Open Access Article
Open Access ArticleAn efficient and practical approach for the synthesis of indoloquinolines and indolo/pyrroloquinoxalines via a Cu-catalyzed Ugi-C/Ugi-N-arylation sequence†
Sheiva Iranfara,
Morteza Shiri *a,
Yazdanbakhsh Lotfi Nosoodb,
Zahra Akbari Keleya,
Zahra Tanbakouchiana,
Zahra Aminia,
Ahmed Al-Harrasi*b and
Faiq H. S. Hussain
*a,
Yazdanbakhsh Lotfi Nosoodb,
Zahra Akbari Keleya,
Zahra Tanbakouchiana,
Zahra Aminia,
Ahmed Al-Harrasi*b and
Faiq H. S. Hussain *c
*c
aDepartment of Organic Chemistry, Faculty of Chemistry, Alzahra University, Vanak, Tehran, 1993893973, Iran. E-mail: mshiri@alzahra.ac.ir
bNatural and Medical Sciences Research Center, University of Nizwa, P. O. Box 33, Birkat Al Mauz, Postal Code 616, Nizwa, Oman. E-mail: aharrasi@unizwa.edu.om
cMedical Analysis Department, Applied Science Faculty, Tishk International University, Erbil, Kurdistan Region, Iraq. E-mail: faiq.hussain@tiu.edu.iq
First published on 7th June 2024
Abstract
A Cu-catalyzed tandem transformation of Ugi adducts through CH/NH bond functionalization reactions was reported for synthesizing a broad spectrum of indolo/pyrrolo-[1,2-a]quinoxaline-6/4-carboxamide, 7H-indolo[2,3-c]quinoline-6-carboxamide, and 1-(cyclohexylamino)-14H-indolo[2,3-c][1,4]oxazino[4,3-a]quinolin-4(3H)-one derivatives in moderate to excellent yields. In this protocol the Ugi condensation of aromatic aldehydes, anilines, acids, and isocyanides leads to the formation of bis-amides in methanol at room temperature. This approach employed simple reaction conditions, including Ugi product as starting material, CuI, L-proline as a ligand, and cesium carbonate, in DMSO for 8 h. This method demonstrated efficiency in synthesizing fused-nitrogen-containing heterocycles through a convenient pathway.
Introduction
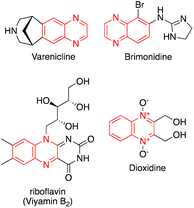
Organic compounds are usually synthesized through several reaction steps. During this process, it is necessary to separate and purify the intermediates before they can be used in the following reaction. However, the overall yield of the final product decreases significantly with each subsequent step of the iterative synthesis.1 Powerful transformations and multicomponent reactions have been effectively utilized for the synthesis of various synthetic intermediates, natural products, and bioactive agents.2–4 Multicomponent reaction techniques enable the rapid and efficient synthesis of complex and diverse molecules, resulting in reduced costs, waste, and time, thus promoting green chemistry. These techniques are now widely utilized to prepare many high-yield compounds.5–7 Multicomponent reactions based on isocyanides are gaining increasing interest due to the availability of various starting materials and the abundance of transformations that can be performed.2 The high efficiency of isocyanides in multicomponent reactions is due to their ability to form reversible bonds by extending their valence.8 In the last decades, the discovery and development of Passerini and Ugi reaction variations have led to immense growth in Isocyanides-based multicomponent reactions.2 The Ugi four-component reaction has been extensively studied for the production of multifunctional adducts due to its wide scope, high variability, and mild reaction conditions.9 N-Heterocycles are one of the most important scaffolds in the structure of natural products, pharmaceuticals, dyes and biologically active molecules.10,11 For instance, many natural and biologically active compounds contain the indole and pyrrole ring.12–14 Quinoline alkaloids, which are typically found in plants of the Haplophyllum A. Juss, exhibit a wide range of biological properties.15,16 Additionally, Quinoxaline derivatives have various pharmacological profiles.17Quinoxalines are found in marketed drugs with antibacterial, antiviral, antibiotic, antitumor, antimicrobial, antifungal and anti-inflammatory activities (Fig. 1).18
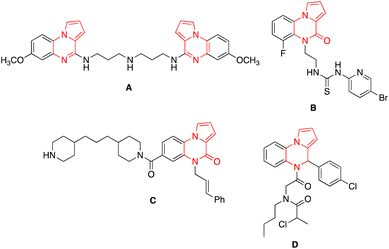
Pyrrole/indole-fused heterocycles are abundant in numerous natural products, as well as medicinal chemistry for lead compounds and drug prospects.19 Amidst the many compounds, pyrrole/indole-fused quinoxaline derivatives have long been of great interest due to their remarkable biological activities and intriguing structures.19 Among them, indolo[1,2-a]quinoxaline analogues have shown promising anti-fungal properties17 and pyrrolo[1,2-a]quinoxaline subunits are present in various biologically and medicinally useful molecules. Several of its derivatives are antimalarial agents A, anti-HIV agents B, anticancer agents C, and antagonist agents D (Fig. 2).20
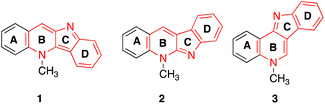
Indolo- and pyrroloquinoline alkaloids can be ascertained among multiple natural products and exhibit a wide range of biological activities.21–23 Indoloquinolines are found in the roots of Cryptolepis sanguinolenta, the west African plant.24 Cryptolepine (1), neocryptolepine (2) and isocryptolepine (3) are three of thirteen identified alkaloids of the root (Fig. 3).25
Pyrroloquinolines, as another class of important N-heterocycles, were discovered in marine natural products.21 Tryptophan has been suggested for many years to yield the pyrroloquinoline alkaloid.26
In the past years, different methods have been reported for the synthesis of indolo[2,3-c]quinoline. These methods include: the chemoselective Suzuki reaction, the Pd-catalyzed intramolecular arylation under microwave irradiation and the third method of including two steps that the first step involved a Cu-catalyzed coupling reaction and the second utilized a Pd-catalyzed intramolecular arylation reaction.21,25,27
Among the various synthetic approaches for the preparation of indolo[1,2-a]quinoxaline, the following methods can be mentioned: the Pictet–Spengler approach using Brønsted acid-catalyzed, the Pd-catalyzed regioselective C–H olefination/cyclization sequence, the transition metal-free process, the Pd-catalyzed intramolecular C–N bond formation, the modified Pictet–Spengler reaction using Lewis acid-catalyzed, the Domino approach involving spirocyclic ring opening, the Pt(IV)-catalyzed hydroamination triggered cyclization, the tandem one-pot reductive cyclization-oxidation, and oxidative reaction.17–20,28–32 A series of 7H-indolo[2,3-c]quinolines was synthesized by Langer et al. by the chemoselective Suzuki reaction followed by a ring-closing two-fold Buchwald–Hartwig reaction of 3-bromo-4-iodoquinoline (Scheme 1a).21 A series of indolo- and pyrrolo[1,2-a]quinoxalines were synthesized by Jayaprakash et al. from the corresponding 2-(1H-indol/pyrrol-1-yl)anilines promoted by molecular iodine (Scheme 1b).33
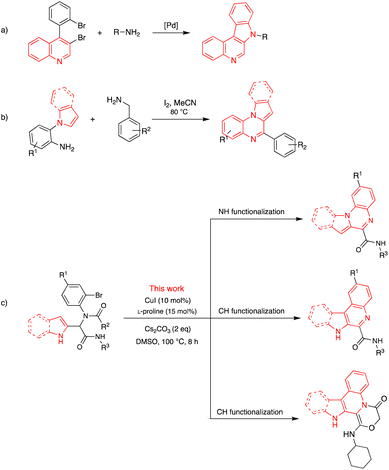 | ||
| Scheme 1 Previous works for the synthesis of 7H-indolo[2,3-c]quinolines (a), indolo- and pyrrolo[1,2-a]quinoxalines (b) and present work (c). | ||
In our ongoing investigation, three different products were synthesized, including indolo/pyrrolo-[1,2-a]quinoxaline-6/4-carboxamides, 7H-indolo[2,3-c]quinoline-6-carboxamides, and 1-(cyclohexylamino)-14H-indolo[2,3-c][1,4]oxazino[4,3-a]quinolin-4(3H)-one, all with a high yield (Scheme 1c).
Results and discussion
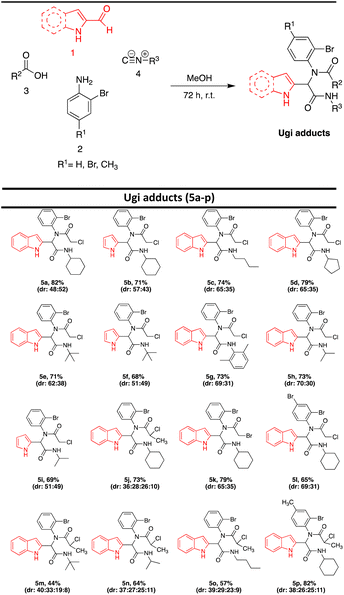
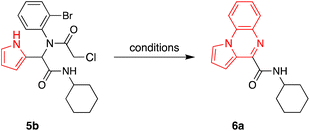
In continuation of our research interest on multicomponent reactions and indole/pyrrol chemistry,5,12,34–45 we focused on the preparation of three different products including indolo/pyrrolo[1,2-a]quinoxaline, 7H-indolo[2,3-c]quinoline, and 14H-indolo[2,3-c][1,4]oxazino[4,3-a]quinolin-4-one, using the post-transformation of Ugi adducts under a copper catalyst. In the first step, the Ugi condensation of aromatic aldehydes, anilines, acids, and isocyanides leads to the formation of bis-amides in methanol at room temperature (Table 1).For the post-transformation of Ugi adducts, the starting material 5b (2-formyl indole, 2-bromoaniline, 2-chloropropanoic acid, and cyclohexyl isocyanide) was reacted with CuI as a catalyst in the presence of L-proline as ligand, Cs2CO3 as base, and DMSO as solvent. The desired product 6a was obtained in 79% yield (entry 1, Table 2).
| Entry | Catalyst | Ligand | Base | Solvent | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| a Conditions: 5b (0.5 mmol), catalyst (10 mol%), ligand (15 mol%), base (2 equiv.), solvent (3 mL).b Isolated yields. | |||||||
| 1 | CuI | L-proline | Cs2CO3 | DMSO | 100 | 8 | 79 |
| 2 | CuCl | L-proline | Cs2CO3 | DMSO | 100 | 24 | 40 |
| 3 | CuSO4 | L-proline | Cs2CO3 | DMSO | 100 | 24 | 53 |
| 4 | CuCl2 | L-proline | Cs2CO3 | DMSO | 100 | 24 | 27 |
| 5 | CuI | L-proline | K2CO3 | DMSO | 100 | 8 | 70 |
| 6 | CuI | L-proline | KOt-Bu | DMSO | 100 | 8 | 75 |
| 7 | CuI | L-proline | KOH | DMSO | 100 | 8 | 67 |
| 8 | CuI | L-proline | Et3N | DMSO | 100 | 8 | — |
| 9 | CuI | Phen | Cs2CO3 | DMSO | 100 | 8 | 22 |
| 10 | CuI | TMEDA | Cs2CO3 | DMSO | 100 | 8 | 72 |
| 11 | CuI | Ph3P | Cs2CO3 | DMSO | 100 | 8 | 64 |
| 12 | CuI | L-proline | Cs2CO3 | Dioxane | 100 | 8 | — |
| 13 | CuI | L-proline | Cs2CO3 | THF | Reflux | 8 | — |
| 14 | CuI | L-proline | Cs2CO3 | CH3CN | Reflux | 8 | — |
| 15 | CuI | L-proline | Cs2CO3 | CH2Cl2 | Reflux | 8 | — |
| 16 | CuI | L-proline | Cs2CO3 | DMSO | Rt | 8 | 12 |
| 17 | CuI | — | Cs2CO3 | DMSO | 100 | 8 | 74 |
| 18 | CuI | L-proline | — | DMSO | 100 | 8 | — |
| 19 | — | — | Cs2CO3 | DMSO | 100 | 24 | 21 |
| 20 | Pd(OAc)2 | Ph3P | Cs2CO3 | DMSO | 100 | 8 | 68 |
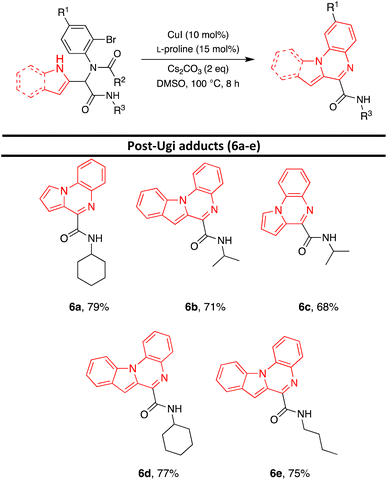
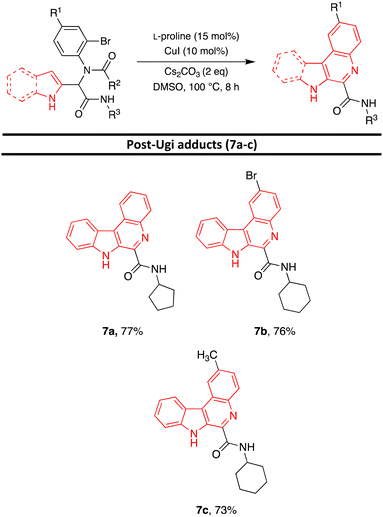
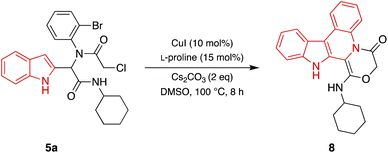
After the identification of compound 6a, the optimization of the reaction conditions including catalyst, base, ligand, solvent, and temperature were investigated. First, various catalysts such as CuI, CuCl, CuSO4, and CuCl2 were investigated and CuI was identified as the best catalyst (entries 1–4, Table 2). Then, various bases such as Cs2CO3, K2CO3, KOt-Bu, KOH, and Et3N were investigated. Cs2CO3 was found to be the best catalyst (entries 1, 5–8, Table 2). Various ligands such as L-proline, 1,10-phenanthroline, TMEDA, and Ph3P were applied for this transformation and the results showed that the best yield was obtained when L-proline was utilized (entries 1, 9–11, Table 2). Running the reaction in the different solvents such as dioxane, THF, CH3CN, CH2Cl2, and DMSO revealed that the best yield was obtained using DMSO as solvent (entries 1, 12–15, Table 2). The impact of temperature on the reaction was also studied and 100 °C was selected as the optimum temperature (entries 1, 15–16, Table 2). Additionally, running the reaction without a ligand or ligand and catalyst, led to a decrease in the reaction efficiency (entries 17,19, Table 2). No product was obtained when the reaction was performed without base (entry 18, Table 2). Furthermore, after changing the catalyst and ligand to Pd(OAc)2 and Ph3P, the desired product was obtained with a lower yield (Entry 20, Table 2). According to the obtained results, the highest yield was detected with CuI as a catalyst, L-proline as a ligand, and Cs2CO3 as a base in DMSO as solvent at 100 °C for 8 hours (entry 1, Table 2). Under optimized reaction conditions, various Ugi adducts were applied to explore the scope of the reaction. Unexpectedly, we procured three different types of products. Post-Ugi adducts 6a–ewere obtained using Ugi adducts 5b, 5h, 5i, 5j, and 5o under optimized reaction conditions (Table 3). On the other side, when Ugi adducts 5d, 5l, and 5p were reacted under mentioned optimized reaction conditions, post-Ugi adducts 7a–cwere formed (Table 4) while using Ugi adduct 5a, final product 8 in 81% yield was generated (Scheme 2). The best results were obtained using less bulky groups on the isocyanide part (8, 6a, 7a) and electron-donating groups on the acid part (6d). Ugi products with bulky isocyanide-like isopropyl showed less reaction efficiency (6c and 6b). A satisfactory result was obtained using electron-withdrawing groups on the amine part (7b).
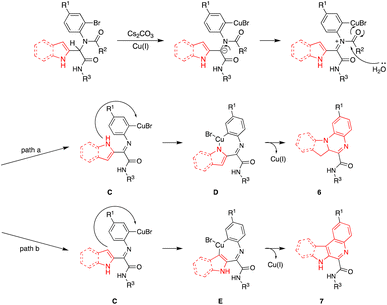
A plausible mechanism for the synthesis of compounds 6 and 7 is presented in Scheme 3. First, by adding Cs2CO3 as base and CuI as catalyzed to compound 5, metal complex A is formed, and that this unstable carbanion forms Intermediate B. In the next step, nucleophilic attacks of H2O in DMSO solvent to carbon of the carbonyl group, and the elimination of carboxylic acid group generates intermediate C. Then the Ullman reaction on intermediate C through path a, leads to C–N bond formation in a new Cu-complex D, which undergoes a reductive elimination to generate the product 6.46 In path b C–C bond formation on intermediate C forms the Cu-complex E, which undergoes reductive elimination leads to the formation of product 7.47
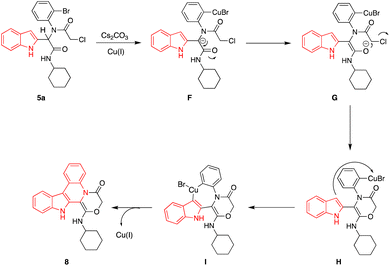
A possible mechanism for the synthesis of compound 8 is presented in Scheme 4. First, the adding of Cs2CO3 and Cu(I) to compound 5a, leads to the formation of metal complex F. The unstable carbanion generates intermediate G via keto–enol tautomerization. Intermediate G forms intermediate H under intramolecular nucleophilic attack by oxygen via cyclization and losing Cl. Nucleophilic attack by carbon atom of the indole part in intermediate H produces a new Cu-complex I. Finally, the corresponding product 8 was released from I through reductive elimination.47
Conclusions
In summary, we presented a copper-catalyzed tandem approach for preparation of three different products, including indolo/pyrrolo-[1,2-a]quinoxaline-6/4-carboxamides, 7H-indolo[2,3-c]quinoline-6-carboxamides and 1-(cyclohexylamino)-14H-indolo[2,3-c][1,4]oxazino[4,3-a]quinolin-4(3H)-one scaffolds found in some biologically active compounds. In this project, Ugi products were synthesized through the Ugi 4CR reaction using aldehydes, amines, isocyanides and acids in methanol as the solvent at room temperature. The Ugi adducts as starting materials were then used in the next step to diversify various classes of fused-nitrogen-containing heterocycles, in a one-pot/multi-steps effective protocol. The identification of the generated compounds was conducted using analytical techniques including FT-IR, 1H-NMR, 13C-NMR and HRMS.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We are grateful for financial support provided by Alzahra University, the Iran National Science Foundation (INSF).References
- I. Ugi, A. Demharter, W. Horl and T. Sehmid, Tetrahedron, 1996, 52, 11657–11664 CrossRef CAS.
- M. Nayak and S. Batra, Tetrahedron Lett., 2010, 51, 510–516 CrossRef CAS.
- D. Singh, S. Sharma, R. K. Thakur, Vaishali, S. Nain, Jyoti, C. C. Malakar and V. Singh, Tetrahedron, 2024, 152, 133809 CrossRef CAS.
- S. Sharma, A. K. Paul and V. Singh, New J. Chem., 2020, 44, 684–694 RSC.
- M. Shiri and Z. Bozorgpour-Savadjani, J. Iran. Chem. Soc., 2015, 12, 389–396 CrossRef CAS.
- S. Sharma, C. C. Malakar and V. Singh, Asian J. Org. Chem., 2020, 9, 1857–1868 CrossRef CAS.
- S. Sharma, D. Singh, S. Kumar, Vaishali, R. Jamra, N. Banyal, Deepika, C. C. Malakar and V. Singh, Beilstein J. Org. Chem., 2023, 19, 231–244 CrossRef CAS PubMed.
- L. El Kaïm, L. Grimaud and J. Oble, Angew. Chem., 2005, 117, 8175–8178 CrossRef.
- U. K. Sharma, N. Sharma, D. D. Vachhani and E. V. Van der Eycken, Chem. Soc. Rev., 2015, 44, 1836–1860 RSC.
- D. Yang, B. An, W. Wei, L. Tian, B. Huang and H. Wang, ACS Comb. Sci., 2015, 17, 113–119 CrossRef CAS PubMed.
- J. Akhtar, A. A. Khan, Z. Ali, R. Haider and M. Shahar Yar, Eur. J. Med. Chem., 2017, 125, 143–189 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, A. Khazaei, T. Faal-Rastegar and H. G. Kruger, J. Iran. Chem. Soc., 2014, 11, 85–90 CrossRef CAS.
- G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875–2911 CrossRef CAS PubMed.
- M. Safarov, Int. J. Early Child. Spec. Educ., 2022, 14, 304–311 Search PubMed.
- V. I. Akhmedzhanova, K. A. Rasulova, I. A. Bessonova, A. S. Shashkov, N. D. Abdullaev and L. Angenot, Chem. Nat. Compd., 2005, 41, 60–64 CrossRef CAS.
- M. B. Yadav, S. G. Balwe, J. T. Kim, B. G. Cho and Y. T. Jeong, Synth. Commun., 2020, 50, 1456–1467 CrossRef CAS.
- A. Preetam and M. Nath, RSC Adv., 2015, 5, 21843–21853 RSC.
- S. A. Trujillo, D. Peña-Solórzano, O. R. Bejarano and C. Ochoa-Puentes, RSC Adv., 2020, 10, 40552–40561 RSC.
- L. Wang, W. Guo, X.-X. Zhang, X.-D. Xia and W.-J. Xiao, Org. Lett., 2012, 14, 740–743 CrossRef CAS PubMed.
- A. Huang, F. Liu, C. Zhan, Y. Liu and C. Ma, Org. Biomol. Chem., 2011, 9, 7351–7357 RSC.
- R. P. Rivera, P. Ehlers, E. T. Rodríguez and P. Langer, ChemistrySelect, 2018, 3, 11177–11179 CrossRef CAS.
- J. Lavrado, R. Moreira and A. Paulo, Curr. Med. Chem., 2010, 17, 2348–2370 CrossRef CAS PubMed.
- L. Shi and B. Wang, Org. Lett., 2016, 18, 2820–2823 CrossRef CAS PubMed.
- P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Curr. Org. Chem., 2011, 15, 1036–1057 CrossRef CAS.
- S. Hostyn, B. U. W. Maes, G. Van Baelen, A. Gulevskaya, C. Meyers and K. Smits, Tetrahedron, 2006, 62, 4676–4684 CrossRef CAS.
- A. Miyanaga, J. E. Janso, L. McDonald, M. He, H. Liu, L. Barbieri, A. S. Eustáquio, E. N. Fielding, G. T. Carter, P. R. Jensen, X. Feng, M. Leighton, F. E. Koehn and B. S. Moore, J. Am. Chem. Soc., 2011, 133, 13311–13313 CrossRef CAS PubMed.
- J. R. Etukala, E. V. K. S. Kumar and S. Y. Ablordeppey, J. Heterocycl. Chem., 2008, 45, 507–512 CrossRef CAS.
- G. Abbiati, E. M. Beccalli, G. Broggini, G. Paladino and E. Rossi, Synthesis, 2005, 17, 2881–2886 Search PubMed.
- A. K. Verma, R. R. Jha, V. K. Sankar, T. Aggarwal, R. P. Singh and R. Chandra, Eur. J. Org Chem., 2011, 2011, 6998–7010 CrossRef CAS.
- S. Mandal and A. Pramanik, J. Org. Chem., 2021, 86, 5047–5064 CrossRef CAS PubMed.
- N. T. Patil, R. D. Kavthe, V. S. Shinde and B. Sridhar, J. Org. Chem., 2010, 75, 3371–3380 CrossRef CAS PubMed.
- C. Wang, Y. Li, R. Guo, J. Tian, C. Tao, B. Cheng, H. Wang, J. Zhang and H. Zhai, Asian J. Org. Chem., 2015, 4, 866–869 CrossRef CAS.
- M. Ramamohan, R. Sridhar, K. Raghavendrarao, N. Paradesi, K. Chandrasekhar and S. Jayaprakash, Synlett, 2015, 26, 1096–1100 CrossRef CAS.
- M. Tajik, M. Shiri, F. H. Hussain, Y. Lotfi Nosood, B. Baeiszadeh, Z. Amini, R. Bikas and A. Pyra, RSC Adv., 2023, 13, 16963–16969 RSC.
- M. Shiri, Z. Gholami-Koupaei, F. Bandehali-Naeini, M.-S. Tonekaboni, S. Soheil-Moghaddam, D. Ebrahimi, S. Karami and B. Notash, Synthesis, 2020, 52, 3243–3252 CrossRef CAS.
- V. Zadsirjan, M. Shiri, M. M. Heravi, T. Hosseinnejad, S. A. Shintre and N. A. Koorbanally, Res. Chem. Intermed., 2017, 43, 2119–2142 CrossRef CAS.
- B. Soleymanifard, M. M. Heravi, M. Shiri, M. A. Zolfigol, M. Rafiee, H. G. Kruger, T. Naicker and F. Rasekhmanesh, Tetrahedron Lett., 2012, 53, 3546–3549 CrossRef CAS.
- M. Shiri, R. Pourabed, V. Zadsirjan and E. Sodagar, Tetrahedron Lett., 2016, 57, 5435–5438 CrossRef CAS.
- M. Shiri, S. Z. Mirpour-Marzoni, Z. Bozorgpour-Savadjani, B. Soleymanifard and H. G. Kruger, Monatsh. Chem., 2014, 145, 1947–1952 CrossRef CAS.
- M. Shiri, M. Heydari and V. Zadsirjan, Tetrahedron, 2017, 73, 2116–2122 CrossRef CAS.
- M. Shiri, M. M. Heravi, V. Zadsirjan, M. Ghiasi, S. A. Shintre, N. A. Koorbanally and T. Singh, J. Iran. Chem. Soc., 2019, 16, 1517–1526 CrossRef CAS.
- M. Shiri, B. Farajpour, Z. Bozorgpour-Savadjani, S. A. Shintre, N. A. Koorbanally, H. G. Kruger and B. Notash, Tetrahedron, 2015, 71, 5531–5537 CrossRef CAS.
- M. Shiri, Z. Faghihi, H. A. Oskouei, M. M. Heravi, S. Fazelzadeh and B. Notash, RSC Adv., 2016, 6, 92235–92240 RSC.
- M. Shiri, Chem. Rev., 2012, 112, 3508–3549 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed.
- S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed.
- V. Tyagi, S. Khan and P. M. S. Chauhan, Tetrahedron Lett., 2013, 54, 1279–1284 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03248a |
| This journal is © The Royal Society of Chemistry 2024 |