Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CO2 capturing by self-assembled belt[14]pyridine encapsulated ionic liquid complexes: a DFT study†
Annum Ahsana,
Ahmed Lakhanib,
Muhammad Umair Ashrafc,
Muhammad Yarad,
Sehrish Sarfaraza and
Khurshid Ayub *a
*a
aDepartment of Chemistry, COMSATS University, Abbottabad Campus, KPK 22060, Pakistan. E-mail: khurshid@cuiatd.edu.pk; Tel: +92-992-383591
bDepartment of Biomedical and Health Sciences, Calumet College of St. Joseph, Whiting, Indiana 46394, USA
cInstitute for Applied Physics, Department of Physics, University of Science and Technology Beijing, Beijing 100083, China
dDepartment of Chemistry, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Punjab 63100, Pakistan
First published on 8th October 2024
Abstract
In the current study, CO2 capturing ability of encapsulated ionic liquids (ENILs) i.e., tetramethylammonium chloride (TMACl), 1,3-dimethylimidazolium chloride (MIMCl), and methylpyridinium hexafluorophosphate (MPHP) encapsulated in self assembled belt[14]pyridine (BP) has been studied. The results show that strong van der Waals forces are involved in capturing of CO2 by these encapsulated ionic liquids. Strong attractive forces arise from synergistic effect of ionic liquid (encapsulated) and atoms of belt. The interaction energies (Eint) ranging from −12.54 to −18.64 kcal mol−1 reveal the capturing of CO2 by these systems as thermodynamically feasible process. The type and strength of interactions between CO2 and encapsulated ionic liquids is studied through QTAIM and NCI analyses. NCI analysis clearly shows that capturing of CO2 is assisted by van der Waals forces between CO2 and encapsulated ionic liquid complexes. The same feature is confirmed through QTAIM analysis as well. Natural bond orbital (NBO) analysis' results show the charge transfer between the fragments (encapsulated ionic liquids and CO2) which is validated further through electron density differences (EDD) analysis. Overall, transfer of charge towards CO2 from encapsulated ionic liquids is proved through the charge accumulation over CO2 (i.e., blue isosurfaces on CO2 molecules) through EDD analysis. The FMO analyses show the decrease in H–L gaps of encapsulated ionic liquids after CO2 capturing. The successful charge transfer and reduction in H–L gap indicate better interaction in the designed systems thus revealing these systems as a potential candidates for CO2 capturing. Overall, the best results for CO2 capture i.e., the highest interaction energy, the lowest H–L gap, and the strongest forces of interactions are shown by methylpyridinium hexafluorophosphate (MPHP) encapsulated belt[14]pyridine (BP–MPHP) system. This is due to the larger anion of methylpyridinium hexafluorophosphate as compared to the other two encapsulated ionic liquids with Cl− as anion which enables it to develop strong interactions with CO2. The designed belt[14]pyridine based encapsulated ionic liquid systems are promising prospects with better CO2 capture performance and represent a new entrant in the CO2 capturing systems.
1. Introduction
Climate change and global warming issues have now become one of the worldwide topics of interest. The main factor contributing to global warming is increase in concentration of greenhouse gases in atmosphere. The major emission of these gases arises from human activities like automotive vehicles, industrial activities and deforestation etc. CO2 is one of the major greenhouse gases responsible for global warming1 and the methods for Carbon Capture and Storage (CCS) have been under consideration from the last few decades. According to the report presented by National Oceanic and Atmospheric Administration (in November, 2022), the concentration of CO2 has reached to 416 ppm in atmosphere. The concentration of CO2 is noticeably higher than it was during the pre-industrial revolution era (280 ppm).2 CO2 is not only responsible for global warming but also considered as an acute toxin that reduces human cognitive ability3 and can result in critical physiological symptoms.4–6 In order to solve this problem, efforts have been concentrated on exploration of new ways along with improvement in currently used methods for better and more effective capturing and separation of CO2. Some of the methods used for capturing of carbon and its further utilization technologies include adsorption,6,7 absorption,8 membrane separation,9 calcium looping,10 oxyfuel combustion11 and conversion of CO2 into fuels and chemicals12,13 etc. Nevertheless, the search for yet better methods and technologies for CO2 capture are in progress. Out of all the methods used for CO2 capture, absorption by aqueous alkanolamines is considered as benchmark for the industrial processes.8 But the limitations associated with this method are corrosivity,14 degradation of alkanolamines15–17 when used under high temperature and high solvent losses. For the purpose of overcoming these challenges associated with the usage of amines, ionic liquids (ILs) (known as green solvents) have received considerable attention and are considered as the most suitable candidates for CO2 capture.One of the advantages of use of ILs for CO2 capturing is their lower energy of regeneration because of the physical absorption mechanism involved. Due to physical absorption, CO2 sorption enthalpy for ILs (about 10 to 20 kJ mol−1) is much lower than energy required by the standard amine solutions.18 Additional benefits of using ILs are, their high thermal stability,19 high chemical stability,20 recyclability,20 non-volatility,20 non-flammability and their tunable physicochemical properties.20 All these characteristics award ionic liquids great potential for use as absorbents for capturing of CO2. Both experimentally and theoretically, CO2 capturing by ionic liquids has been studied in detail. One of the reported studies include the interaction between CO2 and di-cationic ionic liquids (DILs) showing the effect of cation's symmetry and the length of side chains on interactions between ILs and CO2. It is concluded that the symmetric cation with longer side chains tend to interact more strongly with CO2 molecules.21 Another theoretical study reports the ionic liquids (ILs) and deep eutectic solvents (DESs) as good sources to capture gases. In their study, the environmental friendly and cost-effective cholinium geranate ([Cho][Ger]) IL and cholinium geranate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) geranic acid ([Cho][Ger]
geranic acid ([Cho][Ger]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ger acid) DES are investigated for carbon dioxide (CO2) capture. The same study concludes that the interaction of CO2 is stronger with IL than DES thus introducing a renewable and green IL as an interesting candidate for CO2 capture.22
Ger acid) DES are investigated for carbon dioxide (CO2) capture. The same study concludes that the interaction of CO2 is stronger with IL than DES thus introducing a renewable and green IL as an interesting candidate for CO2 capture.22
Another theoretical study on the mechanism of CO2 absorption is reported where dual functional ionic liquids with the combinations of diethylenetriamine cation ([DETAH]+) or 1-ethanolamine-ethylenediamine cation ([1-AOEt-EDAH]+) and 4-fluorobenzoate anion ([4-F-PhO]) are used.23 This study provides detailed explanation on the absorption mechanism of CO2 by these ILs. Yet another study involves comparison of CO2 absorption by 1,2,4-triazolium-based and imidazolium-based ionic liquids of various anions, namely tetrafluoroborate, bis(trifluoromethylsulfonyl)imide and glycinate. The results reveal that the triazolium-based ionic liquids show higher CO2 solubility as compared to imidazolium cation based ionic liquids of different anions.
Despite extensive use of ionic liquids for CO2 adsorption, certain disadvantages also exist, including their high viscosity.24 The high viscosity of ILs causes low mass transfer rate and may reduce the rate of absorption of CO2. Moreover, the high viscosity causes corrosion of equipment, the maintenance costs to be higher,25–28 and also increase power consumption. In this regard, a related concept i.e., encapsulation of ionic liquids (ENILs) is considered as a feasible substitute in order to overcome the rate limitation of mass transfer for separation processes that depend upon ionic liquids.29 Encapsulated ionic liquids have large surface area and are quite easy to handle.30 Moreover, they have improved energy storage, solubility of gases, and extraction capability as compared to un-encapsulated ionic liquids.30 They contain solid support having ionic liquids incorporated in the form of micro drops.31 They are advanced materials that can be applied in well-established technologies.32 The applications of encapsulated ionic liquids include their use in sewage purification,33 in gas separation34 and as catalysts.35 They are also used for capturing of CO2. For example, for the first time, Shirato and Satoh36 prepared encapsulated microcapsules by the blending of 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide with hydrophobic silica nanoparticles at a very high speed. While, Romanos prepared nanoparticles of silica encapsulating ammonium ionic liquids.37 According to Romanas' study, ENILs with 40% ILs loading show potential to separate CO2 from N2. It was concluded that encapsulated ionic liquids show higher absorption capacity for CO2 i.e., 1.5–3.3 mmol g−1 as compared to the conventional ILs.37 Ionic liquids encapsulated in assembled belt[14]pyridine (BP) have been investigated recently but have not been used for capturing of gases. The specialty of belt molecules is the fully conjugated π-system with outstanding complexation properties. They play a vital role in supramolecular chemistry.38 The defined space/cavity inside the belt molecules awards them capability of forming complexes with various molecules that fit the size of the cavity. Moreover, the space/cavity inside the belts can be deepened through stacking or assembly of belts. Keeping in view the literature studies that encapsulated ILs provide better results for capturing of gases, we have used the newly designed encapsulated ILs i.e., assembled belt[14]pyridine (BP) encapsulated ionic liquids (BP–ILs) for CO2 capture in the current study. Our work involves detailed study on interaction of CO2 with BP–ILs.
2. Methodology
Geometry optimization of all the BP–IL–CO2 complexes (encapsulated ionic liquids with CO2 captured) has been performed by using Gaussian 09.39 DFT method i.e., Becke's three parameter hybrid with Lee–Yang–Parr correlation (B3LYP) along with Pople split valence basis set 6-311G(d,p) are used. The previous studies reveal this level of theory as the most suitable one for ionic liquids23,40 because a good agreement to the experimental data was found.41–43 Nanobelts have also been studied theoretically frequently at this level of theory as per the literature review.44,45 However, keeping in mind the noteworthy dispersion forces present in the designed structures, we've calculated the interaction energies by taking dispersion correction into account. For this purpose, we used Grimme's DFT-D3 method and finally optimized the structures at B3LYP-D3/6-311 G(d,p) level of theory. For the purpose of visualization of structures, generation of input files and for analyzing the output files, software GaussView 5.0 is used.46 The presence of true minima and stable optimized structures was ensured by the absence of imaginary frequencies in the calculated vibrational frequencies. The interaction energies (Eint) can be used to understand the strength of interaction between encapsulated ionic liquids (BP–ILs) and CO2 which is defined as the difference between the total energy of the complex (BP–IL–CO2) and sum of the energies of encapsulated ionic liquids (BP–IL) and CO2 molecule. The formula used for ΔEint calculation is,| ΔEint = Ecomplex − (EBP–IL + ECO2) | (1) |
Quantum chemical calculations performed on the complexes containing fragments in the structure (interacting with each other) are more susceptible towards basis set superposition error (BSSE). Hence, such cases demand corrections. In this regard, counter poise method has been considered as the appropriate method to correct the energy. The following equation is used in this method,
| ΔEint,CP = Eint − EBSSE | (2) |
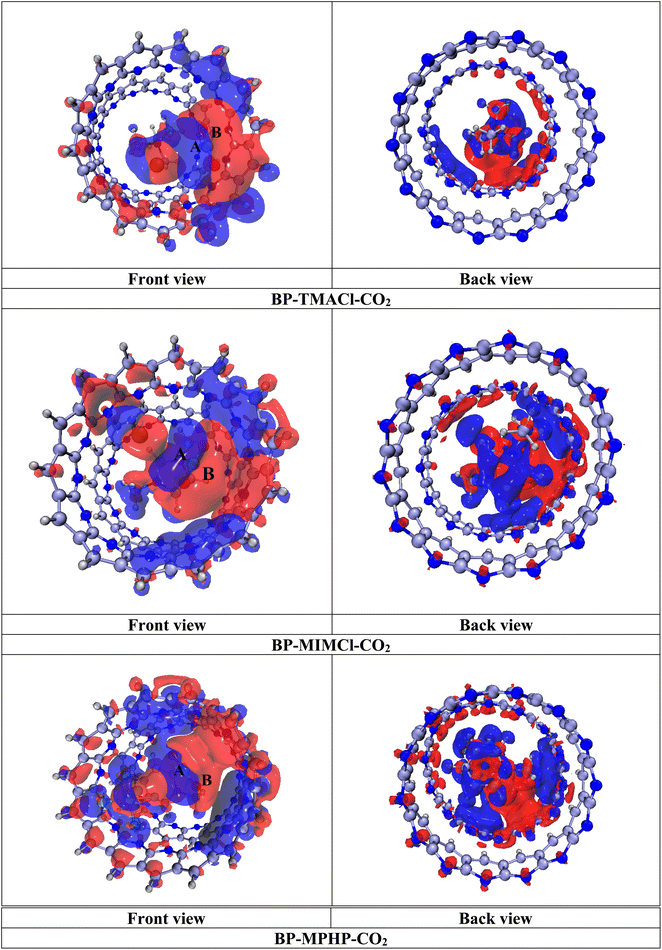
For detailed investigation of transfer of charge between the fragments (BP–ILs and CO2), the analysis of natural bond orbitals (NBOs) is carried out. Moreover, for the purpose of further investigation and visualization of the interactions between fragments in terms of the accumulation and depletion of charge, the electron density differences (EDD) analysis is employed. Furthermore, for examination of changes in electronic properties after BP–ILs and CO2 interact with each other, frontier molecular orbitals (FMOs) analysis is performed.47,48
In the capturing of CO2, major role is played by noncovalent interactions between BP–ILs and CO2. Therefore, it is important to estimate the interactions involved in CO2 capturing. For this purpose, the non-covalent interaction (NCI) analysis is used which distinguishes and visualizes different nonbonding interaction forces i.e., repulsive forces, van der Waals forces, and electrostatic interactions. Through NCI analysis, we get 2-D reduced density gradient (RDG) plots along with 3-D isosurfaces of BP–ILs–CO2 which are generated with the help of Multiwfn 3.8 software.49 The 2-D RDG graphs depend on electron density (ρ) and reduced density gradient.50 Their mathematical relationship is given as:
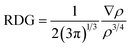 | (3) |
The noncovalent interactions' nature is evaluated by the help of color scheme, which depends on the value of sign(λ2)ρ. In 3-D NCI plots, green, blue, and red isosurfaces are associated with small negative value, higher negative value, and higher positive value of sign(λ2)ρ in RDG plots, respectively. The green color indicates weak van der Waals interactions, blue color indicates strong electrostatic interactions, and red isosurfaces show repulsive forces.
For further exploration of noncovalent interactions' nature between CO2 and BP–ILs, quantum theory of atoms in molecules (QTAIM) analysis is used. In QTAIM, different topological parameters i.e., electron density (ρ), Laplacian of electron density (∇2ρ), kinetic energy density (Lagrangian) G(r), potential energy density V(r), and total energy density H(r) are calculated in order to understand nature of interactions through bond critical points (BCPs).51,52
3. Results and discussion
3.1 Geometric properties
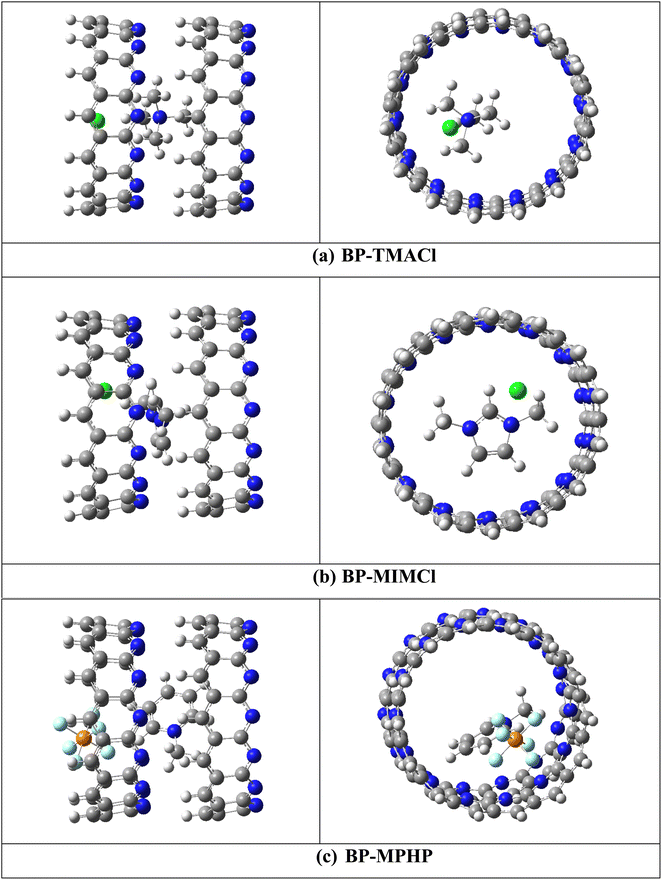
Assembled nanobelt[14]pyridine encapsulated ionic liquids (ENILs) are used for carbon dioxide (CO2) capture in the current study. These ENILs are, tetramethylammonium chloride (TMACl) encapsulated belt[14]pyridine (BP–TMACl), 1,3-dimethylimidazolium chloride (MIMCl) encapsulated belt[14]pyridine (BP–MIMCl), and methylpyridinium hexafluorophosphate (MPHP) encapsulated belt[14]pyridine (BP–MPHP). The mentioned encapsulated ionic liquids have been designed by our group recently. Fig. 2 shows the encapsulated ionic liquids while Fig. 1 presents the geometries of non-encapsulated ionic liquids.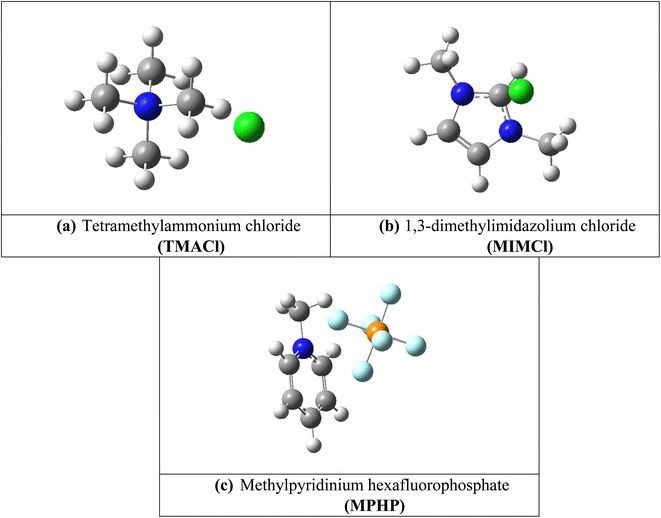 | ||
| Fig. 1 The optimized structures of ILs; (a) tetramethylammonium chloride (TMACl), (b) 1,3-dimethylimidazolium chloride (MIMCl) and (c) methylpyridinium hexafluorophosphate (MPHP). | ||
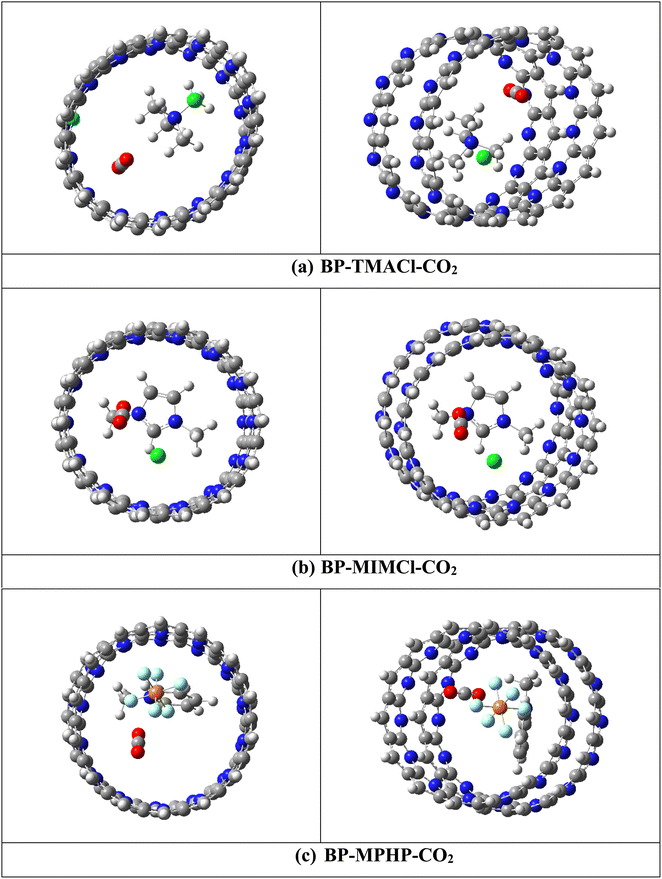
For capturing of CO2 by these encapsulated ionic liquids, two different initial orientations have been selected for CO2 with respect to the encapsulated ionic liquids (Fig S1†). The first orientation contains CO2 near cation of ionic liquid while second orientation contains CO2 near anion of ionic liquid inside the belts' cavity. In case of tetramethylammonium chloride encapsulated belt[14]pyridine, the results show that the orientation containing CO2 near cation is more stable one and interaction is more exothermic for CO2 capturing in this orientation. The results in case of 1,3-dimethylimidazolium chloride and methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine show that better capturing properties along with more exothermic interactions are displayed when CO2 is kept near anions (oriented more towards anion and oriented slightly away from cation). The cation with concentrated charge (tetramethylammonium) attracts CO2 more strongly as compared to anion as in case of tetramethylammonium chloride encapsulated belt[14]pyridine. The reason can be the two electronegative electron rich oxygen atoms of CO2 which show more attraction towards the cation (with concentrated charge) as compared to anion). While, in case of 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine and methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine, the cations are 1,3-dimethylimidazolium and methylpyridinium which contain a ring structure with charge delocalized over it. The delocalization affects the intensity of the charge of cation i.e., lowers its impact and reduces the interaction between CO2 and cation. Hence, anions in these cases show stronger interaction with CO2. The optimized structures with the stable orientations for CO2 capturing are presented in Fig. 3.
Overall, the process of capturing CO2 by all the three encapsulated ionic liquids is thermodynamically feasible as revealed through negative interaction energies ranging from −12.54 to −18.61 kcal mol−1 (calculated using eqn (1)). The negative interaction energies point toward the exothermic nature and experimental feasibility of these reactions. The trend of interaction energies followed by BP–ILs–CO2 complexes is BP–MPHP–CO2 (−18.61 kcal mol−1) > BP–MIMCl–CO2 (−14.43 kcal mol−1) > BP–TMACl–CO2 (−12.64 kcal mol−1). CO2 captured by methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine shows largest interaction energy due to the larger anion of methylpyridinium hexafluorophosphate as compared to the other two encapsulated ionic liquids with Cl− as anion. Larger anion of methylpyridinium hexafluorophosphate enables it to develop strong interaction with CO2, both cation and anion play their role in capturing of CO2 hence awarding better CO2 capturing properties to methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine.
As the designed complexes contain fragments, hence basis set superposition error (BSSE) corrected interaction energies have been calculated (Table 1). The BSSE corrected interaction energies involved in CO2 capturing process range from −10.36 to −12.63 kcal mol−1. Comparing BSSE energies with uncorrected energies (Eint), we see that the absolute values of BSSE energies are altered slightly.
| BP–IL–CO2 | Eint (BP–IL–CO2) | BSSE | OCO bend angle | EH | EL | H–L gap |
|---|---|---|---|---|---|---|
| CO2 | — | — | — | −10.25 | 0.80 | 9.45 |
| BP–TMACL | — | — | — | −5.39 | −5.12 | 0.27 |
| BP–TMACL–CO2 | −12.64 | −10.36 | 174 | −5.40 | −5.14 | 0.26 |
| BP–MIMCL | — | — | — | −5.39 | −5.12 | 0.27 |
| BP–MIMCL–CO2 | −14.43 | −12.63 | 173 | −5.40 | −5.14 | 0.26 |
| BP–MPHP | — | — | — | −5.41 | −5.12 | 0.29 |
| BP–MPHP–CO2 | −18.61 | −11.22 | 178 | −5.39 | −5.14 | 0.25 |
Moreover, the adsorbed CO2 also shows bending after interacting with the encapsulated ionic liquids. The angle of CO2 is changed from 180° to 173°, 174° and 178° for BP–MIMCl–CO2, BP–TMACl–CO2 BP–MPHP–CO2, respectively. The change in angle shows better interaction of CO2 with encapsulated ionic liquid systems.
3.2 Electronic properties
In case of CO2 capturing by tetramethylammonium chloride encapsulated belt[14]pyridine (BP–TMACl), the belt atoms in BP–TMACl show the negative charge ranging from −0.417 to −0.422|e| on all the nitrogen atoms that are pointing outward (outer nitrogen) whereas the nitrogen atoms that lie at the joining points of two nanobelt units (inner nitrogen) bear negative charges of −0.510 to −0.525|e|. After introducing CO2, the NBO charges on belt atoms decrease slightly. The negative charge ranges from −0.417 to −0.421|e| and −0.509 to −0.525|e| on outer and inner nitrogen atoms, respectively in BP–TMACl–CO2 complex. Discussing the interaction between ionic liquid and CO2, the charge on O atoms of CO2 has increased from −0.499|e| to −0.529|e| and −0.508|e| while the negative charge on the chloride (Cl−) of ionic liquid tetramethylammonium chloride has decreased from −0.941|e| to −0.934|e|. Moreover, the positive charge on carbon atom of CO2 has increased from 0.998|e| (uncaptured carbon) to 1.028|e| (in captured carbon). This shift of charge from carbon can be seen on the carbon atoms of the belt surrounding captured CO2 molecule. CO2 molecule has not only interacted with ionic liquid but its interaction with the belt atoms in terms of charge transfer can also be observed. Overall, the charge analysis shows that CO2 has accumulated the charge from tetramethylammonium chloride encapsulated belt[14]pyridine.
After introducing CO2 to 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine (BP–MIMCl), the nitrogen atoms show slight increase in negative charge from −0.41–−0.416|e| to −0.414–−0.417|e| on outer nitrogen atoms of the assembled belts. Discussing the interaction between 1,3-dimethylimidazolium chloride and CO2, the charge on O atoms of CO2 has increased from −0.499|e| to −0.516|e| and −0.524|e| while the negative charge on the Cl− of ionic liquid (1,3-dimethylimidazolium chloride) has decreased from −0.943|e| to −0.942|e|. Moreover, the positive charge on carbon atom of CO2 has increased from 0.998|e| (uncaptured carbon) to 1.033|e| (in captured carbon). Overall, the charge analysis shows that CO2 molecule has accumulated the charge from 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine.
In case of BP–MPHP–CO2 complex (methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine with captured CO2), the outer nitrogen atoms of belts show an increase in magnitude of negative charge from −0.413 to −0.419|e| (for BP–MPHP) to the range of −0.416 to −0.419|e| while inner nitrogen atoms show an increase in charge up to −0.533 to −0.512|e| from −0.503 to −0.534|e| (for BP–MPHP) after capturing CO2. Discussing the interaction between methylpyridinium hexafluorophosphate and CO2, the charge on O atoms of CO2 has increased from −0.499|e| to −0.524|e| and −0.505|e| while the negative charge on the F-atoms of anion of ionic liquid has decreased. Moreover, the positive charge on carbon atom of CO2 has increased from 0.998|e| (uncaptured carbon) to 1.026|e| (in captured carbon). In this case, again CO2 accumulates the charge from methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine.
The NBO analysis shows that CO2 molecule has not just interacted with ionic liquid but its interaction with the atoms of belt is also observed through charge transfer between them. This is why, in the current study, the interaction energies of encapsulated ionic liquids (BP–ILs) with captured CO2 are quite higher than the systems where pure or un-encapsulated forms of ILs are used for CO2 capturing.23,40
Additionally, there is overall slight change observed in NBO charges over the atoms of complex, although strong interactions have been detected between the fragments. The reason is transfer of charge taking place in both the directions i.e., from CO2 towards the atoms of belt & ionic liquid and vice versa. When certain amount of charge is transferred from CO2 molecule towards the atoms of encapsulated ionic liquids and at the same time, charge is transferred backwards as well, then overall change in NBO charge becomes lower. Hence, we can say that exchange of charges is taking place which leads to the net effect of slight change in charge. Moreover, despite overall slight change in NBO charges, CO2 molecule shows more charge accumulation as compared to the depletion of charge.
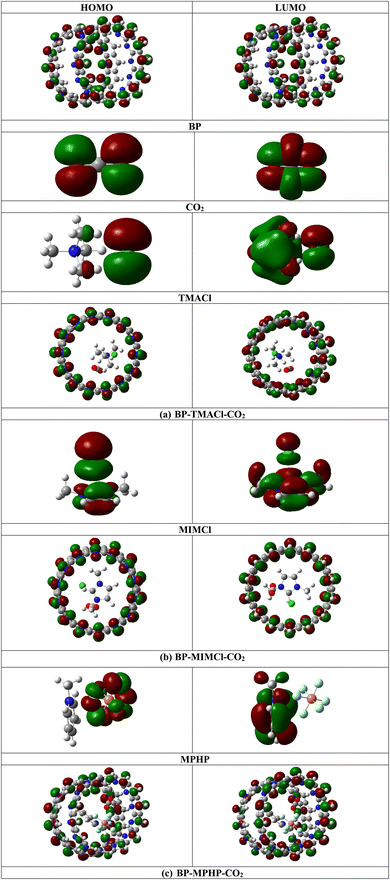
The H–L gap of encapsulated ionic liquids decreases slightly after CO2 capturing. For BP–TMACl–CO2, H–L gap decreases to 0.26 eV from 0.27 eV (for tetramethylammonium chloride encapsulated belt[14]pyridine). For BP–MIMCl–CO2 and BP–MPHP–CO2, the H–L gaps decrease to 0.26 eV and 0.25 eV from 0.27 eV (for 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine) and 0.29 eV (for methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine), respectively. All the three systems show very slight change in H–L gap. The comparatively greater change in H–L gap is shown by methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine (BP–MPHP) after capturing of CO2, showing better interaction of CO2 with BP–MPHP as compared to the other two encapsulated ionic liquids. In case of methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine, the energy of HOMO (EH) is equal to −5.41 eV while its LUMO energy (EL) is equal to −5.12 eV. After CO2 capturing, the EH increases to −5.39 eV and EL decreases to −5.14 eV. This leads to overall decrease in energy gap. On the other hand, 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine doesn't show any change in EL before and after CO2 capturing but shows an increase in EH from −5.39 eV (for BP–MIMCl) to −5.38 eV. The increase in EH after CO2 capture results in overall reduction in H–L gap. For BP–TMACl–CO2 complex (tetramethylammonium chloride encapsulated belt[14]pyridine with CO2 captured), the EH shows decrease in value from −5.39 eV (tetramethylammonium chloride encapsulated belt[14]pyridine) to −5.40 eV. On the other hand, the decrease in EL from −5.12 eV to −5.14 eV results in reduction in H–L gap. The values of EH, EL and H–L gaps for uncaptured CO2, encapsulated ionic liquids (BP–ILs) and encapsulated ionic liquid with CO2 captured (BP–ILs–CO2) complexes are given in Table 1 and the densities of HOMO and LUMO are shown in Fig. 5.
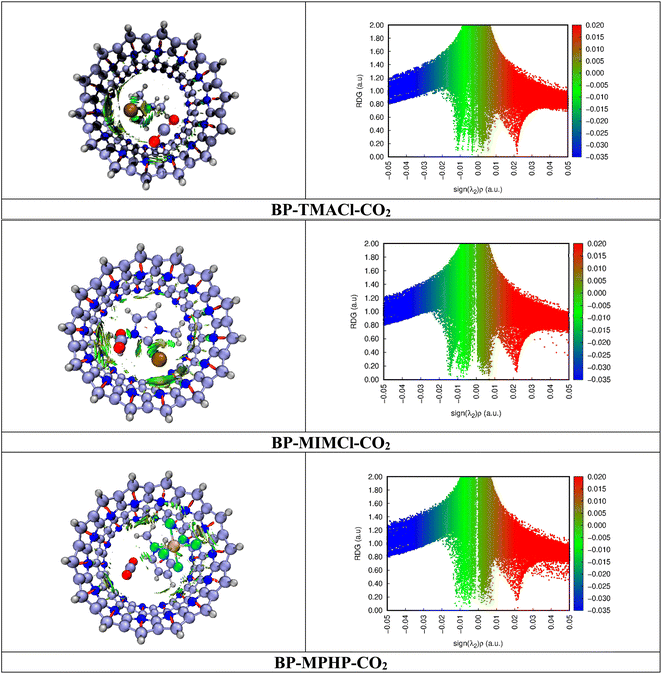
3.3 Reduced density gradient analysis
Noncovalent interaction (NCI) analysis is also known as reduced density gradient (RDG) analysis. It is basically a visual approach NCI analysis used for studying the nonbonding interactions in complexes. The analysis evaluates the nature of nonbonding interaction forces in complexes i.e., repulsive forces, van der Waals interactions and hydrogen bonding. Fig. 6 presents the scatter 2-D plots and 3-D topological graphs of studied BP–ILs–CO2 complexes (assembled belt[14]pyridine encapsulated ionic liquids with CO2 captured). The generated 2-D RDG scattered spectra present green, red, and blue colors as a function of λ2(ρ) in the range of −0.05 a.u. to 0.05 a.u. Blue colored spikes are not observed in these spectra showing the absence of strong electrostatic interactions or hydrogen bonding involved in CO2 capturing. The green colored spikes can be seen in the range of 0.01 a.u. to −0.02 a.u. of λ2(ρ). They depict London dispersion forces in the system. Moreover, the red colored spikes present in the spectra at the region of above 0.02 a.u. of λ2(ρ) depict the steric repulsive forces present in the complexes. Fig. 6 shows the 3-D topologies where the light brown and green patches are present. Light brown and green patches are observed in the complex around CO2 molecule. The patches are present between CO2 and ionic liquids indicating van der Waals interactions between them. These patches are also present between CO2 and BP showing CO2's interaction with BP through van der Waals forces. Moreover, in 2-D RDG plots of all the three CO2 captured encapsulated ionic liquid (BP–ILs) complexes, the green spikes appear at up to −0.02 a.u., which is indication of strong van der Waals forces having main role in CO2 capturing. NCI analysis clearly shows that capturing of CO2 is assisted by van der Waals forces between CO2 and both ionic liquid and belt[14]pyridine atoms.3.4 QTAIM analysis
For the detailed evaluation of noncovalent interactions further, Bader's QTAIM analysis is considered. The nature of nonbonding interactions is estimated on the basis of bond critical points with the help of a number of topological parameters i.e., electron density (ρ), Laplacian ∇2(ρ), and total electron density H(r) which is the sum of local kinetic energy G(r) and local potential energy V(r).| H(r) = V(r) + G(r) | (4) |
The values of these parameters decide the type and strength of interaction forces in complexes. When the values of total energy density H(r) and Laplacian ∇2ρ are positive, the interactions between the fragments forming complexes are noncovalent in nature, whereas negative values of these parameters reveal the presence of covalent bonding. Moreover, total energy density H(r) less than zero (H(r) < 0) and greater than zero (H(r) > 0) reveal shared shell and closed-shell interactions, respectively. Furthermore, the strength of noncovalent interactions is revealed through electron density (ρ) i.e., for strong forces of attraction (covalent interactions), the value of electron density has to be positive always (ρ > 0.1 a.u.) while for weak forces of attraction (noncovalent interactions), the value of electron density has to be negative always (ρ < 0.1 a.u.). Additionally, interaction energy (Eint) of individual bonds also helps in evaluation of nature of bonding (computed via Espinosa approach).
| Eint (a.u.) = ½V(r) | (5) |
The values of Eint ranging from 3 to 10 kcal mol−1 show existence of hydrogen bonding (strong electrostatic interactions). Similarly, another parameter i.e., the ratio −V/G also helps in evaluation of the nature of interactions, the values of −V/G < 1 and −V/G > 2 show nonbonding and covalent interactions, respectively.
Fig. 7 shows the topologies calculated with the help of QTAIM analysis while Table 2 contains the BCP parameters. The QTAIM analysis of the BP–ILs–CO2 complexes i.e., BP–TMACl–CO2, BP–MIMCl–CO2 and BP–MPHP–CO2 shows that total numbers of BCPs found for BP–TMACl–CO2 are four (4), for BP–MIMCl–CO2 are five (5) and for BP–MPHP–CO2 are three (3). The BCPs are related to the possible number of nonbonding interactions between the CO2 molecule and encapsulated ionic liquids, BP–ILs.
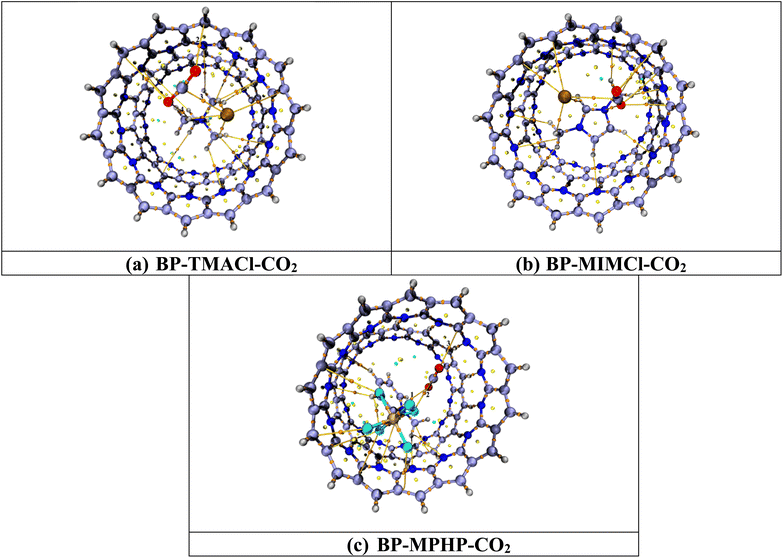 | ||
| Fig. 7 QTAIM analysis results of BP–ILs–CO2 complexes. Bond paths are shown by lines between CO2 and BP–IL, whereas bond critical points (BCPs) are presented by colored dots. | ||
| BP–ILs–CO2 | BP–ILs–CO2 | ρ (a.u.) | ∇2ρ (a.u.) | G(r) (a.u.) | V(r) (a.u.) | H(r) (a.u.) | −V/G | Eint (kcal mol−1) |
|---|---|---|---|---|---|---|---|---|
| BP–TMACl–CO2 | C159⋯C158 | 0.009 | 0.031 | 0.006 | −0.005 | 0.0016 | −3.125 | −1.569 |
| O160⋯C90 | 0.004 | 0.012 | 0.002 | −0.002 | 0.0006 | −3.333 | −0.628 | |
| O161⋯C112 | 0.002 | 0.008 | 0.002 | −0.001 | 0.0004 | −2.500 | −0.314 | |
| O161⋯H146 | 0.003 | 0.011 | 0.002 | −0.002 | 0.0005 | −4.000 | −0.628 | |
| BP–MPHP–CO2 | C163⋯F161 | 0.009 | 0.044 | 0.009 | −0.008 | 0.0016 | −0.889 | −2.510 |
| O164⋯C141 | 0.008 | 0.029 | 0.006 | −0.005 | 0.0012 | −0.833 | −1.569 | |
| O165⋯C88 | 0.007 | 0.026 | 0.006 | −0.005 | 0.0009 | −0.833 | −1.569 | |
| BP–MIMCl–CO2 | C158⋯Cl155 | 0.010 | 0.034 | 0.007 | −0.005 | 0.0016 | −0.71 | −1.569 |
| O159⋯N147 | 0.007 | 0.027 | 0.006 | −0.005 | 0.0009 | −0.83 | −1.569 | |
| O159⋯C7 | 0.005 | 0.019 | 0.004 | −0.003 | 0.0008 | −0.75 | −0.941 | |
| O159⋯C82 | 0.006 | 0.020 | 0.004 | −0.003 | 0.0008 | −0.75 | −0.941 | |
| O160⋯C87 | 0.006 | 0.019 | 0.004 | −0.003 | 0.0008 | −0.75 | −0.941 |
The topological parameters presented in Table 2 show that the CO2 capturing by assembled belt[14]pyridine encapsulated ionic liquids is assisted by strong van der Waals interaction forces. For all the three systems designed for CO2 capturing i.e., tetramethylammonium chloride encapsulated belt[14]pyridine, 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine, and methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine, both total energy density H(r) and Laplacian ∇2ρ are positive showing noncovalent interactions involved in CO2 capturing. The electron density values for BP–ILs–CO2 complexes (encapsulated ionic liquid complexes with CO2 captured) are less than 0.1 a.u. but not negative i.e., ranging from 0.002–0.010 a.u. These values for electron density show presence of strong noncovalent interactions involved in CO2 capturing and among three systems, the values are overall higher for CO2 captured methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine (BP–MPHP–CO2) revealing the strongest interaction in this system as compared to the other two. Moreover, the calculated Eint (values via Espinosa approach) for our designed systems range from −0.314 to −2.510 kcal mol−1 indicating presence of van der Waals forces. These values are greatest i.e., ranging from −1.569–2.510 kcal mol−1 in case of BP–MPHP–CO2 (similar to the Eint calculated through eqn (1)). In the same way, the values of other topological parameters for BP–MPHP–CO2 are also higher as compared to CO2 captured 1,3-dimethylimidazolium chloride encapsulated belt[14]pyridine complex (BP–MIMCl–CO2) and CO2 captured tetramethylammonium chloride encapsulated belt[14]pyridine complex (BP–TMACl–CO2). Hence, the strongest van der Waals forces of attraction are present in BP–MPHP–CO2. Likewise, the higher values calculated for Laplacian ∇2(ρ) and electron density (ρ) also point toward the stronger interaction between CO2 and methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine. We conclude through QTAIM analysis that stronger van der Waals forces are playing vital role in CO2 capturing in our designed systems and methylpyridinium hexafluorophosphate encapsulated belt[14]pyridine is the best choice for CO2 capturing. The results computed with the help of QTAIM analysis can be strongly correlated to the results of interaction energies and NCI analysis.
4. Conclusions
In the current study, three different ILs encapsulated in self-assembled belt[14]pyridine have been studied for CO2 capturing. The encapsulated ionic liquids are, tetramethylammonium chloride (TMACl), 1,3-dimethylimidazolium chloride (MIMCl) and methylpyridinium hexafluorophosphate (MPHP) encapsulated in self-assembled belt[14]pyridine. The interaction energies ranging from −12.54 to −18.61 kcal mol−1 show thermodynamic feasibility of capturing of CO2 by these systems. Moreover, the detailed study of CO2 capturing involves NBO and FMO analysis. NBO analysis shows the charge transfer between fragments (BP–ILs and CO2) which is further confirmed through EDD analysis. FMO analysis reveals slight reduction in H–L gap after capturing of CO2 by encapsulated ionic liquids (BP–ILs). With the largest Eint calculated for BP–MPHP–CO2, the maximum reduction in H–L gap is also shown by the same system after CO2 capturing i.e., from 0.29 eV to 0.25 eV. Furthermore, the nature and strength of forces involved in CO2 capturing process are studied through NCI and QTAIM analysis. The results of both the analyses show that strong van der Waals forces are involved in capturing process in all the systems. The strongest interactions are observed in BP–MPHP–CO2 system. Overall, the system showing largest interaction energy (−18.61 kcal mol−1), the lowest H–L gap (0.25 eV) and strongest forces of interactions for CO2 capturing is methylpyridinium hexafluorophosphate encapsulated self-assembled belt[14]pyridine based system. The study provides satisfactory results for CO2 capturing by the novel encapsulated ionic liquids systems.Data availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.References
- M. R. Qader, et al., Forecasting carbon emissions due to electricity power generation in Bahrain, Environ. Sci. Pollut. Res., 2021, 1–12 Search PubMed.
- C. Pistidda, Preface to the special section on materials for chemical and electrochemical energy storage, J. Mater. Sci., 2022, 57(22), 9937–9938 CrossRef.
- H. Thakkar, et al., 3D-printed zeolite monoliths for CO2 removal from enclosed environments, ACS Appl. Mater. Interfaces, 2016, 8(41), 27753–27761 CrossRef PubMed.
- T. Rogers, J. de Leon and D. Atcher, Possible interaction between warfarin and quetiapine, J. Clin. Psychopharmacol., 1999, 19(4), 382–383 CrossRef PubMed.
- U. Satish, et al., Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance, Environ. Health Perspect., 2012, 120(12), 1671–1677 CrossRef CAS PubMed.
- T. Vehviläinen, et al., High indoor CO2 concentrations in an office environment increases the transcutaneous CO2 level and sleepiness during cognitive work, J. Occup. Environ. Hyg., 2016, 13(1), 19–29 CrossRef.
- I. M. Saeed, et al., Opportunities and challenges in the development of monoethanolamine and its blends for post-combustion CO2 capture, Int. J. Greenhouse Gas Control, 2018, 79, 212–233 CrossRef CAS.
- A. Baghban, et al., Prediction of CO2 loading capacities of aqueous solutions of absorbents using different computational schemes, Int. J. Greenhouse Gas Control, 2017, 57, 143–161 CrossRef CAS.
- N. H. Solangi, et al., A review of recent trends and emerging perspectives of ionic liquid membranes for CO2 separation, J. Environ. Chem. Eng., 2021, 9(5), 105860 CrossRef CAS.
- D. Wei, et al., Process simulation and economic analysis of calcium looping gasification for coal to synthetic natural gas, Fuel Process. Technol., 2021, 218, 106835 CrossRef CAS.
- Y. Zhu, et al., Life cycle water consumption for oxyfuel combustion power generation with carbon capture and storage, J. Cleaner Prod., 2021, 281, 124419 CrossRef CAS.
- M. Usman, et al., Trends and Prospects in UiO-66 Metal-Organic Framework for CO2 Capture, Separation, and Conversion, Chem. Rec., 2021, 21, 1771–1791 CrossRef CAS PubMed.
- S. A. Mazari, et al., An overview of catalytic conversion of CO2 into fuels and chemicals using metal organic frameworks, Process Saf. Environ. Prot., 2021, 149, 67–92 CrossRef CAS.
- N. Kladkaew, et al., Corrosion behavior of carbon steel in the monoethanolamine− H2O− CO2− O2− SO2 system: products, reaction pathways, and kinetics, Ind. Eng. Chem. Res., 2009, 48(23), 10169–10179 CrossRef CAS.
- A. Bello and R. O. Idem, Pathways for the formation of products of the oxidative degradation of CO2-loaded concentrated aqueous monoethanolamine solutions during CO2 absorption from flue gases, Ind. Eng. Chem. Res., 2005, 44(4), 945–969 CrossRef CAS.
- B. R. Strazisar, R. R. Anderson and C. M. White, Degradation pathways for monoethanolamine in a CO2 capture facility, Energy Fuels, 2003, 17(4), 1034–1039 CrossRef CAS.
- J. Davis and G. Rochelle, Thermal degradation of monoethanolamine at stripper conditions, Energy Procedia, 2009, 1(1), 327–333 CrossRef.
- C. Cadena, et al., Why is CO2 so soluble in imidazolium-based ionic liquids?, J. Am. Chem. Soc., 2004, 126(16), 5300–5308 CrossRef PubMed.
- S. A. Forsyth, J. M. Pringle and D. R. MacFarlane, Ionic liquids—an overview, Aust. J. Chem., 2004, 57(2), 113–119 CrossRef.
- J. Huang and T. Rüther, Why are ionic liquids attractive for CO2 absorption? An overview, Aust. J. Chem., 2009, 62(4), 298–308 CrossRef.
- M. Torkzadeh and M. Moosavi, CO2 capture using dicationic ionic liquids (DILs): molecular dynamics and DFT-IR studies on the role of cations, J. Chem. Phys., 2023, 158(2), 024503 CrossRef PubMed.
- J. Płotka-Wasylka, et al., Deep eutectic solvents vs. ionic liquids: similarities and differences, Microchem. J., 2020, 159, 105539 CrossRef.
- B. Wang, et al., Design of novel dual functional ionic liquids and DFT study on their CO2 absorption mechanism, J. Mol. Liq., 2022, 366, 120340 CrossRef CAS.
- W. Kunz and K. Häckl, The hype with ionic liquids as solvents, Chem. Phys. Lett., 2016, 661, 6–12 CrossRef CAS.
- S. Zheng, et al., State of the art of ionic liquid-modified adsorbents for CO2 capture and separation, AIChE J., 2022, 68(2), e17500 CrossRef CAS.
- J. Haider, et al., State-of-the-art process simulations and techno-economic assessments of ionic liquid-based biogas upgrading techniques: challenges and prospects, Fuel, 2022, 314, 123064 CrossRef CAS.
- C. Ma, et al., Techno-economic analysis and performance comparison of aqueous deep eutectic solvent and other physical absorbents for biogas upgrading, Appl. Energy, 2018, 225, 437–447 CrossRef CAS.
- S. Zeng, et al., Ionic-liquid-based CO2 capture systems: structure, interaction and process, Chem. Rev., 2017, 117(14), 9625–9673 CrossRef CAS PubMed.
- C. P. Mehnert, Supported ionic liquid catalysis, Chem.–Eur. J., 2005, 11(1), 50–56 CrossRef.
- Q. Luo, et al., Pickering emulsion-templated encapsulation of ionic liquids for contaminant removal, ACS Appl. Mater. Interfaces, 2019, 11(9), 9612–9620 CrossRef CAS.
- L. Rubén Santiago, Sistemas avanzados basados en líquidos iónicos para la captura y conversión de gases, PhD diss., Universidad Autónoma de Madrid, 2019.
- J. Palomar, et al., Encapsulated ionic liquids (ENILs): from continuous to discrete liquid phase, Chem. Commun., 2012, 48(80), 10046–10048 RSC.
- D.-x. Chen, et al., Adsorption of caprolactam from aqueous solution by novel polysulfone microcapsules containing [Bmim][PF6], Colloids Surf., A, 2014, 441, 72–76 CrossRef CAS.
- V. V. Chaban and O. V. Prezhdo, Nanoscale carbon greatly enhances mobility of a highly viscous ionic liquid, ACS Nano, 2014, 8(8), 8190–8197 CrossRef CAS PubMed.
- R. Santiago, et al., From kinetics to equilibrium control in CO2 capture columns using Encapsulated Ionic Liquids (ENILs), Chem. Eng. J., 2018, 348, 661–668 CrossRef CAS.
- K. Shirato and M. Satoh, Dry ionic liquid” as a newcomer to “dry matter, Soft Matter, 2011, 7(16), 7191–7193 RSC.
- G. E. Romanos, et al., CO2 capture by novel supported ionic liquid phase systems consisting of silica nanoparticles encapsulating amine-functionalized ionic liquids, J. Phys. Chem. C, 2014, 118(42), 24437–24451 CrossRef.
- K. Tahara and Y. Tobe, Molecular loops and belts, Chem. Rev., 2006, 106(12), 5274–5290 CrossRef.
- R. Carlsen, J. R. Jenkins and D. H. Ess, Direct dynamics analysis of the cationic Cp*(PMe 3) Ir (CH 3) methane C–H activation mechanism, Faraday Discuss., 2019, 220, 414–424 RSC.
- Y. S. Sistla and V. Sridhar, Molecular understanding of carbon dioxide interactions with ionic liquids, J. Mol. Liq., 2021, 325, 115162 CrossRef.
- L. T. Costa and M. C. Ribeiro, Molecular dynamics simulation of polymer electrolytes based on poly (ethylene oxide) and ionic liquids. I. Structural properties, J. Chem. Phys., 2006, 124(18), 184902 CrossRef PubMed.
- L. T. Costa, et al., Raman spectra of polymer electrolytes based on poly (ethylene glycol) dimethyl ether, lithium perchlorate, and the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate, Vib. Spectrosc., 2010, 54(2), 155–158 CrossRef.
- B. Bhargava and S. Balasubramanian, Probing anion–carbon dioxide interactions in room temperature ionic liquids: gas phase cluster calculations, Chem. Phys. Lett., 2007, 444(4–6), 242–246 CrossRef CAS.
- H. Sato, et al., N-doped nonalternant aromatic belt via a six-fold annulative double N-arylation, Chem. Sci., 2022, 13, 9947–9951 RSC.
- T.-H. Shi, et al., A Theoretical Study on the Macrocyclic Strain of Zigzag Molecular Belts, Org. Mater., 2020, 2(04), 300–305 CrossRef CAS.
- A. B. Shah, et al., Remarkable Single Atom Catalyst of Transition Metal (Fe, Co & Ni) Doped on C2N Surface for Hydrogen Dissociation Reaction, Nanomaterials, 2022, 13(1), 29 CrossRef.
- I. Bayach, et al., Hydrogen dissociation reaction on first-row transition metal doped nanobelts, Materials, 2023, 16(7), 2792 CrossRef.
- N. M. O'boyle, A. L. Tenderholt and K. M. Langner, Cclib: a library for package-independent computational chemistry algorithms, J. Comput. Chem., 2008, 29(5), 839–845 CrossRef PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33(5), 580–592 CrossRef PubMed.
- S. Pan, et al., Selectivity in gas adsorption by molecular cucurbit [6] uril, J. Phys. Chem. C, 2016, 120(26), 13911–13921 CrossRef.
- S. Sarfaraz, et al., DFT investigation of adsorption of nitro-explosives over C2N surface: Highly selective towards trinitro benzene, J. Mol. Liq., 2022, 352, 118652 CrossRef.
- Y. S. Al-Faiyz, et al., Efficient Detection of Nerve Agents through Carbon Nitride Quantum Dots: A DFT Approach, Nanomaterials, 2023, 13(2), 251 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03394a |
| This journal is © The Royal Society of Chemistry 2024 |