Open Access Article
Open Access ArticleDetection of sulfur mustard simulant by trisaryl phosphoric triamide-based resin using a quartz crystal microbalance sensor†
Jaeyoung Heoa,
Jin Hyun Parka,
Sun Gu Songa,
Seongwoo Leeb,
Seongyeop Limc,
Chang Young Lee c,
Han Yong Bae
c,
Han Yong Bae a and
Changsik Song
a and
Changsik Song *a
*a
aDepartment of Chemistry, Sungkyunkwan University, Suwon 16419, Republic of Korea. E-mail: hjyoung99@g.skku.edu; qkrwlsgus512@gmail.com; mukhon12@naver.com; hybae@skku.edu; songcs@skku.edu
bDepartment of Materials Science and Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea. E-mail: lsw9112128@unist.ac.kr
cSchool of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea. E-mail: lsy981202@unist.ac.kr; cylee@unist.ac.kr
First published on 6th March 2024
Abstract
Chemical warfare agents (CWAs) pose a persistent threat to human safety, and bis(2-chloroethyl) sulfide, or sulfur mustard (SM) is one of the most dangerous substances and is able to cause serious harm. Detecting SM gas is vital, but current methods have high-temperature requirements and limited selectivity, mainly because of the lack of CWA receptor development, and this makes them challenging to use. To address this issue, we present a trisaryl phosphoric triamide-based resin receptor that preferentially interacts with a SM simulant 2-chloroethyl ethyl sulfide (2-CEES) through dipole interactions. The receptor was synthesized through a facile process using an amine and a triethyl phosphate and the properties of its coating were enhanced using epoxy chemistry. The receptor's superior triamide structure was evaluated using a quartz crystal microbalance and reactivity was confirmed by observing the variations in reactivity according to the number of phosphoramides. The receptor showed better reactivity to 2-CEES vapor than to the known poly(epichlorohydrin) and showed selectivity to other volatile organic compounds. Moreover, its durability was evident even 30 days post-coating. The applicability of this receptor extends to array sensors, sound acoustic wave sensors, and chemo-resistive and chemo-capacitive sensors, and it promises advances in chemical warfare agent detection.
1. Introduction
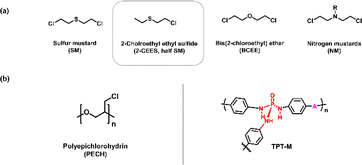
Chemical warfare agents (CWAs), which were created and developed during World Wars I and II, can be classified into several types that include nerve agents, choking agents, and blister agents.1 Nerve agents are chemical compounds that impact the nervous system and induce health effects that are comparable to certain pesticide exposures. Some notable examples of nerve agents are sarin (GB), soman (GD), tabun (GA), and venomous agent X (VX).2 Choking agents, which include substances like ammonia, chlorine, and phosgene, induce intense irritation and inflammation of the respiratory tract and affect the eyes, nose, throat, and lungs.3 These agents produce symptoms such as coughing, wheezing, and difficulty breathing, which are commonly referred to as irritant gas syndrome. Blister agents have corrosive effects on the skin and induce systemic toxicity. Among the blister agents, sulfur mustard (SM) gas stands out as one of the most prevalent and well-known examples (shown in Fig. 1(a)). Exposure to SM can cause extensive harm to the human body, including profound cutaneous blisters, ocular lesions that may lead to permanent blindness, pulmonary injuries that may culminate in respiratory failure, and other severe consequences. Furthermore, the reactivity of SM is predicated on its capacity to alkylate guanine nucleotides within nucleic acids such as DNA and RNA molecules, which impedes cellular division and ultimately induces mortality in living organisms. Nevertheless, current advancements have yet to yield efficacious treatments or antidotes for the toxic effects of SM exposure. Hence, the swift and accurate detection of SM gas is of paramount importance to ensure human safety.In recent years, a range of analytical methodologies has emerged to identify SM and its analogs, including techniques such as gas chromatography/mass spectrometry, immunochemical assays, ion mobility spectrometry, and approaches that involve molecularly imprinted polymers.4,5 However, existing approaches face barriers such as demanding high-temperature conditions and constrained selectivity, which are primarily attributable to the lack of development of superior receptors.6
To date, the availability of receptors for SM gas detection remains considerably limited relative to the field of nerve agent detection.7–13 According to the findings of prior research, the interactions between the probe molecules and SM or 2-chloroethyl ethyl sulfide (CEES) in a nucleophilic attack have been observed to require a relatively long duration.14 Noncovalent interactions, such as sulfur–π and dipole–dipole interactions, may offer a rapid and sensitive detection method, although they have yet to be used for the detection of SM.15,16 Additionally, research has been conducted to detect SM through a reaction that forms a macrocycle using SM's two reactive groups.17 However, interest in gas sensors is growing, and particularly for sensors that are built from metal oxides, primarily due to their compact dimensions, straightforward manufacturing processes, and cost-efficient attributes.18–20 As an example, Yoo et al. engineered ZnO nanoparticles that exhibited a response time of 34 seconds to 1 ppm of 2-CEES at 250 °C.21 Fan et al. synthesized a 2-CEES sensor using WO3/WS2 nanocomposites, and they achieved a response of 1.81 ppm to 5.7 ppm of 2-CEES within a response time of 20 seconds at 240 °C.22 As evident from these examples, the metal oxide-based sensors developed thus far require high operating temperatures. Furthermore, the lack of selectivity can be a huddle to make superior sensor, we necessitating good receptor molecules for SM. Although several investigations are underway, the prevailing choice at present for a receptor material that exhibits optimal performance is PECH (shown in Fig. 1(b)). In prior research endeavors, PECH displayed considerable reactivity toward SM gas when it was incorporated into quartz crystal microbalance (QCM) and surface acoustic wave (SAW) sensors.23,24 Nevertheless, it has shown limited selectivity and has yet to attain a level of performance that is suitable for practical applications. Notwithstanding notable gas detection technology's advancements, the majority of existing sensors continue to exhibit delayed response times, which limits their suitability for real-time gas monitoring. Consequently, the development of novel SM (or its simulant) sensing materials with rapid response capabilities is of utmost importance for timely hazard detection.
Frequently chosen as a simulant for SM gas is 2-CEES, which is also known as “half mustard,” due to its having relevant chemical properties that mirror the actual agent, while being devoid of the associated toxicity (shown in Fig. 1(a)). Hence, 2-CEES exhibits structural and characteristic traits that are comparable to those of SM, yet it demonstrates less reactivity toward the human body. This adequately positions it as a viable surrogate for SM in experimental settings.
In this study, we designed and synthesized a resin receptor having a trisaryl phosphoric triamide structure (shown in Fig. 1(b)). This receptor is designed to detect 2-CEES gas by engaging in possible hydrogen bonding and dipole-induced dipole interactions with 2-CEES, which functions as a mimic of SM agonists. The synthesis of this receptor involved an amine-triethyl phosphate reaction, which made the receptor notably simpler than existing receptors. Moreover, the use of epoxy chemistry significantly enhanced its coating capabilities. Relative to the previously identified poly(epichlorohydrin) receptor, this receptor exhibited a notably heightened responsive to 2-CEES gas when it was evaluated using a QCM instrument. It was subsequently verified that the superior performance of this receptor was attributed to its phosphoramide structure, which is a pivotal factor in detect 2-CEES gas. Furthermore, this receptor displayed notable selectivity toward various volatile organic compounds (VOCs), which could pose a challenge in chemical agent detection. Additionally, it was confirmed that the initial heightened reactivity remained robust, and the receptor exhibited a prolonged lifespan even 30 days following the application of the receptor to the QCM cell.
2. Materials and methods
2.1 Materials and measurements
4,4′-Diaminodiphenyl methane and triethyl phosphate were received from Tokyo Chemical Industry (TCI), and were used as received without further purification. Epichlorohydrin(±) was purchased from Sigma Aldrich and used as purchased without further purification. 1,6-Diaminohexane was obtained from Alfa Aesar. Dimethylformamide (DMF) was purchased from Samchun Chemicals (Seoul, Korea). 2-CEES gas was supplied by Research institute of Gas Analytical Science (RIGAS).Fourier transform infrared (FT-IR) spectra were recorded on a Vertex70 spectrometer (Bruker Optics, MA, USA) equipped with a diamond attenuated total reflection unit. QCM was recorded on a CHI400C.
2.2 Methods
3. Results and discussion
3.1 Synthesis of receptors
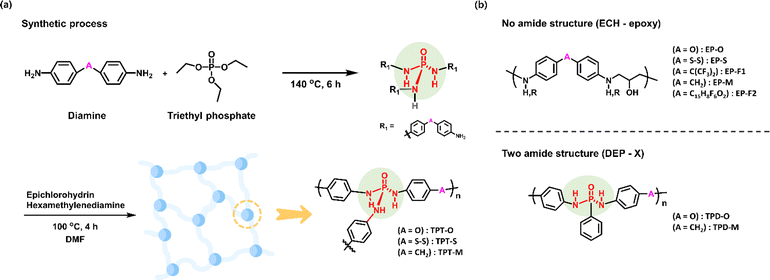
We previously demonstrated the outstanding performance of the phosphotriamide structure in detecting dimethyl methylphosphonate (DMMP), which is a simulant for nerve gas agents.27 The triamide structure of this N-triflyl phosphoric triamide (N-TPT) receptor showed a very fast and sensitive response to the detection of DMMP, proving its excellence as a sensor. Inspired by its chemical structure, we applied these insights in the development of a receptor tailored for the detection of SM or 2-CEES. In particular, we designed the receptor to have a triaryl phosphotriamide structure (shown in Fig. 1(b)) as the key moiety, which could be obtained easily through the reaction between a variety of bis(aryl amine)s and triethyl phosphate (shown in Fig. 2(a)). However, the product obtained by using this synthetic method exhibited poor coating properties when applied to quartz surfaces. To address this limitation, we introduced epichlorohydrin, which is a common epoxy material, and hexamethylenediamine to enhance the coating characteristics of the product. The resultant receptor demonstrated significantly enhanced adhesion when it was applied to quartz surfaces. By following the above-mentioned methods, we prepared triaryl phosphotriamide receptors TPT-O, -S, and -M starting from 4,4′-diaminodiphenyl ether, 4,4′-dithioaniline, and 4,4′-diaminodiphenyl methane, respectively. After the first synthetic process, a yellow-brown sticky liquid was produced. The product was confirmed by Fourier transform infrared spectroscopy (FT-IR) (shown in Fig. S2†). Next, in the second synthetic step, the cured resin was coated onto the QCM cell, and FT-IR analysis confirmed the occurrence of an epoxy curing reaction (shown in Fig. S3†). To validate the superiority of the newly devised receptor structure, additional receptors were synthesized to assess their comparative reactivity. As the phosphoric triamide structure served as the central element of our receptor, we formulated and synthesized a set of comparable receptors by varying the number of amides (shown in Fig. 2(b)). More specifically, the control receptors were categorized into two groups: those lacking an amide, and those featuring a diamide structure. In the case of the receptors that did not have an amide structure, their synthesis and coating involved the introduction of epichlorohydrin into various diamines, which resulted in a product having a structure in which a linear polymer formed due to the reaction of epichlorohydrin with diamine. For the receptors that had a diamide structure, their synthesis was conducted using various diamines in conjunction with diethyl phenylphosphonate, which yielded a product having a structure with two amide groups connected to phenyl groups.3.2 Performance of the TPT-M receptor as a sensor
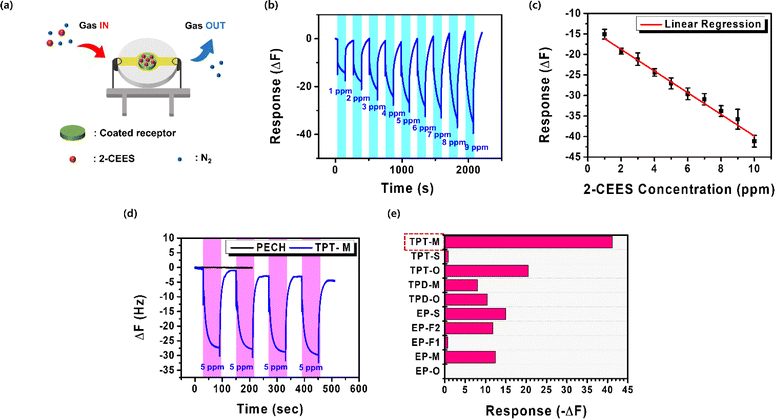
By utilizing a QCM instrument, we assessed the reactivity toward 2-CEES of the triaryl phosphotriamide receptors (TPT-M, -O, and -S) and other control receptors (TPD-M, TPT-O, EP-M, EP-O, EP-S, EP-F1, and EP-F2) (shown in Fig. S4†). Fig. 3(a) illustrates the interaction of the quartz substrate (8 MHz) that was coated with a receptor within the QCM device during exposure to the injected gas, which was a mixture of 2-CEES and N2 gas as a carrier. To enhance the quality of the coating, we combined the receptors that contained terminal amines, epichlorohydrin, and hexamethylenediamine. This mixture was dissolved in DMF, 4 μL of which was subsequently applied to the pre-cleaned QCM quartz cell. The coating process was completed by curing the QCM cell in an oven set to 100 °C for 4 h. We then conducted the measurement of QCM reactivity to 2-CEES using the prepared cells.As illustrated in Fig. 3(b), the instantaneous frequency changes on the QCM were graphed during the exposure of the TPT-M receptor to varying concentrations of 2-CEES, which ranged from 1 to 9 ppm. From the first encounter with 2-CEES at a concentration of 1 ppm, a substantial frequency change of −14.6 Hz was observed within 2.2 min. Subsequently, at concentrations of 2–9 ppm, we observed a gradual increase in frequency changes (−17.6 to −38.3 Hz). From these results, we confirmed that the responsiveness of TPT-M increased proportionally with an increase in the concentration of 2-CEES. Multiple measurements were taken for each concentration, and the average values of the responses were plotted Fig. 3(c), which confirmed that TPT-M responded well to 2-CEES and showed a proportional increase in its reactivity to 2-CEES's concentration (−2.64056 Hz ppm−1). The experimental results demonstrated that our trisaryl phosphoric triamide receptor can rapidly and sensitively detect the SM simulant even under ambient temperature and pressure, unlike other previously developed sensors like MOF or metal oxide,21,22 which necessitated high temperatures ranging from 250 to 240 °C. Additionally, it was confirmed to exhibit higher sensitivity than PECH, which was previously noted for its good 2-CEES detection capability. Furthermore, the TPT-M receptor demonstrated an effective response to 2-CEES gas by consistently exhibiting robust recovery and reproducibility, even under repetitive exposure to 5 ppm of 2-CEES gas (shown in Fig. 3(d)). To validate the superior performance of TPT-M, the triaryl phosphotriamide receptor, we experimented to compare its reactivity with that of PECH, which is a well-established material that is commonly used for the detection of 2-CEES. A same condition of PECH (∼2 mg/1 mL DMF) was applied to a fresh QCM quartz cell, and PECH's reactivity toward 2-CEES (5 ppm) was subsequently assessed. The experimental results indicated that PECH exhibited negligible reactivity to 2-CEES (shown in Fig. 3(d)). However, relative to the established 2-CEES receptor PECH, the TPT-M receptor displayed very high performance and registers significantly improved responses for equivalent 2-CEES gas concentrations.
Furthermore, to assess the structure–property relationship of the phosphotriamide structure we formulated, we conducted a comparative analysis using various control receptors and the QCM reactivity to 2-CEES gas. Our analysis encompassed a group of receptors in which the number of amides was varied, as well as the receptor that featured the triamide structure. A QCM analysis experiment was performed by applying an equal volume (4 μL) of the receptor solutions in DMF (∼0.25 mmol/3 mL) onto separate QCM quartz cells. Subsequently, the cells were exposed to 2-CEES gas having equivalent concentrations (10 ppm), and their responses were compared (shown in Fig. 3(e)). The experimental results revealed that the receptors that lacked an amide structure, namely EP-O, EP-M, EP-F1, EP-F2, and EP-S, displayed frequency changes of 0 Hz, −12.4 Hz, −0.7 Hz, −11.8 Hz, and −14.9 Hz, respectively, which were considerably smaller than that of TPT-M (−41.2 Hz). For the receptors that featured two amide structures, namely TPD-O and TPD-M, the observed frequency changes were −10.0 Hz and −8.0 Hz, respectively, when the receptors were exposed to 2-CEES gas at 10 ppm. In contrast, the receptor TPT-O and TPT-S which featured a triamide structure but a different linker moiety (i.e., ether rather than methylene), exhibited frequency changes of −20.5 Hz and −0.9 Hz in response to 10 ppm of 2-CEES gas, which was a greater change than that of any other control receptors. These results demonstrated the significantly superior performance of the triaryl phosphotriamide structure with 2-CEES relative to control receptors that lacked the triamide structure. Remarkably, the TPT-M structure exhibited at least twice the level of frequency change of the triamide TPTs.
We also calculated the adsorption affinity of the receptors we developed for 2-CEES using the simple Langmuir isotherm. We utilized a linear fitting method28 for QCM responses (ΔF, Hz) per 2-CEES concentration (ppm), and we obtained the maximum response (ΔFmax), equilibrium constant for adsorption (K), and adsorption free energy (ΔG°). According to the derived results, the TPT-M receptor, which exhibited outstanding performance in our study, demonstrated the highest ΔFmax value than others with a reasonably high equilibrium constant K (Fig. S5 and Table S1†). Although further investigations must be performed to derive more reliable data, fitting analysis based on Langmuir isotherms indicates that TPT-M may provide more available binding sites than other receptors. In summary, our findings provide definitive confirmation of the exceptional efficacy of the triamide structure in detecting 2-CEES gas.
3.3 Interaction between 2-CEES gas and the triamide structure through density functional theory (DFT) analysis
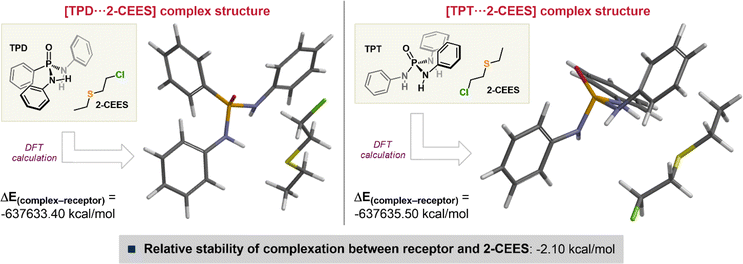
To gain mechanistic insight into the recognition of 2-CEES, we explored the DFT computations for receptor molecules, such as TPD (i.e., phosphonamide) and TPT (i.e., phosphoramide). These molecules are considered to be the key moieties for the TPD and TPT receptors. The DFT calculations were carried out utilizing Spartan ′14 for Windows (MM/MMFF//DFT/EDF2/6-31G*/Vacuum), and the energy differences between the receptor molecule and its complex with 2-CEES were compared to probe the stabilization energies (shown in Fig. 4). According to the results, the complexation of TPT·2-CEES was found to be 2.10 kcal mol−1 more stable than that of TPD·2-CEES. It should be noted that no conspicuous hydrogen-bonding interaction was observed, despite the use of hydrogen-bonding donor receptor molecules.29–31 This phenomenon might be due to the offset of electron density on the sulfur atom by the electron-withdrawing 2-chloroethyl group(s) and the entirely-dispersed molecular softness. Hence, the dipole-induced dipole interaction plays a major role (noncovalent interaction) in the binding mode of receptor molecules toward 2-CEES, the SM simulant.3.4 Receptor selectivity and lifetime evaluation
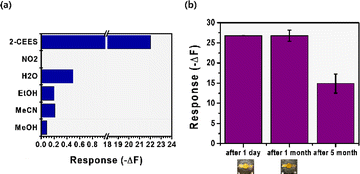
The selectivity of the phosphotriamide TPT-M toward 2-CEES was tested using various VOCs and H2O (shown in Fig. 5(a)). These tests were intended to assess the potential interference of other gases when the phosphotriamide receptor was used for 2-CEES gas detection. We first conducted QCM measurements by exposing the TPT-M receptor to 3 ppm of NO2 from a gas cylinder. The results confirmed that the TPT-M receptor exhibited no reactivity to NO2, which thus validated its selectivity. The receptor's reactivity toward other VOCs and water was assessed by generating the saturated vapor of each substance through the head-space method32,33 and exposing the TPT-M receptor to it. The QCM reactivities of the TPT-M to the VOCs of NO2, H2O, EtOH, MeCN, and MeOH (3 ppm each) and H2O were determined to be −0.0 Hz, −0.5 Hz, −0.2 Hz, −0.2 Hz, and −0.1 Hz, respectively. Consequently, our receptor TPT-M, which was characterized by the phosphotriamide structure, demonstrated the most pronounced reactivity to 2-CEES gas. Furthermore, it exhibited greatly superior reactivity when compared with other VOCs, thereby confirming its selectivity toward 2-CEES gas. These findings confirm the TPT-M's ability to detect 2-CEES without being influenced by potential disturbances, including other VOCs.We conducted a durability test for our proposed TPT-M receptor to assess its operational lifespan under atmospheric conditions (shown in Fig. 5(b)). The TPT-M receptor was applied onto a QCM quartz cell, and its responsiveness to 2-CEES (5 ppm) was evaluated at one-month and 5 month intervals after the coating process. The results of the evaluation indicated that the TPT-M sensor maintained exceptional efficacy in detecting 2-CEES even after 1 month (∼100%). Although its responsiveness diminished after 5 months (∼55.7% responsiveness), the sensor retained sufficient reactivity to detect 2-CEES. The outstanding performance exhibited by the triaryl phosphotriamide receptor TPT-M for 2-CEES detection over the previously reported PECH was verified and was confirmed to last for at least 1 month under atmospheric conditions.
4. Conclusions
In this study, we presented a new receptor for detecting SM gas. We designed and synthesized a receptor that featured a triamide structure, and we subsequently carried out an evaluation of the synthesized receptor using QCM analysis. The experimental results unequivocally established that the receptor featuring the proposed triamide structure displayed exceptional reactivity toward 2-CEES, which was the intended target detection substance. Upon exposing this receptor to different concentrations of 2-CEES, it was observed that the QCM reactivity correspondingly increased with increasing concentrations of injected 2-CEES. This observation confirmed that the developed receptor was responsive to 2-CEES specifically, and that its reactivity was attributed to the interaction with 2-CEES rather than to any other factors. Furthermore, when the receptor we developed was compared with PECH, which is a widely recognized substance for detecting SM, in the presence of 2-CEES gas under identical conditions, its QCM reactivity was notably more sensitive than that of PECH. Additionally, DFT calculations were carried out to provide evidence of the interaction between the triamide structure and the 2-CEES gas. The DFT calculations unequivocally verified that a receptor featuring a triamide structure forms a more stable reaction with 2-CEES through dipole-induced dipole interactions than does a receptor with two amide structures. Furthermore, in anticipation of the real-world application of this receptor in future sensors, we conducted assessments of its selectivity for 2-CEES gas by measuring its reactivity to potential interference factors and other VOCs. The results of these assessments confirmed that its reactivity toward these substances was low, which underscores the excellent selectivity of our receptor for 2-CEES gas detection. After we conducted a comprehensive durability test on the receptor, we confirmed that there had been almost no decrease in its reactivity following its coating onto the QCM cell for up to 1 month. Impressively, the receptor maintained its detection capability even after 5 months, which provides robust evidence of its outstanding performance in 2-CEES detection. We believe that the receptor's potential applicability will extend to array sensors, SAW sensors, and chemo-resistive and chemo-capacitive sensors, and promises advancements for the detection of CWAs.Author contributions
Conceptualization, C. S.; methodology, J. H., J. H. P., S. G. S., S. L., and S. L.; validation, J. H. and J. H. P.; writing original draft preparation, J. H. and J. H. P.; writing – review and editing, C. S., H. Y. B., and C. Y. L.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Institute of Civil-Military Technology Cooperation Center funded by the Defense Acquisition Program Administration and Ministry of Trade, Industry and Energy, Republic of Korea under Grant No. 20-CM-BR-05. This work was also supported by the Tech Incubator Program for Startup funded by the Ministry of SMEs and Startups, Republic of Korea (S3229478).References
- K. Kim, O. G. Tsay, D. A. Atwood and D. G. Churchill, Destruction and Detection of Chemical Warfare Agents, Chem. Rev., 2011, 111(9), 59 CrossRef PubMed.
- S. Costanzi, J.-H. Machado and M. Mitchell, Nerve Agents: What They Are, How They Work, How to Counter Them, ACS Chem. Neurosci., 2018, 9, 13 Search PubMed.
- T. Zellner and F. Eyer, Choking agents and chlorine gas – History, pathophysiology, clinical effects and treatment, Toxicol. Lett., 2020, 320, 7 CrossRef PubMed.
- M. A. Makinen, O. A. Anttalainen and M. E. T. Sillanpää, Ion Mobility Spectrometry and Its Applications in Detection of Chemical Warfare Agents, Anal. Chem., 2010, 82(23), 7 CrossRef PubMed.
- T. Kondo, R. Hashimoto, Y. Ohruia, R. Sekiokaa, T. Nogami, F. Muta and Y. Seto, Analysis of chemical warfare agents by portable Raman spectrometer with both 785 nm and 1064 nm excitation, Forensic Sci. Int., 2018, 291, 16 CrossRef PubMed.
- V. V. Singh, V. Kumar, U. Biswas, M. Boopathi, K. Ganesan and A. K. Gupta, Luminol-Based Turn-On Fluorescent Sensor for Selective and Sensitive Detection of Sulfur Mustard at Ambient Temperature, Anal. Chem., 2021, 93(2), 7 CrossRef PubMed.
- E. Climent, M. Biyikal, K. Gawlitza, T. Dropa, M. Urban, A. M. Costero, R. Martinez-Manez and K. Rurack, A Rapid and Sensitive Strip-Based Quick Test for Nerve Agents Tabun, Sarin, and Soman Using BODIPY-Modified Silica Materials, Chem.–Eur. J., 2016, 22(32), 6 CrossRef PubMed.
- B. H. W. Hendriks, A. J. R. Balthasar, G. W. Lucassen, M. v. d. Voort, M. Mueller, V. V. Pully, T. M. Bydlon, C. Reich, A. T. M. H. van Keersop and J. Kortsmit, et al., Nerve detection with optical spectroscopy for regional anesthesia procedures, J. Transl. Med., 2015, 13(380), 11 Search PubMed.
- L. M. Eubanks, T. J. Dickerson and K. D. Janda, Technological advancements for the detection of and protection against biological and chemical warfare agents, R. Soc. Chem., 2006, 36, 13 Search PubMed.
- J. P. Novak, E. S. Snow, E. J. Houser, D. Park, J. L. Stepnowski and R. A. McGill, Nerve agent detection using networks of single-walled carbon nanotubes, Am. Inst. Phys., 2003, 83(19), 4 Search PubMed.
- C. Sun, W. Xiong, W. Ye, Y. Zheng, R. Duan, Y. Che and J. Zhao, Fast and Ultrasensitive Detection of a Nerve Agent Simulant Using Carbazole-Based Nanofibers with Amplified Ratiometric Fluorescence Responses, Anal. Chem., 2018, 90, 4 Search PubMed.
- V. Kumar, G. Raviraju, H. Rana, V. Rao and A. K. Gupta, Highly selective and sensitive chromogenic detection of nerveagents (sarin,tabunandVX) : a multi analyte detection approach, R. Soc. Chem., 2017, 53, 4 Search PubMed.
- A. Yamaguchi, H. Miyaguchi, A. Ishida and M. Tokeshi, Paper-Based Analytical Device for the On-Site Detection of Nerve Agents, ACS Appl. Bio Mater., 2021, 4(8), 7 Search PubMed.
- J. Hwang, P. Li, M. D. Smith, C. E. Warden, D. A. Sirianni, E. C. Vik, J. M. Maier, C. J. Yehl, C. D. Sherrill and K. D. Shimizu, Tipping the Balance between S-π and O-πInteractions, J. Am. Chem. Soc., 2018, 140(41), 7 CrossRef PubMed.
- C. Qiu, X. Liu, C. Cheng, Y. Gong, W. Xiong, Y. Guo, C. Wang, J. Zhao and Y. Che, Ultrasensitive Detection of Sulfur Mustard via Differential Noncovalent Interactions, Anal. Chem., 2019, 91, 5 Search PubMed.
- R. F. Zetterberg, K. Peterson, R. E. Johnsson, T. Brimert, M. Håkansson, D. T. Logan, H. Leffler and U. J. Nilsson, Monosaccharide Derivatives with Low-Nanomolar Lectin Affinity and High Selectivity Based on Combined Fluorine–Amide, Phenyl–Arginine, Sulfur–π, and Halogen Bond Interactions, ChemMedChem, 2018, 13, 5 CrossRef PubMed.
- V. Kumar and H. Rana, Selective and sensitive chromogenic and fluorogenic detection of sulfur mustard in organic, aqueous and gaseous medium, RSC Adv., 2015, 5(112), 5, 10.1039/C5RA18641B.
- M. Kang, I. Cho, J. Park, J. Jeong, K. Lee, B. Lee, D. D. O. Henriquez, K. Yoon and I. Park, High Accuracy Real-Time Multi-Gas Identification by a Batch-Uniform Gas Sensor Array and Deep Learning Algorithm, ACS Sens., 2022, 7(2), 11 CrossRef PubMed.
- J. Ma, Y. Li, X. Zhou, X. Yang, F. A. Alharthi, A. A. Alghamdi, X. Cheng and Y. Deng, SiO2–WO3 Hybrid Materials with Improved Pore Connectivity for Ultratrace Ethanol Detection at Low Operating Temperature, Small, 2020, 16(46), 10 CrossRef PubMed.
- Q.-Y. Zheng, M. Yang, X. Dong, X.-F. Zhang, X.-L. Cheng, L.-H. Huo, Z. N. Major and Y.-M. Xu, ZnO/PANI nanoflake arrays sensor for ultra-low concentration and rapid detection of NO2 at room temperature, Rare Met., 2023, 42(2), 9 Search PubMed.
- R. Yoo, C. Oh, M.-J. Song, S. Cho and W. Lee, Sensing Properties of ZnO Nanoparticles for Detection of 2-Chloroethyl Ethyl Sulfide as a Mustard Simulant, J. Nanosci. Nanotechnol., 2018, 18(2), 5 Search PubMed.
- Y. Fan, K. Li, X. Ren, W. Yan, C. Zhu, Y. Zhao, W. Zeng, Z. Chena and S. Wang, A highly selective gas sensor based on the WO3/WS2 van der Waals heterojunction for the 2-chloroethyl ethyl sulfide (2-CEES) sensing application, J. Mater. Chem. C, 2021, 9, 8 Search PubMed.
- R. Bunkar, R. Asrey, K. D. Vyas, V. K. Rao, S. Kumar, A. R. Srivastava and M. P. Kaushik, Polyepichlorohydrin modified quartz crystal microbalance sensor for sulfur mustard vapor detection, Indian J. Sci. Technol., 2010, 3(2), 4 Search PubMed.
- Y. Pan, L. Zhang, B. Cao, X. Xue, W. Liu, C. Zhangc and W. Wang, Effects of temperature and humidity on the performance of a PECH polymer coated SAW sensor, RSC Adv., 2020, 10, 8 Search PubMed.
- X. Tan, L. Zeng, Q. Liao, G. Zhang, X. Wu, J. Wang and R. Xu, Model-fitting kinetic analysis of novel phosphorus-containing curing agent for epoxy resin, Thermochim. Acta, 2017, 657, 6 CrossRef.
- R. Bunkar, K. D. Vyas, V. K. Rao, S. Kumar, B. Singh and M. P. Kaushik, Epoxy Resin Modified Quartz Crystal Microbalance Sensor for Chemical Warfare Agent Sulfur Mustard Vapor Detection, Sens. Transducers J., 2010, 113(2), 7 Search PubMed.
- J. H. Park, S. G. Song, M. H. Shin, C. Song and H. Y. Bae, N-Triflyl Phosphoric Triamide: A High-Performance Purely Organic Trifurcate Quartz Crystal Microbalance Sensor for Chemical Warfare Agent, ACS Sens., 2022, 7(2), 7 Search PubMed.
- S. Ha, M. Lee, H. O. Seo, S. G. Song, K.-S. Kim, C. H. Park, I. H. Kim, Y. D. Kim and C. Song, Structural Effect of Thioureas on the Detection of Chemical Warfare Agent Simulants, ACS Sens., 2017, 2(8), 6 Search PubMed.
- J. Abelard, A. R. Wilmsmeyer, A. C. Edwards, W. O. Gordon, E. M. Durke, C. J. Karwacki, D. Troya and J. R. Morris, Adsorption of 2-Chloroethyl Ethyl Sulfide on Silica: Binding Mechanism and Energy of a Bifunctional Hydrogen-Bond Acceptor at the Gas–Surface Interface, J. Phys. Chem. C, 2015, 119(1), 8 CrossRef.
- D. Panayotov and J. T. Yates, Bifunctional Hydrogen Bonding of 2-Chloroethyl Ethyl Sulfide on TiO2-SiO2 Powders, J. Phys. Chem. B, 2003, 107(38), 5 Search PubMed.
- L. Yang, Q. Han, S. Cao, F. Huang, M. Qin, C. Guo and M. Ding, Research on the Interaction of Hydrogen-Bond Acidic Polymer Sensitive Sensor Materials with Chemical Warfare Agents Simulants by Inverse Gas Chromatography, Sensors, 2015, 15(6), 7 CrossRef PubMed.
- M. Gad, H. Zaazaa, S. Amer and M. Korany, Static headspace gas chromatographic method for the determination of residual solvents in cephalosporins, RSC Adv., 2015, 5, 10 RSC.
- M. J. George, L. Marjanovic and D. B. G. Williams, Solvent-Assisted Headspace Sampling Using Solid Phase Microextraction for the Analysis of Phenols in Water, Anal. Chem., 2015, 87(19), 4 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08852a |
| This journal is © The Royal Society of Chemistry 2024 |