Open Access Article
Open Access ArticleRecent advances in microwave-assisted multicomponent synthesis of spiro heterocycles
Ramin Javahershenas
 *a,
Ata Makarem
b and
Karel D. Klika
*a,
Ata Makarem
b and
Karel D. Klika c
c
aDepartment of Organic Chemistry, Faculty of Chemistry, Urmia University, Urmia, Iran. E-mail: jshbco@yahoo.com
bInstitute of Pharmacy, University of Hamburg, 20146, Hamburg, Germany
cMolecular Structure Analysis, German Cancer Research Center (DKFZ), 69120 Heidelberg, Germany
First published on 13th February 2024
Abstract
Spiro heterocycle frameworks are a class of organic compounds that possesses unique structural features making them highly sought-after targets in drug discovery due to their diverse biological and pharmacological activities. Microwave-assisted organic synthesis has emerged as a powerful tool for assembling complex molecular architectures. The use of microwave irradiation in synthetic chemistry is a promising method for accelerating reaction rates and improving yields. This review provides insights into the current state of the art and highlights the potential of microwave-assisted multicomponent reactions in the synthesis of novel spiro heterocyclic compounds that were reported between 2017 and 2023.
1. Introduction
Heterocyclic compounds, which are extensively found in nature, serve as a framework for numerous biologically active molecules and medicinal compounds. This includes antibiotics, antiviral agents, and anticancer drugs.1–4 Various natural products contain these moieties, making the development of efficient and environmentally friendly synthetic methodologies for heterocycles an important goal for chemists. Medicinal chemistry and drug discovery heavily rely on heterocycles, which are organic compounds with at least one ring structure that does not contain carbon atoms.5–7 Traditionally, the synthesis of heterocycles has relied on multi-step processes involving distinct reaction steps and purification procedures. However, these conventional methods often encounter drawbacks such as decreased productivity, extended reaction times, and the production of by-products. In recent years, researchers have focused on developing more sustainable and efficient synthetic methodologies.8–10Multicomponent reactions (MCRs) have revolutionized the field of organic synthesis. Unlike traditional reactions, which typically involve the combination of two reactive species, MCRs involve the reaction of three or more starting materials to form a product in a single reaction step.11,12 The product contains most, if not all, of the atoms of the starting materials, making MCRs efficient and atom economical. MCRs proceed through a series of steps in which different functional groups react with each other in a specific order.13,14 MCRs have numerous applications in organic synthesis, particularly in the exploration and development of novel drugs and bioactive molecules. MCRs can also be used to create combinatorial libraries of potential drug candidates that can be screened for biological activity. This ability to combine multiple reactants in a single process step offers several advantages such as increased efficiency and atom economy, a wide scope of reactant diversity, high complexity generation, low waste production, and diversity in chemical space.15,16
Microwave-assisted MCRs have emerged as a powerful tool for the synthesis of heterocycles, offering several advantages over traditional synthetic methods and has been applied to the synthesis of a wide variety of complex compounds.17,18 Reviews (REFS) on microwave-assisted synthesis of heterocycles showcase the advancements in the total synthesis of such compounds, emphasizing the role of microwave irradiation in the efficient construction of complex heterocyclic frameworks. Furthermore, the synthesis of medium-sized heterocycles has been a focus of microwave-assisted techniques and also showcases the progress made in this area together with emphasis on the use of dedicated microwave synthesizers for the synthesis of these compounds.19,20 The efficient and green strategy for the improved synthesis of biologically and pharmaceutically interesting annulated heterocycles using microwave irradiation has further highlighted the potential of this approach in accessing novel heterocyclic systems. Microwave-assisted MCR of heterocycles has demonstrated the significant impact of microwave irradiation in the rapid, efficient, and sustainable construction of diverse heterocyclic scaffolds. These developments underscore the potential of microwave-assisted techniques in the synthesis of complex molecular architectures with broad applications in medicinal chemistry and pharmaceutical research.21–23
Microwave-assisted MCRs not only make the synthesis of complex molecules more efficient and sustainable, MCR. But in addition to pharmaceuticals, agrochemicals, and materials, researchers have also been able to obtain biologically active molecules using microwave-assisted MCRs. In particular, microwave-assisted synthesis also shows great promise for the rapid and selective synthesis of spiro heterocycles, as demonstrated by several recent studies.24–34
Due to their diverse biological activities and pharmaceutical relevance, spiro heterocycles have received considerable attention in recent years. Because of their diverse structural features, unique properties, and wide range of applications, spiro heterocycles are a fascinating class of organic compounds. These compounds consist of two or more rings connected by a single atom, which can be a carbon atom or another element. In addition to their rich chemical reactivity and ability to adopt various conformations, spiro heterocycles possess an array of intriguing biological functions due to their unique structural arrangements.35–40
In this review, we report on the recent progress in MCRs41–46 and summarize a comprehensive overview of the recent advances in the microwave-assisted MCR of spiro heterocycles, highlighting key developments and methodologies that have contributed to the rapid expansion of the technique applied the synthesis of spiro heterocycles. In addition to providing some perspectives for future research, we also outline the benefits of this method and some challenges it faces. The article thus discusses microwave-assisted MCR as an important method for synthesizing spiro heterocyclic compounds that were reported in the literature between 2017 and 2023.
2. Background to microwave-assisted synthesis
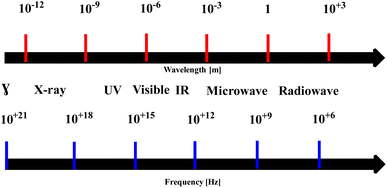
Microwave-assisted synthesis involves heating and accelerating chemical reactions using microwave radiation which causes molecules to vibrate or rotate as a result of absorbing electromagnetic energy. There are several compelling advantages to using this method over conventional organic synthesis methods and47–50 the method has found widespread application in various fields of chemistry such as pharmaceutics, materials, fine chemicals, and nanoparticles, rendering it an invaluable tool for the research of materials. Microwave electromagnetic radiation with a frequency range of 300 MHz to 300 GHz.51–54 Microwave radiation heats materials with electric charges, such as polar molecules or ions, by making them rotate or move in the electric field. This can result in faster and more efficient heating than conventional methods which rely on convection from the heating surface to the system (Fig. 1).55–59The history of microwave synthesis can be traced back to the 1940s, when Percy Spencer, an engineer at Raytheon Corporation, accidently discovered the heating effect of microwaves while working on a radar system. He noticed that a chocolate bar in his pocket had melted when he was near a magnetron, a device that generates microwaves. He then experimented with other foods, such as popcorn and eggs, and realized the potential of microwave heating for cooking. He filed a patent for the first microwave oven in 1945.47,50,52,57
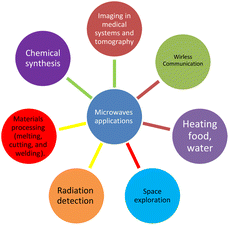
It was not until the 1980s that microwave-assisted synthesis became popular in the field of chemistry after two groups of researchers, Gedye et al.60 and Giguere et al.61 in 1986, independently published papers on the use of microwave irradiation to perform organic reactions. They showed that microwave heating could accelerate the reaction rate, increase the yield, and improve the selectivity of various organic transformations. Since then, microwave-assisted synthesis has been widely applied in various branches of chemistry, such as inorganic chemistry, polymer chemistry, material science, nanotechnology, and biochemistry.62–69 Microwaves can be used for a variety of applications70–77 as shown in Fig. 2.
There are several ways to perform microwave-assisted synthesis, including open-vessel, closed-vessel, and continuous-flow methods. For reactions that do not require high temperatures or pressure, such as solvent extraction or digestion, the open-vessel mode is suitable whereas closed-vessel processes are ideal for reactions that require high temperatures or pressures such as organic synthesis and hydrothermal synthesis. Continuous-flow methods are ideal for reactions requiring rapid and precise heating, such as the synthesis of peptides or the preparation of nanoparticles.78–80 To conduct microwave-assisted synthesis, a microwave reactor is required, which generates, concentrates, and delivers microwave radiation to a reaction mixture. Domestic microwave ovens and dedicated microwave reactors are the two main types of microwave reactors. Domestic microwave ovens when used for chemical synthesis have several limitations, such as uneven heating, inability to control temperature and pressure, and safety concerns. Open-vessel mode is the only option for domestic microwave ovens, which means that the reaction mixture is exposed to the air inside the oven. Consequently, volatile components may be oxidized, contaminated, or lost. Compared to dedicated microwave reactors, domestic microwave ovens have a low power density and a short reaction time (Fig. 2).81–87
Dedicated microwave reactors come in two types, single-mode and multimode. Single-mode reactors produce homogeneous pockets of energy that are highly reproducible as they have small cavities that concentrate the microwave field in the vessel containing the reaction mixture to provide uniform heating distribution and are ideal for thin materials and liquids. Fast and uniform heating can be achieved for reactions up to 20 mL with these devices. On the other hand, multimode reactors feature larger cavity geometries for the simultaneous processing of multiple samples, ranging in size from 30–100 L, but consequently, the microwave field is chaotically distributed because of their large cavities. Multimode reactors are used for thicker materials and solids because the microwaves can penetrate the sample with more energy.88–97
Power density is the amount of microwave energy per unit volume of the reaction mixture. In microwave-assisted synthesis, the temperature, pressure, and heating rate all affect the reaction rate and an increase in power density is generally associated with a faster and more efficient reaction. High power density, however, has some limitations and challenges. In addition, there are issues related to uneven heating, thermal runaway, safety hazards, and scalability.98–102 Studies have shown that the power density can also influence the reaction rate by modifying the hydrodynamics of the system. It is therefore crucial to optimize and control the power density to enhance reaction rates and product quality.103–107
To control the power density, the microwave power output, the reaction volume, and the geometry of the reaction vessel must all be adjusted as there is a direct correlation between the power density and the reaction volume. The geometry of the reaction vessel too has a significant effect on the distribution and concentration of microwave fields inside the vessel. Generally, the higher the power density of a microwave field, the more uniform and focused it is. A variety of reactants, solvents, and additives may also be used to achieve different results.97–107
Materials with electric charges, such as polar molecules or ions, are heated by microwave radiation by being caused to rotate or move in the electric field of the microwaves. Compared with conventional heating methods, which rely on convection to transfer heat from the surface of the heater to the system, microwave irradiation can be faster and more effective.97–106 There are two main interactions for microwave heating, dielectric and ionic.
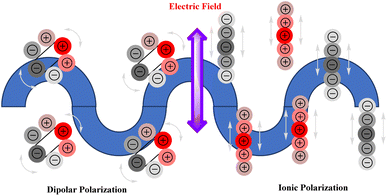
Water and other polar molecules are heated by dielectric heating when they align themselves with the oscillating electric field of microwave radiation. By rotating rapidly, the molecules produce friction and heat. As a result of the asymmetric distribution of electrons within their structure, polar molecules, such as those found in solvents and reactants, possess an electric dipole. Polar molecules strive to align themselves with the microwaves' rapidly oscillating electric field when exposed to microwaves and, as a consequence, the sample is heated. Friction between the molecules is generated by this constant realignment, which produces heat. The microwave field intensity and polarity of molecules are directly related to the extent of heat generation.
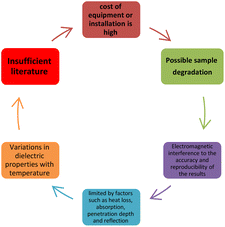
When microwave irradiation is applied to materials with free ions, such as ionic liquids or electrolytes, the ions accelerate as they are subjected to an oscillating electric field. For a material to undergo ionic conduction, the ions must be mobile, and the microwave radiation of a specific frequency is subsequently required. An electric field induces ionic conduction when sodium or chloride ions move back and forth. During collisions, they generate heat as they collide with other ions and molecules. Dielectric properties, such as the dielectric constant and loss factor, determine the microwave heating of a material. These properties determine a material's ability to absorb and dissipate microwave energy (Fig. 3).
In comparison to traditional chemical synthesis methods, microwave-assisted synthesis offers several potential benefits and disadvantages which are briefly shown in Fig. 4 and 5.107–111
The future of microwave-assisted synthesis looks promising as it gains greater popularity. With advancements in technology, microwave-assisted synthesis will likely become more efficient, precise, and cost-effective.47–50 This could lead to its increased use in various industries such as pharmaceuticals, materials science, and chemical manufacturing. There may be further developments in microwave reactor design and the development of new microwave-assisted synthesis techniques, thereby possibly leading to the discovery of novel reactions and the production of novel materials with unique properties.53–56 Thus, scientists are still continuing to uncover the full potential of microwave-assisted synthesis, which is expected to lead to new and exciting discoveries.58–65
3. Spiro heterocycles
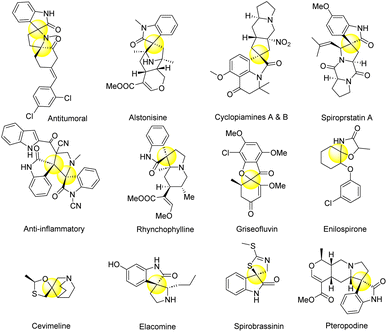
Many organisms can synthesize spiro heterocycles, including plants, bacteria, and marine invertebrates. They play a vital role in regulating hormones, communication, and defence (REF) as well as being bioactive. For example, a soil bacterium produces spinosyn D, a highly insecticidal spiro heterocycle (REF) while the compound spiro tryptamine found in certain fungi acts as a hallucinogen. A remarkable surge in spiro heterocycles has also been observed in the synthetic realm.112–116 In medicinal chemistry, these compounds have proven to be valuable scaffolds. They have the potential to treat infections, cancers, cardiovascular diseases, and more. Since they modulate different biological targets and it is possible to tune their pharmacokinetic properties, they make for promising therapeutic agents.117–122The spirooxindole class of naturally occurring heterocycles is considered a privileged class and spirooxindole forms the core structural unit in several alkaloids such as spirotryprostatin A and B,123,124 elacomine,125 marcfortine B,126 formosanine,127 coerulescine,128,129 rychnophyilline,130 alstonisine,131 mitraphylline,132 pteropodine,133 strychnofoline,134 spirobrassinin,135 horsfiline,136,137 notoamide A.138 3-Spiro[adamantane-2,3′-isoquinolines],139 and spiropyrazoles, spiroisoxazoles, spiropyranes, and spirothiadiazols.140 Some naturally occurring spiro heterocyclic compounds are depicted in Fig. 6.
Spiro heterocycles can also be useful in a variety of material science applications in addition to their medicinal applications. The structure and electronic properties of these materials make them promising candidates for organic semiconductors, light-emitting diodes, and liquid crystals.167–170 They can be tailored optically and electronically due to their molecular properties, which allows their potential application in a wide range of technological fields to expand. The unique properties and diverse applications of spiro heterocycles make them an intriguing class of organic compounds with enormous potential. As a natural product, a potential drug candidate, and a promising material science candidate, they hold significance in a variety of scientific areas.171–174
Spiro heterocycles can be classified according to their structural characteristics, which facilitate their analysis into four categories. These include spiro indole frameworks, sprio pyrrolidine frameworks, spiro pyran frameworks, and miscellaneous compounds. Each of these categories will now be discussed in turn as a result of microwave-assisted MCRs.
3.1. Spiroindole frameworks
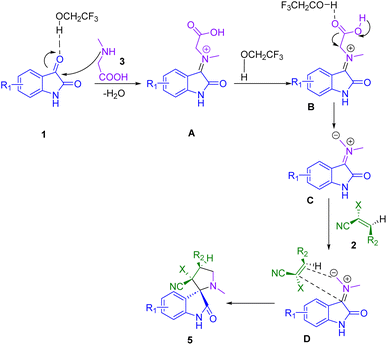
Of note, spirooxindoles possess a wide range of biological activities and pharmaceutical properties such as anti-anxiolytic,139 antibacterial,140–142 antitubercular,143 antimigraine activity,144 antioxidant,145 antitumoral,146 antiprotozoal,147 anti-inflammatory,148 anticonvulsant,149 antihelmintic activity,150 antiepileptic,151 antimycobacterial,152 antimalarial,153 acetyl-cholinesterase inhibitory activities,154,155 anticancer activities,156–160 anesthetic,161 HIV-1 N-NRT inhibitor,162 anti-coronavirus,163 antileishmanial,164 as well as antitubercular.165,166Dandia and co-workers developed a simple, environmentally benign, and highly proficient protocol for the efficient synthesis of antimicrobial spiro pyrrolidines (5) and thiapyrrolizidine oxindole derivatives (6). The molecular frameworks were obtained via a three-component 1,3-dipolar cycloaddition reaction between substituted isatin (1), sarcosine (3), 1,3-thiazole-4-carboxylic acid (4) and 2-cyano-3-phenyl-acrylic acid ethyl ester or 2-benzylidene-malononitrile (2) in 2,2,2-trifluoroethanol (TFE) as a reusable green solvent under microwave irradiation (Scheme 1). Additionally, functional group tolerance and a broad scope of useable substrates are other prominent features of the present methodology with a high degree of chemio-, regio-, and stereoselectivity. Single-crystal X-ray analyses were used to confirm the stereochemistries of the synthesized compounds.175
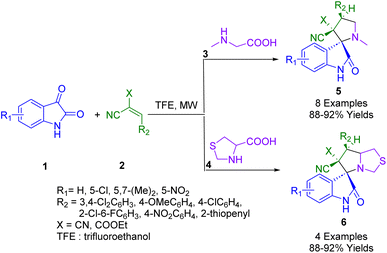 | ||
| Scheme 1 Synthesis of spiro indoline-3,2-pyrrolidine derivatives 5 and spiro indoline-3,5-pyrrolo[1,2-c]thiazole derivatives 6. | ||
The synthesized compounds were also evaluated for their antimicrobial potential. The synthesized compounds containing pyrrolothiazole moieties demonstrated excellent activity against Klebsiella pneumonia, even outperforming drugs in present use in terms of inhibiting activity. According to computational docking studies (3R,7′R)-7′-(3,4-dichlorophenyl)-2-oxo-7′,7a′-dihydro-3′H-spiro[indoline-3,5′-pyrrolo[1,2-c]thiazole]-6′,6′(1′H)-dicarbonitrile has a high affinity for New Delhi metallo-β-lactamase-1.
A tentative mechanism for the formation of the cycloadducts is given in Scheme 2. A significant role is played by 2,2,2-trifluoroethanol (TFE) in organic transformations because of its high polarity, low nucleophilicity, high hydrogen bond strength, Brønsted acidity, and high ionizing power. Furthermore, 2,2,2-trifluoroethanol can be used as an acid catalyst. In the present cycloaddition reaction, 2,2,2-trifluoroethanol forms a hydrogen bond with the carbonyl group of isatin (1) to increase the electrophilicity of the C-3 carbon which easily forms nonstabilized azomethineylide (A) with sarcosine (3) and which consequently behaves as a 1,3-dipolar species. The azomethineylide (A) that is formed subsequently undergoes a 1,3-dipolar cycloaddition reaction involving nucleophilic attack on the carbon atom at the β position of the Knoevenagel adduct (2) by the carbon atom of the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C) moiety in the azomethine ylide. The only products of the cycloadditions are pyrrolidine heterocyclic rings. This mechanism shows regiospecificity in the presented pathway.
N(C) moiety in the azomethine ylide. The only products of the cycloadditions are pyrrolidine heterocyclic rings. This mechanism shows regiospecificity in the presented pathway.
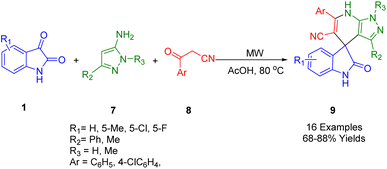
A three-component microwave procedure was developed by Tu and co-workers to synthesize new spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines] (9). By using readily available isatins (1), pyrazol-5-amines (7), and ketonitriles (8), the spiro scaffolds (9) were synthesized. In Scheme 3, HOAc plays both the role of a reaction media and an acid promoter. Microwave irradiation was used to carry out the reaction at 80 °C. Library generation using this strategy is a useful and attractive process of drug discovery due to its flexibility, broad functional group compatibility, and mild reaction conditions.176
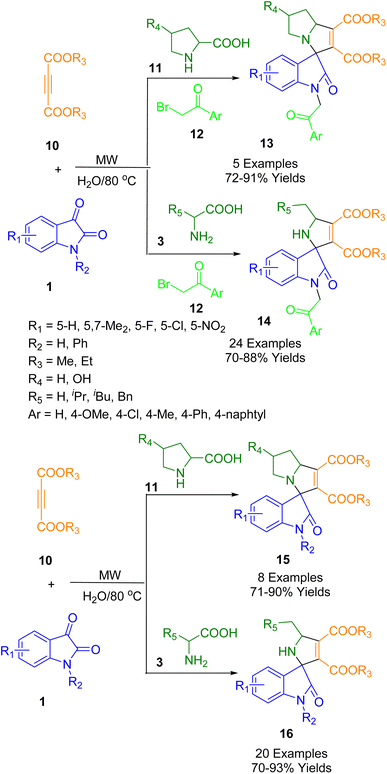
Meshram and colleagues synthesized various functionalized spirooxindoles (13–16) (Scheme 4). Using microwave irradiation under catalyst-free conditions in an aqueous medium, an MCR was developed between isatin (1), a series of amino acids (3, 11), but-2-ynedioates (10), and phenacyl bromide (12). Various functionalized spirooxindole derivatives can be synthesized using this protocol. The MCRs exhibited excellent yields and short reaction times, as well as a broad substrate range. Three human cancer cell lines were used to assess the anticancer activity of the synthesized spirooxindole derivatives: MCF-7 (breast), A549 (lung), and HeLa (cervical). Most of the compounds were found to be moderately-to-strongly cytotoxic.177
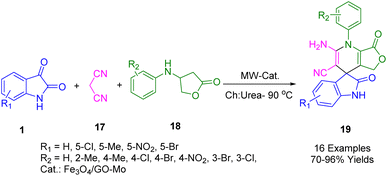
As part of heterogeneous catalysis, spirooxindole dihydropyridines (19) were produced using microwave-assisted methods in choline chloride (ChCl)/urea as a green solvent combining isatins (1), malononitrile (17), and anilinolactones (18) in the one pot under microwave irradiation at 90 °C (Scheme 5). This catalytic system can also be recovered and reused eight times without significant loss of activity, which makes it suitable for industrial applications.178 Spirooxindole dihydropyridines were synthesized with high-to-excellent yields using this method.
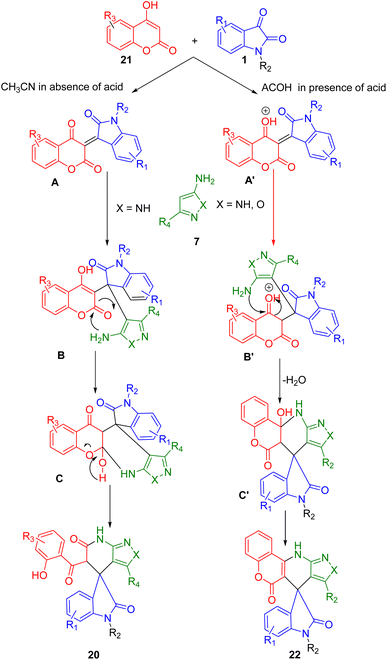
Choudhury and his co-workers developed an MCR in which isatin (1), 4-hydroxycoumarin (21), and aminopyrazole/aminoisoxazole (7) are combined using acetonitrile as a solvent at 85 °C for the synthesis of two different derived spirooxindoles (20, 22) (Scheme 6). By fusing pyrazolo-tetrahydropyridinones with spirooxindoles in a microwave-irradiated medium, pyrazolo-tetrahydropyridinones are formed. A similar combination when treated in acetic acid resulted in spirooxindoles with a tetracyclic coumarin-dihydropyridine-pyrazole/isoxazole moiety and spirooxindoles fused with oxindoles. Under metal-free conditions, spiroxindole-fused pyrazolo-tetrahydropyridinones and coumarin-dihydropyridine-pyrazole/isoxazole tetracycles were synthesized using the switchable MCR. This methodology has the following salient characteristics: medium-dependent response path switching, two types of products can be generated with the same starting materials, metal-free reaction conditions, a wide range of substrates, good yields, and all products contain multiple bioactive moieties.179
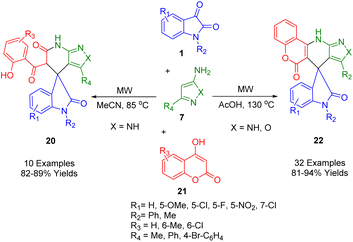 | ||
| Scheme 6 Synthesis of spirooxindoles (22) fused with pyrazolo tetrahydropyridinone and coumarin dihydropyridine pyrazole tetracycles. | ||
A possible reaction pathway for the formation of compounds (20, 22) is depicted in Scheme 7. The reaction of 4-hydroxycoumarin (21) with isatin derivatives (1) produces intermediate (A) followed by the 1,4-addition of amino pyrazole (7) to give (B). As (B) exists in the enol form in non-acidic conditions, its reactivity is less than that of the ester moiety. Intramolecular cyclization thus occurs at the ester carbonyl of the coumarin moiety and intermediate (C) is formed. This is followed by the formation of a stable product (23) through the opening of the coumarin ring. As a result, intermediate (B′) exists in the protonated form (C′) in the presence of an acid, which has a very reactive protonated ketone carbonyl. Thus, intramolecular cyclization occurs when the coumarin ring ketone carbonyl reacts with the ester moiety rather than the ester moiety.
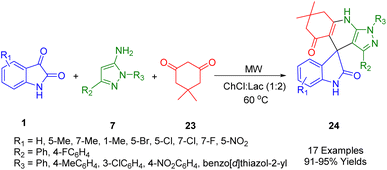
Zhang et al. reported a metal-free, green protocol involving the synthesis of spiro indoline-3,4′-pyrazolo[3,4-b]pyridines (24) (Scheme 8) in choline chloride and lactic acid, a natural deep eutectic solvent (NDDES) which is environmentally friendly. The reaction consisted of the microwave irradiation at 60 °C of a three-component mixture of 1H-pyrazol-5-amine (7), isatin (1), and dimedone (23) in excellent yields. Besides being clean, inexpensive, simple, and high yielding, this method is also scalable and doesn't require chromatography. It is considered a sustainable and environmentally benign process when choline chloride and lactic acid are used as a biodegradable, and reusable media.180
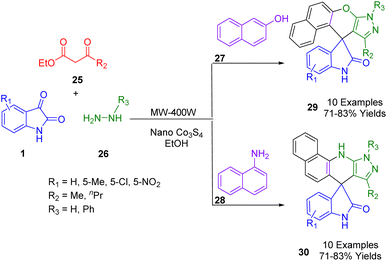
For an efficient and rapid method for the synthesis of a novel series of 10-methyl-8H-spiro[benzo[5,6]chromeno[2,3-c]pyrazole-11,3′-indol]-2′(1′H)-ones (29) and 8-methyl-10-phenyl-10,11-dihydrospiro[pyrazolo[3,4-b]benzo[h]quinolin-7,3′-indol]-2′(1′H)-one (30) using a microwave-assisted MCR, Shahbazi-Alavi et al. utilized Co3S4 nanoparticles as a heterogeneous catalyst (Scheme 9). A microwave irradiation at 90 °C was used to activate the reaction with phenylhydrazine (26), isatin (1), ketoesters (25), and naphthylamine (28) or 2-naphthol (27) in ethanol. There are numerous advantages to this protocol, including the rapid synthesis of novel PPCDs, inexpensive catalysts, ecological safety, and the use of green chemistry protocols.181
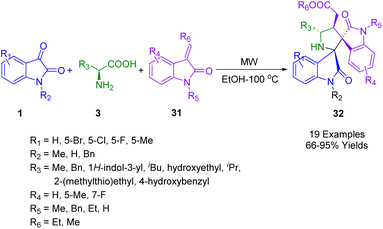
For an efficient one-pot synthesis of pyrrolidine fused bis-spiro oxindole derivatives (32) (Scheme 10) using a highly efficient and catalyst-free green synthetic protocol, Shankaraiah's group used isatins (1) and primary α-amino acids 3 and subsequently performed the [3 + 2] cycloaddition of 3-alkenyl oxindoles (31) using in situ generated azomethine ylide from decarboxylative condensation under microwave-assisted conditions. Yields were good-to-high. The protocol is atom economical, environmentally friendly, and doesn't require additives or catalysts for basic reactions. Single-crystal X-ray analyses of the pyrrolidine-fused bis-spirooxindoles unambiguously confirmed their structures and stereochemistries.182
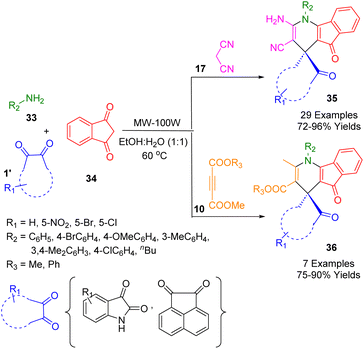
Mukhopadhyay et al. reported a rapid method for the one-pot, chromatography-free synthesis of diversified dihydrospiro[indeno[1,2-b]pyridine]-4,3′-indolines (35) or the analogous spiro acenaphthylene-1,4′-indeno[1,2-b]pyridines (36). A viable microwave-assisted MCR in minimal aqueous ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the green solvent without any metal catalyst was demonstrated by using a mixture of a series of substituted amines (33) and isatins (1) or acenaphthenequinone (1′), malononitrile (17) or acyclic-1,3-diketone (10), and indane-1,3-dione (34) (Scheme 11). The reaction was evaluated using microwave irradiation at 60 °C. The compounds were designed as a fluorescence probe at physiological pH for selective detection of Zn2+, even in the presence of other competitive ions. When a 1
1, v/v) as the green solvent without any metal catalyst was demonstrated by using a mixture of a series of substituted amines (33) and isatins (1) or acenaphthenequinone (1′), malononitrile (17) or acyclic-1,3-diketone (10), and indane-1,3-dione (34) (Scheme 11). The reaction was evaluated using microwave irradiation at 60 °C. The compounds were designed as a fluorescence probe at physiological pH for selective detection of Zn2+, even in the presence of other competitive ions. When a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal–ligand complex was used, the fluorescent signal was enhanced. Additionally, due to its cell permeability, this spiro sensor was successfully used in biomedical studies to detect intracellular Zn2+ within human liver carcinoma cells (HepG2).183
1 metal–ligand complex was used, the fluorescent signal was enhanced. Additionally, due to its cell permeability, this spiro sensor was successfully used in biomedical studies to detect intracellular Zn2+ within human liver carcinoma cells (HepG2).183
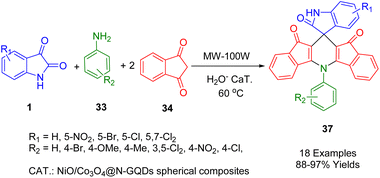
Safaei-Ghomi et al. synthesized with microwave irradiation a series of spiro diindenopyridine-indoline trione derivatives (37) using NiO/Co3O4@N-GQDs spherical composites as a nanocatalyst (Scheme 12). With water as a green solvent at 80 °C, they obtained the corresponding products from mixtures of aniline derivatives (33), substituted isatins (1), and two equivalents of 1,3-indanedione (34) in the presence of NiO/Co3O4@N-GQDs spherical composites. The reaction was rapid, high yielding, and a non-chromatographic purification protocol was used.184
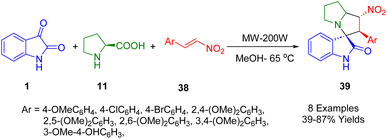
In 2021, Maniam et al. designed a green and rapid methodology for the synthesis of spirooxindole pyrrolizidines (39) employing a microwave-assisted one-pot, three-component 1,3-dipolar cycloaddition of azomethine ylides from L-proline (11) and isatin (1) with various β-nitrostyrenes (38) in methanol as the solvent at 65 °C to obtain products in reasonable-to-high yields (Scheme 13).185
The structure of (1′R,2′R,3R)-2′-(2,4-dimethoxyphenyl)-1′-nitro-1′,2′,5′,6′,7′,7a′-hexahydrospiro[indoline-3,3′-pyrrolizin]-2-one (39) was confirmed by single-crystal X-ray analysis. In primary thioflavin T (ThT) screening, certain nitro-spirooxindoles inhibit HEWL amyloid fibril formation. Throflavin T (ThrT) was used for determining fluorescence intensity in the majority of the compounds. These promising candidates for activity against amyloid misfolding, a pathology associated with Alzheimer's disease, were examined further with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays and molecular docking.
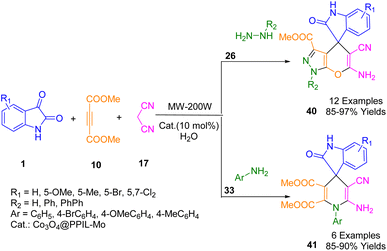
A molybdenum complex supported on cross-linked poly(1-aminopropyl-3-vinylimidazolium bromide) entrapped in cobalt oxide nanoparticles was fabricated by Safaei Ghomi and co-workers was used as a novel heterogeneous nanocatalyst for the synthesis of spiro 1,4-dihydropyridines (41) and spiro pyranopyrazoles (40) (Scheme 14). The three-component condensation reaction was conducted with various isatins (1), malononitrile (17), dimethyl acetylene dicarboxylate (DMAD) (10), various amines (26, 33) (hydrate hydrazine, phenyl hydrazine, and aromatic amines), treated catalyst (10 mol%), and water as a green solvent, and irradiated in a microwave oven using 200 W of power.186
A rapid and effective microwave-assisted solvent-free method for synthesizing polyfunctionalized spiro[indoline-3,4′-pyrazolo[3,4-b]pyridines] (44) with remarkable pharmacological properties was proposed by Ali et al. (Scheme 15). Using a simple and highly efficient microwave-assisted MCR strategy comprising hydrazines (26), DMADs (42), isatin (1), and active methylene compounds (43), reactions were carried out to afford satisfying yields of products.187
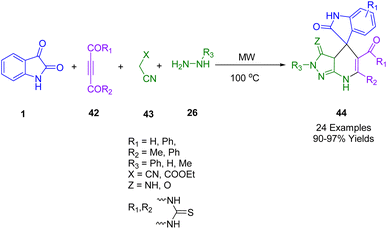 | ||
| Scheme 15 Synthesis of pyrano[2,3-d]pyrimidine, pyrano[3,2-b]pyran, and chromeno[2,3-d]pyrimidine derivatives 44. | ||
The antimicrobial activity of the novel compounds was evaluated against Staphylococcus aureus (G +ve), Pseudomonas aeruginosa (G −ve) Candida albicans (yeast), and Aspergillus niger (fungus) using the agar diffusion method. The majority of the compounds exhibited moderate-to-high antimicrobial activity. A phosphomolybdenum antioxidant technique was used to estimate each compound's total antioxidant capacity. A cytotoxic test of the compounds was conducted against three human tumor cell lines: HePG2 H, CT-116, and MCF-7. Cytotoxicities varied from weak to strong.
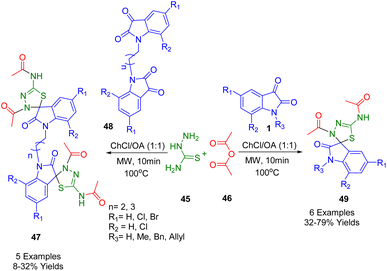
As shown in Scheme 16, microwave irradiation combined with deep eutectic solvents (DES) was used to synthesize 3,4-thiadialzolinic spiro heterocycles (47) and (49). This was achieved using a straightforward and effective three-component approach. Microwave-assisted, one-pot reactions were conducted using choline chloride/oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent for the reaction between monomeric and dimeric isatin derivatives (1, 48). Reactions were conducted at 100 °C for ten minutes and reactions yielded close to or greater yields than reported for two-step reaction sequences. It appears that thiadiazolines with strong cytotoxic properties can be synthesized in a one-pot reaction more effectively, cleaner, and faster than using several steps.188
1) as the solvent for the reaction between monomeric and dimeric isatin derivatives (1, 48). Reactions were conducted at 100 °C for ten minutes and reactions yielded close to or greater yields than reported for two-step reaction sequences. It appears that thiadiazolines with strong cytotoxic properties can be synthesized in a one-pot reaction more effectively, cleaner, and faster than using several steps.188
The effects of spiro compounds on cancer-causing cells were tested using three cancer cell lines: A-549 (small cell lung cancer), HL-60 (promyelocytic leukemia), and K-562 (chronic myeloid leukemia). Spiro thiadiazoline monomers were found to have a significantly higher IC50 than their dimeric counterparts against K-562 cells, but limited activity against HL-60 cells. Notably, the monomers produced similar results to the reference medication against strain K-562. However, non-chlorinated dimers were approximately three times more potent than chlorinated monomers.
Leishmaniasis is a neglected tropical disease and its treatment desperately needs improving. Thus, Coghi et al. developed a green method consisting of a three-component reaction to synthesize novel compounds with antileishmanial properties. A novel series of functionalized spiro[indoline-3,2′-pyrrolidin]-2-one/spiro[indoline-3,3′-pyrrolizin]-2-ones (53–55) were prepared from natural product-inspired pharmaceutically privileged bioactive sub-structures, i.e., isatins (1), various substituted chalcones (50), and amino acids (11, 51, 52) via 1,3-dipolar cycloaddition reactions in MeOH at 80 °C using a microwave-assisted approach with good-to-excellent yields (Scheme 17).189
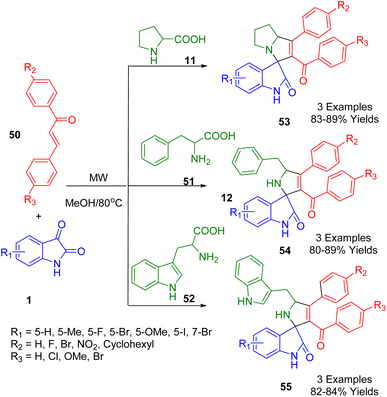 | ||
| Scheme 17 Synthesis of functionalized spiro[indoline-3,2′-pyrrolidin]-2-one/spiro[indoline-3,3′-pyrrolizin]-2-ones 53–55. | ||
The authors reported in vitro antileishmanial activity and SAR studies against Leishmania donovani and the compounds proved to be very active. Compared to Amphotericin B (IC50 = 0.060 m), their IC50 values were 2.43 m, 0.96 m, 1.62 m, and 3.55 m, respectively. Relative to the standard drug Camptothecin, all compounds were tested for their ability to inhibit the Leishmania DNA topoisomerase type IB enzyme. Molecular docking studies were also performed to understand how these compounds bind. Single-crystal X-ray analysis was used to confirm the stereochemistries of the spirooxindoles.
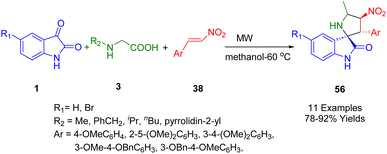
In 2023, Wong and co-workers developed a highly efficient microwave-assisted MCR for the synthesis of a series of nitrostyrene-based spirooxindoles (56) in a chemo/regio-selective manner using [3 + 2] cycloaddition (Scheme 18). By this Huisgen reaction, treatment of readily available substituted isatins (1), several α-amino acids (3), and (E)-2-aryl-1-nitroethenes (38) under microwave irradiation using 300 W of power in methanol as solvent afforded the corresponding desired spiro adducts in good-to-high yield.190
The stereochemical arrangements around the pyrrolidine ring of the spiro pyrrolidine-2,3′-oxindoles (56) were determined using single-crystal X-ray analysis. The compounds were also shown to have promising anticancer activity when tested against human lung cancer A549, human liver cancer HepG2, and immortalized normal human lung and liver cell lines. Five of the synthesized spiro pyrrolidine-2,3′-oxindoles showed significant anticancer in vitro activity (IC50 = 34.99–47.92 μM, SI = 0.96–2.43) against the human lung cancer A549 cell line, while six compounds showed promising anticancer in vitro activity against the human liver cancer HepG2 cell line. The compounds showed greater efficiency and selectivity than the standard reference artemisinin (IC50 = 100 μM, SI: 0.03) in the human lung cancer A549 cell line. However, none of them were active against lung (A549) and liver (HepG2) cancer cells.
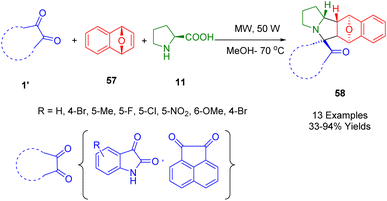
In another study, Zhao et al. synthesized spirooxindoles using a microwave-assisted MCR. Using economical and easily available starting materials, environmentally friendly conditions, and microwave irradiation, they synthesized oxygen-bridged spirooxindoles (58) by reacting various diketones (1′) (isatins or acenaphthenequinone), α-amino acids (11), and 1,4-dihydro-1,4-epoxynaphthalene (57) in methanol as solvent at 70 °C. The reaction yielded reasonable-to-excellent yields (Scheme 19).191
3.2. Spiropyrrolidine frameworks
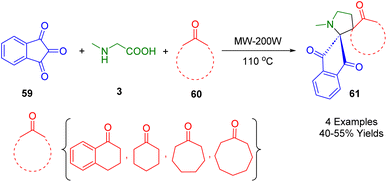
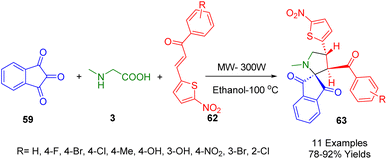
In 2017, Kumar and coworkers developed an efficient microwave-assisted, three-component synthesis of novel dispiro heterocycle derivatives (61) using cycloketone (60), ninhydrin (59), and methylglycine (3) (Scheme 20). This protocol, which is both catalyst- and solvent-free, allows for expansion of the reaction scope using substituted cyclic ketones. The products were obtained in high yields.192Kalluraya and co-workers in 2018 employed an efficient and environmentally friendly microwave-assisted method for the synthesis of diversely functionalized nitrothiophene-containing spiro pyrrolidines (63) based on a catalyst-free approach with high regioselectivity in moderate-to-excellent yields (Scheme 21). The one-pot, three-component system consisted of ninhydrin (59), sarcosine (3), and 1-(tolyl)-3-(5-nitrothienyl)prop-2-en-1-one (62) in ethanol. The reaction produced spiropyrrolidines with novel regiochemistry containing a nitrothiophene moiety using a simplified work-up process with increased reaction rates and improved yield.193
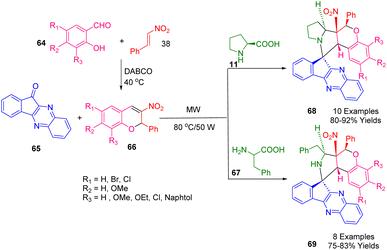
For the construction of sets of spiro indenoquinoxaline pyrrolidine-fused nitrochromenes (68, 69) in good-to-high yields (Scheme 22), Nayak and his team developed an environmentally friendly, rapid, convenient, and highly advantageous microwave-assisted MCR (68, 69). The first step in the microwave-assisted with 50 W of power preparation of the 3-nitro-2H-chromene derivatives (66) at 80 °C was the 1,3-dipolar cycloaddition of trans-β-nitro styrene (38) with salicylaldehydes (64) in the presence of DABCO under solvent-free conditions followed by an oxo-Michael–aldol reaction. After the condensation of indenoquinoxalone (65) and an α-amino acid, L-proline (11) or L-phenyl alanine (67), the 1,3-dipolar cycloaddition of these azomethine ylides with 3-nitrochromenes (66) as dipolarophiles occurred. The synthesis could also be accomplished using classical methods conditions but metal-free conditions were used in this microwave-assisted approach. In the latter approach, the reaction conditions are mild, the yield of the products was high, the regioselectivity was high, and the procedures are simple to follow. Using spectroscopic and single-crystal X-ray analysis, the cycloaddition reaction was studied regioselectively and stereochemically.194
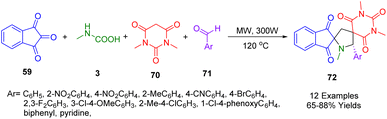
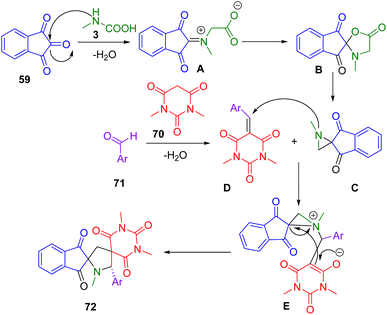
In 2019, Koodlur and his team developed a series of regioselective dispiro pyrrolidine analogues (72) by using an environmentally friendly approach. The one-pot, four-component condensation reaction consisted of the condensation of aromatic aldehydes (71), N,N-dimethyl barbituric acid (70), ninhydrin (59), and sarcosine (3) under microwave irradiation using magnesium silicate as catalyst to provide moderate-to-considerable yields (Scheme 23). Single-crystal X-ray analysis provided unambiguous evidence of stereochemistry. There are several salient features of this green methodology, including easy isolation, high atom economy, mild reaction conditions, no need for column chromatography, use of a mild catalyst, ease of performance, broad substrate scope, and low E-factor.195
The antibacterial and antiproliferative activities were also assessed for the synthesized compounds. Against Gram-positive and Gram-negative bacteria, compounds (Ar = C6H5, and 4-NO2C6H5) showed potent antibacterial properties. The antiproliferative activity of (S)-1′,1′′,3′′-trimethyl-5′-phenyl-2′′H-dispiro[indene-2,2′-pyrrolidine-4′,5′′-pyrimidine]-1,2′′,3,4′′,6′′(1′′H,3′′H)-pentaone was also found to be the strongest against the tested cell lines.
In Scheme 24 is depicted a possible mechanism for this four-component, one-pot reaction. Dimethyl barbituric acid (70) and ninhydrin to form the Knoevenagel adduct (A) and then the spiro aziridine (B). Spiro aziridine (B) reacts by a stereoselective aza-Michael reaction to form aziridinium ion (C), which in turn yields an enolate (D). The desired product is obtained by opening up the aziridinium ions with the enolate. An aza-Michael addition justified the formation of the product which is primarily driven by electronic factors.
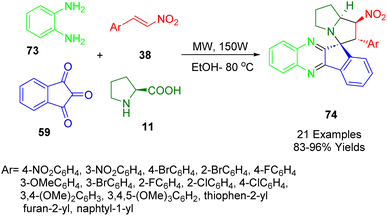
An investigation of spiro quinoxaline derivatives (74) was conducted by Trivedi et al. Using a four-component cascade protocol, spiro indeno[1,2-b]quinoxaline-11,3′-pyrrolizine derivatives (74) can be synthesized in short reaction times (88–98%) with excellent yields (Scheme 25). Microwave irradiation of ninhydrin (59), phenylenediamine (73), L-proline (11), and nitrostyrene derivatives (38) was conducted under green solvent conditions. All of the synthesized compounds were screened for acetylcholinesterase (AChE) inhibitory activity and some showed significant activity with low micromolar IC50.196
From the established chemistry of ninhydrin (59) and indenoquinoxaline (A), it is reasonable to assume that the indenoquinoxaline-11-one intermediate (A) was formed when ninhydrin (59) and 1,2-phenylenediamine (73) were condensed with L-proline (11) to form 1,3-dipolar azomethine ylides (B). As a result of the 1,3-dipole (B) being formed, trans-β-nitrostyrenes (38) react as dipolarophiles to produce (C), a stereoselective adduct (74) (Scheme 26).
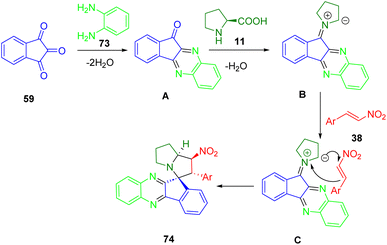 | ||
| Scheme 26 Proposed mechanism for the preparation of spiro indeno[1,2-b]quinoxaline-11,3′-pyrrolizines 74. | ||
In their green, one-pot procedure, Perumal et al. demonstrated the efficient stereoselective synthesis of dihydro-2′H-spiro[indene-2,1′-pyrrolo[3,4-c]pyrrole]-tetraone derivatives (76). An in situ reaction of ninhydrin (59) and sarcosine (3) with a series of 1-aryl-1H-pyrrole-2,5-diones (75) was described using microwaves at 100 °C through 1,3-dipolar cycloaddition (Scheme 27). To determine whether the synthesized compounds inhibited AChE (acetylcholinesterase) and mycobacterial growth, the compounds were screened for antimycobacterial activity. Compared to cycloserine and pyrimethamine, compound 4b (IC50 1.30 mM) has twelvefold stronger antimycobacterial activity than compound 4a. The AchE inhibitory activity of compound (R = 4-MeOC6H4) is evidenced by its IC50 value of 0.78 ± 0.01 mmol L−1. A single-crystal X-ray analysis of one compound (R = cyclohexyl) provided unambiguous evidence of the stereochemistry.197
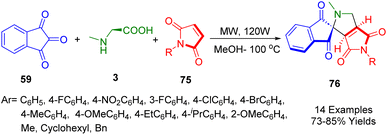 | ||
| Scheme 27 Synthesis of dihydro-2′H-spiro[indene-2,1′-pyrrolo[3,4-c]pyrrole]-tetraone derivatives 76. | ||
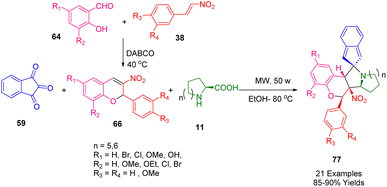
Various spiro indanone pyrrolidine/piperidine-fused nitrochromene derivatives (77) were synthesized by Nayak et al. using a highly efficient, simple, environmentally friendly, three-component method (Scheme 28). Using readily available starting materials, the 1,3-dipolar cycloaddition of azomethine ylides generated in situ by the condensation of ninhydrin (59) and an α-amino acid, L-proline (11) or L-phenyl alanine (67), reacted with 3-nitrochromenes (66) as dipolarophiles under microwave irradiation. The cycloaddition reaction was highly regioselective and diastereoselective. The protocol was operationally simple using mild reaction conditions and provided a high yield.198 A comparison was also made to classical methodology.
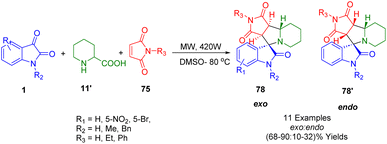
Methods mediated by microwave irradiation were developed by Jaidi's group (Scheme 29) to prepare spiro indoloindolizidines (78, 78′), a significant class of compounds with biological activity. They were synthesized through the decarboxylative condensation of pipecolinic acid (11′) and isatin (1) with maleimide (75) under reflux and microwave irradiation. Using column chromatography, both endo and exo diastereomeric spiro indoloindolizidines (78, 78′) were obtained with exo-selectivity and high yields. Various 2D NMR spectroscopic techniques and single-crystal X-ray analyses were used to determine the stereochemistry and structure of the synthesized cycloadducts. Density functional theory (DFT) calculations were also employed to investigate the reaction mechanism.199
3.3. Spiro pyran frameworks
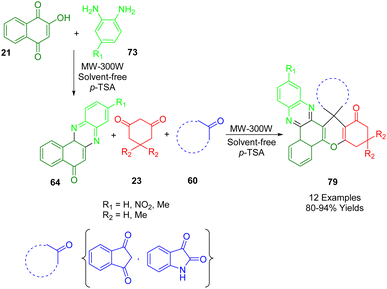
During their search for environmentally friendly synthetic protocols, Mohebat and co-workers developed a heterogeneous, non-toxic nanocatalyst with p-toluenesulfonic acid which facilitated the preparation of novel spiro [benzo[a]chromene[2,3-c]phenazine] derivatives (79). p-Toluenesulfonic acid was used as an acid catalyst for the multicomponent reaction of 2-hydroxy-1,4-naphthaquinone (21), benzene-1,2-diamine (73), cyclic 1,3-dicarbonyl compounds (23) and isatin (1) or ninhydrin (59) at 100 °C with microwave irradiation. The two-step reaction was high yielding and constituted a clean and environmentally friendly method (Scheme 30). By forming five new bonds (two C–C bonds, two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, and one C–O bond) and two additional rings in a single operation, this solvent-free process produced biologically significant spiro heterocycles. In addition to its operational simplicity and short reaction times, this effective green process is also high yielding, eliminates tedious purification processes, and avoids hazardous reagents and solvents.200
N bonds, and one C–O bond) and two additional rings in a single operation, this solvent-free process produced biologically significant spiro heterocycles. In addition to its operational simplicity and short reaction times, this effective green process is also high yielding, eliminates tedious purification processes, and avoids hazardous reagents and solvents.200
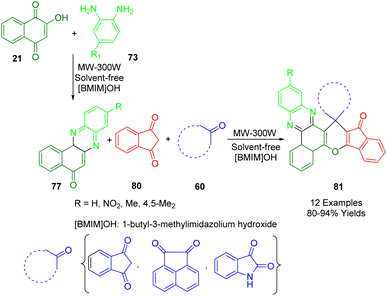
Yazdani-Elah-Abadi and colleagues proposed a technique for the production of biologically and pharmaceutically relevant highly functionalized diverse novel spiro benzo[a]phenazine annulated heterocycles (81) through the use of a basic ionic liquid (1-butyl-3-methylimidazolium hydroxide) as an expedient, environmentally friendly, and reusable catalyst (Scheme 31). The ionic liquid and a heteropolyacid were used to functionalize halloysite nanotubes and synthesize the catalyst. Microwave-assisted methods were employed to achieve high yields and short reaction times when 2-hydroxynaphthalene-1,4-dione (21), benzene-1,2-diamines (73), cyclic carbonyl compounds (60), and 1,3-indandione (80) were condensed, resulting in the formation of spiro[benzo[a]indeno[2′,1′:5,6]pyrano[2,3-c]phenazine] derivatives (81) in high yields under solvent-free conditions.201
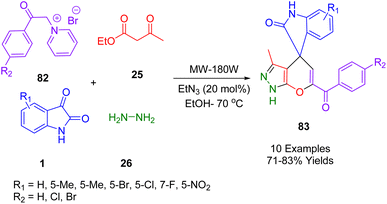
In 2019, Yazdani-Elah-Abadi et al. devised an efficient one-pot, four-component reaction protocol to obtain spirooxindole-furo[2,3-c]pyrazole derivatives (83) (Scheme 32) containing biologically active pyrazole and spirooxindole–furan moieties. The mixture consisted of hydrazine (26), β-keto ester (25), isatin derivatives (1), and pyridinium ylide (82) catalyzed by triethylamine (20 mol%) in ethanol under microwave irradiation. The highly reactive base catalyst Et3N (20 mol%) was used in conjunction with N-phenacyl bromide and pyridine to produce pyridinium ylide. This environmentally friendly method is characterized by high yields, short reaction times, simplicity of operation, lack of tedious workup or purification, and the absence of hazardous or toxic reagents or solvents.202
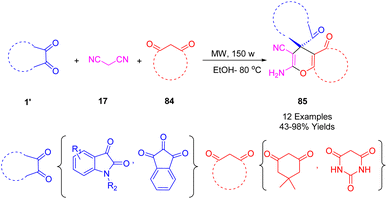
Greco et al. synthesized a novel spiro pyrano[2,3-d]pyrimidine derivative (85) (Scheme 33) using a microwave-assisted Knoevenagel/Michael/cyclization multicomponent. Ethanol was used as the solvent and the ionic liquid 1-methylimidazolium chloride was used as the catalyst. Various starting materials (isatin, malononitrile, dimedone, and barbituric acid) were used in the microwave-assisted MCR at 80 °C.203
The single-crystal X-ray structures of 7′-amino-5-bromo-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile and 7,2-amino-5-bromo-2,2′,4′-trioxo-1′,2′,3′,4′ were determined. In addition, the antiproliferative activities of the spiro heterocycles against human colon carcinoma HCT116, prostate carcinoma PC3, promyelocytic leukemia HL60, and astrocytoma SNB19 cell lines were screened using an MTT-based assay. The spiro compound 7′-amino-6-bromo-2,2′,4′-trioxo-1′,2′,3′,4′-tetrahydrospiro[indoline-3,5′-pyrano[2,3-d]pyrimidine]-6′-carbonitrile showed significant inhibition against all four cell lines with IC50 values of 52.81 M for HCT116, 74.40 M for PC3, 101 M for SNB19, and 49.72 M for HL60.
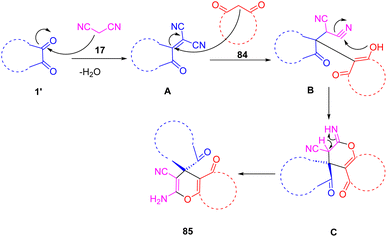
A proposed mechanism for the synthesis of (85) is depicted in Scheme 34. Initially, Knoevenagel condensation forms the isatylidene-malononitrile intermediate (A). As a result of a Michael addition between (A) and (84), followed by cyclization and tautomerization, (B) is formed. Intermediate (A) was able to be isolated and characterized, thereby supporting the mechanistic proposal. In the infrared spectrum of (A), a band at 2230 cm can be observed which is attributed to the stretching of the bond between the nitrile carbons and the central carbon of malononitrile (17) attached to isatin (1).
3.4. Miscellaneous frameworks
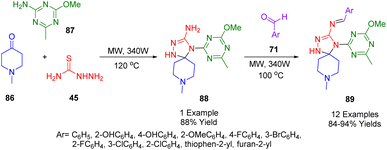
Using a microwave-assisted reaction between 1-methylpiperidin-4-one (86), 2-amino-4-methoxy-6-methyl-1,3,5-triazin-2-yl (87), and thiosemicarbazide (45), Patel and co-workers synthesized 4-(4-methoxy-6,6-methyl-1,3,5-triazin-2-yl)-8-methyl-1,2,4,8-tetraazaspiro[4.5]dec-2-en-3-amine (88). As depicted in Scheme 35, they then used this spiro adduct (88) to synthesize a novel type of spiro compound (89) by reacting it with various aldehydes.204Antibacterial tests indicated that some of the compounds are promising antibacterial agents. Indeed, some compounds were even more effective antimycobacterial agents than the conventional drug Rifampicin. Docking studies using Escherichia coli DNA gyrase reflected the biological results with appropriate docking scores. Drug solubility, hydrogen bonding, and cell permeability are all ideal according to in silico ADME analysis.
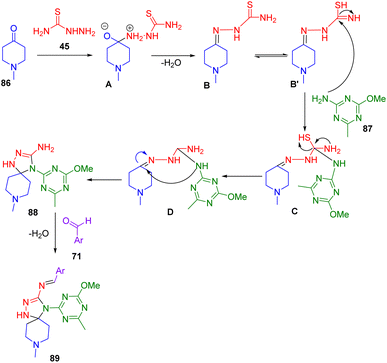
A reaction is mechanism leading to the formation of (88, 89) is depicted in Scheme 36. An intermediate imine is initially formed which then subsequently undergoes a cycloaddition reaction with 2-amino-4-methoxy-6-methy-1,3,5-triazine (87).
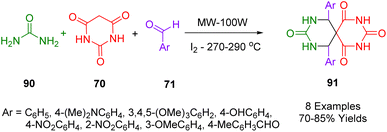
Mehta and his coworkers synthesized environmentally friendly spiro heterocyclic compounds 91 as part of a Biginelli-type condensation process by condensing barbituric acid (70), substituted aldehyde (71), and urea (90) using iodine as a catalyst at 270–290 °C in a solvent-free microwave irradiation approach (Scheme 37).205
The compounds (Ar = 4-NO2C6H4, and 2-NO2C6H4) demonstrated promising antimicrobial activity against bacterial strains Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli as well as fungal strains Candida albicans and Aspergillus niger. The specific binding mode of compounds (Ar = 4-NO2C6H4, and 2-NO2C6H4) was predicted by molecular docking simulations. The anthelmintic activity of compound (Ar = 4-NO2C6H4) was compared to the drug albendazole using the Indian adult earthworm Eisenia fetida where by (Ar = 4-NO2C6H4) showed effective pharmacophore activity.
4. Conclusions
Various heterocyclic compounds, including spiro heterocycles, can be synthesized using microwave-assisted MCRs which is a powerful and efficient method. This review article provides an overview of recent developments in this field covering various types of spiro heterocycles. Additionally, it discusses the potential mechanisms and reactions involved in MCRs, as well as the advantages and limitations of microwave irradiation. Spiro heterocycles are a versatile and diverse class of molecules, and microwave MCRs can be employed to construct them. However, there are challenges and opportunities in this field that warrant further research.Conflicts of interest
There are no conflicts to declare.References
- N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- A. D. Jangale and D. S. Dalal, Synth. Commun., 2017, 47, 2139 CrossRef CAS.
- A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611 RSC.
- T. Y. Zhang, in Advances in Heterocyclic Chemistry, Academic Press, 2017, vol. 121, p. 1 Search PubMed.
- J. Jampilek, Molecules, 2019, 24, 3839 CrossRef CAS PubMed.
- M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265 CrossRef PubMed.
- M. Koley, J. Han, V. Soloshonok, S. Mojumder, R. Javahershenas and A. Makarem, RSC Med. Chem., 2024, 15, 10 RSC.
- A. Al-Mulla, Der Pharma Chem., 2017, 9, 141 CAS.
- P. Arora, V. Arora, H. S. Lamba and D. Wadhwa, Int. J. Pharma Sci. Res., 2012, 3, 2947 Search PubMed.
- R. Nishanth Rao, S. Jena, M. Mukherjee, B. Maiti and K. Chanda, Environ. Chem. Lett., 2021, 19, 3315–3358, DOI:10.1007/s10311-021-01232-9.
- B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323 CrossRef CAS PubMed.
- O. Ghashghaei, F. Seghetti and R. Lavilla, Beilstein J. Org. Chem., 2019, 15, 521 CrossRef CAS PubMed.
- J. Zhu, Q. Wang and M. X. Wang, Multicomponent Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2015 Search PubMed.
- T. J. J. Müller, Multicomponent Reactions General Discussion and Reactions Involving a Carbonyl Compound as Electrophilic Component Science of Synthesis Series, Georg Thieme Verlag KG, Stuttgart, 2014 Search PubMed.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC.
- J. A. dos Santos, P. P. de Castro, K. T. de Oliveira, T. J. Brocksom and G. W. Amarante, Curr. Top. Med. Chem., 2023, 23, 990 CrossRef CAS PubMed.
- M. Henary, C. Kananda, L. Rotolo, B. Savino, E. Owens and G. Cravotto, RSC Adv., 2020, 10, 14170 RSC.
- G. Singh and Z. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed.
- J. Soni, S. Joshi, K. Chemitikanti and N. Shankaraiah, Eur. J. Inorg. Chem., 2021, 10, 1476 CrossRef.
- M. Kamboj, S. Bajpai, M. Yadav and S. Singh, Curr. Green Chem., 2023, 10, 57 CrossRef CAS.
- T. Damera, R. Pagadala, S. Rana and S. B. Jonnalagadda, Catalysts, 2023, 13, 1034 CrossRef CAS.
- S. B. Adhikari and T. Ghosh, Tetrahedron, 2022, 126, 133085 CrossRef.
- S. Gulati, S. E. John and N. Shankaraiah, Beilstein J. Org. Chem., 2021, 17, 819 CrossRef CAS PubMed.
- K. Kamanna and Y. Amaregouda, Curr. Organocatal., 2023, 10, 160 CrossRef CAS.
- D. Amariucai-Mantu, V. Mangalagiu, R. Danac and I. I. Mangalagiu, Molecules, 2020, 25, 716 CrossRef CAS PubMed.
- T. Sengoku, A. Shirai, A. Takano, T. Inuzuka, M. Sakamoto, M. Takahashi and H. Yoda, J. Org. Chem., 2019, 84, 12532 CrossRef CAS PubMed.
- G. Meera, K. R. Rohit, S. Saranya and G. Anilkumar, RSC Adv., 2020, 10, 36031 RSC.
- N. Z. Kiss, E. Bálint and G. Keglevich, Microwave-Assisted Syntheses in Organic Chemistry, ed. G. Keglevich, Milestones in Microwave Chemistry, SpringerBriefs in Green Chemistry for Sustainability, Springer International Publishing, Switzerland, 2016, ch. 2, DOI:10.1007/978-3-319-30632-2_2.
- A. Kumar, Y. Kuang, Z. Liang and X. Sun, Mater. Today Nano, 2020, 11, 100076 CrossRef.
- D. Garella, E. Borretto, A. Di Stilo, K. Martina, G. Cravotto and P. Cintas, MedChemComm, 2013, 4, 1323 RSC.
- J. Fairoosa, S. Saranya, S. Radhika and G. Anilkumar, ChemistrySelect, 2020, 5, 5180 CrossRef CAS.
- G. Tiwari, A. Khanna, V. K. Mishraa and R. Sagar, RSC Adv., 2023, 13, 32858 RSC.
- S. Das, Synth. Commun., 2022, 52, 637 CrossRef CAS.
- N. Sharma, U. K. Sharma and E. V. Van der Eycken, Microwave-Assisted Organic Synthesis: Overview of Recent Applications, Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. W. Zhang and W. Berkeley Cue, John Wiley & Sons Ltd, 2nd edn, 2018, ch. 17 Search PubMed.
- R. Javahershenas, H. Mei, M. Koley, V. A. Soloshonok and A. Makarem, Synthesis, 2024 DOI:10.1055/s-0042-1751526.
- Y. Liu, X. Zhang, R. Zeng, Y. Zhang, Q.-S. Dai, H.-J. Leng, X.-J. Gou and J.-L. Li, Molecules, 2017, 22, 1882 CrossRef PubMed.
- R. G. Soengas, G. Da Silva and J. C. Estévez, Molecules, 2017, 22, 2028 CrossRef PubMed.
- S. Majhi and P. K. Mondal, Curr. Microwave Chem., 2023, 10, 135 CrossRef CAS.
- P. Zhang, X.-L. Yuan, Y.-M. Du, H.-B. Zhang, G.-M. Shen and Z.-F. Zhang, J. Agric. Food Chem., 2019, 67, 11994 CrossRef CAS PubMed.
- A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, Chem. Soc. Rev., 2018, 47, 5946 RSC.
- Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673 CrossRef CAS PubMed.
- R. Javahershenas and S. Nikzat, Ultrason. Sonochem., 2024, 102, 106741 CrossRef CAS PubMed.
- S. Imeni, A. Makarem and R. Javahershenas, Asian J. Org. Chem., 2023, e202300303 CrossRef CAS.
- R. Javahershenas and S. Nikzat, RSC Adv., 2023, 13, 16619 RSC.
- R. Javahershenas, J. Mol. Liq., 2023, 385, 122398 CrossRef CAS.
- R. Javahershenas, Mol. Diversity, 2023, 27, 2399 CrossRef CAS PubMed.
- R. Mannhold, H. Kubinyi and G. Folkers, Microwaves in Organic and Medicinal Chemistry, Wiley-VCH Verlag & Co. KGaA, Boschstr, 2012 Search PubMed.
- A. De La Hoz, J. Alcázar, J. Carrillo, M. A. Herrero, J. D. M. Muñoz, P. Prieto, A. De Cózar and A. Diaz-Ortiz, Reproducibility and Scalability of Microwave-Assisted Reactions, 2011, vol. 1 Search PubMed.
- G. Stefanidis and A. Stankiewicz, Alternative Energy Sources for Green Chemistry, Microwave-Assisted Green Organic Synthesis, RSC Green chemistry, 2016, ch. 1 Search PubMed.
- V. K. Saxena and U. Chandra, Physical Concept, 2011, vol. 1 Search PubMed.
- Microwave-Enhanced Chemistry, ed. S. J. Haswell, Fundamentals, Sample Preparation and Applications, American Chemical Society, Washington DC, 1997 Search PubMed.
- Microwave Techniques and Protocols, ed. R.T. Giberson and R.S. Demaree, Humana Press, Totowa, New Jersey, 2001 Search PubMed.
- W. E. Prentice, Therapeutic Modalities for Physical Therapists, McGraw-Hill, New York, 2002 Search PubMed.
- B. L. Hayes, Microwave Synthesis-Chemistry with the Speed of Light, CEM Publishing, 2002 Search PubMed.
- N. E. Leadbeater, Microwave Chemistry as a Tool of Sustainable Chemistry, CRC Press, 2010 Search PubMed.
- J. P. Tierney and P. Lidstroem, Microwave Assisted Organic Synthesis, CRC Press, 2005 Search PubMed.
- C. O. Kappe, A. Stadler and D. Dallinger, Microwaves in Organic and Medicinal Chemistry, Wiley-VCH-Verlag, 2012 Search PubMed.
- J. M. Kremsner and A. Stadler, A Chemist's Guide to Microwave Synthesis, Anton Paar GmbH, 2013 Search PubMed.
- L. Favretto, Basic Guidelines for Microwave Organic Chemistry Applications, Milestone, 2004 Search PubMed.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279 CrossRef CAS.
- R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Tetrahedron Lett., 1986, 27, 4945 CrossRef CAS.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127 RSC.
- P. Lidström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225 CrossRef.
- C. O. Kappe, Angew. Chem., Int. Ed. Engl., 2004, 43, 6250 CrossRef CAS PubMed.
- G. B. Dudley, A. E. Stiegman and M. R. Rosana, Angew. Chem., Int. Ed. Engl., 2013, 52, 7918 CrossRef CAS PubMed.
- C. O. Kappe, B. Pieber and D. Dallinger, Angew. Chem., Int. Ed. Engl., 2013, 52, 1088 CrossRef CAS PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem. Lett. Rev., 2008, 1, 85 Search PubMed.
- C. T. Chou, T. H. Liang and C. P. Hsieh, J. Food Eng., 2001, 49, 211 Search PubMed.
- G. P. Sharma and S. Prasad, Food Res. Int., 2009, 42, 516 CrossRef.
- A. C. Metaxas, and R. J. Meredith, Industrial Microwave Heating, Peter Peregrinus Ltd, London, 1983 Search PubMed.
- T. S. Awad, H. A. Moharram and O. E. Shaltout, J. Food Sci., 2012, 77, 1161 Search PubMed.
- H. Li, Q. Yu and X. Yan, Crit. Rev. Food Sci. Nutr., 2020, 60, 967 Search PubMed.
- V. C. Pawar and V. T. Thakkar, Int. J. Pharma Sci. Res., 2012, 3, 2469 Search PubMed.
- M. Negash, A. Osman and K. C. Das, Renewable Sustainable Energy Rev., 2017, 72, 1151 Search PubMed.
- X. Chen, X. Zhang and Y. Wu, Mater. Chem. Phys., 2009, 113, 355 Search PubMed.
- G. Cravotto and P. Cintas, Microwave Chemistry, De Gruyter, 2017, DOI:10.1515/9783110479935-001.
- C. O. Kappe, Chem. Rec., 2019, 19, 15 CrossRef CAS PubMed.
- L. Rinaldi, D. Carnaroglio, L. Rotolo and G. Cravotto, J. Chem., 2015, 879531 CAS.
- M. Nüchter, B. Ondruschka, W. Bonrath and A. Gum, Green Chem., 2004, 6, 128 RSC.
- G. B. Dudley, R. Richert and A. E. Stiegman, Chem. Sci., 2015, 6, 2144 RSC.
- C. Strauss and R. Trainor, Aust. J. Chem., 1995, 48, 1665 CrossRef CAS.
- M. Kidwai, Pure Appl. Chem., 2001, 73, 147 CrossRef CAS.
- R. Cecilia, U. Kunz and T. Turek, Chem. Eng. Process., 2007, 46, 870 CrossRef CAS.
- J. Martín-Gil, F. J. Martín-Gil, M. José-Yacamán, L. Carapia-Morales and T. Falcón-Bárcenas, Pol. J. Chem., 2005, 1399 Search PubMed.
- J. Prado-Gonjal, M. E. Villafuerte-Castrejón, L. Fuentes and E. Morán, Mater. Res. Bull., 2009, 44, 1734 CrossRef CAS.
- J. Zhao and W. Yan, Modern Inorganic Synthetic Chemistry, 2011, ch. 8, vol. 173 Search PubMed.
- R. K. Sahu, M. L. Rao and S. S. Manoharan, J. Mater. Sci., 2001, 36, 4099 CrossRef CAS.
- X. Zhang, D. O. Hayward, C. Lee and D. M. P. Mingos, Appl. Catal., B, 2001, 33, 137 CrossRef CAS.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6285 CrossRef CAS PubMed.
- H. Ahmad and M. K. Hossaina, Mater. Adv., 2022, 3, 859 RSC.
- D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed.
- P. Lidström and J.-C. Halls, Tetrahedron, 2001, 57, 9225 CrossRef.
- C. O. Kappe, Angew. Chem., Int. Ed. Engl., 2013, 52, 7924 CrossRef CAS PubMed.
- A. de la Hoz, A. Díaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164 RSC.
- S. H. Zhang and Z. C. Feng, J. Mol. Struct., 2010, 977, 62 CrossRef CAS.
- J. M. H. Levy and M. A. Levy, Chem. Rev., 2017, 117, 5859 Search PubMed.
- A. A. Zhang and J. L. Lippard, Chem. Rev., 2017, 117, 5837 Search PubMed.
- A. O. Adeola, M. P. Duarte and R. Naccache, Front. Carbon, 2023, 2, 2023 Search PubMed.
- E. Berrino and C. T. Supuran, Expert Opin. Drug Discovery, 2018, 13, 861 CrossRef CAS PubMed.
- M. Beller and C. Bolm, Transition Metals for Organic Synthesis, Wiley-VCH, 2004, pp. 254–257 Search PubMed.
- J. Y. Ying, Sci. China: Chem., 2019, 62, 416 Search PubMed.
- C. O. Kappe, Molecules, 2014, 19, 12087 Search PubMed.
- N. E. Leadbeater, Angew. Chem., Int. Ed. Engl., 2006, 45, 1678 CrossRef.
- N. Kuhnert, Angew. Chem., Int. Ed. Engl., 2002, 41, 1863 CrossRef CAS PubMed.
- A. A. Strekalova, A. A. Shesterkina, A. L. Kustov and L. M. Kustov, Int. J. Mol. Sci., 2023, 24, 8272 CrossRef CAS PubMed.
- R. W. Wagner, Drug Discov. World, 2006, 7, 59 Search PubMed.
- M. Driowya, A. Saber, H. Marza, L. Demange, R. Benhida and K. Bougrin, Molecules, 2016, 21, 492 CrossRef PubMed.
- Z. Zhao, H. Li and X. Gao, Chem. Rev., 2023 DOI:10.1021/acs.chemrev.3c00794.
- M. A. Al-Hassan and S. A. El-Sayed, Int. J. Mol. Sci., 2018, 19, 5179 Search PubMed.
- A. Lew, P. O. Krutzik, M. E. Hart and A. R. Chamberlin, J. Comb. Chem., 2002, 4, 95 CrossRef CAS PubMed.
- G. B. Evans, R. H. Furneaux, B. Greatrex, A. S. Murkin, V. L. Schramm and P. C. Tyler, J. Med. Chem., 2008, 51, 948 CrossRef CAS PubMed.
- S. Dhadda, S. Sharma, P. Jakhar and H. Sharma, RSC Adv., 2023, 13, 3723 RSC.
- S. Askri, A. Dbeibia, C. Mchiri, S. Boudriga, M. Knorr, E. Roulland, O. Laprévote, N. Saffon-Merceron and R. Gharbi, Appl. Sci., 2022, 12, 360 CrossRef CAS.
- A. R. Liandi, A. H. Cahyana, D. N. Alfariza, R. Nuraini, R. W. Sari and T. P. Wendari, Green Synth. Catal., 2023 DOI:10.1016/j.gresc.2023.08.001.
- M. Asif, T. Azaz, B. Tiwari and M. Nasibullah, Tetrahedron, 2023, 134, 133308 CrossRef CAS.
- M. Mamaghani and R. Hossein Nia, J. Heterocycl. Chem., 2017, 54, 1700 CrossRef CAS.
- P. Khanna, L. Khanna, S. J. Thomas, A. M. Asiri and S. S. Panda, Curr. Org. Chem., 2018, 22, 67 CrossRef CAS.
- T. P. I. Saragi, T. Spehr, A. Siebert, T. F. Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS PubMed.
- P. Saraswat, G. Jeyabalan, M. Z. Hassan, M. U. Rahman and N. K. Nyola, Synth. Commun., 2016, 46, 1643 CrossRef CAS.
- M. Zaki, A. Oukhrib, M. Akssira and S. B. Raboin, RSC Adv., 2017, 7, 6523 RSC.
- R. R. Kumar, S. Perumal, P. Senthilkumar, P. Yogeeswari and D. Sriram, J. Med. Chem., 2008, 51, 5731 CrossRef CAS PubMed.
- C. B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1996, 52, 12651 CrossRef CAS.
- C. B. Cui, H. Kakeya and H. Osada, J. Antibiot., 1996, 49, 832 CrossRef CAS PubMed.
- C. Pellegrini, M. Weber and H.-J. Borschberg, Helv. Chim. Acta, 1996, 79, 151 CrossRef CAS.
- B. M. Trost, N. Cramer and H. Bernsmann, J. Am. Chem. Soc., 2007, 129, 3086 CrossRef CAS PubMed.
- Y. Ban, N. Taga and T. Oishi, Tetrahedron Lett., 1974, 15, 187 CrossRef.
- S. M. Colegate, N. Anderton, J. Edgar, C. A. Bourke and R. N. Oram, Aust. Vet. J., 1999, 77, 537 CrossRef CAS PubMed.
- S. Lee, J. Yang, S. Yang, G. Lee, D. Oh, M. W. Ha and H. Park, Front. Chem., 2020, 8, 577371 CrossRef CAS PubMed.
- J.-S. Shi, J.-X. Yu, X.-P. Chen and R.-X. Xu, Acta Pharmacol. Sin., 2003, 24, 97 CAS.
- K. Ghedira, M. Zeches-Hanrot, B. Richard, G. Massiot, L. Le Men-Olivier, T. Sevenet and S. H. Goh, Phytochemistry, 1988, 27, 3955 CrossRef CAS.
- E. G. Prado, M. D. G. Gimenez, R. De la Puerta Vázquez, J. L. E. Sánchez and M. T. S. Rodríguez, Phytomedicine, 2007, 14, 280 CrossRef CAS PubMed.
- K. C. Chan, F. Morsingh and G. B. Yeoh, J. Chem. Soc., Perkin Trans. 1, 1966, 24, 2245 RSC.
- A. Lerchner and E. M. Carreira, J. Am. Chem. Soc., 2002, 124, 14826 CrossRef CAS PubMed.
- M. Budovská, P. Kutschy, T. Kožár, T. Gondová and J. Petrovaj, Tetrahedron, 2013, 69, 1092 CrossRef.
- A. Jossang, P. Jossang, B. Bodo, H. A. Hadi, T. Sévenet and B. Bodo, J. Org. Chem., 1991, 56, 6527 CrossRef CAS.
- M. G. Kulkarni, A. P. Dhondge, S. W. Chavhan, A. S. Borhade, Y. B. Shaikh, D. R. Birhade, M. P. Desai and N. R. Dhatrak, Beilstein J. Org. Chem., 2010, 6, 876 CrossRef CAS PubMed.
- H. Kato, T. Yoshida, T. Tokue, Y. Nojiri, H. Hirota, T. Ohta, R. M. Williams and S. Tsukamoto, Angew. Chem., Int. Ed., 2007, 46, 2254 CrossRef CAS PubMed.
- L. Anikina, Y. B. Vikharev, Y. S. Rozhkova and Y. V. Shklyaev, Pharm. Chem. J., 2013, 46, 707 CrossRef CAS.
- M. El-Hashash, S. Rizk and S. Atta-Allah, Molecules, 2015, 20, 22069 CrossRef CAS PubMed.
- G. Bhaskar, Y. Arun, C. Balachandran, C. Saikumar and P. T. Perumal, Eur. J. Med. Chem., 2012, 51, 79 CrossRef CAS PubMed.
- A. Nandakumar, P. Thirumurugan, P. T. Perumal, P. Vembu, M. N. Ponnuswamy and P. Ramesh, Bioorg. Med. Chem. Lett., 2010, 20, 4252 CrossRef CAS PubMed.
- K. A. Badiola, D. H. Quan, J. A. Triccas and M. H. Todd, PLoS One, 2014, 9, e111782 CrossRef PubMed.
- C. A. Stump, I. M. Bell, R. A. Bednar, J. G. Bruno, J. F. Fay, S. N. Gallicchio, V. K. Johnston, E. L. Moore, S. D. Mosser and A. G. Quigley, Bioorg. Med. Chem. Lett., 2009, 19, 214 CrossRef CAS PubMed.
- M. M. Youssef and M. A. Amin, Molecules, 2010, 5, 8827 CrossRef PubMed.
- A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 91 CrossRef CAS PubMed.
- J. Heilmann, S. Mayr, R. Brun, T. Rali and O. Sticher, Helv. Chim. Acta, 2000, 83, 2939 CrossRef CAS.
- E. Rajanarendar, S. Ramakrishna, K. G. Reddy, D. Nagaraju and Y. N. Reddy, Bioorg. Med. Chem. Lett., 2013, 23, 3954 CrossRef CAS PubMed.
- J.-M. Receveur, J. S. Bryans, M. J. Field, L. Singh and D. C. Horwell, Bioorg. Med. Chem. Lett., 1999, 9, 2329 CrossRef CAS PubMed.
- E. W. Zinser, M. L. Wolf, S. J. Alexander-Bowman, E. M. Thomas, J. P. Davis, V. E. Groppi, B. H. Lee, D. P. Thompson and T. G. Geary, J. Vet. Pharmacol. Ther., 2002, 25, 241 CrossRef CAS PubMed.
- H. Potschka, T. J. Feuerstein and W. Löscher, Naunyn-Schmiedeberg's Arch. Pharmacol., 2000, 361, 200 CrossRef CAS PubMed.
- S. M. Rajesh, S. Perumal, J. C. Menéndez, P. Yogeeswari and D. Sriram, MedChemComm, 2011, 2, 626 RSC.
- A. Jarrahpour, E. Ebrahimi, E. De Clercq, V. Sinou, C. Latour, B. L. Djouhri and J. M. Brunel, Tetrahedron, 2011, 67, 8699 CrossRef CAS.
- M. A. Ali, R. Ismail, T. S. Choon, Y. K. Yoon, A. C. Wei, S. Pandian, R. S. Kumar, H. Osman and E. Manogaran, Bioorg. Med. Chem. Lett., 2010, 20, 7064 CrossRef CAS PubMed.
- Y. Kia, H. Osman, R. S. Kumar, V. Murugaiyah, A. Basiri, S. Perumal, H. A. Wahab and C. S. Bing, Bioorg. Med. Chem. Lett., 2013, 21, 1696 CrossRef CAS PubMed.
- Y. Arun, K. Saranraj, C. Balachandran and P. T. Perumal, Eur. J. Med. Chem., 2014, 74, 50 CrossRef CAS PubMed.
- B. Yu, D. Q. Yu and H. M. Liu, Eur. J. Med. Chem., 2015, 97, 673 CrossRef CAS PubMed.
- D. Bora, A. Kaushal and N. Shankaraiah, Eur. J. Med. Chem., 2021, 215, 113263 CrossRef CAS PubMed.
- M. A. Ameen, E. K. Ahmed, M. Ramadan, H. A. Abd El-Naby and A. A. Abdel-Haseeb, Monatsh. Chem., 2017, 148, 1513 CrossRef CAS.
- A. Barakat, M. S. Islam, H. M. Ghawas, A. M. Al-Majid, F. F. El-Senduny, F. A. Badria, Y. A. M. Elshaier and H. A. Ghabbour, RSC Adv., 2018, 8, 14335 RSC.
- M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892 CrossRef CAS PubMed.
- T. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Y.-H. Wu and Y. He, Bioorg. Med. Chem. Lett., 2006, 16, 2105 CrossRef CAS PubMed.
- Ç. B. Apaydın, N. Cesur, A. Stevaert, L. Naesens and Z. Cesur, Arch. Pharm., 2019, 352, 1800330 CrossRef PubMed.
- J. Leañez, J. Nuñez, Y. García-Marchan, F. Sojo, F. Arvelo, D. Rodriguez, I. Buscema, A. Alvarez-Aular and X. Serrano-Martín, Exp. Parasitol., 2019, 198, 31 CrossRef PubMed.
- M. Sapnakumari, B. Narayana, K. S. Shashidhara, B. K. Sarojini and J. Taibah, J. Taibah Univ. Sci., 2017, 11, 1008 CrossRef.
- V. Pogaku, V. S. Krishna, D. Sriram, K. Rangan and S. Basavoju, Bioorg. Med. Chem. Lett., 2019, 29, 1682 CrossRef CAS PubMed.
- A. N. Gray, B. M. Ramirez, S. K. Mawugbe, J. F. Mar, Y.-L. C. Wong and K. S. Huang, J. Visualized Exp., 2021, 168, e61950 Search PubMed.
- T. Thaima, A. Yazici, C. Auranwiwat, A. C. Willis, U. Wille, T. Limtharakul and S. G. Pyne, Org. Biomol. Chem., 2021, 19, 259 RSC.
- T. A. King, H. L. Stewart, K. T. Mortensen, A. J. P. North, H. F. Sore and D. R. Spring, Eur. J. Org Chem., 2019, 2019, 5219 CrossRef CAS PubMed.
- S. Ullah, R. Haider and H. M. Siddiqi, Arabian J. Chem., 2020, 13, 7210 Search PubMed.
- S. Kumar, S. Ponnusamy, J. Dash and R. Ramesh, Arabian J. Chem., 2017, 10, 338 Search PubMed.
- P. K. Sahu and S. Sabde, Curr. Top. Med. Chem., 2019, 19, 472 Search PubMed.
- G. Kaur and S. Kumar, J. Mater. Chem., 2019, 7, 1240 Search PubMed.
- K. Nakano, K. Takase and K. Noguchi, Molecules, 2022, 27, 5103 CrossRef CAS PubMed.
- A. Dandia, S. Khan, P. Soni, A. Indora, D. K. Mahawar, P. Pandya and C. S. Chauhan, Bioorg. Med. Chem. Lett., 2017, 27, 2873 CrossRef CAS PubMed.
- X. Gao, Z. Yang, W.-J. Hao, B. Jiang and S.-J. Tu, J. Heterocycl. Chem., 2017, 54, 2434 CrossRef CAS.
- P. R. Mali, P. K. Shirsat, N. B. Khomane, V. L. Nayak, J. B. Nanubolu and H. M. Meshram, ACS Comb. Sci., 2017, 19, 633 CrossRef CAS PubMed.
- M. Zhang, Y.-H. Liu, Z.-R. Shang, H.-C. Hu and Z.-H. Zhang, Catal. Commun., 2017, 88, 39 CrossRef CAS.
- R. Mishra, A. Jana, A. Panday and M. L. H. Choudhury, New J. Chem., 2019, 43, 2920 RSC.
- W.-H. Zhang, M.-N. Chen, Y. Hao, X. Jiang, X.-L. Zhou and Z.-H. Zhang, J. Mol. Liq., 2019, 278, 124 CrossRef CAS.
- S. Khojasteh-Khosro and H. Shahbazi-Alavi, J. Chem. Res., 2019, 43, 107 CrossRef CAS.
- S. Bhandari, S. Sana, B. Sridhar and N. Shankaraiah, ChemistrySelect, 2019, 4, 1727 CrossRef CAS.
- A. Mondal, B. Naskar, S. Goswami, C. Prodhan, K. Chaudhuri and C. Mukhopadhyay, Mol. Diversity, 2020, 24, 93 CrossRef CAS PubMed.
- J. Safaei-Ghomi, P. Babaei and Z. Elyasi, ChemistrySelect, 2021, 6, 8402 CrossRef CAS.
- N. H. de Silva, S. Pyreddy, E. W. Blanch, H. M. Hügel and S. Maniam, Bioorg. Chem., 2021, 114, 105128 CrossRef CAS PubMed.
- Z. Elyasi, J. Safaei Ghomi and G. R. Najaf, Ultrason. Sonochemistry, 2021, 75, 105614 CrossRef CAS PubMed.
- F. H. Mahmoud Naglaa, S. A. Rizk, A. Elsayed Galal and A. K. Ali, J. Iran. Chem. Soc., 2022, 19, 3711 CrossRef.
- A. Castro, I. M. G. Andrade, M. C. Coelho, D. P. da Costa, D. d. N. Moreira, R. A. Maia, G. d. S. Lima, G. F. dos Santos, B. G. Vaz, G. C. G. Militao, P. B. N. da Silva, M. L. Araujo, A. de Vasconcellos and C. G. Lima-Junior, J. Heterocycl. Chem., 2023, 60, 392 CrossRef CAS.
- N. K. Sahu, R. Sharma, K. P. Suhas, J. Joshi, K. Prakash, R. Sharma, R. Pratap, X. Hu, S. Kaur, M. Jain, C. Coluccini, P. Coghi and S. Chaudhary, Molecules, 2023, 28, 4817 CrossRef CAS PubMed.
- R. Sharma, L. Yadav, A. A. Nasim, R. K. Yadav, R. H. Chen, N. Kumari, F. Ruiqi, A. Sharon, N. K. Sahu, S. K. Ippagunta, P. Coghi, V. K. W. Wong and S. Chaudhary, Molecules, 2023, 28, 6503 CrossRef CAS PubMed.
- Y. Shi, H. Zhao and Y. Zhao, Molecules, 2023, 28, 3508 CrossRef CAS PubMed.
- S. Maharani, S. V. Kumar, A. I. Almansour, R. S. Kumar, A. Kandasamy and R. R. Kumar, New J. Chem., 2017, 41, 11009 RSC.
- B. Kalluraya, S. Mallya and A. Kumar, J. Heterocycl. Chem., 2018, 55, 2075 CrossRef CAS.
- S. Nayak, P. Pattanaik, S. Mohapatra, D. R. Mishra, P. Panda, B. Raiguru, N. P. Mishra, S. Jena and H. S. Biswal, Synth. Commun., 2019, 49, 1823 CrossRef CAS.
- S. G. Hegde, L. Koodlur and M. Narayanarao, Synth. Commun., 2019, 49, 1 CrossRef.
- A. M. Akondi, S. Mekala, M. L. Kantam, R. Trivedi, L. R. Chowhane and A. Das, New J. Chem., 2017, 41, 873 RSC.
- C. Bharkavi, S. V. Kumar, M. A. Ali, H. Osman, S. Muthusubramanian and S. Perumal, Bioorg. Med. Chem. Lett., 2017, 27, 3071 CrossRef CAS PubMed.
- S. Nayak, P. Panda, S. Mohapatra, B. Raiguru and N. Baral, J. Heterocycl. Chem., 2019, 56, 1757 CrossRef CAS.
- H. Arvinnezhad, F. Ghorbani, H. Khosravi, K. Jadidi, B. Notash and S. Naderi, J. Heterocycl. Chem., 2020, 57, 3222 CrossRef CAS.
- R. Mohebat, N. Simin and A. Yazdani-Elah-Abadi, Polycyclic Aromat. Compd., 2017, 40, 159 Search PubMed.
- A. Yazdani-Elah-Abadi, R. Mohebat and M. Kangani, J. Chin. Chem. Soc., 2017, 64, 690 CrossRef CAS.
- A. Yazdani-Elah-Abadi, N. Simin, R. Morekian and H. Heydari-Dahoei, Polycyclic Aromatic Compounds, 2019, DOI:10.1080/10406638.2019.1570948.
- R. Westphal, E. V. Filho, L. B. Loureiro, C. F. Tormena, C. Pessoa, C. de Jesus Guimarães, M. P. Manso, R. G. Fiorot, V. R. Campos, J. A. Lamounier, C. Resende, F. Medici and S. J. Greco, Molecules, 2022, 27, 8051 CrossRef CAS PubMed.
- P. P. Patel, N. B. Patel, M. S. Tople, V. M. Patel, I. Ahmed and H. Patel, Mol. Diversity, 2023, 6, 1 Search PubMed.
- R. Das, D. K. Mehta, S. Gupta, S. Mujwar, V. Sharma, A. Goyal, S. Patel and A. Patel, Lett. Org. Chem., 2023, 20, 1182 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |