadeh and B. Habibi, Spectrochim. Acta, Part A, 2019, 209, 118–125 CrossRef CAS PubMed.
- J. Mu, J. Li, X. Zhao, E.-C. Yang and X.-J. Zhao, Sens. Actuators, B, 2018, 258, 32–41 CrossRef CAS.
- S. Karmakar, S. Barman, F. A. Rahimi and T. K. Maji, Energy Environ. Sci., 2021, 14, 2429–2440 RSC.
- R. Chadha, A. Das, A. K. Debnath, S. Kapoor and N. Maiti, Colloids Surf., A, 2021, 615, 126279 CrossRef CAS.
- N. Zohora, D. Kumar, M. Yazdani, V. M. Rotello, R. Ramanathan and V. Bansal, Colloids Surf., A, 2017, 532, 451–457 CrossRef CAS.
- Y.-C. Hsieh, J.-L. Chir, S.-T. Yang, S.-J. Chen, C.-H. Hu and A.-T. Wu, Carbohydr. Res., 2011, 346, 978–981 CrossRef CAS PubMed.
- F. Yan, D. Kong, Y. Luo, Q. Ye, J. He, X. Guo and L. Chen, Microchim. Acta, 2016, 183, 1611–1618 CrossRef CAS.
- K. Jia, K. Yi, W. Zhang, P. Yan, S. Zhang and X. Liu, Sens. Actuators, B, 2022, 370, 132443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08723a |
| This journal is © The Royal Society of Chemistry 2024 |