 Open Access Article
Open Access ArticleRapid synthesis of aminal-linked covalent organic frameworks for CO2/CH4 separation†
Jianwei Yang,
Xianghao Han* and
Xiao Feng *
*
Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Key Laboratory of Cluster Science, Ministry of Education, Advanced Technology Research Institute (Jinan), Frontiers Science Center for High Energy Material, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, China. E-mail: fengxiao86@bit.edu.cn; hanxh5@126.com
First published on 4th July 2024
Abstract
As an emerging category of crystalline porous materials, covalent organic frameworks (COFs) are primarily synthesized via solvothermal methods. However, achieving rapid synthesis of COFs through this approach poses a significant challenge. To address the issue of slow synthesis, we studied the crystallization process of aminal-linked COFs via the condensation of a cost-effective aldehyde and secondary amine, and successfully expedited the synthesis of COFs within a one-hour duration. Furthermore, gram-scale aminal-linked COFs with abundant ultra-microporous channels demonstrated promising potential for CO2/CH4 separation. This study enables the rapid synthesis of aminal-linked COFs from cheap raw materials, which lays a foundation for their practical applications.
Introduction
Covalent organic frameworks (COFs) are an emerging class of crystalline porous materials constructed by stitching organic building units together with covalent bonds, featuring advantages such as low density, designable structures, and facilely tailored functionalities.1–3 These unique attributes endow COFs with exceptional potential for a wide range of applications in adsorption,4–6 separation,7,8 catalysis,9–13 sensing,14,15 drug delivery,16–18 energy storage,19–21 and so on. Since the pioneering work reported by Yaghi and co-workers in 2005,1 this field has undergone a rapid expansion over the past decade. The formation of crystalline COFs primarily entails a dynamic “self-healing/error-correction” process facilitated by reversible covalent bonds.22,23 This process typically requires high energy input and prolonged reaction durations to drive the reversible process to form thermodynamically stable crystalline products. For instance, the widely used solvothermal method employed for COF synthesis often spans several days or even months.24,25 However, the lengthy reaction times, complex procedures, and high energy consumption associated with this method hinder the functionalization and industrial application of COFs. Efforts to achieve simple and rapid synthesis approaches have long persisted, with various alternative energy sources, including microwave,26,27 ultrasound,28,29 mechanical agitation,30,31 light irradiation,32 and electron beam,33 have been utilized to substantially shortened reaction times from multiple days to several hours, and even seconds in extreme cases. Moreover, the optimization of the reaction system (such as solvent, catalyst, temperature, etc.) holds promise for expediting COF formation by accelerating the bond formation and enhancing the error correction process during the crystallization process.34–36 However, these endeavors mainly concentrated on specific types of COFs, while the utilization of alternative energy sources frequently entails the procurement of costly equipment and adherence to stringent operational protocols. Hence, it is necessary to develop feasible and practical new methods and new materials to rapidly synthesize COFs.It is well known that the aminal linker is a very promising linkage because of its good reversibility, which is comparable to that of the imine bond.37,38 This property enables facile dynamic structural adjustment and defect repair, making aminal linker the promising candidates for rapid synthesis of highly crystalline COFs. Nevertheless, this field remains relatively underexplored with only five reported instances since the first pioneering work reported by Zhao in 2019.37,39,40 This scarcity and unknown attract our attention and prompt us to explore the properties of aminal-linked COFs. Hence, in this study, we mainly focused on exploring the feasibility of rapid synthesis of aminal-linked COFs.
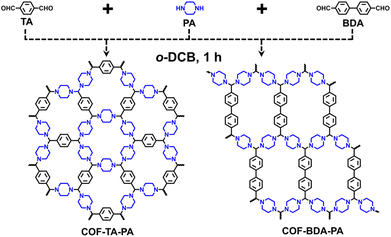
To realize the above idea, we chose readily available and cost-effective terephthalaldehyde (TA, $23 per kg) and piperazine (PA, $18 per kg) as monomers to study the crystallization process of aminal-linked COFs (COF–TA–PA, Scheme 1). We used powder X-ray diffraction (PXRD) patterns and Brunauer–Emmett–Teller (BET) surface areas, which were acquired under uniform data collection parameters, as a benchmark to evaluate the growth of COFs synthesized under different conditions.
Experimental
Materials
All the chemicals commercially available were used directly without further purification. TA, 4,4′-biphenyldicarboxaldehyde (BDA), and PA were purchased from Energy Chemical. Glacial acetic acid, mesitylene (Mes), toluene (Tol), 1,4-dioxane (Dio), and o-dichlorobenzene (o-DCB), dimethylacetamide (DMAc), and n-butanol (n-BuOH) other common solvents were purchased from Energy Chemical and Sigma-Aladdin.Synthetic procedures
Characterization
Powder X-ray diffraction analyses were performed using a Rigaku MiniFlex 600 diffractometer operating at 40 kV voltage and 15 mA current with Cu-Kα X-ray radiation (λ = 0.154056 nm) with a step size of 0.02° in 2θ from 1.5 to 30°. Fourier transform infrared (FT-IR) spectra were collected in the range of 450–4000 cm−1 on Bruker ALPHA spectrometer equipped with an ATR cell. Solid state 13C CP-MAS nuclear magnetic resonance (NMR) spectroscopy was carried out on an Agilent DD2 spectrometer operating at 150.78 MHz for 13C. A double resonance 4 mm T-3 MAS probe was used for all the experiments. The 13C CP/MAS spectra were acquired using a tancpxtoss sequence, with a contact time of 1 ms and a recycle interval of 2 s. The spinning rate was set to 8 kHz. Nitrogen sorption measurements were performed on Quantachrome Instrument ASiQMVH002-5. The isotherms were collected from 0 to 1 atm at 77 K under liquid nitrogen bath. The Brunauer–Emmett–Teller (BET) method was utilized to calculate the specific surface areas. By using the quenched-solid density functional theory (QSDFT) model, the pore size distribution profile was derived from the adsorption data. Field-emission scanning electron microscopy (SEM) images were acquired from a JEOL model JSM-7500F scanning electron microscope. High-resolution transmission electron microscopy (TEM) was performed on an FEI Tecnai G2 F30. X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Scientific K-Alpha+ monochromatic Al K-α source with an electron emission angle of 0 degrees. The analysis region was a circle with a diameter of 400 μm. The work function (ΦSA) of COFs is marked as 4.84 eV,41 so the calculated C 1s peak (EB = 289.58 − ΦSA)42 is 284.74 eV as the internal standard. Thermal gravimetric analyses (TGA) were carried out on the Netzsch STA449F5 analyzer by heating the samples from 25 to 800 °C under nitrogen atmosphere at a heating rate of 10 °C min−1. Elemental analysis was performed on a Thermo FLASH 2000.Static adsorption and breakthrough tests
The static CO2 and CH4 sorption isotherms were conducted on Belsorp Max II at 273, 283, and 298 K. The breakthrough separation experiments were conducted in a home-built breakthrough apparatus under ambient conditions (298 K, 1 atm). The separation of CO2/CH4 was carried out in a fixed bed. Aminal-linked COFs (1.0 g) were packed in a stainless column (6 mm inner diameter, 200 mm length) and purged with He flow (20 mL min−1) for 0.5 hours at 298 K for the activation process. Meanwhile, as a carrier gas, the He was also used to clean the system. The flow rate (2.0 mL min−1 at 298 K and 1 atm) of CO2/CH4 with volume rations of 50/50 was regulated by mass flow controllers. Outlet gas from the sample holder was monitored by using chromatography (GC-9860-5C-NJ). After the breakthrough experiment, the adsorbent was in situ regenerated by flowing He for 30 min at 298 K to finish three cycles of the experimental breakthrough tests.Results and discussion
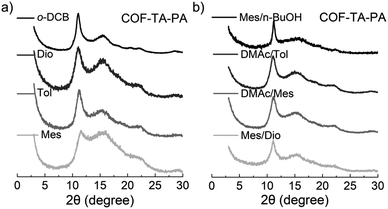
Initially, we examined the influence of solvents on the crystallinity of COF materials. To this end, COF–TA–PA was prepared in eight different solvents without Lewis acid catalysts, and the condensation reaction was carried out in a sealed tube at 120 °C for 3 days. After work-up, the PXRD patterns of the as-obtained products were recorded. As shown in Fig. 1, crystalline COF–TA–PA was obtained not only in monophasic organic solvent systems (e.g., Mes, Tol, Dio, and o-DCB), but also in a range of mixed solvent compositions such as Mes/n-BuOH, Mes/Dio, DMAc/Mes, and DMAc/Tol (Table S1†). Experimental findings indicate exceptional solvent adaptability of COF–TA–PA, with a preference observed for the o-DCB system, which is different from previously reported aminal-linked COFs.40 Further investigations into the effects of temperature indicated that lower temperatures do not foster the self-healing process of the framework. While it is feasible to attain an ordered structure under such conditions, achieving a highly ordered structure proves challenging (Table S2 and Fig. S1†). Examination of the influence of monomer concentration revealed minimal impact on the crystallinity of the material, indicating its potential suitability for scale-up synthesis (Table S3 and Fig. S2†). Moreover, increasing the equivalents of the PA monomer manifests a nuanced yet positive impact on crystallinity, and the presence of excess PA does not restrict the formation process of the material (Table S4 and Fig. S3†).To further elucidate the formation process, a time-dependent transformation experiment was performed. Suspensions containing the monomers PA and TA in o-DCB were subjected to heating at 120 °C for varying durations of 0.5, 1, 2, 6, and 12 hours, respectively. As illustrated in Fig. 2a, PXRD analysis reveals the presence of characteristic peaks corresponding to COF–TA–PA (2θ = 11.1° and 15.6°) within 0.5 hour, indicating the formation of crystalline materials. Upon prolonging the reaction time to 1 hour, a slight enhancement in the intensity of the XRD diffraction peaks was observed, while extending the reaction duration to 12 or even 72 hours did not yield a notable enhancement in the intensity of the primary diffraction peak (Fig. S4†), indicating that the framework had been established within the initial 1 hour period. Their Fourier transform infrared (FT-IR) spectra obtained at different conversion times displayed negligible changes during the 1 hour conversion process (Fig. 2b and Table S5†), thus demonstrating the consistency of their chemical composition. Additionally, nitrogen sorption experiments were conducted to assess their surface areas. As shown in Fig. S5 and S6,† their BET surface areas of the material obtained after a 1 hour reaction were nearly twice as high as those obtained after 0.5 hours. Moreover, there was only a slight increase in the BET surface area with prolonged reaction durations. The aforementioned findings indicate that aminal-linked COF–TA–PA exhibits excellent solvent compatibility, and promising scalability, enabling rapid synthesis within a timeframe as short as 1 hour.
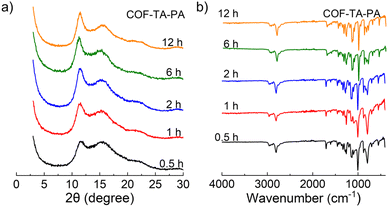 | ||
| Fig. 2 (a) PXRD patterns and (b) FT-IR spectra of COF–TA–PA obtained during different reaction times. | ||
The FT-IR spectra of COF–TA–PA indicate the absence of C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–H (2803 and 2756 cm−1 for TA), C
O)–H (2803 and 2756 cm−1 for TA), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1687 cm−1 for TA), and N–H (3217 cm−1 for PA) vibrations, suggesting a significant degree of polymerization by the near-complete consumption of TA and PA (Fig. S7†). Moreover, the identification of vibration peak attributed to CH–N bonds at 1103 cm−1 provides evidence supporting the formation of aminal-linked COF.43 Furthermore, the absence of a vibration peak corresponding to C
O (1687 cm−1 for TA), and N–H (3217 cm−1 for PA) vibrations, suggesting a significant degree of polymerization by the near-complete consumption of TA and PA (Fig. S7†). Moreover, the identification of vibration peak attributed to CH–N bonds at 1103 cm−1 provides evidence supporting the formation of aminal-linked COF.43 Furthermore, the absence of a vibration peak corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N suggests the absence of imine bonds in the resultant material. The solid-state 13C cross-polarization magic-angle-spinning nuclear magnetic resonance (CP/MAS NMR) spectra of COF–TA–PA provide additional evidence supporting the formation of aminal units, which is indicated by the resonance signals observed at 88 ppm (Fig. S8†). X-ray photoelectron spectroscopy (XPS) analysis further confirmed the structure of COF–TA–PA, which showed a single peak at 398.78 eV (N 1s), corresponding to the aminal C–N bond (Fig. S9†).
N suggests the absence of imine bonds in the resultant material. The solid-state 13C cross-polarization magic-angle-spinning nuclear magnetic resonance (CP/MAS NMR) spectra of COF–TA–PA provide additional evidence supporting the formation of aminal units, which is indicated by the resonance signals observed at 88 ppm (Fig. S8†). X-ray photoelectron spectroscopy (XPS) analysis further confirmed the structure of COF–TA–PA, which showed a single peak at 398.78 eV (N 1s), corresponding to the aminal C–N bond (Fig. S9†).
To elucidate the adaptability of this linkage, an additional aminal-linked COF, denoted as COF–BDA–PA, was synthesized (Scheme 1). First, 4,4′-biphenyldicarboxaldehyde (BDA) and PA underwent reaction within five different solvent systems, thereby demonstrating their adeptness in yielding the target COF structures characterized by significant crystallinity (Table S6 and Fig. S10†). The preferred solvent system is o-DCB, aligning consistently with the synthesis of COF–TA–PA. Additionally, a time-dependent transformation experiment was conducted, and their PXRD analysis showed characteristic peaks corresponding to COF–BDA–PA (2θ = 7.3° and 10.4°) emerging within 0.5 hours indicative of crystalline structure formation (Fig. S11†). With the extension of the reaction period to 1 hour, crystalline COF materials were observed. This observation was substantiated by the FT-IR spectrum (Fig. S12†), consequently affirming the outstanding synthesis efficiency of aminal-linked COFs.
The FT-IR, solid-state 13C CP/MAS NMR, and XPS (Fig. S13–S15 and Table S7†) spectra for COF–BDA–PA also confirmed the formation of an aminal-linked framework, which is similar to COF–TA–PA. The elemental analyses conducted on COF–TA–PA and COF–BDA–PA revealed their C, H, and N contents in proximity with the corresponding theoretical values calculated from anticipated polymerization products (see Experimental section). The thermogravimetric analyses (TGA) demonstrated that COF–TA–PA and COF–BDA–PA exhibited thermally stable up to 255 and 228 °C, respectively (Fig. S16†). Field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses revealed that both COF–TA–PA and COF–BDA–PA manifested nanoparticle aggregate morphology with the size of about 50 nm (Fig. S17 and S18†).
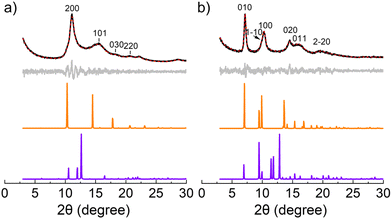
The crystallographic characteristics of the as-prepared aminal-linked COFs were investigated utilizing powder X-ray diffraction (XRD), as illustrated in Fig. 3. The PXRD patterns exhibited discernible peaks at 2θ = 11.1° (200), 15.6° (101), 18.3° (030), and 20.6° (220) for COF–TA–PA, and at 2θ = 7.3° (010), 9.8° (1–10), 10.4° (100), 14.5° (020), 16.0° (011), and 19.6° (2−20) for COF–BDA–PA. Structure modelling and Pawley refinement, conducted using Materials Studio software, facilitated the generation of their plausible structures characterized by 2D AA stacking and AB stacking. A comparative assessment between the experimental PXRD patterns (Fig. 3 black) and simulated ones revealed a closer correspondence of experimental diffraction peaks with simulated patterns featuring AA stacking (Fig. 3 orange), as opposed to those exhibiting AB stacking (Fig. 3 purple). The disparity plots (Fig. 3 gray) suggested that the refined PXRD patterns (Fig. 3 red) aligned well with the experimental ones. Pawley refinements were carried out to determine the unit cell parameters. For COF–TA–PA, the refined parameters were established as follows: a = 17.41 Å, b = 16.23 Å, c = 5.86 Å, α = β = 90°, and γ = 120°, yielding residuals Rp = 2.13% and Rwp = 2.70% (Tables S9 and S11†). Likewise, for COF–BDA–PA, the parameters were determined as a = 9.79 Å, b = 13.96 Å, c = 6.45 Å, α = β = 90°, and γ = 114.6°, resulting in residuals Rp = 3.73% and Rwp = 4.75% (Tables S10 and S12†). These parameters closely align with the corresponding structural models, thereby serving as additional validation for the successful synthesis of aminal-linked COFs utilizing AA stacking, consistent with prior research findings.40
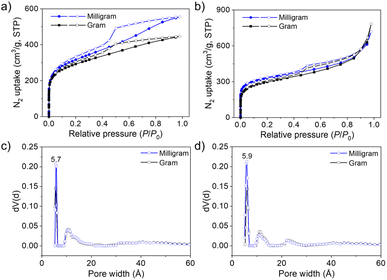
Nitrogen sorption measurements were conducted at 77 K to assess the porosity of aminal-linked COFs. As shown in Fig. 4 (blue line), the sorption profiles of COF–TA–PA and COF–BDA–PA exhibit a type I isotherm with a rapid N2 uptake at the low relative pressure range P/P0 < 0.05, which is characteristic of microporous materials. Their BET surface areas were calculated based on the N2 adsorption data in the range of P/P0 between 0.05 and 0.30 (Fig. S19†). The BET surface areas were calculated to be 1076 and 1088 m2 g−1, while the total pore volumes (at P/P0 = 0.98) were measured to be 0.86 and 1.11 cm3 g−1 for COF–TA–PA and COF–BDA–PA, respectively. The pore size distributions (PSDs), calculated by quenched solid density functional theory (QSDFT), were evaluated to be 0.57 and 0.59 nm for COF–TA–PA and COF–BDA–PA, respectively (Fig. 4c and d). These values are in good agreement with the theoretical frameworks with AA stacking (0.56 nm for COF–TA–PA, 0.69 nm for COF–BDA–PA, as estimated by Zeo++ calculations) (Fig. S20†).44,45 Other minor peak distributions indicate that defects exist in the obtained COFs.
The scale-up synthesis of COFs (see Experimental section) and simultaneous characterization were performed to study the feasibility of scale-up synthesis. Experimental results show that their crystallinity is well preserved (Fig. S21†) and their BET surface area and PSD are only slightly reduced compared to samples prepared at the milligram scale (Fig. 4 black line).
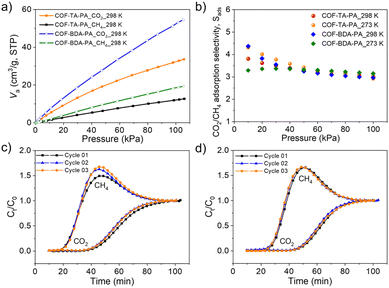
The aminal-linked COFs obtained in this study showcase microporous channels and possess a heightened nitrogen content, thus establishing their prominence as noteworthy candidates for gas adsorption applications. Methane (CH4) is a crucial component of natural gas, serving as a primary energy source for electricity generation, heating systems, and industrial processes.46,47 However, the presence of carbon dioxide (CO2) in natural gas diminishes its calorific efficacy and engenders corrosion within pipelines, thereby augmenting both economic expenditures and safety hazards.48 Purification processes are imperative to ensure that the methane extracted from natural gas is of high quality, maximizing its energy potential. Hence, the separation of CO2/CH4 to improve the purity of CH4 is of significant importance for the development and application of nature technologies. In this study, we conducted an investigation to assess the performance of aminal-linked COFs in the separation of CO2/CH4. The single-component sorption isotherms of CO2 and CH4 were first collected (Fig. S22†). At 298 K, COF–TA–PA shows capacities of 33 and 12 cm3 g−1 for CO2 and CH4 uptake (Fig. 5a), respectively. Conversely, the corresponding values of COF–BDA–PA are 53 and 19 cm3 g−1, respectively. These two aminal-linked COFs did not exhibit excellent CO2 adsorption capacity as expected, possibly attributed to the smaller pore size. The isosteric heat of adsorption (Qst), calculated by the Clausius–Clapeyron equation, suggested that COF–TA–PA had a higher binding affinity for CO2 (29.27 kJ mol−1) than for CH4 (17.47 kJ mol−1), while the binding affinity of CO2 and CH4 for COF–BDA–PA was 18.24 and 6.51 kJ mol−1, respectively (Fig. S23†).
We assessed the CO2/CH4 adsorption selectivity for the CO2/CH4 (v/v = 50/50) mixed gas using the ideal adsorbed solution theory (IAST) by fitting CO2 and CH4 adsorption isotherms at pressures up to 100 kPa at 273 and 298 K (Fig. S24 and Table S8†). The CO2/CH4 selectivity of COF–TA–PA is determined to be 3, which is almost the same as that of COF–BDA–PA (Fig. 5b). Then we performed the experimental breakthrough studies for aminal-linked COFs at room temperature and pressure, in which the CO2/CH4 (v/v = 50/50) gas mixtures flowed over a packed column of the activated COF samples with the flow rate of 2 mL min−1. As shown in Fig. 5c and d, CH4 was eluted from the column first and reached high purity with no CO2 detected. CO2 was preferentially adsorbed by aminal-linked COFs and broke through the column after a substantial delay (16 min for COF–TA–PA and 19 min for COF–BDA–PA). The adsorbents exhibit facile regeneration through purging with helium at 298 K owing to their relatively low Qst values. Recycling tests did not evidence a notable decline in separation efficacy.
Conclusions
In summary, we have deeply studied the characteristics of COFs based on aminal linkages and achieved an expedited synthesis of COFs within a one-hour duration. Two aminal-linked COFs exhibit excellent solvent compatibility and scalability, possess ultra-microporous channels, and have structures with high nitrogen content. Those unique properties highlight the application potential of these materials in gas separation and purification, and experiment results have verified that the material has a certain separation ability for CO2/CH4. Moreover, this study offers a novel pathway for the expeditious synthesis of large-scale, high-quality COF materials, which will facilitate the investigation of practical applications of this unique type of aminal-linked COFs.Data availability
Data for this article, including scheme and figures are available at https://doi.org/10.1039/d4ra02505a. The data supporting this article have been included as part of the ESI.†Author contributions
X. Feng and X. Han conceived and supervised the project. J. Yang and X. Han carried out the experiment. J. Yang, X. Han and X. Feng wrote and revised the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21922502 and 22171022), National Key Research and Development Program of China (2020YFB1506300), Natural Science Foundation of Shandong Province (Grant No. ZR2021QB170), China Postdoctoral Science Foundation (Grant No. 2021M700416), Beijing Municipal Science and Technology Commission No. Z211100002421013, Beijing Institute of Technology Research Fund Program and Analysis and Testing Center of Beijing Institute of Technology.Notes and references
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed
.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC
.
- R. Liu, K. T. Tan, Y. Gong, Y. Chen, Z. Li, S. Xie, T. He, Z. Lu, H. Yang and D. Jiang, Chem. Soc. Rev., 2021, 50, 120–242 RSC
.
- C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed
.
- L. Zhu and Y.-B. Zhang, Molecules, 2017, 22, 1149–1178 CrossRef PubMed
.
- X. H. Han, K. Gong, X. Huang, J. W. Yang, X. Feng, J. Xie and B. Wang, Angew. Chem., Int. Ed., 2022, 61, e202202912 CrossRef CAS PubMed
.
- Z. Wang, S. Zhang, Y. Chen, Z. Zhang and S. Ma, Chem. Soc. Rev., 2020, 49, 708–735 RSC
.
- Z. Kang, Y. Peng, Y. Qian, D. Yuan, M. A. Addicoat, T. Heine, Z. Hu, L. Tee, Z. Guo and D. Zhao, Chem. Mater., 2016, 28, 1277–1285 CrossRef CAS
.
- S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed
.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomacker, A. Thomas and J. Schmidt, J. Am. Chem. Soc., 2018, 140, 1423–1427 CrossRef CAS PubMed
.
- J. Guo and D. Jiang, ACS Cent. Sci., 2020, 6, 869–879 CrossRef CAS PubMed
.
- Y. Yusran, H. Li, X. Guan, Q. Fang and S. Qiu, EnergyChem, 2020, 2, 100035 CrossRef
.
- S. S. A. Shah, M. S. Javed, T. Najam, M. A. Nazir, A. U. Rehman, A. Rauf, M. Sohail, F. Verpoort and S. J. Bao, Mater. Today, 2023, 67, 229–255 CrossRef CAS
.
- X. Liu, D. Huang, C. Lai, G. Zeng, L. Qin, H. Wang, H. Yi, B. Li, S. Liu, M. Zhang, R. Deng, Y. Fu, L. Li, W. Xue and S. Chen, Chem. Soc. Rev., 2019, 48, 5266–5302 RSC
.
- L. Wei, T. Sun, Z. Shi, Z. Xu, W. Wen, S. Jiang, Y. Zhao, Y. Ma and Y.-B. Zhang, Nat. Commun., 2022, 13, 7936 CrossRef CAS PubMed
.
- Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS PubMed
.
- S. Das, T. Sekine, H. Mabuchi, T. Irie, J. Sakai, Y. Zhao, Q. Fang and Y. Negishi, ACS Appl. Mater. Interfaces, 2022, 14, 48045–48051 CrossRef CAS PubMed
.
- M.-X. Wu and Y.-W. Yang, Chin. Chem. Lett., 2017, 28, 1135–1143 CrossRef CAS
.
- C. R. DeBlase, K. E. Silberstein, T. Thanh-Tam, H. D. Abruna and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS PubMed
.
- F. Xu, H. Xu, X. Chen, D. Wu, Y. Wu, H. Liu, C. Gu, R. Fu and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 6814–6818 CrossRef CAS PubMed
.
- J. Li, X. Jing, Q. Li, S. Li, X. Gao, X. Feng and B. Wang, Chem. Soc. Rev., 2020, 49, 3565–3604 RSC
.
- S. J. Lyle, P. J. Waller and O. M. Yaghi, Trends Chem., 2019, 1, 172–184 CrossRef CAS
.
- X. Han, R.-R. Liang, Z.-B. Zhou, Q.-Y. Qi and X. Zhao, Chem. Commun., 2023, 59, 2461–2464 RSC
.
- T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L. H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed
.
- M. S. Lohse and T. Bein, Adv. Funct. Mater., 2018, 28, 1705553 CrossRef
.
- N. L. Campbell, R. Clowes, L. K. Ritchie and A. I. Cooper, Chem. Mater., 2009, 21, 204–206 CrossRef CAS
.
- W. Ji, Y.-S. Guo, H.-M. Xie, X. Wang, X. Jiang and D.-S. Guo, J. Hazard. Mater., 2020, 397, 122793 CrossRef CAS PubMed
.
- S.-T. Yang, J. Kim, H.-Y. Cho, S. Kim and W.-S. Ahn, RSC Adv., 2012, 2, 10179–10181 RSC
.
- J. Yoo, S. Lee, S. Hirata, C. Kim, C. K. Lee, T. Shiraki, N. Nakashima and J. K. Shim, Chem. Lett., 2015, 44, 560–562 CrossRef CAS
.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed
.
- N. Brown, Z. Alsudairy, R. Behera, F. Akram, K. C. Chen, K. Smith-Petty, B. Motley, S. Williams, W. Y. Huang, C. Ingram and X. L. Li, Green Chem., 2023, 25, 6287–6296 RSC
.
- S. Kim, C. Park, M. Lee, I. Song, J. Kim, M. Lee, J. Jung, Y. Kim, H. Lim and H. C. Choi, Adv. Funct. Mater., 2017, 27, 1700925 CrossRef
.
- M. Zhang, J. Chen, S. Zhang, X. Zhou, L. He, M. V. Sheridan, M. Yuan, M. Zhang, L. Chen, X. Dai, F. Ma, J. Wang, J. Hu, G. Wu, X. Kong, R. Zhou, T. E. Albrecht-Schmitt, Z. Chai and S. Wang, J. Am. Chem. Soc., 2020, 142, 9169–9174 CrossRef CAS PubMed
.
- X. Li, C. Yang, B. Sun, S. Cai, Z. Chen, Y. Lv, J. Zhang and Y. Liu, J. Mater. Chem. A, 2020, 8, 16045–16060 RSC
.
- M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder and W. R. Dichtel, J. Am. Chem. Soc., 2017, 139, 4999–5002 CrossRef CAS PubMed
.
- J. Lu, F. Lin, Q. Wen, Q.-Y. Qi, J.-Q. Xu and X. Zhao, New J. Chem., 2019, 43, 6116–6120 RSC
.
- S.-Y. Jiang, S.-X. Gan, X. Zhang, H. Li, Q.-Y. Qi, F.-Z. Cui, J. Lu and X. Zhao, J. Am. Chem. Soc., 2019, 141, 14981–14986 CrossRef CAS PubMed
.
- R. W. Layer, Chem. Rev., 1963, 63, 489–510 CrossRef CAS
.
- Q. Y. Wang, J. Liu, M. Cao, J. H. Hu, R. Pang, S. Wang, M. Asad, Y. L. Wei and S. Q. Zang, Angew. Chem., Int. Ed., 2022, 61, e202207130 CrossRef CAS PubMed
.
- H. Yun, M. Kang, D. W. Kang, H. Kim, J. H. Choe, S. Y. Kim and C. S. Hong, Small, 2023, 19, e2303640 CrossRef PubMed
.
- P. Peng, L. Shi, F. Huo, S. Zhang, C. Mi, Y. Cheng and Z. Xiang, ACS Nano, 2019, 13, 878–884 CrossRef CAS PubMed
.
- G. Greczynski and L. Hultman, Appl. Surf. Sci., 2022, 606, 154855 CrossRef CAS
.
- M. K. Dezfuli and M. R. Saidi, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 89–96 CrossRef CAS
.
- R. L. Martin, B. Smit and M. Haranczyk, J. Chem. Inf. Model., 2012, 52, 308–318 CrossRef CAS PubMed
.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS
.
- T. Yan, Y. Lan, M. Tong and C. Zhong, ACS Sustainable Chem. Eng., 2019, 7, 1220–1227 CrossRef CAS
.
- P. Hu, H. Wang, C. Xiong, H. Liu, J. Han, J. Zhou, Z. Zhao, Y. Wang and H. Ji, ACS Sustain. Chem. Eng., 2021, 9, 15897–15907 CrossRef CAS
.
- H. Tan, Q. Chen, T. Chen, Z. Wei and H. Liu, Chem. Eng. J., 2020, 391, 123521 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02505a |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: