Open Access Article
Open Access Articleβ-Enaminonitrile in the synthesis of tetrahydrobenzo[b]thiophene candidates with DFT simulation, in vitro antiproliferative assessment, molecular docking, and modeling pharmacokinetics†
Amna S. Elgubbi a,
Eman A. E. El-Helw
a,
Eman A. E. El-Helw b,
Motaleb S. Abousiksakaa,
Abdullah Y. A. Alzahranic and
Sayed K. Ramadan
b,
Motaleb S. Abousiksakaa,
Abdullah Y. A. Alzahranic and
Sayed K. Ramadan *b
*b
aChemistry Department, Faculty of Science, Misurata University, 2478, Misurata, Libya
bChemistry Department, Faculty of Science, Ain Shams University, Cairo, 11566, Egypt. E-mail: sayed.karam2008@sci.asu.edu.eg
cChemistry Department, Faculty of Science and Arts, King Khalid University, Abha, Mohail Assir, Saudi Arabia
First published on 10th June 2024
Abstract
Among sulfur-including heterocycles, the benzothiophene skeleton is one of the worthy structure fragments that exhibit structural similarities with active substrates to develop various potent lead molecules in drug design. Thus, some tetrahydrobenzo[b]thiophene candidates were prepared from the β-enaminonitrile scaffold via reactions with diverse carbon-centered electrophilic reagents and supported with DFT studies. The in vitro antiproliferative effect was screened against MCF7 and HePG2 cancer cell lines, and the results displayed the highest potency of imide 5, Schiff base 11, and phthalimido 12 candidates. A molecular docking study was operated to explore the probable binding modes of interaction, and the results revealed the good binding affinity of compounds 5, 11, and 12 toward the tubulin protein (PDB ID 5NM5) with respect to paclitaxel (a tubulin inhibitor) and co-crystallized ligand (GTP). Besides, modeling pharmacokinetics analyses displayed their desirable drug-likeness and bioavailability properties.
Introduction
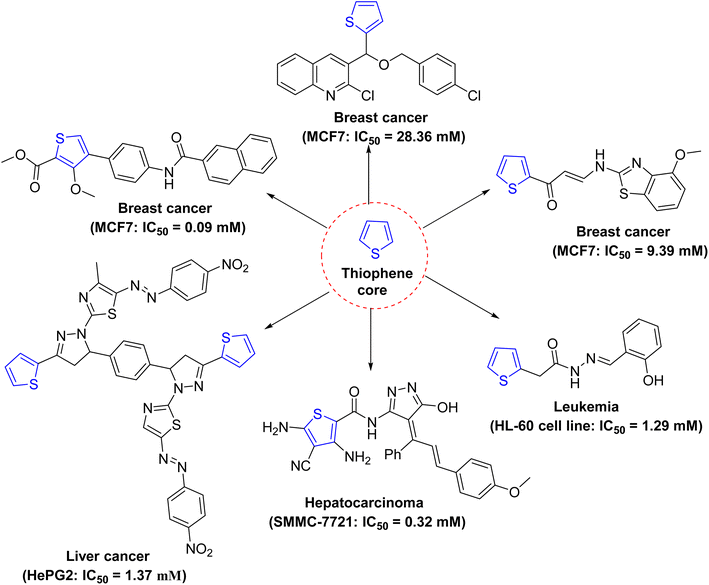
In nature, heterocyclic cores are widely distributed, and many heterocyclic compounds synthesized in laboratories have been utilized as clinical agents.1–6 The most characteristic and important constituents of living cells, DNA and RNA, contain heterocyclic scaffolds. Sulfur-including heterocycles exhibit various biological properties due to the similarities with many natural and synthetic molecules of known potentials.7–10 Thus, the sulfur heterocyclic family involves highly stable aromatic compounds which show physicochemical properties with relevance in the design of new materials.11In literature, various thiophene derivatives have been reported as antitumor agents as depicted in Fig. 1.12–14 In turn, a tetrahydrobenzo[b]thiophene core is one of the privileged structures in drug discovery as this core exhibits diverse biological effects allowing them to act as antitumor, anti-inflammatory, antibacterial, and analgesic agents.15–18 Further, several benzothiophenes, as clinical drugs, have been extensively utilized for treating various types of diseases with high therapeutic potency. Therefore, their structure–activity relationships (SAR) have generated more interest among medicinal chemists, and this has concluded in the discovery of various lead molecules against many diseases.
In particular, 4,5,6,7-tetrahydrobenzo[b]thiophene candidates have been recognized as promising anticancer agents.19 Numerous candidates showed their antiproliferative effects through targeting metalloproteinases 2 and 9 (MMP 2 and 9), HIF-1α and the vascular endothelial growth factor receptor,20 the epidermal growth factor receptor (EGFR), human EGFR-related receptor 2 (HER2),21 and the colon cancer-related genes, namely, collagen type X α1 (COL10A1) and collagen type XI α1 (COL11A1) (cf. Fig. 2).22
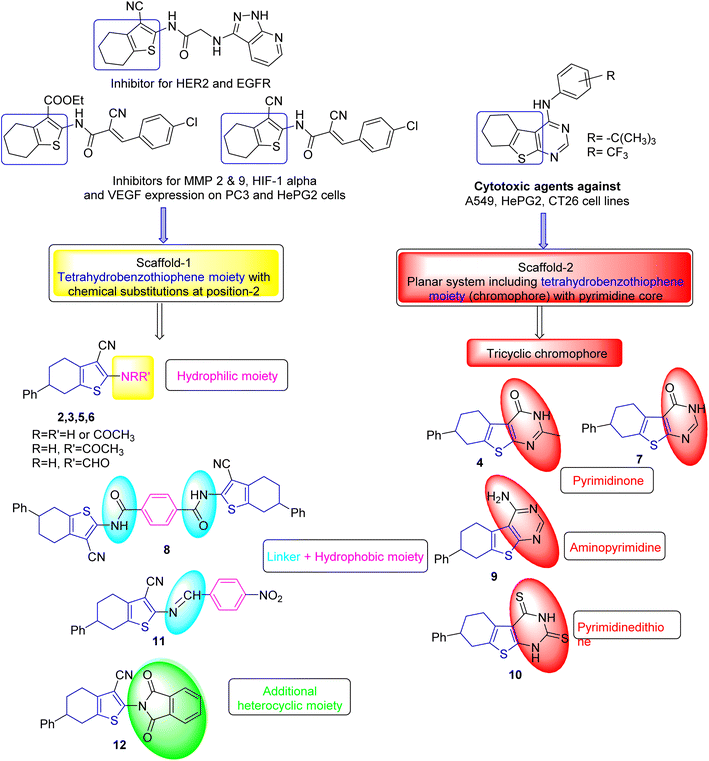 | ||
| Fig. 2 Examples of certain reported tetrahydrobenzo[b]thiophene derivatives as anticancer agents and rationale of molecular design of the chemical structures of the target substrates. | ||
On the other side, microtubules, with diverse roles within the cell, are cellular polymers including chromosomal segregation, cytokine, and chemokine secretion, protein trafficking, maintenance of cell structure, cell migration, and division. Therefore, targeting tubulin dynamics is a promising attempt for new chemotherapeutic agents. Substances capable of disturbing microtubules by either stabilizing or destabilizing them would exhibit antiproliferative effects via enhancing mitotic arrest and cell apoptosis. At least three main binding sites in tubulin protein, including colchicine, taxane, and vinca alkaloid, have been recognized as targets for the anticancer drugs.18,23,24
Therefore, tetrahydrobenzo[b]thiophene scaffold was selected as a central heterocyclic motif for the present work and a phenyl ring attached at position-6 to keep its common structural integrity. However, another fused heterocyclic ring or benzene ring connected via amide, imide, and imine linker offer various insight with new molecular scaffold resulting in a stronger interaction with key role amino acid residues. These hybrids were an attempt to reach some antitumor agents with potent effect. Continuing our attempts for diverse heterocyclic candidates with pharmaceutical interest,25–32 this work targeted to design and prepare a new series of tetrahydrobenzo[b]thiophenes to evaluate their in vitro antiproliferative potency and supported by DFT, molecular docking, and modeling pharmacokinetics studies.
Rationale and design
The rationale of our molecular design was dependent on the generation of two scaffolds of tetrahydrobenzo[b]thiophenes. The first one consisted of tetrahydrobenzo[b]thiophene moiety with chemical substitutions at position-2 as compounds 2, 3, 5, 6, 8, 11, and 12. The substitution pattern on the tetrahydrobenzo[b]thiophene moiety was chosen to ensure various electronic and lipophilic environments manipulating the potency of the target substrates. The second scaffold consisted of a planar system including tetrahydrobenzo[b]thiophene core (chromophore) with pyrimidine skeletons like compounds 4, 7, 9, and 10 (Fig. 2). Also, owing to the reported significant anticancer effects of electron-withdrawing groups, the nitrobenzylidene moiety was selected.33,34 The two designed scaffolds aimed to include the main pharmacophoric features; the planar polyaromatic system (chromophore) was represented by tetrahydrobenzo[b]thiophene core and its side chains or fused rings. Furthermore, most designed substances include basic nitrogen atoms that may be protonated at physiological pH to form cationic centers enhancing the affinity and selectivity of these substances.Results and discussion
Synthesis
The frequent reactivity modes of bifunctional β-enaminonitriles come from the presence of highly nucleophilic center (NH2) and electrophilic center (CN). Also, to design and develop new potential antitumor agents, 2-amino-6-phenyltetrahydrobenzo[b]thiophene-3-carbonitrile 2 (ref. 35) was prepared through one-pot three components approach via interaction of 4-phenylcyclohexanone with malononitrile and elemental sulfur under basic conditions (Scheme 1). Thus, the β-enaminonitrile 2 was allowed to react with diverse carbon-centered electrophiles.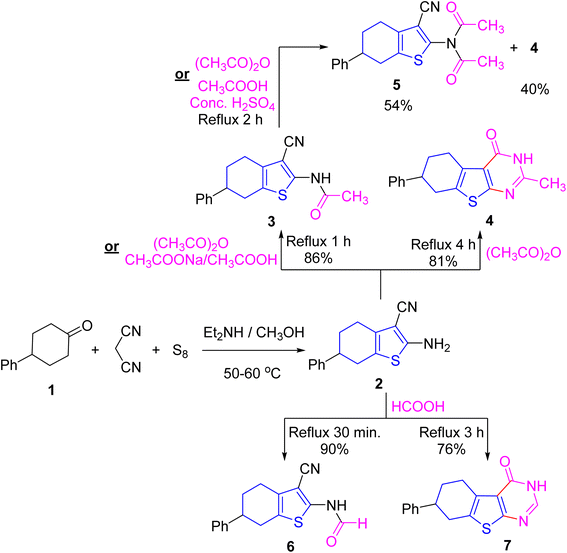 | ||
| Scheme 1 Synthesis and reactions of 2 with ethanoic anhydride and methanoic acid under diverse conditions. | ||
Indeed, treating the enaminonitrile 2 with ethanoic anhydride for 1 or 4 h produced the N-acetyl derivative 3 and the heteroannulated product, tetrahydrobenzo[b]thienopyrimidine derivative 4, respectively. In IR of 3, cyano group was retained and a carbonyl absorption band was detected. While in IR of 4, the cyano absorption disappeared and a carbonyl absorption band appeared. Their 1H NMR displayed a singlet signal for methyl protons. Moreover, the mass spectrum of 3 furnished peaks at m/z = 296.09 (12%) corresponding to its molecular weight (with molecular formula: C17H16N2OS), m/z = 268.11 (14) corresponding to [M–CO]+˙, m/z = 229.09 (20%) due to loss of (N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–CH
C–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and the base peak at m/z = 228.07 (100%) corresponding to the elimination of ketene molecule (CH2
O), and the base peak at m/z = 228.07 (100%) corresponding to the elimination of ketene molecule (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) followed by loss of ˙CN fragment. The 13C NMR of 4 offered signals for carbonyl carbon at δ 163.35 ppm and C
O) followed by loss of ˙CN fragment. The 13C NMR of 4 offered signals for carbonyl carbon at δ 163.35 ppm and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N carbon at δ 158.53 ppm.
N carbon at δ 158.53 ppm.
Otherwise, conducting 3 with ethanoic anhydride or ethanoic acid glacial/sulfuric acid acquired a mixture of the annulated product 4 and N,N-diacetyl derivative 5, which have been separated via fractional recrystallization. In the IR chart of imide 5, NH absorption was absent, and two absorption bands appeared according to vibrational coupling. Its 1H NMR offered singlet signal integrated to six protons at δ 2.34 ppm corresponding to two symmetrical methyl protons.
In turn refluxing 2 with methanoic acid for 30 min and 3 h afforded N-formyl derivative 6 and the annulated product 7 as white crystals, respectively (cf. Scheme 1). IR of 6 conserved cyano group (ν 2212 cm−1) and displayed NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption bands at ν 3185 and 1684 cm−1, respectively. Whilst IR of 7 was devoid of cyano absorption band but offered absorptions for NH band ν 3190 cm−1and C
O absorption bands at ν 3185 and 1684 cm−1, respectively. Whilst IR of 7 was devoid of cyano absorption band but offered absorptions for NH band ν 3190 cm−1and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at ν 1659 cm−1. Also, 1H NMR of 6 displayed a singlet signal for CHO proton at δ 8.50 ppm and an exchangeable singlet for NH proton at δ 8.67 ppm. In turn, 1H NMR of 7 disclosed singlet signals at δ 8.02 and 12.34 ppm for CH
O at ν 1659 cm−1. Also, 1H NMR of 6 displayed a singlet signal for CHO proton at δ 8.50 ppm and an exchangeable singlet for NH proton at δ 8.67 ppm. In turn, 1H NMR of 7 disclosed singlet signals at δ 8.02 and 12.34 ppm for CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and NH protons, respectively.
N and NH protons, respectively.
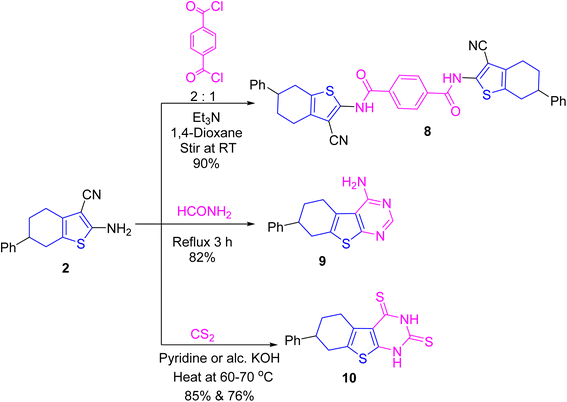
Stirring a solution of the β-enaminonitrile 2 with terephthaloyl dichloride in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, in 1,4-dioxane including triethylamine acquired bis-terephthalamide candidate 8. In its IR, the amide carbonyl absorption was detected. Also, its 1H NMR disclosed an exchangeable singlet signal for NH proton at δ 11.97 ppm. In turn, refluxing 2 with formamide produced tetrahydrobenzothienopyrimidine derivative 9. Its IR lacked both carbonyl and nitrile absorptions, but it displayed an absorption for primary amino group at ν 3285, 3200 cm−1, in addition to C
1, respectively, in 1,4-dioxane including triethylamine acquired bis-terephthalamide candidate 8. In its IR, the amide carbonyl absorption was detected. Also, its 1H NMR disclosed an exchangeable singlet signal for NH proton at δ 11.97 ppm. In turn, refluxing 2 with formamide produced tetrahydrobenzothienopyrimidine derivative 9. Its IR lacked both carbonyl and nitrile absorptions, but it displayed an absorption for primary amino group at ν 3285, 3200 cm−1, in addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption at ν 1636 cm−1. Further, its 1H NMR offered exchangeable singlet signal for NH2 protons at δ 6.84 ppm, besides a singlet signal for methine (CH
N absorption at ν 1636 cm−1. Further, its 1H NMR offered exchangeable singlet signal for NH2 protons at δ 6.84 ppm, besides a singlet signal for methine (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) proton at δ 8.20 ppm.
N) proton at δ 8.20 ppm.
Heating 2 with carbon disulfide in pyridine or alcoholic potassium hydroxide at 60–70 °C led to the construction of tetrahydrobenzothienopyrimidinedithione derivative 10 (cf. Scheme 2). In its IR, both primary amino and nitrile groups were disappeared while NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S were detected at ν 3119 and 1190 cm−1, respectively. Also, its 13C NMR displayed signals of C
S were detected at ν 3119 and 1190 cm−1, respectively. Also, its 13C NMR displayed signals of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups at δ 181.65 and 169.22 ppm. Further, its 1H NMR detected two NH protons at δ 13.22 and 13.89 ppm.
S groups at δ 181.65 and 169.22 ppm. Further, its 1H NMR detected two NH protons at δ 13.22 and 13.89 ppm.
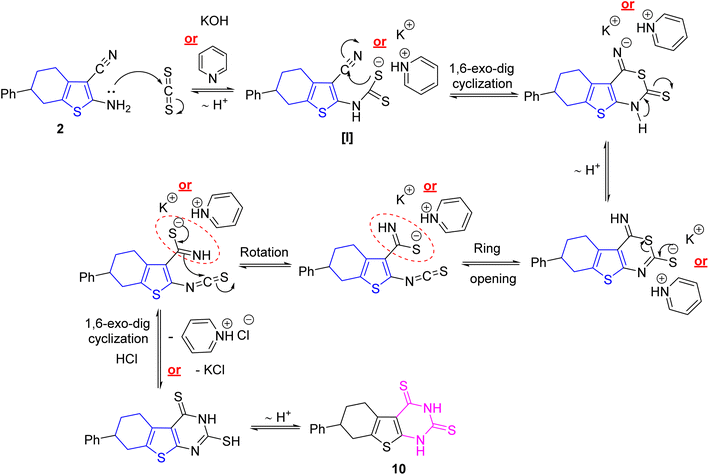
Perhaps, the utility of pyridine or alcoholic potassium hydroxide were responsible for the existence of thiocarbamate intermediate [I] which subsequently attacks the nitrile core via 1,6-exo–dig cyclization to furnish pyrimidine or potassium salt followed by rearrangement to isothiocyanate derivative which underwent 1,6-exo–dig cyclization and eliminate pyridinium hydrochloride or potassium chloride (cf. Scheme 3).
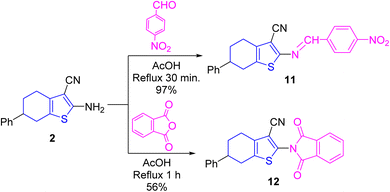
Condensation of enaminonitrile 2 with 4-nitrobenzaldehyde in refluxing ethanoic acid produced the Schiff base candidate 11. The NH2 group was absent in its IR and 1H NMR spectra. As for its IR spectrum, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N group was conserved (at ν 2218 cm−1), whilst the C
N group was conserved (at ν 2218 cm−1), whilst the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety was detected at ν 1600 cm−1, in addition to the NO2 functionality was appeared at ν 1556 and 1336 cm−1. As for its 1H NMR, the methine proton (CH
N moiety was detected at ν 1600 cm−1, in addition to the NO2 functionality was appeared at ν 1556 and 1336 cm−1. As for its 1H NMR, the methine proton (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) appeared at δ 8.84 ppm. Further, its mass spectrum displayed the molecular ion peak at m/z = 387.35 (38%) in addition to other abundant peaks.
N) appeared at δ 8.84 ppm. Further, its mass spectrum displayed the molecular ion peak at m/z = 387.35 (38%) in addition to other abundant peaks.
Treating 2 with phthalic anhydride in refluxing ethanoic acid achieved the phthalimide derivative 12 (cf. Scheme 4). In its IR spectrum, the NH2 moiety disappeared, the nitrile absorption was retained, and the carbonyls of imide linkage were detected at ν 1788 and 1730 cm−1 (due to vibrational coupling between symmetrical and asymmetrical stretching absorptions). Further, its mass spectrum offered M+˙ peak at m/z = 384.31 (22%) and the base at m/z = 77 (100) corresponding to phenyl cation (cf. ESI Experimental section†). All methods of synthesis gave the mentioned compounds with different % yields as displayed in schemes and experimental part, without isolation of by-products.
DFT (density functional theory) study
The DFT simulation assists in demonstrating the chemical reactions of substrate with various reagents and shows high accuracy for producing of experimental results,36,37 as well as expecting the appropriate biological applications. DFT simulation was conducted to optimize the molecular structures of the produced substances and identify both nucleophilic and electrophilic centers. The optimization of molecular structures of produced benzo[b]thiophene candidates were carried out to create a stable geometry.The electrophilic-attacking centers are illustrated by HOMO regions of largest electron density (considered as a donor), while the nucleophilic-attacking sites are represented by LUMO areas (considered as an acceptor). The optimized, HOMO, and LUMO structures of substances 2–12 were drawn employing ChemBio3D Ultra 14.0 and portrayed in Fig. S1 (cf. ESI†). The HOMOs are dispersed around thiophene unit and LUMOs are focused on benzene moiety. The high EHOMO values are likely to signify a molecule's strong tendency for donating electrons.
To demonstrate how the β-enaminonitrile 2 reacted with some reagents to produce substances 3–12, DFT simulation was employed to calculate quantum chemical properties including global hardness (η), global softness (ς), chemical potential (μo), global electrophilicity index (ω), nucleophilicity index (n), ionization potential (IP), electron affinity (EA), and electronegativity (x) (cf. Table S1 in ESI†). Spectral and analytical data confirmed the assigned structures. The results of parameters calculations were in good agreement with the anticancer efficiency. The lower values of energy gap (ΔE = ELUMO − EHOMO) means low energy needed to remove an electron from the last occupied orbital.38,39
Substrates of small ΔE values are mostly referred to as soft compounds, which are more reactive towards radical surface interactions; being efficient of donating electrons easily to hole surface, and therefore might display strong inhibition efficiency. The results showed that energy gap values (ΔE) follow the order: 11 < 12 < doxorubicin < 5 < 9 < 2 < 4 < 7 < 10 < 8 < 6 < 3 (cf. Fig. 3). Thus, Schiff base 11 exhibited the lowest ΔE value (1.540 eV) compared to others.
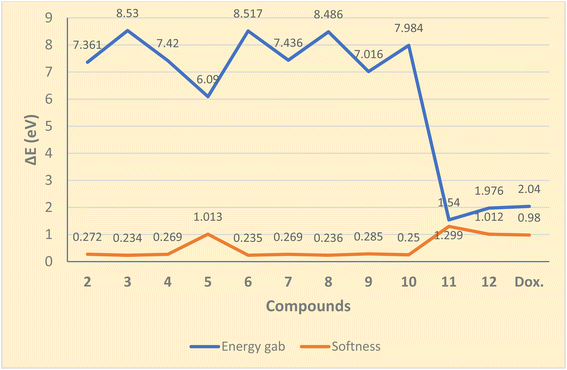 | ||
| Fig. 3 Energy gap (ΔE, eV) and softness (ς, eV−1) values of the synthesized compounds and doxorubicin. | ||
Chemical softness values (σ, eV−1) decrease in the order of 11, 5, 12, 9, 2, 4 = 7, 10, 3, 8, and 6, respectively (cf. Fig. 3), while the hardness values rise in the same order. That is, lower values of softness and higher chemical hardness establish a good stability of the N-formyl derivative 6 which might exhibit high molecular hardness. According to the ionization potential (IP) values of these compounds, the acetamide derivative 3 exhibited the highest IP value (8.164 eV) which means that it is more difficult to remove an electron from its HOMO orbitals. Besides, the N,N-diacetyl derivative 5 showed the lowest electron affinity (1.975 eV) indicating the highest molecular reactivity to nucleophiles. The dipole moments showed that Schiff base 11 exhibited the highest value (3.458 debye) which indicates stronger intermolecular interaction than other compounds and interacting more with the surrounding environment.
The scavenging ability toward positive hole, radical, tumor, and oxygen removable not only depend upon EHOMO values but also, the surface area, number of heteroatoms, electron distributions, and lipophilicity should be considered.40 The results for most potent compounds encompassing hydrophobic moieties were agreed to an outstanding correlation with oxidation inhibition efficiencies. Compatibly, substrates of higher binding energy showed higher potency due to strong interaction with the receptors' active sites.
Cytotoxic activity assessment
The cytotoxic activity of the substrates was examined against mammary gland breast carcinoma (MCF7) and hepatocellular cancer (HePG2) cell lines utilizing MTT assay and expressed in IC50 (growth inhibitory concentration) compared with the untreated controls.41 The findings indicated variable degrees of inhibitory effect ranged from 71.21 to 9.54 μM by these substrates (Table 1). As for MCF7, the relative highest potency was provided by substances 5 (IC50 = 11.42 ± 1.4 μM), 11 (IC50 = 12.93 ± 1.5 μM), and 12 (IC50 = 20.51 ± 2.3 μM), respectively in comparison to doxorubicin and paclitaxel42 as reference agents. As for HePG2, the highest potency was furnished by substrates 11 (IC50 = 9.54 ± 0.7 μM), 5 (IC50 = 13.73 ± 1.4 μM), and 12 (IC50 = 18.16 ± 1.1 μM), respectively. Meanwhile, other substrates offered moderate and weak potencies.| Compds | In vitro cytotoxicity: IC50a,b (μM) | |
|---|---|---|
| MCF7 | HePG2 | |
| a IC50 (μM): 1–10 (very strong), 11–20 (strong), 21–50 (moderate), 51–100 (weak), above 100 (non-cytotoxic).b Results are means ± S.D. (standard deviation, n = 3).c According to ref. 42. | ||
| Doxorubicin | 4.50 ± 0.3 | 4.17 ± 0.2 |
| Paclitaxelc | 2.50 ± 0.2 | 4.00 ± 0.3 |
| 3 | 65.20 ± 4.0 | 71.21 ± 3.5 |
| 4 | 43.21 ± 4.2 | 50.22 ± 3.9 |
| 5 | 11.42 ± 1.4 | 13.73 ± 1.4 |
| 10 | 30.45 ± 2.1 | 25.53 ± 2.6 |
| 11 | 12.93 ± 1.5 | 9.54 ± 0.7 |
| 12 | 20.51 ± 2.3 | 18.16 ± 1.1 |
These findings indicate that significant potency was achieved via conjugation of the parent thiophene core with imide and nitrobenzylidine linkages.33,43 Also, the enhanced potency of substrates 11 (bearing nitro group) and 12 (bearing phthalimido core) may be attributable for the significant effect of the electron-withdrawing group which improves the ability to inhibit cancer cell proliferation and forming hydrogen bonding with the nucleobases of DNA and causes damage.34
Molecular docking study
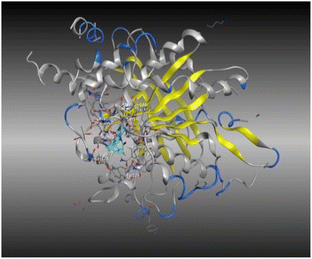
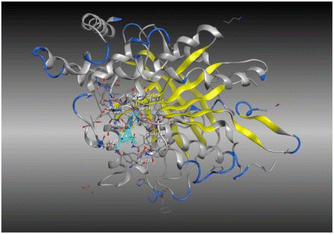
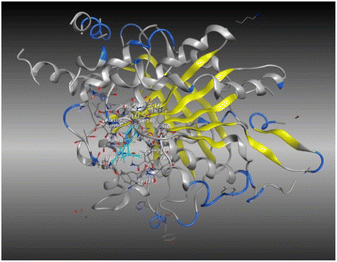
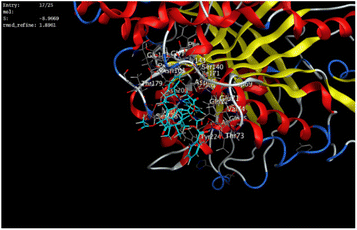
A molecular docking study of a chemical inhibitor in the active pocket of proper target is widely applied as an approach to disclose the possible antitumor mechanism of action. Microtubules, with diverse roles within the cell, are cellular polymers including chromosomal segregation, cytokine, and chemokine secretion, protein trafficking, maintenance of cell structure, cell migration, and division. A tubulin inhibitor is a drug that inhibits mitosis or cell division and is used in treating cancer. Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Therefore, targeting tubulin dynamics is a promising attempt for new chemotherapeutic agents. Substances capable of disturbing microtubules by either stabilizing or destabilizing them would exhibit antiproliferative effects via enhancing mitotic arrest and cell apoptosis. At least three main binding sites in tubulin protein, including colchicine, taxane, and vinca alkaloid, have been recognized as targets for the anticancer drugs.18,23,24 Examples of mitotic inhibitors frequently used in the treatment of cancer include paclitaxel, docetaxel, vinblastine, vincristine, and vinorelbine.Noteworthy, the promising cytotoxic candidates 5, 11, and 12 are tetrahydrobenzo[b]thiophenes with diacetyl, Schiff base, and phthalimido functionalities, where these structural units were found to inhibit tubulin polymerization leading to irreversible damage to the tumor vasculature and causes mitotic arrest and tumor necrosis.44,45 Therefore, the inhibitory potentials of these candidates against tubulin (TUB) domain were investigated via molecular docking analysis utilizing molecular operating environment (MOE 2014.0901) to disclose its probable antitumor mechanism of action, examine the binding free energies of the prepared substances and paclitaxel (as a tubulin stabilizing agent) toward tubulin protein (PDB ID 5NM5) and display the interactions between the prepared ligands and receptors.
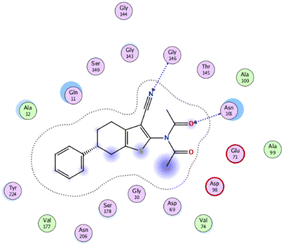
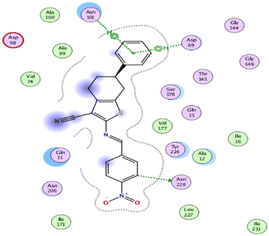
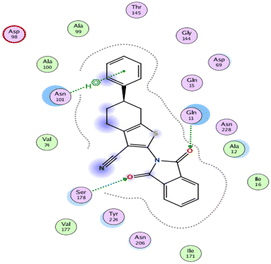
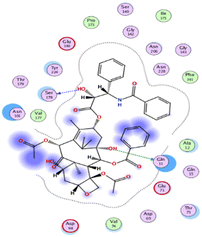
The binding affinities were measured by the binding energies (S-score, kcal mol−1) and hydrogen bonds. All complexes obtained were docked in the same groove of binding site of the native co-crystallized ligand (GTP) (Tables 2 and 3). Further, Table 2 mentions the specific amino acids involved in binding interactions between each compound and its respective target protein through inclusive information concerning the binding amino acids and types of bonds formed like H–acceptor, pi–cation, and among others.
| Compd | S-Score (kcal mol−1) | RMSD (Å) | Binding amino acids | Interacting groups | Receptor | Type of interaction | Bond length (Å) |
|---|---|---|---|---|---|---|---|
| 5 | −6.5483 | 1.0374 | ASN 101 | O 25 | N | H–acceptor | 2.95 |
| ASN 101 | O 25 | ND2 | H–acceptor | 2.87 | |||
| GLY 146 | N 42 | N | H–acceptor | 3.39 | |||
| 11 | −6.2768 | 1.6047 | ASN 228 | C 26 | OD1 | H–donor | 3.25 |
| ASP 69 | 6-Ring | OD2 | Pi–H | 3.32 | |||
| ASN 101 | 6-Ring | ND2 | Pi–H | 3.55 | |||
| ASN 101 | 6-Ring | N | Pi–H | 4.31 | |||
| 12 | −5.7105 | 1.6102 | GLN 11 | O 30 | NE2 | H–acceptor | 2.73 |
| SER 178 | O 31 | OG | H–acceptor | 2.98 | |||
| ASN 101 | 6-Ring | ND2 | Pi–H | 3.33 | |||
| Paclitaxel | −8.9669 | 1.8961 | SER 178 | O 32 | O | H–donor | 2.79 |
| GLN 11 | O 51 | OE1 | H–donor | 2.91 | |||
| Co-crystallized ligand (GTP) | −11.9309 | 1.4869 |
The data presented in Table 2 revealed that the binding energies of the synthesized ligands were near to that of co-crystallized ligand (GTP) with RMSD values lower than 2 Å. Noteworthy, the co-crystallized ligand (GTP) and paclitaxel exhibited binding energies of −11.9309 and −8.9669 kcal mol−1 with RMSD values of 1.8961 and 1.4869 Å, respectively. The inhibition efficiency of paclitaxel was attributed to the hydrogen bonding interaction with some amino acids (SER 178 and GLN 11) of tubulin protein. The best docking score was displayed by compound 5 as it showed binding energy of −6.5483 kcal mol−1 with the lowest RMSD of 1.0374 Å indicating to its tightly hydrogen bonding to some key nucleobases and amino acids (ASN 101, GLY 146) of tubulin protein, which disclose its potential utilization as tubulin stabilizing agents like paclitaxel. Also, compounds 11 and 12 exhibited binding energies of −6.2768 and −5.7105 kcal mol−1 with RMSD of 1.6047 and 1.6102 Å, respectively, which display notably strong binding affinities toward tubulin protein through hydrogen bonding and pi–hydrogen interactions. The orientation and hydrogen bond pattern of these compounds in the active sites correspond to that of paclitaxel, as a tubulin stabilizing agent.
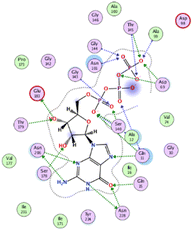
The outcomes of docking analysis of compounds 5, 11, and 12 with the tubulin protein (PDB ID 5NM5) were displayed in graphical representations designated as 2D and 3D visualizations (cf. Table 3). The 3D visualization displays the binding interactions between these substances and tubulin protein, indicating hydrogen bonding interactions highlighted in red. Also, 2D depiction illustrates detailed insights into the molecular interactions between compounds and proteins.
Modeling pharmacokinetics
The ADME (absorption, distribution, metabolism, and excretion) properties of the compelling substances, which involve their physicochemical properties, lipophilicity, and drug-likeness, have been prophesied by SwissADME free web tool to lessen the time of choosing substrates from an enormous collection of substances in the early phases of drug discovery, biological effects, and development for an effective drug.18,46 Compounds 5, 11, and 12 were found to obey with Lipinski's rule of five with a total polar surface area (TPSA) of 89.41, 110.21, and 89.41 Å, respectively (cf. Table S2 and Fig. S2–S12 in ESI†).12,47Also, these substances displayed suitable physicochemical properties, which was examined through the following six parameters: lipophilicity (LIPO), size, polarity (POLAR), insolubility (INSOL), unsaturation (UNSAT), and flexibility (FLEX). Concerning the absorption property, they displayed gastrointestinal tract (GIT) absorption due to their existence in the BOILED-EGG chart white area (Fig. S13 in ESI†). They showed good lipophilicity as presented by the consensus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w which were in 3.45, 4.39, and 4.37, respectively. The good oral bioavailability (0.55) of these substances was clear through the bioavailability radar chart. Thus, compounds 5, 11, and 12 were fully included in the pink area and this supported their well-predicted oral bioavailability. They showed a high GI absorption. Their skin permeation (log
Po/w which were in 3.45, 4.39, and 4.37, respectively. The good oral bioavailability (0.55) of these substances was clear through the bioavailability radar chart. Thus, compounds 5, 11, and 12 were fully included in the pink area and this supported their well-predicted oral bioavailability. They showed a high GI absorption. Their skin permeation (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KP) parameters were −5.94, −4.83, and −5.23 cm s−1, which rendered the bioactive compounds easier to access through skin. Also, their cytochrome P450 isoenzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4), which perform a significant role in biotransformation of medicines through O-type oxidation processes, have also been estimated. In turn, it is expected to penetrate the blood–brain barrier (existed inside the chart yellow area). Further, they are not potential substrates for permeability glycoprotein (PGP), which is indicated by red. Finally, all compounds showed no violations to Lipinski's rule of five except for compound 8 as displayed in Table S2 (ESI†). Jointly, compounds 5, 11, and 12 exhibit desirable drug-likeness and oral bioavailability properties.
KP) parameters were −5.94, −4.83, and −5.23 cm s−1, which rendered the bioactive compounds easier to access through skin. Also, their cytochrome P450 isoenzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4), which perform a significant role in biotransformation of medicines through O-type oxidation processes, have also been estimated. In turn, it is expected to penetrate the blood–brain barrier (existed inside the chart yellow area). Further, they are not potential substrates for permeability glycoprotein (PGP), which is indicated by red. Finally, all compounds showed no violations to Lipinski's rule of five except for compound 8 as displayed in Table S2 (ESI†). Jointly, compounds 5, 11, and 12 exhibit desirable drug-likeness and oral bioavailability properties.
Conclusion
Certain tetrahydrobenzo[b]thiophene candidates were prepared starting from the corresponding β-enaminonitrile scaffold. The in vitro antiproliferative activity of the examined substances against MCF7 and HePG2 cell lines displayed the relative highest potency of imide 5, Schiff base 11, and phthalimido 12 compared with doxorubicin and paclitaxel. The DFT results were coherent with the cytotoxicity of tested compounds by displaying the lowest ΔE values of 1.540, 1.976, and 6.090 eV and highest softness of 1.299, 1.012, and 1.013 eV−1 for compounds 11, 12, and 5, respectively, compared with other compounds. A molecular docking study was performed employing MOE to investigate the binding free energies of paclitaxel (as a tubulin inhibitor) and potent substrates toward tubulin protein (PDB ID 5NM5) and determined the interactions between ligands obtained and receptors. The binding energies of ligands obtained were near to that of co-crystallized ligand (GTP). The best docking score was given by imide derivative 5 (−6.5483 kcal mol−1) indicating its tightly bounding through hydrogen bonding with some key nucleobases and amino acids (ASN 101 and GLY 146) of tubulin protein and revealing its potential utility as tubulin inhibitor. Also, compounds 11 and 12 showed notably strong binding affinity towards tubulin protein. A modeling pharmacokinetics study of all prepared compounds showed that substances 5, 11, and 12 were fully included in the pink area supporting their well-predicted oral bioavailability. The most potent candidates may serve as useful lead substrates in looking for selective and powerful antiproliferative agents.Materials and methods
All solvents and reagents were purified and dried employing standard approaches. The melting points of all substances were taken on a GRIFFIN and GEORGE melting-point apparatus. Infrared (IR) spectra (ν, cm−1) were determined at a NICOLET iS10 infrared spectrophotometer employing KBr disks. 1H and 13C NMR spectra (δ, ppm) were acquired at 400 and 100 MHz, respectively on a Bruker Avance III NMR spectrometer employing deuterated dimethyl sulfoxide (DMSO-d6) solvent with tetramethyl silane (TMS) internal standard. Mass spectra were run on a Shimadzu GC-MSQP-1000 EX mass spectrometer (Thermo Scientific GCMS MODEL (ISQ LT)) running at 70 eV using the Thermo X-CALIBUR software at Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Elemental analyses were recorded at a CHN analyzer, and values obtained were within ±0.4 of the theoretical values. The homogeneity of the substances obtained was controlled by TLC (thin-layer chromatography) on plates with employing aluminum sheet silica gel 60 F254 (Merck, Darmstadt, Germany) with different solvent systems as mobile phases.2-Amino-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile (2)
To a solution of 4-phenylcyclohexanone (5.22 g, 30 mmol), malononitrile (1.98 g, 30 mmol) and elemental sulfur (0.96 g, 30 mmol) in methyl alcohol (10 mL), diethylamine (2 mL) was added dropwise with stirring at 50–60 °C for 10 min. Then heating for 1 h at the same temperature. The solution was allowed to stand at room temperature and the solid formed was filtered off, washed with ethanol several times, dried, and crystallized from ethanol to give beige crystals, mp 181–182 °C [lit. 35 mp 180–182 °C], yield (5.64 g, 74%).N-(3-Cyano-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)acetamide (3)
A solution of β-enaminonitrile 2 (1.27 g, 5 mmol) in ethanoic anhydride (10 mL) or glacial ethanoic acid (10 mL) involving fused sodium acetate (2 g) was refluxed for 1 h. The formed solid after cooling was collected and crystallized from ethyl alcohol to obtain white crystals, mp 234–235 °C, yield (1.27 g, 86%). Rf = 0.41 (Et2O/EtOAc, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3265 (NH), 2216 (C
1). IR (ν, cm−1): 3265 (NH), 2216 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1695 (C
N), 1695 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (δ, ppm): 1.87–1.93 (m, 2H, CH2), 2.19 (s, 3H, CH3), 2.62–2.71 (m, 2H, CH2), 2.83–2.88 (m, 2H, CH2), 2.93–2.99 (m, 1H, CH), 7.22 (t, 1H, Ar–H, J = 7.5 Hz), 7.29–7.34 (m, 4H, Ar–H), 11.57 (br.s, 1H, NH, exchangeable). EIMS, m/z (%): 296.09 (M+˙, 12), 268.11 (14), 229.09 (20), 228.07 (100), 227.04 (61), 211.09 (5), 194.09 (6), 155.07 (15), 140.06 (21), 119.05 (15), 93.08 (27), 77.07 (55), 65.07 (22). Anal. calcd for C17H16N2OS (296.39): C, 68.89; H, 5.44; N, 9.45; found: C, 68.79; H, 5.38; N, 9.43%.
O). 1H NMR (δ, ppm): 1.87–1.93 (m, 2H, CH2), 2.19 (s, 3H, CH3), 2.62–2.71 (m, 2H, CH2), 2.83–2.88 (m, 2H, CH2), 2.93–2.99 (m, 1H, CH), 7.22 (t, 1H, Ar–H, J = 7.5 Hz), 7.29–7.34 (m, 4H, Ar–H), 11.57 (br.s, 1H, NH, exchangeable). EIMS, m/z (%): 296.09 (M+˙, 12), 268.11 (14), 229.09 (20), 228.07 (100), 227.04 (61), 211.09 (5), 194.09 (6), 155.07 (15), 140.06 (21), 119.05 (15), 93.08 (27), 77.07 (55), 65.07 (22). Anal. calcd for C17H16N2OS (296.39): C, 68.89; H, 5.44; N, 9.45; found: C, 68.79; H, 5.38; N, 9.43%.
2-Methyl-7-phenyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one (4)
A solution of 2 (1.27 g, 5 mmol) in ethanoic anhydride (10 mL) was refluxed for 6 h. The formed solid after cooling was collected and crystallized from 1,4-dioxane to obtain white crystals, mp > 300 °C, yield (1.20 g, 81%). Rf = 0.45 (Et2O/EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3200 (NH), 1669 (C
1). IR (ν, cm−1): 3200 (NH), 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 13C NMR (δ, ppm): 163.35, 158.53, 154.43, 145.59, 130.45 (2), 130.19, 128.42 (2), 126.87, 126.31, 119.95, 38.82, 32.05, 29.15, 25.61, 20.82. 1H NMR (δ, ppm): 1.91–2.01 (m, 2H, CH2), 2.33 (s, 3H, CH3), 2.80–2.87 (m, 2H, CH2), 2.96–3.03 (m, 2H, CH2), 3.11–3.15 (m, 1H, CH), 7.23 (t, 1H, Ar–H, J = 7.4 Hz), 7.32–7.33 (m, 4H, Ar–H), 12.25 (br.s, 1H, NH, exchangeable). Anal. calcd for C17H16N2OS (296.39): C, 68.89; H, 5.44; N, 9.45; found: C, 68.80; H, 5.39; N, 9.47%.
O). 13C NMR (δ, ppm): 163.35, 158.53, 154.43, 145.59, 130.45 (2), 130.19, 128.42 (2), 126.87, 126.31, 119.95, 38.82, 32.05, 29.15, 25.61, 20.82. 1H NMR (δ, ppm): 1.91–2.01 (m, 2H, CH2), 2.33 (s, 3H, CH3), 2.80–2.87 (m, 2H, CH2), 2.96–3.03 (m, 2H, CH2), 3.11–3.15 (m, 1H, CH), 7.23 (t, 1H, Ar–H, J = 7.4 Hz), 7.32–7.33 (m, 4H, Ar–H), 12.25 (br.s, 1H, NH, exchangeable). Anal. calcd for C17H16N2OS (296.39): C, 68.89; H, 5.44; N, 9.45; found: C, 68.80; H, 5.39; N, 9.47%.
Action of ethanoic anhydride or ethanoic/sulfuric acids on acetamide derivative 3
A solution of acetamide derivative 3 (1.48 g, 5 mmol) in ethanoic anhydride (10 mL) or ethanoic acid (10 mL) containing concentrated sulfuric acid (0.1 mL) was refluxed for 2–3 h. After cooling, the reaction mixture was poured onto cold-water to get a mixture of heteroannulated product 4 and imide derivative 5 which was filtered off, dried, and fractionally recrystallized from ethyl alcohol to obtain the imide derivative 5. The insoluble residue was recrystallized from dioxane to achieve heteroannulated product 4 (identity: mp, mixed mp, TLC), yield (0.59 g, 40%).N-Acetyl-N-(3-cyano-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)acetamide (5)
White crystals, mp > 300 °C, yield (0.91 g, 54%). Rf = 0.52 (Et2O/EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 2219 (C
1). IR (ν, cm−1): 2219 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1737, 1712 (C
N), 1737, 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational coupling). 1H NMR (δ, ppm): 1.95–2.07 (m, 2H, CH2), 2.34 (s, 6H, 2 CH3), 2.64–2.78 (m, 2H, CH2), 2.82–2.89 (m, 2H, CH2), 3.03–3.08 (m, 1H, CH), 7.24 (t, 1H, Ar–H, J = 7.5 Hz), 7.31–7.35 (m, 4H, Ar–H). EIMS, m/z (%): 338.31 (M+˙, 12), 306.32 (37), 283.40 (100), 228.20 (41), 200.30 (32), 172.21 (17), 132.36 (21), 98.52 (35). Anal. calcd for C19H18N2O2S (338.43): C, 67.43; H, 5.36; N, 8.28; found: C, 67.32; H, 5.29; N, 8.31%.
O vibrational coupling). 1H NMR (δ, ppm): 1.95–2.07 (m, 2H, CH2), 2.34 (s, 6H, 2 CH3), 2.64–2.78 (m, 2H, CH2), 2.82–2.89 (m, 2H, CH2), 3.03–3.08 (m, 1H, CH), 7.24 (t, 1H, Ar–H, J = 7.5 Hz), 7.31–7.35 (m, 4H, Ar–H). EIMS, m/z (%): 338.31 (M+˙, 12), 306.32 (37), 283.40 (100), 228.20 (41), 200.30 (32), 172.21 (17), 132.36 (21), 98.52 (35). Anal. calcd for C19H18N2O2S (338.43): C, 67.43; H, 5.36; N, 8.28; found: C, 67.32; H, 5.29; N, 8.31%.
N-(3-Cyano-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)formamide (6)
A solution of 2 (1.27 g, 5 mmol) in formic acid (10 mL) was refluxed for 30 min. The formed solid was collected, washed well with water, dried, and crystallized from ethanol to offer white crystals, mp 251–253 °C, yield (1.27 g, 90%). Rf = 0.48 (Et2O/EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3185 (NH), 2212 (C
1). IR (ν, cm−1): 3185 (NH), 2212 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1684 (C
N), 1684 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (δ, ppm): 1.58–1.80 (m, 2H, CH2), 1.99–2.22 (m, 2H, CH2), 2.74–2.84 (m, 2H, CH2), 2.95–3.06 (m, 1H, CH), 7.21–7.28 (m, 4H, Ar–H), 7.36 (t, 1H, Ar–H, J = 7.4 Hz), 8.50 (s, 1H, CHO), 8.67 (br.s, 1H, NH, exchangeable). Anal. calcd for C16H14N2OS (282.36): C, 68.06; H, 5.00; N, 9.92; found: C, 67.99; H, 4.96; N, 9.94%.
O). 1H NMR (δ, ppm): 1.58–1.80 (m, 2H, CH2), 1.99–2.22 (m, 2H, CH2), 2.74–2.84 (m, 2H, CH2), 2.95–3.06 (m, 1H, CH), 7.21–7.28 (m, 4H, Ar–H), 7.36 (t, 1H, Ar–H, J = 7.4 Hz), 8.50 (s, 1H, CHO), 8.67 (br.s, 1H, NH, exchangeable). Anal. calcd for C16H14N2OS (282.36): C, 68.06; H, 5.00; N, 9.92; found: C, 67.99; H, 4.96; N, 9.94%.
7-Phenyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one (7)
A solution of 2 (1.27 g, 5 mmol) in formic acid (10 mL) was refluxed for 3 h. The excess solvent was evaporated under vacuum. The residue was triturated with light petroleum, filtered, and recrystallized from 1,4-dioxane/DMF mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get white crystals, mp >360 °C, yield (1.07 g, 76%). Rf = 0.46 (Et2O/EtOAc, 3
1) to get white crystals, mp >360 °C, yield (1.07 g, 76%). Rf = 0.46 (Et2O/EtOAc, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3190 (NH), 1659 (C
1). IR (ν, cm−1): 3190 (NH), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (δ, ppm): 1.96–2.07 (m, 2H, CH2), 2.74–2.89 (m, 2H, CH2), 3.00–3.14 (m, 2H, CH2), 3.35–3.57 (m, 1H, CH), 7.23 (t, 1H, Ar–H, J = 7.3 Hz), 7.32–7.40 (m, 4H, Ar–H), 8.02 (s, 1H, CH
O). 1H NMR (δ, ppm): 1.96–2.07 (m, 2H, CH2), 2.74–2.89 (m, 2H, CH2), 3.00–3.14 (m, 2H, CH2), 3.35–3.57 (m, 1H, CH), 7.23 (t, 1H, Ar–H, J = 7.3 Hz), 7.32–7.40 (m, 4H, Ar–H), 8.02 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 12.34 (br.s, 1H, NH, exchangeable). Anal. calcd for C16H14N2OS (282.36): C, 68.06; H, 5.00; N, 9.92; found: C, 67.95; H, 4.94; N, 9.95%.
N), 12.34 (br.s, 1H, NH, exchangeable). Anal. calcd for C16H14N2OS (282.36): C, 68.06; H, 5.00; N, 9.92; found: C, 67.95; H, 4.94; N, 9.95%.
N1,N4-Bis(3-cyano-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)terephthalamide (8)
To a stirred solution of 2 (1.27 g, 5 mmol) in 1,4-dioxane (15 mL) containing triethylamine (0.1 mL), terephthaloyl dichloride (0.51 g, 2.5 mmol) was added portion wise. The reaction mixture was further stirred for 5 h at ambient temperature. The solid obtained was collected and recrystallized from 1,4-dioxane to produce beige crystals, mp >300 °C, yield (2.87 g, 90%). Rf = 0.56 (Et2O/EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3235 (NH), 2223 (C
1). IR (ν, cm−1): 3235 (NH), 2223 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1664 (C
N), 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (δ, ppm): 1.17–1.23 (m, 4H, 2CH2), 1.95–2.05 (m, 4H, 2CH2), 2.70–2.81 (m, 4H, 2CH2), 2.94–3.17 (m, 2H, 2CH), 7.22–7.43 (m, 10H, Ar–H), 8.04–8.13 (m, 4H, Ar–H), 11.97 (br.s, 2H, 2NH, exchangeable). Anal. cald. for C38H30N4O2S2 (638.80): C, 71.45; H, 4.73; N, 8.77; found: C, 71.32; H, 4.65; N, 8.80%.
O). 1H NMR (δ, ppm): 1.17–1.23 (m, 4H, 2CH2), 1.95–2.05 (m, 4H, 2CH2), 2.70–2.81 (m, 4H, 2CH2), 2.94–3.17 (m, 2H, 2CH), 7.22–7.43 (m, 10H, Ar–H), 8.04–8.13 (m, 4H, Ar–H), 11.97 (br.s, 2H, 2NH, exchangeable). Anal. cald. for C38H30N4O2S2 (638.80): C, 71.45; H, 4.73; N, 8.77; found: C, 71.32; H, 4.65; N, 8.80%.
7-Phenyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-amine (9)
A solution of 2 (1.27 g, 5 mmol) in formamide (10 mL) was refluxed for 3 h. The solid obtained during reflux was filtered and recrystallized from 1,4-dioxane to get colorless crystals, mp 252–253 °C, yield (1.15 g, 82%). Rf = 0.38 (Et2O/EtOAc, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3285, 3200 (NH2), 1636 (C
1). IR (ν, cm−1): 3285, 3200 (NH2), 1636 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 13C NMR (δ, ppm): 158.19, 153.09 (2), 145.54, 130.61 (3), 128.50 (3), 126.97, 126.45, 40.38, 32.48, 29.38, 25.83. 1H NMR (δ, ppm): 1.98–2.05 (m, 2H, CH2), 2.87–2.99 (m, 2H, CH2), 3.01–3.10 (m, 2H, CH2), 3.37–3.55 (m, 1H, CH), 6.84 (br.s, 2H, NH2, exchangeable), 7.23 (t, 1H, Ar–H, J = 7.4 Hz), 7.32–7.41 (m, 4H, Ar–H), 8.20 (s, 1H, CH
N). 13C NMR (δ, ppm): 158.19, 153.09 (2), 145.54, 130.61 (3), 128.50 (3), 126.97, 126.45, 40.38, 32.48, 29.38, 25.83. 1H NMR (δ, ppm): 1.98–2.05 (m, 2H, CH2), 2.87–2.99 (m, 2H, CH2), 3.01–3.10 (m, 2H, CH2), 3.37–3.55 (m, 1H, CH), 6.84 (br.s, 2H, NH2, exchangeable), 7.23 (t, 1H, Ar–H, J = 7.4 Hz), 7.32–7.41 (m, 4H, Ar–H), 8.20 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). Anal. calcd for C16H15N3S (281.38): C, 68.30; H, 5.37; N, 14.93; found: C, 68.20; H, 5.32; N, 14.95%.
N). Anal. calcd for C16H15N3S (281.38): C, 68.30; H, 5.37; N, 14.93; found: C, 68.20; H, 5.32; N, 14.95%.
7-Phenyl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-2,4(1H,3H)-dithione (10)
A mixture of 2 (1.27 g, 5 mmol) and carbon disulfide (10 mL) in pyridine or alcoholic potassium hydroxide (10%, 10 mL) was heated at 65–75 °C for 8 h on water bath. The solution was poured onto crushed ice/cold-water and acidified with diluted hydrochloric acid (10%). The formed solid was filtered, washed well with water, dried, and crystallized from ethanol/1,4-dioxane mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give pale-yellow crystals, mp >300 °C, yield (1.40 g, 85%) and (1.25 g, 76%), respectively. Rf = 0.43 (Et2O/EtOAc, 1
1) to give pale-yellow crystals, mp >300 °C, yield (1.40 g, 85%) and (1.25 g, 76%), respectively. Rf = 0.43 (Et2O/EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 3119 (NH), 1190 (C
1). IR (ν, cm−1): 3119 (NH), 1190 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). 13C NMR (δ, ppm): 181.65, 169.22, 149.99, 148.25, 145.50, 129.54, 128.50 (2), 126.99, 126.42 (2), 125.17, 40.39, 32.15, 29.13, 27.91. 1H NMR (δ, ppm): 1.85–2.00 (m, 2H, CH2), 2.74–2.81 (m, 2H, CH2), 2.81–2.98 (m, 2H, CH2), 3.02–3.42 (m, 1H, CH), 7.21 (t, 1H, Ar–H, J = 7.3 Hz), 7.24–7.32 (m, 4H, Ar–H), 13.22 (br.s, 1H, NHCS, exchangeable), 13.89 (br.s, 1H, NHCS, exchangeable). Anal. calcd for C16H14N2S3 (330.48): C, 58.15; H, 4.27; N, 8.48; found: C, 58.07; H, 4.21; N, 8.51%.
S). 13C NMR (δ, ppm): 181.65, 169.22, 149.99, 148.25, 145.50, 129.54, 128.50 (2), 126.99, 126.42 (2), 125.17, 40.39, 32.15, 29.13, 27.91. 1H NMR (δ, ppm): 1.85–2.00 (m, 2H, CH2), 2.74–2.81 (m, 2H, CH2), 2.81–2.98 (m, 2H, CH2), 3.02–3.42 (m, 1H, CH), 7.21 (t, 1H, Ar–H, J = 7.3 Hz), 7.24–7.32 (m, 4H, Ar–H), 13.22 (br.s, 1H, NHCS, exchangeable), 13.89 (br.s, 1H, NHCS, exchangeable). Anal. calcd for C16H14N2S3 (330.48): C, 58.15; H, 4.27; N, 8.48; found: C, 58.07; H, 4.21; N, 8.51%.
(E/Z)-2-((4-Nitrobenzylidene)amino)-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile (11)
A mixture of 2 (1.27 g, 5 mmol) and 4-nitrobenzaldehyde (0.75 g, 5 mmol) in glacial ethanoic acid (neat condition) was refluxed for 15 min. The solid formed while refluxing was filtered and recrystallized from 1,4-dioxane/DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give orange crystals, mp >300 °C, yield (1.88 g, 97%). Rf = 0.47 (Et2O/EtOAc, 1
1) to give orange crystals, mp >300 °C, yield (1.88 g, 97%). Rf = 0.47 (Et2O/EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 2218 (C
1). IR (ν, cm−1): 2218 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1600 (C
N), 1600 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1556, 1336 (NO2). 1H NMR (δ, ppm): 1.97–2.10 (m, 2H, CH2), 2.78–2.89 (m, 2H, CH2), 2.92–3.09 (m, 2H, CH2), 3.17–3.25 (m, 1H, CH), 7.24 (t, 1H, Ar–H, J = 7.2 Hz), 7.26–7.37 (m, 4H, Ar–H), 8.22 (d, 2H, –C6H4NO2, J = 8.7 Hz), 8.40 (d, 2H, –C6H4NO2, J = 8.7 Hz), 8.84 (s, 1H, CH
N), 1556, 1336 (NO2). 1H NMR (δ, ppm): 1.97–2.10 (m, 2H, CH2), 2.78–2.89 (m, 2H, CH2), 2.92–3.09 (m, 2H, CH2), 3.17–3.25 (m, 1H, CH), 7.24 (t, 1H, Ar–H, J = 7.2 Hz), 7.26–7.37 (m, 4H, Ar–H), 8.22 (d, 2H, –C6H4NO2, J = 8.7 Hz), 8.40 (d, 2H, –C6H4NO2, J = 8.7 Hz), 8.84 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). EIMS, m/z (%): 387.35 (M+˙, 38), 383.00 (76), 365.46 (26), 364.68 (31), 361.33 (52), 344.67 (93), 338.69 (37), 323.07 (23), 278.75 (35), 252.03 (51), 236.28 (65), 224.91 (47), 216.42 (39), 156.91 (55), 144.09 (57), 133.34 (37), 118.91 (54), 103.15 (31), 96.25 (100), 80.83 (38), 73.99 (51), 67.09 (35). Anal. calcd for C22H17N3O2S (387.46): C, 68.20; H, 4.42; N, 10.85; found: C, 68.11; H, 4.36; N, 10.83%.
N). EIMS, m/z (%): 387.35 (M+˙, 38), 383.00 (76), 365.46 (26), 364.68 (31), 361.33 (52), 344.67 (93), 338.69 (37), 323.07 (23), 278.75 (35), 252.03 (51), 236.28 (65), 224.91 (47), 216.42 (39), 156.91 (55), 144.09 (57), 133.34 (37), 118.91 (54), 103.15 (31), 96.25 (100), 80.83 (38), 73.99 (51), 67.09 (35). Anal. calcd for C22H17N3O2S (387.46): C, 68.20; H, 4.42; N, 10.85; found: C, 68.11; H, 4.36; N, 10.83%.
2-(1,3-Dioxoisoindolin-2-yl)-6-phenyl-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbonitrile (12)
A mixture of 2 (1.27 g, 5 mmol) and phthalic anhydride (0.74 g, 5 mmol) in glacial ethanoic acid (2 mL) was refluxed for 1 h or neat condition. The solid formed during reflux was filtered and recrystallized from ethyl alcohol to get bright-yellow crystals, mp 277–278 °C, yield (1.07 g, 56%). Rf = 0.53 (Et2O/EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). IR (ν, cm−1): 2225 (C
1). IR (ν, cm−1): 2225 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), 1788, 1730 (C
N), 1788, 1730 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational coupling). 1H NMR (δ, ppm): 2.03–2.10 (m, 2H, CH2), 2.22–2.29 (m, 2H, CH2), 2.86–2.97 (m, 2H, CH2), 3.07–3.12 (m, 1H, CH), 7.27-7.32-7.40 (m, 7H, Ar–H), 7.89 (d, 1H, Ar–H, J = 7.2 Hz), 8.03 (d, 1H, Ar–H, J = 7.2 Hz). EIMS, m/z (%): 384.31 (M+˙, 22), 309.06 (3), 284.17 (4), 230.09 (7), 229.09 (20), 228.09 (84), 227.08 (57), 155.09 (30), 140.07 (60), 115.06 (37), 114.08 (34), 89.08 (18), 77.07 (100), 59.01 (96), 51.07 (50). Anal. calcd for C23H16N2O2S (384.45): C, 71.86; H, 4.20; N, 7.29; found: C, 71.80; H, 4.16; N, 7.30%.
O vibrational coupling). 1H NMR (δ, ppm): 2.03–2.10 (m, 2H, CH2), 2.22–2.29 (m, 2H, CH2), 2.86–2.97 (m, 2H, CH2), 3.07–3.12 (m, 1H, CH), 7.27-7.32-7.40 (m, 7H, Ar–H), 7.89 (d, 1H, Ar–H, J = 7.2 Hz), 8.03 (d, 1H, Ar–H, J = 7.2 Hz). EIMS, m/z (%): 384.31 (M+˙, 22), 309.06 (3), 284.17 (4), 230.09 (7), 229.09 (20), 228.09 (84), 227.08 (57), 155.09 (30), 140.07 (60), 115.06 (37), 114.08 (34), 89.08 (18), 77.07 (100), 59.01 (96), 51.07 (50). Anal. calcd for C23H16N2O2S (384.45): C, 71.86; H, 4.20; N, 7.29; found: C, 71.80; H, 4.16; N, 7.30%.
Conflicts of interest
No potential conflict of interest was reported by the authors.References
- S. Higazy, N. Samir and A. El-Khouly, et al., Identification of thienopyrimidine derivatives tethered with sulfonamide and other moieties as carbonic anhydrase inhibitors: design, synthesis and anti-proliferative activity, Bioorg. Chem., 2024, 144, 107089 CrossRef CAS PubMed.
- A. H. Abadi, T. M. Ibrahim and K. M. Abouzid, et al., Design, synthesis and biological evaluation of novel pyridine derivatives as anticancer agents and phosphodiesterase 3 inhibitors, Bioorg. Med. Chem., 2009, 17(16), 5974–5982 CrossRef CAS PubMed.
- A. El-Sewedy, E. A. El-Bordany and N. F. Mahmoud, et al., One-pot synthesis, computational chemical study, molecular docking, biological study, and in silico prediction ADME/pharmacokinetics properties of 5-substituted 1H-tetrazole derivatives, Sci. Rep., 2023, 13(1), 17869 CrossRef CAS PubMed.
- S. K. Ramadan, D. R. Abdel Haleem and H. S. Abd-Rabboh, et al., Synthesis, SAR studies, and insecticidal activities of certain N-heterocycles derived from 3-((2-chloroquinolin-3-yl)methylene)-5-phenylfuran-2(3H)-one against Culex pipiens L. larvae, RSC Adv., 2022, 12(22), 13628–13638 RSC.
- M. M. Kaddah, A. A. Fahmi, M. M. Kamel, S. A. Rizk and S. K. Ramadan, Rodenticidal Activity of Some Quinoline-Based Heterocycles Derived from Hydrazide-Hydrazone Derivative, Polycyclic Aromat. Compd., 2023, 43(5), 4231–4241 CrossRef CAS.
- S. K. Ramadan, H. S. Abd-Rabboh, N. M. Gad, W. S. I. Abou-Elmagd and D. S. Haneen, Synthesis and Characterization of Some Chitosan-Quinoline Nanocomposites as Potential Insecticidal Agents, Polycyclic Aromat. Compd., 2023, 43(8), 7013–7026 CrossRef CAS.
- M. A. Aziz, R. A. Serya and D. S. Lasheen, et al., Discovery of potent VEGFR-2 inhibitors based on furopyrimidine and thienopyrimidne scaffolds as cancer targeting agents, Sci. Rep., 2016, 6(1), 24460 CrossRef CAS PubMed.
- M. Asran, E. A. E. El-Helw, M. E. Azab, S. K. Ramadan and M. H. Helal, Synthesis and Antioxidant Activity of Some Benzoquinoline-Based Heterocycles Derived from 2-((3-Chlorobenzo[f]quinolin-2-yl)methylene)hydrazine-1-carbothioamide, J. Iran. Chem. Soc., 2023, 20(12), 3023–3032 CrossRef CAS.
- E. A. E. El-Helw, A. Y. Alzahrani and S. K. Ramadan, Synthesis and antimicrobial activity of thiophene-based heterocycles derived from thiophene-2-carbohydrazide, Future Med. Chem., 2024, 16(5), 439–451 CrossRef CAS PubMed.
- C. J. Shishoo and K. S. Jain, Synthesis of some novel azido/tetrazolothienopyrimidines and their reduction to 2,4-diaminothieno[2,3-d]pyrimidines, J. Heterocycl. Chem., 1992, 29, 883–893 CrossRef CAS.
- S. K. Ramadan, N. A. Ibrahim, S. A. El-Kaed and E. A. E. El-Helw, New potential fungicides pyrazole-based heterocycles derived from 2-cyano-3-(1,3-diphenyl-1H-pyrazol-4-yl)acryloyl isothiocyanate, J. Sulfur Chem., 2021, 42(5), 529–546 CrossRef CAS.
- A. S. Elgubbi, E. A. E. El-Helw, A. Y. Alzahrani and S. K. Ramadan, Synthesis, computational chemical study, antiproliferative activity screening, and molecular docking of some thiophene-based oxadiazole, triazole, and thiazolidinone derivatives, RSC Adv., 2024, 14(9), 5926–5940 RSC.
- A. Archna, S. Pathania and P. A. Chawla, Bioorg. Chem., 2020, 101, 104026 CrossRef PubMed.
- P. Kavaliauskas, B. Grybaite and M. V. ickevicius, et al., Synthesis, ADMET properties, and in vitro antimicrobial and antibiofilm activity of 5-nitro-2-thiophenecarbaldehyde N-((E)-(5-nitrothienyl) methylidene) hydrazone (KTU-286) against Staphylococcus aureus with defined resistance mechanisms, Antibiotics, 2020, 9, 612 CrossRef CAS PubMed.
- K. A. El-Sharkawy, H. M. El-Sehrawi and R. A. Ibrahim, The Reaction of 2-Amino-4,5,6,7-tetrahydrobenzo[b]thiophenes with Benzoyl-Isothiocyanate: Synthesis of Annulated Thiophene Derivatives and Their Antitumor Evaluations, Int. J. Org. Chem., 2012, 2(2), 126–134 CrossRef CAS.
- C. K. Khatri, K. S. Indalkar, C. R. Patil, S. N. Goyal and G. U. Chaturbhuj, Novel 2-phenyl-4,5,6,7-tetrahydro[b]benzothiophene analogues as selective COX-2 inhibitors: design, synthesis, anti-inflammatory evaluation, and molecular docking studies, Bioorg. Med. Chem. Lett., 2017, 27, 1721–1726 CrossRef CAS PubMed.
- R. M. Mohareb and F. M. Manhi, Reaction of Ethyl 2-Diazo-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate with 3-Iminobutyronitrile: Synthesis of Pyridazines, Thiophenes, and Their Fused Derivatives, Heteroat. Chem., 2000, 11(6), 403–412 CrossRef CAS.
- S. Kamal, H. A. Derbala, S. S. Alterary, A. B. Bacha, M. Alonazi, M. K. El-Ashrey and N. N. E. El-Sayed, Synthesis, Biological, and Molecular Docking Studies on 4,5,6,7-Tetrahydrobenzo[b]thiophene Derivatives and Their Nanoparticles Targeting Colorectal Cancer, ACS Omega, 2021, 6, 28992–29008 CrossRef CAS PubMed.
- Q. Han, Z. Yin, J. Sui, Q. Wang and Y. Sun, A Microwave-Enhanced Synthesis and Biological Evaluation of N-Aryl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-amines, J. Braz. Chem. Soc., 2019, 30, 1483–1497 Search PubMed.
- M. F. Mohamed, Y. M. Attia, S. A. Shouman and I. A. Abdelhamid, Anticancer activities of new N-Hetaryl-2-Cyanoacetamide derivatives incorporating 4,5,6,7-Tetrahydrobenzo[b]Thiophene moiety, Anti-Cancer Agents Med. Chem., 2017, 17, 1084–1092 CAS.
- R. Elrayess, Y. M. Abdel Aziz, M. S. Elgawish, M. Elewa, A. S. Yassen, S. S. Elhady, H. A. Elshihawy and M. M. Said, Discovery of Potent Dual EGFR/HER2 Inhibitors Based on Thiophene Scaffold Targeting H1299 Lung Cancer Cell Line, Pharmaceuticals, 2021, 14, 9 CrossRef CAS PubMed.
- F. M. Sroor, M. M. Aboelenin, K. F. Mahrous, K. Mahmoud, A. H. M. Elwahy and I. A. Abdelhamid, Novel 2-cyanoacrylamido-4,5,6,7-tetrahydrobenzo[b]thiophene derivatives as potent anticancer agents, Arch. Pharm., 2020, 353, 2000069 CrossRef CAS PubMed.
- T. Weinert, N. Olieric and R. Cheng, et al., Serial millisecond crystallography for routine room-temperature structure determination at synchrotrons, Nat. Commun., 2017, 8, 542 CrossRef PubMed.
- W. Li, Y. Yin, W. Shuai, F. Xu, H. Yao, J. Liu, K. Cheng, J. Xu, Z. Zhu and S. Xu, Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain, Bioorg. Chem., 2019, 83, 380–390 CrossRef CAS PubMed.
- A. M. Abdelrahman, A. A. Fahmi, S. A. Rizk and E. A. E. El-Helw, Synthesis, DFT and Antitumor Activity Screening of Some New Heterocycles Derived from 2,2′-(2-(1,3-Diphenyl-1H-pyrazol-4-yl)ethene-1,1-diyl)bis(4H-benzo[d][1,3]oxazin-4-one), Polycyclic Aromat. Compd., 2023, 43(1), 721–739 CrossRef CAS.
- M. M. Kaddah, A. R. Morsy and A. A. Fahmi, et al., Synthesis and biological activity on IBD virus of diverse heterocyclic systems derived from 2-cyano-N′-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)acetohydrazide, Synth. Commun., 2021, 51(22), 3366–3378 CrossRef CAS.
- A. I. Hassaballah, S. K. Ramadan, S. A. Rizk, E. A. E. El-Helw and S. S. Abdelwahab, Ultrasonic Promoted Regioselective Reactions of the Novel Spiro 3,1-Benzoxazon-Isobenzofuranone Dye Toward Some Organic Base Reagents, Polycyclic Aromat. Compd., 2023, 43(4), 2973–2989 CrossRef CAS.
- E. A. E. El-Helw, A. M. Abdelrahman, A. A. Fahmi and S. A. Rizk, Synthesis, Density Functional Theory, Insecticidal Activity, and Molecular Docking of Some N-Heterocycles Derived from 2-((1,3-Diphenyl-1H-pyrazol-4-yl)methylene)malonyl diisothiocyanate, Polycyclic Aromat. Compd., 2023, 43(9), 8265–8281 CrossRef CAS.
- Y. M. Youssef, M. E. Azab, G. A. Elsayed, A. A. El-Sayed and E. A. E. El-Helw, Synthesis and Antiproliferative Screening of Some Heterocycles Derived from 4-((5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)-2-phenyloxazol-5(4H)-one, Polycyclic Aromat. Compd., 2023, 43(8), 7152–7163 CrossRef CAS.
- H. A. Khatab, S. F. Hammad, E. M. El-Fakharany, A. I. Hashem and E. A. E. El-Helw, Synthesis and cytotoxicity evaluation of novel 1,8-acridinedione derivatives bearing phthalimide moiety as potential antitumor agents, Sci. Rep., 2023, 13(1), 15093 CrossRef CAS PubMed.
- A. M. Abdelrahman, A. A. Fahmi, E. A. E. El-Helw and S. A. Rizk, Facile Synthesis, Biological Evaluation, DFT Studies and in Silico Prediction ADME/Pharmacokinetics Properties of N-(1-(2-Chlorobenzo[h]quinolin-3-yl)-1-substituted-vin-2-yl)benzamide Derivatives, Polycyclic Aromat. Compd., 2023, 43(7), 6597–6614 CrossRef CAS.
- A. M. Youssef, A. K. El-Ziaty, W. S. I. Abou-Elmagd and S. K. Ramadan, Novel Synthesis of Some Imidazolyl-, Benzoxazinyl-, and Quinazolinyl-2,4-dioxothiazolidine Derivatives, J. Heterocycl. Chem., 2015, 52(1), 278–283 CrossRef CAS.
- C. H. Tseng, et al., Discovery of 2-[2-(5-nitrofuran-2-yl)vinyl]quinoline derivatives as a novel type of antimetastatic agents, Bioorg. Med. Chem., 2015, 23(1), 141 CrossRef CAS PubMed.
- K. Miura, T. Oohashi, S. Matsuda and Y. Igarashi, Chemical and Chemotherapeutical Studies on the Furan Derivatives. XXX. Syntheses and Antibacterial Activities of 2-(5-Nitro-2-furyl)vinyl Heterocyclics, Yakugaku Zasshi, 1963, 83, 771–777 CrossRef CAS PubMed.
- Z. Puterová, A. Krutošíková and D. Végh, Gewald reaction: synthesis, properties and applications of substituted 2-aminothiophenes, ARKIVOC, 2010, 2010, 209 Search PubMed.
- F. Akman and N. Çankaya, A study of experimental and theoretical analysis of N-cyclohexylmethacrylamide monomer based on DFT and HF computations, Pigm. Resin Technol., 2016, 45(5), 301–307 Search PubMed.
- F. Akman, Experimental and theoretical investigation of molecular structure, vibrational analysis, chemical reactivity, electrostatic potential of benzyl methacrylate monomer and homopolymer, Can. J. Phys., 2016, 94(9), 853–864 CrossRef CAS.
- K. N. Halim, S. A. Rizk, M. A. El-Hashash and S. K. Ramadan, Straightforward synthesis, antiproliferative screening and density functional theory study of some pyrazolylpyrimidine derivatives, J. Heterocycl. Chem., 2021, 58(2), 636–645 CrossRef CAS.
- E. A. E. El-Helw, M. Asran, M. E. Azab, M. H. Helal and S. K. Ramadan, Synthesis, Cytotoxic, and Antioxidant Activity of Some Benzoquinoline-Based Heterocycles, Polycyclic Aromat. Compd., 2023 DOI:10.1080/10406638.2023.2270767.
- M. Jabbari and A. Jabbari, Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles, Colloids Surf., A, 2016, 489, 392–399 CrossRef CAS.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS PubMed.
- J. E. Liebmann, J. A. Cook, C. Lipschultz, D. Teague, J. Fisher and J. B. Mitchell, Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines, Br. J. Cancer, 1993, 68, 1104–1109 CrossRef CAS PubMed.
- S. K. Ramadan, H. S. Abd-Rabboh, A. A. Hafez and W. S. I. Abou-Elmagd, Some pyrimidohexahydroquinoline candidates: synthesis, DFT, cytotoxic activity evaluation, molecular docking, and in silico studies, RSC Adv., 2024, 14(23), 16584 RSC.
- R. S. Herbst and F. R. Khuri, Mode of action of docetaxel-a basis for combination with novel anticancer agents, Cancer Treat. Rev., 2003, 29, 407–415 CrossRef CAS PubMed.
- R. S. Keri, K. Chand, S. Budagumpi, S. B. Somappa, S. A. Patil and B. M. Nagaraja, et al., An overview of benzo[b]thiophene based medicinal chemistry, Eur. J. Med. Chem., 2017, 138, 1002–1033 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed.
- C. A. Lipinski, Lead-and drug-like compounds: the rule-of-five revolution, Drug Discovery Today: Technol., 2004, 1, 337–341 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, tables, figures, and spectroscopic data. See DOI: https://doi.org/10.1039/d4ra03363a |
| This journal is © The Royal Society of Chemistry 2024 |