Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Adsorption and sensor performance of transition metal-decorated zirconium-doped silicon carbide nanotubes for NO2 gas application: a computational insight†
Ismail O. Amodu ab,
Faith A. Olaojotulea,
Miracle N. Ogbogua,
Oluwatobi A. Olaiyab,
Innocent Benjamin
ab,
Faith A. Olaojotulea,
Miracle N. Ogbogua,
Oluwatobi A. Olaiyab,
Innocent Benjamin ac,
Adedapo S. Adeyinka
ac,
Adedapo S. Adeyinka d and
Hitler Louis
d and
Hitler Louis *ae
*ae
aComputational and Bio-Simulation Research Group, University of Calabar, Calabar, Nigeria. E-mail: louismuzong@gmail.com
bDepartment of Mathematics, University of Calabar, Calabar, Nigeria
cDepartment of Research Analytics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
dDepartment of Chemical Sciences, University of Johannesburg, Pretoria, South Africa
eSchool of Chemistry, University of Leeds, Leeds LS2 9JT, UK
First published on 12th February 2024
Abstract
Owing to the fact that the detection limit of already existing sensor-devices is below 100% efficiency, the use of 3D nanomaterials as detectors and sensors for various pollutants has attracted interest from researchers in this field. Therefore, the sensing potentials of bare and the impact of Cu-group transition metal (Cu, Ag, Au)-functionalized silicon carbide nanotube (SiCNT) nanostructured surfaces were examined towards the efficient detection of NO2 gas in the atmosphere. All computational calculations were carried out using the density functional theory (DFT) electronic structure method at the B3LYP-D3(BJ)/def2svp level of theory. The mechanistic results showed that the Cu-functionalized silicon carbide nanotube surface possesses the greatest adsorption energies of −3.780 and −2.925 eV, corresponding to the adsorption at the o-site and n-site, respectively. Furthermore, the lowest energy gap of 2.095 eV for the Cu-functionalized surface indicates that adsorption at the o-site is the most stable. The stability of both adsorption sites on the Cu-functionalized surface was attributed to the small ellipticity (ε) values obtained. Sensor mechanisms confirmed that among the surfaces, the Cu-functionalized surface exhibited the best sensing properties, including sensitivity, conductivity, and enhanced adsorption capacity. Hence, the Cu-functionalized SiCNT can be considered a promising choice as a gas sensor material.
1 Introduction
Prevalent diseases such as cancer and stroke account for about a million deaths every year due to the inhalation of microscopic pollutants, which can enter the respiratory and circulatory tracts, causing severe damage to major organs and glands.1,2 In 2018, the World Health Organization (WHO) reported that air pollution is one of the major risks to health.3 Pollutants are chemical or biological substances that disintegrate the quality of useful resources when introduced in the environment and are known to pose a significant risk to public health.4 Nitrogen oxides, a group of highly reactive gases such as nitrogen oxide (NO) and nitrogen dioxide (NO2), serve as major contributors to the depleting air quality.5 Many anthropogenic activities such as the combustion of fossil fuels significantly contributes to the presence of toxic forms of NO gases and exposure to an enormous amount of NO2 has a severe effect on wildlife and humans.6,7 Alternatively, a trace amount of NO2 is suitable as a fingerprint for tracking chronic obstructive pulmonary disease (COPD).8 Nitrogen dioxide (NO2) is also a major contributor to the formation of acid rain and photochemical smog and is the major component in the formation of fine particles. It is a primary air pollutant, which also affects the tropospheric ozone.9 Human activities including oil spillage, explosives, burning, refining of petrol, and manufacturing of food and natural sources such as volcanoes, oceans, and forest fires significantly contribute to the increasing NO2 level. This has caused numerous health problems, especially, respiratory issues such as swelling of the tissues in the throat, reduced oxygenation, and rapid burning, which occur when the lining of the lungs gets inflamed and often triggered by these activities due to the generation of high concentrations of NO2 gas molecules.10,11Nanotechnology is the science and technology of creating nanoparticles and manufacturing materials with sizes less than 100 nm.12 It incorporates different fields such as physics, chemistry, materials science, and biology to develop the production of biomedical products, high-performance products, and consumer articles.13 For example, drug carriers and electrochemical sensors were developed in previous studies using different nanomaterial such as carbon nanotubes and inorganic metal oxides, providing versatile compositions.14,15 Additionally, it aids the engineering of the physical properties ranging from the size, morphology, and surface characteristics to the structure of atoms.16–18 Heightened therapeutic efficiency and enhanced clinical benefits in surviving cancer have been achieved using nanomedicines such as Doxil for the treatment of sarcomas, which increases the stability and solubility of drugs.19 Nano-composite materials are significantly known to include a wide variety of systems including one-dimensional, three-dimensional and amorphous materials.20–22 Nanocages are cage-like assemblies of proteins, having three engineerable surfaces, which can be used as potential carriers of molecules for therapeutic and diagnostic purposes.23,24 These nanomaterials in form of cages, tubes, and wires also facilitate the safer transportation, delivery, trapping and sensing of different molecules and minimize the premature degradation or interactions of doped atoms in the biological or physical environment.25–27
In-depth studies on metal oxide-based NO2 sensors have been done by researchers in the past. The active sites on metal oxides are responsible for their sensing characteristics. This implies that the sensing mechanism of different gases depends on the crystallinity and number of defects present in metal oxide-like nanoparticles. Also, these nanostructures can be used for the fabrication of NO2 gas sensors due to their specific attributes such as high thermal stability, non-toxicity and high chemical sensitivity.28–30 Resistive gas sensors are employed for the detection of variations and concentrations of different ambient gases. In this case, the active layer is the main part of a sensor, which can be composed of various materials including inorganic, organic and hybrid materials.31,32 However, the major disadvantages of resistive gas sensors are their limited sensitivity and poor selectivity. Thus, several strategies have been introduced to overcome these shortcomings, including varying the operating temperature of the sensor.33,34 In recent studies, Cheng et al. reported that gas sensors based on hybrid 1D/3D-structured perovskite provide a new pathway for the highly sensitive and stable detection of NO2 gas.35 Nwagu et al.36 reported that K, Ca, Mg and late transition metal dopants enhance the adsorption of SO2, which is attributed to the improvement in surface properties. In another study, Inah et al. revealed that GQD_N and GQD-S are good adsorbents for the adsorption of C2H4, CH4, and H2 gases that exhibit non-covalent interactions, indicating the weak intermolecular interactions between the surface of the adsorbents and gas molecules.37
Therefore, the present work focused on adopting more effective and reliable methods for the detection of NO2 gas molecules in different sectors ranging from automobiles and industrial plants to farming. Recently, the process of measuring the air quality has been considered to be unaffordable due to the high cost of laboratory equipment and large monitoring devices, which also requires professional operators. Accordingly, the development of alternative devices, procedures and equipment with relatively small size, low cost and promising performances in terms of sensitivity, fast response and low energy consumption will have a positive impact on curbing various pollutants especially NO2 gases. Hence, systems with high miniaturization, low cost, and simple production techniques will be of significant interest because the improvement in nanotechnology using engineered nanoparticles has resulted in exceptional efficacy in the biomedical field and designing future sensor devices.
Herein, the current investigation endeavored to provide new insight into the sensing potential of bare and Cu-group transition metal (Cu, Ag, Au)-functionalized silicon carbide nanotube (SiCNT) nanostructured surfaces through a comprehensive computational study. The aim of this investigation was twofold, i.e., to design advanced sensor materials capable of detecting NO2 gas molecules with intriguing sensitivity and obtaining detailed knowledge on the nature of the interactions and the stabilization of these materials, which are essential for their gas adsorption mechanisms. Initially, we performed structural optimization to uncover the most stable geometric configurations and the structural properties of the materials under investigation. Furthermore, to probe the sensing properties of the materials, various analyses such as adsorption energy, frontier molecular orbital (FMO), stabilization energy, density of states (DOS), atoms-in-molecules (AIM), reduced density gradient (RDG), molecular dynamic (MD) simulations, and sensor mechanisms were thoroughly performed.
2 Computational details
All theoretical calculations were performed using Kohn–Sham (KS) density functional theory (DFT) computation. To obtain the most stable geometric structures, ground-state geometric optimization was carried out for all the structures using the B3LYP-D3(BJ)/def2svp level of theory.38 Gaussian 16,39 together with its Gauss View 6.0.16 (ref. 40) software graphical interphase was used for sketching, optimization and extraction. Insight into the nature of the interactions, electronic properties, and adsorption energy were gained using the visual study, quantum descriptors, and adsorption energy calculations. The visual study, employing the quantum theory of atoms-in-molecules (QTAIM), and its visual extension, which is the non-covalent interaction (NCI) analysis, were carried out using the Multiwfn 3.7 program,41 where the visualization of NCI was done using the visual molecular dynamics (VMD) software.42 Also, the density of states (DOS) was computed using the Multiwfn 3.7 program, and subsequently the generated data was plotted using the Origin software. Upon adsorption, the adsorption energy of the resulting complexes was determined using eqn (1), as follows:| Ead = EComplex − (ESurface + EMolecule) | (1) |
3 Results and discussion
3.1 Optimizations and molecular geometry
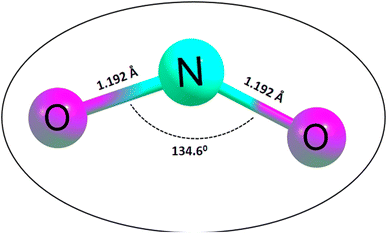
Initially, the geometries of the molecular structures were equilibrated, and the energy minimization was carried out to ensure the stability and sensitivity of the studied systems.44 The importance of optimization in the calculation of adsorption energies cannot be overstated, given that it facilitates the calculation of the adsorption energies of the systems of interest. Initially, the silicon carbide nanotube was doped with zirconium, and then further functionalized with transition metals (Cu, Ag, and Au). The surfaces were optimized separately as well as the NO2 gas molecule, before being placed together, and then optimized again to form complexes. The morphology of the surfaces was reengineered to obtain a new surface with enhanced functionalities towards sensing. The bond length of the optimized NO2 gas molecule was computed, as presented in Fig. 1.Easy glance into the molecular geometries of the structures was possible, as shown in Fig. 1, where the bond lengths between two atoms are shown. Clearly, the bond lengths between two atoms in the NO2 gas molecule and that within the rings of the functionalized SiCNT surfaces were visualized. Considering the NO2 gas molecule, the bond lengths for the N–O bonds were determined to be 1.192 and 1.192 Å, which were found to be consistent with previous computational studies with a bond length of 1.21 and 1.16 Å in ref. 45 and 46. Also, before functionalizing the surface of SiCNT, the carbon–zirconium bond lengths were computed and found to be 2.082, 2.088, and 2.105 Å for the C32–Zr100, C34–Zr100 and C39–Zr100 bonds, respectively. Similarly, computed the bond lengths are consistent with previous theoretically calculated bond lengths.47
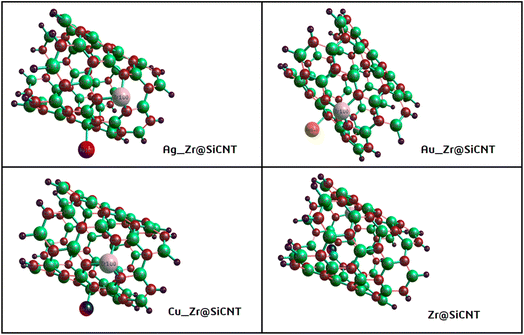
Given that the selectivity of a material used as a sensor device is dependent on its properties, it is necessary to enhance the sensing performance of nanomaterials. Thus, the nanotube surface was reengineered with transition metals such as Ag, Au, and Cu, thereby resulting in the formation of Ag_Zr@SiCNT, Au_Zr@SiCNT, and Cu_Zr@SiCNT surfaces respectively. The functionalization was achieved by adding each transition metal to the carbon atom of the already doped Zr@SiCNT surface, as shown in Fig. 2. Also, Fig. 3 presents the optimized structures of the complexes formed as a result of adsorption.
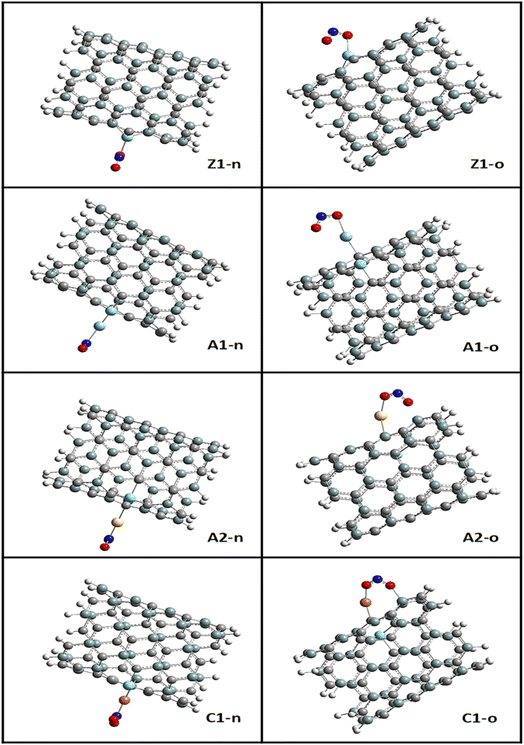
The bond lengths between carbon and zirconium (C–Zr) and that of carbon and silver (C–Ag) were computed before and after adsorption for all the surfaces to gain insight into the effect of adsorption on the morphology of the tubes. This was to determine whether adsorption stretches or shortens the bond lengths. Furthermore, the adsorption distance was computed, which was observed to be between the n-site or o-site of adsorption and transition metals for the functionalized surfaces and between zirconium for the Zr@SiCNT surface. The sites of adsorption were also considered to obtain the best adsorption configurations. The results of the structural geometries are summarized in Table 1. The bond lengths observed for the Z1-X complexes (X = n and o sites) were found to be slightly elongated as a result of adsorption, leading to stretching in the nanotube. For example, the C32–Zr100 bond increased from 2.082 to 2.119 and 2.217 Å, corresponding to the n-site and o-site of adsorption, respectively. However, the functionalized surfaces exhibited different behavior in their structures. For example, adsorption on the A1-X complexes (X = n and o sites) showed a slight or no effect on their bond lengths, with the same bond length values of 2.083 and 2.089 Å before and after adsorption, and then a slight change from 2.103 to 2.101 and 2.100 Å for the n-site and o-site of adsorption respectively. In the labelled A2-X and C1-X complexes, slight changes were observed in the n-site and o-site of adsorption, depicting that adsorption led to an almost insignificant shift in their geometric structures. Overall, the observed changes in the bond lengths indicate that a reaction occurred, thus establishing the adsorption process. In most cases, a shorter bond length suggests a weak interaction between the adsorbate and adsorbent.48 The shortest interaction distances of 1.920 and 1.916 Å, corresponding to the n-site and o-site of adsorption, were found in the Cu-functionalized surfaces, indicating the weakest interactions among the studied surfaces. Alternatively, the strongest interactions were observed in the complex corresponding to the Zr@SiCNT surface, which was not functionalized.
| System | Bond label | Bond length (Å) | ||
|---|---|---|---|---|
| Before ads. | After ads. | |||
| n-site | o-site | |||
| a X denotes the n-site and o-site of adsorption. Q denotes the N102 and O104 atoms present between the surfaces and the NO2 gas molecule. | ||||
| NO2_X_Zr@SiCNT (Z1-X) | Q–Zr100 | — | 2.214 | 2.268 |
| C32–Zr100 | 2.082 | 2.119 | 2.217 | |
| C34–Zr100 | 2.088 | 2.120 | 2.113 | |
| C39–Zr100 | 2.105 | 2.213 | 2.132 | |
| NO2_X_Ag_Zr@SiCNT (A1-X) | Q–Ag101 | — | 2.118 | 2.205 |
| C23–Ag101 | 2.629 | 2.197 | 2.204 | |
| C32–Zr100 | 2.083 | 2.083 | 2.083 | |
| C34–Zr100 | 2.089 | 2.089 | 2.089 | |
| C39–Zr100 | 2.103 | 2.101 | 2.100 | |
| NO2_X_Au_Zr@SiCNT (A2-X) | Q–Au101 | — | 2.080 | 2.133 |
| Si79–Au101 | 2.466 | 2.642 | 2.645 | |
| C32–Zr100 | 2.085 | 2.085 | 2.097 | |
| C34–Zr100 | 2.090 | 2.091 | 2.083 | |
| C39–Zr100 | 2.100 | 2.097 | 2.093 | |
| NO2_X_Cu_Zr@SiCNT (C1-X) | Q–Cu101 | — | 1.920 | 1.916 |
| Si79–Cu101 | 2.376 | 1.966 | 1.961 | |
| C32–Zr100 | 2.084 | 2.084 | 2.096 | |
| C34–Zr100 | 2.089 | 2.089 | 2.080 | |
| C39–Zr100 | 2.102 | 2.099 | 2.095 | |
3.2 Adsorption study
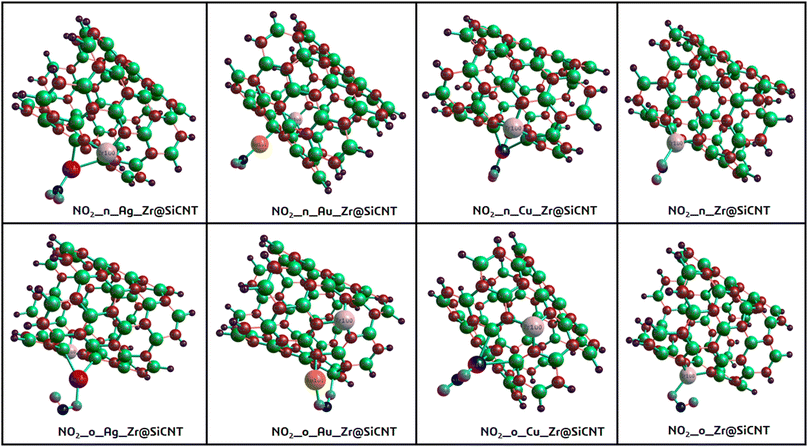
The most stable adsorption configurations were ensured in the adsorption of NO2 gas molecules on the bare and functionalized SiCNT surfaces. To determine the minimum energy configurations, the bare and functionalized surfaces were optimized.49 Initially, in a cluster, structural equilibration was carried out using the def2svp basis set. For the best adsorption conformation, two sites of adsorption were considered, namely, the nitrogen and oxygen sites, which were denoted as n-site and o-site, respectively. It is necessary to determine the adsorption energy to know how well an adsorbate is adsorbed by an adsorbent. In this study, the adsorption energy was calculated using the mathematical expression in eqn (1) of the computational details.50 Energy calculation was performed for each complex and surface, and then for the NO2 gas molecule. Table 2 summarizes the calculated adsorption energy values for all the complexes. The n-site and o-site of adsorption are visualized in Fig. 4. According to Table 2, the adsorption energy values of −2.190 and −2.422 eV were obtained for the Z1-n and Z1-o complexes, respectively. Greater adsorption energies were obtained after functionalizing the surface with transition metals, with the greatest adsorption energies of −3.780 and −2.925 eV corresponding to the C1-o and C1-n complexes, respectively. Similarly, relatively greater energy values were recorded for the A1-n and A1-o complexes, reflecting the adsorption energies of −2.248 and −2.578 eV, respectively. Also, the adsorption energies of −2.512 and −2.265 eV, corresponding to the A2-o and A2-n complexes, respectively, were observed. The o-site of adsorption again exhibits the best adsorption configuration due to the greater adsorption energies recorded. Evidently, the Cu-functionalized surface exhibits the best adsorption potential compared to the Ag- and Au-functionalized Zr@SiCNT surfaces. The basic set superposition error (BSSE) was determined by running BSSE calculations. This was done to correct the adsorption energy calculated from the initial optimization.51,52 However, according to this result, it was remarkable that there is no difference between the adsorption energy and the corrected BSSE plus the adsorption energy. This occurrence left the BSSE values at zeros in all the complexes, which was validated by maintaining the decimal places as they appeared during the calculations. Hence, the reliability of the B3LYP-D3(BJ)def2svp computational method and the BSSE method was confirmed.| System | Ead | EBSSE + Ead | EFL (eV) | Qt (eV) | ΔN (eV) | % ΔN | ΔE back-donation |
|---|---|---|---|---|---|---|---|
| NO2_n_Zr@SiCNT | −2.19016 | −2.19016 | 3.862522 | 1.06451 | −0.00913 | −0.91298 | −0.9656 |
| NO2_n_Ag_Zr@SiCNT | −2.24815 | −2.24815 | 3.973817 | −0.03452 | 0.624818 | 62.48175 | −0.3113 |
| NO2_n_Au_Zr@SiCNT | −2.26472 | −2.26472 | 4.162256 | −0.1166 | 1.196062 | 119.6062 | −1.3731 |
| NO2_n_Cu_Zr@SiCNT | −2.92501 | −2.92501 | 3.777895 | 0.17807 | 0.645692 | 64.56919 | −0.2617 |
| NO2_o_Zr@SiCNT | −2.42173 | −2.42173 | 3.885788 | 0.61595 | −0.60000 | −60.0000 | −0.9714 |
| NO2_o_Ag_Zr@SiCNT | −2.57823 | −2.57823 | 4.112867 | 0.0417 | 0.644919 | 64.49187 | −1.3700 |
| NO2_o_Au_Zr@SiCNT | −2.51232 | −2.51232 | 3.990688 | −0.02583 | 1.773026 | 177.3026 | −0.3113 |
| NO2_o_Cu_Zr@SiCNT | −3.7801 | −3.78010 | 3.955041 | 0.20522 | 0.574286 | 57.42862 | −0.3121 |
3.3 Electronic property investigation
| System | EHOMO | ELUMO | Eg | IP | EA | μ | η | S | ω |
|---|---|---|---|---|---|---|---|---|---|
| SiCNT | −5.333 | −1.878 | 3.456 | 5.333 | 1.878 | 3.606 | 1.728 | 0.289 | 3.762 |
| Zr@SiCNT | −5.252 | −2.531 | 2.721 | 5.252 | 2.531 | 3.891 | 1.361 | 0.367 | 5.564 |
| Ag_Zr@SiCNT | −4.408 | −2.613 | 1.796 | 4.408 | 2.612 | 3.510 | 0.898 | 0.557 | 6.861 |
| Au_Zr@SiCNT | −5.089 | −2.667 | 2.422 | 5.089 | 2.667 | 3.878 | 1.211 | 0.413 | 6.209 |
| Cu_Zr@SiCNT | −3.946 | −2.612 | 1.333 | 3.946 | 2.612 | 3.279 | 0.667 | 0.750 | 8.064 |
| NO2_n_Zr@SiCNT (Z1-n) | −5.442 | −2.286 | 3.157 | 5.442 | 2.286 | 3.864 | 1.578 | 0.317 | 4.730 |
| NO2_n_Ag_Zr@SiCNT (A1-n) | −5.470 | −2.748 | 2.721 | 5.469 | 2.748 | 4.109 | 1.361 | 0.367 | 6.204 |
| NO2_n_Au_Zr@SiCNT (A2-n) | −5.225 | −2.748 | 2.476 | 5.225 | 2.748 | 3.986 | 1.238 | 0.404 | 6.418 |
| NO2_n_Cu_Zr@SiCNT (C1-n) | −5.197 | −2.694 | 2.504 | 5.197 | 2.694 | 3.946 | 1.252 | 0.399 | 6.219 |
| NO2_o_Zr@SiCNT (Z1-o) | −5.425 | −2.346 | 3.079 | 5.425 | 2.346 | 3.886 | 1.539 | 0.325 | 4.904 |
| NO2_o_Ag_Zr@SiCNT (A1-o) | −5.225 | −2.721 | 2.504 | 5.225 | 2.721 | 3.973 | 1.252 | 0.399 | 6.305 |
| NO2_o_Au_Zr@SiCNT (A2-o) | −5.497 | −2.830 | 2.667 | 5.497 | 2.829 | 4.163 | 1.333 | 0.375 | 6.499 |
| NO2_o_Cu_Zr@SiCNT (C1-o) | −4.816 | −2.721 | 2.095 | 4.816 | 2.721 | 3.769 | 1.048 | 0.477 | 6.779 |
| IP = −EHOMO | (2) |
| EA = −ELUMO | (3) |
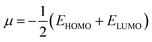 | (4) |
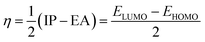 | (5) |
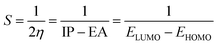 | (6) |
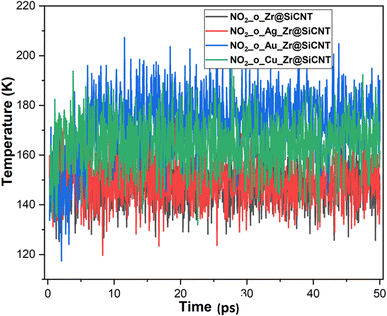
The electrophilicity index (ω) was calculated using the following equation:
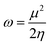 | (7) |
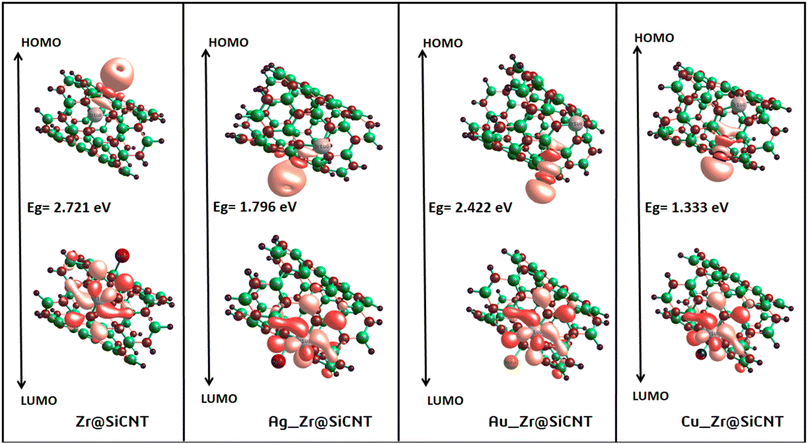
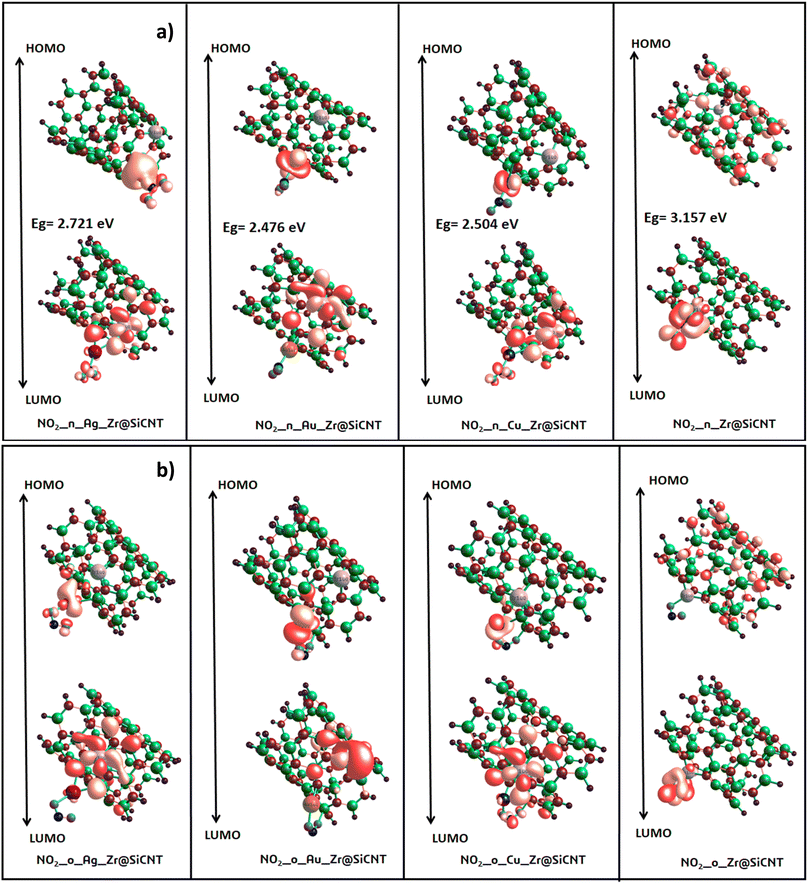
The behavior of a material to resist a charged particle in its environment can be understood using chemical hardness.60 The related literature on theoretical computations showed that the higher the chemical hardness and chemical potential, the higher the stability and lower the reactivity.61 A summary of the global reactivity indices is presented in Table 3. In all cases for the functionalized surfaces, an increase in the chemical potential and hardness values was observed. For example, the values for the Cu-functionalized surface increased from 3.279 eV to 3.946 and 3.769 eV, the values for the Au-functionalized surface increased from 3.986 eV to 3.986 and 4.163 eV, and finally the Ag-functionalized surface showed an increase from 3.510 eV to 4.109 and 3.973 eV, respectively. This increment indicates the higher stability and lower reactivity of the functionalized surfaces. However, the Zr@SiCNT surface, which was not functionalized, showed a decrease in it chemical potential value, thereby indicating its lower stability and higher reactivity. Among the surfaces, the values for the Cu-functionalized surface increased significantly from 0.667 to 1.252 and 1.048 eV for both the n- and o-sites of adsorption, respectively. This again confirmed the inherent stability of the functionalized surfaces, and in particular the Cu-functionalized surface outperformed the studied surfaces due to its relatively large margin. Regarding the site of adsorption, the o-site is the most favorable given that the increment occurred most in the o-site of adsorption. To know whether a chemical species is a strong or weak electrophile, the electrophilicity index (ω) is used. Thus, after functionalizing the surface, the ω value of 5.564 for the Zr@SiCNT surface increased to 6.861, 6.209, and 8.064 eV for the Ag_Zr@SiCNT, Au_Zr@SiCNT, and Cu_Zr@SiCNT surfaces, respectively, indicating that they are strong electrophiles. Also, due to the relatively higher values observed, the o-site of adsorption is a stronger electrophile than the n-site.
3.4 Stabilization energy
As an indispensable approach to gaining useful and reliable knowledge into the pattern of charge transfer within molecules, the natural bond orbital (NBO) analysis, which was developed by Weinhold et al., was employed in this section. To gain further insight into the electronic wavefunction, the localized orbitals such as non-Lewis and anti-bond orbitals were considered. Their applications decipher the knowledge behind the electron density delocalization, re-hybridization, and intra-molecular charge transfer.62 The stabilization of the donor–acceptor interactions is due to the exchange of electron densities between the orbitals.In the present study, we employed the NBO analysis to unravel the pattern of intermolecular delocalization or hyper-conjugation in the adsorption of NO2 gas molecules on the bare and functionalized SiCNT surfaces. This analysis enabled us to gain knowledge on the intra and intermolecular interactions in both the donor and acceptor orbitals. Furthermore, the measure of the donor–acceptor interaction stabilization energy, which is called the second-order perturbation energy (E(2)), leads insights into the type and strength of interaction. Generally, a higher stabilization energy value is associated with strong interactions between electrons and donors and acceptors, indicating a greater degree of conjugation in the system.63 Table 4 presents the most useful results of the NBO calculation including the second-order perturbation energies, donors and acceptors. According to Table 4, before adsorption, the major charge resonance is that from the anti-bonding charge orbital to the same anti-bonding charge orbital of σ-type (σ* → σ*). However, after adsorption, the major charge delocalization was observed to be the lone pairs to the anti-bonding charge orbital of π-type (LP → π*) in the n-site of adsorption, while in the o-site of adsorption, the charge resonance resulting from the charge transfer from the lone pair to the anti-bonding character of the lone pair (LP → LP*). Additionally, the charge delocalization resulting from charge transfer from the anti-bonding natural orbital between the carbon and zirconium atoms to the same anti-bonding natural orbital between the zirconium and nitrogen atoms (σ*C34–Zr100 → σ*Zr100–N101) in the C1-n complex reflects the highest stabilization energy of 1178.00 kcal mol−1. The higher energies observed in most cases indicate strong interactions and great stabilization upon adsorption. Therefore, the Cu-functionalized surface exhibits relatively greater stability, and hence easily stabilized compared to its studied counterparts.
| System | Donor | Acceptor | E(2)/kcal mol−1 | E(i) − E(j) | F(i,j) |
|---|---|---|---|---|---|
| Zr@SiCNT | σ*C34–Zr100 | LP*(3)Zr100 | 561.03 | 0.03 | 0.450 |
| Ag_Zr@SiCNT | σ*C34–Zr100 | σ*C39–Zr100 | 266.28 | 0.04 | 0.422 |
| Au_Zr@SiCNT | σ*C34–Zr100 | σ*C39–Zr100 | 646.89 | 0.02 | 0.442 |
| Cu_Zr@SiCNT | σ*C32–Zr100 | σ*C39–Zr100 | 350.38 | 0.03 | 0.429 |
| NO2_n_Zr@SiCNT (Z1-n) | LP(3)O104 | π*N102–O103 | 128.32 | 0.22 | 0.152 |
| NO2_n_Ag_Zr@SiCNT (A1-n) | LP(3)O103 | π*N102–O104 | 111.03 | 0.23 | 0.143 |
| NO2_n_Au_Zr@SiCNT (A2-n) | LP(3)O103 | π*N102–O104 | 94.59 | 0.26 | 0.141 |
| NO2_n_Cu_Zr@SiCNT (C1-n) | σ*C34–Zr100 | σ*Zr100–N101 | 1178.00 | 0.03 | 0.602 |
| NO2_o_Zr@SiCNT (Z1-o) | LP(3)O104 | LP*(6)Ag101 | 313.70 | 0.82 | 0.48 |
| NO2_o_Ag_Zr@SiCNT (A1-o) | LP(2)O104 | LP*(6)Au101 | 203.84 | 0.69 | 0.371 |
| NO2_o_Au_Zr@SiCNT (A2-o) | LP*(6)Cu101 | LP*(6)Cu101 | 140.30 | 1.03 | 0.341 |
| NO2_o_Cu_Zr@SiCNT (C1-o) | LP(3)O103 | LP*(1)Zr100 | 52.17 | 1.44 | 0.350 |
3.5 Visual study of weak interactions
| System | Bond | CP | P(r) | ∇2ρ(r) | G(r) | V(r) | H(r) | G/|V| | ELF | LOL | λ1 | λ2 | λ3 | λ1/λ3 | ε |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr@SiCNT | C39–Zn100 | 147 | 0.388 | −0.188 | 0.608 | −0.471 | −0.471 | 1.291 | 1.000 | 0.998 | −0.633 | −0.617 | −0.633 | 1.026 | 0.000 |
| C31–Zn100 | 121 | 0.117 | −0.514 | 0.105 | −0.128 | −0.128 | 0.820 | 0.999 | 0.998 | −0.634 | −0.631 | −0.634 | 1.005 | −0.249 | |
| Ag_Zr@SiCNT | C46–Si88 | 99 | 0.166 | −0.427 | 0.348 | −0.106 | −0.107 | 3.283 | 1.000 | 0.995 | −0.143 | −0.142 | −0.142 | 1.007 | 0.000 |
| C49–S94 | 156 | 0.109 | 0.435 | 0.149 | −0.189 | −0.399 | 0.788 | 0.188 | 0.324 | −0.143 | 0.715 | −0.137 | −0.200 | 0.045 | |
| Au_Zr@SiCNT | Si79–Au101 | 141 | 0.703 | −0.457 | 0.244 | −0.602 | −0.358 | 0.405 | 0.665 | 0.585 | −0.501 | 0.518 | −0.474 | −0.967 | 0.057 |
| C48–Si79 | 150 | 0.108 | 0.443 | 0.150 | −0.190 | −0.396 | 0.790 | 0.183 | 0.321 | 0.720 | −0.134 | −0.143 | −5.373 | 0.067 | |
| Cu_Zr@SiCNT | Cu101–Si23 | 132 | 0.771 | 0.262 | 0.809 | −0.962 | −0.154 | 0.841 | 0.201 | 0.331 | −0.690 | −0.594 | 0.390 | 1.162 | 0.161 |
| C52–Si75 | 135 | 0.109 | 0.428 | 0.148 | −0.189 | −0.412 | 0.783 | 0.193 | 0.328 | −0.142 | −0.135 | 0.705 | 1.052 | 0.136 | |
| NO2_n_Zr@SiCNT | Zr100–O102 | 267 | 0.475 | −0.112 | 0.335 | −0.951 | −0.616 | 0.352 | 0.860 | 0.713 | −0.117 | 0.127 | −0.122 | −0.921 | 0.043 |
| Zr100–N101 | 261 | 0.637 | 0.305 | 0.786 | −0.811 | −0.245 | 0.969 | 0.121 | 0.271 | −0.824 | 0.469 | −0.821 | −1.757 | 0.004 | |
| NO2_n_Ag_Zr@SiCNT | C47–Ag101 | 162 | 0.911 | 0.355 | 0.110 | −0.132 | −0.215 | 0.833 | 0.187 | 0.325 | 0.549 | −0.918 | −0.101 | −0.598 | 0.103 |
| C36–Si65 | 135 | 0.892 | 0.441 | 0.850 | −0.595 | 0.254 | 1.429 | 0.165 | 0.115 | 0.273 | 0.215 | −0.465 | 1.270 | −1.216 | |
| NO2_n_Au_Zr@SiCNT | C28–Au101 | 81 | 0.166 | −0.427 | 0.348 | −0.106 | −0.107 | 3.283 | 1.000 | 0.995 | −0.142 | −0.142 | −0.142 | 1.000 | 0.000 |
| C34–Zr100 | 107 | 0.262 | −0.937 | 0.431 | −0.320 | −0.278 | 1.347 | 0.980 | 0.876 | −0.707 | 0.493 | −0.723 | −1.434 | 0.022 | |
| NO2_n_Cu_Zr@SiCNT | C23–Cu101 | 34 | 0.117 | −0.515 | 0.105 | −0.129 | −0.129 | 0.814 | 1.000 | 0.999 | −0.171 | −0.172 | −0.172 | 0.994 | 0.000 |
| N102–Cu101 | 123 | 0.988 | 0.476 | 0.142 | −0.166 | −0.232 | 0.855 | 0.153 | 0.299 | −0.109 | 0.734 | −0.148 | −0.149 | 0.358 | |
| NO2_o_Zr@SiCNT | Zr100–N101 | 263 | 0.308 | 0.173 | 0.387 | −0.342 | 0.456 | 1.132 | 0.481 | 0.184 | 0.113 | 0.911 | −0.309 | 0.124 | −1.339 |
| Zr100–O102 | 262 | 0.473 | 0.214 | 0.517 | −0.500 | 0.169 | 1.034 | 0.106 | 0.256 | −0.618 | 0.331 | −0.552 | −1.867 | 0.120 | |
| NO2_o_Ag_Zr@SiCNT | C23–Ag101 | 169 | 0.831 | 0.205 | 0.724 | −0.937 | −0.212 | 0.773 | 0.282 | 0.385 | 0.364 | −0.787 | −0.805 | −0.463 | 0.023 |
| O104–Ag101 | 147 | 0.641 | 0.315 | 0.854 | −0.921 | −0.665 | 0.927 | 0.106 | 0.256 | −0.713 | 0.452 | −0.651 | −1.577 | 0.095 | |
| NO2_o_Au_Zr@SiCNT | C23–Au101 | 156 | 0.106 | 0.241 | 0.946 | −0.130 | −0.354 | 7.277 | 0.342 | 0.419 | 0.447 | −0.100 | −0.105 | −4.47 | 0.050 |
| Si76–O103 | 105 | 0.105 | 0.151 | 0.946 | −0.151 | −0.570 | 6.265 | 0.335 | 0.415 | −0.133 | 0.421 | −0.137 | −0.316 | 0.026 | |
| NO2_o_Cu_Zr@SiCNT | Si79–Cu101 | 132 | 0.105 | 0.355 | 0.115 | −0.142 | −0.268 | 0.810 | 0.249 | 0.365 | 0.590 | −0.114 | −0.121 | −5.175 | 0.056 |
| Si76–O103 | 169 | 0.101 | 0.372 | 0.130 | −0.166 | −0.366 | 0.783 | 0.191 | 0.327 | −0.127 | −0.124 | 0.624 | 1.024 | 0.022 |
The results obtained can be further confirmed using the values of the ratio of λ1/λ3. Similar to that reported in ref. 69, the values of λ1/λ3 were observed to be less than one in the case of the C1-n complex with λ1/λ3 values of 0.994 and −0.149 for the C23–Cu101 and N102–Cu101 bonds, respectively, indicating the presence of strong intermolecular interactions. Similarly, a small λ1/λ3 value of −5.175 was calculated for the Si79–Cu101 bond, which is less than one, indicating the presence of intermolecular interactions. Furthermore, to confirm the results of the characteristic interactions, the G/|V| ratio was utilized. The nature of interactions is classed as covalent, partial covalent, and non-covalent when the G/|V| ratios are greater 1, between 1 and 0.5, and less than 0.5, respectively.70,71 Again, the partial covalent type of interaction was confirmed by the calculated G/|V| ratio values of between 1 and 0.5 in most cases. Insight into the stability of bonds and the regions of charge density accumulation can be gained using the ellipticity of electron density (ε).72 A stable interaction is depicted by a small bond ellipticity value (ε < 1) at the bond critical points. Small ε values less than unity were found for the C1-o complex (0.056, 0.022) and C1-n complex (0.000 and 0.358), depicting the stability of the Cu-functionalized surface in both sites of adsorption, respectively. In all cases, as shown in Table 5, the ellipticity values are less than one, suggesting the stability of the surfaces.
3.6 Density of states (DOS) analysis
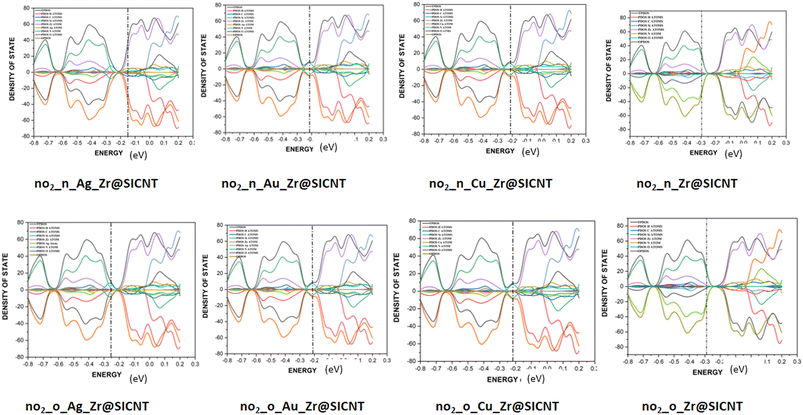
The description of the energy difference between the highest occupied orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of a complex, using the Fermi energy level, TDOS and PDOS peaks can be done in the context of density of states (DOS) analysis.76 The changes that occur due to interactions between the NO2 gas molecule and the surfaces can be visualized using DOS. Insight into the electronic pattern and the most permissible energy for the HOMO to LUMO transition as a result of interactions can be gained using the DOS plots.77 Upon adsorption, the changes in electronic properties of the adsorbent materials can be understood from the total density of states (TDOS). The changes in the conductivity of a material can be shown by the shift in the TDOS peaks and changes in their intensity. The Multiwfn function analyser 3.7 and Origin software package were used to plot the DOS for the nano-surfaces after adsorption, as shown in Fig. 8. It can be seen from the plot in Fig. 8 that the functionalization of the surface leads to an increase in the electronic state near the Fermi energy level. The drop around the Fermi energy level after adsorption suggests an increase in conductivity and sensitivity of the surfaces to NO2 gas.78 The morphology of the functionalized surfaces and the non-overlapping area are the reasons for the similarity in their DOS plots. The wide region of the DOS near the Fermi energy level is an indication that there are more electrons in the HOMO that are readily available to conduct energy upon their transition. Furthermore, higher conductivity was observed for the transition metal-functionalized surfaces, which can be attributed to the high Fermi energy level computed. Thus, the functionalized surfaces have an advantage over the bare Zr@SiCNT surface. Lastly, the individual contribution of the atomic orbitals of the complexes results in the total density of states (TDOS) and the partial density of state (PDOS) in the plot. Due to the unfavorable overlapping within the orbital phase, the anti-bonding characteristics are shown using the overlap partial density of states (OPDOS).3.7 Molecular dynamic simulations
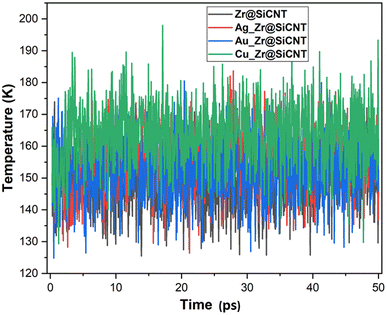
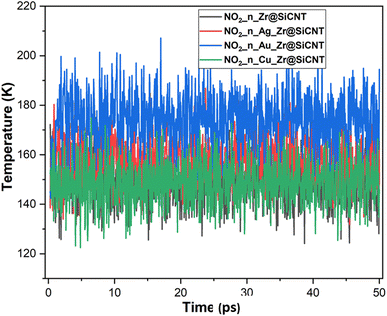
The structural, dynamical and thermodynamic behaviors of the studied systems were further studied using molecular dynamic (MD) simulations. Information on how the NO2 molecule approaches, engages, and adheres to the surface of the bare and TM-functionalized zirconium-doped silicon carbide nanotubes (Zr@SiCNT) was obtained using MD simulations.79,80 Given that metal dopants have a strong influence on the sensing properties of surfaces, structural modifications using these dopants becomes a key part in deigning materials with enhanced functionalities. Hence, MD simulations were performed to gain further insight into how enhanced adsorption potential and stability of these materials can be attained through structural modifications. Briefly, MD simulations were performed to determine the optimal NO2 detection attributes. The results of the simulation calculations such as total, potential, and kinetic energies are presented in Tables S1–S3 of the ESI,† corresponding to the surfaces, n-site, and o-site of adsorption, respectively. It can be observed that upon adsorption, the total energy and potential energy values increase (see Tables S1–S3†). According to Table S2,† the initial total energies of 288.863, 361.520, 222.790, and 187.956 kcal mol−1 were determined for the Z1-n, A1-n, A2-n, and C1-n complexes in the n-site of adsorption, respectively. Similarly, for the o-site of adsorption, the Z1-o, A1-o, A2-o, and C1-o complexes reflect the initial total energy corresponding to 146.233, 305.996, 213.363, and 329.161 kcal mol−1. Furthermore, the initial potential energy values were 199.146, 270.915, 132.186 and 97.351 kcal mol−1 for the Z1-n, A1-n, A2-n, and C1-n complexes, respectively. However, upon simulation, the potential energy increased, where the Z1-n, A1-n, A2-n, and C1-n complexes exhibited values of 241.591, 316.830, 163.826, and 143.941 kcal mol−1, respectively (see Table S1†). Similarly, as observed for the o-site of adsorption, the potential energy increased from the initial values of 56.516, 215.391, 122.758, and 238.556 kcal mol−1 for the Z1-o, A1-o, A2-o, and C1-o complexes to the final values of 102.623, 264.480, 163.526, and 280.427 kcal mol−1, respectively. Due to the above-mentioned adjustment from initial to final values for the potential energy, a favorable interaction is considered to have occurred between the NO2 gas and the labelled surfaces. Initially, the kinetic energy values were found to be as follows: Z1-n complex: 89.716 kcal mol−1; A1-n complex: 90.605 kcal mol−1; A2-n complex: 90.605 kcal mol−1; and C1-n complex: 90.605 kcal mol−1. Alternatively, in the final stage of simulation, the kinetic energy decreased in value to the final value, as follows: Z1-n complex: 47.656 kcal mol−1 A1-n complex: 44.959 kcal mol−1. Similarly, a decrease to 59.131 and 44.342 kcal mol−1 was observed for the A2-n, and C1-n complexes, respectively. At the o-site of adsorption, a consistent decrease from the initial to final value was observed, where the Z1-o, A1-o, A2-o, and C1-o complexes showed a decrease in kinetic energy values from 89.716, 90.605, 90.605, and 90.605 kcal mol−1 to 43.978, 42.037, 50.332, and 49.098 kcal mol−1, respectively (see Table S3†). These variations, as observed during the simulations, can used in the design of new surfaces that are suitable for the fabrication of sensors and drug carriers. These changes can be related to the molecular motion, where a decrease in kinetic energy leads to a decrease in the molecular motion of the system. Fig. 9 shows the dynamic temperature fluctuation for the studied surfaces.The visualization of the fluctuation in dynamic temperature for the adsorption of NO2 gas molecules on the nanotubes is presented in Fig. 10 and 11. The temperature for all the systems was initially recorded as 298 K, with a consistent decrease across all the resulting complexes. In the case of the Z1-n, A1-n, A2-n, and C1-n complexes, a decrease to 158.294, 147.872, 194.483, and 145.840 K, respectively, was confirmed in the n-site of adsorption. Similarly, a consistent decrease was observed for the Z1-o, A1-o, A2-o, and C1-o complexes, where their temperature decreased to 146.078, 138.261, 165.543, and 161.485 K, respectively. These results reveal the thermodynamic characteristics and stability of the studied nanotubes. The mean square displacement (MSD) was employed to determine the bond strength and restoring forces of the nanotubes, wherein high restoring forces are associated with a low MSD, and then decrease in atomic mobility.81 Additionally, a strong and firm bond are attributes of a lower MSD and atom mobility. The small MSD values observed across the systems indicate that the adsorption of NO2 gas will force the structure to deform over time. In summary, the inter-atomic interaction results in a fluctuation in temperature, which indicates the presence of thermal energy.
 | ||
| Fig. 10 Dynamic fluctuation in temperature for the adsorption of NO2 gas molecule on the nanotube at n-site of adsorption. | ||
 | ||
| Fig. 11 Dynamic fluctuation in temperature for the adsorption of NO2 gas molecule on the nanotube at the o-site of adsorption. | ||
3.8 Sensor mechanisms
Here, the parameters that determine the effectiveness of sensor materials are discussed. These parameters are known as sensor parameters or sensor mechanisms. One of the essential parts of developing a sensor device is the knowledge of materials with the potential to sense or detect the targeted adsorbate. Owing to this reason, this section further aims to substantiate and solidify the insights gained on the studied surfaces in the previous sections using the sensor parameters such as charge transfer (Qt), Fermi level (EFL), electrical conductivity (σ), fraction of electron transfer, back donation, and sensor reusability, which is usually measured using the recovery time.| Qt = Qadsorption − Qisolated | (8) |
The portion or part of electrons that are transferred from the adsorbent to the adsorbate as a result of the adsorption process can be quantified by the FET values. Also, the extent of electron donation from a gas molecule to the adsorbent material is known as back donation. In fact, the relationship between the FET and back donation is that back donation is the reverse process of FET, which is the transfer of electrons back from the gas molecule to the surface. Gómez and team shed light on how the contact between the NO2 gas molecule and bare and functionalized SiCNT surfaces may be controlled by an electrical back-donation mechanism.86 The fraction of electron transferred (ΔN) and its back donation was computed based on the Pearson theory. The fraction of electron transferred (ΔN) is calculated using eqn (9), as follows:
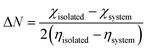 | (9) |
 | (10) |
In a sensor material, back-donation is considered good when η > 0 and ΔEBack-donation < 0. Based on the contact with the surface, the stability can be compared given that it increases with an increase in the chemical hardness of the system. A higher FET value reflects the transfer of electrons from the adsorbent to the adsorbate, resulting in strong adsorption, with an increase in the adsorption potential. Similarly, a higher back donation value suggests good electron donation from the NO2 gas molecule back to the SiCNT surfaces, thereby making the complex stable. The calculated FET (ΔN) and ΔEBack-donation values are summarized in Table 2. According to Table 2, all the complexes reflect relatively high ΔEBack-donation values. The ΔEBack-donation value of −0.262 eV was observed for adsorption on Cu_Zr@SiCNT in the n-site of adsorption. Similarly, the Cu-functionalized Zr@SiCNT surface has a ΔE value of −0.312 eV in the o-site of adsorption. According to Table 2, all the studied complexes exhibit high FET values. The FET values of 64.569 and 57.429 eV were recorded for the C1-n and C1-o complexes, respectively, suggesting efficient electron transfer from the nanotube to the NO2 gas molecule and strong adsorption and enhanced adsorption capacity.
| σ = AT2/3e(Eg/2KT) | (11) |
| τ = V0−1e−Ead/KT | (12) |
| System | Ead (eV) | τ (s) |
|---|---|---|
| NO2_n_Zr@SiCNT (Z1-n) | −2.19016 | 2.6227 × 1028 |
| NO2_n_Ag_Zr@SiCNT (A1-n) | −2.24815 | 3.0827 × 1029 |
| NO2_n_Au_Zr@SiCNT (A2-n) | −2.26472 | 6.2335 × 1028 |
| NO2_n_Cu_Zr@SiCNT (C1-n) | −2.92501 | 9.554 × 1041 |
| NO2_o_Zr@SiCNT (Z1-o) | −2.42173 | 4.9238 × 1032 |
| NO2_o_Ag_Zr@SiCNT (A1-o) | −2.57823 | 3.806 × 1035 |
| NO2_o_Au_Zr@SiCNT (A2-o) | −2.51232 | 2.3127 × 1034 |
| NO2_o_Cu_Zr@SiCNT (C1-o) | −3.7801 | 5.7626 × 1057 |
3.9 Comparative study on previous adsorption of NO2 gas pollutant
This section focuses on the properties of a state-of-the-art NO2 detecting material to show how much the Cu-functionalized SiCNT outperforms the state-of-the-art material. Related theoretical literature was gathered, analyzed, and compared to the present work. In most cases, the Cu-functionalized SiCNT outperforms the state-of-the-art material (see Table 7).| Previous studies on NO2 gas adsorption | Ref. |
|---|---|
| Title: A first-principle investigation of NO2 adsorption behavior on Co, Rh, and Ir-embedded graphitic carbon nitride: looking for highly sensitive gas sensor | 90 |
| There are cases where the adsorption energy was moderate (−0.231, −2.63, and −1.69 eV for pristine/NO2, Co/NO2, and Rh/NO2, respectively); however, the large negative adsorption energy of −4.47 eV for the Ir/NO2 complex, with the recovery time of 3.94 × 1063 s will prolong the recovery of the NO2 gas pollutant, thereby affecting the reusability of device | |
| Title: Adsorption of CO, NH3, NO, and NO2 on pristine and defective g-GaN: improved gas sensing and functionalization | 91 |
| The adsorption energy of −0.364 eV was obtained for NO2 gas on the pristine g-GaN surface. However, the adsorption energy was enhanced when the NO2 gas was absorbed on defective g-GaN surface. Due to the aforementioned small energy, the present work has a relatively higher adsorption as compared to this, showing that Cu-functionalized SiCNT outperforms the studied pristine and defective g-GaN surfaces | |
| Title: Enhancing the sensing performance of zigzag graphene nanoribbon to detect NO, NO2, and NH3 gases | 92 |
| Upon NO2 gas adsorption on ZGNR, ZGNR–O, ZGNR–OH, and ZGNR–O–OH, the adsorption energy obtained was −0.225, −0.212, −0.618, and −0.953 eV respectively. However, although the adsorption was improved via functionalization with small molecules, it was lower than that obtained in the present work. The Cu-functionalized SiCNT surface shows stronger adsorption towards NO2 | |
| Title: Selective adsorption function of B16C16 nano-cage for H2O, CO, CH4 and NO2 | 93 |
| NO2@B16C16 complex does not only reflect a very high adsorption energy of −16.6 eV, indicating very strong adsorption, but also exhibits the highest energy gap of 3.69 eV. This does not correlate with the mechanistic property of a sensor material, according to its lowest conductivity among the studied systems. Besides, the NO2 gas will be prolonged by over four-times than that on the Cu-functionalized SiCNT surface during recovery. Hence, the Cu-functionalized SiCNT surface exhibits a better sensing performance |
4 Conclusions
The adsorption of NO2 gas molecules on bare and transition metal-functionalized SiCNT surfaces was investigated using density functional theory (DFT) computation at the B3LYP-D3(BJ)def2svp level of theory. To determine the best adsorption configurations, different sites of adsorption were considered, wherein the n-site and o-site of adsorption were used. Insights into the sensing attributes of the bare and transition metal-functionalized SiCNT surfaces were gained through electronic descriptors such as the FMO and NBO analyses; the study of weak interactions such as QTAIM and RDG analyses; the knowledge of the electronic pattern via TDOS and PDOS peaks, DOS analysis; and sensor mechanisms to ascertain the results obtained from these investigations. Herein, the following conclusions were drawn:(1) Due to adsorption, the morphology of the nanotubes are altered, as seen from the shortened and elongated bond lengths.
(2) The adsorption phenomenon is best described as chemisorption, owing to the negative adsorption enthalpy. The Cu-functionalized surface reflects the greatest adsorption energies of −3.780 and −2.925 eV, corresponding to the o-site and n-site of adsorption, respectively, which implies that the Cu-functionalized surface is the best at adsorbing NO2 gas among the studied surfaces.
(3) The C1-o complex exhibits the lowest energy gap of 2.095 eV, indicating that the Cu-functionalized surface is the most stable at the o-site of adsorption. Also, a relatively smaller energy gap was observed in the other complexes such as A2-n and C1-n, depicting their stability compared to the other surfaces.
(4) In most cases, partial covalent interactions were observed, except when strong and non-covalent interactions were recorded. The stability of the Cu-functionalized surface in both sites of adsorption was due to the small ellipticity (ε) values found in the C1-o complex (0.056, 0.022) and C1-n complex (0.000 and 0.358), respectively.
(5) The green colour of the 3D RDG maps indicates that all the complexes have low van der Waals interactions.
(6) The sensor mechanisms confirmed that the Cu-functionalized surface exhibits the best sensing attribute, through its superior sensitivity, conductivity, and enhanced adsorption capacity.
Ethical statement
We want to clarify that our research did not require ethical approval, and we have accordingly updated the statement to reflect this information.Data availability
All information regarding to this study are presented in this document and the ESI.†Author contributions
Ismail O. Amodu: investigation, methodology, supervision. Faith O. Akor: writing, analysis and manuscript first draft: Innocent Benjamin and Oluwatobi A. Olaiya: visualization, analysis and writing. Miracle N. Ogbogu: analysis, reviewing, writing, and editing. Adedapo S. Adeyinka: resources and software, Hitler Louis: project conceptualization, design, resources and supervision.Conflicts of interest
All authors declare zero financial or inter-personal conflict of interest that could have influenced the research work or results reported in this research paper.Acknowledgements
The authors would like to acknowledge the Centre for High Performance Computing (CHPC), South Africa for providing computational resources for this research project.References
- C. Mattiuzzi and G. Lippi, Worldwide disease epidemiology in the older persons, Eur. Geriatr. Med., 2020, 11, 147–153 CrossRef PubMed.
- Z. Yang, M. Wang, Z. Feng, Z. Wang, M. Lv, J. Chang and C. Wang, Human Microplastics Exposure and Potential Health Risks to Target Organs by Different Routes: A Review, Curr. Pollut. Rep., 2023, 9(3), 468–485 CrossRef CAS.
- G. P. Bălă, R. M. Râjnoveanu, E. Tudorache, R. Motișan and C. Oancea, Air pollution exposure—the (in) visible risk factor for respiratory diseases, Environ. Sci. Pollut. Res., 2021, 28, 19615–19628 CrossRef PubMed.
- H. Hettiarachchi and J. N. Meegoda, Microplastic pollution prevention: the need for robust policy interventions to close the loopholes in current waste management practices, Int. J. Environ. Res. Public Health, 2023, 20(14), 6434 CrossRef PubMed.
- R. Nieder and D. K. Benbi, Reactive nitrogen compounds and their influence on human health: an overview, Rev. Environ. Health, 2022, 37(2), 229–246 CrossRef CAS PubMed.
- P. Maciejczyk, L. C. Chen and G. Thurston, The role of fossil fuel combustion metals in PM2. 5 air pollution health associations, Atmosphere, 2021, 12(9), 1086 CrossRef CAS.
- I. Manisalidis, E. Stavropoulou, A. Stavropoulos and E. Bezirtzoglou, Environmental and health impacts of air pollution: a review, Front. Public Health, 2020, 8, 14 CrossRef PubMed.
- S. K. Bhavneek Sarwal and H. Singh, Synthesis of iron-based metal-organic framework for heavy metal adsorption from water, Recent Trends in Chemistry and Futuristic Catalysts, 2022, vol. 1 Search PubMed.
- I. B. Abaje, Y. Bello and S. A. Ahmad, A review of air quality and concentrations of air pollutants in Nigeria, J. Appl. Sci. Environ. Manage., 2020, 24(2), 373–379 CAS.
- P. Kumar, A. B. Singh, T. Arora, S. Singh and R. Singh, Critical review on emerging health effects associated with the indoor air quality and its sustainable management, Sci. Total Environ., 2023, 872, 162163 CrossRef CAS PubMed.
- G. D. Albano, R. P. Gagliardo, A. M. Montalbano and M. Profita, Overview of the mechanisms of oxidative stress: impact in inflammation of the airway diseases, Antioxidants, 2022, 11(11), 2237 CrossRef CAS PubMed.
- Y. Khan, H. Sadia, S. Z. Ali Shah, M. N. Khan, A. A. Shah, N. Ullah and M. I. Khan, Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: a review, Catalysts, 2022, 12(11), 1386 CrossRef CAS.
- K. Ghosal and S. Ghosh, Biodegradable polymers from lignocellulosic biomass and synthetic plastic waste: an emerging alternative for biomedical applications, Mater. Sci. Eng., R, 2023, 156, 100761 CrossRef.
- F. Mollarasouli, E. Zor, G. Ozcelikay and S. A. Ozkan, Magnetic nanoparticles in developing electrochemical sensors for pharmaceutical and biomedical applications, Talanta, 2021, 226, 122108 CrossRef CAS PubMed.
- M. D. Ganji, S. Jameh-Bozorgi and M. Rezvani, A comparative study of structural and electronic properties of formaldehyde molecule on monolayer honeycomb structures based on vdW-DF prospective, Appl. Surf. Sci., 2016, 384, 175–181 CrossRef CAS.
- S. Panda, C. K. Biswas and S. Paul, A comprehensive review on the preparation and application of calcium hydroxyapatite: a special focus on atomic doping methods for bone tissue engineering, Ceram. Int., 2021, 47(20), 28122–28144 CrossRef CAS.
- D. Guo, H. Li, Z. Xu and Y. Nie, Development of pyrene-based MOFs probe for water content and investigations on their mechanochromism and acidochromism, J. Alloys Compd., 2023, 968, 172004 CrossRef CAS.
- H. Li, X. Xu, Y. Liu, Y. Hao and Z. Xu, Fluorophore molecule loaded in Tb-MOF for dual-channel fluorescence chemosensor for consecutive visual detection of bacterial spores and dichromate anion, J. Alloys Compd., 2023, 944, 169138, DOI:10.1016/j.jallcom.2023.169138.
- M. J. Nirmala, U. Kizhuveetil, A. Johnson, G. Balaji, R. Nagarajan and V. Muthuvijayan, Cancer nanomedicine: a review of nano-therapeutics and challenges ahead, RSC Adv., 2023, 13(13), 8606–8629 RSC.
- M. D. Ganji and M. Rezvani, Boron nitride nanotube based nanosensor for acetone adsorption: a DFT simulation, J. Mol. Model., 2013, 19, 1259–1265 CrossRef CAS PubMed.
- (a) T. Banibairami, S. Jamehbozorgi, R. Ghiasi and M. Rezvani, Sensing behavior of hexagonal-aluminum nitride to phosgene molecule based on van der Waals-density functional theory and molecular dynamic simulation, Russ. J. Phys. Chem. A, 2020, 94, 581–589 CrossRef CAS; (b) S. Bhaskar and S. Lim, Engineering protein nanocages as carriers for biomedical applications, NPG Asia Mater., 2017, 9(4), e371 CrossRef PubMed.
- H. Li, Y. Wang, F. Jiang, M. Li and Z. Xu, A dual-function [Ru(bpy)3]2+ encapsulated metal organic framework for ratiometric Al3+ detection and anticounterfeiting application, Dalton Trans., 2023, 52(12), 3846–3854, 10.1039/D2DT03388G.
- S. A. Mazari, E. Ali, R. Abro, F. S. A. Khan, I. Ahmed, M. Ahmed and A. Shah, Nanomaterials: applications, waste-handling, environmental toxicities, and future challenges-a review, J. Environ. Chem. Eng., 2021, 9(2), 105028 CrossRef CAS.
- D. Song, X. Chen, M. Wang, Z. Wu and X. Xiao, 3D-printed flexible sensors for food monitoring, Chem. Eng. J., 2023, 474, 146011, DOI:10.1016/j.cej.2023.146011.
- R. A. Dickman and D. S. Aga, A review of recent studies on toxicity, sequestration, and degradation of per-and polyfluoroalkyl substances (PFAS), J. Hazard. Mater., 2022, 436, 129120 CrossRef CAS PubMed.
- R. Paul, B. Das and R. Ghosh, Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: a recent progress, J. Alloys Compd., 2023, 168943 CrossRef CAS.
- X. Wang, H. Feng, T. Chen, S. Zhao, J. Zhang and X. Zhang, Gas sensor technologies and mathematical modelling for quality sensing in fruit and vegetable cold chains: a review, Trends Food Sci. Technol., 2021, 110, 483–492, DOI:10.1016/j.tifs.2021.01.073.
- R. Saeed, H. Feng, X. Wang, X. Zhang and Z. Fu, Fish quality evaluation by sensor and machine learning: a mechanistic review, Food Control, 2022, 137, 108902, DOI:10.1016/j.foodcont.2022.108902.
- C. V. Sudheep, A. Verma, P. Jasrotia, J. J. L. Hmar, R. Gupta, A. S. Verma and T. Kumar, Revolutionizing gas sensors: the role of composite materials with conducting polymers and transition metal oxides, Results Chem., 2023, 101255 Search PubMed.
- M. Jiang, C. Wang, X. Zhang, C. Cai, Z. Ma, J. Chen and D. Chen, A cellular nitric oxide sensor based on porous hollow fiber with flow-through configuration, Biosens. Bioelectron., 2021, 191, 113442, DOI:10.1016/j.bios.2021.113442.
- R. Paul, B. Das and R. Ghosh, Novel approaches towards design of metal oxide based hetero-structures for room temperature gas sensor and its sensing mechanism: a recent progress, J. Alloys Compd., 2023, 168943 CrossRef CAS.
- Z. Li, Y. Chen, Z. Wang, Y. Zhao, Q. Xia, J. Qiu and J. Wang, Ionic liquid hybrid metal–organic frameworks for efficient adsorption and selective separation of ammonia at high temperature, Chem. Eng. J., 2023, 464, 142728, DOI:10.1016/j.cej.2023.142728.
- H. Xie, X. Yao, X. Yu, L. Mao, Y. Zeng, F. Wu and G. He, Flotation Performance and Adsorption Mechanism of Cerussite with Phenylpropenyl Hydroxamic Acid Collector, Minerals, 2023, 13(10), 1315, DOI:10.3390/min13101315.
- P. Xu, X. Liu, Y. Zhao, D. Lan and I. Shin, Study of graphdiyne biomimetic nanomaterials as fluorescent sensors of ciprofloxacin hydrochloride in water environment, Desalin. Water Treat., 2023, 302, 129–137, DOI:10.5004/dwt.2023.29723.
- A. Cheng, J. Zhao, X. A. Wang, Z. Lu, Y. Qi and J. Sun, Hybrid 1D/3D-Structured Perovskite as a Highly Selective and Stable Sensor for NO2 Detection at Room Temperature, Molecules, 2023, 28(6), 2615 CrossRef CAS PubMed.
- A. D. Nwagu, H. Louis, H. O. Edet, I. Benjamin, V. N. Osabor and A. S. Adeyinka, Computational study on nickel doped encapsulated Mg, K, Ca on pristine C24 nanocage for gas sensing applications, Mater. Sci. Semicond. Process., 2023, 157, 107334 CrossRef CAS.
- B. E. Inah, J. F. Eze, H. Louis, H. O. Edet, T. E. Gber, E. A. Eno and A. S. Adeyinka, Adsorption and gas-sensing investigation of oil dissolved gases onto nitrogen and sulfur doped graphene quantum dots, Chem. Phys. Impact, 2023, 7, 100265 CrossRef.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView, Version 6.1, Semichem Inc., Shawnee Mission, KS, 2016 Search PubMed.
- T. Lu and F. Chen, Multiwfn: A Multifunctional Wavefunction Analyzer, J. Comput. Chem., 2012, 33, 580–592, DOI:10.1002/jcc.22885.
- W. Humphrey, A. Dalke and K. Schulten, VMD - Visual Molecular Dynamics, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- Chemcraft – Graphical Software for Visualization of Quantum Chemistry Computations, Version 1.8, Build 682, https://www.chemcraftprog.com Search PubMed.
- H. Louis, D. E. Charlie, I. O. Amodu, I. Benjamin, T. E. Gber, E. C. Agwamba and A. S. Adeyinka, Probing the reactions of thiourea (CH4N2S) with metals (X = Au, Hf, Hg, Ir, Os, W, Pt, and Re) anchored on fullerene surfaces (C59X), ACS Omega, 2022, 7(39), 35118–35135 CrossRef CAS PubMed.
- M. D. Esrafili, Boron and nitrogen co-doped graphene nanosheets for NO and NO2 gas sensing, Phys. Lett. A, 2019, 383(14), 1607–1614 CrossRef CAS.
- M. Ali and N. Tit, Adsorption of NO and NO2 molecules on defected-graphene and ozone-treated graphene: first-principles analysis, Surf. Sci., 2019, 684, 28–36 CrossRef CAS.
- T. O. Unimuke, H. Louis, W. Emori, P. S. Idante, E. C. Agwamba, I. C. Nwobodo and C. A. Anyama, Spectroscopic and molecular electronic property investigation of 2-phenylpyrimidine-4, 6-diamine via 1H NMR, UV-vis, FT-Raman, FT-IR, and DFT approach, J. Mol. Struct., 2022, 1263, 133195 CrossRef CAS.
- T. E. Gber, H. Louis, O. C. Ngana, I. O. Amodu, E. E. Ekereke, I. Benjamin and A. Adeyinka, Yttrium-and zirconium-decorated Mg12O12–X (X = Y, Zr) nanoclusters as sensors for diazomethane (CH2N2) gas, RSC Adv., 2023, 13(36), 25391–25407 RSC.
- W. Q. Lin, S. T. Xiao, H. B. Li, Y. G. Ouyang, L. Y. Wang, Q. Wang and G. H. Chen, Theoretical study of NO2 adsorption on SiCNT and P-doped SiCNT, Phys. E, 2021, 127, 114519 CrossRef CAS.
- L. Sun, T. Liang, X. Sun, C. Li and C. Zhang, Temperature self-compensating and high-sensitivity FBG inclination sensor based on the sliding mass principle, Opt. Fiber Technol., 2023, 81, 103539, DOI:10.1016/j.yofte.2023.103539.
- H. Pasdar, N. Elmi Fard and M. Rezvani, Fabrication of MoS2/Bi2S3 heterostructure for photocatalytic degradation of Metronidazole and Cefalexin and antibacterial applications under NIR light: experimental and theoretical approach, Appl. Phys. A: Mater. Sci. Process., 2023, 129(5), 380 CrossRef CAS.
- A. Rahmanzadeh, M. Rezvani, M. D. Ganji and M. T. Moghim, Corrosion protection performance of laurhydrazide N′-propan-3-one (LHP) adsorbed on zinc surface: a DFT-MD simulation investigation, Mater. Today Commun., 2023, 36, 106946 CrossRef CAS.
- T. E. Gber, H. Louis, O. C. Ngana, I. O. Amodu, E. E. Ekereke, I. Benjamin and A. Adeyinka, Yttrium-and zirconium-decorated Mg12O12–X (X = Y, Zr) nanoclusters as sensors for diazomethane (CH2N2) gas, RSC Adv., 2023, 13(36), 25391–25407 RSC.
- R. Kartika, F. H. Alsultany, A. T. Jalil, M. Z. Mahmoud, M. N. Fenjan and H. Rajabzadeh, Ca12O12 nanocluster as highly sensitive material for the detection of hazardous mustard gas: density-functional theory, Inorg. Chem. Commun., 2022, 137, 109174 CrossRef CAS.
- A. A. Tagiling, W. M. Khairul, R. Rahamathullah, V. Sevakumaran, M. Mohammed and S. Saidin, Computational and Experimental Investigation of Antibacterial Properties of Some Fluorinated Thioureas, Polycyclic Aromat. Compd., 2023, 1–19 Search PubMed.
- N. Montazeri, I. Salahshoori, P. Feyzishendi, F. S. Miri, M. M. Mohseni and H. A. Khonakdar, pH-sensitive adsorption of gastrointestinal drugs (famotidine and pantoprazole) as pharmaceutical pollutants by using the Au-doped@ZIF-90-glycerol adsorbent: insights from computational modeling, J. Mater. Chem. A, 2023, 11(47), 26127–26151 RSC.
- V. Choudhary, A. Bhatt, D. Dash and N. Sharma, DFT calculations on molecular structures, HOMO–LUMO study, reactivity descriptors and spectral analyses of newly synthesized diorganotin (IV) 2-chloridophenylacetohydroxamate complexes, J. Comput. Chem., 2019, 40(27), 2354–2363 CrossRef CAS PubMed.
- A. Mondal, L. R. Domingo and N. Acharjee, Revealing the influence of tether length on the intramolecular [3+ 2] cycloaddition reactions of nitrones from the molecular electron density theory perspective, J. Phys. Org. Chem., 2024, 37(1), e4574 CrossRef CAS.
- P. Zhao, J. Zhu, M. Li, G. Shao, H. Lu, H. Wang and J. He, Theoretical and experimental investigations on the phase stability and fabrication of high-entropy monoborides, J. Eur. Ceram. Soc., 2023, 43(6), 2320–2330, DOI:10.1016/j.jeurceramsoc.2023.01.026.
- M. Khalid, H. M. Lodhi, M. U. Khan and M. Imran, Structural parameter-modulated nonlinear optical amplitude of acceptor–π–D–π–donor-configured pyrene derivatives: a DFT approach, RSC Adv., 2021, 11(23), 14237–14250 RSC.
- M. A. Grishina and V. A. Potemkin, Topological analysis of electron density in large biomolecular systems, Curr. Drug Discovery Technol., 2019, 16(4), 437–448 CrossRef CAS PubMed.
- X. An, G. Başar, M. Stephanov and H. U. Yee, Fluctuation dynamics in a relativistic fluid with a critical point, Phys. Rev. C, 2020, 102(3), 034901 CrossRef CAS.
- S. R. Gadre, C. H. Suresh and N. Mohan, Electrostatic potential topology for probing molecular structure, bonding and reactivity, Molecules, 2021, 26(11), 3289 CrossRef CAS PubMed.
- R. F. W. Bader, Atoms in molecules, Acc. Chem. Res., 1985, 18(1), 9–15 CrossRef CAS.
- C. Gharbi, H. Louis, I. O. Amodu, I. Benjamin, W. Fujita, C. B. Nasr and L. Khedhiri, Crystal structure analysis, magnetic measurement, DFT studies, and adsorption properties of novel 1-(2,5-dimethyphenyl)piperazine tetrachlorocobaltate hydrate, Mater. Today Commun., 2023, 34, 104965 CrossRef CAS.
- P. L. Popelier, Non-covalent interactions from a Quantum Chemical Topology perspective, J. Mol. Model., 2022, 28(9), 276 CrossRef PubMed.
- D. Wang, X. Wang, M. L. Jin, P. He and S. Zhang, Molecular level manipulation of charge density for solid-liquid TENG system by proton irradiation, Nano Energy, 2022, 103, 107819, DOI:10.1016/j.nanoen.2022.107819.
- M. A. Mumit, T. K. Pal, M. A. Alam, M. A. A. A. A. Islam, S. Paul and M. C. Sheikh, DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2,4,5-trimethoxyphenylmethylene)hydrazinecarbodithioate, J. Mol. Struct., 2020, 1220, 128715 CrossRef CAS PubMed.
- H. Xie, X. Yao, X. Yu, L. Mao, Y. Zeng, F. Wu and G. He, Flotation Performance and Adsorption Mechanism of Cerussite with Phenylpropenyl Hydroxamic Acid Collector, Minerals, 2023, 13(10), 1315, DOI:10.3390/min13101315.
- M. Rezvani, M. Astaraki, A. Rahmanzadeh and M. D. Ganji, Theoretical assessments on the interaction between amino acids and the g-Mg3N2 monolayer: dispersion corrected DFT and DFT-MD simulations, Phys. Chem. Chem. Phys., 2021, 23(32), 17440–17452 RSC.
- P. Niknam, S. Jamehbozorgi, M. Rezvani and V. Izadkhah, Understanding delivery and adsorption of Flutamide drug with ZnONS based on: dispersion-corrected DFT calculations and MD simulations, Phys. E, 2022, 135, 114937 CrossRef CAS.
- L. Luo, L. Chu and T. F. Fwa, Molecular dynamics analysis of oxidative aging effects on thermodynamic and interfacial bonding properties of asphalt mixtures, Constr. Build. Mater., 2021, 269, 121299 CrossRef CAS.
- D. Hu, J. Pei, R. Li, J. Zhang, Y. Jia and Z. Fan, Using thermodynamic parameters to study self-healing and interface properties of crumb rubber modified asphalt based on molecular dynamics simulation, Front. Struct. Civ. Eng., 2020, 14, 109–122 CrossRef.
- I. Benjamin, H. Louis, G. A. Okon, S. W. Qader, L. E. Afahanam, C. F. Fidelis and A. L. E. Manicum, Transition metal-decorated B12N12–X (X = Au, Cu, Ni, Os, Pt, and Zn) nanoclusters as biosensors for carboplatin, ACS Omega, 2023, 8(11), 10006–10021 CrossRef CAS PubMed.
- R. A. Timothy, H. Louis, E. A. Adindu, T. E. Gber, E. C. Agwamba, O. E. Offiong and A. M. Pembere, Elucidation of collagen amino acid interactions with metals (B, Ni) encapsulated graphene/PEDOT material: insight from DFT calculations and MD simulation, J. Mol. Liq., 2023, 390, 122950 CrossRef CAS.
- C. Tang, R. Chen, J. Zhang, X. Peng, B. Chen and L. Zhang, A review on the research progress and future development of nano-modified cellulose insulation paper, IET Nanodielectrics, 2022, 5(2), 63–84 CrossRef.
- H. Louis, I. O. Amodu, T. O. Unimuke, T. E. Gber, B. B. Isang and A. S. Adeyinka, Modeling of Ca12O12, Mg12O12, and Al12N12 nanostructured materials as sensors for phosgene (Cl2CO), Mater. Today Commun., 2022, 32, 103946 CrossRef CAS.
- H. Louis, M. Patrick, I. O. Amodu, I. Benjamin, I. J. Ikot, G. E. Iniama and A. S. Adeyinka, Sensor behavior of transition-metals (X = Ag, Au, Pd, and Pt) doped Zn11-X-O12 nanostructured materials for the detection of serotonin, Mater. Today Commun., 2023, 34, 105048 CrossRef CAS.
- M. H. Mahmoudian, A. Azari, A. Jahantigh, M. Sarkhosh, M. Yousefi, S. A. Razavinasab and M. Ghasemian, Statistical modeling and optimization of dexamethasone adsorption from aqueous solution by Fe3O4@NH2-MIL88B nanorods: isotherm, kinetics, and thermodynamic, Environ. Res., 2023, 236, 116773 CrossRef CAS PubMed.
- A. Benbella, H. Jabraoui, I. Matrane and M. H. Mazroui, Exploring adsorption behavior of sulfur and nitrogen compounds on transition metal-doped Cu (100) surfaces: insights from DFT and MD simulations, Phys. Chem. Chem. Phys., 2023, 25(40), 27553–27565 RSC.
- S. K. Tiwari, S. K. Pandey, R. Pandey, N. Wang, M. Bystrzejewski, Y. K. Mishra and Y. Zhu, Stone–Wales Defect in Graphene, Small, 2023, 19(44), 2303340 CrossRef CAS PubMed.
- J. Zhang, L. Liu, Y. Yang, Q. Huang, D. Li and D. Zeng, A review on two-dimensional materials for chemiresistive-and FET-type gas sensors, Phys. Chem. Chem. Phys., 2021, 23(29), 15420–15439 RSC.
- C. D. Gómez-Carmona, D. Rojas-Valverde, M. Rico-González, V. De Oliveira, L. Lemos, C. Martins and J. Pino-Ortega, Crucial workload variables in female-male elite Brazilian Beach Handball: an exploratory factor analysis, Biol. Sport, 2023, 40(2), 345–352 CrossRef PubMed.
- S. Sharma, A. Verma, S. M. Rangappa, S. Siengchin and S. Ogata, Recent progressive developments in conductive-fillers based polymer nanocomposites (CFPNC’s) and conducting polymeric nanocomposites (CPNC’s) for multifaceted sensing applications, J. Mater. Res. Technol., 2023, 5921–5974 CrossRef CAS.
- A. Paghi, S. Mariani and G. Barillaro, 1D and 2D field effect transistors in gas sensing: a comprehensive review, Small, 2023, 19(15), 2206100 CrossRef CAS PubMed.
- R. Gómez, J. Solla-Gullón, J. M. Pérez and A. Aldaz, Nanoparticles-on-electrode approach for in situ surface-enhanced Raman spectroscopy studies with platinum-group metals: examples and prospects, J. Raman Spectrosc., 2005, 36(6–7), 613–622 CrossRef.
- H. Louis, E. E. Ekereke, B. B. Isang, A. I. Ikeuba, I. O. Amodu, T. E. Gber and E. C. Agwamba, Assessing the performance of Al12N12 and Al12P12 nanostructured materials for alkali metal ion (Li, Na, K) batteries, ACS Omega, 2022, 7(50), 46183–46202 CrossRef CAS PubMed.
- H. Louis, B. B. Isang, T. O. Unimuke, T. E. Gber, I. O. Amodu, A. I. Ikeuba and A. S. Adeyinka, Modeling of Al12N12, Mg12O12, Ca12O12, and C23N nanostructured as potential anode materials for sodium-ion battery, J. Solid State Electrochem., 2023, 27(1), 47–59 CrossRef CAS.
- X. Yu, L. Mao, H. Xie, X. Yao, G. He and Z. Huang, Flotation Behavior and Adsorption Mechanism of Phenylpropyl Hydroxamic Acid As Collector Agent in Separation of Fluorite from Calcite, Langmuir, 2023, 39(16), 5936–5943 CrossRef CAS PubMed.
- H. Basharnavaz, A. Habibi-Yangjeh and S. H. Kamali, A first-principle investigation of NO2 adsorption behavior on Co, Rh, and Ir-embedded graphitic carbon nitride: looking for highly sensitive gas sensor, Phys. Lett. A, 2020, 384(2), 126057 CrossRef CAS.
- Z. Cui, X. Wang, Y. Ding, E. Li, K. Bai, J. Zheng and T. Liu, Adsorption of CO, NH3, NO, and NO2 on pristine and defective g-GaN: improved gas sensing and functionalization, Appl. Surf. Sci., 2020, 530, 147275 CrossRef CAS.
- E. Salih and A. I. Ayesh, Enhancing the sensing performance of zigzag graphene nanoribbon to detect NO, NO2, and NH3 gases, Sensors, 2020, 20(14), 3932 CrossRef CAS PubMed.
- S. Ariaei, H. Basiri and M. Ramezani, Selective adsorption function of B16C16 nano-cage for H2O, CO, CH4 and NO2, Adv. J. Chem., Sect. B, 2020, 2, 18–25 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08796d |
| This journal is © The Royal Society of Chemistry 2024 |