Open Access Article
Open Access ArticleMedium-temperature calcination and acid pressure leaching extract the Al2O3 from coal gangue: activation mechanism and kinetic analysis
Zhiyong Yang†
ab,
Yunsheng Zheng† *c,
Zhijun Ma
*c,
Zhijun Ma *cd,
Liang Chengc,
Guangming Wangc and
Ying Qinc
*cd,
Liang Chengc,
Guangming Wangc and
Ying Qinc
aSchool of Mechanical Engineering, Liaoning Technical University, Fuxin 123000, China
bZhundong Energy Research Institute, Xinjiang Tianchi Energy Sources Co., Ltd, 831100, Changji, China
cCollege of Mining, Liaoning Technical University, Fuxin 123000, China. E-mail: zhijunma0930@126.com; zys_lgd@126.com; Tel: +86-139-4188-1359; Fax: +86-183-4284-7818
dCollege of Materials Science & Engineering, Liaoning Technical University, Fuxin 123000, China
First published on 9th April 2024
Abstract
Bauxite is an important strategic resource, and it is facing with the problem of balance between high demand of bauxite ore and low resource of bauxite reserves in China. This research takes the Fuxin coal gangue as the object and extracts Al2O3 by medium-temperature calcination and acid pressure leaching process. The results show that at a calcination temperature of 650 °C, calcination time of 2 h, acid pressure leaching temperature of 160 °C and acid pressure leaching time of 6 h, the extraction ratio of Al2O3 reaches 80.19%. Furthermore, the research finding that the complete activation temperatures of kaolinite and muscovite are 650 °C and 850 °C, respectively, and the decomposition reactions of active Si, active Al, and metakaolinite occur above 800 °C, which leads to a low extraction ratio of Al2O3. The acid pressure leaching process can directly destroy the muscovite structure at a calcination temperature of 650 °C. The acid pressure leaching kinetic equations are studied by three kinetic models, and the apparent activation energies of the reactions are calculated by the Arrhenius formula. The results show that acid pressure leaching is subject to solid residue in-layer diffusion control, and the kinetic equation is “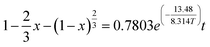 ”. The apparent activation energy is 13.48 kJ mol−1.
”. The apparent activation energy is 13.48 kJ mol−1.
1 Introduction
Bauxite is an important strategic resource, and China is the world's largest producer of alumina. Bauxite is faced with the problem of balance between the high demand of bauxite ore and the low resource of bauxite reserves. The dependence on foreign bauxite in China exceeded 55% at this stage, the import ratio is expected to reach 80% by 2030.1,2 China's bauxite import demand is mainly for higher-quality overseas gibbsite bauxite, which accounts for 75% of global bauxite imports.3 Therefore, Seeking alternatives to bauxite is important for the sustainable development of China's aluminum industry. Coal gangue is a mixture of carbonaceous, muddy, and sandy shale, which is a solid waste thrown out by coal through the process of mining and washing.4,5 It is considered to be a potential mineral resource and is elemental composed primarily of SiO2 and Al2O3, which account for approximately 50–80% elemental composition of coal gangue.6–8 Coal gangue can be used for the production of chemical products such as alumina,9 aluminum sulfate,10 and polymeric aluminum chloride,11 as well as for the preparation of molecular sieve materials.12 Therefore, coal gangue can be used as a substitute for bauxite to provide raw materials for the development of the downstream aluminum industry. Currently, the amount of coal gangue accumulation has reached 45–50 Bt.13 Massive long-term accumulation of coal gangue leads to environmental pollution, landslides, and other problems, threatening the safety of human life and property.14,15 Fuxin is one of the important energy bases in China and is known as the “city of coal and electricity”. According to the statistics, there are 23 coal gangue storage sites, covering an area of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 acres, with a stockpile of 2 Bt in Fuxin. The accumulation of large amounts of coal gangue puts great pressure on land resources and the ecological environment. In addition, the Al2O3 content of coal gangue is in the range of 13.34–18.74% in Fuxin, and it is of great significance to carry out Al2O3 extraction studies.16,17
037 acres, with a stockpile of 2 Bt in Fuxin. The accumulation of large amounts of coal gangue puts great pressure on land resources and the ecological environment. In addition, the Al2O3 content of coal gangue is in the range of 13.34–18.74% in Fuxin, and it is of great significance to carry out Al2O3 extraction studies.16,17
At present, the commonly used methods for extracting Al2O3 from coal gangue include an acid method and an alkali fusion method. The alkali fusion method has the shortcomings of high alkali consumption, complex processing, and a large amount of waste residue, while the acid method of extracting Al2O3 from coal gangue has the advantages of high efficiency and simplicity. Guan et al.,18 extracted Al2O3 directly under a hydrochloric acid system by adding sodium fluoride, and the extraction ratio of Al2O3 only reached only 70.40%. The structures of kaolinite, muscovite, and orthoclase within coal gangue are stable, and hydrochloric acid is not reactive and cannot efficiently extract Al2O3. Therefore, activation treatment is needed before the acid method,19–22 with thermal activation being the most common among these methods. Under the action of thermal activation, internal and external hydroxyl groups are removed from kaolinite, generating the more active product metakaolinite, which is beneficial to increase the extraction ratio of Al2O3. Jia et al.,16 used coal gangue with a high mineral content of kaolinite and a high chemical element content of Al as the raw material, and applied a calcination process and atmospheric pressure “one-step acid dissolution method” to extract Al2O3, for which the extraction ratio reached 94.09%. The alumina product index reached the international metallurgical grade standard. In contrast, Li17 subjected coal gangue from the Fuxin area to the “one-step acid dissolution method” to extract Al2O3, and the extraction ratio reached only 35%. The reason is that the Al2O3 extracted by the calcination activation method and one-step acid dissolution method mainly exist in the form of kaolinite, and the content of kaolinite in the Fuxin coal gangue is low. Therefore, the “one-step acid dissolution method” has strict requirements for the content of Al2O3 and the mineral composition of coal gangue. However, the aluminum content of coal gangue in China is low, so the above research is not universal. In this paper, the acid pressure leaching process is adopted based on a one-step acid dissolution method. Compared with the above process, the acid pressure leaching provides pressure to the reaction system, and H+ more easily penetrates within the pores of the coal gangue, improving the extraction ratio of Al2O3.
This research addresses the problem of a low Al2O3 extraction ratio when performing a “one-step acid dissolution method” with coal gangue in Fuxin. The complete activation temperatures of kaolinite and muscovite are 650 °C and 850 °C respectively, and the decomposition of active Si, active Al, and metakaolinite occurs above 800 °C, which leads to a low extraction ratio of Al2O3. Therefore, the key to improving the leaching rate of alumina from coal gangue in the Fuxin area is to solve the problem that kaolinite and muscovite cannot be activated simultaneously. In this paper, the methods of medium temperature calcination and acid pressure leaching were used to extract Al2O3. The structure of kaolinite can be destroyed directly by a medium-temperature calcination process at a calcination temperature of 650 °C, and the structure of muscovite can be destroyed directly by an acid pressure leaching process. It has solved the problem that kaolinite and muscovite in coal gangue cannot be activated at the same temperature, and the extraction ratio of Al2O3 is significantly improved. This study provides basic data for the high-value utilization of coal gangue and sustainable development of the aluminum industry in China.
2 Materials and methods
2.1 Materials
Coal gangue was taken from the Hengda Coal Processing Plant of the Liaoning Fuminous Group. The coal gangue was passed through a 200 mesh sieve (0.074 mm) and called CG. The CG elemental analysis data are shown in Table 1.| Component | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | CaO | TiO2 | P2O5 | Loss on ignition |
|---|---|---|---|---|---|---|---|---|---|
| Contents (wt%) | 46.19 | 12.60 | 7.13 | 2.19 | 1.34 | 2.06 | 0.74 | 0.17 | 25.19 |
2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixture was stirred evenly, placed in a high-pressure reactor, immersed in a drying oven at 120 °C (pressure value 0.11 MPa), 140 °C (pressure value 0.32 MPa), 160 °C (pressure value 0.55 MPa), 180 °C (pressure value 0.82 MPa) under self-generated pressure for 2–10 h, and then allowed to naturally cool to room temperature. The sample was then washed with distilled water (homemade in the laboratory) to neutralize, and dried in a 100 °C drying oven for 12 h to obtain acid leaching samples, called SJZ-T-H, where T represents the calcination temperature, and H represents the calcination time.
10. The mixture was stirred evenly, placed in a high-pressure reactor, immersed in a drying oven at 120 °C (pressure value 0.11 MPa), 140 °C (pressure value 0.32 MPa), 160 °C (pressure value 0.55 MPa), 180 °C (pressure value 0.82 MPa) under self-generated pressure for 2–10 h, and then allowed to naturally cool to room temperature. The sample was then washed with distilled water (homemade in the laboratory) to neutralize, and dried in a 100 °C drying oven for 12 h to obtain acid leaching samples, called SJZ-T-H, where T represents the calcination temperature, and H represents the calcination time.
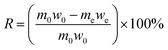 | (A.1) |
2.3 Sample characterization
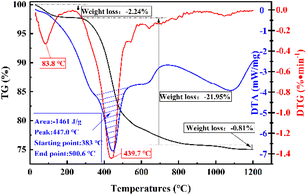
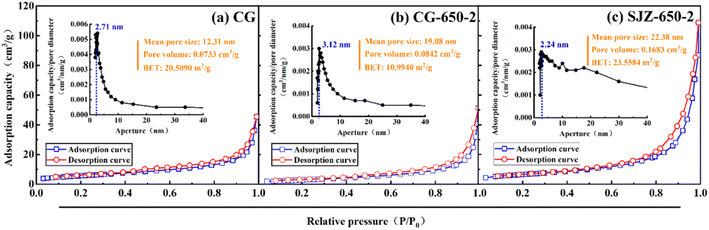
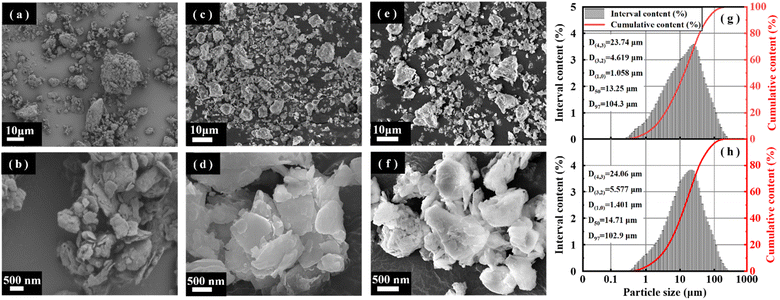
An X-ray diffractometer (XRD) (D8 ADVANCE, Bruker, Germany) was used to determine the phase structure of the samples under the following test conditions: Cu targeted K α-ray, 1.5406 Å wavelength, 40 kV working voltage, 40 mA tube current, 0.05 s step length, and 5–70° scanning range. Fourier transform infrared spectroscopy (FT-IR) (Tensor II, Bruker, Germany) was used to analyze the chemical bonds of the samples under the following test conditions: wave number range of 400–4000 cm−1; a fully automated volumetric vapor adsorption instrument (3H-2000PMV, BEST) was used to analyze the specific surface area, pore size distribution, and pore volume of the samples under the following test conditions: degassing temperature of 573.15 K and degassing time of 4 h. Additionally, the influence of medium temperature calcination and acid pressure leaching on the pore structure of coal gangue was studied; TG-DSC (STA 449F3, Netzsch, Germany) was used to study phase changes during sample warming. The test conditions were as follows: temperature increase rate of 10° per min, temperature range of 0 to 1200 °C, and atmosphere of air; SEM (Sigma, ZEISS, Germany) was used to analyze the surface morphology of the samples.3 Results and discussion
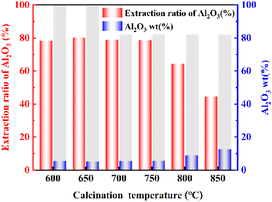
3.1. Effect of calcination temperature on the extraction ratio of Al2O3
The effect of calcination temperature on the extraction ratio of Al2O3 was studied. The analysis in Fig. 1 shows that the extraction ratio of Al2O3 tends to increase and then decrease with increasing calcination temperature. The extraction ratio of Al2O3 was highest at a calcination temperature of 650 °C, under which it reached 80.19%. When the calcination temperature reached 800 °C, the extraction ratio of Al2O3 decreased significantly. When the calcination temperature was 850 °C, the extraction ratio of Al2O3 decreased to its lowest value. This was due to the metakaolin further decomposing to form amorphous SiO2, γ-Al2O3, and sillimanite (Al2O3·SiO2) at 800 °C, which is not reactive with hydrochloric acid, resulting in the extraction ratio of Al2O3 decreased.233.2. Effect of calcination time on the extraction ratio of Al2O3
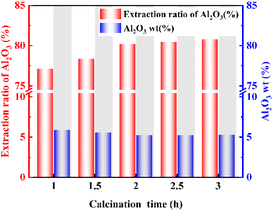
The effect of calcination time on the extraction ratio of Al2O3 was also investigated. The analysis in Fig. 2 shows that the extraction ratio of Al2O3 tends to increase first and then stabilize with increasing calcination time. This is because the calcination time is short, and the calcination process is not sufficient, thus leading to a low extraction ratio of Al2O3. When the calcination time reached 2 h, the extraction ratio of Al2O3 was stable and reached 80.19%. The acid pressure leaching process is accompanied by the leaching of Fe, the extraction ratio is 98.11%, so the iron removal problem should be fully considered in industrial production.3.3 Activation mechanism
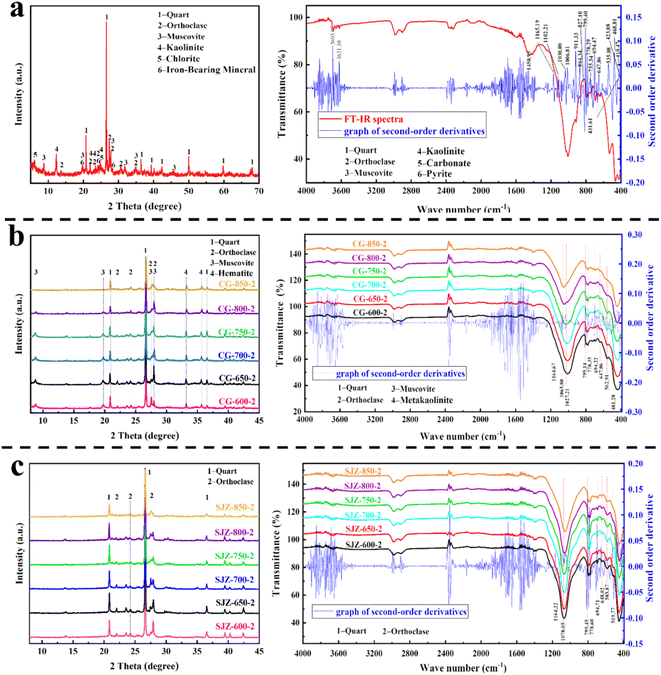
The XRD and FT-IR analyses in Fig. 3a show that the main phase components of coal gangue are 38.1% quartz, 16.1% kaolinite, 21.4% orthoclase, 10.3% muscovite, 13.7% chlorite, and 0.3% iron-bearing minerals (magnetite, hematite, and pyrite). Based on elemental analysis, the main elemental components of coal gangue are SiO2, Al2O3, and Fe2O3, which means that Si exists primarily in the form of quartz. Al and a portion of Si exist in the form of aluminosilicate minerals. And Fe exists in the form of chlorite.25,26
The analysis in Fig. 3b shows that the kaolinite diffraction peak completely disappears at 2θ = 8.89° in the XRD pattern, and the metakaolinite absorption peaks appear at 1065.80 cm−1, 562.91 cm−1, and 481.20 cm−1 in the FT-IR pattern, under calcination temperature at 650 °C. These results indicate that the phase structure of kaolinite is transformed to generate more active metakaolinite at a calcination temperature of 650 °C.27 When the calcination temperature reaches 800 °C, these three absorption peaks of metakaolinite weaken in the FT-IR pattern. These results indicate that the metakaolinite further decomposes to form amorphous SiO2, γ-Al2O3, and sillimanite (Al2O3·SiO2) at a calcination temperature of 800 °C. Moreover, hematite diffraction peaks appear at 2θ = 33.12° and 35.64°, indicating that the phase structure of the iron-bearing minerals transforms to hematite. On the one hand, this is due to the oxidation of Fe2+ to Fe3+ in iron-bearing minerals;28 on the other hand, the dehydroxylation of iron-bearing minerals transforms into hematite under calcination conditions. When the calcination temperature is 850 °C, the muscovite diffraction peak completely disappears at 2θ = 19.88° in the XRD pattern, and the muscovite absorption peaks disappear at 827.10 cm−1, 468.81 cm−1, and 423.08 cm−1 in the FT-IR pattern. These results indicate that the phase structure of muscovite is transformed at 850 °C. To summarize, it can be found that kaolinite and muscovite cannot be activated at the same time by calcination alone, so the “one-step acid dissolution method” has a low extraction ratio of Al2O3 for coal gangue in Fuxin.
The analysis in Fig. 3c shows that the method of acid pressure leaching is further adopted at a calcination temperature of 650 °C, at which point only quartz and orthoclase diffraction peaks remain, and hematite and muscovite diffraction peaks disappear in the XRD and FT-IR patterns. These results indicate that acid pressure leaching can destroy the muscovite structure within coal gangue. The reason is that acid pressure leaching provides pressure to the reaction system, and H+ more easily penetrates within the pores of the coal gangue, improving the extraction ratio of Al2O3. With an increase in calcination temperature to 800 °C, further decomposition of metakaolinite sillimanite (Al2O3–SiO2), which is not easily leached by acid pressure leaching due to their lower activity. These changes reduced the extraction ratio of Al2O3 and increased the residual amount of Al2O3,29 resulting in a reduced diffraction peak intensity for quartz and orthoclase in the XRD pattern.
In summary, phase structure transformation during the process of medium-temperature calcination and acid pressure leaching of coal gangue was analyzed. The phase structure of kaolinite could be transformed into metakaolinite at a calcination temperature of 650 °C, and the phase structure of muscovite was destroyed by acid pressure leaching, which could increase the extraction ratio of Al2O3. The problem that the phase structure of kaolinite and muscovite cannot be activated at the same time in the “one-step acid dissolution method” is solved.
In summary, under medium calcination conditions, the dehydroxylation of kaolinite and the decomposition of organic matter lead to the collapse of the skeleton structure and the production of defects, which increase the pore volume and pore size. Under pressure acid leaching, the active components, metakaolinite, and muscovite are leached, leading to channel dredging and a large number of pores while increasing the pore volume and specific surface area.
3.4 Kinetic analysis of acid pressure leaching
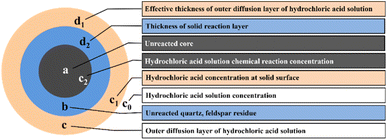
The macrokinetic research method is used to study the kinetic model and kinetic control type of the coal gangue pressurized acid leaching process. The coal gangue pressurized acid leaching reaction is a typical liquid–solid noncatalytic reaction. Quartz and feldspar are not reactive when mixed with hydrochloric acid, and only active substances are selectively leached. Therefore, there will be a certain amount of solid residual products in the acid pressure leaching process. This result is in line with the kinetic principle of hydrometallurgy33 of the “unreacted shrinking core model” (Fig. 7).Solid–liquid leaching reaction based on steady state, and according to Fick's first law, the total reaction rate of hydrochloric acid solution through the boundary layer can be expressed by the amount of Al2O3 leached per unit time (see eqn (A.2)).
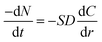 | (A.2) |
It is assumed that the density of coal gangue is ρ, the radius is r, the surface area is S, and the relative molecular weight of the material involved in the leaching reaction is M. The amount of substance at time t of reaction between surface area and alumina is expressed by eqn (A.3) and (A.4).
| S = 4πr2 | (A.3) |
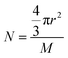 | (A.4) |
Because the radius of the coal gangue particle is not easy to obtain during the reaction process, the relationship between the extraction ratio X of Al2O3 and reaction time is chosen to express the kinetic equation. N0 was set as the amount of initial substance in the reaction, and the Al2O3 extraction ratio is shown in eqn (A.5).
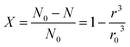 | (A.5) |
When the acid pressure leaching process is controlled by the diffusion boundary layer, C1 ≈ 0, then the amount of substance leached Al2O3 per unit time is shown in eqn (A.6).
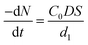 | (A.6) |
It is assumed that the reaction particle size during the process of Al2O3 acid pressure leaching does not change with the reaction and that S is a constant. The kinetic equation of external diffusion control is shown in eqn (A.7).
| X = k1t | (A.7) |
When the acid pressure leaching process is controlled by internal diffusion of the solid residual layer, then the amount of substance leached Al2O3 per unit time is shown in eqn (A.8).
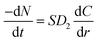 | (A.8) |
It is assumed that the volume of the residual layer is equal to the volume of coal gangue particles and that r0 remains unchanged; then, C1 ≈ C0 and C2 ≈ 0. Eqn (A.3) can be substituted into eqn (A.8) and integrated to obtain eqn (A.9).
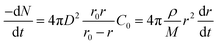 | (A.9) |
To construct eqn (A.9), r is represented by X to obtain the internal diffusion equation of the solid residual layer, which is shown in eqn (A.10).
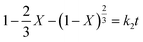 | (A.10) |
When the acid pressure leaching process is controlled by a chemical reaction, the chemical reaction is a first-order reaction, and the amount of substance leached Al2O3 per unit time is shown in eqn (A.11).
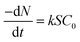 | (A.11) |
Eqn (A.3) and (A. 5) are substituted into eqn (A.11) and the integral result is shown in eqn (A.12).
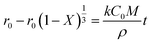 | (A.12) |
The equation for chemical reaction control is shown in eqn (A.13).
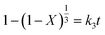 | (A.13) |
The results of fitting the temperature and time of the acid pressure leaching process on the Al2O3 extraction ratio and kinetic analysis are shown in Fig. 8.
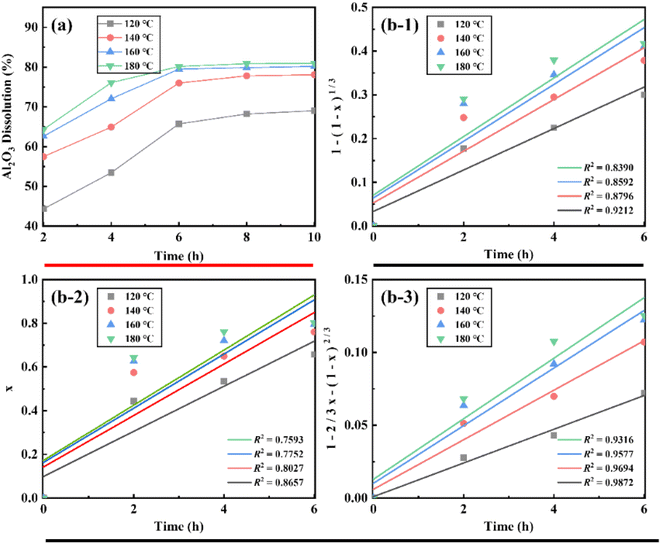 | ||
| Fig. 8 Acid pressure leaching data and kinetic fitting results ((a) – acid pressure leaching temperature and time relationship, (b) – ccid pressure leaching kinetics fitting results). | ||
After 6 h of acid pressure leaching of coal gangue, the extraction ratio of Al2O3 no longer increased, so the kinetic equation was fitted to the data of the Al2O3 extraction ratio from 0–6 h. The results of the boundary layer diffusion control, solid residue in-layer diffusion control, and chemical reaction control fit are shown in Fig. 8. The data were fitted to give the reaction rate constant k and the correlation coefficient R2. Among them, the internal diffusion reaction control equation fits the best, the linear correlation coefficient R2 > 0.93, and the fitted straight line tends to be close to the origin. Therefore, it can be judged that the process of acid pressure leaching Al2O3 from coal gangue is likely controlled by solid residue in-layer diffusion control.
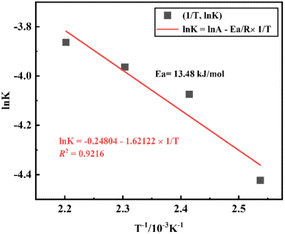
The activation energy of the acid pressure process was calculated according to the “Arrhenius” formula (A.14) and (A.15):
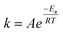 | (A.14) |
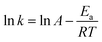 | (A.15) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k − 1/T” for Al2O3 are shown in Fig. 9. The apparent activation energies can be calculated from the slope of the fitting equations based on the Arrhenius formula, where the slope is “−Ea/RT” and the intercept is “ln
k − 1/T” for Al2O3 are shown in Fig. 9. The apparent activation energies can be calculated from the slope of the fitting equations based on the Arrhenius formula, where the slope is “−Ea/RT” and the intercept is “ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A”.
A”.
The fitted linear equation in Fig. 9, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −0.24808 − 1.62122 × 1/T, the activation energy Ea = 13.48 kJ mol−1 and A = 0.7803. Therefore, the kinetic equation of the coal gangue acid pressure leaching process is:
k = −0.24808 − 1.62122 × 1/T, the activation energy Ea = 13.48 kJ mol−1 and A = 0.7803. Therefore, the kinetic equation of the coal gangue acid pressure leaching process is:
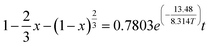 | (A.16) |
Based on the “one-step acid dissolution method”, the extraction ratio of Al2O3 reached 80.19% by the acid pressure leaching process, which has certain advantages compared with the alkali method and atmospheric pressure acid leaching method (Table 2). First of all, the alkali method has a higher extraction ratio of Al2O3, but the sintering temperature is much higher than that of the acid pressure leaching process, and a higher energy consumption is required. In addition, the alkaline process produces a large amount of calcium silicate waste residue in the production process, resulting in low environmental benefits. Although the submolten salt method and supercritical water method reduces the activation energy consumption, it needs to consume a lot of strong alkali and cannot be recycled, resulting in high cost and difficult to achieve large-scale production. In addition, supercritical water method uses strong alkali for extraction at high temperature and pressure, resulting in simultaneous leaching of Si and Al, resulting in difficult separation in later stage. The acid pressure leaching provides pressure to the reaction system, and H+ more easily penetrates within the pores of the coal gangue and extracts the Al2O3 inside the muscovite and other minerals that are not fully activated. The extraction ratio of Al2O3 from Fuxin coal gangue is increased from 35% to 80.19%. The problem of simultaneous activation of muscovite and kaolinite was solved without increasing the complexity of the process, and the extraction ratio of Al2O3 was improved. It has been calculated that 84.56 kg of Al2O3 can be extracted for every 1000 kg of coal gangue consumed, and at the same time, high silica waste residue will be left, which can prepare molecular sieve materials.34 In addition, acid pressure leaching is self-generated pressure in the heating process, without external pressure treatment, which can adapt to industrial scale production. China's bauxite imports more than 60 million tons, to achieve the industrial production of coal gangue extraction Al2O3, can reduce the import pressure of bauxite, with high economic value and environmental benefits. However, the extraction process will produce HCl gas, which has a certain impact on the environment. The HCl can be condensation recovered and then recycled. At the same time, hydrochloric acid is corrosive, easy to cause the erosion of pipelines and equipment, the production of pipelines and equipment materials have higher requirements.
| Number | Technology | Method | Origin of raw materials | Calcination/sintering temperature (°C) | Alumina extraction ratio (%) | Reference source |
|---|---|---|---|---|---|---|
| 1 | Alkali process | Limestone sintering method | Datong | 1260 | 85.6 | 35 |
| 2 | — | 1340–1360 | 80 | 36 | ||
| 3 | Submolten salt method | Pingshuo | 280 | 87.47 | 37 | |
| Supercritical water method | Shanyin | 400 | 78.9 | 38 | ||
| 4 | Acid process | Acid atmospheric leaching | Luan | 800 | 62.4 | 39 |
| 5 | Fuxin | 700 | 35 | 17 | ||
| 6 | Acid pressure leaching | Fuxin | 750 | 80.19 | This work |
4 Conclusions
(1) Medium-temperature calcination and acid pressure leaching effectively improved the extraction ratio of Al2O3 from the Fuxin coal gangue. At a calcination temperature of 650 °C, calcination time of 2 h, HCl concentration of 8 mol L−1, acid pressure leaching temperature of 160 °C, and acid pressure leaching time of 6 h, the dissolution ratio of Al2O3 reached 80.19%.(2) The complete activation temperatures of kaolinite and muscovite are 650 °C and 850 °C, respectively. The decomposition reaction of active Si, active Al, and metakaolinite occurs above 800 °C, and these minerals decompose to form amorphous SiO2, γ-Al2O3, and sillimanite (Al2O3–SiO2), which leads to a low extraction ratio of Al2O3. The research used medium temperature calcination and acid pressure leaching process, the phase structure of kaolinite could be transformed into metakaolinite at a calcination temperature of 650 °C, and the phase structure of muscovite was destroyed by acid pressure leaching, which could achieve the purpose of increasing the extraction ratio of Al2O3. The problem that the phase structure of kaolinite and muscovite cannot be activated at the same time in the “one-step acid dissolution method” is solved.
(3) The acid pressure leaching process of Al2O3 can be described by the “unreacted shrinking core model”, and its reaction rate is controlled by solid residue in-layer diffusion control. The apparent activation energy of the acid pressure leaching Ea = 13.48 kJ mol−1, and the kinetic rate equation of the acid pressure leaching is “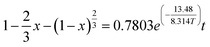 ”.
”.
Author contributions
Conceptualization, Z. Y., Y. Z. and Z. M.; methodology, Z. Y., Y. Z., L. C.; validation, L. C., G. W., and Y. Q.; formal analysis, Z. Y. Y. Z., L. C., G. W., and Y. Q.; investigation, Z. M., G. W., and Y. Q.; resources, Z. M.; data curation, Z. Y., Y. Z.; writing-original draft preparation, Z. Y., Y. Z.; writing-review and editing, Z. Y., Y. Z.; visualization, G. W., and Y. Q.; supervision, L. C.; project administration, Z. M.; funding acquisition, Z. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Science and Technology Research Project of the Liaoning Provincial Department of Education (entry name: “Research on Key Technologies for Preparation of Molecular Sieve Materials Based on Coal Gangue Resource Attribute in Fuxin” (grant number LJ2020ZD006)).Notes and references
- I. Smičiklas, M. Jović, M. Janković, S. Smiljanić and A. Onjia, Water, Air, Soil Pollut., 2021, 232, 324–338 CrossRef.
- J. Ding, S. H. Ma, S. Shen, Z. L. Xie, S. L. Zheng and Y. Zhang, Waste Manage., 2017, 60, 357–387 CrossRef PubMed.
- S. T. Cao, Y. F. Zhang and Y. Zhang, Hydrometallurgy, 2009, 98, 298–303 CrossRef CAS.
- Y. L. Zhang and T. C. Ling, Constr. Build. Mater., 2020, 234, 117424 CrossRef CAS.
- Z. N. Su, X. H. Li and Q. Zhang, J. Cleaner Prod., 2022, 363, 132408 CrossRef CAS.
- I. Baic and K. B. Witkowska, Rocz. Ochr. Sr., 2011, 13, 1315–1325 Search PubMed.
- J. Y. Li, L. F. You and Y. Wei, Adv. Mater. Res., 2012, 1792, 915 Search PubMed.
- I. Hilary, D. John, O. Frank and S. Sue, Min. Sci. Technol., 2010, 20, 215–223 Search PubMed.
- Y. X. Guo, K. Z. Yan, L. Cui and F. Q. Cheng, Powder Technol., 2016, 302, 33–41 CrossRef CAS.
- D. K. Zeng, Preparation of polyaluminum ferric sulfate from acid leaching of coal gangue and its application, Kunming University of Science and Technology, 2021 Search PubMed.
- D. H. Kong, Z. H. Zhou, S. J. Song, S. Feng, M. L. Lian and R. L. Jiang, Materials, 2022, 15(6), 2253 CrossRef CAS PubMed.
- Y. S. Zheng, J. X. Zhou, Z. J. Ma, X. Y. Weng, L. Cheng and G. R. Tang, Materials, 2023, 16(12), 4338 CrossRef CAS PubMed.
- X. D. Wang, Y. Zhang, S. Chen, H. M. Ding and Y. Zhang, Chem. Eng., 2021, 35(04), 68 CAS.
- M. L. Sheng, Energy and Energy Conservation, 2020, 05, 62–63 Search PubMed.
- B. Lv, Z. Y. Zhao, X. W. Deng, C. J. Fang, B. B. Dong and B. Zhang, J. Mater. Cycles Waste Manage., 2022, 24, 1579–1590 CrossRef CAS.
- M. Jia and L. Yang, Multipurp. Util. Miner. Resour., 2020, 02, 140–144 Search PubMed.
- J. Y. Li, Y. Guo, X. W. Lu, K. Wu, H. Z. Cheng and K. Tang, Tianjin Chemical, 2017, 31, 16–19 Search PubMed.
- C. P. Guan and C. H. Yan, Sichuan Nonferrous Metals, 2011, 4, 35–39 Search PubMed.
- G. Mucsi, N. H. Papné, C. Ulsen, P. O. Figueiredo and F. Kristály, ACS Sustain. Chem. Eng., 2021, 9(9), 3416–3427 CrossRef CAS.
- S. Ravaszová and K. Dvořk, IOP Conference Series: Materials Science and Engineering, 2021, 1205, 012003 CrossRef.
- A. Mitrović and M. Zdujić, Int. J. Miner. Process., 2014, 132, 59–66 CrossRef.
- Y. X. Guo, K. Z. Yan, L. Cui and F. Q. Cheng, Powder Technol., 2016, 302, 33–41 CrossRef CAS.
- L. Cao, L. Zhang, Y. Guo and F. Cheng, Clean Coal Technol., 2020, 26, 203–208 Search PubMed.
- Y. Zhang, Study on mineral transformation during pulverized coal combustion by INFRARED spectroscopy, Changsha University of Science and Technology, 2017 Search PubMed.
- S. L. Cui, Q. P. Zhang, J. Y. Zhang and L. K. Gu, Non-Met. Mines, 2010, 33, 25–27 CAS.
- S. C. Liu, Q. Zhou, G. Li, L. H. Feng, Q. Zhang, X. Y. Wang, J. Zhang and Z. J. Ma, Fuel, 2022, 326, 124971 CrossRef CAS.
- H. H. Zhang, F. L. Yu and J. J. Tang, Chemical Minerals and Processing, 2019, 48, 57–61 Search PubMed.
- V. Strezov, J. A. Lucas, T. J. Evans and L. Strezov, J. Therm. Anal. Calorim., 2004, 78, 385–397 CrossRef CAS.
- L. Q. Cao, L. H. Zhang, Y. X. Guo and F. Q. Cheng, Clean Coal Technol., 2020, 26, 203–208 Search PubMed.
- D. D. Zhang, S. Q. Wu and S. Hu, Non-Met. Mines, 2014, 37, 47–49 CAS.
- C. C. Zhou, G. J. Liu, Z. C. Yan, T. Fang and R. W. Wang, Fuel, 2012, 97, 644–650 CrossRef CAS.
- Z. J. Ma, Q. Zhang and H. T. Zhao, Journal of Liaoning University of Engineering and Technology, 2018, 37, 575–579 Search PubMed.
- H. G. Li, Hydrometallurgy, Zhongnan University Press, Changsha, 1998 Search PubMed.
- J. F. Han, Y. Ha, M. Y. Guo, P. P. Zhao, Q. L. Liu, C. X. Liu, C. F. Song, N. J, X. B. Lu, D. G. Ma and Z. G. Li, Ultrason. Sonochem., 2019, 59, 104703 CrossRef CAS PubMed.
- G. K. Ren, D. L. Zhu and C. Tan, J. Saf. Environ., 2014, 14(1), 160–163 CAS.
- J. Y. Zhang, J. W. Tong and P. M. Sun, Hydrometall. China, 2011, 30(4), 316–319 CAS.
- Q. C. Yang, Research on extraction of alumina from coal gangue and preparation of functional materials, China University of Mining and Technology, Beijing, 2020 Search PubMed.
- L. Han, W. G. Ren, B. Wang, X. X. He, L. J. Ma, Q. H. Huo, J. C. Wang, W. R. Bao and L. P. Chang, Fuel, 2019, 253, 1184–1192 CrossRef CAS.
- K. Z. Yan, Y. X. Guo, J. C. Zhang, Q. Zhang and F. Q. Cheng, Coal Convers., 2014, 37(4), 85–90 CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |