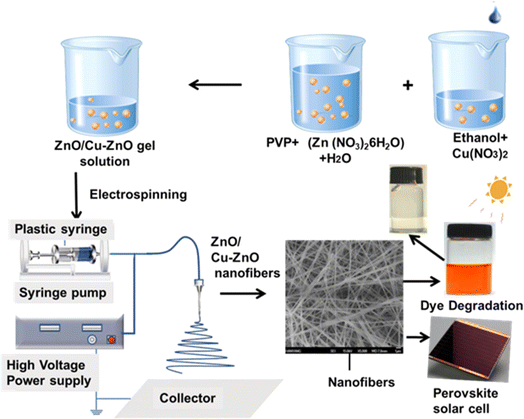
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimized Cu-doping in ZnO electro-spun nanofibers for enhanced photovoltaic performance in perovskite solar cells and photocatalytic dye degradation
Kang Hoon Leea,
Rabeea Farheenb,
Zafar Arshad *c,
Mumtaz Alicg,
Hamza Hassan*d,
Mubark Alshareef
*c,
Mumtaz Alicg,
Hamza Hassan*d,
Mubark Alshareef e,
A.Dahshanf and
Usama Khalidc
e,
A.Dahshanf and
Usama Khalidc
aDepartment of Energy and Environment Engineering, Catholic University, Korea
bDepartment of Physics, Government College Women University Faisalabad, Pakistan
cSchool of Engineering and Technology, National Textile University, 37640, Faisalabad, Pakistan. E-mail: zafarnubii@gmail.com
dDepartment of Chemical Engineering, University of Engineering and Technology Peshawar, Pakistan
eDepartment of Chemistry, Faculty of Applied Science, Umm Al Qura University, Makkah 24230, Saudi Arabia. E-mail: mmshreef@uqu.edu.sa
fDepartment of Physics, College of Science, King Khalid University, Abha, Saudi Arabia
gDepartment of Organic and Nano Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea
First published on 13th May 2024
Abstract
Perovskite solar cells (PSCs) compete with conventional solar cells regarding their low-temperature processing and suitable power conversion efficiency. In PSCs, the electron transport layer (ETL) plays a vital role in charge extraction and avoiding recombination; however, poor charge transport of ETL leads to high internal resistance and associated low fill factors. To successfully resolve this challenge, copper-doped zinc oxide nanofibers as an electron transport layer are prepared with various doping levels of 1, 2, and 3 wt% using the electrospinning sol–gel method. The 3 wt% doping of Cu revealed the optimum performance as an ETL, as it offers an electrically efficient transporting structure. SEM images revealed a randomly oriented distribution of nanofibers with different sizes having mesoporous uniformity. Optical properties of doped nanofibers examined using UV-visible analysis showed an extended light absorption due to heteroatom-doping. Adding Cu into the ZnO leads to enhanced charge mobility across the electron transport material. According to Hall measurements, dopant concentration favors the conductivity and other features essentially required for charge extraction and transport. The solar cell efficiency of ZnO doped with 0%, 1%, 2%, and 3% Cu is 4.94%, 5.97%, 6.89%, and 9.79%, respectively. The antibacterial and photocatalytic activities of the prepared doped and undoped ZnO are also investigated. The better light absorption of Cu–ZnO showed a pronounced improvement in the photocatalytic activity of textile electrodes loaded with doped ZnO. The dye degradation rate reaches 95% in 180 min under visible light. In addition, these textile electrodes showed strong antibacterial activity due to the production of reactive oxygen species under light absorption.
1. Introduction
Energy is crucial for driving modern technological devices, and its consumption is growing exponentially over time. Based on the latest energy demand trends and current consumption rate, energy shortages will be faced in the future.1 As a solution, solar energy has emerged as a sustainable ingredient in building a stable global community. While Si-solar cell based technologies have been developed to use solar energy aggressively, there is still a need for competitive renewable energy devices to overcome their bottleneck of high production cost.2,3 Considering which, perovskite solar cells (PSCs) have emerged as low-cost and efficient photovoltaic devices.4Research on solar cells is frequently conducted in the field of clean energy. The most crucial element in nanomaterials use in solar cells is their size-dependent bandgap tuning property. Due to their better dispersion, regulated band gap, excellent chemical stability, and low toxicity, 2-D materials have many benefits over other materials used in solar cells, such as silicon and perovskite. 2-D materials are perfect for solar cells since they also exhibit the quantum confinement effect and the tunable band edge alignment. The use of hole transport layer (HTL) material, silicon heterojunction solar cells, semiconductor solar cells, and conductive polymer-doped solar cells are just a few of the 2-D materials based solar cells that have been described thus far. In the research of Li et al.,5 solution chemistry was used to create with a uniform size distribution from doublewalled CNTs. The 2-D materials in chlorobenzene produce a dazzling blue glow when exposed to UV light. Power conversion efficiency (PCE) is greatly increased when these are added to a bulk heterojunction polymer solar cell (PSC) made of poly(3-hexylthiophene):(6,6)-phenyl-C61 butyric acid methyl ester (P3HT:PCBM). The efficiency can be raised even more by modifying the PCBM content in the active layer, leading to a maximum PCE of 5.24%.
PSCs are a potential alternative to traditional silicone and thin-film solar cells due to their efficient and economical conversion of solar energy into electricity.6 PSCs have shown a remarkable increase in performance due to advancements in structural design and optimized processing parameters.7 PSCs generally consist of inverted p-i-n or regular n-i-p-based structures, where perovskite (absorber) is sandwiched between an electron transport layer (ETL: commonly TiO2, ZnO, SnO2) and a hole transportation layer (HTL).8,9 The energy alignment for each layer is crucial excitement and transportation of charge through both ETL and HTL after the photoexcitation in the absorber layer. Photoexcited electrons are injected into the conduction band of ETL, while the holes are transported into the HOMO level of HTL (commonly used spiro-OMeTAD).10 The suitable band alignment is responsible for charge carriers' separation and suppressed recombination. Despite the facile processing and simple structure of PSCs, the major challenge of low fill factor (FF) exists due to high internal resistance.11 By enhancing the FF and associated efficiency of PSCs, superior performance with lower costs can be achieved, as compared to first- and second-generation photovoltaic devices.12
Zinc oxide is a familiar semiconductor from group (II–IV) served as an electron transport material (ETM), having unique electron mobility 120 cm2 V−1 s−1 comparable with others ETMs.13 In general, ZnO has trace phase crystalline material as cubic rock salt, hexagonal wurtzite structure and cubic zinc blend. Wurtzite ZnO crystals are demanding due to their suitable band gap of 3.37 eV at room temperature and high binding energy.14 Moreover, ZnO is also an important component for electron transport materials in optoelectronic devices.15 Strong piezoelectric properties, unique optical response, and antibacterial characteristics of ZnO have expanded its circle of applications. To further widen the applications of ZnO, several bottlenecks related to its lower conductivity and limited light absorbance should be addressed.16
In recent times, several reports on tuning the electronic properties of ZnO by heteroatom doping have highlighted significant improvements.17 The addition of metal ions into ZnO leads to the reduction of interfacial defects to approach high operating efficiency as well as decreasing the rate of recombination.18 Doped-ZnO also creates quasi-Fermi levels on the interfaces and built-in potential (Vbi) while contacting each other, essentially required in thin film solar cells.19 This split in quasi level creates a higher open-circuit voltage (Voc) by various metal dopants, such as calcium-doped ZnO,20 magnesium-doped ZnO,21 and aluminum-doped ZnO,22 to enhance the performance of pristine ZnO. Doping with these metals influences carrier collection, quick photo-generated current harvesting, and efficient thermal stability. For higher photovoltaic performance, there were multiple passivation strategies to suppress the deep trap state for the compensation of dangling bonds at the perovskite surface. Also, inducing metal doping into ZnO creates a continuous change of energy alignment and tailors the nucleation process for its layer growth.23,24
An efficient low-temperature, Zn-doped TiO2 compact layers were synthesized by solution processing and used as ETL for efficient perovskite solar devices. This doped thin layers reduced the fermi levels and series resistance by Zn doping. High electrons conductivity and efficiency upto 27% was achieved with these PSCs.25 Recently, fabricated ZnO thin films with a dopant concentration of more than 5% are not feasible via the sol–gel method, as excessive doping inhibits crystal growth.26 In addition, for device application, Al-doped zinc oxide thin films showed potential for the fabrication of highly efficient PSCs with the benefits of low energy consumption and low cost. Its thermal stability is limited to 450 °C; previous studies also showed the thermal stability range of material is 300–680 °C.27 Cu/ZnO has a large surface area, little defect density, and appealing grain boundaries with ideal porosity without sacrificing the material's porosity.28 Cu atoms in the ZnO matrix of dye-sensitized solar cells (DSSC) improve the efficiency (6.5%) and interface charge transfer characteristics of the device.29 The Fermi levels in ZnO's hexagonal wurtzite structure are greatly enhanced by the addition of Cu, moving them in closer proximity to the conduction band edge and indicating larger open-circuit potentials. When compared to pristine ZnO, the photoconversion efficiency of the Cu-doped ZnO material-based cells was four times higher.30 Cu dopant-created shallow intermediate energy levels aligned with the conduction band of ZnO nanostructure, promoting a quick charge transfer rate, easier for electrons to move forward, causing higher performance in the case of solar cells based on doped photoelectrode.31
Another important application of metal oxide nanostructures with reduced band gaps and low recombination rates is working as a photocatalyst. Metal oxide nanostructures were expected to enhance photocatalytic activity because of their increased surface area and functional groups with the incorporation of dopant ions. Using metal oxide nanostructures as a photocatalyst metal oxide is supported by its many fascinating features such as wide band gap, unique physical and chemical properties, environmental stability, and non-toxicity.28 For photocatalysis, ZnO nanostructure is regarded as suitable material due to abundant availability, low cost and comparable band gap as for TiO2.32 Moreover, it exhibits better performance in the degradation of organic dye molecules in both acidic and basic media. Furthermore, the addition of cationic dopants, such as Cu2+, Ni2+, Co2+, and Mn2+, can be used to modify the structural, optical, chemical, electrical, and magnetic properties of ZnO. In comparison to undoped ZnO, cation-doped ZnO has a smaller band gap energy value.33 The doping of Cu in ZnO was expected to modify absorption and other physical or chemical properties of ZnO because of the different structure of the electronic shell and the similar size of Cu and Zn.34
Scientists also worked on ZnO-based nanofibers; metal oxide nanofibers possess outstanding properties such as the surface area being significantly better, tuned electronic band, photoactive, and better for photons conduction surface area is significantly better, tuned electronic band, photoactive, and better for photons conduction. Indium-doped ZnO improved the structural properties and tuned the electronic band structure and consequently showed an improvement in photocatalytic activity, using an optimum dopant concentration of 3%.35 By careful addition of Mg nanofibers into the ZnO substrate, charge extraction was promoted without sacrificing the charge transport efficiency leading to enhanced photovoltaic efficiency. Based on the information that was presented herein, Cu-doped ZnO nanofibers are synthesized by electrospinning sol–gel solution for the application as a transporting layer for solar cells and as a photocatalyst. That on the spirit of copper is a metal having an electron cloud that can facilitate the conductivity and photoexcitation of the ZnO. With this aim, we have doped ZnO with a trace wt% of Cu as applied the doped structures for PSC for performance enhancement.
There has been a significant increase in scientific interest in photoactive fabrics. Antimicrobial and antibacterial substances break cellular walls, which ultimately harms the microorganism and prevents it from growing.36 Reactive oxygen species (ROS) are formed by exciton due to photon absorption in semiconductor semiconductors. These ROS are in charge of the photocatalytic microbial and organic material destruction. The photoactive textiles can be created by covering the doped metals with textile substrates. These ROS have antibacterial qualities as well as the ability to quickly breakdown organic contaminants from water when exposed to sunshine. Even while mobile phase photocatalysts are better at photocatalytically degrading dyes, recovering nanomaterials from treated water presents another difficulty. An alternative to this issue is the passivation of active photocatalysts on porous textile substrates, which can easily be recycled.9 A high surface area for aggregation-free assembly photocatalysts is provided by the high surface roughness of fibrous textile surfaces. Performance is not severely hindered as a result. For applications in water treatment, Cu-doped ZnO with an improved structure and increased photoactivity was put onto a textile substrate.
In this work, ZnO nanofibers with 1, 2, and 3 wt% of Cu-doping were synthesized by electrospinning and deposited onto indium-doped tin-oxide (ITO) glass substrate. PSCs were fabricated with pristine and Cu-doped ZnO as an ETL. The structural characteristics of these nanofibers were then evaluated, together with the impact of the dopant on the ZnO lattice, using XRD and Raman spectroscopy. Additionally, analyses of surface influence and optoelectronic characteristics demonstrated a significant impact of doping on the electrical structure. Additionally, improvements in the ZnO fibers electrical characteristics were calibrated using Hall effect and I–V measurements. The current density and fill factor were significantly improved as a result of the optimized Cu doping (3%) in solar cells, raising their ability to convert sunlight into electricity. Cu-doped ZnO has increased photoactivity for antibacterial and dye degradation effects when applied to fabrics, as compared to pristine ZnO.
2. Experimental
2.1. Chemical reagents
All reagents, zinc acetate dihydrate (Zn (CH3CO2)2·2H2O, 99%), absolute ethanol (C2H6O, ≥99.8%), deionized water, and copper nitrate (Cu(NO3)2·4H2O, 94%) were acquired from Sigma-Aldrich.2.2 ETM synthesis
The synthesis of ZnO and ZnO doped with Cu proceeded as follows: a fixed proportion (8 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g) of zinc acetate and polyvinylpyrrolidone (PVP) was used to make electrospinnable gel in deionized water (DI) and ethanol mixture, with the ratio (8
2 g) of zinc acetate and polyvinylpyrrolidone (PVP) was used to make electrospinnable gel in deionized water (DI) and ethanol mixture, with the ratio (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 V
3 V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V). In this gel, PVP serves as a binder and thickening agent to assist in the fiber formation process from Zn/Cu precursors. The solution is kept under continuous stirring for 2 h at 60 °C and at 450 rpm, followed by the addition of a few nitric acid drops, which produced a white-colored gel. For Cu-doped ZnO, copper nitrate (Cu(NO3)2·4H2O) was measured as Zn1−xCuxO (where x = 0.01, 0.02, and 0.03). The experimental procedure for the preparation of pristine ZnO was the same as doped nano-fibers, except without the addition of copper nitrate. For the electrospinning setup, the applied voltage was set to 15.6 kV. Temperature was 25 °C. The feeding rate was uniformly controlled as 1.0 mL min−1 for uniform thickness of nanofibers.
V). In this gel, PVP serves as a binder and thickening agent to assist in the fiber formation process from Zn/Cu precursors. The solution is kept under continuous stirring for 2 h at 60 °C and at 450 rpm, followed by the addition of a few nitric acid drops, which produced a white-colored gel. For Cu-doped ZnO, copper nitrate (Cu(NO3)2·4H2O) was measured as Zn1−xCuxO (where x = 0.01, 0.02, and 0.03). The experimental procedure for the preparation of pristine ZnO was the same as doped nano-fibers, except without the addition of copper nitrate. For the electrospinning setup, the applied voltage was set to 15.6 kV. Temperature was 25 °C. The feeding rate was uniformly controlled as 1.0 mL min−1 for uniform thickness of nanofibers.
2.3 Solar cell fabrication
We have fabricated solar cells based on doped and doped ZnO nanofibers as electron-transporting layers. The fabrication steps are as follows: first, ethanol and distilled water were used for cleaning of indium doped tin oxide (ITO) glass substrate with a sheet resistance of 10 Ω sq−1 [aldrich], sequentially. Substrates were then dried using an air stream, and then organic material is removed by ultraviolet ozone treatment for time duration 20 minutes. After the successful cleaning of the substrate, the ZnO nanofibers were deposited on the glass substrate by electrospinning, followed by annealing at 450 °C for 2 h in muffle furnance. For the active layer deposition, formamidinium iodide (FAI, 1 mM), 20 ML of PbBr2 (0.2 mM), methyl ammonium bromide (MABr, 0.2 mM), and lead iodide (PbI2, 1.1 mM) was mixed uniformly into 0.2 mL dimethylsulfoxide (DMSO, 0.2 mL) and dimethyl formamide (DMF, 0.8 mL) on a stirrer at 400 rpm and 25 °C. The obtained uniform mixture was deposited on nanofibers coated ITO by fast crystallization method while keeping the spin rate 4500 rpm and time 7 s for uniform surface coverage of the substrate in the glove box. It was coated for almost seven times to get dense dispersion and adhesion to reduce cracks. For the crystallization of perovskite, 90 μL of anhydrous dichlorobenzene was allowed to fall during spin coating in the glove box. Then it was allowed to dry for 20 minutes at 100 °C and then cooled to room temperature. For HTL deposition, the material was spiro-OMeTAD (100 mg) and chlorobenzene (1.094 mL): synthesized using the sol gel synthesis method. Again, it was spin-coated after successive absorber layers at 2000 rpm for 320 seconds (350 nm) in the glove box. Finally, gold back electrodes (80 nm) were deposited under 10–7 Torr vacuum pressure by thermal evaporation. The scan rate was 0.45 V s−1 for current–voltage curves (Fig. 1).2.4 Characterization
For structural characterization and to reveal the effect of dopant on crystallinity and structure, X-ray diffraction (XRD) analysis was carried out by D8 Advance Bruker GmbH (Karlsruhe, Germany) having Braggs angle arrangement with radiation frequency of 1.5418 Å. Diffraction measurements were recorded in the range of 10° to 80°, and sweep time 0.05° per 5 seconds for each scan. Then the peaks were identified by JAD program and crystallite size was calculated by following the Scherrer equation (eqn (1)).37
G = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
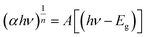 | (2) |
| η = Voc·Jsc·FF/Pin | (3) |
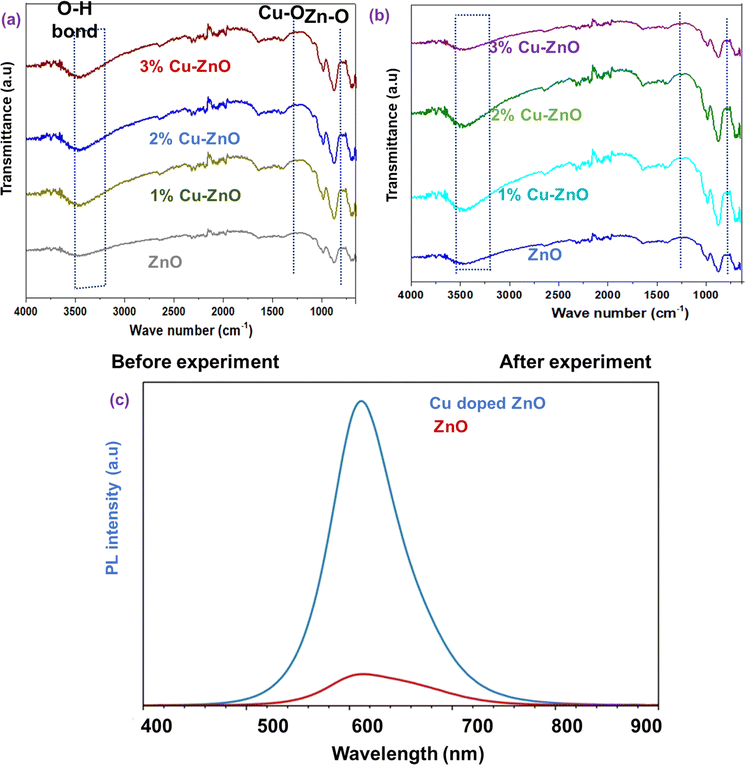
Antibacterial measurements for pristine and Cu–ZnO fibers were measured by (standard AATCC 147 quantitative and qualitative) using Escherichia coli (E.coli) and Staphylococcus aureus (S. aureus). It was immersed for an antibacterial immunity test and then incubated at 37 °C for 24 h. For qualitative analysis, photocatalyst doped fabric were brought towards UV light for the time period half hour. These were then shifted to the bacterial lawn for 24 h at 37 °C after activation. For photocatalysis and to check the dye degradation analysis for pristine ZnO trace Cu–ZnO nanofibers, coated fabrics were interacted with methyl blue (MB) dye by dipping into the solution having aqueous solution (10 ppm) by using a lux meter. To remove the adsorption effect, the electrode was submerged in a dye solution and left in the dark for forty-five minutes. The reference solution (highest concentration) was determined following adsorption. A mixture of visible and UV light (12 W, Phillips 4) (400–900 nm) and UV light (12 W, Phillips 1) (360 nm) was then turned on to replicate the natural solar spectrum. Then, for duration of 75 minutes, the decrease in the dye solution's absorbance was plotted to determine the photocatalytic degradation caused by the light. To ensure that the rate of diffusion was not a limiting factor in the degradation process, the solution was also maintained under slow magnetic stirring. An intriguing use for such low bandgap active material is photocatalysis; to this end, the proposed electrode was examined for the removal of methylene blue from water. UV–Visible spectrometer recorded the maximum absorption intensity of MB. 2 × 2 cm of the active material loaded fabric sample was immersed into 150 mL dye solution and the catalyst-dye adsorption–desorption equilibrium was investigated for 30 minutes under dark conditions. The adsorption effect was excluded to study the photocatalysis performance. The aqueous dye solution was imperiled to visible light irradiation to record the UV absorption spectra for a total of 180 min while ZnO-coated fabric was remained immersed in the dye solution. Following (eqn (4)) was used to measure the dye degradation as:
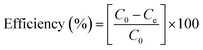 | (4) |
3. Results and discussion
3.1 Material characterizations
The crystal structure of undoped ZnO nanofibers and doped with 1, 2, and 3 wt% of copper were investigated using wide-angle X-ray diffraction XRD, as shown in Fig. 2a. Strong three peaks positioned at diffraction angles (2θ) around 31.70°, 34.36°, and 36.18° related to (100), (002), and (101) planes of ZnO. Other peaks with low intensity at higher diffraction angles are positioned at angles (2θ) of 47.49°, 56.53°, 62.81°, 67.90°, 69.15°, 72.52°, and 76.88° correspond to (102), (110), (103), (112), (201), (004), and (202) ZnO having JPD card number 36-1451.38 All detected diffraction lines match well with the hexagonal wurtzite structure of ZnO. Furthermore; the obtained structures possess high crystallization, and no peak corresponding to CuO. This result is expected for low doping content of Cu due to comparable ionic radii of Cu and zinc ions.39 There is no observable shift of diffraction lines after replacing the Zn ion with Cu within the lattice. The crystallite size was calculated as 22 nm for pure ZnO, and for doped with 1, 2, and 3 wt% of copper are 19.5, 18, and 17 nm, respectively.40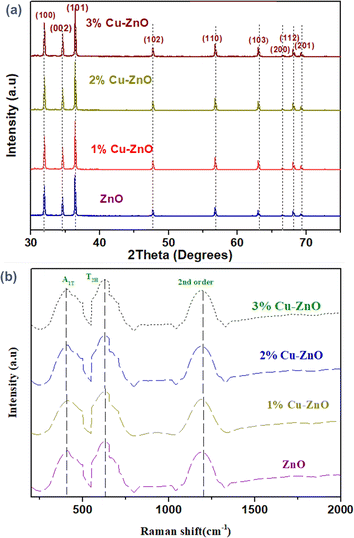 | ||
| Fig. 2 Crystal structure characterization of pristine and Cu-doped fibers using (a) XRD and (b) Raman spectra. | ||
Further crystallite size, microstrain and dislocation density were calculated by using the Sherrer's equation and Williamson and Smallman's relation as presented in eqn (5) and (6)
 | (5) |
 | (6) |
Strain analysis and crystallite size was calculated by using the eqn (5) and (6) and presented in Table 1.
| Sample name | Diffraction angle 2θ (°) | FWHM | Crystallite size D (nm) | Microstrain E | Dislocation density, δ (1014 lines per m2) |
|---|---|---|---|---|---|
| ZnO | 25.62 | 0.37 | 25 | 0.37 | 15.2 |
| 1 wt% Cu ZnO | 25.62 | 0.39 | 24 | 0.31 | 17.3 |
| 2 wt% Cu ZnO | 25.62 | 0.42 | 22 | 0.43 | 18.4 |
| 3 wt% Cu ZnO | 25.62 | 0.431 | 20 | 0.47 | 26.3 |
Thus crystallite size reduces with the addition of effective dopant concentration. It is because dopant induces resultant drag forces against growth of crystallite size. It leads towards smaller crystallite size. As in case of ETMs, crystallite size reduces from 25 nm to 20 nm after Cu doping.
For the samples doped with Cu content of more than 3 wt%, the structure is distorted, and crystallinity is also disturbed; elucidation of this point is explained in SEM investigation. So, loading of dopant into zinc oxide lattice is chosen wisely to achieve essentially required properties. Fig. 2b is Raman scattering for pristine and trace wt% doped Cu–ZnO nanofibers in the range of 200–3000 cm−1. There was a dominant peak at 434.5 cm−1 detected, representing the non-polar optical phonons E2 (high) modes of the ZnO fibers. The E2 phonon is shifted toward higher energy, from 434.5 to 437 cm−1, with increasing doping concentration. Another peak appeared at 570 cm−1, attributed to the LO phonon of ZnO. The appearance of LO of (A1)∥c confirmed the result of X-ray diffraction that the formed nanocrystallites are hexagonal wurtzite structures. The peaks at 522 and 303 cm−1 are due to microscopic silicon slides used as a substrate. The change of Raman peak position with doping corresponds to a small decrease in tensile stress generated into the crystal lattice via doping. The grain boundaries are high-energy sites able to capture the maximum quantity of carriers; therefore, doping is critical in the modification of the crystal size and grain boundaries in nanofibers. These grain boundaries, after capturing charge carriers form an interface barrier and resistant strains and other impurities distortions due to the substitution of Cu in the ZnO.41
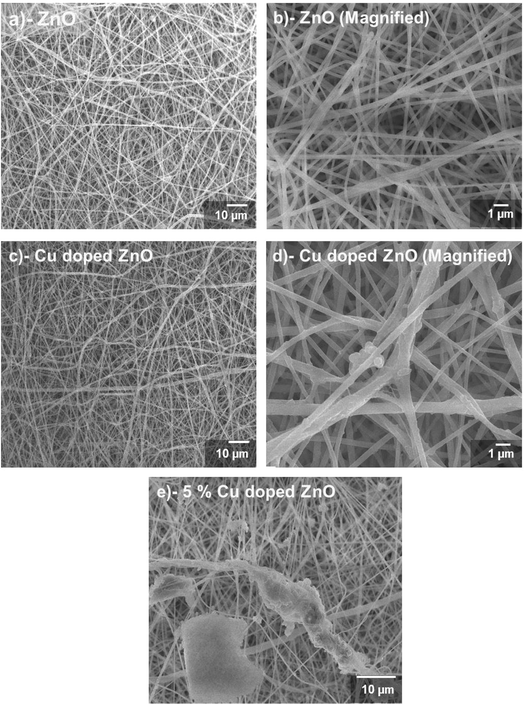
The surface morphology of pristine ZnO and Cu-doped ZnO nanofibers was analysed using SEM at different resolutions, as shown in Fig. 3a–e. ZnO nanofibers are uniformly dispersed onto the ITO substrate as representing in Fig. 3a and b. SEM images revealed a randomly oriented distribution of nanofibers with different sizes having mesoporous uniformity. The deposited ZnO sheet has a large number of nanofibers having a diameter of less than 0.5 μm, providing a suitably high interface for charge extraction from the absorber layer. Similarly, nanofibers showed an entangled network, providing multidimensional channels for charge transport.42 Up to 3%-doping concentration, the agglomeration of nanofibers was not observed. Cu is doped into the ZnO lattice uniformly, arising from proper dispersion of the dopant in the electrospinning gel. The morphology of the nanofibers remained similar after the Cu doping in the lattice of ZnO, which favored the formation of a rod-like structure, as shown in Fig. 3c and d. However, the crystal size of doped fibers was slightly decreased, as confirmed by XRD results. Fibers morphology has a significant effect on the photo anode performance, where long fibers with dense packing and excessive interconnections offer high PSC performance.43 The porous interconnected network of nanofibers appears to be highly correlated with the required structure, even after dopant incorporation in the ZnO lattice. The thickness of the electrospun web plays a crucial role in determining the performance and functionality. The electrospun nanofibers sheet thickness was around ≈10.5 μm in the fabricated devices.
ZnO wurtzite structure of nanofibers is obtained after 450 °C annealing temperature, which causes the transformation from amorphous to polycrystalline phase. Doped ZnO fibers offer large surface areas, as required for suitable photocatalytic activity and the current density of PSCs. Nanofibers offer a porous network, which allows easier transmission of light to the absorber layer. Structural and morphological characterizations confirmed that 3 wt% of Cu retains the crystallinity, along with improving the electronic structure. Excessive Cu-doping, i.e., more than 3 wt% causes structural distortions and unwanted aggregations, as shown in Fig. 3e. The fibers show unwanted aggregations, which are not feasible for the photoconversion process.
Because Cu2+ ionic radius (0.73 A) is so close to Zn2+'s (0.74 A) ionic radius, Cu can readily enter the ZnO/crystal lattice. Nevertheless, very weak peaks corresponding to CuO emerged with an increase in Cu doping up to 5%, and it has been discovered that the intensity of these peaks increases with additional Cu doping. Therefore, CuO began to segregate above 5% Cu doping. Therefore, at lower doping concentrations of Cu, its ions can effectively replace Zn ions; but, as the concentration of Cu rises, CuO begins to form clusters and is isolated as an impurity phase.44
FTIR analysis was performed for pristine and trace wt% ZnO to analyze functional groups in the respective samples, as shown in Fig. 4a. The vibration mode of the O–H bond is presented in the range of 3020–3650 cm−1, originating from bond stretching.45 The peaks around 3500 cm−1 are due to the hydroxyl component presence. The hydroxyl peaks are more dominant after copper doping, which contributes positively to enhancing photoactivity. Undoped-ZnO shows the lowest hydroxyl peak concentration as compared to doped structures. The absorption bands that appeared near 800 cm−1 are mainly due to Zn–O bonding, serving as a strong, cohesive forces agent. Similarly, other symmetric bands of the fingerprint region showed an increase in intensity due to the incorporation of Cu into the lattice, which is related to amorphous or defect-rich structure formation. Adding Cu into the ZnO leads to enhanced hydroxyl content forming; this hydroxyl content is proportional to enhanced charge mobility across electron transport material. Sufficient hydroxyl content promotes electron transport as well as restricts electron–hole combinations in the photoactive material. The intensity of the peaks around 975 cm−1 is also due to O–H bending, which is consistent with the stretching peak at high wavelength increased after increasing the Cu content in doped ZnO, related to increased defects in nano-fibers.46 To measure the total organic carbon (TOC) after the experiments, we tested the chemical characterization (FTIR) before photocatalysis and after photocatalysis. There are no additional peaks and compounds formation as a by-product as shown in the following Fig. 4a and b.
 | ||
| Fig. 4 (a) FTIR analysis of undoped ZnO, 1% Cu–ZnO, 2% Cu–ZnO, and 3% Cu–ZnO before photocatalysis, (b) after photocatalysis FTIR samples measurements. (c) PL Spectra of nanofibers ZnO, Cu doped ZnO. | ||
Photoluminescence (PL) spectra were used to examine the electron and hole recombination of ZnO and Cu-doped ZnO, as illustrated in Fig. 4c. Higher ZnO peak intensity indicates more electron–hole pair directs recombination. On the other hand, optimal Cu doping reduces charge recombination, improving charge separation and related photocatalytic activity. Charges can engage in photocatalytic reactions for extended periods of time because they have longer dwell durations.
3.2 Optoelectronic characterizations
Optical absorption of the nanofibers was studied by UV-vis spectroscopy, as demonstrated in Fig. 5a. Optical absorption was measured for the suspension of nanocrystallites of undoped and ZnO doped with 1, 2, and 3% of Cu. It was observed Cu-doping causes a shift of the absorption edge towards the visible range. The doping of Cu in ZnO creates shallow donor and trap states, thereby decreasing the Fermi level. Decreasing Fermi level energy could lead to better band alignment and consequently better charge extraction,46 which makes the structures suitable for application in solar cell and photocatalysis.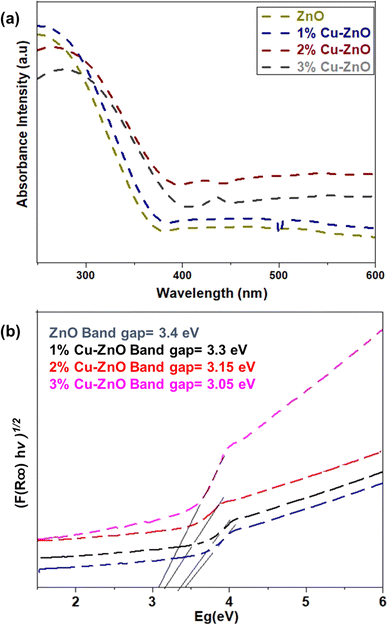 | ||
| Fig. 5 Optical characterizations of nanofibers using (a) UV-Visible absorption spectra and (b) Tuac plot. | ||
The band gap of the pristine ZnO, 1, 2, and 3 wt% Cu doped ZnO fibers were calculated using Tauc plot, as shown in Fig. 5b. It is noteworthy that Eg values estimated from the absorption edges vary depending on the Cu doping level and decrease to ∼3.05 eV for the 3 wt% Cu-doped ZnO fibers. The decrease of the band gap is due to the formation of intermediate states due to Cu loading.33 These intermediate states enable photoelectrons in the valence band to move from lower energies into the conduction band, extending the photo-absorbance range. This bandgap tuning causes the bandgap to drop significantly from 3.4 to 3.05 eV, enabling the electron to transition to an excited state at lower energies. Additionally, the creation of Zn vacancies due to Cu doping limits the electrons to recombine, which may be advantageous for photocatalysis and PSCs.21,47 The obtained result indicates that the higher absorption of the optical band gap can be obtained by controlling the Cu concentration in the Zn1−xCuxO nanocrystalline fibers.
3.3 Hall measurements
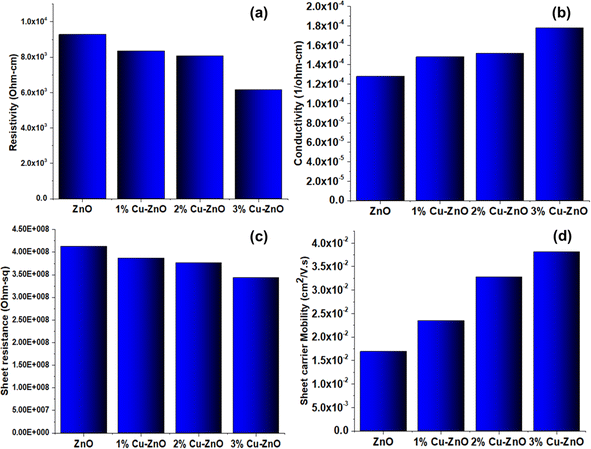
Hall measurements were characterized to access the semiconducting behavior, as shown in Fig. 6. These measurements were conducted with photoanodes spin-coated on the microscopic glass side. Resistivity was decreased with an increase in Cu content up to 3%, where the fibers' resistivity is minimum at 7.16 × 103 Ohm cm. The improvement in the conductive is what causes the resistance to drop with doping concentration. Similar to this, the conductivity rises in relation to the dopant addition. Similar to this, the addition of a dopant increases the increase in carrier mobility. With an increase in doping concentration, mobility increased as well, which is related to the growth of Fermi states and semicrystalline structures.48 This can be calculated as a decrease in the grain size of the fibers with increasing Cu-doping, along with enhanced n-type conductivity of ZnO due to the presence of sensitive strains such as oxygen and interstitial zinc defects. The incorporation of Cu into ZnO improves electrical mobility due to the creation of a single ion Cu substituted at Zn sites. On the other hand, an increase in the concentration of carriers indicates that the copper doping atoms are well substituted for the zinc sites in the ZnO lattice. A significant increase in mobility was found with the increase in dopant concentration, from 1.69 × 10−2 cm2 V−1 s−1 for undoped ZnO fibers to 3.25 × 10−2 cm2 V−1 s−1, 3.28 × 10−2 cm2 V−1 s−1, 3.31 × 10−2 cm2 V−1 s−1 for 1%, 2%, 3% Cu doped ZnO respectively. As a result, resistivity has significantly reduced, specifically for the 3% doping. Higher resistivity of undoped ZnO is related to charge entrapment at grain boundaries; however, Cu-doping causes an increase in conductivity. From this data, we can conclude that dopant concentration has favored the conductivity and other features essentially required for charge extraction and transport.493.4 Characteristics of J–V for PSCs
J–V curves for PSCs with undoped ZnO and trace wt% Cu–ZnO are shown in Fig. 7a. The parameters summary for these curves, including Jsc, FF, and Voc, are tabulated in below Table 2.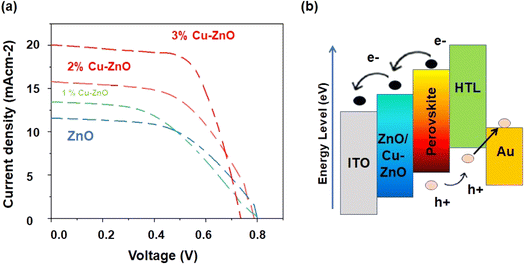 | ||
| Fig. 7 (a) Characteristics of J–V curves PSCs undoped ZnO, 1% Cu doped ZnO, 2% Cu doped ZnO, 3% Cu doped ZnO photoanodes, (b) energy band diagram for Cu doped ZnO photoanode. | ||
| PSCs | Current density Jsc (mA cm−2) | Open circuit voltage Voc (V) | Fill factor FF | Efficiency η (%) |
|---|---|---|---|---|
| Pristine ZnO | 11.59 | 0.80 | 0.53 | 4.94 |
| 1% Cu–ZnO | 13.59 | 0.80 | 0.55 | 5.97 |
| 2% Cu–ZnO | 15.79 | 0.79 | 0.56 | 6.89 |
| 3% Cu–ZnO | 20.08 | 0.73 | 0.66 | 9.79 |
Undoped ZnO shows the least current density and efficiency compared to doped samples. As we doped Cu into ZnO, it caused an increase in photoactivity by reducing charge recombination. Cu plays a vital role in enhancing carrier mobility, current densities, and open circuit voltage in a positive manner. The current density value for undoped ZnO is 11.58 mA, which is increased up to 20.07 mA after 3% Cu-doping. The obtained values of current densities for various cells based on doped and undoped ZnO are listed in Table 2. Obliviously there is continuous enhancement of current density with increasing doping up to 3%. As we incorporate 3% Cu into ZnO, the presence of Cu enhances the charge mobility, more and more charge conductions occurs and the charge recombination is reduced.50 For effective charge extraction and overall device performance, the energy band alignment in a perovskite solar cell with a zinc oxide (ZnO) electron transport layer is essential. The perovskite absorber material may efficiently absorb sunlight since its bandgap is usually between 1.1 and 1.7 eV. One typical application for zinc oxide is as an electron transport layer. Electrons move from the perovskite to the ZnO because of its conduction band (CB) energy level, which is higher (more negative) than the perovskite absorber's. This encourages effective extraction of electrons. Enhancement between the LUMO of the perovskite and the ETL layer results from localized states forming in the ZnO layer's forbidden gap, which enhances carrier electron transport.51
As Cu percentage increases into ZnO, intermediate states are formed, which supports electron extraction.52 At higher dopant concentration, metallic centres of Cu decreases due to formation of recombination sites. They recombine electrons and holes, decreasing efficiency. Processing of nanofibers is also difficult due to agglomeration and higher concentration does not support electrons conduction. Visible light absorption is enhanced, and light cannot access towards perovskite. That's why it is not feasible to dope higher concentration of Cu. The formation of localized states in the forbidden gap of the Cu–ZnO layer causes enhancement between the LUMO of perovskite and ETL layer, consequently improving carrier electron transportation.13,53 Furthermore, the cell based on ZnO doped with 3% Cu doping showed an enhancement of FF, which was attributed to the decrease of the internal resistance of the solar cells. We can conclude that loading ZnO with Cu is effective for increasing absorption rate, band gap reduction, and electron mobility, which resulting an increase the efficiency. The efficiency of ZnO doped with 0%, 1%, 2%, and 3% Cu is 4.94%, 5.97%, 6.89%, and 9.79%, respectively.
The performance of our proposed Cu-doped ZnO nano-fibers was comparable to that of previously reported nano-fibers with other metal dopants as shown in Table 3. Compared to other metals, low cost and earth abundant nature of Cu provides an advantage in commercial application.
| PSC | Jsc (mA cm−2) | Voc (mV) | FF | η (%) | Ref. |
|---|---|---|---|---|---|
| Pure ZnO nanofibers | 22.0 | 990 | 68 | 14.81 | 54 |
| In-doped ZnO nanofibers | 23.0 | 1000 | 70 | 16.10 | 55 |
| Pure ZnO | 18.4 | 1040 | 78.78 | 15.13 | 56 |
| Al doped Cu–ZnO | 18.6 | 1080 | 70.77 | 14.18 | 57 |
| TiO2 planar | 15.88 | 980 | 63.0 | 9.82 | 58 |
| TiO2 fiber | 4.02 | 1060 | 73.0 | 3.11 | 59 |
| Pristine–TiO2 NFs | 23.32 | 1013 | 67.0 | 15.82 | 60 |
| rGO2–TiO2 NFs | 23.25 | 1020 | 68.0 | 16.12 | 61 |
| Ag doped CuO NFs | 17.8 | 890 | 53.8 | 8.7 | 62 |
| ZnO | 18.1 | 670 | 58.1 | 7.05 | 63 |
| 3% Mg–ZnO PSC | 25.06 | 830 | 0.65 | 13.52 | 13 |
| Cu doped ZnO | 20.08 | 730 | 0.66 | 9.79 | Our work |
3.5 Antibacterial activity
Due to their ability to prevent disease spread, particularly for medical applications, photoactive fabrics are in high demand. Undoped and Cu-doped Zinc oxide nanofibers were used for this purpose, and they were attached to the fabric by dipping and then curing in binder into a 2.5% solution.21 For quantitative antibacterial measurements, Staphylococcus aureus and Escherichia coli: both Gram-positive and Gram-negative bacteria; known to negatively impact human health, were tested.64 These both served as model organisms for testing the biological self-cleaning abilities of coated fabrics. Bacterial cell counting with time duration t = 0, and after t = 24 h measured to estimate the coated fabric's quantitative antibacterial properties, and the results are shown in Fig. 8a. This change in bacterial growth with time is indicative of the effect the fabric had on the incubation process. Fig. 8a shows that after being contaminated with Staphylococcus and E. coli, the cell count of uncontaminated cotton fabric increased from 4.25 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL to 4.86 log
CFU mL to 4.86 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL. This proved that the sterile cotton fabric was vulnerable to germs and may be harmed by their degrading effects after 24 hours of incubation with Staphylococcus and E. coli bacterial strains. A decrease in cell count was seen on the cloth that had been coated with modified nano ZnO. At t = 0, there was 4.25 log
CFU mL. This proved that the sterile cotton fabric was vulnerable to germs and may be harmed by their degrading effects after 24 hours of incubation with Staphylococcus and E. coli bacterial strains. A decrease in cell count was seen on the cloth that had been coated with modified nano ZnO. At t = 0, there was 4.25 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 of Staphylococcus on the unwashed cloth. By t = 24 hours later, that number had dropped to 2 log
CFU mL−1 of Staphylococcus on the unwashed cloth. By t = 24 hours later, that number had dropped to 2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1. Staphylococcus count on the same coated cloth decreased from 4.25 log
CFU mL−1. Staphylococcus count on the same coated cloth decreased from 4.25 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 to 3.8 log
CFU mL−1 to 3.8 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 following only one cycle through the washing machine. After 5 washing cycles, the coated fabric's Staphylococcus count decreased from 4.25 log
CFU mL−1 following only one cycle through the washing machine. After 5 washing cycles, the coated fabric's Staphylococcus count decreased from 4.25 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 to 3.33 log
CFU mL−1 to 3.33 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1. After being washed 10 times, the coated cloth exhibited a decrease in the number of Staphylococcus bacteria, from 4.25 log
CFU mL−1. After being washed 10 times, the coated cloth exhibited a decrease in the number of Staphylococcus bacteria, from 4.25 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1 to 3.86 log
CFU mL−1 to 3.86 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU mL−1. This reduction in the bacterial count before and after washing cycles demonstrated the antimicrobial qualities of the coated fabrics.65
CFU mL−1. This reduction in the bacterial count before and after washing cycles demonstrated the antimicrobial qualities of the coated fabrics.65
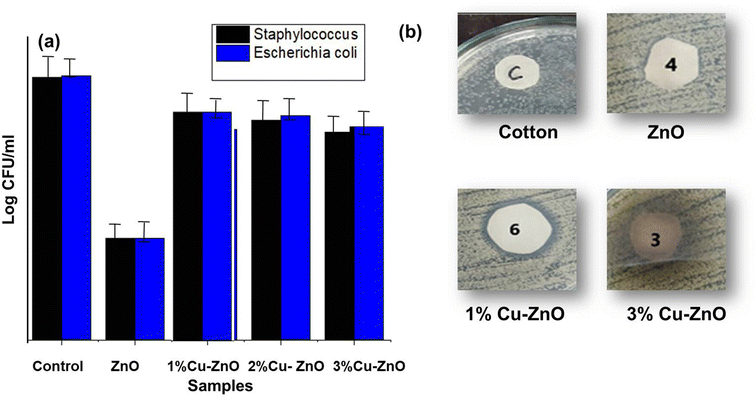 | ||
| Fig. 8 (a) Quantitative antibacterial measurements, (b) qualitative antibacterial measurements of photoactive samples. | ||
Bacterial growth was limited in case of coated washed sample as shown in (Fig. 8) following by ten washing cycles for coated fabric in evaluation of activity. This is because of modified ZnO present on the fabric surface produces ROS (reactive oxygen species), which inhibits bacterial growth. In addition, the results obtained showed that the coated cotton fabrics antibacterial activity was greater against E. coli and less effective against Staphylococcus. The coated, unwashed fabric was shown to be 99.6% effective against Staphylococcus aureus and E. coli. Ten washes in the washing machine killed 78.251% of the Staphylococcus and 79.4% of the E. coli on the coated cloth. The bacterial growth is decreased due to limited mechanical activities and detergent solution is also accessible with fabric thereby removing modified nano zinc oxide from surface. Coated fabric has ability to cope itself against bacteria even after a small amount of the modified zinc oxide was removed. As a result, the modified nano zinc oxide was able to stick to the fabric surface, and the antibacterial qualities remained even after several washing cycles.66
Fabrics were treated with pristine ZnO and Cu-doped ZnO to evaluate qualitative antibacterial activity, as shown in Fig. 8b. The antibacterial characterizations revealed that Cu doping enhances antibacterial activity in ZnO, where 1 wt% Cu–ZnO showed the highest antibacterial activity. The zone of inhibition formed by 1% Cu-doped ZnO is 3.05 mm, whereas the zone of inhibition formed by pristine ZnO is 2.32 mm. 1% Cu-doped ZnO possesses a greater zone of inhibition due to the stronger photocatalytic effect of Cu-doped ZnO nanofibers relative to pure ZnO. As a result of photoabsorption, ROS possesses a strong destruction potential against bacteria. Cu-doped ZnO gives a leaching effect in bacterial activity not suitable for photocatalytic activity.67,68 There is higher vicinity surrounding active material as a result of strong photocatalytic activity; hence bacterial growth was suppressed. As a result, Cu–ZnO showed a wider inhibition zone and a great ability to thwart microbial development. Bacterial growth at bare cotton fabric substrate may be seen in Fig. 8b since the reference cotton sample had a negligible zone of inhibition.
3.6 Photocatalytic dye degradation test
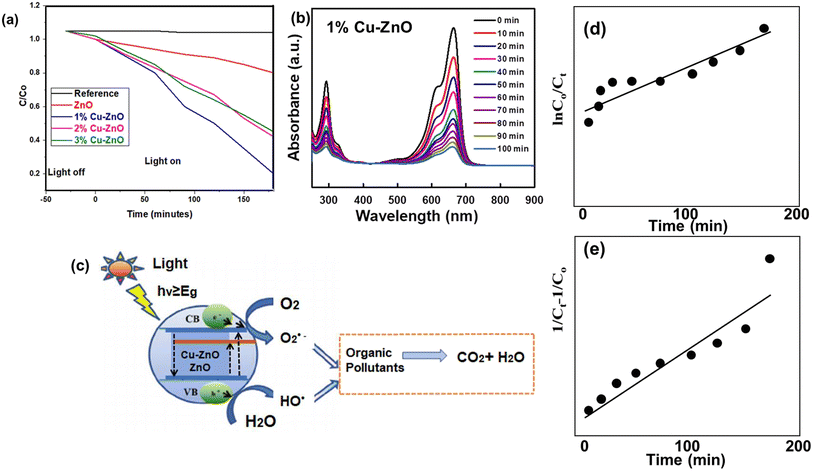
Photocatalytic degradation allows for the efficient conversion of solar energy into chemical energy. The photoabsorption characteristic has a strong influence on photocatalytic activity. Because of its low cost, long-term stability, outstanding photocatalytic efficacy, and environmental friendliness, ZnO can be an effective catalyst in the degradation of organic contaminants and water splitting. Photocatalytic dye degradation for Cu-doped ZnO nanofibers coated fabrics are shown in Fig. 9a and b. Fabric electrodes were then immersed in the dye solution, and then allowed to keep them to sunlight for degradation evaluation. For the degradation evaluation, Methlylene blue dye solution was prepared with 10 ppm solution and subjected to UV-Visible spectrometer for determining the maximum absorption intensity of MB degradation at 464 nm.69 The electrode adsorption is monitored in dark and then displayed in the negative time to zero time range. When the adsorption of dyed sample was reached to saturation, it was exposed to sunlight. When the photocatalysis dye degradation for all photcatalysts were compared, the undoped ZnO electrode only destroyed 20% of the starting concentration, while the 1% Cu–ZnO electrode only degraded 80%. This optimized 1% Cu–ZnO electrode structure's significant role in the photocatalytic activity of dye is demonstrated by the 60% higher photocatalytic degradation of dyes utilizing our recommended electrode.70 The dye degradation of the 62% and 58% dyes occurs at 2% and 3% Cu–ZnO electrodes, respectively. As also observed in Fig. 9b, 1% Cu–ZnO has optimized dye degradation also suitable for photocatalytic activity. Additionally, a test was run only in the absence of a photocatalyst to examine dye photolysis induced by irradiation source, which was shown to be quite minimal.Increased light absorption caused by the Cu-doped ZnO electrode led to a significant amount of excitons being produced for photodegradation, as seen in Fig. 9c. Copper functions as efficient electrons sinker supporting dye degradation. This action also leads to an improvement in charge separation, which prolongs the dwell period of excitons for low doping levels. While excessive Cu doping promotes exciton recombination, Cu-centers with zero bandgaps can contain both holes and electrons. Using 2% and 3% Cu-doped ZnO electrodes results in reduced photocatalytic activity of dyes due to somewhat faster recombination.71 When light absorbs at photocatalyst, excitons are created and photons are absorbed. Higher excitons can improve photocatalytic activity by raising the possibility that electron–hole separation will occur and that ROS will subsequently be produced as a result. The electron and hole of the excitons can then be separated, and they can engage in redox reactions with molecules that have been adsorbed on the surface of the photocatalyst. The process efficiency is raised by the photocatalysis occurring in the mobile phase, which encourages the generation of ROS. The photocatalysis is slower in the passivated state, or coated on cloth, than it is in the mobile phase. Due to the less complicated recovery of active materials, careful planning, and optimisation of the photocatalyst's capabilities with little secondary pollution, the suggested method is closer to practical applications.
In photocatalysis and antibacterial characterizations, 1% Cu-doped ZnO possesses a greater zone of inhibition due to the stronger photocatalytic effect of Cu-doped ZnO nanofibers relative to pure ZnO. As a result of photoabsorption, ROS possesses a strong destruction potential against bacteria. 2.3% Cu-doped ZnO gives a leaching effect in bacterial activity not suitable for photocatalytic activity. There is higher vicinity surrounding active material as a result of strong photocatalytic activity; hence bacterial growth was suppressed.72 When the photocatalysis dye degradation for all photcatalysts were compared, the undoped ZnO electrode only destroyed 20% of the starting concentration, while the 1% Cu–ZnO electrode only degraded 80%. This optimized 1% Cu–ZnO electrode structure's significant role in the photocatalytic activity of dye is demonstrated by the 60% higher photocatalytic degradation of dyes utilizing our recommended electrode. 1% Cu–ZnO has optimized dye degradation also suitable for photocatalytic activity. Additionally, a test was run only in the absence of a photocatalyst to examine dye degradation brought on by the light source, which was shown to be quite minimal.61
When metal dopants are doped into ZnO, ROS generation is increased due to which antibacterial activity is improved. In case of photovoltaic, charge flow and charge transfer resistances are crucial. For higher mobility, Cu dopant up to 3% is recommended for enhanced charge flow and electrons conduction for higher efficiency. But for photocatalysis and antibacterial, charge suppression is needed. Hence metal dopants are linked together for both applications; photovoltaic as well as photocatalysis.13,73
Here are the approximate energy levels (in electron volts, eV) for the conduction band (CB), valence band (VB), and a few common redox couples (O2/˙O2− and H2O/˙OH) referenced to vacuum for zinc oxide (ZnO), a wide-bandgap semiconductor that is frequently used in various optoelectronic applications. The conduction band edge of ZnO is approximately −4.3 eV to −4.5 eV relative to vacuum. ZnO valence band edge is situated between −7.8 and −8.0 eV from vacuum. Oxygen's (˙O2−) energy level in ZnO varies with its shape and surroundings. In a number of redox processes, the superoxide ion (˙O2−) is an intermediate species. The ˙O2− species in ZnO is generally found at a lower energy level (about −4.8 to −5.2 eV relative to vacuum) than the ZnO CB edge. The atmosphere can also affect the energy level of the hydroxyl radical (˙OH) in zinc oxide (ZnO). Generally speaking, the ˙OH species is at a higher energy level roughly −6.2 to −6.5 eV relative to vacuum – than the ZnO VB.74
Fig. 9d presents the linear relations of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/Ct versus time, and Fig. 9e presents the linear relationship of (1/Ct − 1/C0) versus time as first and second order kinetic models, using Cu doped ZnO 1% optimized against MB dyes. The values of K1 and K2 are calculated from the slope of first and second order kinetics as 0.0068 min−1 and 0.0005 L μmol−1 min−1, respectively. The R2 value and rate constant, K, for dye degradation are shown in Table 4. The R2 value for first order kinetics is best-fitted relative to the second kinetics model, suggesting the proposed reaction kinetic follows first order model.
C0/Ct versus time, and Fig. 9e presents the linear relationship of (1/Ct − 1/C0) versus time as first and second order kinetic models, using Cu doped ZnO 1% optimized against MB dyes. The values of K1 and K2 are calculated from the slope of first and second order kinetics as 0.0068 min−1 and 0.0005 L μmol−1 min−1, respectively. The R2 value and rate constant, K, for dye degradation are shown in Table 4. The R2 value for first order kinetics is best-fitted relative to the second kinetics model, suggesting the proposed reaction kinetic follows first order model.
| Dye | 1st order kinetics | 2nd order kinetics | ||
|---|---|---|---|---|
| R2 | K1 (min−1) | R2 | K2 (L μmol−1 min−1) | |
| MB | 0.92 | 0.0068 | 0.81 | 0.005 |
3.7 Radical scavenger measurements and reproducibility analysis
Photocatalysis of organic contaminants is attained by multiple reactive and toxic species like electrons (e−), holes (h+), hydroxyl radicals (HO˙) etc. The present study used the radical scavengers Butylated Hydroxytoluene (BHT) for hydroxyl radicals, EDTA (ethylene-diamine-tetra-acetate) for holes, and ascorbic acid for e trapping to assess the amount of these types of radicals that activate photocatalysis and ROS formation. For each scavenger, a total of 15 mM concentration was added to 100 mL of dye solution. Every experiment was carried out in Phillips visible and UV light with ideal solution pH concentration, reaction time, and other circumstances. The outcomes of employing 1%Cu–ZnO as radical trapping scavengers against MB dyes are shown in Fig. 10a. The results indicated that 91% of the MB dye was degraded in the absence of a scavenger. On the other hand, dye degradation efficiency dropped from 91% to 68.5% upon the addition of EDTA. Scavenger for h+ ions is EDTA in this study.75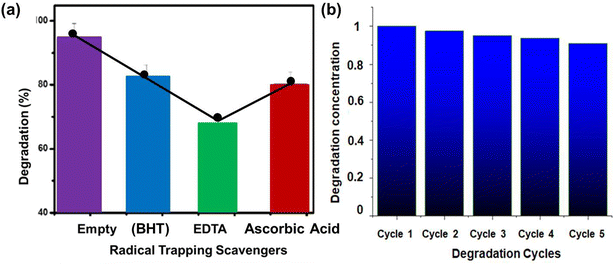 | ||
| Fig. 10 (a) Comparison of radical trapping scavengers for different electrodes, (b) comparison of degradation cycles for photocatalysts. | ||
To detect the recyclability of the material, following five consecutive cycles, photocatalytic activity was assessed, as shown in Fig. 10b. The electrode's cyclic stability and reusability is one of the goals of growing on a fabric substrate. The electrode maintained 98, 97.5, 95, 93, and 91.25 percent of its initial photocatalytic activity after five consecutive cycles, according to reusability results, as shown in Fig. 10b. This demonstrates the electrode's stability for real-world uses where separation can be facilitated more easily without the need for filtration or centrifugation – processes that are frequently used with mobile phase photocatalysts. When compared to the usage of binders, direct growth has the benefit of high performance and stability. The binders impede the interaction between active materials and pollution molecules by passivating on their surface.76
4. Conclusion
In this research, we synthesized undoped, and 1–3 wt% Cu–ZnO nanofibers by electrospinning method, and solar cell devices were fabricated. The 3 wt% doping of nanofibers tuned the electronic structure to support efficient charge transporting structure. Due to better porosity, the structure offers higher optical transmission, despite having a lower bandgap. Optical properties of doped nanofibers examined using UV-visible analysis showed a red shift in UV-vis absorption spectra due to heteroatom-doping. The electrical measurements suggested increased values of electron mobility and conduction with Cu doping. J–V results described that Cu-doped ZnO shows high efficiency, i.e., undoped ZnO and 3% Cu–ZnO showed 4.937% and 9.79% power conversion efficiency, respectively. 3% Cu–ZnO electrode has the highest photocatalytic activity because of its stable and conductive structure. Also, Cu-doped ZnO is intact with fabric which is more photo-active against bacterial growth inhibition and photocatalytic dye degradation. In contrast to the charge transport characteristics of 3% doping, the photocatalysis was better for the 1% Cu-doping.5. Future works and limitations
Efficient charge transport and reduced recombination at interfaces within the perovskite solar cell structure are crucial for achieving high efficiency. Optimizing interfaces between the perovskite layer, electron/hole transport layers, and electrodes is an ongoing challenge to improve device performance and stability. There is need to work on improvement of performance and stability for metal doped ZnO nanofibers.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors extend their application to Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number RGP2/207/45.References
- K. H. Lee, U. Arfa, Z. Arshad, E.-J. Lee, M. Alshareef and M. M. Alsowayigh, et al., The Comparison of Metal Doped TiO2 Photocatalytic Active Fabrics under Sunlight for Waste Water Treatment Applications, Catalysts, 2023, 13, 1293 CrossRef CAS
.
- S.-K. Jung, N.-G. Park and J.-W. Lee, Light management in perovskite solar cells, Mater. Today Energy, 2023, 101401 CrossRef CAS
.
- W. Bergesen, Optimization of the Energy Management System of a Solar Car, NTNU, 2023 Search PubMed
.
- S. Ye, J. Zhu, S. Zhu, Y. Zhao, M. Li and Z. Huang, et al., Design strategies for perovskite-type high-entropy oxides with applications in optics, ACS Appl. Mater. Interfaces, 2023, 15, 47475–47486 CrossRef CAS PubMed
.
- M. Batmunkh, M. Bat-Erdene and J. G. Shapter, Black phosphorus: synthesis and application for solar cells, Adv. Energy Mater., 2018, 8, 1701832 CrossRef
.
- M. Hu, B. Gao, Y. Wang, Z. Xie, F. Cai and Y. Zhang, et al., Dithiols enhance the photovoltaic performance and stability of perovskite solar cells and modules by elongating the carrier lifetime, Mater. Today Energy, 2023, 37, 101392 CrossRef CAS
.
- L. Yang, T. Xu, Z. Bai and S. Qin, Improved Open-Circuit Voltage of AZO/CsPbBr3/Carbon Structure Perovskite Solar Cells by an Al-Doped ZnO Electron Transport Layer, J. Phys. Chem. C, 2023, 127, 7492–7500 CrossRef CAS
.
- G. Dong, T. Ye, Y. Yang, L. Sheng, D. Xia and J. Wang, et al., SiW12–TiO2 Mesoporous Layer for Enhanced Electron-Extraction Efficiency and Conductivity in Perovskite Solar Cells, ChemSusChem, 2017, 10, 2218–2225 CrossRef CAS PubMed
.
- X. Li, S. Aftab, S. Hussain, F. Kabir, A. Henaish and A. G. Al-Sehemi, Dimensional diversity (0D, 1D, 2D, 3D) in Perovskite solar cells: Exploring the potential of mix-dimensional integrations, J. Mater. Chem. A, 2024, 37, 4122–4125 Search PubMed
.
- H. Liu, H. Li, J. Tao, J. Liu, J. Yang and J. Li, et al., Single Crystalline Transparent Conducting F, Al, and Ga Co-Doped ZnO Thin Films with High Photoelectrical Performance, ACS Appl. Mater. Interfaces, 2023, 15, 22195–22203 CrossRef CAS PubMed
.
- G. Dong, W. Chi, D.-f. Chai, Z. Zhang, J. Li and M. Zhao, et al., A novel Ag3BiO3/ZnO/BC composite with abundant defects and utilizing hemp BC as charge transfer mediator for photocatalytic degradation of levofloxacin, Appl. Surf. Sci., 2023, 619, 156732 CrossRef CAS
.
- B. Sun, G. Dong, J. Ye, D.-f. Chai, X. Yang and S. Fu, et al., Selenium anion substitution endows manganese sulfide as a bifunctional electrocatalyst for efficient water splitting in alkaline solutions, Chem. Eng. J., 2023, 459, 141610 CrossRef CAS
.
- Z. Arshad, S. Wageh, T. Maiyalagan, M. Ali, U. Arshad and M. B. Qadir, et al., Enhanced charge transport characteristics in zinc oxide nanofibers via Mg2+ doping for electron transport layer in perovskite solar cells and antibacterial textiles, Ceram. Int., 2022, 48, 24363–24371 CrossRef CAS
.
- J. Luo, Y. Wang and Q. Zhang, Progress in perovskite solar cells based on ZnO nanostructures, Sol. Energy, 2018, 163, 289–306 CrossRef CAS
.
- R. Chen, J. Cao, Y. Duan, Y. Hui, T. T. Chuong and D. Ou, et al., High-efficiency, hysteresis-less, UV-stable perovskite solar cells with cascade ZnO–ZnS electron transport layer, J. Am. Chem. Soc., 2018, 141, 541–547 CrossRef PubMed
.
- P. Zhang, J. Wu, T. Zhang, Y. Wang, D. Liu and H. Chen, et al., Perovskite solar cells with ZnO electron-transporting materials, Adv. Mater., 2018, 30, 1703737 CrossRef PubMed
.
- M. K. Mohammed and M. Shekargoftar, Surface treatment of ZnO films with carbon nanotubes for efficient
and stable perovskite solar cells, Sustainable Energy Fuels, 2021, 5, 540–548 RSC
.
- M. Manabeng, B. S. Mwankemwa, R. O. Ocaya, T. E. Motaung and T. D. Malevu, A review of the impact of zinc oxide nanostructure morphology on perovskite solar cell performance, Processes, 2022, 10, 1803 CrossRef CAS
.
- P. Chandrasekhar, A. Dubey and Q. Qiao, High efficiency perovskite solar cells using nitrogen-doped graphene/ZnO nanorod composite as an electron transport layer, Sol. Energy, 2020, 197, 78–83 CrossRef CAS
.
- D. R. Kulkarni, S. J. Malode, K. K. Prabhu, N. H. Ayachit, R. M. Kulkarni and N. P. Shetti, Development of a novel nanosensor using Ca-doped ZnO for antihistamine drug, Mater. Chem. Phys., 2020, 246, 122791 CrossRef CAS
.
- V. Etacheri, R. Roshan and V. Kumar, Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis, ACS Appl. Mater. Interfaces, 2012, 4, 2717–2725 CrossRef CAS PubMed
.
- V. Musat, B. Teixeira, E. Fortunato, R. Monteiro and P. Vilarinho, Al-doped ZnO thin films by sol–gel method, Surf. Coat. Technol., 2004, 180, 659–662 CrossRef
.
- F. Maldonado and A. Stashans, Al-doped ZnO: Electronic, electrical and structural properties, J. Phys. Chem. Solids, 2010, 71, 784–787 CrossRef CAS
.
- L. Sha, B.-B. Sui, P.-F. Wang, Z. Gong, Y.-H. Zhang and Y.-H. Wu, et al., 3D network of zinc powder woven into fibre filaments for dendrite-free zinc battery anodes, Chem. Eng. J., 2024, 481, 148393 CrossRef CAS
.
- X. Liu, Z. Wu, Y. Zhang and C. Tsamis, Low temperature Zn-doped TiO2 as electron transport layer for 19% efficient planar perovskite solar cells, Appl. Surf. Sci., 2019, 471, 28–35 CrossRef CAS
.
- F. Shan and Y. Yu, Band gap energy of pure and Al-doped ZnO thin films, J. Eur. Ceram. Soc., 2004, 24, 1869–1872 CrossRef CAS
.
- K.-J. Chen, T.-H. Fang, F.-Y. Hung, L.-W. Ji, S.-J. Chang and S.-J. Young, et al., The crystallization and physical properties of Al-doped ZnO nanoparticles, Appl. Surf. Sci., 2008, 254, 5791–5795 CrossRef CAS
.
- V. Vaiano, G. Iervolino and L. Rizzo, Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light, Appl. Catal., B, 2018, 238, 471–479 CrossRef CAS
.
- K. R. Aneesiya and C. Louis, Localized surface plasmon resonance of Cu-doped ZnO nanostructures and the material's integration in dye sensitized solar cells (DSSCs) enabling high open-circuit potentials, J. Alloys Compd., 2020, 829, 154497 CrossRef CAS
.
- M. W. Alam, M. Z. Ansari, M. Aamir, M. Waheed-Ur-Rehman, N. Parveen and S. A. Ansari, Preparation and characterization of Cu and Al doped ZnO thin films for solar cell applications, Crystals, 2022, 12, 128 CrossRef CAS
.
- Z. Ge, C. Wang, T. Chen, Z. Chen, T. Wang and L. Guo, et al., Preparation of Cu-doped ZnO nanoparticles via layered double hydroxide and application for dye-sensitized solar cells, J. Phys. Chem. Solids, 2021, 150, 109833 CrossRef CAS
.
- G. Colón, M. Maicu, M. s. Hidalgo and J. Navío, Cu-doped TiO2 systems with improved photocatalytic activity, Appl. Catal., B, 2006, 67, 41–51 CrossRef
.
- R. Mohan, K. Krishnamoorthy and S.-J. Kim, Enhanced photocatalytic activity of Cu-doped ZnO nanorods, Solid State Commun., 2012, 152, 375–380 CrossRef CAS
.
- G. Z. Xing, J. B. Yi, J. G. Tao, T. Liu, L. M. Wong and Z. Zhang, et al., Comparative study of room-temperature ferromagnetism in Cu-doped ZnO nanowires enhanced by structural inhomogeneity, Adv. Mater., 2008, 20, 3521–3527 CrossRef CAS
.
- J.-A. Park, J. Moon, S.-J. Lee, S.-C. Lim and T. Zyung, Fabrication and characterization of ZnO nanofibers
by electrospinning, Curr. Appl. Phys., 2009, 9, S210–S212 CrossRef
.
- R. C. Nonato, L. H. Mei, B. C. Bonse, C. V. Leal, C. E. Levy and F. A. Oliveira, et al., Nanocomposites of PLA/ZnO nanofibers for medical applications: Antimicrobial effect, thermal, and mechanical behavior under cyclic stress, Polym. Eng. Sci., 2022, 62, 1147–1155 CrossRef CAS
.
- R. Siddheswaran, R. Sankar, M. Ramesh Babu, M. Rathnakumari, R. Jayavel and P. Murugakoothan, et al., Preparation and characterization of ZnO nanofibers by electrospinning, Cryst. Res. Technol., 2006, 41, 446–449 CrossRef CAS
.
- X. Yang, C. Shao, H. Guan, X. Li and J. Gong, Preparation and characterization of ZnO nanofibers by using electrospun PVA/zinc acetate composite fiber as precursor, Inorg. Chem. Commun., 2004, 7, 176–178 CrossRef CAS
.
- I. M. P. Silva, G. Byzynski, C. Ribeiro and E. Longo, Different dye degradation mechanisms for ZnO and ZnO doped with N (ZnO: N), J. Mol. Catal. A: Chem., 2016, 417, 89–100 CrossRef CAS
.
- M. Wang, J. Yi, S. Yang, Z. Cao, X. Huang and Y. Li, et al., Electrodeposition of Mg doped ZnO thin film for the window layer of CIGS solar cell, Appl. Surf. Sci., 2016, 382, 217–224 CrossRef CAS
.
- M. A. Vargas, E. M. Rivera-Muñoz, J. E. Diosa, E. E. Mosquera and J. E. Rodríguez-Páez, Nanoparticles of ZnO and Mg-doped ZnO: Synthesis, characterization and efficient removal of methyl orange (MO) from aqueous solution, Ceram. Int., 2021, 47, 15668–15681 CrossRef CAS
.
- Z. Huang, P. Luo, Q. Wu and H. Zheng, Constructing one-dimensional mesoporous carbon nanofibers loaded with NaTi2 (PO4) 3 nanodots as novel anodes for sodium energy storage, J. Phys. Chem. Solids, 2022, 161, 110479 CrossRef CAS
.
- S. Sitthichai, A. Phuruangrat, T. Thongtem and S. Thongtem, Influence of Mg dopant on photocatalytic properties of Mg-doped ZnO nanoparticles prepared by sol–gel method, J. Ceram. Soc. Jpn., 2017, 125, 122–124 CrossRef CAS
.
- D. Toloman, A. Popa, M. Stan, M. Stefan, G. Vlad and S. Ulinici, et al., Visible-light-driven photocatalytic degradation of different organic pollutants using Cu doped ZnO-MWCNT nanocomposites, J. Alloys Compd., 2021, 866, 159010 CrossRef CAS
.
- M. N. H. Mia, M. F. Pervez, M. K. Hossain, M. R. Rahman, M. J. Uddin and M. A. Al Mashud, et al., Influence of Mg content on tailoring optical bandgap of Mg-doped ZnO thin film prepared by sol-gel method, Results Phys., 2017, 7, 2683–2691 CrossRef
.
- N. Guo, X. Wei, R. Zhao and X. Xu, Preparation and optical properties of Mg-doped ZnO nanorods, Appl. Surf. Sci., 2014, 317, 400–404 CrossRef CAS
.
- C. Wang, P. Shi, C. Guo, R. Guo and J. Qiu, CuCo2O4/CF cathode with bifunctional and dual reaction centers exhibits high RhB degradation in electro-Fenton systems, J. Electroanal. Chem., 2024, 956, 118072 CrossRef CAS
.
- S. Suwanboon and P. Amornpitoksuk, Preparation of Mg-doped ZnO nanoparticles by mechanical milling and their optical properties, Procedia Eng., 2012, 32, 821–826 CrossRef CAS
.
- L. Umaralikhan and M. J. M. Jaffar, Green synthesis of ZnO and Mg doped ZnO nanoparticles, and its optical properties, J. Mater. Sci.: Mater. Electron., 2017, 28, 7677–7685 CrossRef CAS
.
- M. Rouchdi, E. Salmani, B. Fares, N. Hassanain and A. Mzerd, Synthesis and characteristics of Mg doped ZnO thin films: Experimental and ab-initio study, Results Phys., 2017, 7, 620–627 CrossRef
.
- X. Xu, H. Zhang, J. Shi, J. Dong, Y. Luo and D. Li, et al., Highly efficient planar perovskite solar cells with a TiO 2/ZnO electron transport bilayer, J. Mater. Chem. A, 2015, 3, 19288–19293 RSC
.
- E. Vinoth, S. Gowrishankar and N. Gopalakrishnan, Effect of Mg doping in the gas-sensing performance of RF-sputtered ZnO thin films, Appl. Phys. A: Mater. Sci. Process., 2018, 124, 1–8 CrossRef CAS
.
- N. Kılınç, L. Arda, S. Öztürk and Z. Öztürk, Structure and electrical properties of Mg-doped ZnO nanoparticles, Cryst. Res. Technol., 2010, 45, 529–538 CrossRef
.
- K. Mahmood, A. Khalid, S. W. Ahmad and M. T. Mehran, Indium-doped ZnO mesoporous nanofibers as efficient electron transporting materials for perovskite solar cells, Surf. Coat. Technol., 2018, 352, 231–237 CrossRef CAS
.
- S. Ilican, Y. Caglar, M. Caglar and F. Yakuphanoglu, Electrical conductivity, optical and structural properties of indium-doped ZnO nanofiber thin film deposited by spray pyrolysis method, Phys. E, 2006, 35, 131–138 CrossRef CAS
.
- H. Yang, H.-C. Kwon, S. Ma, K. Kim, S.-C. Yun and G. Jang, et al., Energy level-graded Al-doped ZnO protection layers for copper nanowire-based window electrodes for efficient flexible perovskite solar cells, ACS Appl. Mater. Interfaces, 2020, 12, 13824–13835 CrossRef CAS PubMed
.
- M. Manjula, B. Karthikeyan and D. Sastikumar, Cu-doped zinc oxide fiber optic sensor for acetone detection at room temperature, Appl. Phys. A: Mater. Sci. Process., 2020, 126, 1–7 CrossRef
.
- S. Dharani, H. K. Mulmudi, N. Yantara, P. T. T. Trang, N. G. Park and M. Graetzel, et al., High efficiency electrospun TiO 2 nanofiber based hybrid organic–inorganic perovskite solar cell, Nanoscale, 2014, 6, 1675–1679 RSC
.
- Z. Wang, W. Fu, L. Hu, M. Zhao, T. Guo and D. Hrynsphan, et al., Improvement of electron transfer efficiency during denitrification process by Fe-Pd/multi-walled carbon nanotubes: Possessed redox characteristics and secreted endogenous electron mediator, Sci. Total Environ., 2021, 781, 146686 CrossRef CAS
.
- J. V. Patil, S. S. Mali, A. P. Patil, P. S. Patil and C. K. Hong, Highly efficient mixed-halide mixed-cation perovskite solar cells based on rGO-TiO2 composite nanofibers, Energy, 2019, 189, 116396 CrossRef CAS
.
- Q. A. Alsulami, Z. Arshad, M. Ali and S. Wageh, Efficient Tuning of the Opto-Electronic Properties of Sol–Gel-Synthesized Al-Doped Titania Nanoparticles for Perovskite Solar Cells and Functional Textiles, Gels, 2023, 9, 101 CrossRef CAS PubMed
.
- Z. Zhuang, L. Qiu, L. Dong, Y. Chen, Z. Chu and X. Ma, et al., Preparation of high-efficiency perovskite solar cells via doping Ag into CuO nanofibers as hole buffer layer, Polym. Compos., 2020, 41, 2145–2153 CrossRef CAS
.
- F. Mohtaram, S. Borhani, M. Ahmadpour, P. Fojan, A. Behjat and H.-G. Rubahn, et al., Electrospun ZnO nanofiber interlayers for enhanced performance of organic photovoltaic devices, Sol. Energy, 2020, 197, 311–316 CrossRef CAS
.
- S. Ghosh, N. A. Kouamé, L. Ramos, S. Remita, A. Dazzi and A. Deniset-Besseau, et al., Conducting polymer nanostructures for photocatalysis under visible light, Nat. Mater., 2015, 14, 505–511 CrossRef CAS PubMed
.
- K. H. Lee, Z. Arshad, A. Dahshan, M. Alshareef, Q. A. Alsulami and A. Bibi, et al., Porous Aerogel Structures as Promising Materials for Photocatalysis, Thermal Insulation Textiles, and Technical Applications: A Review, Catalysts, 2023, 13, 1286 CrossRef CAS
.
- M. Shoaib, Z. Latif, M. Ali, A. Al-Ghamdi, Z. Arshad and S. Wageh, Room Temperature Synthesized TiO2 Nanoparticles for Two-Folds Enhanced Mechanical Properties of Unsaturated Polyester, Polymers, 2023, 15, 934 CrossRef CAS PubMed
.
- Z. Arshad and S. S. Alharthi, Enhancing the Thermal Comfort of Woven Fabrics and Mechanical Properties of Fiber-Reinforced Composites Using Multiple Weave Structures, Fibers, 2023, 11, 73 CrossRef
.
- H. Lü, X. Chen, Q. Sun, N. Zhao and X. Guo, Uniform Garnet Nanoparticle Dispersion in Composite Polymer Electrolytes, Acta Phys.-Chim. Sin., 2024, 2305016 Search PubMed
.
- A. ur Rehman, M. Aadil, S. Zulfiqar, P. O. Agboola, I. Shakir and M. F. A. Aboud, et al., Fabrication of binary metal doped CuO nanocatalyst and their application for the industrial effluents treatment, Ceram. Int., 2021, 47, 5929–5937 CrossRef CAS
.
- S. Lee, J. H. Noh, H. S. Han, D. K. Yim, D. H. Kim and J.-K. Lee, et al., Nb-doped TiO2: a new compact layer material for TiO2 dye-sensitized solar cells, J. Phys. Chem. C, 2009, 113, 6878–6882 CrossRef CAS
.
- H. Peng, R. Guo and H. Lin, Photocatalytic reduction of CO2 over Sm-doped TiO2 nanoparticles, J. Rare Earths, 2020, 38, 1297–1304 CrossRef CAS
.
- Y. Xue, X. Liu, N. Zhang, Y. Shao and C. C. Xu, Enhanced photocatalytic performance of iron oxides@ HTCC fabricated from zinc extraction tailings for methylene blue degradation: Investigation of the photocatalytic mechanism, Int. J. Miner., Metall. Mater., 2023, 30, 2364–2374 CrossRef CAS
.
- Z. Arshad, M. Ali, E.-J. Lee, M. Alshareef, M. M. Alsowayigh and K. Shahid, et al., Comparison of Electrospun Titania and Zinc Oxide Nanofibers for Perovskite Solar Cells and Photocatalytic Degradation of Methyl Orange Dye, Catalysts, 2023, 13, 1062 CrossRef CAS
.
- K. Noori and F. Giustino, Ideal Energy-Level Alignment at the ZnO/P3HT Photovoltaic Interface, Adv. Funct. Mater., 2012, 22, 5089–5095 CrossRef CAS
.
- L. Zhu, Z. Li and K. Hou, Effect of radical scavenger on electrical tree in cross-linked polyethylene with large harmonic superimposed DC voltage, High Voltage, 2023, 8, 739–748 CrossRef
.
- X.-j. Yang, W. Shu, H.-m. Sun, X.-b. Wang and J.-s. Lian, Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles, Trans. Nonferrous Met. Soc. China, 2015, 25, 504–509 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |