Open Access Article
Open Access ArticleInnovative microwave-assisted biosynthesis of copper oxide nanoparticles loaded with platinum(II) based complex for halting colon cancer: cellular, molecular, and computational investigations†
Nada K. Sedky‡
 a,
Iten M. Fawzy‡
a,
Iten M. Fawzy‡ b,
Afnan Hassanc,
Noha Khalil Mahdyd,
Reem T. Attiae,
Samir N. Shammaf,
Mohammad Y. Alfaifig,
Serag Eldin Elbehairig,
Fatma A. Mokhtar
b,
Afnan Hassanc,
Noha Khalil Mahdyd,
Reem T. Attiae,
Samir N. Shammaf,
Mohammad Y. Alfaifig,
Serag Eldin Elbehairig,
Fatma A. Mokhtar h and
Sherif Ashraf Fahmy
h and
Sherif Ashraf Fahmy *i
*i
aDepartment of Biochemistry, School of Life and Medical Sciences, University of Hertfordshire Hosted by Global Academic Foundation, R5 New Garden City, New Administrative Capital, Cairo, Egypt
bDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, Future University in Egypt, Cairo, 11835, Egypt
cBiomedical Sciences Program, Zewail City of Science and Technology, Giza, 12578, Egypt
dDepartment of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Kasr El-Aini Street, 11562 Cairo, Egypt
eDepartment of Pharmacology and Toxicology and Biochemistry, Faculty of Pharmacy, Future University in Egypt, Cairo, 11835, Egypt
fInstitute of Global Health and Human Ecology, School of Sciences & Engineering, The American University in Cairo, AUC Avenue, P.O. Box 74, New Cairo 11835, Egypt
gKing Khalid University, Faculty of Science, Biology Department, Abha 9004, Saudi Arabia
hDepartment of Pharmacognosy, Faculty of Pharmacy, El Saleheya El Gadida University, El Saleheya El Gadida, Sharkia, 44813, Egypt
iDepartment of Chemistry, School of Life and Medical Sciences, University of Hertfordshire Hosted by Global Academic Foundation, R5 New Garden City, New Capital, Cairo 11835, Egypt. E-mail: sheriffahmy@aucegypt.edu; Tel: +20 1222613344
First published on 29th January 2024
Abstract
In the current study, we biosynthesized copper oxide NPs (CuO NPs) utilizing the essential oils extracted from Boswellia carterii oleogum resin, which served as a bioreductant and capping agent with the help of microwave energy. Afterwards, the platinum(II) based anticancer drug, carboplatin (Cr), was loaded onto the CuO NPs, exploiting the electrostatic interactions forming Cr@CuO NPs. The produced biogenic NPs were then characterized using zeta potential (ZP), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction spectroscopy (XRD), and Fourier transform infrared spectroscopy (FTIR) techniques. In addition, the entrapment efficiency and release profile of the loaded Cr were evaluated. Thereafter, SRB assay was performed, where Cr@CuO NPs demonstrated the highest cytotoxic activity against human colon cancer cells (HCT-116) with an IC50 of 5.17 μg mL−1, which was about 1.6 and 2.2 folds more than that of Cr and CuO NPs. Moreover, the greenly synthesized nanoparticles (Cr@CuO NPs) displayed a satisfactory selectivity index (SI = 6.82), which was far better than the free Cr treatment (SI = 2.23). Regarding the apoptosis assay, the advent of Cr@CuO NPs resulted in an immense increase in the cellular population percentage of HCT-116 cells undergoing both early (16.02%) and late apoptosis (35.66%), significantly surpassing free Cr and CuO NPs. A study of HCT-116 cell cycle kinetics revealed the powerful ability of Cr@CuO NPs to trap cells in the Sub-G1 and G2 phases and impede the G2/M transition. RT-qPCR was utilized for molecular investigations of the pro-apoptotic (Bax and p53) and antiapoptotic genes (Bcl-2). The novel Cr@CuO NPs treatment rose above single Cr or CuO NPs therapy in stimulating the p53-Bax mediated mitochondrial apoptosis. The cellular and molecular biology investigations presented substantial proof of the potentiated anticancer activity of Cr@CuO NPs and the extra benefits that could be obtained from their use.
Introduction
Colon cancer is the third most frequent cancer worldwide, causing more than 1 million morbidity cases during the last decade.1 Despite being one of the most diagnosed types of tumors, colon cancer is characterized by high mortality rates due to poor prognosis.2 The pathogenesis of colon cancer involves genetic mutations, environmental factors, wrong food habits with insufficient nutrients, and improper lifestyle habits, including smoking.3 Three main conventional treatment strategies are used to treat tumors and limit their progression; surgical resection (especially in early stages), chemotherapeutic agents, and radiotherapy. Both radiotherapy and chemotherapies lack selectivity to cancer cells in addition to possessing high systematic toxicity.4Carboplatin (Cr), a platinum-based drug analog widely used in cancer treatment, can cause tumor cell apoptosis via creating DNA damage. It is activated inside the cell, causing G2 phase cell cycle arrest by damaging cell DNA molecules via intra- and inter-strand cross-linkage.5 Cr has broad antitumor activity against head and neck cancer cell lines (SCC61, SQ20B and HEP2).6 It was also tested against ovarian cancer cell lines (A2780 and OVCAR-3) in vitro and in vivo and showed significant cancer-killing activities.7 Cr is also active in non-small cell lung cancer (NSCLC), with an overall response rate of just under 10%.8 It was also tested against Swiss albino mice with DMH-induced colon hyperplasia, where they reduced the histologic hyperplasia score, upregulated PI3K/AKT/JNK phosphorylation, and elevated caspase 3 levels inducing apoptosis.9 However, its clinical use is accompanied by several systemic side effects and cancer cells resistance. Thus, several studies have been reported on the nanoformulation of Cr in an attempt to improve its pharmacokinetics properties and pharmacological activity while minimizing its toxic effects.10
Finding a solution for the rapid CRC development and the devastating side effects of the commonly used therapeutic agents became an obligate need. One of the recent strategies used is developing nanoparticles (NPs) for many purposes, like drug delivery, imaging, and diagnosis.11 Various metallic nanoparticles have been fabricated and tested against colon cancer in vitro and in vivo. Among the widely used metallic NPs (MNPs) are gold, silver, copper, copper oxide, iron oxide and zinc oxide, which have shown promising effects against colon cancer.12
Metallic NPs, including copper oxide (CuO) NPs have gained significant attention in nanotechnology and nanomedicine due to their various applications, especially as anticancer agents. CuO NPs showed high cytotoxic effects against HT-29 and HCT-116 cell lines with IC50 of 50 and 25 μg mL−1, respectively.13,14 Nanocomposites formed by loading FDA-approved drugs to metallic NPs have recently been used for targeting therapies for CRC. For instance, 5-fluorouracil (5-FU) was efficiently delivered to CT-26 murine colon.15–17 Gold NPs were used for loading light-sensitive photosensitizer drugs, which showed a higher effect on the HT-29 colon cancer cell line.18 Another study reported the anticancer activity of chitin/copper nanocomposite (CNP/CuNP) against MCF-7 breast cancer cells.19 Indoleamine 2,3-dioxygenase-1 inhibitors (1-MT) loaded copper(I) phosphide nanocomposites (Cu3P/1-MT NPs) induced immunogenic tumor cell death effect by activating CD8+ T cells.20
Three general methods are used for the synthesis of CuO NPs; chemical synthesis (sol–gel, co-precipitation and sonochemical methods), physical synthesis (bar milling, microwave assisted and laser ablation) and green synthesis (plant, micro-organisms, fungi and algae-based methods).21,22 The biosynthesis of CuO (green synthesis) using plant extracts has gained attention due to its eco-friendly nature, safety, cost-effectiveness, and high cytotoxic effect on different cancer cells. For instance, the leaf extract of Pterolobium hexapetalum was used in the biosynthesis of CuO NPs, showing high cytotoxic activities against MDA-MB-231 breast cancer cells with a high safety margin (where its effect on the cancer cells was more than 1.5 fold more than that of normal ones).23 Also, another study reported the antiproliferative effect of CuO NPs, synthesized using spinach leaf extract, against MCF-7 cells with IC50 value of 21 ± 6 g mL−1.24 Biogenic synthesis of CuO NPs utilizing fresh leaves of Ormocarpum cochinchinense was shown to be effective against colon cancer (HCT-116) with reported IC50 of 40 μg mL−1.25
Boswellia carterri extracts were revealed to have anti-inflammatory, antioxidant, and antitumor activities.26 Boswellic acids have been shown to induce apoptosis in colon cancer cells (HT29 cells) via inhibition of PI3K/Akt pathway27 Suhail et al. reported that Boswellia essential oil induces apoptosis and suppresses tumor aggressiveness in breast cancer cells (ERpositive T47D cells, human breast cancer MCF7 cells, MDA-MB-231 E1A-transfected breast cancer cells).28 Boswellia essential oil also decreased β-catenin signaling molecules, suppressing colon cancer stem cell proliferation.29 In addition to its reported anticancer activity, some studies have shown that some BO components can act as reducing, capping, and stabilizing agents and inhibit the aggregation and agglomeration of the MNPs.30,31
In this study, we extracted Boswellia carterri essential oil (BCO), using an eco-friendly approach, which was then used as a reducing and capping agent, supporting the biosynthesis of CuO NPs via a microwave-assisted approach. Then, Cr was loaded on the greenly synthesized CuO NPs (Cr@CuO), and its Entrapment Efficiency percent (EE%) and release rate were investigated. The obtained NPs were analyzed using ZP, UV-vis, FTIR, X-ray diffraction, and TEM and tested against HCT-116 colon cancer cells. Its efficacy was tested via cell viability, apoptosis, and cell cycle analysis assays. Also, computational analysis was carried out to predict Cr and copper oxide interactions. Finally, molecular docking was applied for Cr, CuO, and our prepared CuO–Cr mixture on human alanine aminotransferase 2 to investigate their mechanism of anticancer activity.
Materials and methods
Materials
Copper acetate monohydrate and carboplatin were purchased from Sigma Aldrich, Germany. Dulbecco's Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Thermoscientific, Germany). Phosphate-buffered saline (PBS, pH 7.4) was obtained from Lonza Bioscience (Walkersville, USA). Penicillin–streptomycin mixture was attained from Lonza (Bioscience, Switzerland). Annexin V-FITC apoptosis detection kit was purchased from Abcam Inc. (Cambridge Science Park, Cambridge, UK). Propidium iodide was obtained from Calbiochem (Darmstadt, Germany). The remaining reagents were all of analytical grade.Boswellia carterii essential oil extraction
100 g of fresh Bosweilla carterii oleogum resins were hydro-distilled using Clevenger-apparatus for 90 min to extract the essential oil as described by Elyemni et al.32GC-MS analysis
Boswellia carterii essential oil extract was analyzed with gas chromatography (GC) (7890B), equipped with Agilent 19091S-433 UI, HP-5MS UI column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness), and mass spectrometer detector (MS) (5977B). Helium was chosen to be the carrier gas, flowing with a rate of 1.0 mL min−1 and at a split ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A volume of 1 μL of Boswellia carterii essential oil extract was injected, and the temperature program was set to 40 °C for 1 min then it raised to 150 °C with a rate of 4 °C min−1 and held for 6 min. Then, temperature was raised again to reach 210 °C with a rising rate of 4 °C min−1 and held for another 1 min. Finally, temperatures of 280 °C and 220 °C were set for the injector and detector, respectively. For MS, 70 eV was set for electron ionization (EI) with m/z ranging between 50 and 450 and 6 min for solvent delay. And finally, the sample was run for 50.5 min to identify the extracted oil constituents. Wiley and NIST Mass Spectral Library data was used to identify the sample's constituents via comparing the obtained spectrum fragmentation pattern.33,34
1. A volume of 1 μL of Boswellia carterii essential oil extract was injected, and the temperature program was set to 40 °C for 1 min then it raised to 150 °C with a rate of 4 °C min−1 and held for 6 min. Then, temperature was raised again to reach 210 °C with a rising rate of 4 °C min−1 and held for another 1 min. Finally, temperatures of 280 °C and 220 °C were set for the injector and detector, respectively. For MS, 70 eV was set for electron ionization (EI) with m/z ranging between 50 and 450 and 6 min for solvent delay. And finally, the sample was run for 50.5 min to identify the extracted oil constituents. Wiley and NIST Mass Spectral Library data was used to identify the sample's constituents via comparing the obtained spectrum fragmentation pattern.33,34
Microwave-assisted green synthesis of CuO nanoparticles
CuO NPs were greenly synthesized via a microwave-assisted approach using Boswellia carterii Essential Oil (BCO) and copper acetate monohydrate according to the previously reported with few modifications.35 The oil acts as a reducing and capping agent, supporting nanoparticle preparation. In brief, BCO and copper acetate monohydrate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were added to 20 mL of deionized water and stirred. After that, the mixture was transferred to a microwave and heated for a total duration of 5 min (10 cycles of 30 seconds each). The obtained metal hydroxide paste was then calcined at 600 °C for 5 hours to obtain the desired metal oxide nanoparticles that were used for further characterization.36
10) were added to 20 mL of deionized water and stirred. After that, the mixture was transferred to a microwave and heated for a total duration of 5 min (10 cycles of 30 seconds each). The obtained metal hydroxide paste was then calcined at 600 °C for 5 hours to obtain the desired metal oxide nanoparticles that were used for further characterization.36
Preparation of Cr/CuO NPs (Cr@CuO NPs)
A specific amount of the prepared CuO NPs was added to 15 mL of deionized water and stirred for 15 min. After that, an aqueous solution containing Cr was added dropwise to CuO NPs and kept on a magnetic stirrer at RT for 24 h. Then, the formed solution was sonicated for 15 min, centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 1 h), dialyzed against deionized water for 24 h, and lyophilized.
000 rpm, 1 h), dialyzed against deionized water for 24 h, and lyophilized.
Physicochemical characterization of the prepared nanoparticles
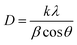 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 2 h, 4 °C); (Hermle Z 326 K, Labortechnik GmbH, Wehingen, Germany). Then, the supernatant was ultrafiltrated, and the unencapsulated Cr was quantified using HPLC, as described in the ESI.† The EE (%) of Cr@CuO NPs was determined using eqn (2).42
000 rpm, 2 h, 4 °C); (Hermle Z 326 K, Labortechnik GmbH, Wehingen, Germany). Then, the supernatant was ultrafiltrated, and the unencapsulated Cr was quantified using HPLC, as described in the ESI.† The EE (%) of Cr@CuO NPs was determined using eqn (2).42
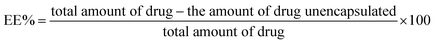 | (2) |
Biological assays
Computational methods
First, the interaction type between Cr and copper oxide was assessed using Discovery Studio 4.1. Cr structure was downloaded from PUBCHEM in a readable extension and copper oxide was built up using the drawing tools of Discovery Studio. Both Cr and copper oxide were subjected to minimization using CHARMM forcefield and Momany-Rone to render metals in a readable energy.Then, Cr was identified as a receiver molecule and copper oxide as a ligand. The favorable charge and molecule percentage were calculated via energy minimization simulation studies and analysis of ligand poses after the interaction.
Later, human alanine aminotransferase 2 was downloaded from PDB with code: 3IHJ. The enzyme was cleaned for missing residues and minimized, applying the same forcefield. Then, the protein was defined as a receptor, and the C-docker protocol was adopted for molecular docking studies between aminotransferase and complexed ligand, copper oxide, Cr alone, and the mixture of Cr and copper oxide. Furthermore, Ramachandran Plot was generated to verify predicted torsion angles in protein during interaction with the mixture.
Statistical analysis
Statistical analysis of the results was performed using SPSS 20.0, while the graphical representations were all plotted with GraphPad Prism 6.0. The data are displayed as the mean ± standard deviation (SD) throughout the study. Statistical analysis was considered at P-values less than 0.05. One-way analysis of variance (ANOVA) with multiple comparisons was used for normally distributed data.Results and discussion
BCO GC-MS chemical composition analysis
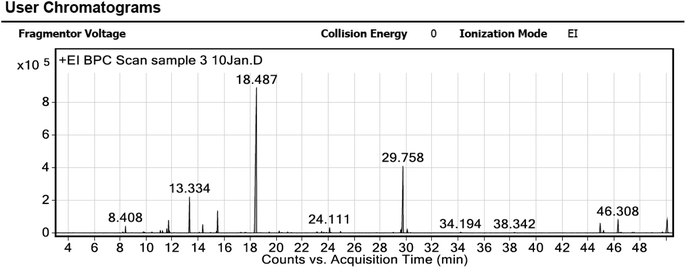
Several peaks for essential oil extract were detected via GC-MS chromatogram analysis, as shown in Fig. 1. The obtained MS was compared to the NIST library to identify essential oil constituents, as listed in Table 1. The most abundant component was trans-nerolidol (16.41%), and the second was verticiol (3.04%) followed by DL-limonene and linalool (1.98% and 1.31%, respectively) as major components; the mass spectra of these components were illustrated in Fig. S1a–d,† respectively.| Peak | RT | Compound | Area sum (%) |
|---|---|---|---|
| 1 | 8.208 | α-Fellandrene | 0.12 |
| 2 | 8.408 | α-PINENE | 0.96 |
| 3 | 9.777 | Sabinene | 0.17 |
| 4 | 9.861 | 2-β-PINENE | 0.14 |
| 5 | 10.433 | β-Myrcene | 0.13 |
| 6 | 11.583 | p-Cymene | 0.66 |
| 7 | 11.723 | DL-Limonene | 1.98 |
| 8 | 11.797 | Eucalyptol | 0.24 |
| 9 | 13.334 | Formic acid, octyl ester(octyl formate) | 6.35 |
| 10 | 14.348 | Linalool | 1.31 |
| 11 | 17.617 | α-Terpineol | 0.1 |
| 12 | 18.487 | Acetic acid, octyl ester(octyl acetate) | 51.83 |
| 13 | 19.465 | CARVONE | 0.14 |
| 14 | 23.496 | Neryl acetate | 0.26 |
| 15 | 23.682 | n-Decanoic acid | 0.13 |
| 16 | 23.859 | α-Copaene | 0.09 |
| 17 | 27.756 | 9-Decen-1-ol | 0.09 |
| 18 | 29.599 | Lilac alcohol | 0.63 |
| 19 | 29.758 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-(trans-nerolidol) | 16.41 |
| 20 | 45.195 | Cembrene | 0.52 |
| 21 | 46.308 | Verticiol | 3.04 |
| 22 | 47.411 | Thunbergol | 0.12 |
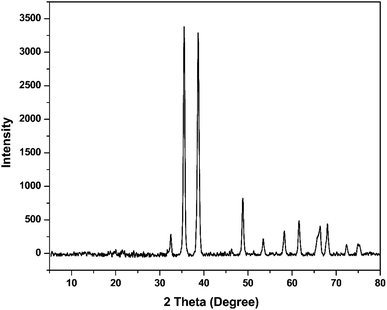
Physicochemical characterization of the prepared nanoparticles
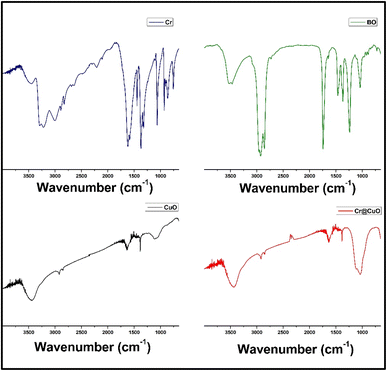
Mimicking the peaks in the CuO NPs' chart, Cr@CuO FTIR chart exhibits the same peak at 532 cm−1 attributed to Cu–O bond. The peaks indicating the remaining BO are found at 3438, 2919, 1621, 1382, and 1112 cm−1. Similarly, these peaks are slightly shifted and with reduced intensity. In addition, a strong peak was detected at 1048 cm−1, which can be attributed to the loaded Cr carbonyl group.
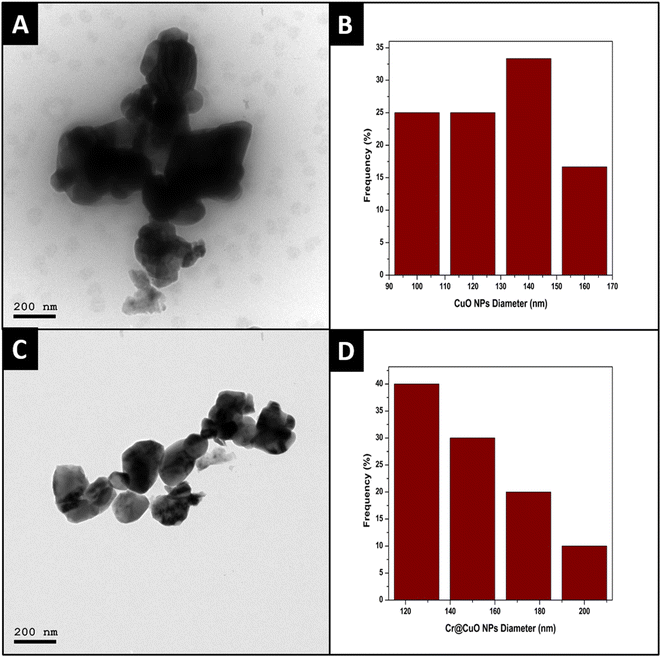 | ||
| Fig. 4 TEM micrographs of CuO NPs (A) and Cr@CuO NPs (C) and their particle diameter distribution histograms (B and D, respectively). | ||
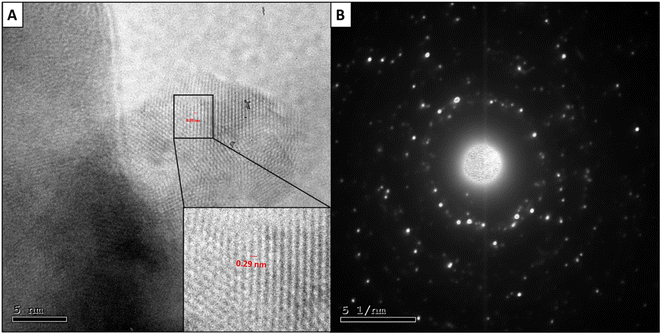
In addition, the interplanar distance between CuO NPs' lattice fringes was found to be 0.29 nm, which is in harmony with previously reported studies,57 confirming the CuO NPs' formation. The magnified lattice fringes of CuO NPs are displayed in Fig. 5A. On the other hand, as shown in Fig. 5B, the selected area electron diffraction (SAED) pattern of CuO NPs presented diffraction patterns involving sharp spots; hence, the structures of these NPs must be ordered.38
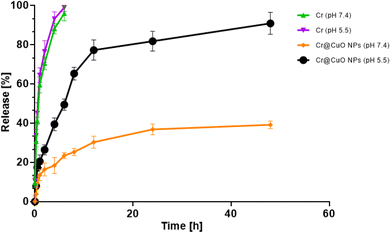
To determine the appropriateness of CuO NPs as chemotherapeutic carriers, in vitro release study of Cr from Cr@CuO NPs, utilizing two dissimilar pH media mimicking the tumor microenvironment (pH 5.4) and the physiological conditions (pH 7.4), was carried out and compared to the free Cr. The release of Cr from Cr@CuO NPs was pH-dependent (Fig. 6). At pH 5.5, a triphasic release behavior was observed for the release of Cr from the Cr@CuO NPs over the 48 h-duration of the release test. Different release mechanisms controlled each of the three phases: dissolution, diffusion, and erosion.59 In the first 2 h, rapid release of Cr was detected (26.42%), which is linked to the Cr molecules present on the CuO NPs' surface. The partition of Cr to be dissolved in water was encouraged by the solubility of Cr in water.60 The small particle size supports the large surface area of the CuO NPs. At this stage, the Cr is only challenged by the dissolution mechanism to be soluble and released in the medium. The next phase extended until 12 h of the release test, where 77.30% was released in the medium. At the second stage, the entrapped Cr in the outer layer of the CuO NPs matrix dissolves and diffuses to reach the release medium. Hence, the second phase is limited by both the drug's dissolution and diffusion to be released from the nanoparticle matrix. The last phase presents the slowest Cr release rate, reaching 90.92% of a cumulative released drug after 48 h since the beginning of the in vitro release assay. This phase is the slowest since it is controlled by an additional mechanism (erosion) besides the previously mentioned mechanisms (dissolution and diffusion). The erosion is added to the release challenges since the drug in the innermost core of the nanoparticle will have to travel a long distance in the CuO NP matrix before reaching the interphase between the medium and the matrix. Cr@CuO NPs showed a sustained release profile in comparison to the free Cr release profile in which 98.95 ± 2.00% of the Cr was released in the first 6 h of the release test. The sustained release of Cr for a prolonged period was tuned by the particle size of CuO NPs.61 In contrast, at physiological conditions (pH 7.4) the release rates of Cr from Cr@CuO NPs at pH 7.4 were much slower as compared to those observed at (pH 5.5). The release rates were 16.5%, 30.3% and 39% at 2 h, 12 h and 48 h, respectively. The higher percentage of drug release in the acidic medium might be attributed to the release of copper ions (Cu2+) in the acidic medium62 and the protonation of Cr, subsequently strengthening the repulsive forces leading to faster release of rates of Cr, as compared to that at physiological pH.63 A triggered pH-dependent behavior is beneficial in cancer therapy. At pH 7.4 (mimicking physiological conditions), it is anticipated that most of the loaded drug will remain adsorbed to the surface of metallic NPs, with no high release and thus providing stability when found in the plasma, with minimum toxic effects on the normal tissues. Thereafter, the drug will rapidly be released when the NPs reach the cancer cells (pH 4.5–6) tumor cells, which will definitely augment the efficacy of the preferential cancer therapy.64
Biological assays
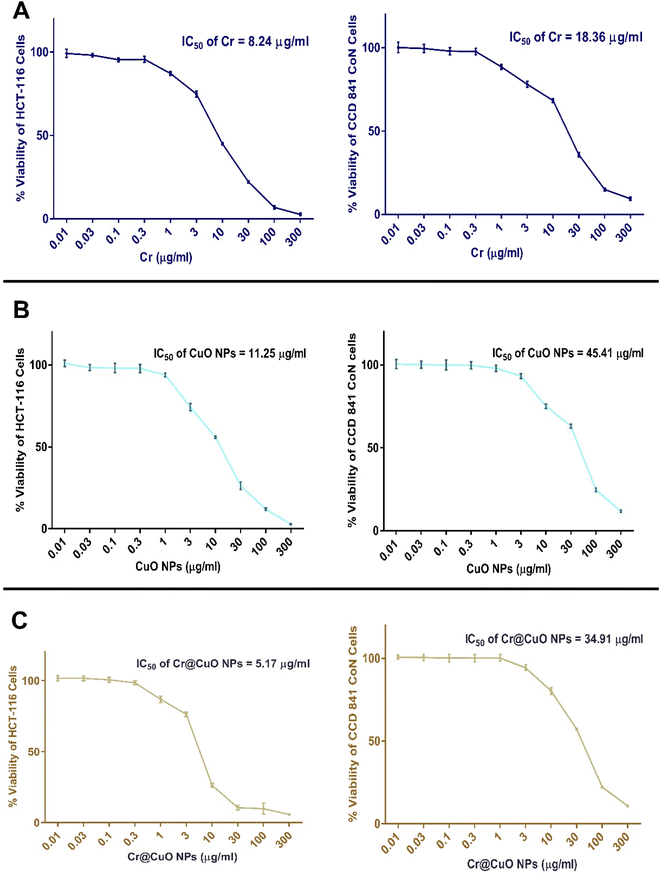
| Test agent | IC50 on HCT-116 cells (μg mL−1) | CC50 on CCD 841 CoN cells (μg mL−1) | SI (CC50/IC50) |
|---|---|---|---|
| a Data presented as the mean IC50 of three trials ± standard deviation (SD). | |||
| Carboplatin (Cr) | 8.24 ± 0.74 | 18.36 ± 1.12 | 2.23 |
| Copper oxide NPs (CuO NPs) | 11.25 ± 1.04 | 45.41 ± 2.16 | 4.04 |
| Copper oxide NPs loaded carboplatin (Cr@CuO NPs) | 5.17 ± 0.48 | 34.91 ± 1.08 | 6.82 |
The IC50 of Cr and CuO NPs were estimated to be 8.24 μg mL−1 and 11.25 μg mL−1 on HCT-116 cancer cells as shown in Table 2 and Fig. 7A, B. Meanwhile, Cr@CuO NPs exhibited the highest antiproliferative activity against HCT-116 cells with an IC50 of 5.17 μg mL−1 which was 1.6 and 2.2 folds higher than that of Cr and CuO NPs treated groups, Table 2 and Fig. 7C. Therefore, loading Cr onto the greenly synthesized CuO NPs proved to be a successful approach for improving the efficacy of Cr where CuO NPs exerted a synergistic effect and potentiated the actions of Cr. Our drug (Cr) has usually been limited by its poor cellular uptake and the demand for high therapeutic doses. In vitro studies showed that the incorporation of Cr in nanoparticles could be a valuable approach to enhance its delivery and reduce the required dose.68 In the modern era, copper nanoparticles have become a rising bio-nanotechnology approach in cancer therapy. Such NPs are believed to function as DNA-cleaving and powerful anticancer agents, which could be attributed to their capacity for binding, ability to modify their surface properties through conjugation with other chemotherapeutics, effectiveness as a nano-drug delivery system with much affordable costs than that of Ag or Au, and their ability to act as molecular doping operators that could manipulate the cell cycle.69
The SI values for pure Cr and CuO NPs were 2.23 and 4.04, respectively. Interestingly, Cr@CuO NPs attained the highest SI (SI = 6.82) towards cancer cells and achieved the uttermost safety profile among all other treatments, Table 2. The loading of Cr onto Cr@CuO NPs improved its selectivity by 3-times compared to the pure Cr treatment. A Similar approach was performed previously where Cr together with doxorubicin (Dox) were dual loaded onto ZnO nanoparticles and proved highly efficient at increasing the sensitivity and selectivity of chemo drugs to human breast adenocarcinoma cells.70
All the above reinforces the idea that our newly synthesized Cr@CuO NPs possess much better therapeutic potential than free Cr or CuO Nps in the treatment of colon cancer.
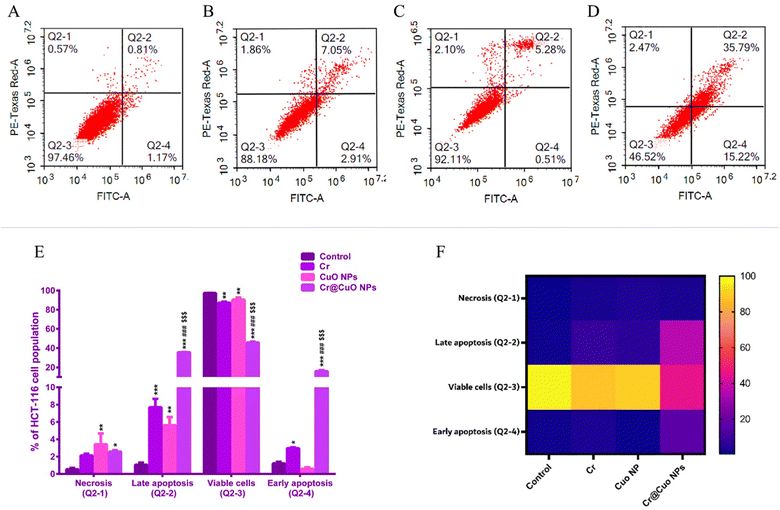
| Apoptotic stage of HCT-116 | Control | Cr (8.24 μg mL−1) | Cuo NPs (11.25 μg mL−1) | Cr@Cuo NPs (5.17 μg mL−1) |
|---|---|---|---|---|
| a Data presented as mean ± SD. (*), (#) and ($) represent significant differences compared to the Control, Cr and Cuo NPs treated groups, respectively. Statistical analysis was performed using one one-way ANOVA test with Tukey post hoc test. | ||||
| Necrosis (Q2-1) | 0.52 ± 0.17 | 2.11 ± 0.22 | 3.41** ± 1.26 | 2.56* ± 0.17 |
| Late apoptosis (Q2-2) | 1.05 ± 0.24 | 7.68*** ± 0.99 | 5.61** ± 0.96 | 35.66***###$$$ ± 0.23 |
| Viable cells (Q2-3) | 97.22 ± 0.24 | 87.24** ± 1.09 | 90.41** ± 2.20 | 45.76***###$$$ ± 0.92 |
| Early apoptosis (Q2-4) | 1.21 ± 0.18 | 2.97* ± 0.09 | 0.58 ± 0.18 | 16.02***###$$$ ± 1.01 |
The apoptotic effects of Cr were previously noted on a few cancer cell lines, including non-small cell lung cancer (A549),71 ovarian (OVCA429),72 and cervical (SiHa)73 cancers. The literature search also revealed considerable apoptotic effects for CuO NPs on breast (MCF-7),74 colorectal (HCT-116),13 and cervical (HeLa)75 cancer cells. Therefore, our apoptosis assay findings were in line with the other studies where both Cr and CuO NPs exerted significant apoptotic effects, while Cr@CuO NPs exceeded their apoptotic activity. Our results also showed that Cr@CuO NPs caused a significant reduction in the viable cells by 41.5% and 44.65% when set against Cr and CuO NPs (P ≤ 0.001). Cr@CuO NPs also caused a 5-fold escalation in the necrotic cell population percentage relative to the control (P ≤ 0.05). As such, our findings confirmed the cytotoxicity assay results and reinforced the ideology of incorporating Cr onto the greenly synthesized CuO NPs.
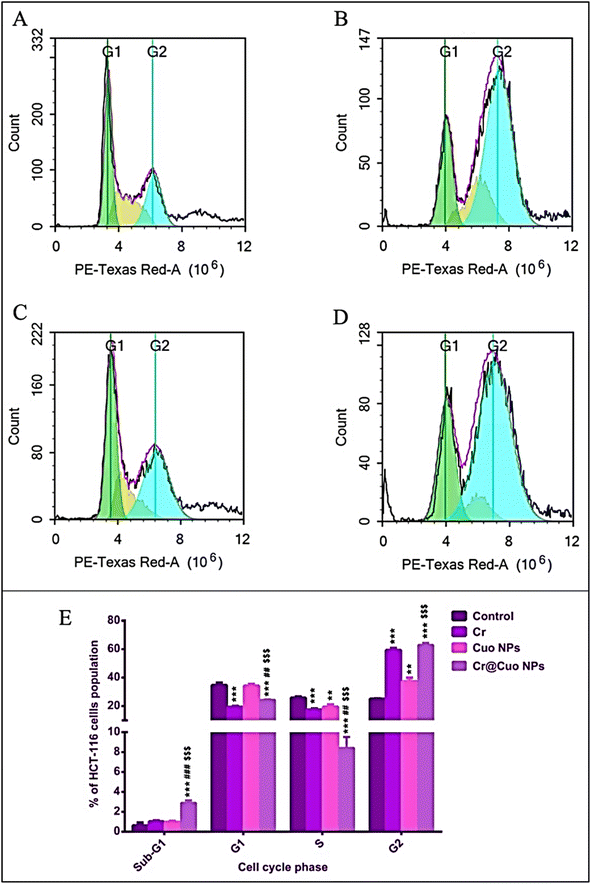
| HCT-116 phase | Control (untreated) | Cr (8.24 μg mL−1) | CuO NPs (11.25 μg mL−1) | Cr@CuO NPs (5.17 μg mL−1) |
|---|---|---|---|---|
| a Data presented as mean ± SD. (*), (#) and ($) represent significant differences compared to the Control, Cr and Cuo NPs treated groups, respectively. Statistical analysis was performed using one one-way ANOVA test with Tukey post hoc test. | ||||
| Sub-G1 | 0.61 ± 0.29 | 1.01 ± 0.10 | 0.96 ± 0.10 | ***###$$$2.88 ± 0.26 |
| G1 | 34.70 ± 1.69 | ***19.24 ± 0.73 | 34.26 ± 1.17 | ***##$$$24.26 ± 0.40 |
| S | 25.74 ± 0.85 | ***17.50 ± 0.79 | **19.49 ± 1.52 | ***###$$$8.42 ± 1.10 |
| G2 | 25.02 ± 0.16 | ***59.27 ± 1.42 | **37.27 ± 2.61 | ***$$$62.95 ± 1.44 |
In the synthetic phase, also known as the “S phase”, the cell produces an exact copy of its genetic material, i.e., the cell duplicates its DNA content.77 Treatment with Cr, CuO, and Cr@CuO NPs significantly reduced cell population at the S phase compared to the control group (untreated). So, all treatment groups displayed a noticeable activity in decreasing DNA synthesis. It is worth emphasizing that Cr@CuO NPs also surpassed free Cr and CuO NPs at reducing the frequency of cells undergoing DNA duplication (P ≤ 0.001).
G2 is the pause stage following S phase during which the cell gets ready to enter mitosis (M). CDK1 usually regulates the transition from G2 to M. However, when the brakes go wrong, uncontrolled cell division occurs and cancer is induced.78 In the current study, all treatment groups significantly arrested the cells at the G2 phase and stopped the G2/M transition relative to the control group. Cr@CuO NPs trapped even more cells at G2 phase than pure CuO NPs. Meanwhile, both Cr@CuO NPs and free Cr arrested a comparable or almost very similar cell population percentage at the G2 phase. Likewise, analysis of colon adenocarcinoma (CT-26) cells revealed a significant increase in the number of G2 phase cells following treatment with free Cr relative to the control (blank) cells.79 Moreover, CuO NPs ability to arrest the cell cycle at G2 phase and prevent G2-M transition were previously reported in human lung cancer (A-549)80 and skin cancer (A-375)81 cells.
In a nutshell, the three treatments successfully decreased the percent cellular population undergoing DNA duplication in the S-phase and stopped the G2/M transition by trapping the cells at G2. Cr@CuO NPs possessed an additional effect by inducing cellular arrest at the Sub-G1 phase and towering up the effects of the other two treatments at both S and G2 phases whereby providing added evidence to the apoptosis assay results.
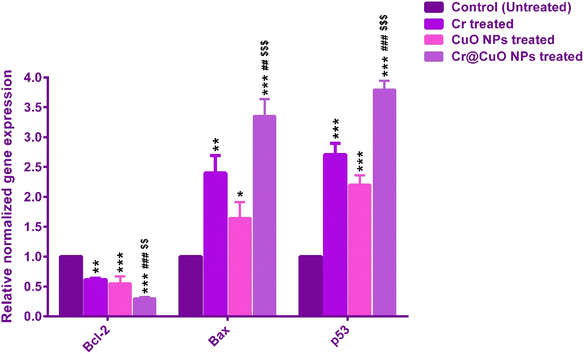
In several cancers, including colon cancer, the repression of Bax gene has been linked to chemotherapy resistance and a bad prognosis.84 The Cr treatment has previously demonstrated a significant ability to elevate the expression of Bax in several tumors, such as the human lymphoma Raji cell line85 and breast cancer (MCF-7) cells.86 CuO NPs were also noticed to induce Bax gene expression in human hepatocarcinoma HepG2,87 and chronic myeloid leukemia K562,88 cell lines. Herein, our study revealed a 2.4-fold and 1.6-fold elevation in the relative normalized gene expression of Bax in HCT-116 cells treated with Cr and CuO NPs, respectively. The synthesized Cr@CuO NPs caused a 3.5-fold increase in Bax expression. As reported in Fig. 10, the expression of Bax in Cr@CuO NPs treated cells significantly surpassed that of Cr (P ≤ 0.01) and CuO NPs (P ≤ 0.001) treated cells. This could be considered an additional proof of the apoptotic effects of Cr@CuO NPs at the molecular level, which is strongly supported by the noticeably increased levels of Bax.
Bcl-2 is a protein that inhibits apoptosis. The increase in the Bcl-2 gene product has been widely associated with resistance to chemotherapeutic agents in multiple colon cancer cell lines.89 As opposed to the Bax gene, Cr treatment for 48 h resulted in a considerable reduction in the expression of Bcl-2 in the human lymphoma Raji cell line85 and breast cancer (MCF-7) cells.86
Additionally, the Bcl-2 expression witnessed a remarkable downregulation in human hepatocarcinoma HepG2,87 and chronic myeloid leukemia K562 cell lines88 following CuO NPs treatment. Likewise, our findings showed that Cr and CuO NPs treatments significantly reduced the normalized gene expression of the anti-apoptotic marker, BCl-2, by 39% and 45%, respectively (Fig. 10). The newly formulated Cr@CuO NPs treatment resulted in a more significant decline in Bcl-2 gene expression relative to Cr (P ≤ 0.001) and CuO NPs (P ≤ 0.01) (Fig. 10).
Since both the gain- and loss-of-its-function mutations have been observed in various tumors, the tumor suppressor gene, p53 has revealed a complex participation in the process of carcinogenesis.90 The p53 gene is known to directly enhance the Bax gene transcription and hence, the production of Bax protein.91 Cumulative studies have pointed to the ability of both Cr86 and CuO NPs87,88 treatments to produce a significant increase in the p53 gene expression, promoting that of the Bax gene in a variety of tumors. In the current study, treatment with both Cr and CuO NPs resulted in a noticeable increase in the relative normalized gene expression of p53 by 2.7 and 2.2 folds (Fig. 10). It is noteworthy that Cr@CuO NPs treatment resulted in a marked increase of p53 expression by 3.8 folds, which exceeded that of the other two treatments (Fig. 10).
The novel Cr@CuO NPs treatment displayed an unprecedented and surplus activity at increasing the p53-Bax mediated mitochondrial apoptosis and downregulating the expression of Bcl-2 compared to the single Cr or CuO NPs therapy. As such, Cr@CuO NPs are depicted to possess favorable therapeutic properties that are worth further preclinical and clinical trials in colon cancer.
Computational findings
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
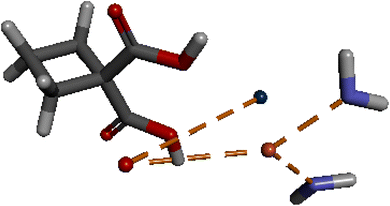
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the unfavorable count of ligands will be zero. The interaction between Cr and CuO (Fig. 12) showed metal–ligand interaction between Pt and oxygen atom and two electrostatic interactions between Cu and each of the NH3 groups of the Cr. The VDW interaction energy of the mixture obtained was −0.0019 kcal mol−1, while the interaction energy of CuO is −18.6092 kcal mol−1 and that of cris −18.4248 kcal mol−1. On the other hand, calculated in situ starting energy was −67,404.2 kcal mol−1, in situ final energy after the interaction was −45.2372 kcal mol−1, in situ gradient was 0.000696528 and free energy was −431.833 kcal mol−1.
1 and the unfavorable count of ligands will be zero. The interaction between Cr and CuO (Fig. 12) showed metal–ligand interaction between Pt and oxygen atom and two electrostatic interactions between Cu and each of the NH3 groups of the Cr. The VDW interaction energy of the mixture obtained was −0.0019 kcal mol−1, while the interaction energy of CuO is −18.6092 kcal mol−1 and that of cris −18.4248 kcal mol−1. On the other hand, calculated in situ starting energy was −67,404.2 kcal mol−1, in situ final energy after the interaction was −45.2372 kcal mol−1, in situ gradient was 0.000696528 and free energy was −431.833 kcal mol−1.
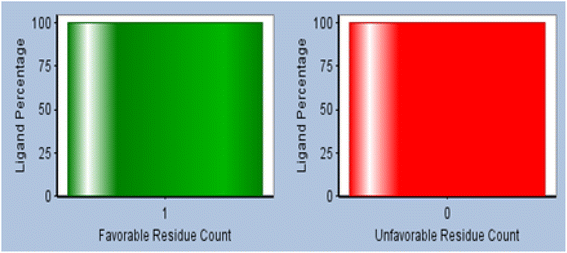 | ||
| Fig. 11 Statistical residue analysis plot of the favorable (green) and unfavorable (orange) ratio of ligand (CuO) interaction with the receiver molecule (Cr). | ||
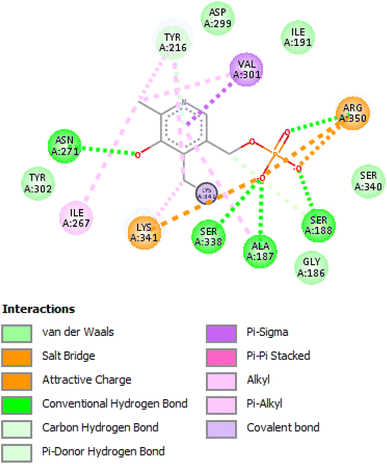
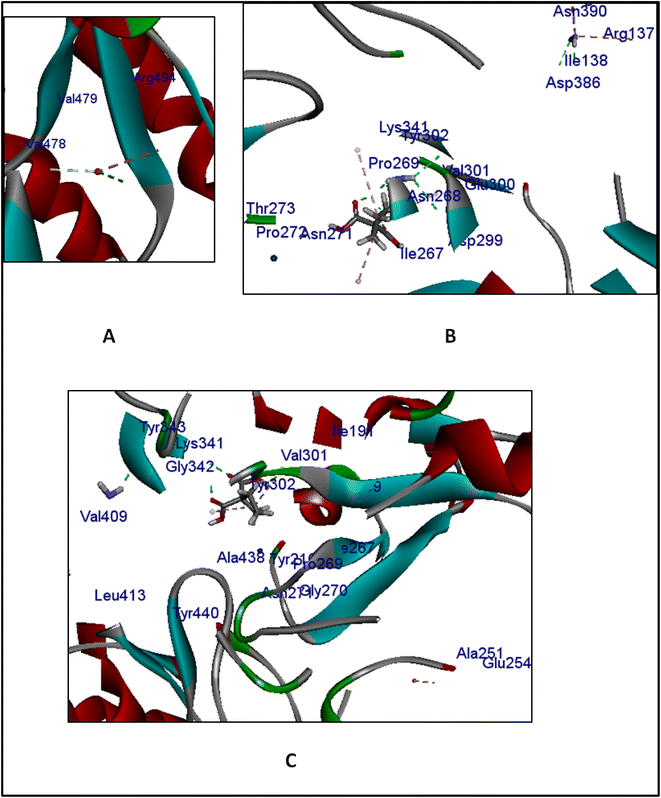
The results revealed that the reference compound PLP had − (C-docker interaction energy) = −22.5467 kcal mol−1, and as shown in Fig. 13 PLP interacted with aminotransferase via attractive charge with Lys 341 and Pi-interactions with Val 301 and Tyr 216. Meanwhile, the interaction energies of CuO, Cr, and mixture were −32.2555, −43.2792, and −68.8381 kcal mol−1, respectively. The 3D interaction diagrams between each ligand and enzyme in Fig. 14 illustrate the interaction of CuO via H-bond with Arg 494, and Val 478 and, another Pi-interaction with Arg 494, and a metal–ligand interaction between Cu and Asp177. This reflected a remote interaction than the binding site of CuO alone. At the same time, Cr showed two H-bonds with Lys 341, two H-bonds with Asp 386, H-bond with Pro 269, Glu 300, and Arg 134, together with Pi-interaction with Arg 134, Arg 137, Val 301, and Tyr 216 and finally Pt-ligand interaction with Pro 214, Asn 271 and Thr 273. That resulted in better interaction within the targeted binding site of the enzyme and better interaction energy. Furthermore, the mixture presented interactions as two H-bonds with Lys 341 and one H-bond with Gly 342, Pi-interaction with Val 301, Tyr 216, and Tyr 302, and metal–ligand interactions in the form of Cu and Gly 254 and Pt and Pro 269 and Gly 270. These results ensured that the binding mode of the mixture was the best among all ligands and had better stability from interaction energy. That correlated much with the in vitro cell line assay against colorectal cell lines that showed the most potent anticancer activity in the mixture form with CuO than individual CuO or Cr.
Conclusions
In this work, we designed spherical NPs utilizing a facile and rapid microwave-assisted approach using Boswellia carterii essential oil extract. GC/MS analysis of the chemical composition of the extracted essential oil showed the abundancy of trans-nerolidol, verticiol, DL-limonene and linalool, which might be involved in the biosynthesis of CuO NPs by acting as reducing and capping agents. The loading of Cr onto the CuO NPs did not affect the spherical morphology of the CuO NPs, and had a high EE% and a triphasic release behavior into acidic pH, which simulated that of the tumor microenvironment. Several cellular and molecular biology assays were performed to assess the antitumor activity of Cr@CuO NPs, as well as its underlying mechanism of action. The results sounded very promising whereby Cr@CuO NPs imposed a superb cytotoxic activity against HCT-116 colon cancer cells as compared to free Cr or CuO NPs treatments. Moreover, Cr@CuO NPs succeeded at conserving the normal CCD841 CoN cells and attaining the highest selectivity index among all the investigated agents, rendering it of high therapeutic potential with minimal toxicity. The newly synthesized Cr@CuO NPs were superior to free Cr or CuO NPs by far in inducing apoptosis and cell cycle arrest at both sub-G1 and G2-phases of the cell cycle. Molecular biology investigations reflected the exquisite ability of Cr@CuO NPs to stimulate the gene expression of the pro-apoptotic markers p53 and Bax while downregulating Bcl-2. The insightful results suggest multiple benefits for using Cr@CuO NPs in the treatment of colon cancer.Data availability
Data is contained within the article.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was funded by the RSC Research Fund grant (ID: R22-3870733873) from the Royal Society of Chemistry to Dr Sherif Ashraf Fahmy. The authors are thankful to the Deanship of Scientific Research at King Khalid University in Abha, Saudi Arabia, for partial funding of parts of this work through large groups (project under grant number R.G.P. 1/430/44).References
- M. Ahmed, Colon Cancer: A Clinician's Perspective in 2019, Gastroenterol. Res., 2020, 13, 1–10, DOI:10.14740/gr1239.
- X. Li, D. Wen, X. Li, C. Yao, W. Chong and H. Chen, Identification of an Immune Signature Predicting Prognosis Risk and Lymphocyte Infiltration in Colon Cancer, Front. Immunol., 2020, 11, 1–13, DOI:10.3389/fimmu.2020.01678.
- A. Esmeeta, S. Adhikary, V. Dharshnaa, P. Swarnamughi, Z. Ummul Maqsummiya, A. Banerjee, S. Pathak and A. K. Duttaroy, Plant-derived bioactive compounds in colon cancer treatment: an updated review, Biomed. Pharmacother., 2022, 153, 113384, DOI:10.1016/j.biopha.2022.113384.
- T. Hu, Z. Li, C. Y. Gao and C. H. Cho, Mechanisms of drug resistance in colon cancer and its therapeutic strategies, World J. Gastroenterol., 2016, 22, 6876–6889, DOI:10.3748/wjg.v22.i30.6876.
- G. F. de Sousa, S. R. Wlodarczyk and G. Monteiro, Carboplatin: molecular mechanisms of action associated with chemoresistance, Braz. J. Pharm. Sci., 2014, 50, 693–702, DOI:10.1590/S1984-82502014000400004.
- N. Aissat, C. Le Tourneau, A. Ghoul, M. Serova, I. Bieche, F. Lokiec, E. Raymond and S. Faivre, Antiproliferative effects of rapamycin as a single agent and in combination with carboplatin and paclitaxel in head and neck cancer cell lines, Cancer Chemother. Pharmacol., 2008, 62, 305–313, DOI:10.1007/s00280-007-0609-2.
- T. Chen, M. Li, R. Zhang and H. Wang, Dihydroartemisinin induces apoptosis and sensitizes human ovarian cancer cells to carboplatin therapy, J. Cell. Mol. Med., 2009, 13, 1358–1370, DOI:10.1111/j.1582-4934.2008.00360.x.
- P. A. Bunn Jr, Review of therapeutic trials of carboplatin in lung cancer, Semin. Oncol., 1989, 16, 27–33 Search PubMed.
- H. I. Bahr, A. T. Ibrahiem, A. M. Gabr, A. M. Elbahaie, H. S. Elmahdi, N. Soliman, A. M. Youssef, M. El-Sherbiny and S. A. Zaitone, Chemopreventive effect of α-hederin/carboplatin combination against experimental colon hyperplasia and impact on JNK signaling, Toxicol. Mech. Methods, 2021, 31, 138–149, DOI:10.1080/15376516.2020.1849483.
- S. Danışman-Kalındemirtaş, F. Kariper, İ. A. Erdemir, G. Sert and E. Erdem-Kuruca, Evaluation of anticancer effects of carboplatin–gelatin nanoparticles in different sizes synthesized with newly self-assembly method by exposure to IR light, Sci. Rep., 2022, 12(1), 10686 CrossRef PubMed.
- F. A. Khan, R. Albalawi and F. H. Pottoo, Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment, Med. Res. Rev., 2022, 42, 227–258, DOI:10.1002/med.21809.
- N. A. Bhaskaran and L. Kumar, Treating colon cancers with a non-conventional yet strategic approach: an overview of various nanoparticulate systems, J. Controlled Release, 2021, 336, 16–39, DOI:10.1016/j.jconrel.2021.06.008.
- S. Tabrez, A. U. Khan, A. A. Mirza, M. Suhail, N. R. Jabir, T. A. Zughaibi and M. Alam, Biosynthesis of copper oxide nanoparticles and its therapeutic efficacy against colon cancer, Nanotechnol. Rev., 2022, 11, 1322–1331, DOI:10.1515/ntrev-2022-0081.
- A. N. Harbi and K. H. Abd, Biosynthesis of CuO NPs and its anticancer activity on human colon cancer cell lines (HT-29), J. Phys.: Conf. Ser., 2021, 1963, 012151, DOI:10.1088/1742-6596/1963/1/012151.
- G. Go, C. S. Lee, Y. M. Yoon, J. H. Lim, T. H. Kim and S. H. Lee, Prpc aptamer conjugated–gold nanoparticles for targeted delivery of doxorubicin to colorectal cancer cells, Int. J. Mol. Sci., 2021, 22, 1–16, DOI:10.3390/ijms22041976.
- Y. Wang, P. Li, L. Chen, W. Gao, F. Zeng and L. X. Kong, Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles, Drug Delivery, 2015, 22, 191–198, DOI:10.3109/10717544.2013.875603.
- P. Gogoi, G. Kaur and N. K. Singh, Nanotechnology for colorectal cancer detection and treatment, World J. Gastroenterol., 2022, 28, 6497–6511, DOI:10.3748/wjg.v28.i46.6497.
- B. Brar, K. Ranjan, A. Palria, R. Kumar, M. Ghosh, S. Sihag and P. Minakshi, Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy, Front. Nanotechnol., 2021, 3, 1–21, DOI:10.3389/fnano.2021.699266.
- D. Solairaj, P. Rameshthangam and G. Arunachalam, Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells, Int. J. Biol. Macromol., 2017, 105, 608–619, DOI:10.1016/j.ijbiomac.2017.07.078.
- J. He, R. Song, F. Xiao, M. Wang and L. Wen, Cu3 P/1-MT Nanocomposites Potentiated Photothermal-Immunotherapy, Int. J. Nanomed., 2023, 18, 3021–3033, DOI:10.2147/IJN.S414117.
- R. S. Lanje, A. S. Sharma, S. J. Pode and R. B. Ningthoujam, Synthesis and optical characterization of copper oxide nanoparticles, Adv. Appl. Sci. Res., 2010, 1, 36–40 Search PubMed.
- S. Naz, A. Gul, M. Zia and R. Javed, Synthesis, biomedical applications, and toxicity of CuO nanoparticles, Appl. Microbiol. Biotechnol., 2023, 107, 1039–1061, DOI:10.1007/s00253-023-12364-z.
- E. Nagaraj, K. Karuppannan, P. Shanmugam and S. Venugopal, Exploration of Bio-synthesized Copper Oxide Nanoparticles Using Pterolobium hexapetalum Leaf Extract by Photocatalytic Activity and Biological Evaluations, J. Cluster Sci., 2019, 30, 1157–1168, DOI:10.1007/s10876-019-01579-8.
- N. Al-Jawhari, H. Bin-Thiyab and H. Elbialy, In vitro antioxidant and anticancer activities of cupric oxide nanoparticles synthesized using spinach leaves extract, Nano-Struct. Nano-Objects, 2022, 29, 100815 CrossRef.
- V. Gnanavel, V. Palanichamy and S. M. Roopan, Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116), J. Photochem. Photobiol., B, 2017, 171, 133–138, DOI:10.1016/j.jphotobiol.2017.05.001.
- K. Huang, Y. Chen, K. Liang, X. Xu, J. Jiang, M. Liu and F. Zhou, Review of the Chemical Composition, Pharmacological Effects, Pharmacokinetics, and Quality Control of Boswellia carterii, Evid.-Based Complementary Altern. Med., 2022, 2022, 6627104, DOI:10.1155/2022/6627104.
- J. J. Liu and R. D. Duan, LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells, Anticancer Res., 2009, 29, 2987–2991 Search PubMed.
- M. M. Suhail, W. Wu, A. Cao, F. G. Mondalek, K. M. Fung, P. T. Shih, Y. T. Fang, C. Woolley, G. Young and H. K. Lin, Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells, BMC Complementary Altern. Med., 2011, 11, 129, DOI:10.1186/1472-6882-11-129.
- E. Becer, H. Kabadayı, K. H. C. Başer and H. S. Vatansever, Boswellia sacra essential oil manages colon cancer stem cells proliferation and apoptosis: a new perspective for cure, J. Essent. Oil Res., 2021, 33, 53–62, DOI:10.1080/10412905.2020.1839586.
- N. O. Makarov, V. V. Love, A. J. Sinitsyna, O. V. Makarova, S. S. Yaminsky, I. V. Taliansky and M. E. Kalinina, “Green” nanotechnologies: synthesis of metal nanoparticles using plants, Acta Nature, 2014, 6, 35–44, DOI:10.4135/9781452231631.n3.
- H. M. E. S. Azzazy, A. Abdelnaser, H. Al Mulla, A. M. Sawy, S. N. Shamma, M. Elhusseiny, S. Alwahibi, N. K. Mahdy and S. A. Fahmy, Essential Oils Extracted from Boswellia sacra Oleo Gum Resin Loaded into PLGA-PCL Nanoparticles: Enhanced Cytotoxic and Apoptotic Effects against Breast Cancer Cells, ACS Omega, 2022, 2–10, DOI:10.1021/acsomega.2c06390.
- M. Elyemni, B. Louaste, I. Nechad, T. Elkamli, A. Bouia, M. Taleb, M. Chaouch and N. Eloutassi, Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation, Sci. World J., 2019, 2019, 3659432, DOI:10.1155/2019/3659432.
- S. E. Stein, P. Ausloos and S. G. Lias, Comparative Evaluations of Mass Spectral Databases, J. Am. Soc. Mass Spectrom., 1991, 2, 441–443, DOI:10.1016/1044-0305(91)85012-U.
- R. Oprean, L. Oprean, M. Tamas, R. Sandulescu and L. Roman, Essential oils analysis. II. Mass spectra identification of terpene and phenylpropane derivatives, J. Pharm. Biomed. Anal., 2001, 24, 1163–1168, DOI:10.1016/S0731-7085(00)00578-1.
- C. Mallikarjunaswamy, V. Lakshmi Ranganatha, R. Ramu, Udayabhanu and G. Nagaraju, Facile microwave-assisted green synthesis of ZnO nanoparticles: application to photodegradation, antibacterial and antioxidant, J. Mater. Sci.: Mater. Electron., 2020, 31, 1004–1021, DOI:10.1007/s10854-019-02612-2.
- S. A. Fahmy, I. M. Fawzy, B. M. Saleh, M. Y. Issa, U. Bakowsky and H. M. E. S. Azzazy, Green synthesis of platinum and palladium nanoparticles using Peganum harmala L. Seed alkaloids: biological and computational studies, Nanomaterials, 2021, 11, 1–15, DOI:10.3390/nano11040965.
- S. A. Fahmy, A. Ramzy, A. M. Sawy, M. Nabil, M. Z. Gad, M. El-Shazly, M. A. M. Aboul-soud and H. M. E. Azzazy, Ozonated Olive Oil: Enhanced Cutaneous Delivery via Niosomal Nanovesicles for Melanoma Treatment, Antioxidants, 2022, 11, 1318, DOI:10.3390/antiox11071318.
- N. K. Mahdy, M. El-Sayed, S. E. D. Al-Mofty, A. Mohamed, A. H. Karaly, M. E. El-Naggar, H. Nageh, W. A. Sarhan and H. M. E. S. Azzazy, Toward Scaling up the Production of Metal Oxide Nanoparticles for Application on Washable Antimicrobial Cotton Fabrics, ACS Omega, 2022, 7, 38942–38956, DOI:10.1021/acsomega.2c04692.
- S. Talam, S. R. Karumuri and N. Gunnam, Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles, ISRN Nanotechnol., 2012, 1–6, DOI:10.5402/2012/372505.
- S. A. Fahmy, M. Y. Issa, B. M. Saleh, M. R. Meselhy and H. M. E. S. Azzazy, Peganum harmala alkaloids self-assembled supramolecular nanocapsules with enhanced antioxidant and cytotoxic activities, ACS Omega, 2021, 6, 11954–11963, DOI:10.1021/acsomega.1c00455.
- S. M. Abdel-Hafez, R. M. Hathout and O. A. Sammour, Tracking the transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy, Int. J. Biol. Macromol., 2018, 108, 753–764, DOI:10.1016/j.ijbiomac.2017.10.170.
- M. X. Chen, T. Li, S. Peng and D. Tao, Supramolecular nanocapsules from the self-assembly of amphiphilic calixarene as a carrier for paclitaxel, New J. Chem., 2016, 40, 9923–9929, 10.1039/c6nj01986b.
- N. K. Sedky, N. M. Abdel-kader, M. Y. Issa, M. M. M. Abdelhady, S. N. Shamma, U. Bakowsky and S. A. Fahmy, Co-Delivery of Ylang Ylang Oil of Cananga odorata and Oxaliplatin Using Intelligent pH-Sensitive Lipid-Based Nanovesicles for the Effective Treatment of Triple-Negative Breast Cancer, Int. J. Mol. Sci., 2023, 24(9), 8392, DOI:10.3390/ijms24098392.
- S. A. Fahmy, N. K. Sedky, A. Ramzy, M. M. M. Abdelhady, O. Abd, A. Alabrahim, S. N. Shamma, H. Mohamed and E. Azzazy, Green extraction of essential oils from Pistacia lentiscus resins : Encapsulation into Niosomes showed improved preferential cytotoxic and apoptotic effects against breast and ovarian cancer cells, J. Drug Delivery Sci. Technol., 2023, 87, 104820, DOI:10.1016/j.jddst.2023.104820.
- K. Sarkar, J. Chakraborty, N. Chatterjee, A. Bhattacharjee, A. Dasgupta and D. Acharya, Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris, Nanomaterials, 2020, 1–27 Search PubMed.
- N. Anandhavalli, B. Mol, S. Manikandan, N. Anusha, V. Ponnusami and K. S. Rajan, Green synthesis of cupric oxide nanoparticles using water extract of Murrya koenigi and its photocatalytic activity, Asian J. Chem., 2015, 27, 2523–2526, DOI:10.14233/ajchem.2015.17966.
- N. M. Aboeita, S. A. Fahmy, M. M. H. El-Sayed, H. M. E. S. Azzazy and T. Shoeib, Enhanced Anticancer Activity of Nedaplatin Loaded onto Copper Nanoparticles Synthesized Using Red Algae, Pharmaceutics, 2022, 14(2), 418, DOI:10.3390/pharmaceutics14020418.
- A. A. A. Go and S. Ronald, Review of the Comparative Pharmacology and Clinical Activity of Cisplatin and Carboplatin, J. Clin. Oncol., 2016, 17, 409–422 CrossRef.
- A. O. Fatimah, R. I. Alharbi, G. Albasher, R. Almeer and N. S. Alsaggabi, Antifungal Potential of Aqueous Extract of Boswellia carteri, J. Pure Appl. Microbiol., 2019, 13, 2375–2381, DOI:10.22207/JPAM.13.4.53.
- F. M. Fakhree, I. F. Waheed and K. M. Mahmoud, Synthesis and characterization of Cuo nanoparticles stabilized by quercetin and its application for anti-breast cancer activity, Egypt. J. Chem., 2021, 64, 2989–2995, DOI:10.21608/ejchem.2021.56260.3207.
- R. I. Iliescu, E. Andronescu, C. D. Ghiţulică, D. Berger and A. Ficai, Montmorillonite-alginate nanocomposite beads as drug carrier for oral administration of carboplatin-preparation and characterization, U.P.B. Sci. Bull., Series B, 2011, 73, 3–16 Search PubMed.
- M. A. Khan, M. Zafaryab, S. H. Mehdi, J. Quadri and M. M. A. Rizvi, Characterization and carboplatin loaded chitosan nanoparticles for the chemotherapy against breast cancer in vitro studies, Int. J. Biol. Macromol., 2017, 97, 115–122, DOI:10.1016/j.ijbiomac.2016.12.090.
- V. V. T. Padil and M. Černík, Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application, Int. J. Nanomed., 2013, 8, 889–898, DOI:10.2147/IJN.S40599.
- D. Das, B. C. Nath, P. Phukon and S. K. Dolui, Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles, Colloids Surf., B, 2013, 101, 430–433, DOI:10.1016/j.colsurfb.2012.07.002.
- S. Sindhwani, A. M. Syed, J. Ngai, B. R. Kingston, L. Maiorino, J. Rothschild, P. MacMillan, Y. Zhang, N. U. Rajesh, T. Hoang, J. L. Y. Wu, S. Wilhelm, A. Zilman, S. Gadde, A. Sulaiman, B. Ouyang, Z. Lin, L. Wang, M. Egeblad and W. C. W. Chan, The entry of nanoparticles into solid tumours, Nat. Mater., 2020, 19, 566–575, DOI:10.1038/s41563-019-0566-2.
- S. M. Narum, T. Le, D. P. Le, J. C. Lee, N. D. Donahue, W. Yang and S. Wilhelm, Passive targeting in nanomedicine: fundamental concepts, body interactions, and clinical potential, in Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications, Elsevier, 2019, pp. 37–53, DOI:10.1016/B978-0-12-816662-8.00004-7.
- A. A. Umar and M. Oyama, A seed-mediated growth method for vertical array of single-crystalline CuO nanowires on surfaces, Cryst. Growth Des., 2007, 7, 2404–2409, DOI:10.1021/cg0602008.
- M. Naz, N. Nasiri, M. Ikram, M. Nafees, M. Z. Qureshi, S. Ali and A. Tricoli, Eco-friendly biosynthesis, anticancer drug loading and cytotoxic effect of capped ag-nanoparticles against breast cancer, Appl. Nanosci., 2017, 7, 793–802, DOI:10.1007/s13204-017-0615-6.
- M. Bruschi, Main mechanisms to control the drug release, in Strategies to Modify the Drug Release from Pharmaceutical Systems, 2015, pp. 37–62, DOI:10.1016/B978-0-08-100092-2.00004-7.
- A. T. Alex, A. Joseph, G. Shavi, J. V. Rao, A. T. Alex, A. Joseph, G. Shavi, J. V. Rao and N. Udupa, Development and evaluation of carboplatin- loaded PCL nanoparticles for intranasal delivery development and evaluation of carboplatin-loaded PCL nanoparticles for intranasal delivery, Drug Delivery, 2016, 23(7), 2144–2153, DOI:10.3109/10717544.2014.948643.
- S. Ranghar, P. Sirohi, P. Verma and V. Agarwal, Nanoparticle-based Drug Delivery Systems: Promising Approaches Against Infections, Braz. Arch. Biol. Technol., 2014, 57, 209–222 CrossRef.
- Y. H. Hsueh, P. H. Tsai and K. S. Lin, pH-dependent antimicrobial properties of copper oxide nanoparticles in Staphylococcus aureus, Int. J. Mol. Sci., 2017, 18(4), 793, DOI:10.3390/ijms18040793.
- M. H. Karimi, G. R. Mahdavinia and B. Massoumi, pH-controlled sunitinib anticancer release from magnetic chitosan nanoparticles crosslinked with κ-carrageenan, Mater. Sci. Eng., C, 2018, 91, 705–714, DOI:10.1016/j.msec.2018.06.019.
- U. Luesakul, S. Puthong, N. Neamati and N. Muangsin, pH-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells, Carbohydr. Polym., 2018, 181, 841–850, DOI:10.1016/j.carbpol.2017.11.068.
- R. B. Badisa, S. F. Darling-reed, P. Joseph, J. S. Cooperwood, L. M. Latinwo and C. B. Goodman, Selective Cytotoxic Activities of Two Novel Synthetic Drugs on Human Breast Carcinoma MCF-7 Cells, Anticancer Res., 2009, 2996, 2993–2996 Search PubMed.
- P. Francielli, D. Oliveira, J. Morais, J. Lopes, R. Aparecida, M. Oliveira, H. Júnior, A. Eduardo, M. Crotti and D. Crispim, Cytotoxicity screening of essential oils in cancer cell lines, Rev. Bras. Farmacogn., 2015, 25, 183–188, DOI:10.1016/j.bjp.2015.02.009.
- Z. S. El Fakharany, Y. M. Nissan, N. K. Sedky, R. K. Arafa and S. M. A. Seri, New proapoptotic chemotherapeutic agents based on the quinolone - 3 - carboxamide scaffold acting by VEGFR - 2 inhibition, Sci. Rep., 2023, 1–22, DOI:10.1038/s41598-023-38264-w.
- T. Sadhukha and S. Prabha, Encapsulation in Nanoparticles Improves Anti-cancer Efficacy of Carboplatin, AAPS PharmSciTech, 2014, 15(4), 1029–1038, DOI:10.1208/s12249-014-0139-2.
- H. Eg and P. Aa, Copper Nanoparticles as Therapeutic Anticancer Agents Copper Nanoparticles as Therapeutic Anticancer Agents, J. Nanomed. Nanotechnol., 2018, 2(1), 119 Search PubMed.
- A. I. Journal, S. Pairoj, P. Damrongsak, B. Damrongsak, R. Kaewkhaw, C. Ruttanasirawit and K. Locharoenrat, Antitumor activities of carboplatin – doxorubicin – ZnO complexes in different human cancer cell lines (breast, cervix uteri, colon, liver and oral) under UV exposition, Artif. Cells, Nanomed., Biotechnol., 2021, 49, 120–135, DOI:10.1080/21691401.2021.1876718.
- S. M. Aborehab, N. M. Abd-Elmawla, M. A. ElSayed, A. M. Sabry and O. Ezzat, Acovenoside A as a novel therapeutic approach to boost taxol and carboplatin apoptotic and antiproliferative activities in NSCLC: interplay of miR-630/miR-181a and apoptosis genes, Bioorg. Chem., 2023, 139, 106743 CrossRef.
- S. Vidot, J. Witham, R. Agarwal, S. Greenhough, H. S. Bamrah, G. J. Tigyi, S. B. Kaye and A. Richardson, Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells, Cell Signal., 2010, 22, 926–935, DOI:10.1016/j.cellsig.2010.01.017.
- S. Singh, A. K. Upadhyay, A. K. Ajay, M. K. Bhat, F. Zschunke, N. Miosge, P. Thelen and T. Schlott, p53 regulates ERK activation in carboplatin induced apoptosis in cervical carcinoma : a novel target of p53 in apoptosis, FEBS Lett., 2007, 581, 289–295, DOI:10.1016/j.febslet.2006.12.035.
- K. Ali, Q. Saquib, B. Ahmed, M. A. Siddiqui, J. Ahmad, M. Al-shaeri, A. A. Al-khedhairy and J. Musarrat, Bio-functionalized CuO nanoparticles induced apoptotic activities in human breast carcinoma cells and toxicity against Aspergillus fl avus : An in vitro approach, Process Biochem., 2020, 91, 387–397, DOI:10.1016/j.procbio.2020.01.008.
- P. C. Nagajyothi, P. Muthuraman, T. V. M. Sreekanth, D. Hwan and J. Shim, Green synthesis: in vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells, Arabian J. Chem., 2017, 10, 215–225, DOI:10.1016/j.arabjc.2016.01.011.
- P. Pozarowski and Z. Darzynkiewicz, Analysis of Cell Cycle by Flow Cytometry, Checkpoint Controls and Cancer 2, Activat, 2004, pp. 301–311 Search PubMed.
- M. Park and S. Lee, Cell Cycle and Cancer, J. Biochem. Mol. Biol., 2003, 36, 60–65 Search PubMed.
- G. H. Williams and K. Stoeber, The cell cycle and cancer, J. Pathol., 2012, 352–364, DOI:10.1002/path.3022.
- X. Zhu, Y. Peng and L. Qiu, Amino-functionalized nano-vesicles for enhanced anticancer efficacy and reduced myelotoxicity of carboplatin, Colloids Surf., B, 2017, 157, 56–64, DOI:10.1016/j.colsurfb.2017.05.041.
- M. Hanagata, N. Zhuang, F. Connolly, S. Li, J. Ogawa and N. Xu, Molecular Responses of Human Lung Epithelial Cells to the Toxicity of Copper Oxide Nanoparticles Inferred from Whole Genom Expression Analysis, J. Am. Chem. Soc., 2011, 9326–9338 Search PubMed.
- S. Shahriary, F. Tafvizi, P. Khodarahmi and M. Shaabanzadeh, Phyto - Mediated Synthesis of CuO Nanoparticles Using Aqueous Leaf Extract of Artemisia Deserti and Their Anticancer Effects on A2780 - CP Cisplatin - Resistant Ovarian Cancer Cells, Biomass Convers. Biorefin., 2022, 14, 2263–2279, DOI:10.1007/s13399-022-02436-x.
- E. Z. Bagci, Y. Vodovotz, T. R. Billiar, G. B. Ermentrout and I. Bahar, Bistability in Apoptosis : Roles of Bax , Bcl-2 , and Mitochondrial Permeability Transition Pores, Biophys. J., 2006, 90, 1546–1559, DOI:10.1529/biophysj.105.068122.
- N. K. Sedky, M. Braoudaki, K. Mahdy, K. Amin, I. M. Fawzy, E. K. Efthimiadou, A. Youness and S. A. Fahmy, Box–Behnken design of thermo-responsive nano- liposomes loaded with a platinum(IV) anticancer complex: evaluation of cytotoxicity and apoptotic pathways in triple negative breast cancer cells, Nanoscale Adv., 2023, 5, 5399–5413, 10.1039/d3na00368j.
- M. Manoochehri and A. Karbasi, Down-Regulation of BAX Gene During Carcinogenesis and Acquisition of Resistance to 5-FU in Colorectal Cancer, Pathol. Oncol. Res., 2014, 301–307, DOI:10.1007/s12253-013-9695-0.
- P. Lin, B. Zhou, H. Yao and Y. Guo, Effect of carboplatin injection on Bcl-2 protein expression and apoptosis induction in Raji cells on om m er ci al us e on m al, Eur. J. Histochem., 2020, 64, 3134 CrossRef.
- N. M. Aborehab, M. R. Elnagar and N. E. Waly, Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF - 7 human breast cancer cell line, J. Biochem. Mol. Toxicol., 2021, 1–11, DOI:10.1002/jbt.22638.
- M. A. Siddiqui, H. A. Alhadlaq, J. Ahmad, A. A. Al-khedhairy, J. Musarrat and M. Ahamed, Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells, PLoS One, 2013, 8(8), e69534, DOI:10.1371/journal.pone.0069534.
- M. Shafagh, F. Rahmani and N. Delirezh, CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53, Iran. J. Basic Med. Sci., 2015, 18, 993–1000 Search PubMed.
- T. Violette, S. Poulain, L. Dussaulx, E. Pepin, D. Faussat, A. M. Chambaz, J. Lacorte, J. M. Staedel and C. Lesuffleur, Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-XL in addition to Bax and p53 status, Int. J. Cancer, 2002, 504, 498–504, DOI:10.1002/ijc.10146.
- A. J. Levine, W. Hu and Z. Feng, The P53 pathway : what questions remain to be explored, Cell Death Differ., 2006, 1027–1036, DOI:10.1038/sj.cdd.4401910.
- M. T. Hemann and S. W. Lowe, The P53 – Bcl-2 Connection, Cell Death Differ, 2006, 1256–1259, DOI:10.1038/sj.cdd.4401962.
- C. M. Moon, K. E. Yun, S. Ryu, Y. Chang and D. Il Park, High serum alanine aminotransferase is associated with the risk of colorectal adenoma in Korean men, J. Gastroenterol. Hepatol., 2017, 32, 1310–1317, DOI:10.1111/jgh.13684.
- M. M. Sohocki, L. S. Sullivan, W. R. Harrison, E. J. Sodergren, F. F. B. Elder, G. Weinstock, S. Tanase and S. P. Daiger, Human glutamate pyruvate transaminase (GPT): localization to 8q24.3, cDNA and genomic sequences, and polymorphic sites, Genomics, 1997, 40, 247–252, DOI:10.1006/geno.1996.4604.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08779d |
| ‡ Nada K. Sedky and Iten M. Fawzy contributed equally to this work (first co-authors). |
| This journal is © The Royal Society of Chemistry 2024 |