Open Access Article
Open Access ArticleDirect synthesis of carbamates, thiocarbamates, and ureas from Boc-protected amines: a sustainable and efficient approach†
Wanyong Li‡
a,
Mengting Lv‡a,
Xiaolin Luoa,
Zhouyu Wang*ab,
Qiao Song*ab and
Xiaoqi Yu ab
ab
aDepartment of Chemistry, Xihua University, Chengdu 610039, China. E-mail: soonq@126.com; zhouyuwang@mail.xhu.edu.cn; zhouyuwang77@163.com
bAsymmetric Synthesis and Chiral Technology Key Laboratory of Sichuan Province, Yibin 644000, China
First published on 1st July 2024
Abstract
In the quest for sustainable and efficient synthetic methodologies within medicinal chemistry, the synthesis of carbamates and their derivatives holds a pivotal role due to their widespread application in bioactive compounds. This investigation unveils a novel methodology for the straightforward transformation of Boc-protected amines into carbamates, thiocarbamates, and ureas, utilizing tert-butoxide lithium as the sole base. This approach effectively obviates the necessity for hazardous reagents and metal catalysts, presenting marked enhancements compared to traditional synthetic pathways. Notably, the method demonstrates facile scalability to gram-level production. This study contributes to the advancement of sustainable synthetic methodologies, offering a more benign and efficient alternative for the synthesis of key chemical intermediates with implications for broad pharmaceutical and agrochemical applications.
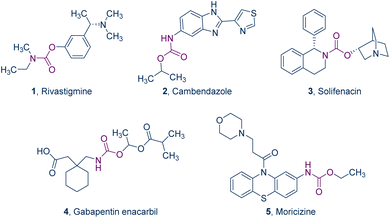
Carbamates, along with their derivatives, are omnipresent in an extensive array of bioactive compounds, including herbicides,1–3 insecticides,4,5 bactericides,6–10 and antiviral agents.11 These compounds are particularly noted for their chemical stability and potential to enhance cellular membrane permeability.12,13 Examples include drugs like rivastigmine for Parkinson's disease14 (Fig. 1, 1), cambendazole for worm infections15 (Fig. 1, 2), solifenacin for overactive bladders16 (Fig. 1, 3), gabapentin enacarbil for restless legs syndrome17 (Fig. 1, 4), and moricizine for cardiac arrhythmia18 (Fig. 1, 5), all of which incorporate this structural motif. This underscores their indispensable role in the realm of drug design and medicinal discovery, highlighting an escalating importance within the field of medicinal chemistry.
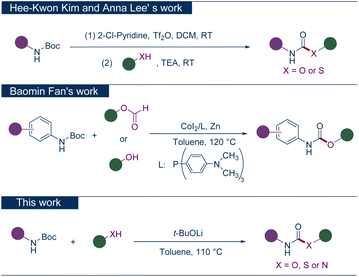
Acknowledging the paramount bioactivity of these compounds,19 the development of novel and efficient synthetic methodologies is deemed crucial. Conventional synthesis pathways that rely on phosgene,20 its derivatives,21,22 and transition metal catalysts (including palladium,23 nickel,24,25 and rhodium26,27) are fraught with challenges, notably the use of toxic reagents and the consequential contamination with residual heavy metals. The widespread use of tert-butoxycarbonyl (Boc) in amine protection renders Boc-protected amines readily accessible reagents, facilitating innovations in synthetic methodologies. Based on this, in 2016, Hee-Kwon Kim and Anna Lee reported a method for the direct synthesis of substituted carbamates from Boc-protected amines, involving the activation with Tf2O to generate isocyanate intermediates, which then react with alcohols to yield the desired carbamates.28 In 2019, Baomin Fan's group achieved the direct conversion of Boc-protected amines with formates or alcohols to carbamates, catalyzed by Co and Zn.29 As part of our program to develop efficient methods for synthesizing pharmaceutical products,30–34 here we introduce a cost-effective and environmentally benign methodology for the direct synthesis of carbamates and their analogs from Boc-protected amines. This novel strategy employs tert-butoxide lithium (t-BuOLi) as the sole base, obviating the need for metal catalysts and eliminating the use of hazardous substances (Scheme 1).
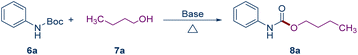
The reaction conditions were optimized using N-Boc aniline (6a) and n-butanol (7a) as the substrates (Table 1). Initially, n-butanol was used as the solvent and t-BuOLi as the base to explore the reaction temperature (Table 1, entries 1–4). The yield of the product increased gradually with the rise in reaction temperature, achieving a 95% yield at 110 °C (Table 1, entry 3). The reaction was virtually unfeasible when the base was switched to Na2CO3, DBU, TEA, or pyridine (Table 1, entries 5–8). With NaOH, t-BuONa, and t-BuOK as the base, the reaction proceeded but with a significantly reduced yield (Table 1, entries 9–11). The direct use of the alcoholic substrate as the solvent presented considerable limitations. Consequently, we experimented with other common solvents (Table 1, entries 12–15). The reaction exhibited low conversion rates in DMSO and DMF (Table 1, entry 12 and 14) and was almost unfeasible in dioxane (Table 1, entry 15). However, satisfactory yields were obtained when the solvent was switched to toluene (Table 1, entry 13).
| Entry | T (°C) | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 6a (0.5 mmol), 7a (2.5 mmol), base (0.6 mmol), heating 2 h.b Obtained yield.c ND means “not detected”. | ||||
| 1 | 80 | t-BuOLi | n-Butanol | 12 |
| 2 | 100 | t-BuOLi | n-Butanol | 70 |
| 3 | 110 | t-BuOLi | n-Butanol | 95 |
| 4 | 120 | t-BuOLi | n-Butanol | 94 |
| 5 | 110 | Na2CO3 | n-Butanol | NDc |
| 6 | 110 | DBU | n-Butanol | ND |
| 7 | 110 | TEA | n-Butanol | ND |
| 8 | 110 | Pyridine | n-Butanol | ND |
| 9 | 110 | NaOH | n-Butanol | 56 |
| 10 | 110 | t-BuONa | n-Butanol | 58 |
| 11 | 110 | t-BuOK | n-Butanol | 42 |
| 12 | 110 | t-BuOLi | DMSO | 22 |
| 13 | 110 | t-BuOLi | Toluene | 95 |
| 14 | 110 | t-BuOLi | DMF | 20 |
| 15 | 110 | t-BuOLi | Dioxane | ND |
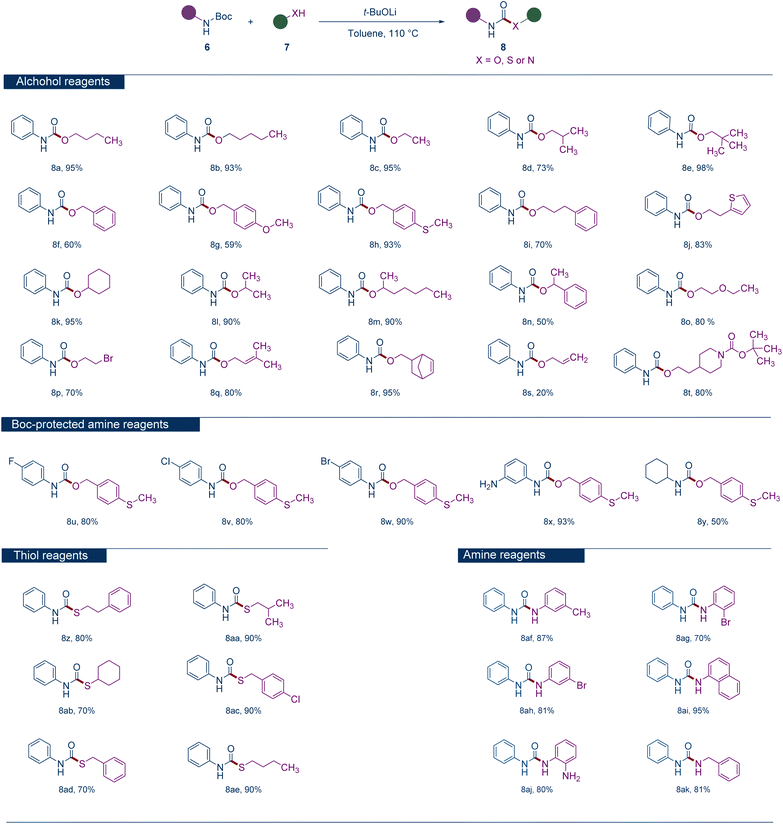
With the optimal conditions established, we delved into the reaction's scope, initially examining various alcohol substrates (Scheme 2). A comprehensive investigation revealed that a broad spectrum of aliphatic primary alcohols, both linear and branched, as well as those adorned with aromatic groups, consistently yielded products with high efficiency (Scheme 2, 8a–8j). Benzyl alcohols, under these conditions, achieved moderate to high yields (Scheme 2, 8f). Cyclic or acyclic secondary alcohols can achieve high yields of products (Scheme 2, 8k–8m), while benzylic secondary alcohol tend to yield lower (Scheme 2, 8n). Ethylene glycol and its derivatives can be smoothly converted into products with moderate to high yields (Scheme 2, 8o). The presence of olefinic bonds in the molecule does not hinder the reaction, although alcohols with terminal olefinic bonds yield lower (Scheme 2, 8q–8s). Interestingly, the N-Boc secondary amine groups within the molecule can be retained intact, with only the N-Boc primary amine groups undergoing reaction (Scheme 2, 8t). Subsequently, various Boc-protected amine substrates were investigated. Aromatic amines containing halogens or amino groups could produce the corresponding carbamates with high yields (Scheme 2, 8u–8x), whereas an aliphatic amine resulted in only moderate yield (Scheme 2, 8y). Substrates with strong electron-withdrawing groups on the aromatic ring, such as 4-nitro-N-Boc aniline, were also investigated. The reaction products were quite complicated, and the target carbamate product was not obtained. The reactivity of various thiol substrates was then explored. Thiols, both cyclic and acyclic, primary or secondary, could be converted into the corresponding thiocarbamates with high yields (Scheme 2, 8z–8ae). The feasibility of various amine substrates reacting with Boc-protected amines under these conditions was also studied. Pleasingly, aromatic and benzyl amines could yield various substituted urea derivatives with high efficiency (Scheme 2, 8af–8ak). The Lamothe group also reported the synthesis of ureas using N-Boc protected primary amines along with amines or anilines in 1996.35 They also attempted to use sodium tert-butoxide as a base, but alkyl lithium and sodium hydride seemed to be more effective in their reaction system.
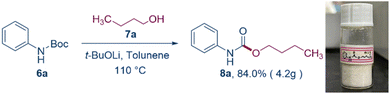
The reaction was also successfully scaled up to a gram level (Scheme 3). At this scale, the synthesis of the specified compound yielded a significant quantity of product, maintaining a high yield even when scaled up nearly 50 times.
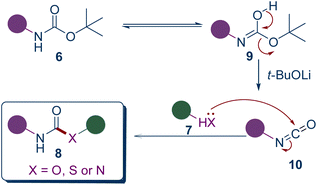
To elucidate the reaction mechanism, control experiments were conducted. Heating compound 6a and t-BuOLi in toluene for 30 minutes and monitoring the reaction via LCMS revealed the formation of isocyanate esters, suggesting that the reaction likely proceeds through an isocyanate intermediate. This is consistent with the observation that the N-Boc secondary amine group in compound 8t is retained because such groups are difficult to form isocyanates. Based on the experimental results and previously reported literature,35,36 we propose the following reaction mechanism. Boc-protected amines (6), under the influence of t-BuOLi, form isocyanates (10), which are then attacked by the corresponding nucleophiles (7) alcohols, thiols, or amines, to produce the respective carbamates, thiocarbamates, and ureas (8, Scheme 4).
In summary, our research presents a method for producing carbamates directly from Boc-protected amines, utilizing t-BuOLi as the sole base. This novel approach eliminates the need for toxic reagents and metal catalysts, aligning with the principles of sustainable chemistry. We successfully optimized the reaction conditions to achieve high yields across a wide range of substrates. Our method also facilitates the synthesis of thiocarbamates and ureas, broadening its applicability. Mechanistic studies indicate the formation of isocyanate esters as intermediates, providing valuable insights into the reaction pathway. This research contributes a significant advance in the sustainable synthesis of carbamates, offering promising implications for drug discovery and agrochemical production.
Data availability
Experimental details and characterization data for the products have been included as part of the ESI† and are available at RSC Advances. DOI: https://doi.org/10.1039/D4RA03683B.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22208268) and Sichuan Science and Technology Program (2023NSFSC1977).References
- D. Chaturvedi, Curr. Org. Chem., 2011, 15, 1593–1624 CrossRef CAS.
- T. Mizuno, I. Nishiguchi, T. Okushi and T. Hirashima, Tetrahedron Lett., 1991, 32, 6867–6868 CrossRef CAS.
- Y. S. Chen, I. Schuphan and J. E. Casida, J. Agric. Food Chem., 1979, 27, 709–712 CrossRef CAS PubMed.
- H. Malhotra, S. Kaur and P. S. Phale, Front. Microbiol., 2021, 12, 648868 CrossRef.
- W. Chin-Hsien, Synthesis, 1981, 8, 622–623 CrossRef.
- K. Bowden and R. S. Chana, J. Chem. Soc., Perkin Trans. 2, 1990, 12, 2163–2166 RSC.
- M. Beji, H. Sbihi, A. Baklouti and A. Cambon, J. Fluor. Chem., 1999, 99, 17–24 CrossRef CAS.
- A. W. Erian and S. M. Sherif, Tetrahedron, 1999, 55, 7957–8024 CrossRef CAS.
- N. N. M. Shahri, H. Taha, M. H. S. A. Hamid, E. Kusrini, J.-W. Lim, J. Hobley and A. Usman, RSC Adv., 2022, 12, 3136–3146 RSC.
- T. S. Lobana, S. Indoria, H. Kaur, D. S. Arora, A. K. Jassal and J. P. Jasinski, RSC Adv., 2015, 5, 14916–14936 RSC.
- A. Goel, S. J. Mazur, R. J. Fattah, T. L. Hartman, J. A. Turpin, M. Huang, W. G. Rice, E. Appella and J. K. Inman, Bioorg. Med. Chem. Lett., 2002, 12, 767–770 CrossRef CAS.
- A. Matošević and A. Bosak, Arch. Ind. Hyg. Toxicol., 2020, 71, 285–299 Search PubMed.
- A. K. Ghosh and M. Brindisi, J. Med. Chem., 2015, 58, 2895–2940 CrossRef CAS PubMed.
- S. Reilly, S. Dhaliwal, U. Arshad, A. Macerollo, N. Husain and A. D. Costa, Eur. J. Neurol., 2024, 31, e16142 CrossRef PubMed.
- E. T. Lyons, J. H. Drudge, S. C. Tolliver and S. Stamper, Am. J. Vet. Res., 1993, 54, 406–410 CAS.
- N. Majdinasab, N. Orakifar, L. Kouti, G. Shamsaei, M. Seyedtabib and M. Jafari, Front. Neurosci., 2023, 17, 1107886 CrossRef PubMed.
- D. O. Lee, M. J. Buchfuhrer, D. Garcia-Borreguero, A. Y. Avidan, M. Ahmed, R. Hays, W. G. Ondo, M. J. Jaros, R. Kim and G. Shang, Sleep Med., 2016, 19, 50–56 CrossRef.
- C. Han, M. Wirianto, E. Kim, M. J. Burish, S.-H. Yoo and Z. Chen, Clocks Sleep, 2021, 3, 351–365 CrossRef PubMed.
- B. V. Varun and K. R. Prabhu, RSC Adv., 2013, 3, 3079 RSC.
- J. S. Nowick, N. A. Powell, T. M. Nguyen and G. Noronha, J. Org. Chem., 1992, 57, 7364–7366 CrossRef CAS.
- R. A. Batey, V. Santhakumar, C. Yoshina-Ishii and S. D. Taylor, Tetrahedron Lett., 1998, 39, 6267–6270 CrossRef CAS.
- P. Majer and R. S. Randad, J. Org. Chem., 1994, 59, 1937–1938 CrossRef CAS.
- E. V. Vinogradova, N. H. Park, B. P. Fors and S. L. Buchwald, Org. Lett., 2013, 15, 1394–1397 CrossRef CAS PubMed.
- M. Abla, J.-C. Choi and T. Sakakura, Green Chem., 2004, 6, 524 RSC.
- I. Dindarloo Inaloo, M. Esmaeilpour, S. Majnooni and A. Reza Oveisi, ChemCatChem, 2020, 12, 5486–5491 CrossRef CAS.
- T. Mizuno, J. Mol. Catal. Chem., 1997, 123, 21–24 CrossRef CAS.
- X. Bu, Z. Wang, Y. Wang, T. Jiang, L. Zhang and Y. Zhao, Eur. J. Org Chem., 2017, 7, 1132–1138 CrossRef.
- H.-K. Kim and A. Lee, Tetrahedron Lett., 2016, 57, 4890–4892 CrossRef CAS.
- S. Li, R. Khan, X. Zhang, Y. Yang, Z. Wang, Y. Zhan, Y. Dai, Y. Liu and B. Fan, Org. Biomol. Chem., 2019, 17, 5891–5896 RSC.
- X.-L. He, Y.-W. Wen, H. Li, S. Qian, M. He, Q. Song and Z. Wang, J. Org. Chem., 2023, 88, 493–503 CrossRef CAS PubMed.
- Q. Song, S. Wang, X. Lei, Y. Liu, X. Wen and Z. Wang, Molecules, 2022, 27, 4698 CrossRef CAS.
- Q. Song, X. Lei, S. Yang, S. Wang, J. Wang, J. Chen, Y. Xiang, Q. Huang and Z. Wang, Molecules, 2022, 27, 5139 CrossRef CAS PubMed.
- X.-L. He, C. Wang, Y.-W. Wen, Y.-B. Zhao, H. Yang, S. Qian, L. Yang and Z. Wang, Org. Chem. Front., 2021, 8, 5847–5851 RSC.
- Q. Song, Y. Liu, L. Cai, X. Cao, S. Qian and Z. Wang, Chin. Chem. Lett., 2021, 32, 1713–1716 CrossRef CAS.
- M. Lamothe, M. Perez, V. Colovray-Gotteland and S. Halazy, Synlett, 1996, 6, 507–508 CrossRef.
- H.-K. Kim and A. Lee, Org. Biomol. Chem., 2016, 14, 7345–7353 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data for the products. See DOI: https://doi.org/10.1039/d4ra03683b |
| ‡ Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |