Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carpesabrolide A, a novel meroterpenoid with anti-inflammatory activity from Carpesium abrotanoides†
Xiao-Fang Zhanga,
Jiao-Xian Dua,
Si-Qiong Tenga,
Hui Liua,
Juan He*ab,
Tao Feng *abc and
Ji-Kai Liu*ab
*abc and
Ji-Kai Liu*ab
aSchool of Pharmaceutical Sciences, South-Central Minzu University, 182 Minzu Road, Wuhan 430074, P.R. China. E-mail: 2015048@mail.scuec.edu.cn; tfeng@mail.scuec.edu.cn; liujikai@mail.scuec.edu.cn
bNational Demonstration Center for Experimental Ethnopharmacology Education, South-Central Minzu University, Wuhan 430074, China
cCollege of Life Science, South-Central Minzu University, Wuhan 430074, China
First published on 5th April 2024
Abstract
Carpesabrolide A (1), featuring an unprecedented fumaric acid–guaiane sesquiterpenoid hybrid, has been isolated from the folk medicinal plant Carpesium abrotanoides. The structure with absolute configuration has been established by spectroscopic methods and single crystal X-ray diffraction analysis. The plausible biosynthetic pathway for 1 is proposed. Compound 1 shows significant anti-inflammatory activity by inhibiting NO production with an IC50 value of 2.7 μM.
Introduction
The genus Carpesium (Asteraceae) contains about 21 species worldwide.1,2 They are mostly perennial herbs and distributed across Asia and Europe, particularly in the mountainous areas of Southwest China.3 Many Carpesium plants have been used in traditional medicines for the treatments of fevers, colds, bruises, insect and snake bites in China, Korea, Japan and other Asian countries.4,5 Previous chemical investigations have demonstrated that Carpesium plants are a good source of monoterpenoids,6,7 sesquiterpenoids,8,9 diterpenoids and phenolic derivatives,10–12 with various biological activities such as anti-inflammatory,13 anti-tumor,14 insecticidal15 and bactericidal effects.16Carpesium abrotanoides L. is a perennial herb widely distributed in East, central, South and southwest China at altitudes below 2000 m.17 Its fruit is used in the insecticidal prescription of traditional Chinese medicine.1,18 C. abrotanoides is also a famous Tujia medicine used for detoxifying toxins, hemostatic, killing worms, tonsillitis, malaria, acute hepatitis and itchy skin rashes for a long time.19 According to literature, sesquiterpenes are the main secondary metabolites found in C. abrotanoides, including guaiane-type, eudesmane-type, eremophilane-type, etc., with diverse pharmacological properties, such as anti-inflammatory,20 antiviral,21 antifungal,22 antibacterial23 and cytotoxic activities.1,24,25
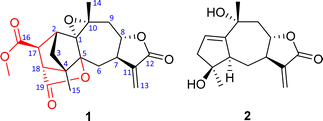
The diversity of structures and biological activities has aroused our great research interest in the chemical composition of C. abrotanoides. In this study, a novel succinic acid–guaiane meroterpenoid, namely carpesabrolide A (1), together with its possible precursor compound 2, has been isolated from the whole grass of C. abrotanoides (Fig. 1). The anti-inflammatory activity for 1 was tested. Herein, the isolation, structural elucidation, possible biosynthetic pathway, and anti-inflammatory activity of these isolates are reported.
Results and discussion
The dried and powdered whole grass of C. abrotanoides (5 kg) was extracted three times by methanol to give a crude extract (800 g). The crude extract was distributed by EtOAc and H2O to give an organic layer (160 g). The latter was separated by column chromatography to give compounds 1 (6 mg) and 2 (12 mg).Compound 1 was obtained as colorless needles (MeOH). Its molecular formula, C20H22O7, was established on the basis of HRESIMS (measured at m/z 375.14395 [M + H]+; calcd for C20H22O7H+, 375.14383), corresponding to ten degrees of unsaturation. The 1H NMR data (Table 1) displayed three singlets at δH 1.33, 1.52, and 3.73, which could be readily assigned as two methyls and one methoxy group, respectively. In addition, two olefinic protons at δH 6.16 and 5.68 with a small coupling constant of J = 3.2 Hz suggested the presence of a terminal double bond, while one signal at δH 4.19 indicated the presence of oxygenated group(s) in the structure. In the 13C NMR spectrum, a total of 20 carbon resonances could be detected (Table 1), which were classified into three CH3, four CH2, five CH, and eight no-protonated carbons by DEPT and HSQC technologies.
| No. | δH | δC |
|---|---|---|
| a Data were assigned by HSQC, HMBC, 1H–1H COSY and ROESY spectra. | ||
| 1 | 75.8, C | |
| 2 | 2.6, brs | 45.5, CH |
| 3a | 1.79, dd (12.1, 1.5) | 35.3, CH2 |
| 3b | 1.67, dd (12.1, 1.5) | |
| 4 | 58.4, C | |
| 5 | 91.3, C | |
| 6a | 2.37, dd (14.8, 2.0) | 33.4, CH2 |
| 6b | 1.84, dd (14.8, 11.5) | |
| 7 | 2.76, m | 45.2, CH |
| 8 | 4.19, ddd (12.4, 9.3, 3.0) | 81.5, CH |
| 9a | 2.75, dd (14.0, 3.0) | 43.8, CH2 |
| 9b | 2.28, dd (14.0, 12.4) | |
| 10 | 68.6, C | |
| 11 | 140.9, C | |
| 12 | 171.2, C | |
| 13a | 6.16, d (3.2) | 120.7, CH2 |
| 13b | 5.68, d (3.2) | |
| 14 | 1.52, s | 25.0, CH3 |
| 15 | 1.49, s | 14.9, CH3 |
| 16 | 173.7, C | |
| 17 | 3.13, d (1.9) | 50.2, CH |
| 18 | 2.96, d (1.9) | 52.1 CH |
| 19 | 179.2, C | |
| OMe | 3.73, s | 53.1, CH3 |
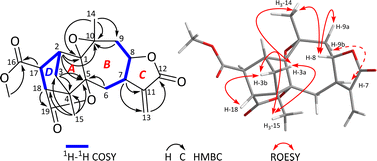
The 1H–1H COSY spectrum revealed the presence of two spin systems, including correlations of H2-3/H-2/H-17/H-18 (a) and H2-6/H-7/H-8/H2-9 (b) as showed in Fig. 1. Based on these data, the HMBC experiment established the planar structure of 1. As shown in Fig. 1, the HMBC correlations from H2-3 to C-4 and C-5, from H-2 to C-1 and C-5, and from H3-15 to C-3, C-4, and C-5 established a five-membered ring A, with a methyl substituent at C-4. The HMBC correlations from H3-14 to C-1, C-9, and C-10, from H-9 to C-10, and from H2-6 to C-1 and C-5 constructed a seven-membered ring B, including a methyl group at C-10. Furthermore, the HMBC correlations from H-7 to C-11 and C-13, and from H-13 to C-12 built a γ-lactone C, with a conjugated exocyclic double bond. So far, a 5/7/5-fused ring system was established, which resembled a guaiane sesquiterpene backbone, with reference to those in the literature.26,27 Further analyses of the NMR and MS data suggested the presence of an epoxy moiety between C-1 and C-10 in ring B. Five remaining carbons were assigned to monomethyl fumarate based on the HMBC correlations from δH 3.73 (3H, s, OMe) to δC 173.7 (s, C-16) and from H-17 and H-18 to C-16 and C-19. Importantly, the HMBC correlations from H3-15 to C-18 and from H-18 to C-4 established a five-membered ring D (Fig. 2). The HMBC correlations from H-17 to C-1, C-3, and C-4, from H-18 to C-5, and H2-3 to C-18 suggested that the ring D was fused together with the ring A via C-2/C-3/C-4. A possible lactone moiety was suggested to be placed between C-5 (δC 91.3, s) and C-19 (δC 179.2, s) according to analyses of the HMBC correlations from H-18 to C-19, C-5, along with MS data analysis. Therefore, the structure of 1 was established as an unprecedented fumaric acid–guaiane meroterpenoid with a complicated polycyclic system.
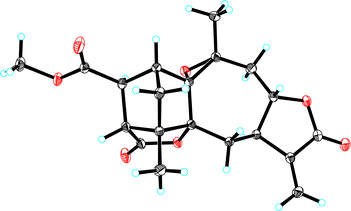
In the ROESY spectrum (Fig. 2), two key ROE correlations of H3-14/H-3a and H3-14/H-8 indicated that H3-14 and H-8 were on the same face, which were assigned as β-orientation tentatively. Accordingly, the ROE correlations of H-8/H-9a and H-9b H−1-7 indicated that H-7 should be α-oriented. In addition, the key ROE correlation between H-18 and H-3b suggested that H-18 to be β-oriented, while the cross peak between H3-15 and H-3 allowed the lactone moiety between C-5 and C-19 to be α-oriented. The small coupling constant of J17,18 = 1.9 Hz suggested the angle between H-7 and H-18 close to 90°, allowing H-17 to be α-oriented. Finally, the single crystal X-ray diffraction (Cu Kα radiation) not only confirmed the planar structure but also clarified the absolute configuration (Fig. 3). Hence, the structure of 1 was identified and trivially named carpesabrolide A.
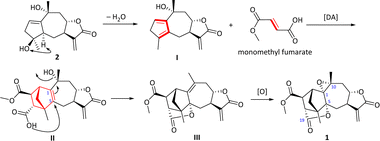
Carpesabrolide A (1) represents hitherto the first example of fumaric acid–sesquiterpene hybrid directly linked by two carbon bonds, which aroused our interest in its plausible biosynthesis pathway. As shown in Scheme 1, compound 2 was isolated in this study, which should be a possible biogenetic precursor of 1. Through dehydration, compound 2 formed a key diene body I, which reacted with monomethyl fumarate through a Diels–Alder cycloaddition to produce II, featuring a novel bicyclo[2.2.1]heptane moiety. Then an epoxide moiety between C-1 and C-10 and a lactone between C-5 and C-19 were established, and compound 1 was finally produced.
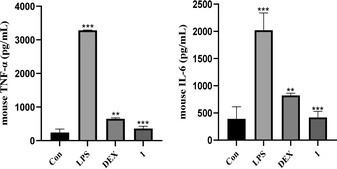
Sesquiterpenoids from C. abrotanoides have been widely studied for their pharmacological activities, and many of them have been demonstrated promising anti-inflammatory activity.20 Therefore, compound 1 was evaluated for its anti-inflammatory activity by inhibiting NO release in LPS-activated RAW264.7 cells. As a result, compound 1 showed significant inhibitory activity against NO production with an IC50 value of 2.7 μM, better than that of dexamethasone (positive control, IC50 = 9.0 μM). Furthermore, the effects of 1 on pro-inflammatory mediators TNF-α and IL-6 were investigated in LPS-stimulated RAW264.7 cells using enzyme linked immunosorbent assay (ELISA) as we reported previously.28 As depicted in Fig. 4, the results suggested that compound 1 visibly suppressed the secretion of TNF-α and IL-6 compared to the LPS-only treatment at the concentration of 5 μM, better than that of dexamethasone (10 μM) as well.
Conclusions
In conclusion, carpesabrolide A (1), an unprecedented fumaric acid–guaiane sesquiterpenoid hybrid, was isolated from the traditional Chinese medicine C. abrotanoides. The novel carbon skeleton and significant anti-inflammatory activity make it a good scaffold for further chemistry study and biological investigation. Although the chemical composition and biological activity of C. abrotanoides have been largely studied previously, the discovery in study suggested that there are still rich resources for this medicinal plant and it is worth exploring further.Experimental section
General experimental procedures
Melting points were measured on a WRX-4 apparatus. Optical rotations were measured with a Horiba SEPA-300 polarimeter. IR spectra were obtained with a Tenor 27 spectrophotometer using KBr pellets. 1D and 2D spectra were run on a Bruker Avance III 600 MHz spectrometer with TMS as an internal standard. Chemical shifts (δ) were expressed in ppm with reference to the solvent signals. Mass spectra were recorded on an Agilent 6200 Q-TOF mass spectrometry system. Column chromatography (CC) was performed on silica gel (200–300 mesh), RP-18 gel (20–45 μm), and Sephadex LH-20. Medium pressure liquid chromatography (MPLC) was performed on a Biotage One equipment packed with RP-18 gel columns. Preparative high performance liquid chromatography (prep-HPLC) was performed on an Agilent 1260 liquid chromatography system equipped with Zorbax SB-C18 columns (5 μM, 9.4 × 250 mm) and a DAD detector. Fractions were monitored by TLC (GF 254), and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH.Plant material
The whole plant of Carpesium abrotanoides L. was collected from Yunnan, China, in August 2022, and identified by Mr Jun Zhang. A voucher specimen (HFG-P-CA20220812.2) was deposited at School of Pharmaceutical Sciences, South-Central Minzu University.Extraction and isolation
The dried and powdered whole grass of C. abrotanoides (5 kg) was extracted three times by methanol to obtain a crude extract (800 g). The crude extract was further extracted with EtOAc to give an organic layer (160 g). The latter was separated by CC over silica gel eluted with CH2Cl2/MeOH (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain eight fractions (A–H). Fraction C (8.1 g) was further separated by CC over silica gel eluted with CH2Cl2/MeOH (12
1) to obtain eight fractions (A–H). Fraction C (8.1 g) was further separated by CC over silica gel eluted with CH2Cl2/MeOH (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give seven subfraction C1–C7. Fraction C6 (620 mg) was subjected MPLC (MeOH/H2O, from 2
1) to give seven subfraction C1–C7. Fraction C6 (620 mg) was subjected MPLC (MeOH/H2O, from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 8
8 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give eight subfractions C61–C68. Fraction C67 (85 mg) was purified by HPLC (MeCN/H2O, from 3
2) to give eight subfractions C61–C68. Fraction C67 (85 mg) was purified by HPLC (MeCN/H2O, from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 4
7 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in 30 min) to give compound 1 (4 mg, tR = 24 min). Fraction D (2.2 g) was subjected to MPLC to give seven subfractions D1–D7. Fraction D4 (490 mg) was purified by HPLC (MeCN/H2O, from 3
6 in 30 min) to give compound 1 (4 mg, tR = 24 min). Fraction D (2.2 g) was subjected to MPLC to give seven subfractions D1–D7. Fraction D4 (490 mg) was purified by HPLC (MeCN/H2O, from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 4
7 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in 35 min) to give compound 2 (12 mg, tR = 26 min).
6 in 35 min) to give compound 2 (12 mg, tR = 26 min).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 210 (2.87) nm; 1H NMR (600 MHz, methanol-d4) and 13C NMR (150 MHz, methanol-d4) data in Table 1, respectively; HRESIMS (Positive) m/z 375.14383 [M + H]+ (calcd for C20H23O7, 375.14395).
ε): 210 (2.87) nm; 1H NMR (600 MHz, methanol-d4) and 13C NMR (150 MHz, methanol-d4) data in Table 1, respectively; HRESIMS (Positive) m/z 375.14383 [M + H]+ (calcd for C20H23O7, 375.14395).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 reflections measured, 3367 independent reflections (Rint = 0.0552). The final R1 values were 0.0295 (I > 2σ(I)). The final wR(F2) values were 0.0723 (I > 2σ(I)). The final R1 values were 0.0310 (all data). The final wR(F2) values were 0.0731 (all data). The goodness of fit on F2 was 1.057. Flack parameter = 0.08(7). The crystal data (cif file) was deposited at the Cambridge Crystal Data Center (CCDC) (http://www.ccdc.cam.ac.uk) under access number 2311963.†
042 reflections measured, 3367 independent reflections (Rint = 0.0552). The final R1 values were 0.0295 (I > 2σ(I)). The final wR(F2) values were 0.0723 (I > 2σ(I)). The final R1 values were 0.0310 (all data). The final wR(F2) values were 0.0731 (all data). The goodness of fit on F2 was 1.057. Flack parameter = 0.08(7). The crystal data (cif file) was deposited at the Cambridge Crystal Data Center (CCDC) (http://www.ccdc.cam.ac.uk) under access number 2311963.†In vitro anti-inflammatory activity
Author contributions
Xiao-Fang Zhang contributed to the isolation and structure elucidation. Jiao-Xian Du and Si-Qiong Teng contributed to the biological activity test. Hui Liu and Juan He contributed to structure determination and X-ray diffraction. Tao Feng guided the experiments and the manuscript preparation. Ji-Kai Liu designed the experiments.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22177138, 22277147, 22177139).Notes and references
- H. B. Wu, H. B. Wu, W. S. Wang, T. T. Liu, M. G. Qi, J. C. Feng, X. Y. Li and Y. Liu, Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides, Ind. Crops Prod., 2016, 92, 77–83 CrossRef CAS.
- C. Yan, Q. Long, Y. D. Zhang, G. Babu, M. V. Krishnapriya, J. F. Qiu, J. R. Song, Q. Rao, P. Yi, M. Sun and Y. M. Li, Germacranolide sesquiterpenes from Carpesium cernuum and their anti-leukemia activity, Chin. J. Nat. Med., 2021, 19, 528–535 CAS.
- J. P. Zhang, G. W. Wang, X. H. Tian, Y. X. Yang, Q. X. Liu, L. P. Chen, H. L. Li and W. D. Zhang, The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology, J. Ethnopharmacol., 2015, 163, 173–191 CrossRef CAS.
- Editorial board of Chinese Materia Medica, Chinese Materia Medica, Shanghai Science & Technology Press, PR China, Shanghai, 1999 Search PubMed.
- C. B. Lee, Illustrated Flora of Korea, https://www.nhbs.com/3/series/flora-of-korea, 1985 Search PubMed.
- O. P. Zee, D. K. Kim and K. R. Lee, Thymol derivatives from Carpesium divaricatum, Arch. Pharmacal Res., 1998, 21, 618–620 CrossRef CAS PubMed.
- C. Yang, Q. X. Zhu, W. Yong and Z. J. Jia, A new eudesmanolide and a new aromatic derivative from Carpesium cernuun, Chin. Chem. Lett., 2001, 12, 597–600 CAS.
- M. Maruyama, A. Karube and K. Sato, Sesquiterpene lactones from Carpesium abrotanoides, Phytochemistry, 1983, 22, 2773–2774 CrossRef CAS.
- M. R. Kim, S. K. Lee, C. S. Kim, K. S. Kim and D. C. Moon, Phytochemical constituents of Carpesium macrocephalum FR- et SAV, Arch. Pharmacal Res., 2004, 27, 1029–1033 CrossRef CAS PubMed.
- X. Gao, C. J. Lin and Z. J. Jia, Cytotoxic germacranolides and acyclic diterpenoides from the seeds of Carpesium triste, J. Nat. Prod., 2007, 70, 830–834 CrossRef CAS PubMed.
- X. Gao, Z. X. Zhang and Z. J. Jia, New acyclic 12-hydroxygeranylgeraniol-derived diterpenoids from the seeds of Carpesium triste, Helv. Chim. Acta, 2008, 91, 1934–1939 CrossRef CAS.
- I. M. Chung, S. H. Seo, E. Y. Kang, W. H. Park, S. D. Park and H. I. Moon, Antiplasmodial activity of isolated compounds from Carpesium divaricatum, Phytother. Res., 2010, 24, 451–453 CrossRef CAS PubMed.
- E. J. Kim, H. K. Jin, Y. K. Kim, H. Y. Lee, S. Y. Lee, K. R. Lee, O. P. Zee, J. W. Han and H. W. Lee, Suppression by a sesquiterpene lactone from Carpesium divaricatum of inducible nitric oxide synthase by inhibiting nuclear factor-κB activation, Biochem. Pharmacol., 2001, 61, 903–910 CrossRef CAS.
- X. W. Li, L. Weng, X. Gao, Y. Zhao, F. Pang, J. H. Liu, H. F. Zhang and J. F. Hu, Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi, Bioorg. Med. Chem. Lett., 2011, 21, 366–372 CrossRef CAS PubMed.
- J. Bero and J. Quetin Leclercq, Natural products published in 2009 from plants traditionally used to treat malaria, Planta Med., 2010, 77, 631–640 CrossRef PubMed.
- S. Sancheti, S. Sancheti and S. Y. Seo, Evaluation of antiglycosidase and anticholinesterase activities of Boehmeria nivea, Pak. J. Pharm. Sci., 2010, 23, 236–240 Search PubMed.
- Institute of Botany, The Chinese Academy of Sciences, Flora of China, https://www.iplant.cn, 2020 Search PubMed.
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China. Pharmacopoeia of PR China. Part I, China Medical Science Press, PR China, Beijing, 2020 Search PubMed.
- Z. X. Fang, H. Zhao and J. H. Zhao, Tujia Medicine, China Medical Science Press, PR China, 2007 Search PubMed.
- Q. G. Qian, L. M. Gong, S. H. Yang, B. Q. Zhao, J. Cai, Z. J. Zhang, Y. H. Wang and X. J. Zhou, Two new compounds from Carpesium abrotanoides, Phytochem. Lett., 2020, 40, 5–9 CrossRef CAS.
- Y. Q. He, L. Cai, Q. G. Qian, S. H. Yang, D. L. Chen, B. Q. Zhao, Z. P. Zhong and X. J. Zhou, Anti-influenza A (H1N1) viral and cytotoxic sesquiterpenes from Carpesium abrotanoides, Phytochem. Lett., 2020, 35, 41–45 CrossRef CAS.
- J. T. Feng, Z. Q. Ma, J. H. Li, J. He, H. Xu and X. Zhang, Synthesis and antifungal activity of carabrone derivatives, Molecules, 2010, 15, 6485–6492 CrossRef CAS.
- C. F. Xie, L. M. Sun, L. H. Meng, M. C. Wang, J. Xu, M. Bartlam and Y. Q. Guo, Sesquiterpenes from Carpesium macrocephalum inhibit Candida albicans biofilm formation and dimorphism, Bioorg. Med. Chem. Lett., 2015, 25, 5409–5411 CrossRef CAS PubMed.
- J. W. Wu, C. Tang, C. Q. Ke, S. Yao, H. C. Liu, L. G. Lin and Y. Ye, Dicarabrol A, dicarabrone C and dipulchellin A, unique sesquiterpene lactone dimers from Carpesium abrotanoides, RSC Adv., 2017, 7, 4639–4644 RSC.
- Q. L. Hu, P. Q. Wu, Y. H. Liu, F. M. Qi, C. X. Yu, Y. Zhao, Y. F. Yu, D. Q. Fei and Z. X. Zhang, Three new sesquiterpene lactones from Carpesium abrotanoides, Phytochem. Lett., 2018, 27, 154–159 CrossRef CAS.
- J. J. Qin, J. X. Zhu, Q. Zeng, X. R. Cheng, Y. Zhu, S. D. Zhang, L. Shan, H. Z. Jin and W. D. Zhang, Pseudoguaianolides and guaianolides from Inula hupehensis as potential anti-inflammatory agents, J. Nat. Prod., 2011, 74, 1881–1887 CrossRef CAS PubMed.
- J. S. Mossa, F. S. El-Feraly, I. Muhammad, K. Zaw, Z. H. Mbwambo, J. M. Pezzuto and H. H. S. Fong, Sesquiterpene lactones and thymol esters from Vicoa pentanema, J. Nat. Prod., 1997, 60, 550–555 CrossRef CAS.
- T. Feng, X. Q. Gan, Y. L. Zhao, S. B. Zhang, H. P. Chen, J. He, Y. S. Zheng, H. Sun, R. Huang, Z. H. Li and J. K. Liu, Tricholopardins A and B, anti-inflammatory terpenoids from the fruiting bodies of Tricholoma pardinum, J. Nat. Prod., 2019, 82, 45–50 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C and 2D NMR, and HRESIMS spectra, the detailed X-ray crystallography data of 1. CCDC 2311963. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra00292j |
| This journal is © The Royal Society of Chemistry 2024 |