Open Access Article
Open Access ArticleImpact of cathodic pH and bioaugmentation on acetate and CH4 production in a microbial electrosynthesis cell†
Emmanuel Nwanebua,
Mara Jezernikb,
Christopher Lawsonb,
Guillaume Bruanta and
Boris Tartakovsky *a
*a
aEnergy, Mining and Environment Research Centre, National Research Council Canada, 6100 Royalmount Avenue, Montreal, Quebec H4P 2R2, Canada. E-mail: Boris.Tartakovsky@cnrc-nrc.gc.ca
bDepartment of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, Canada
First published on 31st July 2024
Abstract
This study compares carbon dioxide conversion in carbonate-fed microbial electrosynthesis (MES) cells operated at low (5.3), neutral (7) and high (8) pH levels and inoculated either with wild-type or bioaugmented mixed microbial populations. Two 100 mL (cathode volume) MES cells inoculated with anaerobic digester sludge were operated with a continuous supply of carbonate solution (5 g L−1 as CO32−). Acetate production was highest at low pH, however CH4 production still persisted, possibly due to pH gradients within the cathodic biofilm, resulting in acetate and CH4 volumetric (per cathode compartment volume) production rates of 1.0 ± 0.1 g (Lc d)−1 and 0.84 ± 0.05 L (Lc d)−1, respectively. To enhance production of carboxylic acids, four strains of acetogenic bacteria (Clostridium carboxidivorans, Clostridium ljungdahlii, Clostridium autoethanogenum, and Eubacterium limosum) were added to both MES cells. In the bioaugmented MES cells, acetate production increased to 2.0 g (Lc d)−1. However, production of other carboxylic acids such as butyrate and caproate was insignificant. Furthermore, 16S rRNA gene sequencing of cathodic biofilm and suspended biomass suggested a low density of introduced acetogenic bacteria implying that selective pressure rather than bioaugmentation led to improved acetate production.
1. Introduction
Sustainable technologies are crucial for mitigating the impact of greenhouse gas emissions on the environment and limiting global warming. Microbial electrosynthesis (MES) is one emerging technology with the potential to combine CO2 sequestration with the production of CH4 and chemicals such as carboxylic acids, typically derived from fossil fuels.1 In a MES cell, CO2 conversion is facilitated by microorganisms capable of cathode utilization as an electron donor through direct or indirect electron transfer mechanisms.2,3 Several previous studies were dedicated to determining operating conditions suitable for CO2 conversion in a MES cell. The main operating and design parameters affecting MES performance include pH,4,5 hydraulic retention time,6 applied voltage,7–9 microbial inoculum,10 reactor design,11 cathode materials12–14 and cathode surface properties.15,16 In addition to operating conditions, cathodic microbial populations play a decisive role in determining the metabolites formed and, therefore, the overall efficiency of this bioelectrochemical process.12,17–21CO2 conversion at a MES cathode can be achieved by wild type mixed microbial populations as well as co-cultures developed using pure strains. Although several CO2 conversion products are simultaneously expected,17 pure cultures can improve product selectivity19,20,22–24 and shorten time required for achieving stable product formation.25 Nevertheless, most of the available MES studies have been conducted using mixed microbial cultures, with limited research on the impact of bioaugmentation with pure acetogenic and chain-elongating strains on MES performance. Meanwhile, bioaugmentation could be advantageous for improving performance and directing CO2 conversion towards high value products, such as caproate26,27 and polyhydroxyalkanoates.28
Most often, microbial electrosynthesis results in the conversion of CO2 to CH4 and short chain fatty acids (SCFAs), mostly acetate.1,19,26 Meanwhile, medium and long chain multi-carbon compounds (e.g. valerate, caproate, polyhydroxyalkanoates) are more valuable because they can be used as precursors for sustainable manufacturing of fuels, lubricants, antimicrobial agents, flavor additives, bioplastics, and other high value chemicals29–31 that are currently produced from fossil fuels.19 With this regard, bioaugmentation of mixed microbial populations combined with optimal selection of cathodic pH can be used to accelerate MES startup,32 increase the rate of CO2 conversion to acetate, and expand the product range, while eliminating or suppressing methanogenic populations.4,5
In this study, two bioaugmentation strategies were compared in an attempt to enhance the rate of CO2 conversion and achieve MCFA production. First, the MES cell cathodic compartment containing a well-developed biofilm originating from a mixed microbial community of an anaerobic digester was bioaugmented by simultaneously introducing Clostridium carboxidivorans, Clostridium ljungdahlii, Clostridium autoethanogenum, and Eubacterium limosum. Notably, C. carboxidivorans, C. ljungdahlii and E. limosum have been observed to directly produce caproate from CO2 and electrons.33,34 In the second bioaugmentation attempted, the four acetogenic populations were combined with the anaerobic sludge and then introduced to the MES cell cathode compartment. It was hypothesized that by introducing acetogenic strains and optimizing the cathode compartment pH in order to suppress or at least restrict methanogenic activity, CO2 conversion to acetate can be improved. Furthermore, SCFA accumulation in the catholyte was expected to facilitate proliferation of indigenous chain-elongating strains in the mixed microbial community leading to microbial chain elongation.
2. Materials and methods
2.1 Analytical measurements
The cathode and anode off-gas composition was measured using gas chromatograph (GC) (HP 6890 GC, Hewlett Packard, USA). The off-gas flow rate from the anode and cathode compartments of each MES cell was determined using U-tube shaped gas bubble counters.13 Concentrations of acetate, propionate, butyrate, valerate and caproate were also determined using a GC (Agilent 6890 N gas chromatograph, USA). Detailed description of both analytical methods can be found elsewhere.35 Formate and alcohol (methanol, ethanol, propanol, butanol) concentrations were measured using an Agilent 6890 N GC (Wilmington, USA). Furthermore, total organic carbon (TOC) measurements were performed to determine the level of conversion of dissolved CO2 and carbonates to soluble organic products. The TOC test was carried out using TOC-VCPH Total Organic Carbon Analyzer (Shimadzu, Kyoto, Japan) with TOC-Control V ver.2 software for data analysis. Gas flow measurements were validated using anode and cathode gas balance calculations based on water electrolysis stoichiometry (see ESI section†).Carbonate concentration in cathodic liquid was measured using a headspace method, which involved addition of a 3 mL liquid sample in a 10 mL air-tight vial followed by sample acidification using injection of 0.5 mL of 3 N HCl solution. The headspace CO2 concentration was analyzed by GC after 15 min. A five-point calibration curve (R2 = 0.99) with predetermined carbonate concentrations was used to estimate carbonate concentration based on the measured headspace CO2 concentration.
To evaluate the energy performance of the MES, the energy consumption (expressed in H2 equivalent) and the coulombic efficiency (CE) based on the major products generated at the cathode (H2, CH4 and acetate) were determined as described elsewhere.36 All measurements were carried out in duplicates or quadriplicates.
2.2 MES cell design and operation
Two identical MES cells (MES-1 and MES-2) were operated. Each setup was constructed with PVC plates arranged to form a two-compartment cell comprising a 50 mL anode chamber and a 100 mL cathode chamber separated by a 7 cm × 5 cm dialysis membrane (14 KD, Biotech, TX0113, BBI) wrapped by a layer of Nylon cloth. The anode chamber contained two 100 × 45 × 1 mm Ti/IrO2 meshes (Magneto Special Anodes, Netherlands) held together by a Ti wire. The cathode chamber was filled with pieces of shredded carbon felt (approximately 10 × 10 × 5 mm). Carbon felt pieces were used to ensure unrestricted liquid flow through the cathode compartment and improve transport of dissolved gases. CO2 supply to the cathode was provided by a continuous feed of carbonate solution composed of (in g L−1): Na2CO3 5, K2CO3 5, NH4Cl 0.25, Na2HPO4 1.25, K2HPO4 1.25, NaCl 0.5, KCl 1.5, and 1 ml L−1 of trace metals solution at a flow rate of 150 mL per day. The detailed composition of the trace metal solution can be found elsewhere.37 The pH of the cathode liquid was controlled using a pH probe installed in the external recirculation loop, and a pH controller, which supplied 0.2 N HCl solution to maintain pH value at a pre-set value. The combined flow of carbonate and pH control solutions resulted in a hydraulic retention time (HRT) of 8–12 hours. A 0.25 N NaOH solution was fed to the anode at the same rate as the cathode feed. The catholyte was recirculated at 18 L per day to facilitate mixing and effective control of pH and temperature in the cathode compartment. The temperature of the cathode liquid was kept constant at 27 ± 2 °C using a rope heater coiled around a section of the external recirculation loop and a temperature controller with temperature probe installed in the cathode chamber. Constant current of 50 mA was supplied to each MES cell using a power source (PW18-1.8AQ, Kenwood Corp, Tokyo, Japan).The experiments were conducted in three main experimental phases (runs). In Run #1, each MES cell was inoculated with 50 mL of homogenized anaerobic sludge with a volatile suspended solids (VSS) content of approximately 40 g L−1. This inoculum originated from an anaerobic reactor treating agricultural waste (Rougemont, QC, Canada). After inoculation MES-1 was first operated at moderate pH 7.2 and then at low (5.0–6) pH, whereas MES-2 was operated at moderate pH and then at high (7.5–8.5) pH.
To evaluate the effect of bioaugmentation on the performance of MES cells, in Runs #2 and #3, the MES cells were inoculated with four strains of acetogenic bacteria: C. carboxidivorans, C. ljungdahlii, C. autoethanogenum, and E. limosum. These strains were grown in 160 mL serum bottles containing 30 mL of culture media and H2/CO2 (80/20 v/v) in the bottle headspace. Detailed media composition and cultivation conditions can be found in Bruant et al.38 All cultures were incubated at 30 °C for 5 days, corresponding to nearly complete consumption of CO2 in the bottle headspace and an optical density (OD600) of 0.2–0.3. For bioaugmentation, the culture broth collected from all bottles was combined resulting in an OD of 0.26.
At the startup of Run #2, the MES cells already containing adapted microbial populations from Run #1 were bioaugmented with the combined culture broth by withdrawing 80 mL of catholyte from each MES cell and replacing it with the combined pure culture broth. In the following Run #3, new carbon felt cathodes were installed and each MES cell was inoculated with 50 mL of the same anaerobic inoculum (homogenized anaerobic sludge) as in Run #1 and 50 mL of the culture broth containing 4 acetogenic strains. Table 1 provides additional details of operating conditions during Runs #1–3.
To determine whether there is a statistically significant difference in CH4 and acetate production rates across different experiments (Runs #1, #2, and #3) conducted in MES cells, the average values of these parameters were calculated and compared using a t-test.
2.3 Cyclic voltammetry
The electrochemical response of the cathode material in the HER region was investigated by cyclic voltammetry (CV) using a three-electrode cell configuration with cathode as a working electrode. An Ag/AgCl electrode (+0.199 V vs. reversible hydrogen electrode, RHE, CH Instrument, USA) was the reference electrode, and a pair of 100 × 45 × 1 mm Ti/IrO2 meshes (anode) served as the counter electrode. During the CV tests, to ensure anaerobic conditions, the reference electrode was inserted through a septum located at the top of the cathode compartment. Cyclic voltammograms were recorded in a potential window from −0.25 V to −0.75 V vs. Ag/AgCl at a scan rate of 5 mV s−1. All measurements were performed using a potentiostat (Model 2273, Princeton Applied Research, USA) controlled by the VersaStudio v2.6 software (Princeton Applied Research, USA).2.4 Microbial community analysis
The microbial community structure of the MES cells was monitored by analyzing the electroactive biofilm developed on the carbon felt cathode. DNA was extracted from the microbial inoculum (anaerobic sludge) and the carbon felt cathodes (250 mg samples) using a DNeasy PowerSoil Pro kit (Qiagen, USA) and following the manufacturer's instructions. DNA quality and quantity assessments were carried out using the Quant-iT PicoGreen assay (Fisher Scientific Ltd). DNAs were then prepared for 16S rRNA gene sequencing, using the 515F-Y/926R primer pair covering the V4 and V5 hypervariable region of the 16S rRNA gene39 and the 2X KAPA HiFi HotStart ReadyMix (Fisher Scientific). After normalization, amplified DNAs were finally shipped to the Centre d'expertise et de services Génome Québec to be sequenced on an Illumina NovaSeq6000 – PE150 system. Data from the 16S rRNA gene amplicon sequencing were treated, analyzed and annotated using NRC-EME internal AmpliconTagger pipeline40 and visualized in Microsoft Excel 2017 (Microsoft Office suite).3. Results
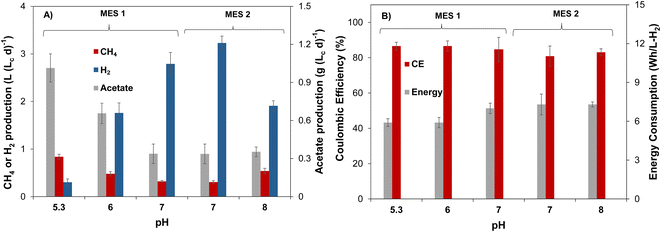
3.1 Impact of catholyte pH on acetate and CH4 production
As mentioned earlier, catholyte pH plays an important role in both shaping the MES cell mixed microbial community and determining CO2 availability in the catholyte. Indeed, while low pH is considered optimal for many acetogenic species, chain-elongating bacteria such as C. carboxidivorans prefer pH values close to neutral. Additionally, CO2 solubility increases at higher pH values. Accordingly, a broad range of pH values (5.5–8.5) was investigated.To evaluate the impact of catholyte pH on the production of volatile fatty acids (VFAs) and CH4 in a microbial electrosynthesis system, in the first experiment (Run #1), MES-1 and MES-2 cells were inoculated with mixed anaerobic microbial community of an anaerobic reactor and operated at several pH levels described in Table 1. These pH settings were based on several previous studies, which demonstrated methanogenesis suppression at pH values below 6 resulting in increased production of volatile fatty acids (VFAs) (e.g. Gavilanes et al.5), while our previous work also showed increased production of acetate and ethanol in a MES cell operated at a pH of 8 and fed with gaseous CO2.36 As described above, at startup both MES cells were operated at a pH setpoint of 7.2 (70 days for MES-1 and 40 days for MES-2) to obtain duplicate measurements. MES cell operation at near neutral pH is expected to promote both hydrogenotrophic and acetoclastic methanogenic populations17,41 therefore leading to increased CH4 production and low concentration of acetate in the cathodic liquid.
Steady state gas production rates and acetate concentrations of MES-1 and MES-2 during the first phase of operation at moderate pH are in a reasonable agreement, as can be seen from the results shown in Fig. 1A. Volumetric rates of CH4 and acetate production stabilized around 0.31 L (Lc d)−1 and 0.34 g (Lc d)−1, respectively, while H2 production averaged 3.0–3.2 L (Lc d)−1. Production of VFAs other than acetate was insignificant in both cells. Some differences, such as higher H2 production in MES-2, are well within the expected variability of microbial populations in the two MES cells and can be also attributed to pH variations, which were somewhat higher during MES-2 operation (see Table 1), resulting in lower microbial consumption of H2 and, accordingly, higher outflow of H2 at the same applied current as in MES-1. Once stable performance in terms of acetate, CH4, and H2 production was observed, in the second part of this experiment the catholyte pH of MES-1 was decreased to 6 (days 95–124) and then to a lower pH of 5.3 (days 125–145), while MES-2 catholyte pH was increased to a setpoint of 8. Following each pH adjustment by the controller, noticeable pH fluctuations were observed, resulting in actual pH values fluctuating within a certain range, e.g. 5.7–6.3 with a pH setpoint of 6.
The analysis of MES-1 effluent composition showed that when the pH of catholyte decreased from 7 to 6, the production of acetate more than doubled achieving a volumetric productivity of 0.8 ± 0.1 g (Lc d)−1, corresponding to acetate concentration of 302 ± 33 mg L−1. Once catholyte pH was further decreased to 5.3, the production of acetate reached 1.0 ± 0.1 g (Lc d)−1 (Fig. 1A). At low pH values, production of other VFAs in MES-1 was once again negligible with propionate always present at concentrations below 20 mg L−1 and other VFAs below a detection limit of 2 mg L−1. Fig. 1 shows that in MES-1, the highest CH4 production rate of 0.84 ± 0.05 L (Lc d)−1 (84 ± 5 mL per day) was obtained at pH 5.3, while at pH 7.2 CH4 production was 0.32 ± 0.03 L (Lc d)−1.
After day 40 of the experiment, MES-2 pH was increased and maintained at around 8 for the remaining 60 days of this experiment. In MES-2, at high catholyte pH the volumetric productivity of acetate remained at 0.54 ± 0.05 g (Lc d)−1, which is slightly higher than the value recorded at near neutral pH in this MES (Fig. 1). Similar to MES-1, production of VFAs other than acetate was insignificant. In particular, propionate concentration remained below 10 mg L−1, while other VFAs were, once again, below the detection limit. The increase of catholyte pH to 8 in MES-2 also resulted in CH4 production increase to 0.54 ± 0.06 L (Lc d)−1, which is statistically higher (t-test p < 0.05) than that at pH 7.2 setpoint.
Several previous studies showed production of propionate and butyrate, in addition to acetate, in MES cells fed with gaseous CO2.33,34,42 In addition to the absence of short chain carboxylic acids other than acetate, in this study for both MES cells catholyte analysis showed the absence of ethanol, indicating that acetate conversion to ethanol through solventogenesis was insignificant. The absence of other products is supported by the comparison of total organic carbon (TOC) and acetate measurements, which confirmed that production of acetate accounted for 92 ± 3% of measured TOC. Also, coulombic efficiency calculations (see ESI†), which accounted for CH4 and acetate production, yielded values in a range of 82–87% (Fig. 1B) thus confirming CH4 and acetate as the two main products of CO2 conversion.
In addition to affecting microbial populations, cathodic pH determined dissolved CO3− availability for microbial conversion. As expected, pH values above 7 considerably increased measured concentration of dissolved carbonate (CO3−) in water with an average measured value of 3.2 ± 0.5 g L−1 in MES-2 operated at pH 8, while in MES-1 at pH 6, the measured dissolved carbonate concentration was one order of magnitude lower (0.2 ± 0.1 g L−1). Furthermore, at pH 5.2 the measured dissolved carbonate concentration approached zero. CO3− availability depends on catholyte pH as well as on the microbial metabolic activity (carbonate consumption rate). Accordingly, higher metabolic activity in MES-1 due to increased acetate production might also contribute to near zero dissolved carbonate concentration at low pH. Interestingly, coulombic efficiency and energy consumption estimations showed that these parameters were not adversely affected at low CO3− concentrations. Indeed, Fig. 1B shows that coulombic efficiency increased slightly from 82 ± 5% to 87 ± 3%, while the energy consumption reduced by 20% to 5.9 ± 0.4 W h LH2−1 as catholyte pH decreased from 8.0 ± 0.2 (MES-2) to 5.2 ± 0.1 (MES-1).
Overall, results of Run #1 suggested that low hydraulic retention time of approximately 10 h implemented during this study in both MES cells to maintain sufficient supply of carbonate promoted formation of acetate-producing bacteria (e.g., Clostridia species) in the cathodic biofilm and catholyte at all tested pH ranges. As expected, acetate production was the highest during MES-1 operation at pH 5.3. At the same time, methanogenic activity also improved at this pH, contrary to the expected suppression at such low pH value.
3.2 Bioaugmentation with acetogens
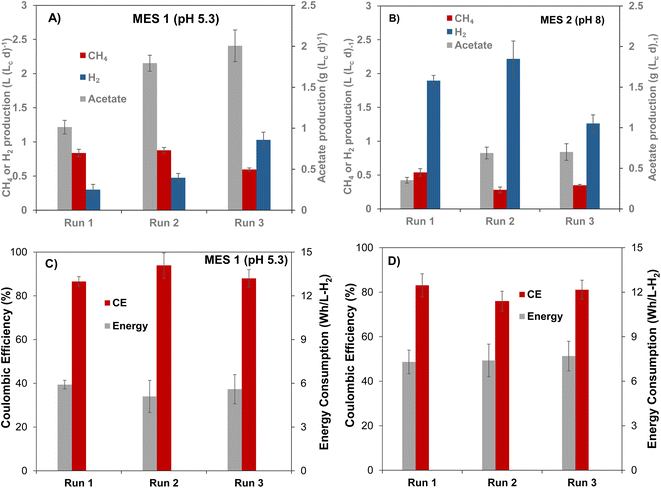
Bioaugmentation is considered an efficient approach for improving biocatalytic performance of a microbial population.16,22,32,43 To evaluate the influence of bioaugmentation on the mixed microbial populations of MES-1 and MES-2, four cultures of acetogenic bacteria (C. carboxidivorans, C. ljungdahlii, C. autoethanogenum, and E. limosum) were grown in batch cultivations with CO2 as a sole source of carbon. All of these cultures convert CO2 to acetate in the presence of H2, while some cultures (e.g. C. Carboxidivorans) were previously shown to produce ethanol and caproate.44,45 Thus, proliferation of C. Carboxidivorans in the cathodic biofilm and/or catholyte was expected to result in ethanol and caproate detection, in addition to increased acetate production. Culture media containing each strain were combined and then added to cathodic compartments of MES-1 and MES-2. Two bioaugmentation attempts were carried out. As mentioned in Materials and methods, during the first bioaugmentation attempt corresponding to Run #2 (Table 1), the four acetogenic strains culture was added to MES-1 and MES-2 cathode compartments at the end of Run #1 without cathode material replacement, i.e. acetogenic cultures were added to the mature cathodic biofilm. In the following experiment (Run #3), the four acetogenic strains culture was mixed with the anaerobic sludge, then the bioaugmented inoculum was introduced to MES-1 and MES-2 cathodic compartments with virgin carbon felt cathodes. This comparison was aimed at evaluating the impact of inoculum composition on population distribution of the mature cathodic biofilm and the resulting MES cell performance in terms of VFA and CH4 production.At the beginning of the first bioaugmentation trial (Run #2), MES-1 operated at around pH 6, showed a rapid increase in acetate production from near zero concentration at the startup (day 0, Fig. S2B†) to 700 mg L−1 (day 22–25), corresponding to a production rate of 1.5 ± 0.1 g (Lc d)−1, as well as increased CH4 production, which at steady state reached 0.88 ± 0.06 L (Lc d)−1. Also, H2 production declined from 3.8 L (Lc d)−1 to 0.4 L (Lc d)−1 (Fig. S2A†). The decrease in released H2 is attributed to its consumption by acetogenic cultures added to the reactor, which may have also stimulated CH4 formation through increased activity of acetoclastic methanogens. Interestingly, once the pH was further decreased to 5.4 in MES-1, CH4 production slightly increased, however, this change was statistically insignificant (t-test, p = 0.03) while acetate production substantially increased to 1.8 g (Lc d)−1 (1.8 folds, p = 0.005) with corresponding CE of 94 ± 5% and reduced energy consumption of 5.1 ± 0.1 W h LH2−1 (Fig. 2). The significant increase in acetate production in Run #2 of MES-1 in comparison to Run #1 (130% at pH 6.2 ± 0.1 and 80% at pH 5.4 ± 0.1, p ≤ 0.005) can be attributed to either the impact of bioaugmentation or to the proliferation of indigenous acetogenic populations introduced via the mixed cathodic biofilm community.
Results of MES-2 operation, which was maintained at a higher pH setpoint of 8 throughout Run #2, also showed improved acetate production (Fig. 2). Acetate production nearly doubled from 0.35 ± 0.03 g (Lc d)−1 in Run #1 (day 70–120) to 0.69 ± 0.07 g (Lc d)−1 in Run #2 (day 120–180), as shown in Fig. 2B. However, high pH values resulted in lower rate of acetate production as compared to MES-1, although concentration of carbonate was an order of magnitude higher.
The impact of bioaugmentation on CO2 conversion was further investigated in Run #3, in which both mixed and pure cultures were simultaneously added to the cathode compartments of MES-1 and MES-2. In this experiment, MES-1 operation was initially started at pH 6. After 40 days of operation, the pH was decreased to 5.1 (Fig. S2†). Shortly after startup of this test, acetate production peaked at 1.6 g (Lc d)−1 and then stabilized at 0.92 ± 0.04 g (Lc d)−1. Subsequently, the catholyte pH was decreased to 5.1, resulting in a statistically significant increase in acetate production (t-test, p = 0.006), which reached 2.2 g (Lc d)−1 at the end of this experiment. This value is higher than that at a similar pH in Run #1 (Fig. 2A).
3.3 Electrochemical characterization
The cyclic voltammograms recorded for MES-1 and MES-2 at the start and end of each experiment are shown in Fig. 3. These results illustrate the electrochemical current response of the systems to cathodic potentials (i.e. energy input) in a range of 0.7–1.0 V (vs. Ag/AgCl reference electrode). The current response recorded is a measure of the volume of H2 generated on the cathode, which is necessary for the CO2 bioelectrochemical conversion to acetate and CH4. Fig. 3 shows that the hydrogen evolution reaction (HER) rate increased exponentially at cathodic (negative) potential above 0.2 V in both MES cells. Further, it can be seen that the HER was lowest under abiotic conditions and increased over time, indicating the presence of additional HER electroactive sites. These additional active sites can be attributed to the development of electroactive biofilm on the cathode surface. Interestingly, hydrogenotrophic microorganisms can thermodynamically favor H2 evolution on the cathode by maintaining low H2 partial pressure on the electrode surface. Lower H2 partial pressure due to H2 consumption has been shown to favor the growth of acetogenic bacteria with high affinity to H2.46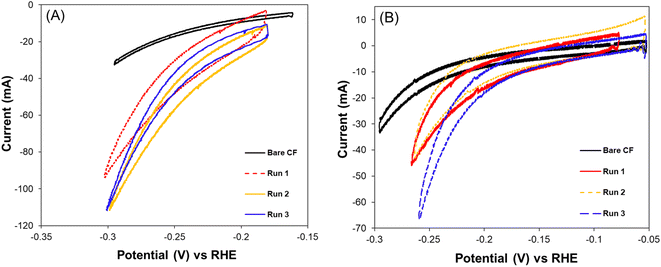 | ||
| Fig. 3 Cyclic voltammograms at the startup (bare carbon felt) and the end of each experimental Run of (A) MES-1 at pH range 5.0–5.5 and (B) MES-2 at pH range 7.5–8.5. | ||
The evidence of microbial growth, in either case, is supported by the broadening of the CV curves recorded under biotic conditions as compared to those under abiotic conditions. The increase of the area between the forward and backward scans of the CV curve over time is due to the increased capacitance of the carbon felt cathode, which is can be attributed to the growth of the cathodic biofilm.13,47
Considering the CV response of the MES-1 biocathodes from Run #1 to Run #3, it can be deduced that at the end of Run #2 at pH 5.1, there was more efficient consumption or removal of H2 from the surface of the cathode. This is in agreement with the CE data showing that the highest CE (94 ± 6%) was recorded at Run #2. Conversely, the lowest CE (87 ± 6%) was observed in Run #1. This indicates that the bioelectroreduction of CO2 using H2 by hydrogenotrophic microorganisms in MES-1 may be related to the cell's HER activity at a given pH. This can be hypothesized since the CE trend in electron utilization efficiency for the production of acetate and CH4 agreed with the trend in HER activity of the cell.
MES-2 CV results indicate the impact of the biofilm on the CO2 bioelectro-reduction at the cathode. Specifically, for Run #2, which yielded the highest energy consumption, the CV shown in Fig. 3B suggests that in this experiment MES-2 had the thickest biofilm, as can be concluded from the largest area under the CV curve. The thick biofilm likely limited proton and carbonate transport to electroactive bacteria inside the biofilm, resulting in decreased cathodic electroactivity relative to Run #3, where less time was given for biofilm development on the cathode and, consequently, a thinner biofilm was expected. Another explanation for the increased capacitive behavior of MES-2 in Runs #2 and #1 is the tendency of thicker microbial biofilms to accumulate more charge, manifested as an increased area under the CV curve. Notably, Run #2, in which MES-2 was operated for 62 days, was the continuation of Run #1, which was already operated for 120 days, resulting in a total time of 182 days for the formation of electroactive biofilm. Contrary to the decreased HER electroactivity in MES-2, there was increased electroactivity of MES-1 during Run #2, which agrees with the experimentally observed increase in acetate production. Overall, the observed differences in CVs agree with acetate and CH4 production rates and confirm the impacts of pH and microbial populations on bioelectrochemical CO2 conversion.
3.4 Microbial community analysis
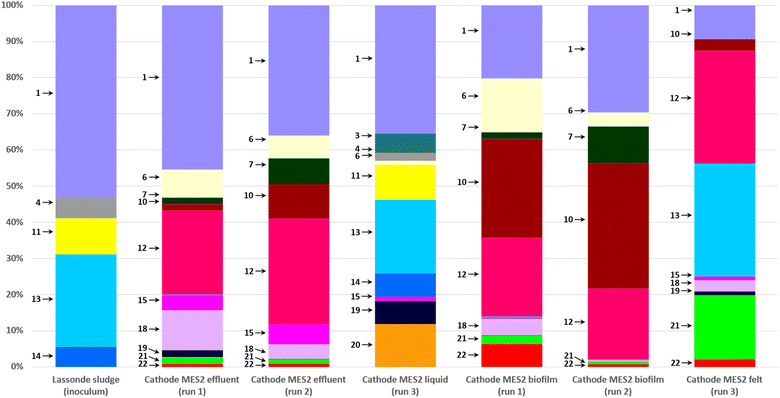
Most abundant phylogenetic groups detected in MES-1 and MES-2 microbial communities and their main characteristics are given in Table 2. Microbial community analyses of MES-1 and MES-2 revealed significant differences between the populations of the inoculum (anaerobic sludge), the cathodic biofilm and effluent of MES cells. A significant reduction of diversity was observed after the first experiment (Run #1). While almost half of the inoculum's population (48%) was composed of multiple species, each representing less than 5% of the total population, this number decreased to 30% and 20% in the adapted populations of the cathodic biofilms of MES-1 and MES-2, respectively (end of Run #1). In Run 2, in which 4 pure strains of acetogenic bacteria were added to the mature cathodic biofilm, these numbers remained similar: 31% and 29%, respectively (Fig. 4 and 5). Furthermore, simultaneous introduction of the mixed microbial consortium of anaerobic sludge and acetogenic strains at the startup of the second bioaugmentation trial (Run #3), also resulted in a reduced microbial diversity at the end of this test with 16% and 9% of microbial populations representing less than 5% of the total population in MES-1 and MES-2, respectively.| ID | Phylogeny | Characteristics of interest | References | |
|---|---|---|---|---|
| 1 | n/a | <5% of the total microbial population | n/a | |
| 2 | Bacteria | Xanthobacter (genus) | Aerobic or microaerophilic, H2-oxidizers, methylotrophic, N2-fixer, acids and alcohols as carbon and energy sources | 48 |
| 3 | Bacteria | Syntrophomonas (genus) | H2-, propionate- and acetate-producer, syntrophic fatty-acids oxidizer, biofilm producer, DIET-related microorganism | 49 |
| 4 | Bacteria | Syntrophobacter (genus) | H2- and acetate-producer, syntrophic propionate oxidizers and sulfate reducers | 49 |
| 5 | Bacteria | Stenotrophomonas (genus) | Electrotrophs, acetate producer, nitrate and nitrite reducer, role in sulphur cycles | 50 |
| 6 | Bacteria | Rikenellaceae (family) | Hydrogen producer in biocathodes, carbohydrate degrader | 51,52 |
| 7 | Bacteria | Pseudomonas (genus) | Role in MES and biocathode processes, extracellular electron transfer | 49,53 |
| 8 | Bacteria | Proteinivoracales (order) | Haloalkaliphilic VFAs and H2 producer from proteinaceous substrates | 54 |
| 9 | Bacteria | Propionispora (genus) | Short-chain fatty acids (e.g. propionate and acetate) producer | 55 |
| 10 | Bacteria | Peptostreptococcales-Tissierellales (order) | Organic matter degraders and acetate producers | 49,53,54 |
| 11 | Archaea | Methanosaeta (genus) | Acetoclastic CH4 producers | 49,56 |
| 12 | Archaea | Methanobrevibacter (genus) | Hydrogenotrophic CH4 producers | 49,56 |
| 13 | Archaea | Methanobacterium (genus) | Hydrogenotrophic CH4 producers, DIET-related microorganism | 49,56 |
| 14 | Bacteria | Longilinea (genus) | Electroactive VFAs producer, enhanced growth in co-cultivation with hydrogenotrophic methanogens | 57 |
| 15 | Bacteria | Eubacterium (genus) | Acetogen, butyrate producer, caproate producer | 51,58,59 |
| 16 | Bacteria | Erysipelothrix (genus) | Capnophilic microorganism with weak fermentative activity, do not produce gas | 53 |
| 17 | Bacteria | Enterobacteriaceae (family) | Heterogeneous family with various functions: H2 producers, organic acids producers, nitrate reducers, electroactive | 53 |
| 18 | Bacteria | Dysgonomonas (genus) | Electroactive, propionate, acetate, lactate and succinate producer | 53,54 |
| 19 | Bacteria | Clostridium sensu stricto 1 (genus) | Heterogeneous genus with various functions: electron-donating bacteria, acidogen, acetogen, alcohols producer | 49,51,53,58,59 |
| 20 | Bacteria | Bacteroides (genus) | Propionic and acetic acids producer, electron-donating bacteria | 49,53 |
| 21 | Bacteria | Acetobacterium (genus) | Acetate producer from CO2 and H2 | 49,51,59 |
| 22 | Bacteria | Acetoanaerobium (genus) | Hyper-ammonia producing anaerobe, organic acids and solvents producer from proteins | 59 |
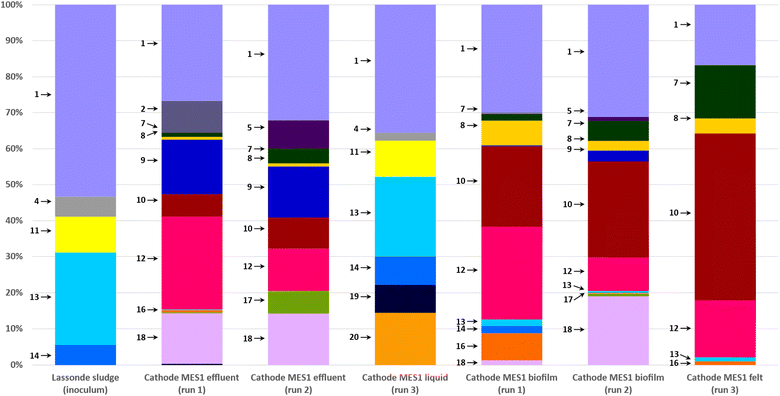 | ||
| Fig. 4 Distribution of microbial populations at the end of MES-1 Runs #1, #2, and #3 based on 16S rRNA amplicon sequencing. Description of microbial populations (numbers 1–20) is provided in Table 2. For a better visualization, only microorganisms representing 5% or more of the total population are identified on the graph. | ||
 | ||
| Fig. 5 Distribution of microbial populations at the end of MES-2 Runs #1, #2, and #3 based on 16S rRNA amplicon sequencing. Description of microbial populations (numbers 1–20) is provided in Table 2. For a better visualization, only microorganisms representing 5% or more of the total population are identified on the graph. | ||
As can be seen from the population analysis distribution shown in Fig. 4 and 5, the population of Methanosaeta (acetoclastic methanogen), which represented 9% of the inoculum populations, was undetectable in the cathodic biofilms of both MES cells. Considering that acetate was readily available at concentrations above 300 mg L−1, it can be suggested that high levels of dissolved H2 or carbonate in combination with low pH in MES-1 and high pH in MES-2 had a negative impact on the acetoclastic methanogenic populations. At the same time, hydrogenotrophic methanogens, which comprised less than 1% of the inoculum population, proliferated comprising 20–30% of microbial populations both in the cathodic biofilms and catholytes of MES-1 and MES-2 cells. Interestingly, in MES-1 this population evolved significantly from a dominance of Methanobacterium spp. to a dominance of Methanobrevibacter spp. (Fig. 4). As expected, acetogenic bacteria, such as Peptostreptococcales-Tissierellales, proliferated due to the presence of CO2 and hydrogen.
Electroactive species, such as Pseudomonas, were also detected, e.g. in MES-1 Run #3 (14% of the total microbial population, Fig. 4). Proliferation of this and other electroactive bacteria supports the observed increase in HER shown in Fig. 3. Interestingly, in both MES cells proliferation of acetate and propionate oxidizing bacteria (e.g. Syntrophobacter and Peptostreptococcales-Tissierellales) was observed. Likely, these populations led to electron “recycling”, which resulted in energy losses and decreased the overall Coulombic efficiency.
In spite of increased acetate production, microbial community analysis suggested poor retention of the acetogenic strains introduced to cathodic compartments in both bioaugmentation trials. While several reasons for poor retention of the newly introduced strains can be suggested, high flow rate with an HRT of only 8–12 h at which MES cells were operated, most likely contributed to the washout of these newly introduced species. It is likely that washout was combined with insufficient attachment of the four acetogenic strains to the existing biofilm, in particular in Run #2.
4. Discussion
In both bioaugmentation tests introduction of pure cultures to the cathodic liquid led to increased acetate production shortly after these cultures were introduced. However, these newly introduced acetogenic cultures did not appear to proliferate in the cathodic biofilm and catholyte, while wild-type acetogenic populations thrived. The absence of significant production of butyrate and caproate can serve as an indirect confirmation of this hypothesis. At the same time, low pH played the decisive role in promoting acetate formation in MES-1 by indigenous acetogenic populations. Interestingly, the highest acetate production was observed in MES-1 Run #3, which had the shortest duration of 56 days as compared to 209 days for the combined Runs #1 and #2. Not surprisingly, CH4 production in MES-1 was the lowest during Run #3 as compared to Run #1, although once again CH4 production persisted throughout the entire MES-1 operation. Overall, acetate was the main product in the catholyte, while the off-gas primarily contained CH4 and H2. If the CO2 conversion efficiency is high leading to near zero off-gas CO2 concentration, the resulting CH4 and H2 mixture, known as hythane, can be considered a valuable biofuel.60In the two MES-2 tests carried out with bioaugmented microbial populations, the steady state acetate concentrations were comparable (Fig. 2B, Runs #2 and #3). A more detailed analysis of the acetate production dynamics shows that a peak in acetate production was observed shortly after the startup of the second bioaugmentation trial (Run #3, Fig. S3†), but not in the first one (Run #2). At the same time CH4 production remained comparable in both experiments. These trends are similar to MES-1 performance and once again suggest that although introduction of acetogenic strains impacted acetate production at the startup of each experiment, indigenous populations of acetogens and hydrogenotrophic methanogens proliferated over time.
Apart from acetate, production of other fatty acids and ethanol was insignificant, even after bioaugmentation that included addition of C. carboxidivorans. This acetogenic bacterium is known for its ability to produce ethanol and caproate from CO2, in addition to acetate. Although low levels of propionate and butyrate were detected after bioaugmentation, similar to Run #1 with the anaerobic sludge inoculum, concentrations of these short chain fatty acids remained below 20 mg L−1 accounting for less than 5% of all measurable VFAs. This observation indicated poor survival of C. carboxidivorans and other pure cultures in MES-1 and MES-2. In addition, although caproic acid was detected in the bioaugmentation trials, its concentration remained at less than 5 mg L−1 in MES-1 catholyte and below the detection limit in MES-2 catholyte. Caproic acid production in a MES cell operated in a similar pH range under substantially longer hydraulic retention time (10–14 days) has been reported elsewhere.15,27 Also, in our previous work,36 high concentrations of acetate and ethanol were observed in a MES operated at a high cathodic pH with continuous supply of gaseous CO2 and a relatively long retention time of 6–8 days. Overall, while it was hypothesized that introduction of species capable of CO2 conversion to acetate and caproate (e.g. C. carboxidivorans) would lead to MCFAs production, this hypothesis was not confirmed.
Operating MES cells at low pH is a well known strategy for suppressing methanogenic activity.5 In microbial electrosynthesis, persistent CH4 production at low pH can be linked to the existence of a pH gradient within the three-dimensional (3D) carbon felt cathode. Indeed, H2 formation at the cathode accompanied by proton consumption increases pH in the cathode surface vicinity. Considering such factors as the dense packing of carbon felt fibers in the carbon felt, biofilm formation around individual carbon fibers, and restricted catholyte flow through the carbon felt, it is reasonable to anticipate a significantly higher pH inside the carbon felt, even when bulk liquid pH values are controlled to be below pH 5.5. This pH gradient created an environmental niche suitable for the growth and metabolic activity of methanogenic populations, particularly hydrogenotrophic methanogens. Additionally, the dissolved H2 concentration is expected to be highest in the cathode surface vicinity. In such scenario, hydrogenotrophic methanogens can utilize the H2 produced at the electrode surface, readily available inside the 3D cathode. 16S analysis of microbial populations in MES-1 operated at low pH confirmed the proliferation of hydrogenotrophic methanogens in the cathodic biofilm (Methanobrevibacter and Methanobacterium spp., Fig. 4 and 5). In spite of high concentration of acetate, the population of acetoclastic methanogens in the cathodic biofilm dropped below the detection limit compared to its abundance in the inoculum. The only exception was the presence of Methanosaeta spp. in the catholyte of MES-1 and MES-2 (but not in the cathodic biofilms) at the end of Run #3 (Fig. 5). Considering shorter duration of this run, the presence of acetoclastic methanogens in the suspended biomass can be explained by the remaining anaerobic sludge inoculum.
In addition to catholyte pH, hydraulic retention time (HRT) appeared to be yet another crucial factor influencing cathodic microbial populations and limiting the CO2 conversion products. Throughout all experiments, a 5 g L−1 (as CO32−) stock solution of carbonate was used as a source of inorganic carbon. Although carbonate concentration can be increased, significant volatilization losses would be expected at pH values below 6. Consequently, carbonate concentration was maintained at 5 g L−1 with a corresponding HRT of 8–12 h. Such short HRT favored the proliferation of fast-growing acetogenic microbial populations in bulk liquid and likely led to the washout of acetogenic cultures introduced in Runs #2 and #3 and wild-type chain elongating bacteria. Nevertheless, the steady state volumetric rate of acetate production achieved in this study (2.0 g (Lc d)−1 in MES-1, Run #3) compares favorably with other results available in the literature. For example, an acetate production rate of 0.2 g (Lc d)−1 was obtained using MoS2 nanoflowers on carbon felt cathode,61 and a rate of 2.1 g (Lc d)−1 was achieved with TiO2 and Rh nanoparticles on a carbon felt cathode.62
In summary, multiple strategies can be proposed to combine microbial electrosynthesis of short chain fatty acids with the production of MCFAs. First of all, additional efforts are required to suppress CH4 formation. Given that low pH was insufficient for suppressing CH4 production and might not be optimal for the metabolic activity of chain-elongating microorganisms, alternative approaches to eliminate methanogenic populations need to be tested. These approaches include thermal or alkaline inoculum pretreatment63 and engineering efficient microbial consortia comprising acetogenic and chain elongating microorganisms.25,64 Additional strategies could involve enhancing mass transfer within the three-dimensional cathode by utilizing novel cathode materials that promote the proliferation and attachment of acetogenic and chain-elongating bacteria. In this regard, improving cathode materials emerges as a promising avenue for research. The use of highly porous three-dimensional cathodes, for instance, can increase the total surface area available for microbial biofilm formation and enhance the transport of substrates and intermediates to and from the cathode surface. Recent advancements in the development of 3D cathodes provide several examples of such improvements.14,65,66 Moreover, beyond using cathode materials with a larger surface area, cathode modification with transition metals can enhance electron exchange efficiency. Specifically, electrodeposition of transition metal alloys, such as NiFeMn, has been shown to increase the rate of CO2 conversion.67 Also, the NiFeMn cathode led to increased production of butyrate.13
5. Conclusion
In this study, we assessed the bioelectrochemical conversion of CO2 to acetate and CH4 in two MES cells supplied continuously with carbonate. To determine the optimal pH for microbial electrosynthesis of fatty acids, the MES cells were operated either at low (5.3), moderate (7), or high (8) pH values. Although these experiments demonstrated enhanced acetate production at low pH, CH4 production persisted. Subsequent 16S sequencing of cathodic biofilms confirmed a significant presence of hydrogenotrophic methanogens, particularly Methanobrevibacter and Methanobacterium spp. Additionally, bioaugmenting the cathodic microbial population with well-known acetogenic Clostridia and Eubacteria strains had a limited impact on the cathodic biofilm's microbial populations. Instead, an effective microbial consortium comprising electroactive, acetogenic, and hydrogenotrophic methanogenic populations was established resulting in a substantial increase of acetate production rate from 0.34 g (Lc d)−1 in Run #1 to 2.0 g (Lc d)−1 in Run #3, in addition to CH4 production. It is hypothesized that the relatively short HRT (8–12 h) used throughout the experiments to ensure sufficient supply of carbonate, led to the washout of the introduced acetogenic cultures. To facilitate the development of cathodic microbial populations capable of CO2 conversion into products beyond acetate, gaseous CO2 supply as a delivery method can be recommended to increase cathodic HRT. Furthermore, to mitigate CH4 production, inoculum pretreatment should be considered.Conflicts of interest
There are no conflicts to declare.References
- P. Dessi, L. Rovira-Alsina, C. Sanchez, G. K. Dinesh, W. Tong, P. Chatterjee, M. Tedesco, P. Farras, H. M. V. Hamelers and S. Puig, Biotechnol. Adv., 2021, 46, 107675 CrossRef CAS PubMed.
- D. Pant, A. Singh, G. Van Bogaert, S. I. Olsen, P. S. Nigam, L. Diels and K. Vanbroekhoven, RSC Adv., 2012, 2, 1248–1263 RSC.
- S. Zhang, J. Jiang, H. Wang, F. Li, T. Hua and W. Wang, J. CO2 Util., 2021, 51, 101640 CrossRef CAS.
- S. Bajracharya, R. Yuliasni, K. Vanbroekhoven, C. J. Buisman, D. P. Strik and D. Pant, Bioelectrochemistry, 2017, 113, 26–34 CrossRef CAS PubMed.
- J. Gavilanes, M. T. Noori and B. Min, Bioresour. Technol. Rep., 2019, 7, 100292 CrossRef.
- L. Jourdin, M. Winkelhorst, B. Rawls, C. J. N. Buisman and D. P. B. T. B. Strik, Bioresour. Technol. Rep., 2019, 7, 100284 CrossRef.
- C. Im, K. Valgepea, O. Modin and Y. Nygård, Bioresour. Technol. Rep., 2022, 19, 101156 CrossRef CAS.
- C. Flores-Rodriguez and B. Min, Bioresour. Technol., 2020, 300, 122624 CrossRef CAS PubMed.
- G. Mohanakrishna, K. Vanbroekhoven and D. Pant, J. CO2 Util., 2016, 15, 57–64 CrossRef CAS.
- J. B. Arends, S. A. Patil, H. Roume and K. Rabaey, J. CO2 Util., 2017, 20, 141–149 CrossRef CAS.
- L. Jourdin and T. Burdyny, Trends Biotechnol., 2021, 39, 359–369 CrossRef CAS PubMed.
- L. Xiao, R. Sun, P. Zhang, S. Zheng, Y. Tan, J. Li, Y. Zhang and F. Liu, Chem. Eng. J., 2019, 378, 122229 CrossRef CAS.
- E. Nwanebu, S. Omanovic, S. Hrapovic, A. G. Vidales and B. Tartakovsky, Int. J. Hydrogen Energy, 2022, 47, 203–215 CrossRef CAS.
- G. Zhen, S. Zheng, X. Lu, X. Zhu, J. Mei, T. Kobayashi, K. Xu, Y.-Y. Li and Y. Zhao, Bioresour. Technol., 2018, 266, 382–388 CrossRef CAS PubMed.
- L. Jourdin, S. Freguia, V. Flexer and J. Keller, Environ. Sci. Technol., 2016, 50, 1982–1989 CrossRef CAS PubMed.
- G. Wang, Q. Huang, T.-S. Song and J. Xie, Energy Fuels, 2020, 34, 8666–8675 CrossRef CAS.
- A. G. Vidales, G. Bruant, S. Omanovic and B. Tartakovsky, Electrochim. Acta, 2021, 383, 138349 CrossRef.
- D. González-Tenorio, K. M. Muñoz-Páez and I. Valdez-Vazquez, Biomass Convers. Biorefin., 2022, 1–11 Search PubMed.
- J. K. Otten, Y. Zou and E. T. Papoutsakis, Front. Bioeng. Biotechnol., 2022, 10, 965614 CrossRef PubMed.
- H. Richter, B. Molitor, H. Wei, W. Chen, L. Aristilde and L. Angenent, Energy Environ. Sci., 2016, 9, 2392–2399 RSC.
- I. Vassilev, P. Dessì, S. Puig and M. Kokko, Bioresour. Technol., 2022, 348, 126788 CrossRef CAS PubMed.
- C. Zhang, H. Liu, P. Wu, J. Li and J. Zhang, Bioresour. Technol., 2023, 369, 128436 CrossRef CAS PubMed.
- S. Esquivel-Elizondo, C. Bağcı, M. Temovska, B. S. Jeon, I. Bessarab, R. B. Williams, D. H. Huson and L. T. Angenent, Front. Microbiol., 2021, 11, 594524 CrossRef PubMed.
- F. Liew, A. M. Henstra, M. Köpke, K. Winzer, S. D. Simpson and N. P. Minton, Metab. Eng., 2017, 40, 104–114 CrossRef CAS PubMed.
- J. S. Deutzmann and A. M. Spormann, ISME J., 2017, 11, 704–714 CrossRef CAS PubMed.
- L. Jourdin, S. M. Raes, C. J. Buisman and D. P. Strik, Front. Energy Res., 2018, 6, 7 CrossRef.
- L. Jourdin, M. Winkelhorst, B. Rawls, C. J. Buisman and D. P. Strik, Bioresour. Technol. Rep., 2019, 7, 100284 CrossRef.
- A. González, E. Salgado, Z. Vanegas, C. Niño-Navarro, O. Cortés, I. Chairez and E. I. García-Peña, Fermentation, 2022, 8, 644 CrossRef.
- C. Zhang, L. Yang, P. Tsapekos, Y. Zhang and I. Angelidaki, Environ. Int., 2019, 127, 134–141 CrossRef CAS PubMed.
- S. Ghysels, S. Buffel, K. Rabaey, F. Ronsse and R. Ganigué, Bioresour. Technol., 2021, 319, 124236 CrossRef CAS PubMed.
- P. San-Valero, H. N. Abubackar, M. C. Veiga and C. Kennes, Bioresour. Technol., 2020, 300, 122659 CrossRef CAS PubMed.
- S. Wang, J. Li, G. Zheng, G. Du and J. Li, Archaea, 2018, 4634898 Search PubMed.
- H.-B. Ding, G.-Y. Tan and J.-Y. Wang, Bioresour. Technol., 2010, 101, 9550–9559 CrossRef CAS PubMed.
- M. V. Reddy, A. ElMekawy and D. Pant, Biofuels, Bioprod. Biorefin., 2018, 966–977 CrossRef CAS.
- B. Tartakovsky, M.-F. Manuel, V. Neburchilov, H. Wang and S. Guiot, J. Power Sources, 2008, 182, 291–297 CrossRef CAS.
- A. Gomez Vidales, G. Bruant, S. Omanovic and B. Tartakovsky, Electrochim. Acta, 2021, 383, 138349 CrossRef CAS.
- A. Gomez Vidales, S. Omanovic and B. Tartakovsky, Bioresour. Technol. Rep., 2019, 8, 100302 CrossRef.
- G. Bruant, M.-J. Lévesque, C. Peter, S. R. Guiot and L. Masson, PLoS One, 2010, 5, e13033 CrossRef PubMed.
- A. E. Parada, D. M. Needham and J. A. Fuhrman, Environ. Microbiol., 2016, 18, 1403–1414 CrossRef CAS PubMed.
- J. Tremblay and E. Yergeau, GigaScience, 2019, 8, giz146 CrossRef PubMed.
- G. Gensollen, A.-M. Pourcher, A.-L. Duedal, S. Picard, S. Le Roux and P. Peu, Bioresour. Technol. Rep., 2022, 20, 101256 CrossRef CAS.
- S. Schlager, M. Haberbauer, A. Fuchsbauer, C. Hemmelmair, L. M. Dumitru, G. Hinterberger, H. Neugebauer and N. S. Sariciftci, ChemSusChem, 2017, 10, 226–233 CrossRef CAS PubMed.
- J. S. Deutzmann and A. M. Spormann, ISME J., 2017, 11, 704–714 CrossRef CAS PubMed.
- Á. Fernández-Naveira, M. C. Veiga and C. Kennes, Bioresour. Technol., 2019, 280, 387–395 CrossRef PubMed.
- J. S.-C. Liou, D. L. Balkwill, G. R. Drake and R. S. Tanner, Int. J. Syst. Evol. Microbiol., 2005, 55, 2085–2091 CrossRef CAS PubMed.
- J. Philips, Front. Microbiol., 2020, 10, 2997 CrossRef PubMed.
- E. Marsili, J. B. Rollefson, D. B. Baron, R. M. Hozalski and D. R. Bond, Appl. Environ. Microbiol., 2008, 74, 7329–7337 CrossRef CAS PubMed.
- E. N. Tikhonova, D. S. Grouzdev and I. K. Kravchenko, Int. J. Syst. Evol. Microbiol., 2021, 71, 004972 Search PubMed.
- R.-Z. Xu, S. Fang, L. Zhang, W. Huang, Q. Shao, F. Fang, Q. Feng, J. Cao and J. Luo, Bioresour. Technol., 2021, 341, 125823 CrossRef CAS PubMed.
- R. P. Ryan, S. Monchy, M. Cardinale, S. Taghavi, L. Crossman, M. B. Avison, G. Berg, D. van der Lelie and J. M. Dow, Nat. Rev. Microbiol., 2009, 7, 514–525 CrossRef CAS PubMed.
- P. Dessì, C. Sánchez, S. Mills, F. G. Cocco, M. Isipato, U. Z. Ijaz, G. Collins and P. N. L. Lens, Bioelectrochemistry, 2021, 137, 107686 CrossRef PubMed.
- X.-L. Su, Q. Tian, J. Zhang, X.-Z. Yuan, X.-S. Shi, R.-B. Guo and Y.-L. Qiu, Int. J. Syst. Evol. Microbiol., 2014, 64, 2986–2991 CrossRef CAS PubMed.
- L. Zhang, M. Yana, T.-H. Tsui, J. T. E. Lee, K.-C. Loh, Y. Dai and Y. W. Tong, Biomass, Biofuels, Biochem., 2022, 16, 367–394 Search PubMed.
- I. Owusu-Agyeman, E. Plaza and Z. Cetecioglu, Bioresour. Technol., 2022, 346, 126621 CrossRef CAS PubMed.
- D. M. Abou-Zeid, H. Biebl, C. Spröer and R. J. Müller, Int. J. Syst. Evol. Microbiol., 2004, 54, 951–954 CrossRef CAS PubMed.
- P. N. Evans, J. A. Boyd, A. O. Leu, B. J. Woodcroft, D. H. Parks, P. Hugenholtz and G. W. Tyson, Nat. Rev. Microbiol., 2019, 17, 219–232 CrossRef CAS PubMed.
- T. Yamada, H. Imachi, A. Ohashi, H. Harada, S. Hanada, Y. Kamagata and Y. Sekiguchi, Int. J. Syst. Evol. Microbiol., 2007, 57, 2299–2306 CrossRef CAS PubMed.
- P. Candry and R. Ganigué, Curr. Opin. Biotechnol., 2021, 67, 99–110 CrossRef CAS PubMed.
- H. L. Drake, A. S. Gößner and S. L. Daniel, Ann. N. Y. Acad. Sci., 2008, 1125, 100–128 CrossRef CAS PubMed.
- S. A. Abdur Rawoof, P. S. Kumar, D.-V. N. Vo, T. Devaraj and S. Subramanian, Renewable Sustainable Energy Rev., 2021, 152, 111700 CrossRef CAS.
- T.-s. Song, L. Fu, N. Wan, J. Wu and J. Xie, J. CO2 Util., 2020, 41, 101231 CrossRef CAS.
- S. Das, S. Das and M. M. Ghangrekar, Process Biochem., 2021, 101, 237–246 CrossRef CAS.
- M. P. G. de Almeida, C. Mondini, G. Bruant, J. Tremblay, D. G. Weissbrodt and G. Mockaitis, J. Chem. Technol. Biotechnol., 2024, 99, 989–1001 CrossRef CAS.
- S. T. Boto, B. Bardl, F. Harnisch and M. A. Rosenbaum, Green Chem., 2023, 25, 4375–4386 RSC.
- V. Flexer and L. Jourdin, Acc. Chem. Res., 2020, 53, 311–321 CrossRef CAS PubMed.
- N. Aryal, F. Ammam, S. A. Patil and D. Pant, Green Chem., 2017, 19, 5748 RSC.
- E. Nwanebu, S. Omanovic, S. Hrapovic, A. Gomez Vidales and B. Tartakovsky, Int. J. Hydrogen Energy, 2021, 47, 203–215 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03906h |
| This journal is © The Royal Society of Chemistry 2024 |