Open Access Article
Open Access ArticleSynergistic effect of KI on the corrosion inhibition of a poly(diallylammonium chloride)-based cyclocopolymer containing bis-cationic motifs for mild steel corrosion in 20% formic acid†
Lipiar K. M. O. Gonia,
Ibrahim Y. Yaagooba,
Mohammad A. J. Mazumder *ab and
Shaikh A. Ali
*ab and
Shaikh A. Ali *ab
*ab
aChemistry Department, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia. E-mail: jafar@kfupm.edu.sa; Fax: +(966) 13 860 4277; Tel: +(966) 13 860 7836
bInterdisciplinary Research Center for Advanced Materials, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia
First published on 22nd March 2024
Abstract
This study entails the syntheses of a homopolymer, poly(diallylammonium chloride) (3), and copolymers (8a–c) containing hydrophilic/hydrophobic pendants and their role in mitigating mild steel in aggressive 20% formic acid, a type of corrosion that is not frequently discussed in the literature. The synthesized homopolymer and copolymers were characterized by FTIR, NMR, viscometry, and TGA. Inhibitor 8b was found to be the most potent, with 81.8% inhibition efficiency (IE) registered via the potentiodynamic polarization method for 100 ppm of inhibitor concentration at 30 °C. Inhibitor 8b, mixed with 2 mmol KI, showed more than 90% IE for a meager 1 ppm inhibitor concentration. For a synergism of 50 ppm inhibitor and 2 mmol KI, the IE reached a high value of 99.1%. The synergism was so good that it helped the inhibitor retain ∼100% of its original IE even after a 24 h weight loss study at 60 °C. The adsorption isotherm study showed that 8b followed the Langmuir adsorption isotherm and adsorbed via chemisorption. A very high value (2.48 × 105 L mol−1) of the equilibrium adsorption constant (Kads) indicated strong adsorption. XPS and SEM surface studies provided evidence of the inhibitor found on the metal surface. Some toxicological parameters, such as LC50, bioaccumulation factor, and developmental toxicity, have been measured computationally. A brief mechanistic insight into how the inhibitors functioned has been offered along with the DFT study.
1. Introduction
The phenomenon of corrosion happens when metals lose electrons upon reacting with a corrosive environment, causing the metals to degrade and ensuing materials and economic losses and claiming lives at times.1,2 Scientifically, corrosion is regarded as an exchange of electrons happening at the interface between a metal and solution.3 It has become an extraordinarily pervasive and perennial problem. Any nation worldwide reportedly spends 5–10% of its GNP on corrosion-related costs.2 It was revealed that the world had to spend 3.4% of its total GDP on the cost of corrosion in 2013.4 Several strategies, namely coating, cathodic or anodic protection, corrosion inhibition, etc., are available to combat corrosion. However, corrosion inhibitors are hugely popular and in-demand approaches.5–7 These substances significantly reduce corrosion when added to a corrosive metal solution. Mild steel has enormous implications in industrial set-ups, and is used to construct machinery parts, pipelines, railways, automobile body parts, etc., owing to its favorable mechanical properties and affordable costs.8 As such, mild steel corrosion study is of great importance.Corrosion caused by organic acids isn't discussed as much as that caused by mineral acids. Even though organic acids have many implications across many industries, the corrosion they cause hasn't been studied widely.9 Carboxylic acids in the form of acetic acid, formic acid, etc., can incur corrosion damage on various metals, such as nickel, copper, zinc, etc., in many indoor atmospheric environments. In many industries, the out-gassing of organic constituents, followed by their gradual oxidation to carboxylic acid, generates these acids.10 Organic acids are weaker than mineral acids but can still be corrosive.11 In oil and gas industries, acidizing is sometimes done where an acidic solution, such as hydrochloric, acetic, formic, sulfamic acid, etc., is injected to promote good productivity. The acids are generally used in the 5–35% concentration range. However, even though this highly concentrated acid injection crushes the mineral build-up in the rocky matrix to boost the well capacity, the metallic components of the process become highly prone to severe corrosion damage from the aggressivity of the acid.12 Therefore, adding inhibitors to the acidic solution is vital in this process. Furthermore, formic acid corrosion has been reported to have happened during the oxidation and burning of methanol fuel, which has been deemed cleaner and quite popular.13 Therefore, formic acid corrosion across many industries can't be ignored.
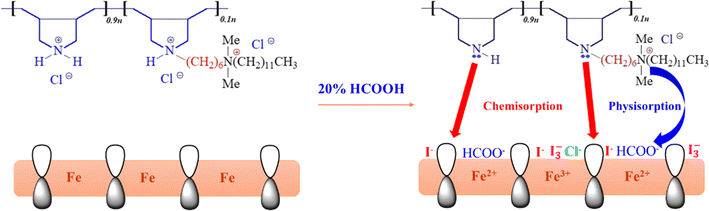
Organic compounds that consist of heteroatoms, lone pairs, π-electrons, multiple bonds, and/or aromatic functionalities adsorb very well on the metal surface.14 Polymers have multiple adsorption centers and an immense ability to cover the metal surface. In addition, nitrogenous compounds are found to be excellent inhibitors because they can trigger physical and chemical adsorption.2 Studies have shown that polymeric surfactants are very effective in arresting corrosion.15–19 The hydrophilic portions of the surfactants interact with the metal surfaces, and the hydrophobic parts spread out toward the water molecules and repel them. This study explores the mild steel corrosion inhibition efficiency of a homopolymer (3), poly(diallylammonium chloride), and some copolymers (8a–c) containing pyrrolidine ring and a long hydrophobic/hydrophilic cationic pendant (5–20%) moieties (Scheme 1) in a 20% formic acid solution. We found earlier that homopolymer (3), poly(diallylammonium chloride), was very efficient in inhibiting 1 M HCl-induced 304L stainless steel corrosion at 30 °C, a condition different from the current one in terms of the electrolyte and metal specimen.20 The rationale behind orchestrating the homopolymer (3) with hydrophilic/hydrophobic pendant is founded upon the facts that nitrogen-containing imidazoles, long-chain aliphatic diamines, and quaternary ammonium-based compounds best tackle the acid-induced and underground pipeline corrosions.21,22 Furthermore, when the same hydrophilic/hydrophobic pendant was added to poly(methyldiallylammonium chloride), a variant of homopolymer (3), the resulting co-polymeric corrosion inhibitor retarded CS 1018 corrosion in 15% HCl by >99%.23 Therefore, this gave us the impression that modifying homopolymer (3) with that hydrophilic/hydrophobic pendant will produce a new, robust, and very efficient co-polymeric corrosion inhibitor to be studied for organic acid corrosion.
As per the recent awareness raised in terms of using conventional, toxic corrosion inhibitors harmful to both human beings and the environment, the current trends in the study of corrosion inhibitors are all about designing, developing, and synthesizing corrosion inhibitors with variable functional motifs (quaternary ammonium salts) that are environmentally benign. A corrosion inhibitor can be declared non-toxic based on several indices, such as environmental toxicity, bioaccumulation, biodegradability, etc. International commissions like Oslo and Paris (OSPAR) and Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) set these parameters.24 The current study aims to cover extensive theoretical investigation to declare the studied corrosion inhibitors safe and non-toxic.
Recently, the synergism of corrosion inhibitors with additives to combat corrosion has become a very useful trend, as it helps reduce the required dosage of corrosion inhibitors and widens their applications in a range of corrosive media. It has been reported that halide ions help organic inhibitors adsorb onto metal surfaces and improve their inhibitive effects.25,26 Solomon and Umoren studied the synergism of polypropylene glycol (PPG) and KI for mild steel corrosion in 0.5 M H2SO4.27 Adding 5 mM KI to 1000 ppm of PPG improved the IE from 83.8% to 98.7%. In another study, the same authors showed an impressive increase in IE for adding 2 mM KI to 1000 ppm of poly(methacrylic acid) (PMAA). While 1000 ppm of PMAA showed only 56.0% IE for mild steel corrosion in 0.5 M H2SO4, the IE increased to 95.9% for a mixture of 1000 ppm of PMAA and 2 mM KI.28 Ansari et al. studied the anticorrosive effect of polyethyleneimine-grafted graphene oxide (PEI-GO) towards carbon steel corrosion in 15% HCl. While 100 ppm of PEI-GO imparted a maximum IE of 90.46%, a blend of 5 mM KI and 50 ppm of PEI-GO improved it to 95.77%.29 Sovizi and Abbasi showed that the addition of 5 mM KI to 0.5 g L−1 of carboxymethyl cellulose (CMC) improved the IE from 65.2 to 80.4% for aluminium corrosion in 2 M H2SO4.30 Haladu et al. studied the anticorrosion behavior of a cyclic cationic polymer (CCP) for low carbon steel corrosion in 15% HCl. Without adding KI, CCP imparted a maximum IE of 73.3% for a very high concentration of 500 ppm. However, when 5 mM KI was added, the IE improved to 95.1%.31
In this study, the inhibitory potential of inhibitor 8b showed the highest inhibitory potential via gravimetric weight loss analysis. Several corrosion measurement techniques, both gravimetric and electrochemical, were employed to study the synergistic effects of the corrosion inhibitor. A computational analysis DFT was utilized to realize the mechanical and interactive functions of the corrosion inhibitors.
2. Experimental
2.1 Materials
Diallylamine (1), N,N-dimethyldodecylamine (4), ammonium persulfate (APS), and 1,6-dibromohexane (5) were purchased from Sigma-Aldrich. Spectra/Por membrane (MWCO: 3.5 kDa) from Spectrum Laboratories Inc. was used to dialyze the synthesized polymers. Mild steel metal samples were obtained from Chrome Contracting Ltd, Riyadh, Saudi Arabia. The composition (wt%) of the mild steel samples was as follows: C (0.21%), Si (0.29%), P (0.06%), Mn (0.49%), S (0.05%), Cu (0.06%), and Fe (balance). The test solution (20% formic acid) was prepared from 98% formic acid purchased from Sigma-Aldrich.2.2 Physical methods
The measurement of the atomic compositions was carried out by an elemental analyzer (2400 Series II CHNS/O Analyzer – PerkinElmer). The recording of the IR spectra was conducted by an FTIR spectrometer (PerkinElmer 16F PC). A 400-MHz Bruker Avance III spectrometer was utilized to record 1H- and 13C-NMR spectra using CD3OD and D2O as the solvents. HOD and 13C dioxane signals at δ 4.65 and 67.4 ppm were used as a reference, respectively. An Ubbelohde viscometer with a viscometer constant of 0.005 cSt s−1 was used to measure the viscosities at 30.0 ± 0.1 °C under N2- and CO2-free water conditions.2.3 Polymer synthesis
Additionally, monomers as white crystals were obtained in a separate experiment by freeze-drying an aqueous solution of 2. δH (D2O) 3.44 (4H, d, J 6.4 Hz), 5.27 (4H, m), 5.69 (2H, m); δC (D2O) 49.49 (2C), 124.62 (2C), 128.19 (2C), (dioxane: δ 67.4 ppm).
δH (D2O) 0.71 (3H, t, J 6.8 Hz), 1.10–1.50 (22H, m), 1.57 (4H, m), 1.76 (2H, m), 2.98 (6H, s), 3.16 (4H, m), 3.39 (2H, J 6.4 Hz); δC (D2O) 14.82 (1C), 22.99 (1C), 23.17 (1C), 23.57 (1C), 25.80 (1C), 26.94 (1C), 28.17 (1C), 30.01 (1C), 30.41 (1C), 30.47 (1C), 30.76 (3C), 32.88 (1C), 33.11 (1C), 35.80 (1C), 52.89 (2C), 63.11 (2C) (dioxane: δ 67.4 ppm).
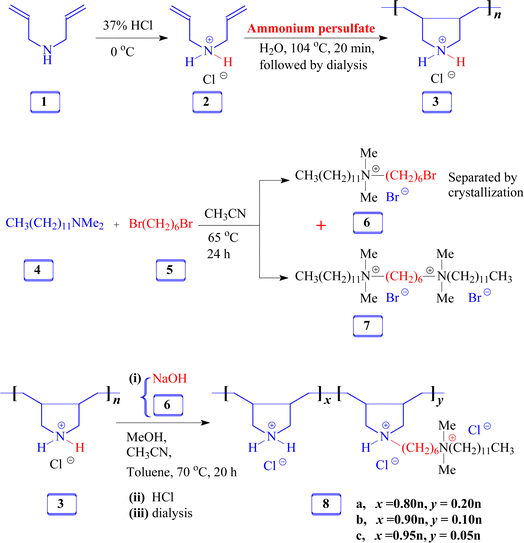
2.3.3.1 Copolymer 8a (x = 0.8n; y = 0.2n). To a mixture of 2.67 g (20 mmol repeating unit) of 3 in 8 mL of methanol, 0.68 g (17 mmol) of powdered NaOH was added. A white paste containing NaCl was obtained following a 30-min stirring of the mixture. Afterward, 40 mL of acetonitrile, 40 mL of toluene, and 1.83 g (4.0 mmol) of quaternary bromide 6 were added successively. Under N2, the mixture was heated at 70 °C for 20 h. Using a rotary evaporator, the solvents were removed at 70 °C. Furthermore, 25 mL of methanol was used to triturate the residual mixture and rotovaped once again to eradicate toluene completely. The residual mixture was transferred to a dialysis tube (MWCO: 3500 Da) using 10 mL of conc. HCl, to exchange Cl− with Br−, and a minimum quantity of methanol. The dialysis was continued for 24 h against distilled water. The dialyzed solution was freeze-dried to afford a creamy white solid of hydrophobically modified copolymer 8a (3.6 g, 90%). νmax (KBr): 3485, 2930, 2855, 2730, 2062, 1626, 1466, 1384, 1256, 1129, 1046, 980, 897, 728, 671, 620, and 464 cm−1. FTIR spectrum is displayed in Fig. S3.†
2.3.3.2 Copolymer 8b (x = 0.9n; y = 0.1n). The reaction and workup described in Section 2.3.3.1 were repeated using polymer 3 (4.0 g, 30 mmol), quaternary bromide 6 (1.37 g, 3.0 mmol), NaOH (25 mmol), acetonitrile (60 mL), and toluene (60 mL). The procedure afforded (8b) (4.45 g, 89%).
2.3.3.3 Copolymer 8c (x = 0.95n; y = 0.05n). The above reaction and workup described in Section 2.3.3.1 were repeated using polymer 3 (4.0 g, 30 mmol), quaternary bromide 6 (0.686 g, 1.5 mmol), NaOH (25 mmol), acetonitrile (60 mL), and toluene (60 mL). The procedure afforded (8c) (3.97 g, 88%).
2.4 Corrosion measurements
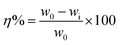 | (1) |
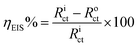 | (2) |
 | (3) |
2.5 Surface tension
The surface tension of inhibitor 8b, both with and without 2 mmol KI, was measured in the test solution. A PHYWE Surface Tensiometer (Germany), following the Du Nouy ring method, was used for this purpose. A torsion dynamometer with a front scale range of 10 mN was associated with the surface tensiometer. The tear-off force was measured with a platinum-iridium ring with a radius of 94 cm. Eqn (4) was used to find out the standard free energy of micellization.
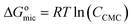 | (4) |
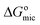 , CCMC, and R represent Gibbs free energy of micellization, critical micelle concentration, and molar gas constant, respectively.
, CCMC, and R represent Gibbs free energy of micellization, critical micelle concentration, and molar gas constant, respectively.
2.6 Surface studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 μm diameter) was used for X-ray photoelectron spectroscopy (XPS) analysis. The base pressure was maintained at 10−10 mbar during the experiments. C 1s peak at 284.8 eV was used as the reference. Avantage software (version 5.51) was used for spectra deconvolution and peak fitting. The metal coupons for XPS analysis were cleaned as described earlier for the weight loss study. In a solution of 20% formic acid containing inhibitor 8b and 2 mmol KI at 30 °C, the coupons were immersed for 3 h. After the study period had elapsed, distilled water was used to wash the coupons, which were then dried in an ambient air stream, followed by the analysis.
650 μm diameter) was used for X-ray photoelectron spectroscopy (XPS) analysis. The base pressure was maintained at 10−10 mbar during the experiments. C 1s peak at 284.8 eV was used as the reference. Avantage software (version 5.51) was used for spectra deconvolution and peak fitting. The metal coupons for XPS analysis were cleaned as described earlier for the weight loss study. In a solution of 20% formic acid containing inhibitor 8b and 2 mmol KI at 30 °C, the coupons were immersed for 3 h. After the study period had elapsed, distilled water was used to wash the coupons, which were then dried in an ambient air stream, followed by the analysis.2.7 Toxicological evaluation
The environmental friendliness of inhibitor 8b was predicted via the Toxicity Estimation Software Tool (TEST) (Developer: USAEPA, version: 5.1).32 To estimate toxicity, the software takes advantage of Quantitative Structure–Activity Relationships (QSARs). QSARs are mathematical models that use molecular descriptors, which are different chemical and physical characteristics, to predict toxicity. In the current investigation, a widely recognized QSAR, the hierarchical clustering method, has been used to predict toxicity. This model estimates the toxicity of a given query by taking the weighted average of the predictions from several different models.2.8 Density functional theory
Quantum chemical calculations were carried out using a single repeating unit (MW: 135.64 and 601.27 Da for 3 and 8b, respectively) of the polymer. To fully optimize the geometries of the chemical structures, quantum chemical calculations were performed using density functional theory (DFT) at Becke-3-parameter-Lee-Yang-Par (B3LYP) level of theory and 6-31+G (d,p) as the basis set,33 implemented in Gaussian software. The electronic properties of an inhibitor dictate how efficiently an inhibitor can undergo adsorption onto a metal surface.34 Therefore, several important parameters, such as the energy gap between the HOMO and LUMO (ΔE), ionization energy (IE), electron affinity (EA), etc., were calculated at the same level of theory. Frontier molecular orbitals (FMO) of inhibitors 3 and 8b were visualized using Gauss View 3.0 and considered to find the molecular sights the metal surface interacts with.3. Results and discussion
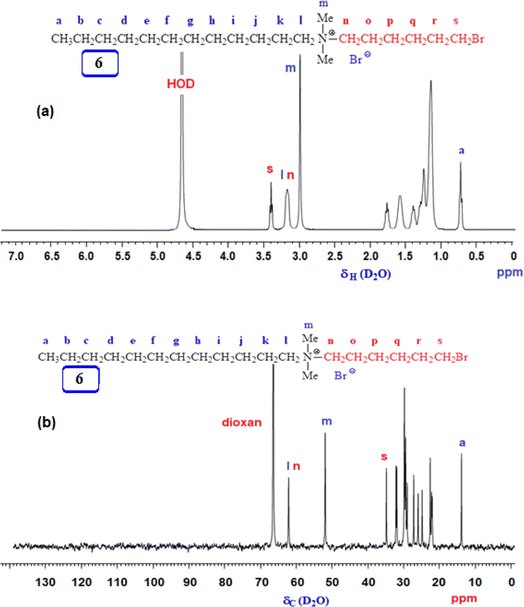
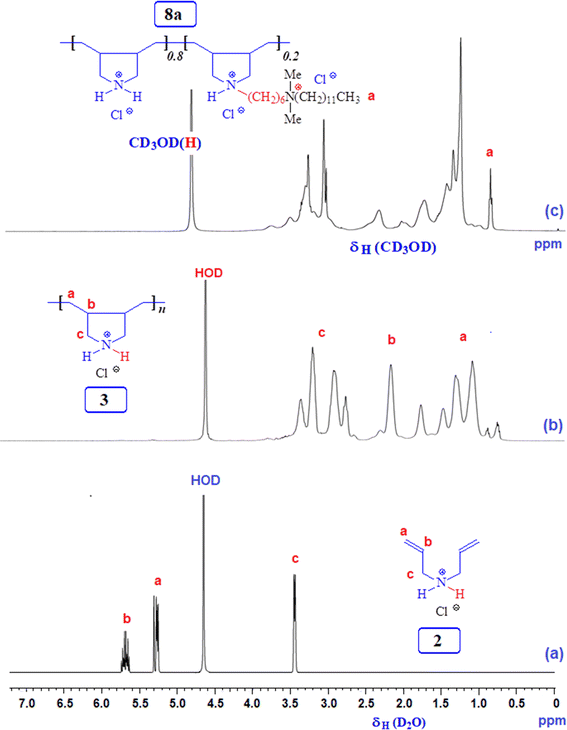
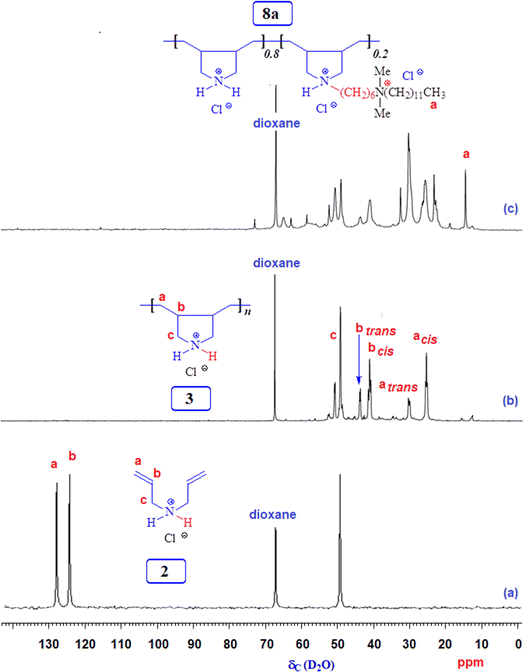
3.1 Polymer synthesis
The FTIR and NMR spectra were utilized to confirm the chemical structures of the compounds synthesized in this study. Fig. 1 shows the 1H and 13C NMR spectra of monocationic salt 6. The proton, carbon count, and area integration of the proton signals confirmed the structure. Besides, the elemental analysis of quaternary ammonium salt 6 closely matches the calculated values, ensuring the synthesis of the structure. Fig. 2 and 3 depict the 1H- and 13C-NMR spectra of monomer 2, cyclopolymer 3, and modified cyclo-copolymer 8a, respectively. The assignments of NMR peaks are based on numerous earlier works.35–37The absence of residual alkene signals for monomer 2 in the 1H- (Fig. 2a) and 13C-NMR spectrum (Fig. 3a) can be ascribed to chain termination via abstraction of allylic proton and/or coupling. Each carbon marked ‘a’ and ‘b’ appeared as two signals, thereby indicating the cis- and trans-dispositions of the vicinal substituents in an approximate ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3b). The presence of methyl protons and carbon (marked as ‘a’) in Fig. 2c and 3c confirm the incorporation of hydrophobic/hydrophilic pendant in 8a.
1 (Fig. 3b). The presence of methyl protons and carbon (marked as ‘a’) in Fig. 2c and 3c confirm the incorporation of hydrophobic/hydrophilic pendant in 8a.
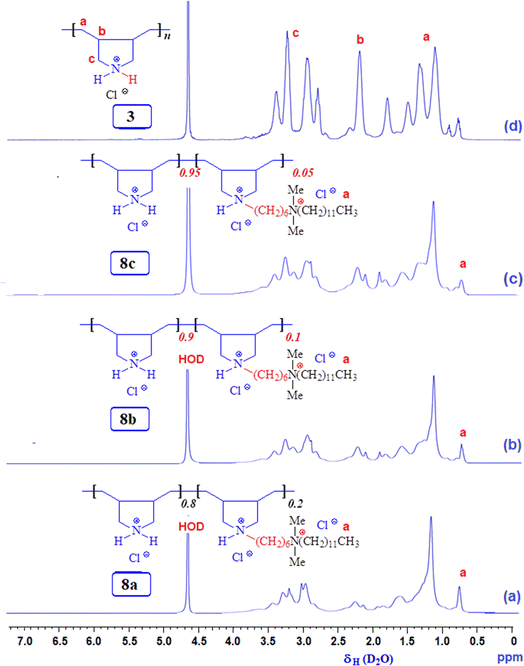
3.2 Compositions of modified copolymers 8a, 8b, and 8c
Three modified cyclo-copolymers, 8a, 8b, and 8c, with a degree of modification of 20%, 10%, and 5%, were successfully synthesized (Scheme 1). Their 1H NMR spectra in D2O are displayed in Fig. 4. Methyl protons marked ‘a’ can be seen in Fig. 4a–c, where the intensity of the protons decreases as the concentration of the hydrophobe is decreased from 20% (Fig. 4a) to 15% (Fig. 4b) to 5% (Fig. 4c). However, the integrations of methyl protons cannot be used to calculate the degree of modifications since the signals overlap with other signals. As illustrated in the case of 8a in Fig. 2c, luckily, the methyl protons appeared as clear signals when CD3OD was used as the solvent. The methyl protons of 8b and 8c were also found to be free of any overlapping signals in solvent CD3OD. The degree of methyl protons incorporation was determined via the 1H NMR spectra recorded in CD3OD. The degree of incorporation almost matched the feed ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 90
20, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 95
10, and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for 8a, 8b, and 8c, respectively.
5 for 8a, 8b, and 8c, respectively.
For quantitative analysis, the full relaxation of 1H NMR signals (which usually have a T1 of 1 s) was ensured by employing a pulse width of 14 μs for 90° pulse (hard pulse) with a power level of 9.9 W and a delay time of 5 s. To calculate the degree of hydrophobe incorporation, the area integration of CH3 of the hydrophobe (Fig. 2c, marked ‘a’) is taken as A, so the area for 1H would be (A/3). The modified ring has 53 Hs (exchangeable NH is not counted), so its area integration is 53 × (A/3). The total area B belongs to all the protons of both rings. So, the area for the protons in the unmodified ring becomes B − 53 × (A/3). Since the unmodified ring has 10 Hs, the area for its 1H becomes [B − 53 × (A/3)]/10. The percent incorporation of the hydrophobe thus becomes:
3.3 Viscosity of the parent polymer 3 and modified cyclocopolymers 8a–c
Viscosity plots of the homopolymer and copolymers are shown in Fig. S4.† The intrinsic viscosity [η] values determined by an Ubbelohde viscometer were 0.105, 0.080, 0.121, and 0.115 dL g−1 for 3, 8a (20% incorporation), 8b (10% incorporation), and 8c (5% incorporation), respectively. An excessive number of hydrophobic pendants in (8a) presumably leads to self-micellization, thereby decreasing its hydrodynamic volume and viscosity. An increase in hydrophobe content is known to enhance intramolecular association.38,39 In the lower hydrophobe content range, the distance between the hydrophobe units in the polymer backbone increases, thereby preventing them from undergoing intramolecular association.3.4 Thermogravimetric analysis
Thermogravimetric (TGA) analysis of parent polymer 3 and cyclo-copolymer 8b was studied in the 20–800 °C temperature range under N2 flow condition (50 mL min−1) and at a temperature increment of 10 °C min−1. The corresponding TGA curves are shown in Fig. S5.† Both 3 and 8b seem to be experiencing ≈5% weight loss until 250 °C. This can be attributed to polymer surfaces having some water molecules attached. Overall, the polymers are stable until 250 °C, encouraging their applications at high temperatures.3.5 Weight loss studies
The efficacies of 3 and 8a–c toward arresting mild steel corrosion at 30 °C are shown in Table 1 and Fig. 5a. The weight loss study was initially conducted at 100 ppm of inhibitors 3 and 8a–c. It was revealed that homopolymeric corrosion inhibitor 3 imparted the lowest IE of 56.1%. On the other hand, among the three modified copolymers 8a, 8b, and 8c, the order of IE was found to be 8b (77.1%) > 8c (67.9%) > 8a (61.1%). The reason for modified copolymers performing better than corresponding homopolymer 3 can be attributed to copolymers containing an additional hydrophilic/hydrophobic bis-quaternary salt pendant, which has helped the copolymers to cover a larger surface area of the mild steel. On the other hand, copolymer 8a was found to be the least efficient among all three copolymers. The presence of an excessing number (20%) of the hydrophobic pendant in 8a has probably caused the copolymer to be less soluble and impart less IE, or the excessive hydrophobic pendant has caused a heavy crowdedness near the metal surface and didn't allow the inhibitor to undergo adsorption to a meaningful extent. Alternately, self-micellization could also have ensued before the copolymer could cover the whole surface due to an excessive hydrophobic pendant. Copolymer 8b, with a good balance between a hydrophobic and hydrophilic portion of the chain, was found to be the most efficient of all three copolymers. Three quaternary ammonium salt moieties (NR4+) have facilitated excellent adsorption. The long hydrophobic chain also repelled the corrosive water molecules away from the surface. Copolymer 8c with a 5% hydrophobic pendant was revealed to take the intermediate stance in terms of IE. Therefore, copolymer 8b was chosen for further study on the effect of concentration on IE and corrosion rate (CR). Table 1 shows that the IE of 8b kept increasing until 100 ppm. For 200 ppm of 8b, IE started decreasing, which can be attributed to inhibitor desorption. As such, 100 ppm of inhibitor 8b concentration was chosen for surface study. Fig. 5b shows the variation in mild steel corrosion rates with respect to inhibitor concentration. Reasonably, 100 ppm of 8b decreased the CR the most.| Sample | Conc. (ppm) | KI (mmol) | Weight loss (mg) | CR (mm year−1) | Surface coverage (θ) | ηWL (%) |
|---|---|---|---|---|---|---|
| Blank | — | — | 45.9 ± 1.05 | 7.09 ± 0.16 | — | — |
| 1 | — | 21.3 ± 0.95 | 3.29 ± 0.15 | 0.536 | 53.6 ± 1.62 | |
| 1 | 2 | 3.58 ± 0.28 | 0.55 ± 0.04 | 0.922 | 92.2 ± 0.44 | |
| 5 | — | 18.7 ± 0.20 | 2.89 ± 0.03 | 0.592 | 59.2 ± 0.78 | |
| 5 | 2 | 2.16 ± 0.06 | 0.33 ± 0.01 | 0.953 | 95.3 ± 0.17 | |
| 10 | — | 16.8 ± 0.15 | 2.60 ± 0.02 | 0.633 | 63.3 ± 0.46 | |
| 10 | 2 | 1.81 ± 0.37 | 0.28 ± 0.06 | 0.961 | 96.1 ± 0.82 | |
| 8b | 20 | — | 15.8 ± 0.95 | 2.44 ± 0.15 | 0.656 | 65.6 ± 1.25 |
| 20 | 2 | 1.38 ± 0.21 | 0.21 ± 0.03 | 0.970 | 97.0 ± 0.26 | |
| 50 | — | 14.6 ± 0.79 | 2.25 ± 0.12 | 0.682 | 68.2 ± 1.20 | |
| 50 | 2 | 0.67 ± 0.12 | 0.10 ± 0.02 | 0.986 | 98.6 ± 0.36 | |
| 100 | — | 10.5 ± 0.36 | 1.63 ± 0.06 | 0.771 | 77.1 ± 0.29 | |
| 100 | 2 | 0.61 ± 0.03 | 0.09 ± 0.00 | 0.987 | 98.7 ± 0.10 | |
| 200 | — | 11.8 ± 0.17 | 1.82 ± 0.03 | 0.743 | 74.3 ± 0.92 | |
| 200 | 2 | 0.86 ± 0.05 | 0.13 ± 0.01 | 0.981 | 98.1 ± 0.10 | |
| 5 | 100 | — | 20.2 ± 0.30 | 3.11 ± 0.05 | 0.561 | 56.1 ± 0.36 |
| 8a | 100 | — | 17.8 ± 0.36 | 2.60 ± 0.06 | 0.611 | 61.1 ± 0.36 |
| 8c | 100 | — | 14.7 ± 0.53 | 2.27 ± 0.08 | 0.679 | 67.9 ± 0.46 |
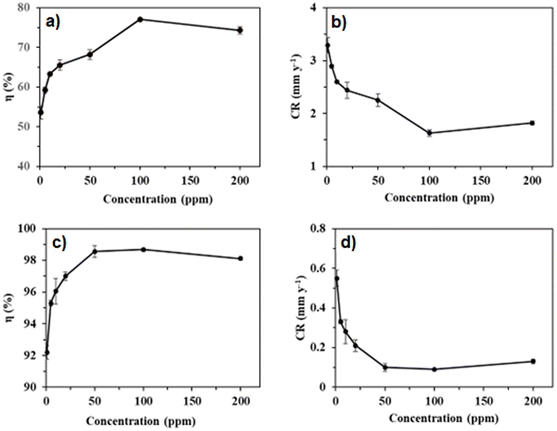 | ||
| Fig. 5 Effect of inhibitor 8b concentrations on the IE (a) and CR (b); synergistic effect of inhibitor 8b concentrations and 2 mmol KI on the IE (c) and CR (d). | ||
Further, the impact of 2 mmol KI as an additive on the IE of inhibitor 8b was studied. The use of additives to enhance key inhibitors' efficacy isn't uncommon. This is also valid when the corrosive solution is much more aggressive, the study temperature is too high, or both. Halide ions are known to stabilize the adsorption of organic inhibitors. This, in turn, increases the inhibitive effect. Ascribed to the polarizability issue, I− ion is the most effective in this regard, followed by Br− and Cl−.25,26 Following the synergistic effect of 2 mmol KI, it was found that, as shown in Table 1, the IE of inhibitor 8b increases significantly, with 1 ppm of the inhibitor and 2 mmol KI imparting more than 90% IE, which is truly remarkable. The IE of 8b for every studied concentration increased slowly upon adding 2 mmol KI. The effect of 2 mmol KI on the IE and CR in the presence of 8b are shown in Fig. 5c and d, respectively. In the presence of KI, the CR decreases dramatically, with the CR for the synergism of 100 ppm 8b and 2 mmol KI reducing by 98.7% from that of control. The synergistic IE didn't increase significantly after reaching 98.6% for 50 ppm of 8b and 2 mmol KI. As such, this combination has been used to gain insights into surface morphologies, thermodynamic parameters, and the effect of temperature.
3.6 Adsorption isotherm
The adsorption of inhibitors, dictated by several factors, such as the structure of the inhibitor, charge distribution, the type of the metal and its surface, etc., releases valuable information about the mechanism.40 The adsorption of the inhibitor at the metal/solution interface is governed by several factors, such as the type of metal and its surface, the inhibitor structure, the charge distribution, etc. A few adsorption isotherms were investigated for the current study. For Langmuir adsorption isotherm, the regression of coefficient (R2) value was found to be nearer to one, indicating that it’s the best-followed isotherm. Eqn (5), where Kads, C, and θ define the equilibrium constant, concentration, and surface coverage, respectively, as a mathematical expression for Langmuir adsorption isotherm. Table 2 summarizes the information obtained from all the studied isotherms. Eqn (6), where R implies molar gas constant and T highlights the experimental temperature (303 K), was used to calculate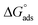 , or the Gibbs free energy of adsorption. The numerical value 55.5 in eqn (6) is the molar concentration of water in the test solution.
, or the Gibbs free energy of adsorption. The numerical value 55.5 in eqn (6) is the molar concentration of water in the test solution.
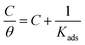 | (5) |
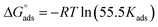 | (6) |
Fig. S6† shows the Langmuir adsorption isotherm plot that was used to find Kads from the intercept. The obtained value of Kads was used to find the value of 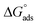 using eqn (6). The values of both Kads and
using eqn (6). The values of both Kads and 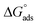 are shown in Table 2. A very high value of 2.48 × 105 L mol−1 for Kads demonstrates the strong adsorption of inhibitor 8b onto mild steel surfaces. The adsorption process can be regarded as physisorption or chemisorption if the absolute value of
are shown in Table 2. A very high value of 2.48 × 105 L mol−1 for Kads demonstrates the strong adsorption of inhibitor 8b onto mild steel surfaces. The adsorption process can be regarded as physisorption or chemisorption if the absolute value of 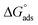 is less than 20 or greater than 40 kJ mol−1, respectively. A mixed physi-chemisorption is considered when the absolute value of
is less than 20 or greater than 40 kJ mol−1, respectively. A mixed physi-chemisorption is considered when the absolute value of 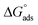 falls between this range.41,42 The value of
falls between this range.41,42 The value of 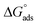 in the current study has been calculated to be −41.5 kJ mol−1, indicating inhibitor 8b has undergone adsorption onto mild steel via chemisorption. Overall, the negative sign associated with the value of
in the current study has been calculated to be −41.5 kJ mol−1, indicating inhibitor 8b has undergone adsorption onto mild steel via chemisorption. Overall, the negative sign associated with the value of 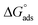 ensures that the adsorption process is spontaneous.19,40
ensures that the adsorption process is spontaneous.19,40
3.7 Effect of temperature and thermodynamic evaluation
Fig. 6a shows the variation in IE of KI (2 mmol) alone and 8b (50 ppm) in the presence of 2 mmol KI measured at different time intervals of 3, 6, 12, and 24 h via WL technique at 60 °C. It is evident that the IE of KI remained unchanged until 12 h and then reduced drastically when measured at 24 h. On the contrary, the synergistic effect of 8b and 2 mmol KI was so strong that its IE remained consistently close to 100% for the entire study period conducted at 60 °C. This study was carried out to point out that the contribution of KI is only to enhance the IE of 8b. KI alone isn't enough as a corrosion inhibitor in the current investigation, as few studies are available demonstrating the usage of KI alone as a corrosion inhibitor.43–45 To demonstrate the impact of temperatures on the IE of 8b (100 ppm) or the corrosion rate, weight loss studies were performed at 303, 313, 323, and 333 K for 3 h. Fig. 6b shows the change of IE and corrosion rate at different temperatures; Table 3 shows various thermodynamic parameters. There is no definitive pattern in the change of IE that is quite the same over the studied temperature range while the corrosion rate increases slightly.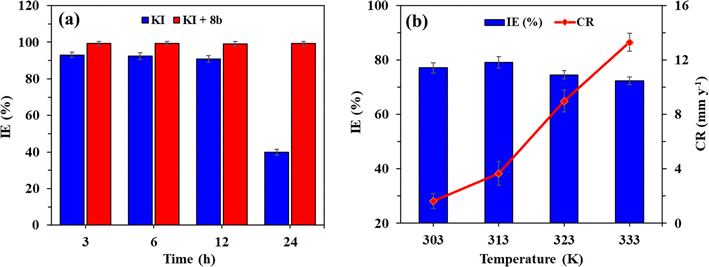 | ||
| Fig. 6 (a) Variation of the IE of KI and 8b (50 pppm) with 2 mmol KI at different time intervals at 60 °C; (b) impact of temperature (303–333 K) on the IE imparted by 8b (100 ppm). | ||
| Temp. (K) | % IE | CR (mm year−1) | Ea (kJ mol−1) | ΔHads (kJ mol−1) | ΔSads (J mol−1 K−1) | |||
|---|---|---|---|---|---|---|---|---|
| Blank | Inhibitor 8b | Blank | Inhibitor 8b | Blank | Inhibitor 8b | |||
| 303 | 77.1 | 1.63 | 54.3 | 60.6 | 51.7 | 58.0 | −57.3 | 6.84 |
| 313 | 79.1 | 3.67 | ||||||
| 323 | 74.5 | 8.98 | ||||||
| 333 | 72.5 | 13.3 | ||||||
The corrosion rate values from different temperatures (303–313 K) can be further used to evaluate the thermodynamic parameters outlined in Table 3. Fig. S7a† shows the Arrhenius plots from which the activation energy (Ea) for the dissolution of mild steel in the presence and absence of the inhibitor was calculated using eqn (7). The transition state plots (Fig. S7b†) were obtained using eqn (8) and were used to find out the enthalpy (ΔHads) and entropy of adsorption (ΔSads) values.
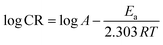 | (7) |
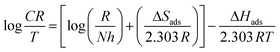 | (8) |
The Ea values were calculated as 54.3 and 60.6 kJ mol−1 in the absence and presence, respectively, of 8b, indicating that the corrosion process is more difficult in the presence of the inhibitor.18 Reportedly, if the Ea value of the inhibited solution is greater than that of blank/control; the inhibition process is believed to follow physisorption. On the contrary, if the value of Ea for the inhibited process is equal to or less than that of blank/control, the inhibitor is believed to have undergone chemisorption.46–48 However, some reports and findings contradict this consensus.49,50 Nevertheless, the Ea parameter, on its own, can't decide the adsorption process. There's stiff competition between the water and inhibitor molecules for the adsorption. Therefore, displacing the water molecules from the adsorption sites takes some activation energy on the part of the inhibitor.51 Moreover, the Ea values for the inhibited process and the blank/control aren't staggeringly different from refuting our earlier finding that inhibitor 8b has undergone chemisorption. It has been reported that if |ΔHads| ≤ 40 kJ mol−1, physisorption is proposed to have taken place. In contrast, chemisorption is thought to have happened for the condition: 40 kJ mol−1 < |ΔHads| < 100 kJ mol−1.52,53 The observed ΔHads value of 58.0 kJ mol−1 for the inhibited process confirms our adsorption study, finding that the inhibition process is chemisorption. Overall, the endothermic nature and bigger value of ΔHads for the inhibited process reconfirms that the corrosion phenomenon becomes more difficult when the inhibitor is present.54 Moreover, an increase in the ΔSads value for the inhibited process means increased randomness resulting from the displacement of the water molecules by the added inhibitor.55,56
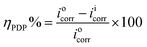
3.8 Potentiodynamic and linear polarization (PDP)
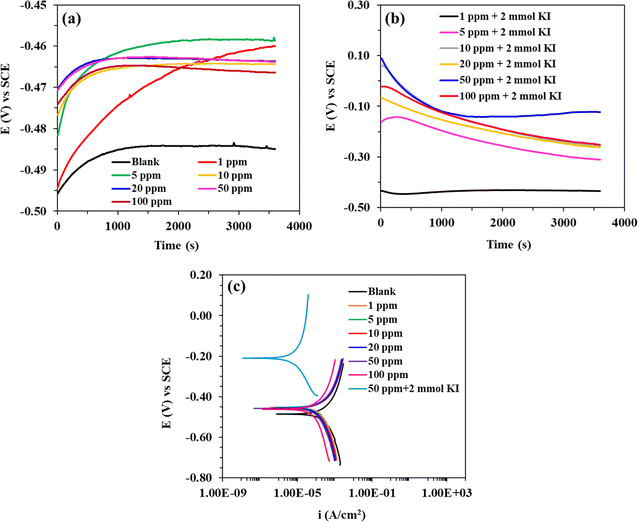
Fig. 7a and b show the OCP vs. time curves for mild steel corrosion in 8b without and with KI, respectively. The straight OCP lines achieved after 50–60 min of dissolution indicate that the surface oxide layer was completely dissolved. It can be observed that the addition of 8b causes the OCP curves to shift toward the more noble positive direction. The case is more prominent for the doses aided by 2 mmol KI. This clearly shows that the addition of 8b, with and without KI, works by the adsorption of the inhibitor at the metal/solution interface. Fig. 7c shows the Tafel curves representing different concentrations of 8b and the case where 50 ppm of 8b was aided by 2 mmol KI. The linear segments from the anodic and cathodic curves were extrapolated to obtain several parameters, such as anodic Tafel slope (βa), cathodic Tafel slope (βc), current density (icorr), corrosion potential (Ecorr), etc. Table 4 enumerates all these parameters, and the IE (ηPDP) values. The icorr values, as easily noticeable, were found to decrease remarkably when the inhibitor was present, confirming that the addition of the inhibitor can retard both half-cell reactions.20,47 This downward shift is more pronounced when 2 mmol KI aids the inhibitor. When the inhibitor is used alone, the maximum ηPDP of 81.7% was obtained for the concentration of 100 ppm of 8b. On the other hand, the ηPDP reached an amazingly high value of 99.1% for a mixture of 50 ppm 8b and 2 mmol KI. The values of both βa and βc decrease with increasing inhibitor concentration regardless of 2 mmol KI addition, indicating a general change in the corrosion mechanism. A corrosion inhibitor is regarded as either anodic or cathodic when Ecorr experiences a shift of more than 85 mV in the respective direction.55,56 Otherwise, the inhibitor is regarded as a mixed type. Interestingly, the shift in Ecorr doesn't exceed 85 mV when inhibitor 8b is used alone, indicating that 8b acts as a mixed type of inhibitor. However, when 8b is used in the presence of 2 mmol KI, the Ecorr values vary markedly, moving mostly toward the noble anodic direction. This implies that 8b, in the presence of 2 mmol KI, acts as an anodic inhibitor, a case reported earlier.57,58 Moreover, LPR experiments were carried out in the presence and absence of 8b and for the cases of 8b aided by 2 mmol KI. The Rp values obtained from each line were used to find out the efficiencies (ηLPR). As expected, Rp values kept increasing with increasing concentration, meaning that the metal surface was experiencing more protection with increasing inhibitor concentration. Commensurately, ηLPR values kept increasing as well, resonating with weight loss findings and PDP studies.| Conc. (ppm) | KI (mmol) | PDP | LPR | |||||
|---|---|---|---|---|---|---|---|---|
| Ecorr (mV) | βa (mV dec−1) | −βc (mV dec−1) | Icorr (μA cm−2) | ηPDP (%) | Rp (Ωcm2) | ηLPR (%) | ||
| Blank | — | −488.6 | 408.6 | 531.4 | 723.3 | — | 142.8 | — |
| 1 | — | −455.6 | 203.9 | 418.5 | 318.5 | 56.0 | 311.2 | 54.1 |
| 1 | 2 | −449.6 | 115.1 | 237.0 | 46.67 | 93.6 | 1645 | 91.3 |
| 5 | — | −455.6 | 210.4 | 408.8 | 284.2 | 60.7 | 358.7 | 60.3 |
| 5 | 2 | −397.0 | 319.7 | 231.2 | 15.46 | 97.9 | 4127 | 96.5 |
| 10 | — | −458.6 | 197.5 | 337.6 | 248.3 | 65.7 | 376.9 | 62.1 |
| 10 | 2 | −321.8 | 313.0 | 207.5 | 14.38 | 98.0 | 4791 | 97.0 |
| 20 | — | −458.6 | 190.3 | 375.3 | 235.7 | 67.4 | 419.8 | 66.0 |
| 20 | 2 | −266.2 | 323.4 | 198.0 | 13.48 | 98.1 | 6550 | 97.8 |
| 50 | — | −458.6 | 193.9 | 372.0 | 207.7 | 71.3 | 482.9 | 70.4 |
| 50 | 2 | −209.0 | 243.0 | 170.9 | 6.813 | 99.1 | 9037 | 98.4 |
| 100 | — | −461.7 | 245.8 | 440.9 | 131.8 | 81.8 | 701.0 | 79.6 |
| 100 | 2 | −324.8 | 181.0 | 156.6 | 7.057 | 99.0 | 9272 | 98.8 |
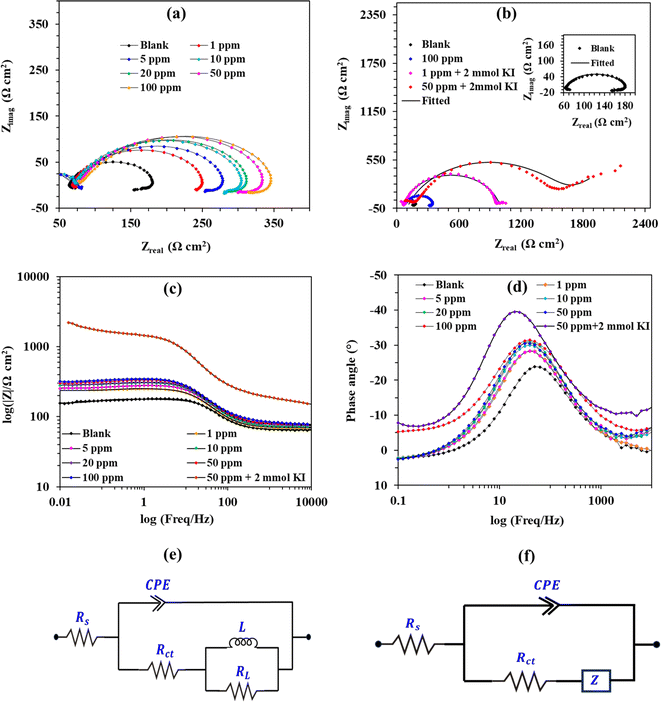
3.9 Electrochemical impedance spectroscopy (EIS)
EIS is an important electrochemical technique that discloses several important information about the interface. Fig. 8a–d show the Nyquist, Bode magnitude, and phase angle plots. Table 5 shows the relevant EIS parameters, which were obtained after fitting the Nyquist and Bode plots. Fig. 8a shows the Nyquist plots corresponding to the different concentrations of 8b. The plots are characterized by depressed semicircles, with the low-frequency region having an inductive loop and the high-frequency region having a capacitive loop. It is easily noticeable that adding an inhibitor causes the inductive loop to increase. The capacitive loop at the high-frequency region corresponds to the double layer and charge transfer corrosion process.| Conc. (ppm) | KI (mmol) | L (H cm2) | RL (Ω cm2) | Rct (Ω cm2) | n | Cdl (μF cm−2) | Zw × 10−3 (Ω cm2) | η (%) |
|---|---|---|---|---|---|---|---|---|
| Blank | — | 45.17 | 21.82 | 45.13 | 0.917 | 50.78 | — | — |
| 1 | — | 12.98 | 19.53 | 97.99 | 0.782 | 41.90 | — | 53.9 |
| 1 | 2 | — | — | 845.9 | 0.844 | 21.85 | 179.1 | 94.7 |
| 5 | — | 24.21 | 31.33 | 111.4 | 0.847 | 39.00 | — | 59.5 |
| 5 | 2 | — | — | 994.1 | 0.769 | 23.87 | 10.07 | 95.5 |
| 10 | — | 19.96 | 36.95 | 139.1 | 0.801 | 39.56 | — | 67.6 |
| 10 | 2 | — | — | 1015 | 0.756 | 22.96 | 8.772 | 95.6 |
| 20 | — | 29.16 | 36.59 | 154.9 | 0.839 | 37.70 | — | 70.9 |
| 20 | 2 | — | — | 1159 | 0.799 | 20.83 | 11.18 | 96.1 |
| 50 | — | 42.43 | 43.05 | 175.9 | 0.830 | 37.40 | — | 74.3 |
| 50 | 2 | — | — | 1381 | 0.760 | 42.21 | 8.038 | 96.7 |
| 100 | — | 38.71 | 47.15 | 192.1 | 0.785 | 42.06 | — | 76.5 |
| 100 | 2 | — | — | 1460 | 0.800 | 26.71 | 8.327 | 96.9 |
On the contrary, the inductive loop at the low frequency is ascribed to the relaxation process stemming from the interactions of the adsorbed H+ and HCOO− ions with inhibitor species.59,60 Fig. 8b shows the Nyquist plots for the use of 1 and 50 ppm of inhibitor 8b in synergism with 2 mmol KI. Interestingly, when KI is used to augment the inhibitory action of 8b, the low-frequency region shows Warburg impedance. The diameter of the semicircles is a general indication of the efficiency of an inhibitor.18,61,62 It is visible that increasing the inhibitor concentration helped the diameter of the semicircles increase, both in the cases of inhibitor 8b aided or not by 2 mmol KI. This gives the impression that increasing inhibitor concentration increases efficacy. A very small semicircle for 100 ppm of 8b, as shown in Fig. 8b, in comparison to those associated with inhibitor 8b aided by 2 mmol KI, validates the findings of the weight loss and potentiodynamic polarization studies. Fig. 8c shows the Bode magnitude plot, and Fig. 8d represents the Bode phase angle plot. Fig. 8c shows that impedance at the low-frequency side increases upon increasing the inhibitor concentrations, reaching the maximum IE for 50 ppm 8b in the presence of 2 mmol KI. At intermediate frequencies in Bode phase angle plots, there's one time constant with one maximum, revealing that one relaxation process is happening. The values of phase angles increasing with increasing inhibitor concentrations are also another indication of increased protection.
Fig. 8e shows the circuit used to fit the Nyquist plots obtained from the immersion of mild steel in 20% formic acid containing corrosion inhibitor 8b only. Fig. 8f shows the equivalent circuit used to fit the Nyquist plots corresponding to mild steel corrosion in the presence of 8b and 2 mmol KI. In the circuits, Rs, Rct, and Wd represent ohmic resistance, charge transfer resistance, and Warburg impedance, respectively. The inductive elements are represented by L and RL. The double-layer capacitance, Cdl, is affected by the heterogeneity of the surface. Therefore, a constant phase element (CPE) is used in the circuit to replace it. The impedance of the CPE, ZCPE, and the Cdl can be expressed in eqn (9) and (10), respectively.59
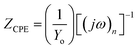 | (9) |
| Cdl = Yo(ωmax)n−1 | (10) |
Table 5 shows that the value of Rct, in comparison to that of blank, goes up with increasing inhibitor concentrations, both for the cases of 8b used with and without KI. This confers the idea that the movement of charges across the metal/solution interfaces gets more difficult with increasing inhibitor concentration. This is another way of saying that the metal surface is experiencing more protection.50 It is noticeable that each value of Rct for inhibitor 8b concentrations in the presence of 2 mmol KI is approximately seven to eight times higher than corresponding 8b concentrations without 2 mmol KI. This approves of what we have seen so far: a blend of inhibitor 8b and 2 mmol KI showing very good synergism. The increase in inhibitor concentration decreased the Cdl values. The reason behind this is the local dielectric constant and/or the exposed area of the metal reducing. Alternately, it can also be said that the increased thickness of the electrical double layer with increased inhibitor concentrations can also lead to a reduction in the values of Cdl. The increase in the values of Rct or decrease in the values of Cdl is strong evidence for the interaction between the inhibitors and the metal surface under study.63,64 Additionally, the values of n were found to be in the range 0.5 < n < 1, which, reportedly, is much like that of a non-ideal polarized electrode system.61,62
3.10 Surface tension
The surface tension vs. the concentration curves for inhibitor 8b in the presence and absence of 2 mmol KI are depicted in Fig. S8.† The critical micelle concentration (CMC) value found from extrapolating the curves is quite helpful in comparing the adsorption pattern of the inhibitor on either side of the CMC.65 The CMC values for inhibitor 8b were found to be 240 μmol L−1 (40 ppm) and 190 μmol L−1 (31.7 ppm) in the absence and presence of 2 mmol KI, respectively. Even before the CMC value was reached when inhibitor 8b was used alone, a considerable portion of the surface was already covered, which was approximately 80% of the maximum IE shown by the inhibitor. Even before the micellization has ensued, a uniform adsorption layer has been constructed already. After that, micelles can adsorb more to form multilayers that provide more protection. The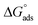 values were calculated to be −23.1 kJ mol−1 and −23.7 kJ mol−1 for inhibitor 8b in the absence and presence of 2 mmol KI, respectively. Adsorption isotherm study (vide supra) showed that the
values were calculated to be −23.1 kJ mol−1 and −23.7 kJ mol−1 for inhibitor 8b in the absence and presence of 2 mmol KI, respectively. Adsorption isotherm study (vide supra) showed that the 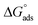 value for inhibitor 8b in the absence of 2 mmol KI was −41.5 kJ mol−1, a value that is much more negative than that of
value for inhibitor 8b in the absence of 2 mmol KI was −41.5 kJ mol−1, a value that is much more negative than that of 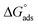 for the same inhibitor, which suggested the formation of a monolayer surface before micellization occurs at the metal surface.18
for the same inhibitor, which suggested the formation of a monolayer surface before micellization occurs at the metal surface.18
3.11 Surface morphology
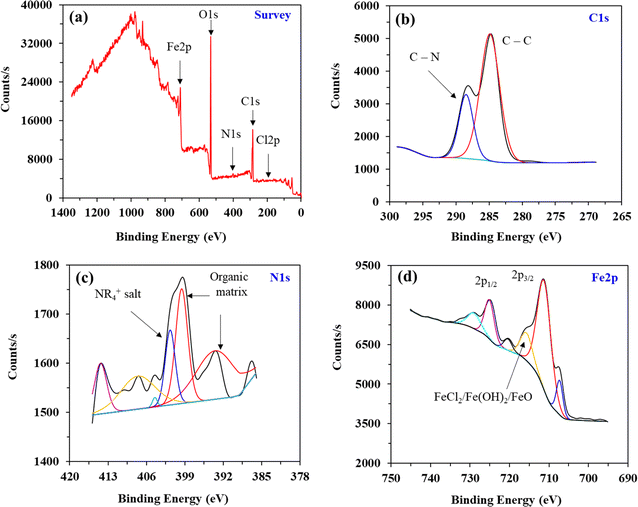 | ||
| Fig. 9 XPS survey (a) and deconvoluted C 1s (b), N 1s (c), and Fe 2p (d) spectra for mild steel corrosion in 20% formic acid containing 100 ppm of 8b at 30 °C. | ||
| Species | Peak BE (eV) | Assignment | Atom% |
|---|---|---|---|
| Fe 2p | 724.98 | Fe–Ox | 13.82 |
| 711.08 | Fe(OH)3/Fe2O3/FeCl3 | 54.69 | |
| 707.18 | Fe–Ox/Fe–OH | 8.03 | |
| 715.58 | FeCl2/Fe(OH)2/FeO | 12.29 | |
| 728.88 | FeCl2 | 7.77 | |
| 720.28 | Fe/Cu | 3.40 | |
| C 1s | 284.78 | C–C | 72.97 |
| 288.38 | C–N | 27.03 | |
| N 1s | 393.38 | Organic matrix | 31.56 |
| 399.48 | Organic matrix | 24.35 | |
| 401.58 | NR4+ salt | 13.10 | |
| 404.38 | — | 0.81 | |
| 407.48 | — | 20.00 | |
| 414.28 | — | 10.18 |
Furthermore, another peak at 288.38 eV position relating to C–N is another confirmation of 8b undergoing adsorption. The deconvoluted spectrum of N 1s (Fig. 9c) is somewhat exciting and confirms that inhibitor 8b underwent chemisorption, as we found through the adsorption study. The molecular structure of 8b shows that every nitrogen present in the compound is part of either a 2°-, 3°-, or 4°-ammonium salt moiety. However, the extent of ammonium salt present in the deconvoluted spectrum is 13.10%, as shown in Table 6. This implies that it’s possible that the protons attached to 2°- and 3°-ammonium salts could be abstracted by the concentrated 20% formic acid solution, leading to the nitrogens owning either a lone pair or two now. Otherwise, the atomic% of ammonium salt would have been much higher. If this happens, these nitrogens can now undergo chemisorption onto the metal surface via these lone pairs on nitrogen.
Nevertheless, two other prominent peaks at 393.38 and 399.48 eV corresponding to the organic matrix confirm the adsorption of the inhibitor. Fig. 9d shows Fe 2p1/2 and 2p3/2 spin orbitals regions without satellite peaks.67,68 Relative percentages of Fe2+ and Fe3+ from both orbitals account for approximately 45 and 55%, respectively. The binding energies at 724.98, 707.18, 715.58, and 728.88 eV are attributed to Fe2+. On the other hand, the binding energy at 711.08 eV is attributed to Fe3+.
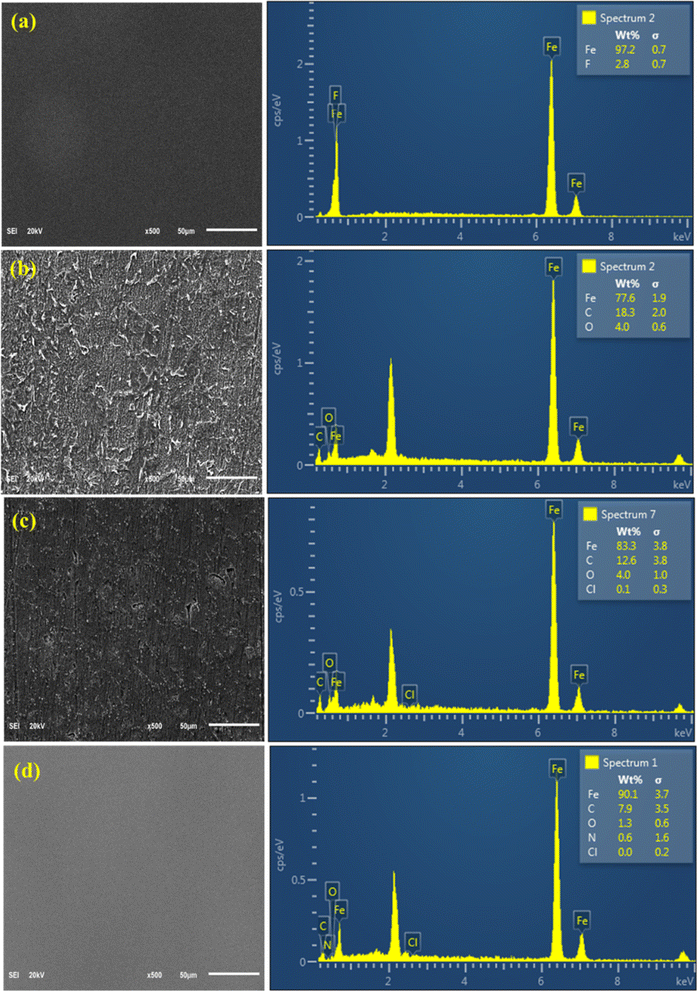 | ||
| Fig. 10 Surface morphologies and EDX spectra of polished mild steel (a), mild steel corroded in the absence (b) and presence (c) of 8b, and in the presence of 8b and 2 mmol KI (d). | ||
However, upon adding 100 ppm of 8b, the mild steel surface, represented by Fig. 10c, experiences less deterioration and shows a much better and smoother surface. Peaks for carbon and chlorine on the corresponding EDX spectrum prove that inhibitor 8b became adsorbed on the surface. Furthermore, 2 mmol KI was added, which augmented the inhibitory action 10b (50 ppm). The metal surface (Fig. 10d) looked incredibly smooth and almost the same as the polished one. This visual inspection strongly supports the extremely high efficiency found for the synergistic effect of 50 ppm 8b and 2 mmol KI. The emergence of a small nitrogen peak and reduction in oxygen peak intensity strongly support this remark.
3.12 Toxicology study
A toxicological study that doesn't entail unnecessary animal testing is a great way to gauge chemical compounds' ecological and health impact. Fathead minnow (Pimephales promelas) LC50 (96 h) is a widely recognized endpoint to assess the aquatic organisms' initial effect.69 The LC50 value refers to the concentration (in the unit of mg L−1) of a chemical that kills 50% of the test animals during the study period. Certain qualities, such as relative hardiness, tolerance of variable temperatures and harsh conditions, adaptability to controlled experimentation, etc., make the Fathead minnow an ideal test organism for toxicology studies. Fathead minnow can also be found in freshwater ecosystems and uninhabitable conditions such as waste drainage sites, rendering this aquatic species a worthy candidate for toxicological studies. A Fathead minnow LC50 (96 h) test simulation for the test compound 8b done via hierarchical clustering QSAR toxicity estimation method predicted the LC50 value to be 1157.99 mg L−1. USEPA states that a chemical compound can be practically non-toxic if the LC50 value exceeds 100 mg L−1.70 Therefore, the test compound 8b can be considered relatively benign. Furthermore, the mean absolute error (MAE), which is the absolute average distance between the actual data and the predicted data, for the Fathead minnow LC50 (96 h) test was 0.48, which is lower than that of the entire set (0.57). This confirms an increased confidence in the predicted value. Moreover, the developmental toxicity of the test compound 8b was also predicted using the hierarchical clustering method. Developmental toxicity indicates whether a particular chemical can interfere with human or animal growth. The test compound 8b was found to be a developmental non-toxicant. Bioconcentration factor (BCF) is another measure of how threatening a chemical is towards aquatic species, and it's a ratio of the chemical concentration in fish to that in water at a steady state. The BCF for the test compound 8b was predicted to be 12.28 (unitless). According to REACH regulations, a chemical is bio-accumulative if its BCF value exceeds 2000 and very bio-accumulative if its BCF value exceeds 5000.71 This implies the tested compound is very, very safe in terms of bioaccumulation.3.13 Density functional theory
The frontier molecular orbitals and the optimized structures of the homopolymer (3) and copolymer (8b) are depicted in Fig. 11. Table 7 enlists the quantum chemical descriptors relevant to the reactivity of these molecules. A close look at the frontier molecular orbitals (Fig. 11c–f) reveals that most of the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) charge-sharing and accepting capabilities, respectively, are located around the chloride counter ions and quaternary nitrogen moieties, observed in some earlier studies.18–20 The phenomena of donation and retrodonation, i.e., charge sharing, are crucial for the bonding between metal and inhibitor.72,73 It is evident that the chloride counterions and pyrrolidinium moieties were important for the adsorption. The remaining long hydrocarbon chain must have acted as a water-repellent and created a hydrophobic barrier.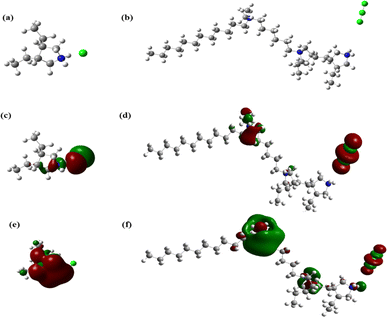 | ||
| Fig. 11 Optimized structures (a and b), HOMO (c and d), and LUMO (e and f) for inhibitors 3 (left) and 8b (right). | ||
| Inhibitor | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | IE (eV) | EA (eV) | η (eV) | χ (eV) | ω (eV) | σ (eV) | ΔN | μ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | −5.576 | 0.555 | 6.130 | 5.576 | −0.555 | 3.065 | 2.511 | 9.659 | 0.326 | 0.377 | 9.350 |
| 8b | −0.204 | 0.013 | 0.217 | 0.204 | −0.013 | 0.108 | 0.096 | 0.0005 | 9.222 | 21.784 | 37.711 |
The FMO energies, EHOMO and ELUMO, have been said to be the most critical parameters to determine if an inhibitor has undergone adsorption to show an anticorrosive effect.74 An inhibitor with a high EHOMO value is more likely to donate its electrons to the empty d-orbitals of the metal atoms and make coordination bonds.18 From Table 7, we can see that EHOMO of the copolymer is much higher than that of the homopolymer. Additionally, ELUMO of the copolymer is much lower than that of the homopolymer, indicating that the copolymer is more potent towards accepting electrons from the metal itself. This phenomenon is known as retrodonation and is important in corrosion inhibition. It's also known that the higher the ΔE, the difference between ELUMO and EHOMO, the more stable the inhibitor is regarding electron donation.75 The ΔE value of the homopolymer is much higher than that of the copolymer, which is likely to show more IE. The electron affinity (EA) values, the negative of ELUMO values that showed the tendency towards accepting electrons and were higher for the copolymer. Moreover, the IE of the copolymer was much lower than that of the homopolymer, indicating that it’s much easier to ionize the copolymer, and it's more likely that the copolymer will donate more electrons than the homopolymer.76 The electronegativity, χ, is another parameter that reveals the efficacy of a corrosion inhibitor. A high value of χ is associated with low efficacy. However, the χ and the global electrophilicity index (ω) values of the copolymer are lower than those of the homopolymer. Additionally, higher global softness (σ) and lower hardness (η) values for 8b resonate with its higher IE.77 The ΔN value, representing the fraction of electron transfer, of the copolymer is much, much higher than that of the homopolymer, which is another indication of its higher anticorrosion potential. Lastly, the dipole moment (μ), which indicates the overall polarity of a molecule, is also higher for the copolymer. Overall, all these DFT-optimized parameters have favored the copolymer, providing support for the experimental findings and enough incentives to explore the copolymerization of the homopolymer.
3.14 Mechanism of corrosion inhibition
XPS and SEM surface studies demonstrated that inhibitor 8b adsorbed on the mild steel surfaces. Fig. 12 illustrates how 8b may have undergone adsorption. DFT studies demonstrated that HOMO and LUMO regions, which indicate charge sharing and charge accepting capabilities, respectively, are located around the pyrrolidinium ring and the chloride counter ions. Since both moieties are charged species, this gives the impression that 8b will undergo an electrostatic interaction with the positively charged metal surface to cause physical adsorption. However, adsorption and thermodynamic studies found the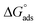 and
and 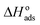 values to be −41.5 kJ mol−1 and 58.0 kJ mol−1, respectively. These values indicated the mode of 8b adsorption was chemisorption or predominantly chemisorption. This could be explained by the fact we already discussed in the XPS investigation that both 2°- and 3°-nitrogen could undergo deprotonation in the test solution. Moreover, chloride and hydroxide ions present in the test solution can deprotonate the pyrrolidinium ring's nitrogen.23 This will lead to both nitrogens having an unshared pair of electrons that can now be used to coordinate to empty the d-orbital of metal atoms. Another 4°-nitrogen atom in the compound can't undergo deprotonation and can be adsorbed via a physisorption mechanism.18–20 It has been reported that the net charge of iron surface in acid is positive.43 Therefore, without KI, the formate ions (HCOO−) from 20% formic acid and the Cl− counter ions from 8b can be adsorbed onto the metal surface. After that, these pre-adsorbed ions can attract the inhibitor toward the metal surface for more protection. When KI is added to the electrolyte, the I− ions can get oxidized to I2 by dissolving oxygen in the solution (eqn (11)). Later on, the I2 combines very fast with I− ions to form soluble yellowish I3− ions (eqn (12)).78
values to be −41.5 kJ mol−1 and 58.0 kJ mol−1, respectively. These values indicated the mode of 8b adsorption was chemisorption or predominantly chemisorption. This could be explained by the fact we already discussed in the XPS investigation that both 2°- and 3°-nitrogen could undergo deprotonation in the test solution. Moreover, chloride and hydroxide ions present in the test solution can deprotonate the pyrrolidinium ring's nitrogen.23 This will lead to both nitrogens having an unshared pair of electrons that can now be used to coordinate to empty the d-orbital of metal atoms. Another 4°-nitrogen atom in the compound can't undergo deprotonation and can be adsorbed via a physisorption mechanism.18–20 It has been reported that the net charge of iron surface in acid is positive.43 Therefore, without KI, the formate ions (HCOO−) from 20% formic acid and the Cl− counter ions from 8b can be adsorbed onto the metal surface. After that, these pre-adsorbed ions can attract the inhibitor toward the metal surface for more protection. When KI is added to the electrolyte, the I− ions can get oxidized to I2 by dissolving oxygen in the solution (eqn (11)). Later on, the I2 combines very fast with I− ions to form soluble yellowish I3− ions (eqn (12)).78| 2I− → I2 + 2e | (11) |
| I2 + I− → I3− | (12) |
Both I− and I3− ions can be adsorbed on the metal surface, replacing some HCOO− ions. Therefore, the addition of KI to the solution causes the surface to be more negatively charged by creating I− and I3− ions. Then this negatively charged surface containing Cl−, HCOO−, I−, I3− ions will pull in the positively charged inhibitor molecule with a great force to provide excellent protection. Therefore, physisorption may have been the initial mode of interaction. However, with the successive deprotonation of the pyrrolidinum ring's nitrogens, chemisorption stands to be the dominant mode of adsorption. Electron donation from 8b to metal atoms can cause a rise in the inter-electronic repulsion that forces the metal atoms to donate their excess electrons to unoccupied p-orbitals of the inhibitor compound, a phenomenon known as retro-donation. The hydrophobic pendant attached to the inhibitor helps to repel the water molecules away.
4. Conclusion
In the current investigation, a homopolymer, poly(diallylammonium chloride) (3), has been synthesized. The homopolymer was modified by adding hydrophilic/hydrophobic bis-cationic motifs that afforded three cyclo-copolymers (8a–c). The synthesized compounds were characterized by IR, 1H- and 13C-NMR, TGA, and viscometric methods. All the synthesized polymers were then evaluated for their efficacies in mitigating mild steel corrosion in 20% formic acid. The cyclo-copolymers showed more inhibitive potential than the homopolymers. Copolymer 8b showed the maximum IE of 81.8% for 100 ppm at 30 °C. Some explanations in support of 8b to show better IE were offered. A good synergism between 8b (50 ppm) and 2 mmol KI improved the IE to >99%. An extended 24 h weight loss study performed at 60 °C didn't affect the synergism at all. Adsorption isotherm studies and thermodynamic parameters were discussed in detail. With increasing inhibitor concentration, the decreasing icorr values found from PDP and the increasing Rct values found from EIS studies supported the findings of weight loss studies. 8b acted as a mixed-type and an anodic inhibitor in the absence and presence of 2 mmol KI, respectively. A toxicological computational survey was performed to render the inhibitor non-toxic. XPS and SEM studies provided solid evidence for the adsorption of 8b on the metal surface. The sights of the inhibitor interacting with the metal surface were found through DFT analysis. Excellent synergism between 8b and KI at an elevated temperature of 60 °C can be studied further in other acid media and/or higher temperatures.Author contributions
Lipiar K. M. O. Goni: writing – original draft, investigation, data curation, formal analysis, validation, visualization Ibrahim Y. Yaagoob: formal analysis, investigation Mohammad A. J. Mazumder: conceptualization, methodology, supervision, project administration, funding acquisition, writing – review & editing Shaikh A. Ali: conceptualization, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research facilities provided by King Fahd University of Petroleum & Minerals (KFUPM) under project # INAM 2112 are gratefully acknowledged. Dr Ibrahim Y. Yaagoob thankfully acknowledges the postdoctoral fellowship under the Distinguished University Professor Award (# DUP23102) provided by KFUPM.References
- U. M. Angst, Challenges and opportunities in corrosion of steel in concrete, Mater. Struct., 2018, 51, 4 CrossRef.
- B. Liao, Z. Luo, S. Wan and L. Chen, Insight into the anti-corrosion performance of Acanthopanax senticosus leaf extract as eco-friendly corrosion inhibitor for carbon steel in acidic medium, J. Ind. Eng. Chem., 2023, 117, 238–246 CrossRef CAS.
- E. Mccafferty, Introduction to Corrosion Science, Springer-Verlag, New York, 2010 Search PubMed.
- R. Ganjoo, S. Sharma, C. Verma, M. A. Quraishi and A. Kumar, Heteropolysaccharides in sustainable corrosion inhibition: 4E (Energy, Economy, Ecology, and Effectivity) dimensions, Int. J. Biol. Macromol., 2023, 235, 123571 CrossRef CAS PubMed.
- W. Qi, Y. Huang, Y. Ma, Z. Yu and X. Zhu, Developing novel imidazoline-modified glucose derivatives as eco-friendly corrosion inhibitors for Q235 steel, RSC Adv., 2023, 13, 13516–13525 RSC.
- A. M. Alsabagh, M. Z. Elsabee, Y. M. Moustafa, A. Elfky and R. E. Morsi, Corrosion inhibition efficiency of some hydrophobically modified chitosan surfactants in relation to their surface active properties, Egypt. J. Pet., 2014, 23, 349–359 CrossRef.
- M. Salman, K. R. Ansari, J. Haque, V. Srivastava, M. A. Quraishi and M. A. J. Mazumder, Ultrasound-assisted synthesis of substituted triazines and their corrosion inhibition behavior on N80 steel/acid interface, J. Heterocycl. Chem., 2020, 57, 2157–2172 CrossRef CAS.
- R. S. Vidyarthy and P. Sivateja, Influence of activating flux tungsten inert gas welding on mechanical and metallurgical properties of the mild steel, Mater. Today: Proc., 2019, 28, 977–981 Search PubMed.
- M. A. Quraishi and H. K. Sharma, Thiazoles as corrosion inhibitors for mild steel in formic and acetic acid solutions, J. Appl. Electrochem., 2005, 35, 33–39 CrossRef CAS.
- M. Forslund, C. Leygraf, P. M. Claesson and J. Pan, Octadecanethiol as corrosion inhibitor for zinc and patterned zinc–copper in humidified air with formic acid, J. Electrochem. Soc., 2014, 161, C330–C338 CrossRef CAS.
- F. A. Ansari and M. A. Quraishi, Inhibitive performance of Gemini surfactants as corrosion inhibitors for mild steel in formic acid, Port. Electrochim., 2010, 28, 321–335 CAS.
- R. C. Nascimento, L. B. Furtado, M. J. O. C. Guimarães, P. R. Seidl, J. C. Rocha, J. A. C. Ponciano and M. T. M. Cruz, Synergistic effect of propargyl alcohol, octadecylamine, and 1,3-dibutyl thiourea for API P110 alloys in acetic and formic acidic solutions used in oil well acidizing, J. Mol. Liq., 2018, 256, 548–557 CrossRef CAS.
- J. Wang, J. Liu, Q. Liu and Y. Chong, The inhibition performance of heterocyclic compounds on Q235 steel in methanol/formic acid medium: experimental and theory, J. Mol. Liq., 2021, 343, 117663 CrossRef CAS.
- Y. Boughoues, M. Benamira, L. Messaadia and N. Ribouh, Adsorption and corrosion inhibition performance of some environmental friendly organic inhibitors for mild steel in HCl solution via experimental and theoretical study, Colloids Surf., A, 2020, 593, 124610 CrossRef CAS.
- E. A. Badr, H. H. H. Hefni, S. H. Shafek and S. M. Shaban, Synthesis of anionic chitosan surfactant and application in silver nanoparticles preparation and corrosion inhibition of steel, Int. J. Biol. Macromol., 2020, 157, 187–201 CrossRef CAS PubMed.
- S. A. Umoren, A. A. AlAhmary, Z. M. Gasem and M. M. Solomon, Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel, Int. J. Biol. Macromol., 2018, 117, 1017–1028 CrossRef CAS PubMed.
- C. Verma, L. K. M. O. Goni, I. Y. Yaagoob, H. Vashisht, M. A. J. Mazumder and A. Alfantazi, Polymeric surfactants as ideal substitutes for sustainable corrosion protection: a perspective on colloidal and interface properties, Adv. Colloid Interface Sci., 2023, 318, 102966 CrossRef CAS PubMed.
- B. Lyu, H. Liu, P. Li, D. Gao and J. Ma, Preparation and properties of polymeric surfactants: a potential corrosion inhibitor of carbon steel in acidic medium, J. Ind. Eng. Chem., 2019, 80, 411–424 CrossRef.
- I. Y. Yaagoob, L. K. M. O. Goni, M. A. J. Mazumder, S. A. Ali, M. A. Quraishi and C. Verma, Synthesis of polymeric surfactant containing bis-cationic motifs as a highly efficient acid corrosion inhibitor for C1018 carbon steel, New J. Chem., 2023, 47, 3445–3461 RSC.
- L. K. M. O. Goni, I. Y. Yaagoob, C. Verma, F. Almustafa, M. Y. I. Alobaid, S. A. Ali, M. A. Quraishi and M. A. J. Mazumder, Comparative corrosion inhibition performance of diallyl amine-based cyclopolymers bearing secondary, tertiary and quaternary nitrogen's motifs in 1 M HCl, J. Mol. Liq., 2023, 375, 121371 CrossRef CAS.
- A. H. Nahlé, T. J. Harvey and F. C. Walsh, Quaternary aryl phosphonium salts as corrosion inhibitors for iron in HCl, J. Alloys Compd., 2018, 765, 812–825 CrossRef.
- A. E. Al-Rawajfeh and E. M. Al-Shamaileh, Inhibition of corrosion in steel water pipes by ammonium pyrrolidine dithiocarbamate (APDTC), Desalination, 2007, 206, 169–178 CrossRef CAS.
- I. Y. Yaagoob, L. K. M. O. Goni, M. A. J. Mazumder, S. A. Ali, A. Alfantazi and C. Verma, Surface and interfacial properties of poly(methyldiallylammonium chloride): effect of hydrophobic pendant and synergism (KI) on corrosion of C1018CS in 15% HCl, J. Taiwan Inst. Chem. Eng., 2023, 149, 105000 CrossRef CAS.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Recent developments in sustainable corrosion inhibitors: design, performance and industrial scale applications, Adv. Mater., 2021, 2, 3806–3850 RSC.
- A. A. Khadom, A. N. Abd and N. A. Ahmed, Synergistic effect of iodide ions on the corrosion inhibition of mild steel in 1 M HCl by Cardaria Draba leaf extract, Results Chem., 2022, 4, 100668 CrossRef CAS.
- N. Sait, N. Aliouane, N. Ait Ahmed, L. Toukal and M. Al-Noaimi, Synergistic effect of potassium iodide on corrosion inhibition of copper by tetraphosphonic acid in hydrochloric acid solution, J. Adhes. Sci. Technol., 2022, 36, 109–133 CrossRef CAS.
- M. M. Solomon and S. A. Umoren, Enhanced corrosion inhibition effect of polypropylene glycol in the presence of iodide ions at mild steel/sulphuric acid interface, J. Environ. Chem. Eng., 2015, 3, 1812–1826 CrossRef CAS.
- M. M. Solomon and S. A. Umoren, Performance evaluation of poly (methacrylic acid) as corrosion inhibitor in the presence of iodide ions for mild steel in H2SO4 solution, J. Adhes. Sci. Technol., 2015, 29, 1060–1080 CrossRef CAS.
- K. R. Ansari, D. S. Chauhan, M. A. Quraishi, A. Y. Adesina and T. A. Saleh, The synergistic influence of polyethyleneimine grafted graphene oxide and iodide for the protection of steel in acidizing conditions, RSC Adv., 2020, 10, 17739 RSC.
- M. R. Sovizi and R. Abbasi, Effect of carboxymethyl cellulose on the corrosion behavior of aluminum in H2SO4 solution and synergistic effect of potassium iodide, J. Adhes. Sci. Technol., 2020, 34, 1664–1678 CrossRef CAS.
- S. A. Haladu, S. A. Umoren, S. A. Ali and M. M. Solomon, Synthesis and characterization of cyclic cationic polymer and its anti-corrosion property for low carbon steel in 15% HCl solution, Int. J. Electrochem. Sci., 2017, 12, 9061–9083 CrossRef CAS.
- U. S. EPA, TEST 5.1 (Toxicity Estimation Software Tool), U.S. Environmental Protection Agency, Cincinnati, OH, 2020 Search PubMed.
- Z. Shariatinia and A. Ahmadi-Ashtiani, Corrosion inhibition efficiency of some phosphoramide derivatives: DFT computations and MD simulations, J. Mol. Liq., 2019, 292, 111409 CrossRef CAS.
- J. Sebhaoui, Y. E. Bakri, Y. E. Aoufir, E. H. Anouar, A. Guenbour, A. A. Nasser and E. M. Essassi, Synthesis, NMR characterization, DFT and anti-corrosion on carbon steel in 1 M HCl of two novel 1,5-benzodiazepines, J. Mol. Struct., 2019, 1182, 123–130 CrossRef CAS.
- S. R. Johns, R. I. Willing, S. Middleton and A. K. Ong, Cyclopolymerization. VII. The 13C NMR spectra of cyclopolymers obtained from N,N-diallyamines, J. Macromol. Sci., Part A, 1976, 10, 875–891 Search PubMed.
- J. E. Lancaster, L. Baccei and H. P. Panzer, The structure of poly(diallyldimethyl-ammonium) chloride by 13C-NMR spectroscopy, J. Polym. Sci., Polym. Lett. Ed., 1976, 14, 549–555 CrossRef CAS.
- I. Y. Yaagoob, M. K. Aldahdooh, A. A. Al-Taq, H. A. Al-Muallem, M. A. J. Mazumder and S. A. Ali, Synthesis of stimuli-responsive ionic cyclopolymers in search of phosphorous-free antiscalants, J. Appl. Polym. Sci., 2021, 138, 50402 CrossRef CAS.
- Y. Chang and C. L. McCormick, Water-soluble copolymers: 57. Amphiphilic cyclocopolymers of diallylalkoxybenzyl-methylammonium chloride and diallyl-dimethylammonium chloride, Polymer, 1994, 35, 3503–3512 CrossRef CAS.
- S. A. Ali, Y. Umar and B. F. Abu-Sharkh, Amphiphilic cycloterpolymers of diallyldimethylammonium chloride, diallyloctadecylammonium chloride and sulfur dioxide, J. Appl. Polym. Sci., 2005, 97, 1298–1306 CrossRef CAS.
- R. K. Mehta, M. Yadav and I. B. Obot, Electrochemical and computational investigation of adsorption and corrosion inhibition behavior of 2-aminobenzohydrazide derivatives at mild steel surface in 15% HCl, Mater. Chem. Phys., 2022, 290, 126666 CrossRef CAS.
- S. K. Gupta, R. K. Mehta, N. Kumari, M. Yadav and I. B. Obot, Study on benzylidine derivatives as corrosion inhibitors for mild steel in 15% HCl medium: experimental & theoretical investigation, J. Phys. Chem. Solids, 2023, 183, 111632 CrossRef CAS.
- U. Mamudu, M. S. Alnarabiji and R. C. Lim, Adsorption isotherm and molecular modeling of phytoconstituents from Dillenia suffruticosa leaves for corrosion inhibition of mild steel in 1.0 M hydrochloric acid solution, Results Surf. Interfaces, 2023, 13, 100145 CrossRef.
- A. A. Khadom, A. N. Abd and N. A. Ahmed, Potassium iodide as a corrosion inhibitor of mild steel in hydrochloric acid: kinetics and mathematical studies, J. Bio- Tribo-Corros., 2018, 4, 17 CrossRef.
- A. S. Yaro, A. A. Khadom and S. M. Lahmod, Kinetics of the corrosion inhibition reaction of steel alloys in acidic media by potassium iodide, React. Kinet., Mech. Catal., 2013, 109, 417–432 CrossRef CAS.
- S. H. Sanad, A. A. Ismail and N. A. Mahmoud, Inhibition effect of potassium iodide on corrosion of stainless steel in hydrochloric acid solution, J. Mater. Sci., 1992, 27, 5706–5712 CrossRef CAS.
- S. Masroor, M. Mobin, A. K. Singh, R. A. K. Rao, M. Shoeb and M. J. Alam, Aspartic di-dodecyl ester hydrochloride acid and its ZnO-NPs derivative, as ingenious green corrosion defiance for carbon steel through theoretical and experimental access, SN Appl. Sci., 2020, 2, 144 CrossRef CAS.
- N. A. Odewunmi, M. A. J. Mazumder, S. A. Ali and I. B. Obot, 1,12-Dodecyldiyl-bis(dimethylalkylammonium bromide) compounds anticorrosion property on C1018/15% HCl solution interface: experimental, molecular dynamics simulation, and DFT studies, J. Mol. Liq., 2022, 346, 118332 CrossRef CAS.
- N. A. Odewunmi, M. A. J. Mazumder and S. A. Ali, Tipping effect of tetra-alkylammonium on the potency of N-(6-(1H-benzo[d]imidazole-1-yl)hexyl)-N, N-dimethyldodecan-1-aminium bromide (BIDAB) as corrosion inhibitor of austenitic 304L stainless steel in oil and gas acidization: experimental and DFT approach, J. Mol. Liq., 2022, 360, 119431 CrossRef CAS.
- M. Yadav, D. Behera and U. Sharma, Nontoxic corrosion inhibitors for N80 steel in hydrochloric acid, Arabian J. Chem., 2016, 9, S1487–S1495 CrossRef CAS.
- A. Yurt, A. Balaban, S. U. Kandemir, G. Bereket and B. Erk, Investigation on some Schiff bases as HCl corrosion inhibitors for carbon steel, Mater. Chem. Phys., 2004, 85, 420–426 CrossRef CAS.
- K. R. Ansari, M. A. Quraishi and A. Singh, Pyridine derivatives as corrosion inhibitors for N80 steel in 15% HCl: electrochemical, surface and quantum chemical studies, Measurement, 2015, 76, 136–147 CrossRef.
- J. Haque, K. R. Ansari, V. Srivastava, M. A. Quraishi and I. B. Obot, Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: a combined experimental and theoretical approach, J. Ind. Eng. Chem., 2017, 49, 176–188 CrossRef CAS.
- S. K. Shukla and E. E. Ebenso, Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium, Int. J. Electrochem., 2011, 6, 3277–3291 CrossRef CAS.
- N. M. El Basiony, A. Elgendy, A. E. El-Tabey, A. M. Al-Sabagh, G. M. Abd El-Hafez, M. A. El-raouf and M. A. Migahed, Synthesis, characterization, experimental and theoretical calculations (DFT and MC) of ethoxylated aminothiazole as inhibitor for X65 steel corrosion in highly aggressive acidic media, J. Mol. Liq., 2020, 297, 111940 CrossRef CAS.
- P. K. Paul, M. Yadav and I. B. Yadav, Investigation on corrosion protection behavior and adsorption of carbohydrazide-pyrazole compounds on mild steel in 15% HCl solution: electrochemical and computational approach, J. Mol. Liq., 2020, 314, 113513 CrossRef CAS.
- N. Tiwari, R. K. Mitra and M. Yadav, Corrosion protection of petroleum oil well/tubing steel using thiadiazolines as efficient corrosion inhibitor: experimental and theoretical investigation, Surf. Interfaces, 2021, 22, 100770 CrossRef CAS.
- M. Tang, X. Li, S. Deng and R. Lei, Synergistic inhibition effect of Mikania micrantha extract with KI on steel corrosion in H2SO4 solution, J. Mol. Liq., 2021, 344, 117926 CrossRef CAS.
- J. Tan, L. Guo, H. Yang, F. Zhang and Y. El Bakri, Synergistic effect of potassium iodide and sodium dodecyl sulfonate on the corrosion inhibition of carbon steel in HCl medium: a combined experimental and theoretical investigation, RSC Adv., 2020, 10, 15163–15170 RSC.
- K. C. D. S. D. Lima, V. M. Paiva, D. Perrone, B. Ripper, G. Simões, M. L. M. Rocco, A. G. D. Veiga and E. D'Elia, Glycine max meal extracts as corrosion inhibitor for mild steel in sulphuric acid solution, J. Mater. Res. Technol., 2020, 9, 12756–12772 CrossRef CAS.
- I. B. D. Barros, M. A. A. Kappel, P. M. D. Santos, V. F. D. V. Junior, E. D'Elia and I. N. Bastos, The inhibitory action of Bauhinia purpurea extracts on the corrosion of carbon steel in sulfuric acid medium, Mater. Res., 2016, 19, 187–194 CrossRef.
- N. A. Odewunmi, M. A. J. Mazumder and S. A. Ali, Tipping effect of tetra-alkylammonium on the potency of N-(6-(1H-benzo[d]imidazole-1-yl)hexyl)-N, N-dimethyldodecan-1-aminium bromide (BIDAB) as corrosion inhibitor of austenitic 304L stainless steel in oil and gas acidization: experimental and DFT approach, J. Mol. Liq., 2022, 360, 119431 CrossRef CAS.
- B. Liao, S. Ma, S. Zhang, X. Li, R. Quan, S. Wan and X. Guo, Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution, Int. J. Biol. Macromol., 2023, 239, 124358 CrossRef CAS PubMed.
- S. E. H. Etaiw, G. S. Hassan, A. A. El-Hossiany and A. S. Fouda, Nano-metal–organic frameworks as corrosion inhibitors for strengthening anti-corrosion behavior of carbon steel in a sulfuric acid environment: from synthesis to applications, RSC Adv., 2023, 13, 15222–15235 RSC.
- X. Luo, C. Ci, C. Zhou, J. Li, W. Xiong, Z. H. Xie, M. Guo, D. Wu, B. Chen and Y. Liu, Dopamine modified natural glucomannan as a highly efficient inhibitor for mild steel: experimental and theoretical methods, Int. J. Biol. Macromol., 2023, 242, 124712 CrossRef CAS PubMed.
- V. N. Ayukayeva, G. I. Boiko, N. P. Lyubchenko, R. G. Sarmurzina, R. F. Mukhamedova, U. S. Karabalin and S. A. Dergunov, Polyoxyethylene sorbitan trioleate surfactant as an effective corrosion inhibitor for carbon steel protection, Colloids Surf., A, 2019, 579, 123636 CrossRef.
- E. A. Flores, O. Olivares, N. V. Likhanova, M. A. Domínguez-Aguilar, N. Nava, D. Guzman-Lucero and M. Corrales, Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution, Corros. Sci., 2011, 53, 3899–3913 CrossRef CAS.
- D. Wilson and M. A. Langell, XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature, Appl. Surf. Sci., 2014, 303, 6–13 CrossRef CAS.
- B. Andrzejewskia, K. Chybczyńska, B. Hilczer, M. Błaszyk, T. Luciński, M. Matczak and L. Kępiński, Controlled growth of bismuth ferrite multiferroic flowers, Mater. Sci., 2014, 1402, 1336, DOI:10.48550/arXiv.1402.1336.
- C. L. Russom, S. P. Bradbury, S. J. Broderius, D. E. Hammermeister and R. A. Drummond, Predicting modes of toxic action from chemical structure: acute toxicity in the fathead minnow (Pimephales Promelas), Environ. Toxicol. Chem., 1997, 16, 948–967 CrossRef CAS.
- United States Environmental Protection Agency (USEPA), https://www3.epa.gov/pesticides/endanger/litstatus/effects/redleg-frog/naled/appendix-i.pdf accessed December 2023.
- M. I. Petoumenou, F. Pizzo, J. Cester, A. Fernández and E. Benfenati, Comparison between bioconcentration factor (BCF) data provided by industry to the European Chemicals Agency (ECHA) and data derived from QSAR models, Environ. Res., 2015, 142, 529–534 CrossRef CAS PubMed.
- D. K. Verma, R. Aslam, J. Aslam, M. A. Quraishi, E. E. Ebenso and C. Verma, Computational modeling: theoretical predictive tools for designing potential organic corrosion inhibitors, J. Mol. Struct., 2021, 1236, 130294 CrossRef CAS.
- C. Fu, C. Liu, T. Li, X. Zhang, F. Wang, J. Yang, Y. Jiang, P. Cui and H. Li, DFT calculations: a powerful tool for better understanding of electrocatalytic oxygen reduction reactions on Pt-based metallic catalysts, Comput. Mater. Sci., 2019, 170, 109202 CrossRef CAS.
- I. Arshad, K. Qureshi, A. S. Saleemi, A. Abdullah, A. A. A. Bahajjaj, S. Ali and A. Bokhari, Melamine–isatin tris Schiff base as an efficient corrosion inhibitor for mild steel in 0.5 molar hydrochloric acid solution: weight loss, electrochemical and surface studies, RSC Adv., 2023, 13, 19301–19311 RSC.
- D. S. Chauhan, K. R. Ansari, A. A. Sorour, M. A. Quraishi, H. Lgaz and R. Salghi, Thiosemicarbazide, and thiocarbohydrazide functionalized chitosan as ecofriendly corrosion inhibitors for carbon steel in hydrochloric acid solution, Int. J. Biol. Macromol., 2018, 107, 1747–1757 CrossRef CAS PubMed.
- N. Baildya, N. N. Ghosh and A. P. Chattopadhyay, Anti-corrosive properties of quercetin and its derivatives on Fe (111) surface: a quantum chemical approach, SN Appl. Sci., 2019, 1, 735 CrossRef.
- M. Rbaa, M. Fardioui, C. Verma, A. S. Abousalem, M. Galai, E. E. Ebenso, T. Guedira, B. Lakhrissi, I. Warad and A. Zarrouk, 8-Hydroxyquinoline based chitosan derived carbohydrate polymer as biodegradable and sustainable acid corrosion inhibitor for mild steel: experimental and computational analyses, Int. J. Biol. Macromol., 2020, 155, 645–655 CrossRef CAS PubMed.
- S. Cao, D. Liu, H. Ding, J. Wang, H. Lu and J. Gui, Corrosion inhibition effects of a novel ionic liquid with and without potassium iodide for carbon steel in 0.5 M HCl solution: an experimental study and theoretical calculation, J. Mol. Liq., 2019, 275, 729–740 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra08959b |
| This journal is © The Royal Society of Chemistry 2024 |