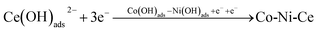Open Access Article
Open Access ArticlePreparation of Co–Ni–Ce/TiC alloy coatings by double-pulse under a sulfamic acid system and a process mechanism study
Jidong Lia,
Hongxuan Xinga,
Senhu Jina,
Yaowu Wang *b and
Jinlin Luc
*b and
Jinlin Luc
aSchool of Materials and Metallurgy, University of Science and Technology Liaoning, Anshan 114051, China
bNortheastern University, School of Metallurgy, Shenyang 110000, China
cGuangzhou Maritime University, Guangzhou 510725, China
First published on 13th March 2024
Abstract
To enhance the protective ability of copper crystallizers and extend their service life, this study explores the use of double pulse co-deposition under a sulfamic acid system to create protective coatings such as Co–Ni. The hardness test and friction wear analysis compare Co–Ni, Co–Ni–Ce, and Co–Ni–Ce/TiC coatings, revealing that the Co–Ni–Ce/TiC coating exhibits the most outstanding protective performance. SEM and XRD techniques are employed to characterize the three protective coatings, demonstrating that the incorporation of rare-earth cerium and nanoparticles improves the coating morphology and modifies their crystalline phase structure. Furthermore, cyclic voltammetry tests on the plating solutions of the three protective coatings indicate that the addition of Ce3+ and nanoparticles influences the deposition potentials. The deposition of Co2+ and Ni2+ follows a two-step, two-electron process, while the deposition of Ce3+ follows a one-step, three-electron process. It is observed that the deposition of all three ions is irreversible. To gain further insights into the nucleation mechanism of Ce3+, a chronoamperometry test is conducted, revealing that the nucleation of Ce3+ is a transient process controlled by diffusion.
1 Introduction
In the steel industry, the copper crystallizer plays a crucial role in the steelmaking process.1 However, the harsh steelmaking environment can cause irreversible damage to the surface of the copper crystallizer, resulting in steel leakage over time and significant losses. To protect the copper crystallizer, manufacturers apply protective coatings on its surface.2 The Co–Ni alloy is commonly used for its excellent wear resistance, corrosion resistance, and thermal stability, effectively extending the service life of the copper crystallizer.3The most common method for applying protective coatings is electrodeposition, which traditionally utilizes direct current deposition technology. However, this approach has drawbacks such as ion concentration polarization and hydrogen precipitation reaction at the cathode interface during electrodeposition. These issues reduce current efficiency and can lead to irregular crystal growth and structural defects in the plating layer.4 Double-pulse plating is a technique that utilizes reverse current to improve the plating process. It helps replenish metal ions near the specimen to be plated, reducing concentration polarization and enhancing the nucleation rate within the plating layer. This technique promotes the refinement of crystal grain.5 Gao et al.6 conducted a study where they prepared a Co–Ni/graphene composite coating on a copper crystallizer substrate. By adjusting the percentage of Co content in the coating, they found that high Co composite coatings exhibited excellent hardness and good wear resistance. Rare earth elements, with their unique atomic structure, are extensively utilized in various sectors such as electronics, machinery, energy, environmental protection, and agriculture. The addition of rare earth elements enhances the surface properties of coatings, impacting factors like deposition rate, surface morphology, wear resistance, and corrosion resistance.7 Cerium (Ce) has emerged as a key element for studying the characteristics of rare earth elements. During the deposition process, Ce3+ can absorb coating defects, enhancing surface morphology and overall performance, thereby extending the lifespan of the workpiece.8 Fu et al. conducted a study where they utilized the jet-electrodeposition method to prepare Ni–W–Ce alloy coatings. Their findings indicated that the inclusion of rare earth Ce significantly improved the hardness and wear resistance of the coatings.7 Additionally, the presence of Ce in the coatings induced lattice distortion within the alloy coatings.7 Another study by Li et al.9 involved the preparation of Ni–Co/ZrO2 composite coatings by adding nano-ZrO2 in a sulfamic acid solution system. The addition of nano-ZrO2 significantly improved the mechanical and chemical properties of the composite coatings, making the electrode reaction more likely to occur. Wang H10 utilized the double pulse electrodeposition process to fabricate Ni–TiC composite coatings. The inclusion of nano TiC particles in the plating exhibited superior resistance to high temperatures compared to other particles. This characteristic makes it highly suitable for application on the surface of the copper crystallizer.
The double-pulse preparation of Co–Ni–Ce/TiC alloys has been scarcely reported. Consequently, this study presents the preparation of Co–Ni–Ce/TiC alloy coating through double-pulse one-step co-deposition on the copper crystallizer's surface. This approach eliminates the need for additives in previous DC electrodeposition and contributes to the lightweighting of equipment. Moreover, this paper not only investigates the process properties of the three alloys but also elucidates the mechanism of the ion deposition process. The combination of these two research aspects facilitates the advancement and exploration of surface functional alloy coatings, thereby expanding the range of applications for copper metal materials.
2 Materials and methods
2.1 Experimental materials
In the experiments, a 20 × 40 × 2 mm copper (Cu) plate was employed as the cathode, while a 5 × 10 × 50 mm nickel (Ni) plate was utilized as the anode, and the distance between the poles was set at 20 mm. The Table 1 provides information on the concentrations of reagents used during the process experiments, along with the corresponding conditions. The experimental concentration in this article is based on the experimental results obtained during multiple process explorations, and the process exploration process is not explained in this article.| Prerequisites | Co–Ni | Co–Ni–Ce | Co–Ni–Ce/TiC | Pure | |
|---|---|---|---|---|---|
| Concentration (g L−1) | Ni (SO3NH2)2·4H2O | 100 | 100 | 100 | AR |
| Co (SO3NH2)2·4H2O | 33.3 | 33.3 | 33.3 | AR | |
| NiCl2·6H2O | 16.6 | 16.6 | 16.6 | AR | |
| H3BO3 | 30 | 30 | 30 | AR | |
| CeCl3·7H2O | — | 2 | 2 | AR | |
| C12H25SO4Na | 0.15 | 0.15 | 0.15 | AR | |
| C6H8O7H2O | 30 | 30 | 30 | AR | |
| NH4Cl | 33 | 33 | 33 | AR | |
| TiC (40 nm) | — | — | 5 | AR | |
| Process parameters | Positive current density (A dm−2) | 4 | 4 | 4 | — |
| Positive duty cycle (%) | 40 | 40 | 40 | — | |
| Positive pulse frequency (kHZ) | 20 | 20 | 20 | — | |
| Reversed current density (A dm−2) | 0.2 | 0.2 | 0.2 | — | |
| Reverse duty cycle (%) | 40 | 40 | 40 | ||
| Reverse pulse frequency (kHZ) | 2 | 2 | 2 | — | |
| pH | 4 | 4 | 4 | — | |
| T (°C) | 40 | 40 | 40 | — | |
| Time (min) | 75 | 75 | 75 | — |
2.2 Composite coating procedure
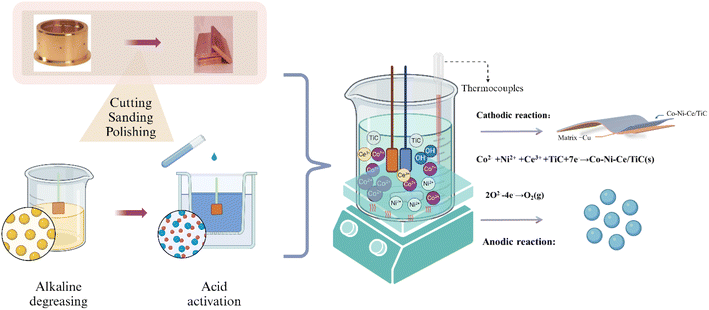
To prepare the surface for the deposition of Co–Ni–Ce/TiC, it underwent a polishing treatment using sandpaper of varying mesh sizes (400, 800, 1200, 1500, and 2000). Subsequently, the Cu surface was subjected to cleansing and activation using a 5% HCl solution to eliminate the surface film, thereby enhancing the bonding strength of the Co–Ni–Ce/TiC coating. Deionized water was used for rinsing purposes following the activation step. Ultimately, the electrodeposition process led to the formation of the Co–Ni–Ce/TiC alloy coating. The schematic representation of this entire sequence is illustrated in Fig. 1.2.3 Surface characterization and mechanism test
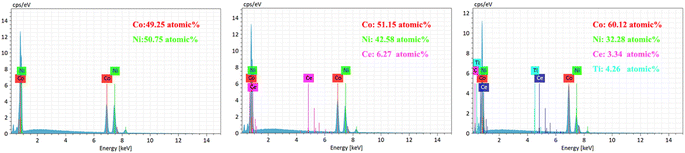
To observe the surface morphology of the sample, a sigma500 scanning electron microscope (SEM) was utilized. Additionally, a Bruker energy dispersive spectrometer (EDS) was employed to analyze the coated sample's chemical composition and elemental distribution. X-ray diffraction (XRD) experiments were conducted on various coatings using an Ultam IV X-ray diffractometer manufactured by Rigaku, Japan. The X-ray tube used CuKα1 radiation with a wavelength (λ) of 0.15405 nm, a tube voltage of 40 kV, and a tube current of 20 mA. The scanning rate was set at 40° min−1, with a scanning range (2θ) of 30° to 90°.The hardness values of both the substrate and coating were examined using an HVS-5 micro-Vickers hardness tester. The force was applied at a speed of 0.05 mm s−1, with a load force of 200 g, and a holding time of 10 s, and five replicates were averaged. The coated samples' abrasion resistance was evaluated using the MS-T3100 ball and disk friction and wear tester manufactured by Huahui Instrument Technology in Lanzhou. In this test, Si3N4 grinding balls with a diameter of 2 mm were employed, with an applied load of 400 g. The tester operated at a rotational speed of 200 r min−1, with a radius of rotation of 4 mm, and a test time of 30 min.
For the mechanical experiment, we employed a copper coating with dimensions of 20 × 40 × 2 mm as the cathode. The anode, on the other hand, consisted of a platinum sheet measuring 10 × 20 × 0.5 mm. To compare electrochemical potentials, we utilized an Ag/AgCl electrode as the reference electrode. Maintaining uniformity, both the cathode-to-reference and anode-to-reference gaps were set at 20 mm. Oxygen is removed from the solution by passing argon gas before each test.
3 Results and discussion
3.1 Characterization of composite plating
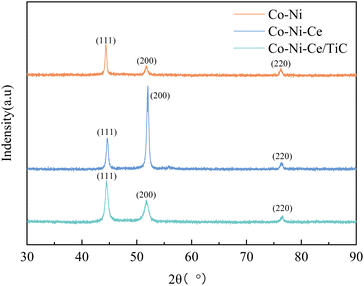
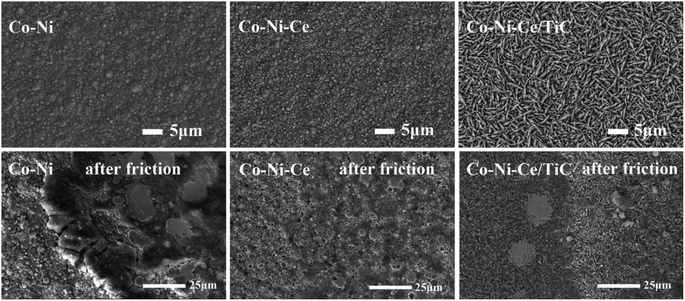
In addition, both Co–Ni–Ce and Co–Ni–Ce/TiC alloys show enhancement of the diffraction peaks at (200) compared to Co–Ni alloys, and the enhancement is most obvious for Co–Ni–Ce alloys, which may be due to the fact that rare earth elements have large atomic radii and high ionic valence, and the addition of Ce affects the grain growth direction of Co–Ni alloys which would cause it to develop more towards the (200) crystal plane, and the addition of TiC would equalize this effect.7,10,12
By observing the XRD, it can be found that there is a small broadening of the peak shape, indicating that the addition of Ce and TiC can effectively inhibit the growth of grains and make the plated layer fine grains. To further obtain the grain size, the Debye–Scherrer eqn (1) (ref. 7) was used for the calculation.
D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
As illustrated in Fig. 5, the Co–Ni, Co–Ni–Ce, Co–Ni–Ce/TiC, and Cu exhibit a two-layer composite structure. The Co–Ni layer has a thickness of 300 μm, the Co–Ni–Ce layer has a thickness of 250 μm, and the Co–Ni–Ce/TiC layer has a thickness of 150 μm, all of which can be regulated by the deposition time during actual production. The reduction in layer thickness following the addition of Ce and TiC is attributed to the decrease in deposition potential caused by these substances, resulting in slower deposition and finer layers.7,14 This decrease in deposition potential is also confirmed through subsequent cyclic voltammetry analysis.
3.2 Performance testing
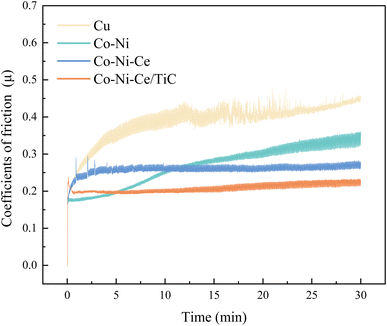
The average friction coefficient of the Co–Ni alloy coating is 0.2693, and the wear rate is 1.99 × 10−5 g m−1. According to Table 2, the average friction coefficient of the Co–Ni–Ce alloy coating is 0.2582, and the wear rate is 1.72 × 10−5 g m−1. The average friction coefficient of the Co–Ni–Ce/TiC composite coating is 0.2079, and the wear rate is 1.06 × 10−5 g m−1, and the wear-resistant properties are all better than those of the Co–Ni–Ce alloy plating.
| Cu | Co–Ni | Co–Ni–Ce | Co–Ni–Ce/TiC | |
|---|---|---|---|---|
| Coefficient of friction-μmax | 0.4807 | 0.3603 | 0.2947 | 0.2373 |
Average coefficient of friction-![[small mu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0cd.gif) |
0.3863 | 0.2693 | 0.2582 | 0.2079 |
| Friction-Fmax/N | 1.8844 | 1.4123 | 1.1551 | 0.9304 |
Average friction-![[F with combining macron]](https://www.rsc.org/images/entities/i_char_0046_0304.gif) /N /N |
1.5142 | 1.0555 | 1.0120 | 0.8145 |
| Abrasion quantity-ΔM/mg | 5.0 | 1.5 | 1.3 | 0.8 |
| Wear rate-W/g m−1 | 6.63 × 10−5 | 1.99 × 10−5 | 1.72 × 10−5 | 1.06 × 10−5 |
3.3 Mechanism of the electrode reaction
As can be seen in Fig. 8, the test curves at different sweep speeds in the negative direction scanning showed a reduction peak within the scanning potential of −0.25 V to −0.5 V. This peak is the reduction peak of H+, and the reduction reaction formula is: 2H2O + 2e− → H2↑ + 2OH−.20 In addition to this, Co2+ and Ni2+ each gain one electron and combine with OH− to become CoOHads and NiOHads.15,16 The current density started to increase in the scanning potential interval from −0.65 V to −0.75 V. This interval potential is the starting potential for the co-deposition of the alloy.21 During the forward scan of the CV test, an oxidation peak in the potential range of 0.1 V to 0.5 V appeared, which was caused by the anodic dissolution of the electrode surface.20
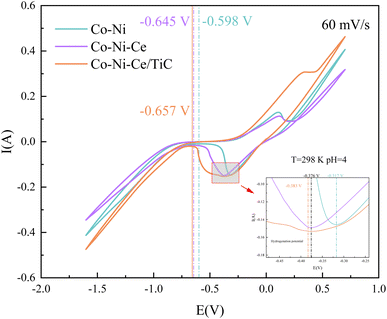
By comparing the CV curves of the three systems, it was observed that the reduction potential for hydrogen precipitation and the reduction potential for metal co-deposition in the Co–Ni–Ce system were negatively shifted compared to the Co–Ni system. This suggests that the addition of Ce3+ increased the cathodic polarization of the system solution and hindered the reduction reaction in the Co–Ni system, but promoted the induced co-deposition of Ce3+ with Co–Ni.22 The negative shift of the co-deposition potential of TiC nanoparticles relative to that of Co–Ni–Ce is not significant, indicating that the addition of nanoparticles has less influence on the deposition effect potential in this system.23 Additionally, when conducting the cyclic voltammetry test with a positive sweep, it was noticed that the potential of the oxidation peak in the CV became more positive with the addition of TiC nanoparticles. This suggests that the Co–Ni–Ce/TiC composite plating layer exhibits greater resistance to oxidation.23 To gain further insights into the deposition mechanism of the ions and the related electrochemical parameters, CV and chronoamperometry tests were conducted at different sweep speeds for the Co–Ni–Ce system.
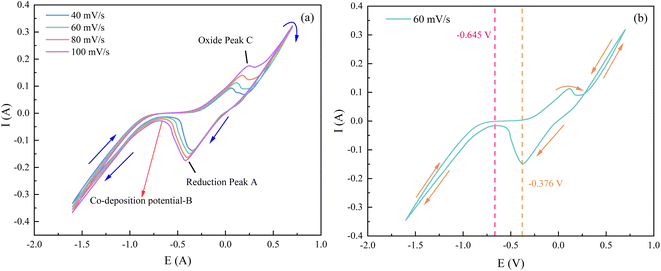
From Fig. 9(a), it can be observed that as the sweep speed increases, the reduction peak potential shifts towards the negative direction and the reduction peak current gradually increases. Similarly, the oxidation peak potential shifts towards the positive direction and the oxidation peak current also gradually increases. To further confirm the number of electrons transferred by ions during the deposition process, four data points were selected at the reduction peak A and at the co-deposition potential B for fitting at a sweep speed of 60 mV s−1. The relevant data calculations were recorded in Table 3, and the electron transfer number was calculated using the Nernst equation.21
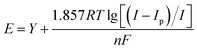 | (2) |
| Ip/mA | I/mA | E/V | lg[|(Ip − I)/I|] | |
|---|---|---|---|---|
| −0.376 V | −145.56 | −134.16 | −0.2783 | −1.0707 |
| −145.56 | −135.28 | −0.2807 | −1.1194 | |
| −145.56 | −136.38 | −0.2832 | −1.1719 | |
| −145.56 | −137.45 | −0.2856 | −1.2291 | |
| −0.645 V | −15.994 | −16.028 | −0.654 | −2.6725 |
| −15.994 | −16.049 | −0.657 | −2.4636 | |
| −15.994 | −16.080 | −0.659 | −2.2695 | |
| −15.994 | −16.113 | −0.662 | −2.1284 |
As can be seen in Fig. 10, the fitting coefficients are 0.998 and 0.976, indicating a good linear relationship of the point data. Based on the fitting slopes in Fig. 10, the number of transferred electrons of the ions can be calculated as follows:21
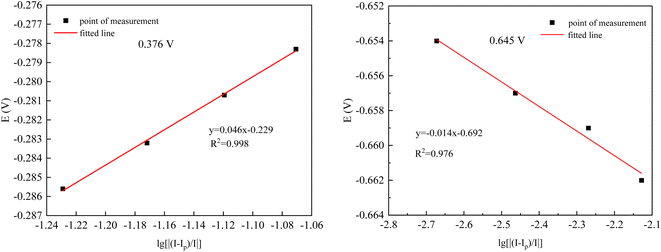 | ||
| Fig. 10 Fitted datas from Table 3. | ||
It was calculated that Co2+ and Ni2+ first gained one electron each in the plating solution and combined with OH− in the solution to form Co (OH)ads and Ni (OH)ads, while Ce3+ combined with OH− directly to form Ce (OH)ads adsorbed on the surface of Cu electrode. In Co–Ni–Ce alloy plating solutions, Ce cannot be deposited alone.24 Instead, Co+–Ni+ induced formation of Ce metal is required.24 The reduction of Ce3+ in aqueous solution at the Cu electrode was calculated to be a one-step, three-electron process, which can be represented by the following electrode reaction:
| Co2+ + OH− + e− → Co(OH)ads and Ni2+ + OH− + e− → Ni(OH)ads |
| Co(OH)ads + Ni(OH)ads → Co(OH)ads − Ni(OH)ads |
To study the reversibility of the reaction, reduction control steps of Ce3+ on the copper electrode. The relevant data in the CV curves of Fig. 9(b) were fitted and calculated, and the relevant data are listed in Table 4 below.
| v/(V s−1) | 0.040 | 0.060 | 0.080 | 0.100 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v/ln(V s−1) v/ln(V s−1) |
−3.219 | −2.813 | −2.526 | −2.303 |
| v1/2/(V s−1)1/2 | 0.200 | 0.245 | 0.283 | 0.316 |
| Epc/V | −0.630 | −0.645 | −0.664 | −0.676 |
| −Ipc/A | 0.003405 | 0.00410 | 0.005315 | 0.00650 |
| Ep/2/V | −0.042 | −0.037 | −0.039 | −0.044 |
| ΔEp | 0.691 | 0.76 | 0.84 | 0.911 |
| ∣Epc − Ep/2∣/V | 0.588 | 0.608 | 0.625 | 0.640 |
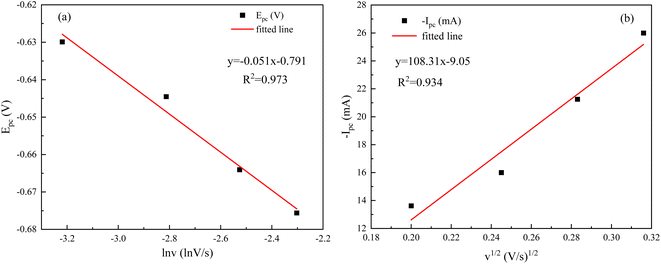
As can be seen from Fig. 11(a), the relationship between the reduction peak potential (Epc) and the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v is line, indicating that the reduction process of Ce3+ in the system plating solution is irreversible.20 The irreversibility in the reduction process of Ce3+ is further demonstrated by the increase in the value of the ΔEp with the v in Table 4. To further determine the steps controlling the redox reactions of Ce3+, the relationships between the peak currents Ipc and v1/2 were investigated. The cathodic peak potential Epc and half peak potential Epc/2 satisfied the following equation:25
v is line, indicating that the reduction process of Ce3+ in the system plating solution is irreversible.20 The irreversibility in the reduction process of Ce3+ is further demonstrated by the increase in the value of the ΔEp with the v in Table 4. To further determine the steps controlling the redox reactions of Ce3+, the relationships between the peak currents Ipc and v1/2 were investigated. The cathodic peak potential Epc and half peak potential Epc/2 satisfied the following equation:25
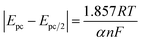 | (3) |
The Nernst equation and CV tests allowed us to determine various parameters during the reduction of Ce3+ on the Cu plate, including the cathodic peak potential (Epc) and half peak potential (Epc/2) in volts, the charge transfer coefficient (α), and the number of transferred electrons (n). We substituted the respective values into eqn (3), and the mean value of α was calculated to be 0.0260 (Fig. 12).
The correlation coefficients of the linear fits of the peak currents Ipc with respect to v1/2 are 0.934, respectively. The redox reaction of Ce3+ is controlled by diffusion, and the diffusion coefficient of Ce3+ can be derived from the Berzins–Delahay equation.26,27
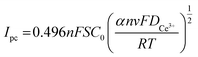 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1, R = 8.314 J (mol K)−1, T = 298 K, α = 0.0260, S = 2 cm2, and C0 = 0.054 and by inserting these into the formula.
500 C mol−1, R = 8.314 J (mol K)−1, T = 298 K, α = 0.0260, S = 2 cm2, and C0 = 0.054 and by inserting these into the formula.
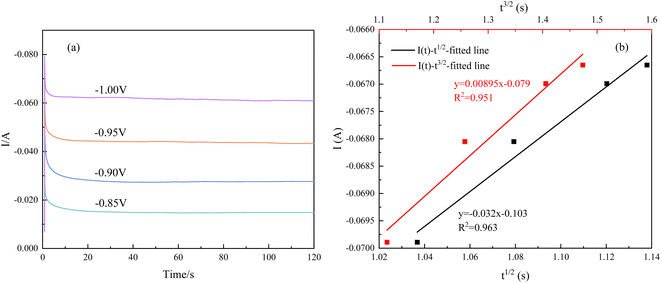
Instantaneous nucleation and continuous nucleation equations can be fitted from four different points arbitrarily taken from the rising portion of the different timing current curves in Fig. 11.25
Instantaneous nucleation:
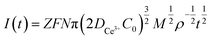 | (5) |
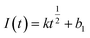 | (6) |
Continuous nucleation:
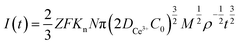 | (7) |
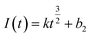 | (8) |
In the above equations, I(t)- electron current corresponding to time/mA; Z-valence; F = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1; N-the nucleation number density/cm−2; DMo6+-diffusion coefficient/cm2 s−1; C0-concentration of Ce3+/mol L−1; M-molar mass/g mol−1; ρ-density/g cm−3; Kn-nucleation rate constant/cm−2 s−1; t-time/s; and π = 3.14. Fig. 10 shows that the fitting degree of I(t)-t1/2 is better than the fitting degree of I(t)-t3/2 at any potential, which indicates that the nucleation mechanism of Ce3+ on Cu plate is instantaneous nucleation.24
500 C mol−1; N-the nucleation number density/cm−2; DMo6+-diffusion coefficient/cm2 s−1; C0-concentration of Ce3+/mol L−1; M-molar mass/g mol−1; ρ-density/g cm−3; Kn-nucleation rate constant/cm−2 s−1; t-time/s; and π = 3.14. Fig. 10 shows that the fitting degree of I(t)-t1/2 is better than the fitting degree of I(t)-t3/2 at any potential, which indicates that the nucleation mechanism of Ce3+ on Cu plate is instantaneous nucleation.24
4 Conclusions
The following conclusions can be drawn from the study of the preparation of the Co–Ni–Ce/TiC alloy coating:(1) XRD tests of Co–Ni–Ce/TiC coatings revealed that the addition of Ce affects the grain growth direction of Co–Ni alloys which would cause it to develop more towards the (200) crystal plane, and the addition of TiC would equalize this effect. SEM characterization confirmed that the introduction of Ce and TiC helps refine the grains and improve the surface quality of the alloy coatings. Furthermore, friction and wear tests demonstrated that the TiC nanoparticles play a lubricating role.
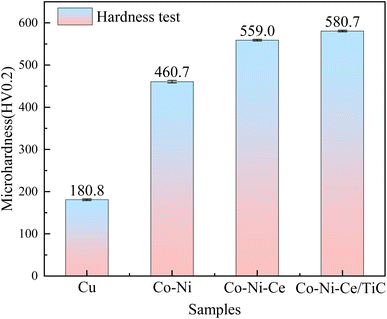
(2) The hardness of the Co–Ni–Ce/TiC alloy coatings prepared by double pulse deposition using aminosulfonic acid reaches 580.71 HV0.2, which is significantly higher than that of the Cu substrate and the Co–Ni coatings. Moreover, the wear rate of the Co–Ni–Ce/TiC alloy coating is 1.06 × 10−5 g m−1, which is 5 times lower than that of the copper substrate, providing effective substrate protection.
(3) Cyclic voltammetry tests showed that the introduction of Ce3+ and TiC negatively shifts the co-deposition potential. The reduction reactions of Co2+ and Ni2+ are two-step, two-electron irreversible reactions, while the reduction reaction of Ce3+ is a one-step, three-electron process. The deposition of Ce3+ follows a diffusion-controlled, transient nucleation process, with a diffusion coefficient of 8.23 × 10−6 cm2 s−1.
Conflicts of interest
All authors disclosed no relevant relationships.Acknowledgements
Supported by National Nature Science Foundation of China (52374352) and Open Project Fund of Provincial Key Laboratory of Metallurgical Engineering, Liaoning University of Science and Technology (2023KFKT-10). Supported by the Graduate science and Technology Innovation Program of University of Science and Technology Liaoning and Key Research (LKDYC202321). Supported by Liaoning Provincial Department of Education Key Public Relations Project (JYTZD2023091). Fig. 1 is Created with https://www.biorender.com (UV26IAQUHC).References
- S. Chen, J. Liang and C. Liu, et al., Preparation of a novel Ni/Co-based alloy gradient coating on surface of the crystallizer copper alloy by laser, Appl. Surf. Sci., 2011, 258(4), 1443–1450 CrossRef CAS.
- X. Yang, X. Lu and W. Zhang, et al., Preparation and application of nano-Ni–Co alloy, J. Nanopart. Res., 2023, 25(7), 152 CrossRef CAS.
- R. M. A. Radadi and M. A. M. Ibrahim, Nickel-cobalt alloy coatings prepared by electrodeposition Part II: Morphology, structure, microhardness, and electrochemical studies, Korean J. Chem. Eng., 2021, 38, 152–162 CrossRef CAS.
- Y. Chen, H. Yang and H. Feng, et al., Electrodeposition and corrosion performance of Ni-Co alloys with different cobalt contents, Mater. Today Commun., 2023, 35, 106058 CrossRef CAS.
- S. Tebbakh, L. Mentar and Y. Messaoudi, et al., Effect of cobalt content on electrodeposition and properties of Co–Ni alloy thin films, Inorg. Nano-Met. Chem., 2021, 51(12), 1796–1802 CrossRef CAS.
- E. Gao, G. Wei and B. Yan, et al., Effect of Graphene Content on the Micromorphology, Microhardness and Micro Frictional Resistance of Co-Ni-Graphene Composite Coating, Mater. Sci. Forum, 2021, 1035, 608–614 Search PubMed.
- X. Fu, Q. Wang and J. Lin, et al., Effect of Ce (SO4) (2) Concentration on Properties of Ni-W-Ce Alloy Coating Prepared by Jet-electrodeposition, Rare Met. Mater. Eng., 2020, 49(9), 2948–2955 CAS.
- X. Zhang, H. Chen and X. Li, et al., Effect of Rare Earth on the Microstructure and Performance of Electrodeposited Ni-W Coatings, Rare Met. Mater. Eng., 2016, 45(10), 2605–2608 CAS.
- B. Li, J. Su and J. Li, et al., Comparison of the Effect of Mechanical and Ultrasonic Agitation on the Properties of Electrodeposited Ni-Co/ZrO2 Composite Coatings, Metals, 2023, 13(3), 478 CrossRef CAS.
- H. Wang, X. Mao and T. Shen, et al., Effect of Nano-TiC Particles on Microstructure and Properties of Ni-TiC Composite Coatings, J. Mater. Eng., 2017, 45(1), 52–57 Search PubMed.
- F. Cheng, Z. Cao and Z. Wen, et, al., Surface of AISI 430 stainless steel Pulsed electrodeposition of Co-Ni alloys, Mater. Prot., 2015, 48(07), 11–14 CAS.
- H. Tian, Fundamental Research on Manufacturing Technology and Properties of Ni-Based Nano-Composite Coatings by Electrophoretic-Electrochemical Deposition, PhD thesis, Nanjing University of Aeronautics and Astronautics, 2008.
- W. Jia, Study of Electroless Co-ni-P Alloy Films with Rare Earth Element Cerium Addition, PhD thesis, Hefei University of Technology, 2007.
- X. Ren, S. Wu and R. Jiang, et, al., Preparation and structural characterization of Ni-nano-TiC composite coating under pulse condition, Ordnance Mater. Sci. Eng., 2019, 42(05), 74–77 Search PubMed.
- L. Burzyńska and E. Rudnik, The influence of electrolysis parameters on the composition and morphology of Co–Ni alloys, Hydrometallurgy, 2000, 54(2–3), 133–149 CrossRef.
- A. N. Correia and S. A. S. Machado, Electrodeposition and characterisation of thin layers of Ni-Co alloys obtained from dilute chloride baths, Electrochim. Acta, 2000, 45(11), 1733–1740 CrossRef CAS.
- P. Zhou, W. Li and Y. Li, et al., Fabrication and corrosion performances of pure Ni and Ni-based coatings containing rare earth element Ce and graphene by reverse pulse electrodeposition, J. Electrochem. Soc., 2017, 164(2), D75 CrossRef CAS.
- J. Zhou, X. Meng and R. Zhang, et al., Progress on electrodeposition of rare earth metals and their alloys, Electrocatalysis, 2021, 1–13 Search PubMed.
- S. You, C. Jiang and L. Wang, et al., Effect of CeO2 nanoparticles on the microstructure and properties of the NiCo-CeO2 composite coatings, Vacuum, 2022, 196, 110765 CrossRef CAS.
- H. Kang, J. Li and C. Zhang, et al., Study of the electrochemical recovery of cobalt from spent cemented carbide, RSC Adv., 2020, 10(37), 22036–22042 RSC.
- H. Xing, J. Li and X. Hu, et al., Repair of soft magnetic properties of wasted silicon steel and Co7Fe3 alloy deposition mechanism, RSC Adv., 2023, 13(47), 33525–33532 RSC.
- X. Zhou, Y. Shen and H. Jin, et al., Microstructure and depositional mechanism of Ni–P coatings with nano-ceria particles by pulse electrodeposition, Trans. Nonferrous Met. Soc. China, 2012, 22(8), 1981–1988 CrossRef CAS.
- D. Escorcia-Díaz, S. García-Mora and L. Rendón-Castrillón, et al., Advancements in Nanoparticle Deposition Techniques for Diverse Substrates: A Review, Nanomaterials, 2023, 13(18), 2586 CrossRef PubMed.
- H. Xing, J. Li and X. Hu, et al., Mechanism study of composite co-deposited Cu/Co-Mo corrosion-resistant coating on 6061 Al alloy, Surf. Coat. Technol., 2024, 476, 130202 CrossRef CAS.
- Z. Hou, J. Li and X. Wang, et al., Effect of Tin Ion on Electrodeposition Behavior of Indium, Electrochemistry, 2022, 90(8), 087007 CrossRef CAS.
- T. Berzins and P. Delahay, Kinetics of fast electrode reactions, J. Am. Chem. Soc., 1955, 77(24), 6448–6453 CrossRef CAS.
- E. Gomez, E. Pellicer and E. Valles, Detection and characterization of molybdenum oxides formed during the initial stages of cobalt–molybdenum electrodeposition, J. Appl. Electrochem., 2003, 33, 245–252 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |