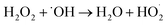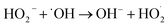Open Access Article
Open Access ArticleThe treatment of high concentration wastewater in the natural gas processing industry
Zi-li Gong†
a,
Wan-jin Hu†a,
Yang Qub,
Ya-lan Yu *a,
Wen-shi Liua and
Zheng Lana
*a,
Wen-shi Liua and
Zheng Lana
aCollege of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu, 610500, PR China. E-mail: yuyalan@swpu.edu.cn
bPetrochina Southwest Oil and Gas Field Company, PR China
First published on 28th June 2024
Abstract
The operation of the Cansolv tail gas treatment device in natural gas plants generates acidic and alkaline wastewater from the venturi unit and amine purification unit (APU), respectively. The APU wastewater is complex in composition and contains hard-to-degrade organic matter, which can adversely impact the normal functioning of the water treatment system. This study assesses the efficacy of three ozone-based advanced oxidation processes (ozone (O3), ozone/hydrogen peroxide (O3/H2O2), and ozone/Fenton (O3/Fenton)) for treating Cansolv wastewater, with chemical oxygen demand (COD) and total organic carbon (TOC) serving as indicators of organic degradation. The findings demonstrate that all three processes effectively eliminate coloration and reducible sulfur, with O3/Fenton exhibiting superior performance in removing organic substances. The treated wastewater has a clarified light-yellow appearance with residual COD levels at 43 mg L−1. Under the optimum Fenton oxidation conditions (initial pH 5, H2O2 dosage 97.8 mmol L−1, FeSO4·7H2O dosage 550 mg L−1), average TOC and COD removal rates reached 50% and 97%, respectively. After a treatment duration of 60 minutes, the wastewater demonstrated an enhanced membrane-specific flux, confirming the effectiveness of the O3/Fenton oxidation process in mitigating membrane fouling while ensuring the stable operation of the wastewater treatment system.
1. Introduction
The Cansolv exhaust gas treatment device has been utilized in various industries, including natural gas processing, petrochemicals, smelting, and coal-fired power plants to selectively remove sulfur dioxide from exhaust gases using organic amine solutions. This technology offers significant technical advantages and environmental benefits.1 However, the pre-wash unit (venturi) and amine purification unit (APU) in the Cansolv device generate large volumes of acidic and alkaline wastewater, respectively. These wastewaters undergo treatment through a flocculation softening – ultrafiltration – ion – exchange – two-stage reverse osmosis process to produce freshwater for reuse and concentrated water for evaporation crystallization. This zero – wastewater discharge system reduces water consumption. Nevertheless, the intake quality of APU wastewater exhibits considerable fluctuations in practical applications due to its complex composition containing numerous recalcitrant organic compounds.2 The treatment of highly alkaline APU wastewater at certain gas purification plants has been explored using conventional electrocatalytic oxidation and biochemical oxidation methods in practical applications. However, the methods were intricate, requiring an additional 2 hours reaction time and pH adjustment within a range of 3–5.The application of advanced oxidation processes (AOPs) in water treatment and reclaimed water reuse has garnered significant attention and implementation in various countries and regions.3–7 Previous studies have investigated the treatment efficacy of the UV-Fenton process within different pH ranges for actual drilling wastewater. Both the acidic UV-Fenton process and the neutral modified UV-Fenton process effectively treated the wastewater, resulting in effluent BOD5/COD ratios of 0.53 and 0.6, respectively.5 In the field of AOPs, especially ozone-based AOPs, integrated with other oxidation techniques, have been extensively employed for the removal of recalcitrant contaminants in diverse water matrices.8–11 Ozone (O3) and its derivative hydroxyl radicals (˙OH) possess remarkable electron affinity and oxidative potential, ranking second only to Fluorine.12–14 The O3 molecule can directly or selectively react with water impurities and undergo decomposition via a chain reaction mechanism, generating ˙OH.15,16 The O3/H2O2 oxidation process, derived from the development of O3 oxidation process, has promising potential in advanced wastewater treatment owing to its non-selectivity and absence of secondary pollution.17 The ˙OH concentration is effectively increased through the addition of H2O2, thereby facilitating the degradation of organic matter in wastewater.18 In previous studies, after O3/H2O oxidation, long-chain unsaturated organic compounds (C > 40, double bond equivalents > 20) were degraded into short-chain aldehydes and low molecular weight fatty acids, and the subsequent Fenton oxidation effectively removed refractory organic matter.3 The O3/Fenton oxidation process is particularly well-suited for the treatment of recalcitrant organic wastewater, as it combines the potent oxidation capabilities of O3 with the highly reactive ˙OH species generated through the Fenton reaction, which exhibits strong electrophilic characteristics.19,20 This synergistic approach enables comprehensive degradation of a wide range of organic compounds present in wastewater. In our previous study, we investigated the effects of O3/H2O2 and O3/Fenton oxidation on the wastewater of APU unit at the organic molecular level, ultimately determining that the O3/Fenton process exhibits superior oxidation efficacy.3
Therefore, this study primarily investigates the impacts of three advanced oxidation processes (AOPs), namely O3 oxidation, O3/H2O2 oxidation, and O3/Fenton oxidation, on the APU wastewater produced by Cansolv device. The focus is mainly on evaluating changes in COD and TOC levels. Specifically, the three oxidation methods were examined to identify the factors influencing the treatment of APU wastewater and determine the optimal process conditions. Key parameters such as initial O3 dosage, H2O2 dosage, FeSO4·7H2O dosage, and pH value were thoroughly examined to achieve this objective.
2. Materials and methods
2.1 Materials
The experimental wastewater came from APU unit of the Cansolv device at a natural gas purification plant in China. The sample was pale yellow and transparent, with a faint pungent smell and a little suspension matter, which might have been due to iron ions and organic matter. The metal content, sulfide, pH, COD, and TOC in wastewater were obtained by different testing methods. 30% H2O2 was guaranteed reagent, and the other reagents such as FeSO4·7H2O, NaOH, concentrated sulfuric acid were the analytical reagents. All agents were purchased from Cologne Chemical Reagent Plant in China. The central laboratory apparatus included an O3 generator (QJ-8006K, Guangzhou Quanju Ozone Technology Co, Ltd), a total organic carbon meter (SHIMADZU TOC-LCP, Japan) et al.2.2 O3 oxidation process
The O3 generator produced ozone by utilizing a pure oxygen stream and introduced it into the liquid bulk through a gas diffuser. The O3 generator maintained a constant concentration of 9 g h−1, and all experiments were conducted under ambient temperature conditions. After a reaction time of 15 minutes, a noticeable improvement in chromaticity was observed, indicating enhanced clarity. O3 treatment durations of 5, 15, 30, 60, and 90 minutes were selected for subsequent analysis of COD and TOC levels.2.3 O3/H2O2 oxidation process
The treatment of a 200 mL wastewater sample with O3 was conducted in a 1 L reactor, where O3 was generated using an O3 generator and continuously introduced into the water at a rate of 9 g h−1. Furthermore, various dosages of H2O2 (1 mL, 2 mL, 3 mL, and 4 mL) were added to the reactor. After 1 h treatment time, any remaining oxidants in the samples were neutralized using sodium thiosulfate. The treated water samples were stored at a temperature of 4 °C for subsequent analysis to evaluate COD and TOC levels as indicators of the reaction oxidation process.2.4 O3/Fenton oxidation process
The O3/Fenton oxidation process was conducted in a 1 L reactor at ambient temperature. Different dosages of H2O2 and FeSO4·7H2O, as well as various pH levels, were employed to determine the optimal conditions for achieving maximum COD and TOC removals. O3 was continuously added into 200 mL wastewater through a gas diffuser, maintaining a constant concentration of 9 g h−1. H2O2 and FeSO4·7H2O were added into the wastewater to investigate the degradation effect on organic matter and determine the most effective dosages. The resulting residue was allowed to settle for 30 minutes, while the treated water was stored at 4 °C for subsequent analysis of water quality parameters, specifically COD and TOC measurements.Gas chromatography-mass spectrometry (GC-MS) was used to study the oxidation of organic matter in wastewater. The surface morphology and chemical composition of the membranes treated with different oxidation wastewater were analyzed by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS).
3. Results and discussion
3.1 Analysis of water quality
The results of water quality tests conducted on the APU wastewater are presented in Table 1. The analysis revealed that the wastewater exhibited an alkaline nature with a high concentration of sodium ions, while other metal ion levels met the standard requirements. Both ammonia nitrogen and chloride levels were found to be within the limits set by the Chinese wastewater discharge standard, which stipulates values below 15 mg L−1 and 1000 mg L−1, respectively. However, sulfide levels and organic content exceeded the prevailing regulations for wastewater effluent in China.| Parameter | Unit | Values |
|---|---|---|
| Na+ | mg L−1 | 344.0 |
| K+ | mg L−1 | 0.0 |
| Ca2+ | mg L−1 | 0.0 |
| Mg2+ | mg L−1 | 0.0 |
| Fe3+ | mg L−1 | 0.0 |
| Ba2+ | mg L−1 | 0.0 |
| pH | — | 11.5 |
| Total alkalinity (CaCO3) | mg L−1 | 2222.2 |
| CO32− | mg L−1 | 10.5 |
| HCO3− | mg L−1 | 25.5 |
| Turbidity | NTU | 21.3 |
| Ammonia nitrogen | mg L−1 | 6.3 |
| Chloride | mg L−1 | 550.2 |
| Sulfide | mg L−1 | 473.8 |
| SO32−, S2O32− | mg L−1 | 1369.4 |
| SO42− | mg L−1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580.1 580.1 |
| COD | mg L−1 | 1838.8 |
| TOC | mg L−1 | 293.6 |
The membrane contamination was primarily attributed to the high organic matter content, which exceeded the design threshold of the Cansolv wastewater treatment unit. Additionally, the elevated sulfur content in the wastewater surpassed standard levels, resulting in a need to remove reduced sulfur along with organic matter and its subsequent conversion into sulfate.
3.2 O3 oxidation process
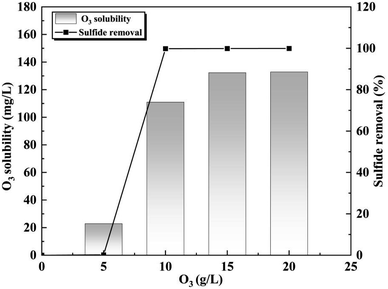
During O3 oxidation process, complex organic matter in APU wastewater is transformed into simpler intermediates, primarily micromolecular esters and phenols. As a result, the pH of the wastewater decreases from 11.52 to 6.78 after undergoing O3 oxidation treatment. The alkaline nature of the wastewater facilitated O3 induction by hydroxide ions (OH−), leading to the generation of hydroxyl radicals (˙OH). These highly reactive radicals react with the organic matter, resulting in the decomposition of macromolecular substances.21,22 Overall, it can be concluded that the application of O3 oxidation process proves to be an advanced and suitable technology for treating APU wastewater.The solubility of O3 in water and the change in sulfide content after a 60 minutes reaction at different O3 concentrations are illustrated in Fig. 1. When the treatment time was 60 minutes and the O3 concentration was 5 g L−1, minimal oxidation effect was observed with sulfide content remaining at 473.1 mg L−1. This can be attributed to the relatively low solubility of O3 in water, approximately 22.9 mg L−1, which is insufficient for effective sulfide deoxidization. However, increasing the O3 concentration from 5 g L−1 to 10 g L−1 resulted in a rapid decrease in sulfide content to less than 1 mg L−1, achieving over 99.9% removal efficiency. At higher O3 concentrations of 15 g L−1 and 20 g L−1, the sulfide content further decreased to only 0.5 mg L−1 and 0.3 mg L−1, respectively. Notably, when exceeding a concentration of 10 g L−1, the amount of dissolved O3 in water increased significantly with saturated solubility reaching about 132.3 mg L−1.
When introduced into the wastewater, O3 partially dissolved and reacted with the organic matter, thereby facilitating the conversion of undissolved O3 into dissolved O3 (Fig. 1). As the reaction progressed, O3 was gradually consumed, leading to its dissolution in water approaching saturation. Notably, it was observed that the equilibrium dissolution of O3 in wastewater exceeded that in deionized water.
The variations in sulfide, sulfate (SO42−), sulfite (SO32−), and thiosulfate (S2O32−) concentrations in the wastewater under experimental conditions of 10 g per L O3 concentration and a treatment time of 60 minutes are presented in Table 2. The initial content of reducible sulfur in the wastewater was found to be similar to the sulfate concentration after treatment. During the O3 oxidation process, all reduced sulfur present in APU wastewater can be completely oxidized to SO42−, without generating any secondary pollutants.
| Index | Unit (mg L−1) | |||||
|---|---|---|---|---|---|---|
| Before O3 oxidation process | After O3 oxidation process | |||||
| Sulfide | SO32− and S2O32− | SO42− | Sulfide | SO32− and S2O32− | SO42− | |
| 473.8 | 1067.4 | 9773.6 | 0.3 | 1.2 | 11303.6 | |
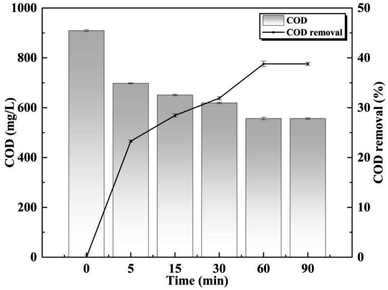
The initial concentration of COD in the wastewater was 909.5 mg L−1, and it gradually decreased with increasing O3 oxidation time. Within the first 5 minutes, there was a significant reduction in COD from 909.4 mg L−1 to 697.8 mg L−1, corresponding to a decrease of 23.3%. Among different treatment durations, the most efficient removal of organic matter occurred during the 60 minutes period. After 60 minutes of treatment, O3 effectively degraded most of the macromolecular organic matter in the wastewater, converting it into micromolecular organic matter. Consequently, the COD level remained stable and decreased to approximately 556.6 mg L−1 (Fig. 2).
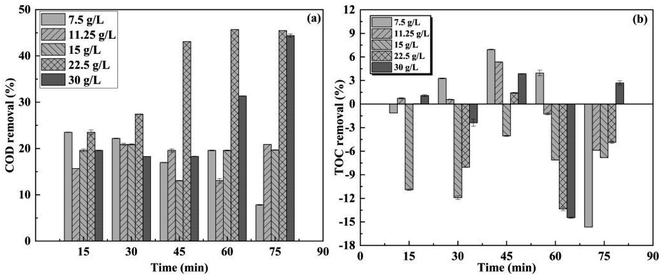
The removals of COD and TOC were investigated at various treatment durations and O3 concentrations. The highest COD elimination was achieved at an O3 concentration of 22.5 g L−1 (Fig. 3a). Within the initial 45 minutes, there was a significant increase in COD removal, reaching a peak removal of 45.7% at 60 minutes. This COD removal process was attributed to the generation of abundant ˙OH by O3 oxidation. However, excessive ˙OH concentration can lead to self-quenching, resulting in a reduced utilization rate of O3. Consequently, when the O3 concentration was increased to 30 g L−1, the COD removal decreased accordingly due to this phenomenon.
The solubility of O3 in wastewater was limited, thus, even with an O3 concentration of 15 g L−1, only approximately 20% COD removal could be achieved. It was found that an appropriate dosage of O3 facilitated the degradation of organic pollutants effectively. During the ozonation process using O3 as an oxidant, macromolecular organic matter present in wastewater underwent transformation into micromolecular organic matter which led to fluctuations in measured TOC levels (Fig. 3b). Although ozonation has the ability to disrupt the structure of the original organic matter and generate intermediates with simpler structures, achieving complete mineralization solely through ozonation remains challenging for most organic matter compounds.3 Therefore, additional oxidation processes need to be integrated alongside O3 oxidation for effective treatment.
3.3 O3/H2O2 oxidation process
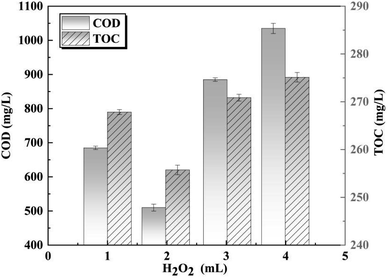
To further enhance the removal of COD and TOC, the impact of different dosages of H2O2 on organic degradation was investigated in the O3/H2O2 oxidation process. Initially, the concentrations of COD and TOC decreased and then increased as the dosage of H2O2 increased (Fig. 4). However, when the dosage exceeded 2 mL, excessive H2O2 and the intermediate product HO2− reacted with ˙OH. The COD decreased to 510.7 mg L−1, achieving a maximum removal efficiency of 60.6% at a dosage of 2 mL H2O2. Furthermore, with a further increase in H2O2 dosage, TOC initially increased but gradually decreased thereafter until reaching its lowest value at a dosage of 255.7 mg L−1.The degradation of organic matter by O3 occurs through both direct and indirect oxidation, while H2O2 primarily acts as a potent oxidant dependent on the ˙OH produced during its decomposition.23 H2O2 exhibits instability under alkaline conditions,23,24 and an optimal H2O2 amount in wastewater enhances the transfer of O3 to water.24,25 This generates a significant quantity of ˙OH that synergistically degrades organic matter with O3, effectively reducing COD and TOC. Nevertheless, excessive H2O2 and its intermediate HO2− react with ˙OH produced by the O3 decomposition, and the main reaction mechanism is as follows.26,27
| H2O2 + H2O → HO2− + H3O+ |
Elevated concentrations of H2O2 and O3 typically result in an enhanced production of ˙OH, but excessive utilization can give rise to detrimental consequences. Hence, it is crucial to assess the optimal H2O2/O3 ratio for attaining maximum degradation of pollutants.
3.4 O3/Fenton oxidation process
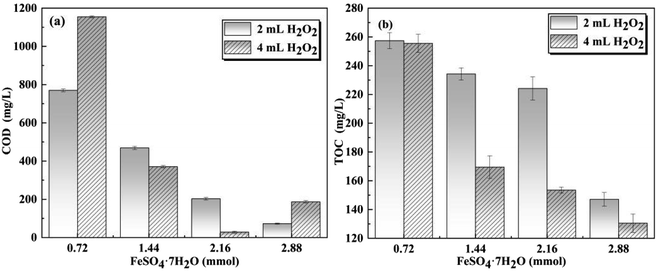
The O3 oxidation process comprises two modes of oxidation, namely direct oxidation under acidic conditions and indirect oxidation under alkaline conditions. These modes generate a significant quantity of ˙OH radicals.27 Considering the highly alkaline nature of the APU wastewater, the primary oxidation mechanism gradually shifted from indirect O3 oxidation to direct ˙OH oxidation. After undergoing the process of O3 oxidation, the water became weakly acidic, thus creating favorable conditions for the occurrence of the Fenton reaction. The Fenton reaction involved the generation of additional ˙OH, and the reaction between Fe2+ and H2O2 produced highly reactive ˙OH, which effectively degraded complex organic matter, including aromatic and heterocyclic unsaturated organic matter.28–30 In the O3/Fenton oxidation process, precise determination of the appropriate dosage is crucial for achieving optimal oxidation and subsequent decolorization. Therefore, it was imperative to investigate the optimum oxidative effect of different dosages of FeSO4·7H2O and H2O2 to determine the ideal agent dosage.The oxidation efficiency of wastewater treated by O3/Fenton oxidation process in the Cansolv device was investigated. As shown in Fig. 5a, an increase in FeSO4·7H2O dosage significantly enhanced COD removal when H2O2 dosage was 2 mL. A COD removal of 95% was achieved with a dosage of 2.88 mmol FeSO4·7H2O dosage, highlighting its crucial role in the organic degradation process within the O3/Fenton system. However, when H2O2 dosage was increased to 4 mL, COD initially increased and then decreased with increasing FeSO4·7H2O content. The highest point of COD removal (98%) was reached at a FeSO4·7H2O content of 2.16 mmol. The decline in COD removal with an addition of 2.88 mmol FeSO4·7H2O can be attributed to excessive ˙OH consumption by Fe2+ ions within the system, leading to impaired oxidation.27
After adding 2 mL and 4 mL of H2O2, the removal of TOC increased with an increase in FeSO4·7H2O content. The optimal TOC removal rates were found to be 49% and 55%, respectively. This suggests that a higher dosage of H2O2 dosage generates more ˙OH, thereby enhancing the organic degradation of organic compounds and improving TOC removal to some extent.31 When the H2O2 dosage was fixed at 4 mL, further increasing the FeSO4·7H2O dosage resulted in a continuous increase in TOC removal without reaching equilibrium. However, excessive FeSO4·7H2O dosage in the O3/Fenton oxidation process led to increased alkali consumption during subsequent decolorization, resulting in significant formation of ferric hydroxide sludge.32,33 Considering COD and TOC removal efficiencies as well as economic factors, it was determined that the optimal dosages for the O3/Fenton oxidation process were 2 mL H2O2 and 2.88 mmol FeSO4·7H2O.
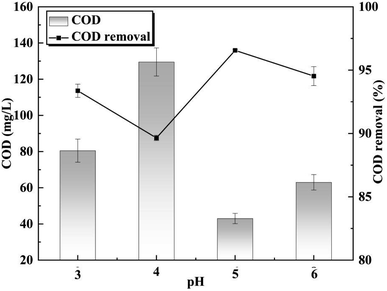
The impact of the O3/Fenton oxidation process on COD removal within the pH range of 3 to 6 was investigated. Fenton exhibited superior oxidation efficacy at a pH of 3, while under alkaline conditions, the O3 oxidation process demonstrated greater effectiveness. Therefore, the optimal performance was achieved when employing the O3/Fenton oxidation process with a value of 5 for pH.
In the O3/Fenton oxidation process, a pH that is too low would hinder the oxidation of O3 in the solution, while a pH that is too high would diminish the effectiveness of Fenton's reagent. Under alkaline conditions, Fe2+ undergoes rapid oxidized to Fe3+, resulting in precipitation of ferric hydroxide.29,33 Additionally, H2O2 acts as a weak acid and easily ionizes under neutral and alkaline conditions. When the wastewater pH exceeds 5, there is a significant reduction in H2O2 concentration, leading to a decrease in ˙OH concentration within the system (Fig. 6). A pH of 5 creates an optimal environment for the O3/Fenton oxidation process, leading to a 97% decrease in COD removal.
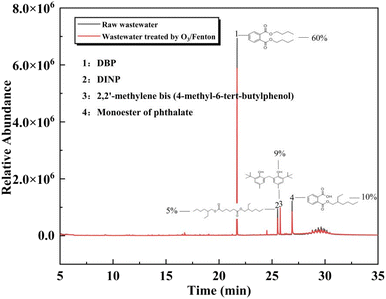
The composition of APU wastewater before and after the O3/Fenton process was compared using GC-MS analysis, as depicted in Fig. 7. The red line represents the organic analysis of the treated APU wastewater. Initially, the wastewater predominantly contained polycyclic aromatic hydrocarbons (PAHs) and long-chain ester organic matter, with dibutyl phthalate (DBP) comprising 60% of the total proportion. After undergoing O3 reaction (electrophilic substitution and cycloaddition), the esters and phenolic organic compounds in the system can be effectively decomposed into more stable intermediates. Subsequently, following Fenton reaction, the intermediates in the wastewater are efficiently mineralized.3,34 Thus, a significant reduction in organic composition within the wastewater was observed after a 1 hour O3/Fenton oxidation process.
In Fig. 8, the wastewater treated by the O3/H2O2 process and O3/Fenton process respectively exhibit high clarity level. The O3/H2O2 process achieved a 61% reduction in COD and a 13% reduction in TOC. The O3/Fenton process exhibited the highest removal efficiency for complex organic matter in APU wastewater, resulting in a significant decrease of 97% in COD and 55% in TOC (Table 3). This process effectively degraded macromolecular organic matter into smaller and simpler structures, and even led to complete mineralization of the organic matter.3
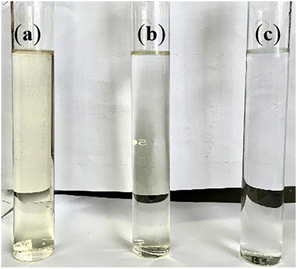 | ||
| Fig. 8 Different chromatic characteristics of raw APU wastewater (a), wastewater treated by O3/H2O2 process (b), wastewater treated by O3/Fenton process (c). | ||
| Parameter (mg L−1) | Different processes | ||
|---|---|---|---|
| O3 | O3/H2O2 | O3/Fenton | |
| Original sulfide | 473.8 | 473.8 | 473.8 |
| Sulfide | 0.32 | 0.25 | 0.2 |
| Sulfide removal (%) | 99.9 | 99.9 | 99.9 |
| Original COD | 1383 | 1383 | 1383 |
| Original TOC | 293.6 | 293.6 | 293.6 |
| COD | 846.4 | 539.4 | 41.5 |
| TOC | 273 | 255 | 132 |
| COD removal (%) | 38.8 | 61 | 97 |
| TOC removal (%) | 7 | 13 | 55 |
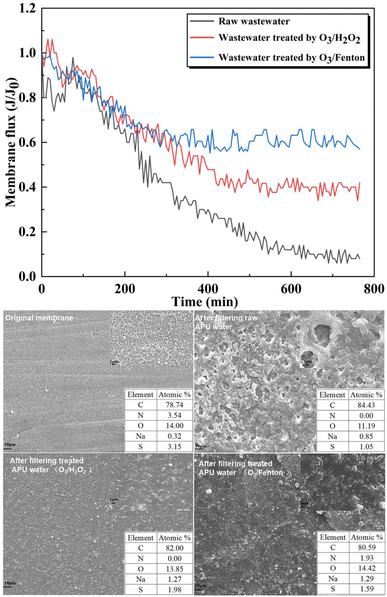
As shown in Fig. 9, membrane flux was assessed using a high-pressure flat membrane apparatus, which compared the effects of different oxidation processes on mitigating membrane fouling. Initially, the membrane flux of various wastewater samples exhibited a gradual decrease within the first 200 minutes, with a relatively consistent decline rate. However, as the filtration time increased, the decline rate in membrane flux became increasingly inconsistent among the different wastewaters. For raw APU wastewater, the membrane flux exhibited a rapid and nearly complete reduction after 600 minutes treatment, indicating the occurrence of severe membrane fouling. The raw APU wastewater caused dense irregularities and micron-sized pores on the surface of the utilized membrane along with a thick layer of contaminants. The O3/H2O2 process resulted in a slight increase in membrane flux by approximately 40%, indicating an improved efficiency of this approach for organic matter treatment. The O3/Fenton oxidation process maintained a consistent state at approximately 60% of the initial membrane flux due to its surface uneven pore structure, which facilitates water flow through reverse osmosis membranes. Furthermore, apparent enhancement in membrane flux was observed during wastewater treatment using O3/Fenton process, suggesting gradual decomposition of organics with enhanced treatment.
From SEM-EDS analysis of membrane surface, the original reverse osmosis membrane is dense and smooth with numerous nanoporous structures, and the C, O, N, S contents of the membrane surface are 78.74%, 14.00%, 3.54% and 3.15%, respectively. After filtering raw APU water, the membrane surface exhibited a multitude of microporous irregular structures within the organic contaminant layer, and the C, O, N, S contents of the membrane surface are 84.43%, 11.19%, 0.00% and 1.05%, respectively. These pollutants persistently accumulated on the membrane during the flushing process, resulting in membrane blockage and reduced lifespan. After O3/H2O2 oxidation process, there was a decrease in C content from 84.43% to 82.00% while an increase in O content from 11.19% to 13.85%, indicating organic degradation on the membrane surface. In the O3/Fenton oxidation process, incorporation of Fenton reagent enhanced the oxidation effect, leading to a reduction in C content from 84.43% to 80.59%, approaching that of the initial membrane at 78.74%. Furthermore, the O and N contents are 14.42% and 1.93%, resulted in an approach towards the initial membrane surface. The decline in C content signifies continuous reduction of organic matter on the membrane surface during Cansolv wastewater treatment. The addition of FeSO4·7H2O in the O3/Fenton oxidation process promotes organic degradation and effectively prevents the formation of an organic pollution layer on reverse osmosis membrane surface.
According to the field test conducted at a natural gas plant, the electrocatalytic oxidation process achieved a remarkable 95% conversion rate of reduced sulfur, while the COD removal rate reached 40%. However, when the wastewater was subjected to biochemical oxidation treatment, it exhibited an unexpected increase in COD levels instead of reduction, indicating poor biodegradability of the APU unit wastewater. In contrast, O3/Fenton oxidation process significantly enhanced the COD removal rate to 97%. These findings unequivocally demonstrate that the O3/Fenton oxidation process represents the most optimal combined approach for treating wastewater originating from APU unit of Cansolv device.
4. Conclusion
This study conducted a comprehensive comparison of the degradation performance of three O3-based oxidation processes (O3 only, O3/H2O2, and O3/Fenton) for APU wastewater treatment. The degradation efficiency towards organic matter exhibited variations among the different O3-based oxidation processes. In the case of the O3/Fenton oxidation process, the addition of Fenton reagent effectively reduced organic matter in wastewater and eliminated the organic layer adhered to the membrane surface. This approach successfully addressed membrane fouling issues during Cansolv wastewater treatment, resulting in a significant reduction in COD (97%) and TOC (55%). The proposed wastewater treatment scheme aligns with circular economy principles and holds potential for pollution reduction, enhancement of industrial environmental performance, improved efficiency, and compliance with ecolabel criteria.Conflicts of interest
There are no known competing financial interests or personal relationships that could affect the work reported in this article.Acknowledgements
The authors gratefully acknowledge the financial support by NSFC (Grant No. 21506175).References
- M. Davoudi, S. Samieirad and H. R. Mottaghi, et al., The main sources of wastewater and sea contamination in the south pars natural gas processing plants: Prevention and recovery, J. Nat. Gas Sci. Eng., 2014, 137–146 CrossRef CAS.
- M. F. V. Dooren, P. Astrup and T. K. Mikkelsen, Influence of Cansolv tail gas treatment unit on natural gas purification plant and discussion on optimization measures, Chem. Eng. Oil Gas, 2022, 138–146 Search PubMed.
- W. J. Hu, Y. Qu and J. Xiong, et al., Wastewater from natural gas Cansolv desulfurization process: Comprehensive characterization and effective removal of organic compounds, Sci. Total Environ., 2024, 911, 168681 CrossRef CAS PubMed.
- Y. Wang, H. Q. Li and L. M. Ren, Organic matter removal from mother liquor of gas field wastewater by electro-Fenton process with the addition of H2O2: effect of initial pH, R. Soc. Open Sci., 2019, 6, 191304 CrossRef CAS PubMed.
- W. Chen, C. Zou and X. Li, et al., The treatment of phenolic contaminants from shale gas drilling wastewater: A comparison with UV-Fenton and modified UV-Fenton processes at neutral pH, RSC Adv., 2016, 90682–90689 RSC.
- Z. G. Liu, W. Zhou and X. L. Liu, et al., Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process, Separations, 2023, 451 CrossRef.
- Z. Liu, K. Demeestere and S. Van Hulle, Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review, J. Environ. Chem. Eng., 2021, 105599 CrossRef CAS.
- C. C. Chang, C. Y. Chiu and C. Y. Chang, et al., Combined photolysis and catalytic ozonation of dimethyl phthalate in a high-gravity rotating packed bed, J. Hazard. Mater., 2009, 287–293 CrossRef CAS PubMed.
- A. C. Mecha and M. N. Chollom, Photocatalytic ozonation of wastewater: a review, Environ. Chem. Lett., 2020, 1491–1507 CrossRef CAS.
- M. Mehrjouei, S. Mueller and D. Moeller, A review on photocatalytic ozonation used for the treatment of water and wastewater, Chem. Eng. J., 2015, 209–219 CrossRef CAS.
- G. H. Zheng, B. T. Xia and X. U. Jing, Comparison of O3, UV/O3, and UV/O3/PS Processes for Marine Oily Wastewater Treatment: Degradation Performance, Toxicity Evaluation, and Flocs Analysis, Water Res., 2022, 119–234 Search PubMed.
- P. Asaithambi, R. Govindarajan and M. B. Yesuf, et al., Enhanced treatment of landfill leachate wastewater using sono-ozone–electrocoagulation process: role of process parameters on color, COD and electrical energy consumption, Process Saf. Environ. Prot., 2020, 212–218 CrossRef CAS.
- W. S. Liu, K. Xiao and J. Li, et al., Efficient removal of organic contaminants in real shale gas flowback water using Fenton oxidation assisted by UV irradiation: Feasibility study and process optimization, Process Saf. Environ. Prot., 2022, 687–697 CrossRef CAS.
- B. Kasprzykhordern, M. Ziolek and J. Nawrock, Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment, Appl. Catal., B, 2003, 639–669 CrossRef CAS.
- M. M. Bello and A. A. A. Raman, et al., A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment, Process Saf. Environ. Prot., 2019, 119–140 CrossRef CAS.
- S. N. Malik, P. C. Ghosh, A. N. Vaidya and A. Asghar, Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review, J. Water Proc. Eng., 2020, 101–193 Search PubMed.
- Z. P. Gu, W. M. Chen and F. Wang, et al., Transformation and degradation of recalcitrant organic matter in membrane bioreactor leachate effluent by the O3/H2O2 process, Environ. Sci.: Water Res. Technol., 2019, 1748–1757 RSC.
- C. L. Jung, Y. Deng and R. Z. Zhao, et al., Chemical oxidation for mitigation of UV-quenching substances (UVQS) from municipal landfill leachate: Fenton process versus ozonation, Water Res., 2017, 260–270 CrossRef CAS PubMed.
- E. C. Catalkaya and F. Kargi, Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: a comparative study, J. Hazard. Mater., 2007, 244–253 CrossRef CAS PubMed.
- W. Sun, Z. L. Lu and Z. Y. Zhang, et al., Ozone and Fenton oxidation affected the bacterial community and opportunistic pathogens in biofilms and effluents from GAC, Water Res., 2022, 118–495 Search PubMed.
- E. Rezae, B. Jafari and M. Abbasi, et al., Hybridized microfiltration-Fenton system for the treatment of greywater, J. Environ. Chem. Eng., 2023, 109–725 Search PubMed.
- T. Lu, Y. Chen and M. Liu, et al., Efficient degradation of evaporative condensing liquid of shale gas wastewater using O3/UV process, Process Saf. Environ. Prot., 2019, 175–183 CrossRef CAS.
- V. Fontanier, V. Farines and J. Albet, et al., Study of catalyzed ozonation for advanced treatment of pulp and paper mill effluents, Water Res., 2006, 303–310 CrossRef CAS PubMed.
- A. Vinayak and G. B. Singh, Biodecolorization of reactive black 5 using magnetite nanoparticles coated Bacillus sp. RA5, Mater. Today: Proc., 2022, 1523–1526 CAS.
- E. M. Aieta, K. M. Reagan and J. S. Lang, et al., Advanced Oxidation Processes for Treating Groundwater Contaminated With TCE and PCE: Pilot-Scale Evaluations, J.–Am. Water Works Assoc., 1988, 64–72 CrossRef CAS.
- I. A. Balcioglu and M. Oetker, Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes, Chemosphere, 2002, 85–95 Search PubMed.
- J. J. Pignatello, E. Oliveros and A. Mac Kay, Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry, Crit. Rev. Environ. Sci. Technol., 2006, 1–84 CrossRef CAS.
- B. Enric, M. Eva and S. Roser, et al, Aniline mineralization by AOPs: anodic oxidation, photocatalysis, electro-Fenton and photoelectro-Fenton processes, Appl. Catal., B, 1998, 31–42 Search PubMed.
- P. V. Nidheesh, Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review, RSC Adv., 2015, 40552–40577 RSC.
- J. Yang, H. Zhang and Z. Zhang, et al., Degradation of 2, 4-dinitrophenol in aqueous solution by microscale Fe0/H2O2/O3 process, Environ. Eng. Sci., 2019, 207–218 CrossRef.
- N. Hassanshahi and A. Karimi-Jashni, Comparison of photo-Fenton, O3/H2O2/UV and photocatalytic processes for the treatment of gray water, Ecotoxicol. Environ. Saf., 2018, 683–690 CrossRef CAS PubMed.
- F. AI Momani, D. W. Smith and M. G. El-Din, Degradation of cyanobacteria toxin by advanced oxidation processes, J. Hazard. Mater., 2008, 238–249 CrossRef PubMed.
- J. H. Ma, W. J. Song and C. C. Chen, et al., Fenton Degradation of Organic Compounds Promoted by Dyes under Visible Irradiation, Environ. Sci. Technol., 2005, 5810–5815 CrossRef CAS PubMed.
- F. Wang, Y. Huang and P. Wen, et al., Transformation mechanisms of refractory organic matter in mature landfill leachate treated using an Fe-participated O3/H2O2 process, Chemosphere, 2021, 128198 CrossRef CAS PubMed.
Footnote |
| † Co-first author. |
| This journal is © The Royal Society of Chemistry 2024 |