Open Access Article
Open Access ArticleA novel strategy combining hydrogenotrophic methanogens' bioaugmentation and biochar biostimulation for simultaneous polycyclic aromatic hydrocarbon biodegradation and bioenergy recovery
Rui Tang,
Min Zhang and
Xin Li *
*
College of Engineering, China Agricultural University (Key Laboratory for Clean Renewable Energy Utilization Technology, Ministry of Agriculture), No. 17 Qinghua Donglu, Haidian District, Beijing 100083, People's Republic of China. E-mail: lxin@cau.edu.cn; Fax: +86 (10) 62737858; Tel: +86 (10) 62737858
First published on 29th July 2024
Abstract
A novel strategy combining bioaugmentation using methanogenic archaea and biostimulation using biochar was proposed for the first time to obtain simultaneous improvement of mixed PAHs' anaerobic biodegradation and bioenergy production. The results showed that the addition of PHAs immediately resulted in inhibition in methane production and accumulation of VFA, indicating that PHAs are more toxic to methanogens than the acetogenic bacteria. The coupling of biochar with hydrogenotrophic methanogen alleviated the inhibitory effects of PAHs, allowing the anaerobic fermentation system to recover its methane production capability rapidly. Compared to the Fe3+ + bioaugmentation group, the biochar + bioaugmentation group exhibited a 7.5% higher restored cumulative methane production. This coupling strategy ultimately facilitated the degradation of most PAHs, achieving a removal rate of over 90%. Moreover, the coupled biochar and bioaugmentation induced significant changes in the archaeal community structure. Direct interspecies electron guilds (i.e., Streptococcus and Methanosarcina) were enriched in the presence of biochar and bioaugmentation, responsible for prominent PAH removal and methane recovery. This study demonstrated the feasibility of simultaneous PAH biodegradation and bioenergy production using electron acceptor and enriched microorganisms.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are carcinogenic and mutagenic compounds, that are generated by human actions such as emissions from burning fossil fuels or wastewater from industrial operations, agricultural practices, and domestic heating. After migrating into wastewater, PAHs may accumulate in sediments due to their limited solubility in water and hydrophobic characteristics, leading to subsequent soil contamination. According to research findings, PAHs have a toxic effect on microorganisms in the subsequent sewage treatment process, and trace amounts of PAHs can lead to microbial inactivation.1 Therefore, correctly and efficiently treating wastewater containing PAHs is necessary and urgent before being discharged into the environment.Many physical and chemical methods, such as volatilization,2 photodegradation,3 chemical oxidation,4 and solubilization-elution technology,5 have been employed for PAH removal. Their application is constrained by the elevated costs, residual pollutants, and the potential secondary pollution. In comparison, microbial degradation of PAHs is gaining attention due to its eco-friendly, sustainable, and cost-effective advantages.
PAHs can be categorized based on benzene ring numbers, with 2–3 rings classified as low molecular weight (LMW-PAHs) and 4–7 rings as high molecular weight (HMW-PAHs). Previous research has primarily focused on the decomposition of LMW-PAHs or a specific HMW-PAH.6 In wastewater, such as petroleum hydrocarbons and pyrolysis oil, PAHs are often a mixture of multiple compounds. The HMW-PAHs exhibit greater resistance and toxicity to living organisms compared to the LMW-PAHs.3 Nevertheless, there is limited documentation on the anaerobic biodegradation of mixed PAHs and scarce literature on the anaerobic degradation of mixed HMW-PAHs.
For PAH biodegradation (biomineralization), a terminal electron acceptor (TEA) is needed. PAHs biomineralization is efficient in aerobic conditions using O2 as TEA. In anaerobic conditions, alternate TEAs such as nitrate,7 sulfate,8 and Fe(III)9 can enhance PAHs degradation efficiently. However, additional TEA should be added to the anaerobic system to treat extensive or high concentrations of PAHs wastewater. This may cause secondary pollution. Alternatively, biochar has been recognized as a conductive material mediating direct interspecies electron transfer (DIET).10 Biochar is an eco-friendly and low-cost conductive material. Microbial degradation of PAHs may be facilitated by biochar.11
Another crucial factor influencing PAHs biodegradation efficiency is the prevalence of functional microorganisms. Implementing a bioaugmentation dosage is a viable approach for efficient biodegradation of PAHs. Previous studies have researched improving PAHs biodegradation by adding specific microbial strains extracted from soils contaminated with PAHs or crude oil.12,13 However, the biodegradation efficiency of these strains was found to be unstable.5 The research community has also focused on using bacteria strains, such as Rhodocccus erythropolis and Pseudomonas stuzeri,2 or a defined bacteria consortium for bioaugmentation. However, pure functional microorganisms are difficult to apply to real PAH-contaminated sites. Researchers have reported that a mixed bacterial consortium shows enhanced degradation of mixed PAHs compared to a single strain, due to its versatile enzymatic and metabolic functions.14 Additionally, Bianco et al. (2020) found that the methanogenic phase has a greater impact on PAH degradation than the acidogenic phase.15 However, there is a lack of literature on studies investigating the evidence of evolution of the archaeal community during PAH anaerobic digestion. Furthermore, no studies have focused on the correlation between PAH degradation and methanogenic archaea bioaugmentation.
Therefore, this study proposed a novel strategy that combines bioaugmentation using methanogenic archaea and biostimulation using biochar for the first time, aiming to achieve simultaneous improvement in mixed PAHs anaerobic biodegradation and bioenergy production. Additionally, the study elucidated the microbial community changes during the anaerobic digestion of PAHs. This study aimed to provide an interdisciplinary overview of the anaerobic biodegradation of mixed PAHs.
2. Materials and methods
2.1 Preparation of PAH solution
In total, 16 PAHs (6 LMW-PAHs and 10 HMW-PAHs) were used in this study. All PAHs were dissolved in n-hexane to make the PAH-contained solution with a final concentration of 10 mg L−1 for each PAH (Table 1). The total concentration of 16 types of PAHs is 160 mg L−1. On day 10, 100 mL of the solution was added to the reactors.2.2 Preparation of biochar and enrichment of bioaugmentation dosages
Cattle manure (CM) was used in the production of biochar. The CM was collected from a dairy farm in Beijing, China. Following washing, CM was dried in an oven at 105 °C for 24 h. Biochar was derived from CM through slow pyrolysis in a tubular furnace, with a final temperature set at 600 °C. The pyrolysis process had a heating rate of 10 °C min−1. After reaching the final pyrolysis temperature, the final temperature was maintained for 1 h. During pyrolysis, a constant N2 stream (0.2 L min−1) was purged to guarantee an oxygen-limited environment. When pyrolysis ended, biochar was cooled below 100 °C and transferred into desiccators before use.According to Wang et al. (2023), the enrichment process of hydrogenotrophic methanogens (HM) serving as bioaugmentation dosage was carried out.16 The enrichment process progressed in a continuous stirred tank reactor (CSTR) using CM as the substrate. In the meantime, external H2 was introduced through an air stone diffuser and circulated along with the generated biogas throughout a 24 hours batch cycle. After 80 days, methane content reached up to 95%, and the HM consortium was obtained. Before the start of the test, the microbial flora was cultivated in a liquid medium, and the concentration of the microorganisms in the medium was determined to be approximately 5 × 103 to 8 × 103 CFU mL−1.
2.3 Anaerobic biodegradation test of PAHs
Anaerobic digestion of PAHs was carried out in 1 L laboratory-scale CSTRs. The temperature was maintained at 37 ± 2 °C with a water bath. Inoculum was collected from a biogas facility supplied with municipal wastewater in Beijing. Before utilization, the inoculum was stored under anaerobic conditions until there was no traceable gas. The specific traits of the inoculum are outlined in Table 2.| Parameters | Unit | Inoculum |
|---|---|---|
| Average ± SD | ||
| a ‘—’: not available. TS: total solid; VS: volatile solid; SS: suspended solid; VSS: volatile suspended solid; TCOD: total chemical oxygen demand. | ||
| TS | g L−1 | 59.08 ± 0.18 |
| VS | g L−1 | 43.26 ± 0.11 |
| SS | g L−1 | 49.45 ± 0.53 |
| VSS | g L−1 | 19.74 ± 0.12 |
| pH | — | 7.84 ± 0.19 |
| TCOD | mg L−1 | 47.24 ± 3.05 |
| SCOD | mg L−1 | 23.37 ± 0.66 |
| NH4+–N | mg L−1 | 857 ± 21 |
Five treatment reactors were established in this experiment. The first treatment reactor was fed with 5 g glucose every day and served as the control reactor (RCK). The second treatment reactor was fed with 5 g glucose every day, and PAHs were added on day 10. Later, FeCl3 was added on day 15 to reach a final concentration of 0.1 mg L−1 and 10 mL HM was added on day 26 (RFe+HM). The third treatment reactor was fed with 5 g glucose every day, and PAHs were added on day 10. Later, BC was added on day 15 to reach a final concentration of 0.5 g L−1, and HM was added on day 26 (RBC+HM). The fourth treatment reactor was fed with 5 g glucose every day; PAHs were added on day 10. Then, the reactors received BC only on day 15 (RBC). The fifth treatment reactor was fed with 5 g glucose every day; PAHs were added on day 10; and then, reactors received HM only on day 26 (RHM). All the reactors were operated for 43 days.
The reactors were operated in a fed-batch mode. The effluent from the reactor was used to dissolve glucose for feeding, with only a minimal quantity of liquid in the reactor being allocated for testing purposes. This was done to prevent the loss of PAHs during feeding and discharge.
2.4 Analytical methods
Gas composition (CH4 and CO2) was analyzed by gas chromatograph (Shimadzum GC-8A, Japan). Liquid samples used for VFA measurement were collected through filtration through a 0.45 μm filter after centrifuging at 8000 rpm for 20 minutes (TGL-16 M, China). VFAs were analyzed by Gas Chromatograph (Shimadzu, GC-2010 Plus, Japan).16After sample collection, 20 g of fresh samples were taken and dehydrated using a vacuum freeze dryer, then ground into fine particles of about 1 mm. PAHs and metabolites were determined as described by Mu et al. (2022). The freeze-dried samples were transferred to a 150 mL conical flask. 50 mL solution of dichloromethane and n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the flask and ultrasonic extraction was carried out with the precipitate for 30 min. Then, the flask was allowed to stand for 10 min to separate into two layers. The supernatant was collected, and the residual samples were supplemented with 50 mL of extract solution and subjected to ultrasonic extraction again. The operation was repeated twice. Eventually, three extracts were combined, purified, and dehydrated with anhydrous sodium sulfate.17
1) was added to the flask and ultrasonic extraction was carried out with the precipitate for 30 min. Then, the flask was allowed to stand for 10 min to separate into two layers. The supernatant was collected, and the residual samples were supplemented with 50 mL of extract solution and subjected to ultrasonic extraction again. The operation was repeated twice. Eventually, three extracts were combined, purified, and dehydrated with anhydrous sodium sulfate.17
Concentrations of PAHs were determined through the following procedure: PAHs were identified using a GC/MS system (Agilent GC/MSD 7890B, USA). Helium was the carrier gas with a 1.2 mL min−1 flow rate. The injector and transfer line temperatures were held at 280 °C and 300 °C, respectively. The mass spectrometer operated in electron ionization (EI) mode at 70 eV, scanning within the range of 30–600 m/z. Standard curves for each compound were established by injecting a mixture of 16 PAHs (Mix A, Sigma Aldrich, Italy). All samples were measured in triplicates, and the results were exhibited as the mean of the triplicates.
2.5 Microbial analysis
Total DNA was extracted using an E.Z.N.A.® soil DNA kit (Omega Biotek, USA). Primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify bacterial 16S rRNA gene V3–V4 variable region. Primers 524F (5′-TGYCAGCCGCCGCGGTAA-3′) and 958R (5′-YCCGGCGTTGAVTCCAATT-3′) were used to amplify archaeal 16S rRNA gene V3–V4 variable region. The obtained products were quantified using the Quantus™ Fluorometer (Promega, USA).The purified PCR products were then used for library construction with the NEXTFLEX Rapid DNA-Seq Kit, followed by sequencing on the MiSeq PE300 platform. Finally, OTU clustering and species classification analyses were performed on the Majorbio Cloud Platform based on the practical data.
2.6 Predicated gene expression analysis
The Phylogenetic Investigation of Communities by Reconstruction (PICRUSt2) tool was utilized to predict the gene expression of the microbial community. All sequences were annotated against the Kyoto Encyclopedia of Genes and Genomes databases using PICRUSt2 to predict gene functions. The presumed function of microbial communities was determined based on their taxonomic composition.3. Results and discussion
3.1 Influence of PAHs on anaerobic digestion performance
The biogas and methane production of each experimental reactor is presented in Fig. 1. During the first ten days before adding the PAHs, there was no significant difference in the biogas and methane production among all the reactors, indicating a comparable performance in all the reactors (P > 0.05). After PAHs addition (on day 10), inhibition occurred immediately in the RBC, RHM, RFe+HM, and RBC+HM. Although reactors received biochar or Fe3+ on day 15, biogas production still declined. During 19–27 days, no detectable amounts of gas were produced from these reactors. Results implied that an external electron acceptor alone cannot rapidly alleviate the inhibitory effects of PAHs on anaerobic digestion processes.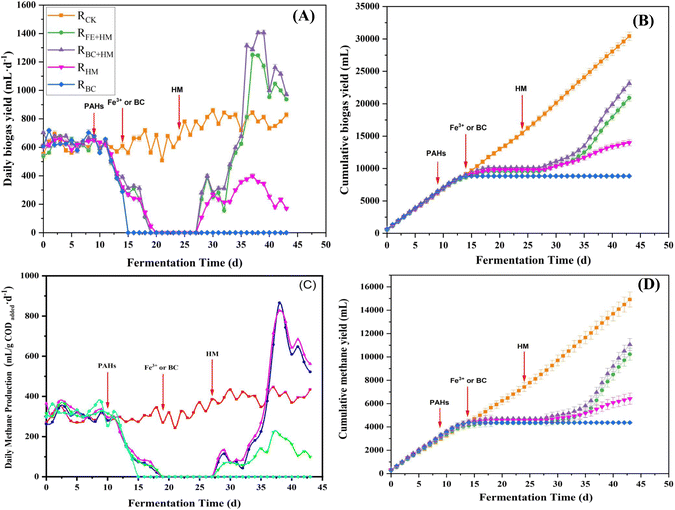 | ||
| Fig. 1 Daily biogas yield (A), cumulative biogas yield (B), daily methane yield (C), and cumulative methane yield (D) variations during the PAH anaerobic digestion process. | ||
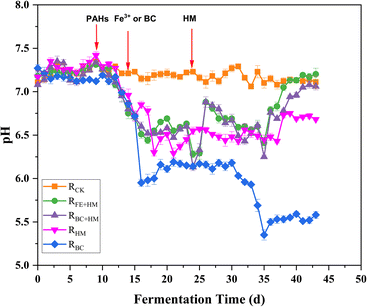
Fig. 3 depicts the variations in VFA concentrations within the anaerobic digesters. In RCK, VFA concentrations remained stable at levels below 500 mg L−1 throughout the experimental period. In contrast, following the introduction of PAHs on day 10, VFA concentrations exhibited a gradual increase, reaching over 1500 mg L−1 on day 15, consistent with a decline in pH in all experimental reactors (Fig. 1 and 2). Concomitantly, VFA concentrations continued to rise significantly, dominated by high concentrations of acetic acid on day 19. The low pH and high total VFA concentration in the reactors receiving PAHs suggested over-acidification and unfavorable conditions as the main reasons for process inhibition.
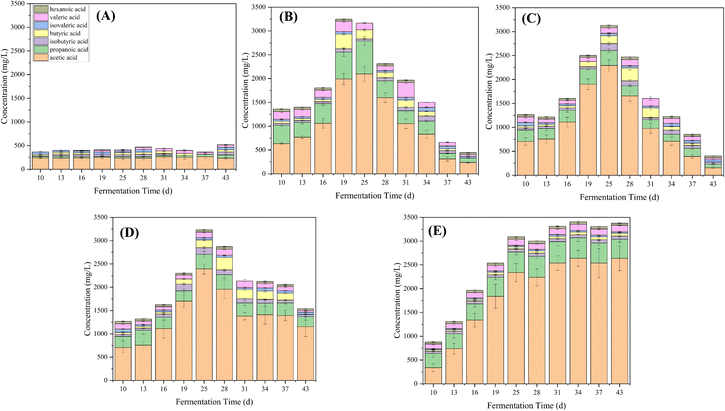 | ||
| Fig. 3 VFA variations of RCK (A), RFE+HM (B), RBC+HM (C), RHM (D), and RBC (E) during the PAH anaerobic digestion process. | ||
The stimulatory effect of PAH on VFA production has been affirmed by Chen et al. (2022), where a reasonable amount of PAHs could promote acidogenesis, acetogenesis, and methanogenesis in the anaerobic co-digestion of food waste and sludge.18 Further, Yao et al. (2022) claimed that adding naphthalene could promote acidogenesis, but an overdose could induce an imbalance between acidogenesis and methanogenesis, causing a pH imbalance and sabotaging methane production.19 Methanogenic archaea are more vulnerable to changes in the surrounding environment than bacteria involved in hydrolysis and acidogenesis.20 Based on the VFA profile analysis, it is suggested that the PAHs may have had a greater toxic effect on the methanogens compared to the acetogenic bacteria (Fig. 3).
3.2 The detoxification effect of combined HM with biochar or Fe3+ on PAH biodegradation
On day 25, RFe+HM, RBC+HM, and RHM were remediated by adding HM. Upon addition, the biogas production in RHM, RFe+HM, and RBC+HM recovered gradually. Notably, the daily biogas production in RFe+HM and RBC+HM increased rapidly (Fig. 1). On day 37, the daily biogas production in RFe+HM and RBC+HM significantly increased, reaching 1249 mL day−1 and 1405 mL day−1, respectively. Such phenomenon was due to the degradation of accumulated VFAs consumed by external HM (Fig. 3). The daily biogas production of RHM also experienced a slight recovery after bioaugmentation with HM, which was significantly lower than that in RFe+HM and RBC+HM, probably because of the deficiency of TEA in the RHM (P < 0.05). While the biogas production in RBC continued to be suppressed, indicating the limited absorption effect of biochar for PAH detoxication (Fig. 1).The cumulative methane production obtained during 43 days of bioremediation in all reactors is shown in Fig. 1. Because of the toxicity of PAHs, the cumulative biomethane production in reactors with PAHs addition was significantly lower than RCK (P < 0.05). Among the PAHs treatment reactors, RBC+HM enjoyed the highest cumulative biomethane production, followed by RFe+HM, indicating that the detoxication effect of biochar was better than Fe3+ in the presence of HM (P < 0.05). We hypothesized that the better detoxification effect of biochar may be attributed to the following reasons: first, biochar acts as a shelter for potential bacterial PAH-degraders, which could expediently use PAHs as the carbon source. Second, the conductive properties of biochar enable it to act as an electron acceptor, facilitating the degradation of PAHs.21
The strategy can be applied in sewage treatment plants, and treatment for surface water, and groundwater contaminated by PAHs. Another scenario is application for preliminary PAHs removal of industrial wastewater before being discharged into the sewage system. When the biogas production is inhibited by PAHs pollution and acid accumulation occurs, biochar and HM microbial agents could be added to the system every two or three days once time until the biogas production recovers.
3.3 The degradation fate of PAHs during AD
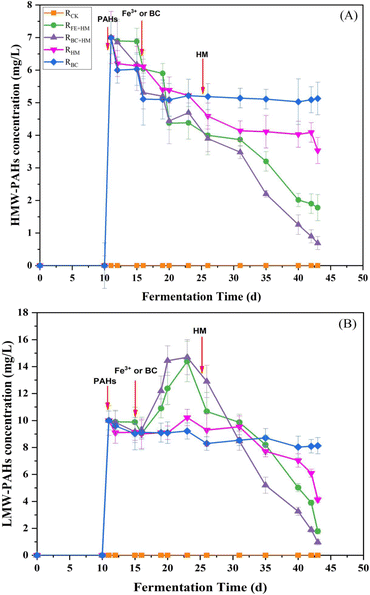
Changes in the concentrations of LMW-PAHs and HMW-PAHs during the anaerobic digestion process are presented in Fig. 4. Results showed that the HMW-PAHs concentration increased to around 7 mg L−1 after the addition of PAHs on day 10 and decreased gradually after the addition of Fe3+ or biochar on day 15. At the same time, the LMW-PAHs concentrations increased more significantly and reached the peak concentration in each experimental group on day 23 (Fig. 4). The increased concentration of LMW-PAHs might be due to the biotransformation of HMW-PAHs to their sub compounds. After bioaugmenting with HM, the concentrations of HMW-PAHs and LMW-PAHs in RFe+HM and RBC+HM decreased rapidly, indicating that the PAHs were mineralized to produce methane and CO2. In the following days, the concentration of PAHs in RFe+HM and RBC+HM further declined. At the end of the experiment, the removal rates of HMW-PAHs and LMW-PAHs in RBC+HM were the highest, reaching 90.1% and 90.3%, respectively, followed by 82.2% and 74.6% of HMW-PAHs and LMW-PAHs in RFe+HM, indicating BC coupled with HM had better removal efficiency of PAHs than individuals. Such observation was in accordance with the corresponding biogas and methane production (Fig. 1). In contrast, the removal rates of HMW-PAHs and LMW-PAHs in RHM were 49.6% and 58.7%, respectively. The results indicated that the HM can only promote the biodegradation of PAHs to a certain extent. The lowest removal rate in PAH concentrations was obtained for RBC (HMW-PAHs: 26.7% LMW-PAHs: 18.7%), indicating the limited effect of biochar absorption in removing PAHs. Previous studies have demonstrated the capacity of biochar to adsorb PAHs; however, this adsorption merely facilitates the transfer of PAHs from one medium to another and does not enhance or eliminate the contaminants.22 Based on the above experimental results, it can be inferred that the degradation of PAHs involved the cleavage of the benzene ring of HMW-PAHs into LMW-PAHs, followed by the cracking of LMW-PAHs into CO2 and methane. The toxic effect of PAHs on methanogens inhibits the final mineralization of PAHs, thereby limiting the further degradation of PAH. While HM bioaugmentation can compensate for the final mineralization step, thereby facilitating the transformation of HMW-PAHs into LMW-PAHs and consequently enhancing the overall removal rate of PAHs.23,24 Additionally, electron transfer occurs among microbial species within anaerobic biota, laying the foundation for cooperative behaviors and community functions. Interspecies electron transfer is considered a vital process in anaerobic conditions. Therefore, improving interspecies electron transfer could be a promising approach to accelerating microbial metabolism in bioenergy applications that require interspecies electron exchange. Numerous studies have highlighted the efficacy of using conductive materials to accelerate interspecies electron transfer in biological processes.25,26 The results obtained in this study indicated that biochar could serve as specific electron transfer mediators to improve and facilitate the electron transfer process.In the analysis of the PAH solution by GC-MS, some PAH metabolic intermediates were detected using NIST 17 spectrum library, as shown in Table 3. The results showed that the PAH cyclic cracking products were detected, so the metabolic pathways of PAHs included substitution reaction and ring-opening cracking reaction. Some studies have demonstrated that the main degradation pathways of PAHs are hydroxylation, methylation, and carboxylation. The aromatic rings in PAH are reduced to produce a cyclohexane ring. The subsequent ring opening process to form aliphatic compounds is followed by multiple steps that lead to CO2 production. The research shows that the degradation of HMW-PAHs may produce LMW-PAHs, which was consistent with that of Yukang, Zhou et al. (2020).27 It mainly undergoes the process from the first to last aromatic ring. The degradation pathway of a specific PAH needs to be further explored using individual PAH.
In order to compare the anaerobic biodegradation performance of PAHs, a review of recent literature was done about the strategies in other studies used for PAHs anaerobic biodegradation. Table 4 shows that most studies have focused on individual PAH, lacking research on mixed PAHs. Moreover, there is relatively little research on methods combining biostimulation and bioaugmentation. The results in this study indicated that the novel strategy combining hydrogenotrophic methanogens bioaugmentation and biochar biostimulation showed excellent performance in anaerobic biodegradation of mixed PAHs with above 90% of removal rate.
| PAHs | Strategy | Removal rates (%) | References |
|---|---|---|---|
| Phenanthrene | Granular biochar & ethanol | 86.2 | 11 |
| Phenanthrene, anthracene, fluoranthene, pyrene and benzo(a)pyrene | HCO3− | 84.98 | 17 |
| NAP, PHE, and PYR | Bioelectrochemical systems | 97.60, 42.90, and 22.00 | 27 |
| Phenanthrene, anthracene, fluoranthene, pyrene, and benzo[a]pyrene | Bicarbonate and acetate | 89.67 | 28 |
| Pyrene, benzo[a]pyrene | Pure sulfate reducing pyrene and benzo[a]pyrene-degrading cultures | 99.6, 99.8 | 29 |
| Naphthalene | Microbial electrolysis cells and bioaugmentation | 94.5 | 30 |
| Phenanthrene | Anaerobic sludge and granular biochar | 81.0 | 31 |
| ∑16PAHs | Nitrogen addition | 36.65 | 32 |
| ∑16PAHs | Nitrate & PAH degrading inoculum | 76 | 33 |
| ∑16PAHs | Methanogenic archaea bioaugmentation using and biochar biostimulation | 90 | This study |
3.4 Microbial community analysis
| Specimens | OTUs | Shannon | Simpson | Chao | Coverage | |
|---|---|---|---|---|---|---|
| Bacteria | RCK | 832 | 3.813 | 0.2130 | 462 | 0.9979 |
| RBC | 806 | 3.112 | 0.0801 | 452 | 0.9969 | |
| RHM | 818 | 3.278 | 0.1326 | 454 | 0.9971 | |
| RBC+HM | 968 | 3.313 | 0.1378 | 654 | 0.9949 | |
| RFE+HM | 925 | 3.363 | 0.1302 | 635 | 0.9949 | |
| Archaea | RCK | 37 | 1.978 | 0.2536 | 23 | 0.9997 |
| RBC | 22 | 0.850 | 0.1871 | 12 | 0.9946 | |
| RHM | 22 | 0.752 | 0.2141 | 15 | 0.9943 | |
| RBC+HM | 60 | 1.972 | 0.2646 | 28 | 0.9969 | |
| RFE+HM | 38 | 1.336 | 0.2531 | 25 | 0.9968 |
Shannon and Simpson indexes comprehensively reflect species richness and evenness. Higher values are associated with greater evenness in species distribution, contributing to increased diversity. The Chao1 index estimates the number of species in the microbial community, indicating species richness in the sample. Coverage refers to microbial coverage, reflecting the accuracy of sequencing results in representing the actual conditions of a sample. Sobs indicate the number of species observed in a sample.
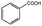
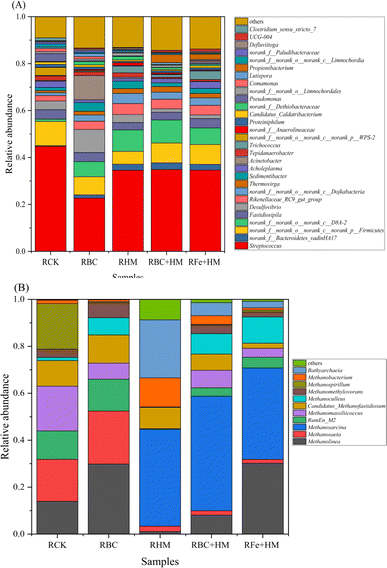
From these diversity indexes, it can be concluded that adding PAHs suppressed both the richness and evenness of the bacterial community. From this perspective, PAH addition will likely intervene in the microbial communities by blocking specific metabolic pathways. When comparing bacteria and archaea within the same group, it is evident that Shannon, Chao, and Sobs of bacteria were significantly higher than those of archaea (Table 5). These results indicated that bacterial communities exhibited higher diversity than archaeal communities under PAH inhibition. Notably, RBC+HM on day 43 presented the highest Shannon index of archaea, demonstrating that combining biochar and HM can increase the diversity and richness of the microbial community.
After the addition of PAHs, the population of Streptococcus decreased. However, reactors that were treated with HM bioaugmentation along with Fe3+ or biochar experienced an increase in the abundance of Streptococcus. Similarly, the presence of norank_c__D8A-2 increased from 1.2% in RCK to 11.3% in RBC+HM after the introduction of HM. It became a significant component of the microbial community in this experiment, which helped facilitate efficient VFA oxidation through DIET.
A shift in dominant genera was observed after adding bioaugmentation dosage. Methanosarcina replaced Methanosaeta, becoming the predominant genus (Fig. 5). In RFe+HM, Methanolinea and Methanosarcina had relative abundances of 30.18% and 39.00%, respectively. In RBC+HM, Methanolinea and Methanosarcina had relative abundances of 8.10% and 48.83%, respectively. This indicated that during the recovery process, Methanosarcina replaced Methanosaeta for methane production, restoring gas and methane production processes, especially in RBC+HM. Biochar promotes syntrophic anaerobic oxidation of VFA and enriches the hydrogenotrophic archaea involved in DIET. These archaea receive electrons through VFA oxidation and reduce CO2 to methane, facilitating methane production.36,39,45
| EC number | RCK | RHM | RFE+HM | RBC | RBC+HM |
|---|---|---|---|---|---|
| a ND: under detection limit. | |||||
| Acetoclastic methanogenesis | |||||
| 2.7.2.1 | UD | UD | UD | 0.0326 ± 0.0021 | 0.0468 ± 0.0015 |
| 2.3.1.8 | UD | UD | UD | 0.0236 ± 0.0008 | 0.0377 ± 0.0016 |
| 6.2.1.1 | 0.5230 ± 0.0036 | 0.5160 ± 0.0061 | 0.5029 ± 0.0011 | 0.4411 ± 0.0031 | 0.3544 ± 0.0022 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Hydrogenotrophic methanogenesis | |||||
| 1.12.98.1 | 0.4046 ± 0.0037 | 0.5497 ± 0.0041 | 0.4248 ± 0.0037 | 0.3194 ± 0.0049 | 0.7090 ± 0.0062 |
| 1.12.98.2 | UD | 0.0002 ± 0.0000 | UD | UD | 0.0007 ± 0.0001 |
| 1.2.7.4 | 0.1509 ± 0.0011 | 0.2430 ± 0.0026 | 0.2275 ± 0.0036 | 0.1761 ± 0.0022 | 0.2652 ± 0.0031 |
| 1.5.98.1 | 0.1057 ± 0.0014 | 0.1110 ± 0.0018 | 0.1228 ± 0.0025 | 0.0954 ± 0.0010 | 0.1450 ± 0.0021 |
| 1.5.98.2 | 0.1308 ± 0.0029 | 0.1574 ± 0.0031 | 0.1469 ± 0.0033 | 0.1127 ± 0.0016 | 0.1584 ± 0.0010 |
| 2.1.1.86 | 0.8934 ± 0.0035 | 1.0061 ± 0.0027 | 1.0983 ± 0.0015 | 0.8282 ± 0.0025 | 1.2282 ± 0.0031 |
| 2.3.1.101 | 0.1076 ± 0.0032 | 0.1335 ± 0.0024 | 0.1253 ± 0.0033 | 0.0965 ± 0.0030 | 0.1537 ± 0.0029 |
| 2.8.4.1 | 0.5456 ± 0.0014 | 0.5852 ± 0.0019 | 0.5172 ± 0.0024 | 0.5113 ± 0.0029 | 0.6286 ± 0.0024 |
| 3.5.4.27 | 0.1751 ± 0.0037 | 0.1436 ± 0.0028 | 0.1592 ± 0.0017 | 0.1196 ± 0.0021 | 0.1655 ± 0.0025 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Wood-Ljungdahl pathway | |||||
| 1.5.1.20 | 0.1551 ± 0.0025 | 0.1963 ± 0.0017 | 0.1218 ± 0.0018 | 0.1478 ± 0.0013 | 0.2194 ± 0.0027 |
| 1.5.1.5 | 0.0041 ± 0.0011 | 0.0353 ± 0.0021 | 0.0137 ± 0.0027 | 0.0027 ± 0.0009 | 0.0520 ± 0.0022 |
| 1.17.1.10 | 0.1551 ± 0.0018 | 0.1963 ± 0.0036 | 0.1218 ± 0.0028 | 0.1803 ± 0.0051 | 0.2662 ± 0.0026 |
| 2.3.1.169 | 0.0755 ± 0.0018 | 0.0968 ± 0.0027 | 0.0541 ± 0.0029 | 0.0888 ± 0.0031 | 0.1305 ± 0.0027 |
| 3.5.4.9 | 0.1233 ± 0.0031 | 0.1138 ± 0.0027 | 0.1010 ± 0.0027 | 0.1338 ± 0.0014 | 0.2039 ± 0.0023 |
| 6.3.4.3 | ND | ND | ND | ND | ND |
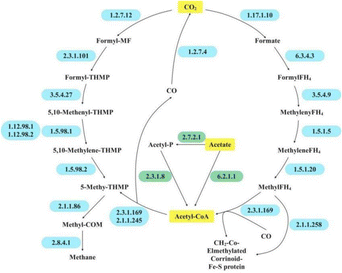
Through acetate methanogenesis, the resulting acetic acid is ultimately converted to methane through a series of steps (Fig. 6). Enzymes involved in acetoclastic methanogenesis were found to be lower in HM-fed reactors than in RCK or RBC, which was due to the origin of HM dosage as it mainly contained hydrogenotrophic methanogens.
For hydrogenotrophic methanogenesis, the process known as the Wood–Ljungdahl (WL) pathway should be emphasized due to its essential role in energy generation and carbon fixation in methanogens. Initially, hydrogenotrophic methanogens gradually reduce CO2 to methyl-H4MPT via the methyl branch of the WL pathway. The methyl group of the formed methyl-H4MPT is then transferred to coenzyme M via N5-methyltetrahydroleaflavin (EC 2.1.1.86) and ultimately reduced to methane and an isodisulfide (CoM-S-S-CoB) by the methyl-CoM reductase complex (EC 2.8.4.1) (Fig. 6 and Table 6).
In this study, hydrogenotrophic methanogenesis-related enzymes flourished in HM-fed reactors, particularly in RBC+HM. Similarly, previous studies have emphasized the stimulating effect of PAH on hydrogenotrophic methanogenesis.49 Based on the microbial profile (Fig. 5), the hydrogenotrophic methanogen could work syntrophically with VFA-degrading bacteria through DIET for PAH inhibition remediation and methane recovery. Notably, RBC+HM enjoyed the most profound hydrogenotrophic methanogenesis-related enzyme activities among the tested samples (Table 6). Consequently, the higher activity of EC 2.8.4.1 was obtained in RBC+HM, which compared well with its highest restored methane yield (Fig. 1).
4. Conclusions
Adding terminal electron acceptors (Fe3+ or biochar) coupled with hydrogenotrophic methanogens rapidly alleviated PAHs inhibition and restored biogas production capability. RBC+HM showed the highest PAHs removal efficiency, with 90.1% for HMW-PAHs and 90.3% for LMW-PAHs, respectively. Consequently, RBC+HM exhibited the highest restored cumulative methane production. The coupling of biochar with hydrogenotrophic methanogens restored the archaeal community's richness, leading to more efficient degradation of PAHs. Biochar and hydrogenotrophic methanogen promoted syntrophic anaerobic oxidation of VFA by enriching DIET-related microbiomes, further enhancing methane production.Data availability
Data for this article, including figures and tables are available within the manuscript and its additional files.Author contributions
Rui Tang: conceptualization, methodology, writing – original draft, investigation. Min Zhang: conceptualization, formal analysis. Xin Li: conceptualization, supervision, writing – original draft, writing – review & editing, funding acquisition.Conflicts of interest
The authors declare there is no conflict.Acknowledgements
The team would like to thank the support from the Key Laboratory of Clean Production and Utilization of Renewable Energy, Ministry of Agriculture and Rural Affairs, China Agricultural University; National Center for International Research of BioEnergy Science and Technology, Ministry of Science and Technology, China Agricultural University; and Beijing Municipal Key Discipline of Biomass Engineering.References
- G. Pagnozzi, S. Carroll, D. D. Reible and K. Millerick, Environ. Pollut., 2021, 268, 115641 CrossRef CAS PubMed.
- R. Forján, I. Lores, C. Sierra, D. Baragaño, J. L. R. Gallego and A. I. Peláez, Appl. Sci., 2020, 10, 2837 CrossRef.
- J. Luo, L. Wu, Y. Chen, L. Feng and J. Cao, J. Hazard. Mater., 2019, 365, 322–330 CrossRef CAS PubMed.
- M. Sharma, A. Nandy, N. Taylor, S. V. Venkatesan, V. Ozhukil Kollath, K. Karan, V. Thangadurai, N. Tsesmetzis and L. M. Gieg, J. Hazard. Mater., 2020, 389, 121845 CrossRef CAS PubMed.
- J. Liu, A. N. Zhang, Y. J. Liu, Z. Liu, Y. Liu and X. J. Wu, Ecotoxicol. Environ. Saf., 2021, 225, 112789 CrossRef CAS PubMed.
- Q. Leng, J. Mu and G. Yang, Environ. Pollut., 2021, 284, 117210 CrossRef CAS PubMed.
- A. M. Himmelberg, T. Brüls, Z. Farmani, P. Weyrauch, G. Barthel, W. Schrader and R. U. Meckenstock, Environ. Microbiol., 2018, 20, 3589–3600 CrossRef CAS PubMed.
- Z. Zhang, H. Guo, J. Sun and H. Wang, J. Hazard. Mater., 2020, 383, 121191 CrossRef CAS PubMed.
- H. Ribeiro, T. de Sousa, J. P. Santos, A. G. G. Sousa, C. Teixeira, M. R. Monteiro, P. Salgado, A. P. Mucha, C. M. R. Almeida, L. Torgo and C. Magalhães, Chemosphere, 2018, 199, 54–67 CrossRef CAS PubMed.
- D. R. Lovley, Annu. Rev. Microbiol., 2017, 71, 643–664 CrossRef CAS PubMed.
- Y. Shi, H. Xue, Y. Yao, C. Jing, R. Liu, Q. Niu and H. Lu, Chem. Eng. J., 2023, 477, 147229 CrossRef CAS.
- N. Haleyur, E. Shahsavari, S. S. Jain, E. Koshlaf, V. B. Ravindran, P. D. Morrison, A. M. Osborn and A. S. Ball, J. Environ. Manage., 2019, 238, 49–58 CrossRef CAS PubMed.
- J. Mu, Q. Leng, G. Yang and B. Zhu, Mar. Pollut. Bull., 2021, 167, 112294 CrossRef CAS PubMed.
- S. Kumari, R. K. Regar and N. Manickam, Bioresour. Technol., 2018, 254, 174–179 CrossRef CAS PubMed.
- F. Bianco, M. Race, S. Papirio and G. Esposito, Sci. Total Environ., 2020, 709, 136141 CrossRef CAS PubMed.
- S. Wang, X. Li, R. Dong, W. Xiong, Y. Li and Y. Zhu, Chemosphere, 2023, 344, 140370 CrossRef CAS PubMed.
- J. Mu, Y. Chen, Z. Song, M. Liu, B. Zhu, H. Tao, M. Bao and Q. Chen, J. Hazard. Mater., 2022, 438, 129569 CrossRef CAS PubMed.
- Y. Chen, Z. Qin, P. Zhang, X. Li and L. Feng, Bioresour. Technol., 2022, 360, 127567 CrossRef CAS PubMed.
- Y. Yao, J. Li, H. Xue, Y. Liu, J. Qiao, J. Tang, R. Liu and Q. Niu, Sustainability, 2022, 14(24), 16377 CrossRef CAS.
- M. Yan, Z. Hu, Z. Duan, Y. Sun, T. Dong, X. Sun, F. Zhen and Y. Li, Water Res., 2023, 246, 120711 CrossRef CAS PubMed.
- J. Zhao, Y. Li and G. J. W. Euverink, Chem. Eng. J., 2022, 428, 131015 CrossRef CAS.
- X. Li, Z. Yu, Q. Chen, C. Wang, L. Ma and G. Shen, Chem. Eng. J., 2022, 430, 132844 CrossRef CAS.
- S. B. Larsen, D. Karakashev, I. Angelidaki and J. E. Schmidt, J. Hazard. Mater., 2009, 164, 1568–1572 CrossRef CAS PubMed.
- A. Ferraro, G. Massini, V. M. Miritana, A. Panico, L. Pontoni, M. Race, S. Rosa, A. Signorini, M. Fabbricino and F. Pirozzi, Chemosphere, 2021, 275, 130091 CrossRef CAS PubMed.
- L. Yu, Y. Yuan, J. Tang, Y. Wang and S. Zhou, Sci. Rep., 2015, 5, 16221 CrossRef CAS PubMed.
- S. Chen, A. E. Rotaru, P. M. Shrestha, N. S. Malvankar, F. Liu, W. Fan, K. P. Nevin and D. R. Lovley, Sci. Rep., 2014, 4(1), 5019 CrossRef CAS PubMed.
- Y. Zhou, Q. Zou, M. Fan, Y. Xu and Y. Chen, J. Hazard. Mater., 2020, 381, 120945 CrossRef CAS PubMed.
- Q. Chen, Z. Li, Y. Chen, M. Liu, Q. Yang, B. Zhu and Z. Chen, Mar. Pollut. Bull., 2024, 199, 115925 CrossRef CAS PubMed.
- Z. Zhang, J. Sun, X. Gong, C. Wang and H. Wang, J. Hazard. Mater., 2023, 459, 132053 CrossRef CAS PubMed.
- Z. Min, T. Rui and L. Yu, Water Sci. Technol., 2024, 89(10), 2716–2731 CrossRef CAS PubMed.
- H. Xue, Y. Shi, J. Qiao, X. Li and R. Liu, Sustainability, 2023, 16(1), 366 CrossRef.
- S. Yuan, X. Han, X. Yin, P. Su, Y. Zhang, Y. Liu and D. Zhang, Sci. Total Environ., 2023, 864, 161034 CrossRef CAS PubMed.
- N. Zhou, Z. Yang, J. Zhang, Z. Zhang and H. Wang, Bioresour. Technol., 2024, 393, 130090 CrossRef CAS PubMed.
- A. Janbandhu and M. H. Fulekar, J. Hazard. Mater., 2011, 187, 333–340 CrossRef CAS PubMed.
- C. E. Achife, U. J. J. Ijah, S. B. Oyeleke, J. D. Bala, O. A. Oyewole, N. R. Maddela and R. Prasad, Appl. Biochem. Biotechnol., 2024, 196, 2819–2838 CrossRef CAS PubMed.
- Y. Lei, D. Sun, Y. Dang, H. Chen, Z. Zhao, Y. Zhang and D. E. Holmes, Bioresour. Technol., 2016, 222, 270–276 CrossRef CAS PubMed.
- Y. Qian, M. Xu, T. Deng, W. Hu, Z. He, X. Yang, B. Wang, D. Song, L. Chen, Y. Huang and G. Sun, J. Hazard. Mater., 2021, 407, 124385 CrossRef CAS PubMed.
- H. Cai, L. Sun, Y. Wang, T. Song, M. Bao and X. Yang, Chem. Eng. J., 2019, 369, 1078–1092 CrossRef CAS.
- F. Zhang, D.-K. Qian, X.-B. Wang, K. Dai, T. Wang, W. Zhang and R. J. Zeng, Sci. Total Environ., 2020, 723, 138080 CrossRef CAS PubMed.
- S. Chen, F. Yao, Z. Pi, L. He, K. Luo, X. Li and Q. Yang, Environ. Manage., 2024, 351, 119911 CAS.
- Y.-Q. Wang, M.-X. Wang, Y.-Y. Chen, C.-M. Li and Z.-F. Zhou, J. Hazard. Mater., 2021, 417, 126086 CrossRef CAS PubMed.
- Y. Feng, J. Lu, Z. Shen, J. Li, H. Zhang, X. Cao, Z. Ye, G. Ji, Q. Liu, Y. Hu and B. Zhang, J. Hazard. Mater., 2023, 451, 131055 CrossRef CAS PubMed.
- C. Berdugo-Clavijo, X. Dong, J. Soh, C. W. Sensen and L. M. Gieg, FEMS Microbiol. Ecol., 2012, 81, 124–133 CrossRef CAS PubMed.
- U. Kunapuli, M. K. Jahn, T. Lueders, R. Geyer, H. J. Heipieper and R. U. Meckenstock, Int. J. Syst. Evol. Microbiol., 2010, 60, 686–695 CrossRef CAS PubMed.
- J. Lee, T. Koo, A. Yulisa and S. Hwang, J. Environ. Manage., 2019, 241, 418–426 CrossRef CAS PubMed.
- Z. Wang, C. Zhang, J. Watson, B. K. Sharma, B. Si and Y. Zhang, Chem. Eng. J., 2022, 435, 135078 CrossRef CAS.
- T. D. Mand and W. W. Metcalf, Microbiol. Mol. Biol. Rev., 2019, 83(4), e000200 CrossRef PubMed.
- S. V. Mohan, T. Kisa, T. Ohkuma, R. A. Kanaly and Y. Shimizu, Rev. Environ. Sci. Bio/Technol., 2006, 5, 347–374 CrossRef CAS.
- H. Wang, L. Lu, X. Chen, Y. Bian and Z. J. Ren, Water Res., 2019, 164, 114942 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |