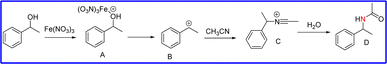
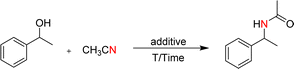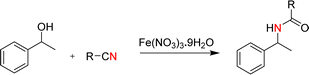Open Access Article
Open Access ArticleEfficient synthesis of amides from secondary alcohols and CH3CN promoted by Fe(NO3)3·9H2O†
Han Li‡
a,
Xiaodan Wang‡ac,
Wenhao Li‡a,
Xinmei Wanga,
Ruijing Chenga,
Danfeng He*a,
Huanjun Xu *a,
Yiying Li*b and
Jinhui Wangac
*a,
Yiying Li*b and
Jinhui Wangac
aSchool of Science, Qiongtai Normal University, Haikou, 571127, China. E-mail: hedanfeng@mail.qtnu.edu.cn; 15798946232@163.com
bCollege of Basic Medicine and Life Sciences, Hainan Medical University, Haikou, China. E-mail: liyiying_hy@163.com
cDepartment of Medicinal Chemistry and Natural Medicine Chemistry, College of Pharmacy, Harbin Medical University, Harbin, China
First published on 18th September 2024
Abstract
The Ritter reaction is the most attractive method for synthesizing amides, and various acids have been used to promote this reaction. Compared to these acids, Fe(NO3)3·9H2O is less toxic and costly, and it shows relatively high Lewis acidity and great catalytic activity. In this study, a simple and efficient protocol involving Fe(NO3)3·9H2O as an additive for the synthesis of amides was developed. Various secondary alcohols could be reacted with CH3CN to obtain their corresponding products, with CH3CN being used as a reactant and solvent. This protocol was found to be applicable to a wide range of alcohols and nitrile substrates. In general, it was found that substrates containing electron-donating-groups offered the corresponding amides in good to excellent yields, while those with electron-withdrawing groups offered low to moderate yields. Meanwhile, this approach was scalable to the gram level, offering an attractive opportunity for further application in organic synthesis.
Introduction
The amide group is an important functional group in medicinal and synthetic chemistry. It is used as a functional molecule in bioactive natural products, clinical drugs, organic catalysts, DNA damage probes, and macromolecules.1 The conventional protocol to form amide bonds involves acylation of amines by various species such as carboxylic acids, acid chlorides, and anhydrides.2 There are alternative protocols to prepare amides, such as the Schmidt reaction,3 Schotten–Baumann reaction,4 Ugi reaction5 and Ritter reaction.6 Among these, the Ritter reaction is the most attractive method and involves the reaction of a nitrile with an alcohol or olefin promoted by a strong acid, like sulfuric acid.7 The major drawback of the traditional Ritter reaction is that it is promoted under harsh conditions with a strong Brønsted acid or a Lewis acid, which results in a narrow substrate scope and side reactions. To avoid the need of using a strong mineral acid, various homogeneous and heterogeneous catalysts have been developed.8 A variety of acidic reagents have been developed for the Ritter reaction from secondary alcohols, such as FeCl3/AgSbF6,8a Ca(OTf)2/Bu4NPF6,8b BiBr3,8c zirconium perfluorooctanesulfonate,8d FeClO4/SiO2,8e γ-Fe2O3@SiO2–HClO4,8f 2,4-dinitrobenzenesulfonic acid,8g tropylium,8h MWCNT-CSP,8i AmberlystⓇ-15(H),8j TfOH,8k I2,8l and PMA-SiO2 (ref. 8m) (Table 1). However, many of these catalysts suffer from at least one of the following or other issues: being corrosive, moisture-sensitive, flammable, expensive, complex to use, and difficult to prepare; requiring extended reaction times; and yielding competing side reactions.| Entry | Additive | Reaction conditions | Yield/% | Reference |
|---|---|---|---|---|
| 1 | FeCl3 (25 mol%), AgSbF6 (75 mol%) | DCE, 100 °C | 87 | 8a |
| 2 | Ca(OTf)2 (5 mol%), Bu4NPF6 (5 mol%) | 5 min, MW, 120 °C | 94 | 8b |
| 3 | BiBr3 (10 mol%) | H2O (30 μL mmol−1), 48 h, 120 °C | 86 | 8c |
| 4 | Bis(pentamethylcyclopentadienyl) zirconium-perfluorooctanesulfonate (5 mol%) | Solvent-free or DCE, 6 h, 80 °C | 88 | 8d |
| 5 | γ-Fe2O3@SiO2–HClO4 (12.5 mmol%), and 2.5 mol% of HClO4 | 4 h, RT | 96 | 8e |
| 6 | SiO2-supported FeClO4 (100 mol%) | 5 min, 100 °C | 95 | 8f |
| 7 | 2,4-Dinitrobenzenesulfonic acid (10 mol%) | 14 h, 80 °C | 82 | 8g |
| 8 | Tropylium (10 mol%) | Water, 150 °C, MW | 92 | 8h |
| 9 | MWCNT-CSP (50 mg) | 24 h, 100 °C | 75 | 8i |
| 10 | AmberlystⓇ-15(H) (5 g) | 4.5 h, 80 °C | 88 | 8j |
| 11 | TfOH (20 mol%), SDS (10 mol%) | Water, 200 °C, 5 h | 82 | 8k |
| 12 | I2 (20%) | H2O (2 eq.), 110 °C, 4 h | 85 | 8l |
| 13 | Phosphomolybdic acid-SiO2 (0.5 mol%) | 70 °C, 5.5 h | 86 | 8m |
| 14 | Fe(NO3)3·9H2O (100 mol%) | 3 h, 80 °C | 98.8 | This work |
Recently, iron catalysis has been considered as an alternative not only because of its lower toxicity and cost compared to other metals but also because its useful properties that have been utilized in many transformations.9 Iron-catalyzed C–C,10 C–N11 and C–O12 bond-forming reactions have recently been developed. In 2009, Sébastien Reymond reported that FeCl3·6H2O (10 mmol%) could catalyze the reaction of alcohol with nitrile to form amide in a sealed tube at 150 °C with H2O (2 equiv.).13 In 2012, Basavaprabhu and Sureshbabu described the reaction of cyanamide with alcohol catalyzed by FeCl3 (30 mol%) in dichloromethane.14 Further, an efficient and mild methodology was reported for the synthesis of amides through the reaction of nitriles with esters catalyzed by Fe(ClO4)3·H2O.15
Fe(NO3)3·9H2O, in particular, displays several advantageous features. It shows relatively high Lewis acidity and great catalytic activity, and it is an inexpensive, non-toxic and readily available inorganic oxidant; it has been used as an efficient oxidant,16 nitro source17 and catalyst in cross-coupling reactions.18 Meanwhile, in 2009, Jonathan M. J. Williams reported that Fe(NO3)3·9H2O could catalyze the formation of amides via the addition of amines to nitriles.19 Thus, we proposed that Fe(NO3)3·9H2O might have the potential to induce the Ritter reaction for alcohols and nitriles. Compared with the previous reports of reactions with other additives, this study shows that the synthesis of amides from secondary alcohols and CH3CN proceeded at a relatively low temperature (80 °C) and short time (3 h) with Fe(NO3)3·9H2O as the additive, giving the corresponding products in high to excellent yields for substrates containing electron-donating groups and low to moderate yields for substrates containing electron-withdrawing groups. In addition, this protocol was readily scaled up to 0.5 grams without loss in its efficiency.
Results and discussion
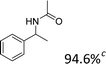
In our initial studies, we chose to compare the performances of various loadings of Fe(NO3)3·9H2O for the reaction of 1-phenylethanol (0.5 mmol) in 3 mL CH3CN. Amide was formed in 89.4%, 98.8%, and 94.6% GC yield for catalyst loading of, respectively, 0.375 mmol, 0.5 mmol and 0.625 mmol at 80 °C with a reaction time of 3 h (Table 2, entries 1–3). That is, loading too much or too little catalyst resulted in a lower yield of product (Tables 2 and S1†). Then we modified the reaction temperature; compared to that at 80 °C, a sharply lower yield occurred at 65 °C. We also tested other ferric salts; FeBr3 offered a low yield and FeCl3 offered a similar level of catalytic activity as did Fe(NO3)3·9H2O (Table 2, entries 7–8). Other types of metallic nitrates were also tested. Some other nitrates could convert 1-phenylethanol to amide. For example, when using Cr(NO3)3·9H2O as the additive, amide was produced but with a yield of 69.2%, which is lower than the yield obtained when Fe(NO3)3·9H2O is used as the additive (Table 2, entry 9). Similarly, Ce(NO3)3·6H2O and Cu((NO3)3·2.5H2O successfully converted the 1-phenylethanol to amide but their catalytic performance was significantly decreased (Table 2, entries 10, 12). Co(NO3)3·6H2O and Cd(NO)3·9H2O showed no catalytic activity (Table 2, entries 11, 13). The order of activity of the different tested nitrates was Fe(NO3)3·9H2O > Cr(NO3)3·9H2O > Ce(NO3)3·6H2O > Cu((NO3)3·2.5H2O > Co(NO3)3·6H2O ≈ Cd(NO)3·9H2O. We tested the reaction under either an O2 or N2 atmosphere; it proceeded smoothly in each case (Table 2, entries 14–15). Overall, Fe(NO3)3·9H2O was found to be the best additive, and the best reaction conditions involved carrying out the reaction at 80 °C for 3 h with 100 mol% Fe(NO3)3·9H2O in open air.| Entry | Catalyst (mmol) | T/oC | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Conditions: substrate (0.5 mmol), CH3CN (3 mL), GC yield.b Isolated yield.c Under O2.d Under N2. | ||||
| 1 | Fe(NO3)3·9H2O(0.375) | 80 | 3 h | 89.4 |
| 2 | Fe(NO3)3·9H2O (0.5) | 80 | 3 h | 98.8/96.2b |
| 3 | Fe(NO3)3·9H2O (0.625) | 80 | 3 h | 94.6 |
| 4 | Fe(NO3)3·9H2O (0.5) | 65 | 3 h | 71.9 |
| 5 | Fe(NO3)3·9H2O (0.5) | 80 | 2h | 81.7 |
| 6 | Fe(NO3)3·9H2O(0.5) | 80 | 4 h | 95.3 |
| 7 | FeBr3 (0.5) | 80 | 3 h | 74.7 |
| 8 | FeCl3 (0.5) | 80 | 3 h | 98.3 |
| 9 | Cr(NO3)3·9H2O (0.5) | 80 | 3 h | 69.2 |
| 10 | Ce(NO3)3·6H2O (0.5) | 80 | 3 h | 39.7 |
| 11 | Co(NO3)3·6H2O (0.5) | 80 | 3 h | NR |
| 12 | Cu((NO3)3.2.5H2O (0.5) | 80 | 3 h | 19.1 |
| 13 | Cd(NO)3·9H2O (0.5) | 80 | 3 h | NR |
| 14 | Fe(NO3)3·9H2O(0.5) | 80 | 3h | 93.5c |
| 15 | Fe(NO3)3·9H2O (0.5) | 80 | 3 h | 94.3d |
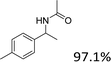
With the standard conditions in hand, we then turned our attention to expanding the scope of this protocol, initially investigating the reaction of various types of secondary alcohols with CH3CN. In general, it was found 1-phenylethanol, having an electron-donating group, offered the corresponding amides in good to excellent yields, while having an electron-withdrawing group offered low to moderate yields.
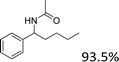
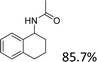
Analogues with different lengths of the alkyl group at the β-position of the methyl group were successfully transformed to the corresponding products with good to excellent yields (Table 3, entries 1–3). The results also showed that the length of alkyl group did not show any obvious effect on the reaction: that is, when increasing the alkyl-group length, the yield of corresponding product did not show an apparent decrease (Table 3, entries 1–3). 1,2,3,4-Tetrahydronaphthalen-1-ol, i.e., the substrate with a cyclic substituent, offered an 85.7% yield of the desired product (Table 3, entry 4).
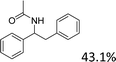
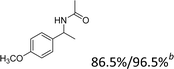
| Entry | Substrate | Product/yield |
|---|---|---|
| a Conditions: substrate (0.5 mmol), Fe(NO3)3·9H2O (0.5 mmol), CH3CN (3 mL), 80 °C, 3 h, isolated yield.b 65 °C, GC yield.c Substrate (4.1 mmol), Fe(NO3)3·9H2O (4.1 mol), CH3CN (15 mL), 80 °C, 3 h, isolated yield. | ||
| 1 |  |
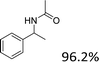 |
| 2 |  |
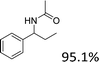 |
| 3 |  |
 |
| 4 | 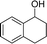 |
 |
| 5 | 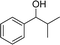 |
 |
| 6 | 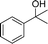 |
 |
| 7 | 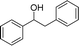 |
 |
| 8 | 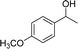 |
 |
| 9 | 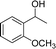 |
 |
| 10 | 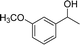 |
 |
| 11 | 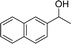 |
 |
| 12 | 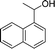 |
 |
| 13 | 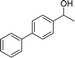 |
 |
| 14 | 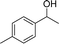 |
 |
| 15 | 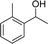 |
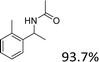 |
| 16 | 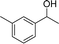 |
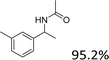 |
| 17 | 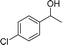 |
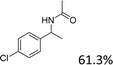 |
| 18 | 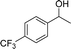 |
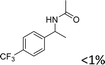 |
| 19 | 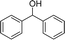 |
 |
| 20 | 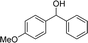 |
 |
| 21 | 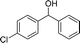 |
 |
| 22 | 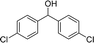 |
 |
| 23 | 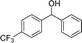 |
 |
| 24 | 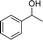 |
 |
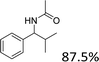
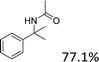
Meanwhile, steric hindrance did apparently affect the reaction: 2-methyl-1-phenylpropanol, with a sterically hindering group, was transformed into the desired product in a relatively low yield (87.5%, Table 3, entry 5); the yield when using 2-phenyl-2-propanol was even lower (77.1%, Table 3, entry 6) due to an even stronger steric hindrance effect. For the analogue with another type of substituent at the position β to the methyl group, the Fe(NO3)3·9H2O-catalyzed Ritter reaction led to the expected amide in low yield (Table 3, entry 7).
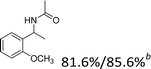
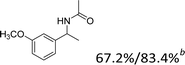
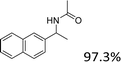
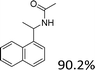
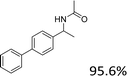
Our results show that reactions with secondary alcohols having strongly electron-donating groups should be carried out at a lower temperature. We observed relatively low product yields when reacting 1-(4-methoxyphenyl) ethanol, 1-(3-methoxyphenyl) ethanol, and 1-(2-methoxyphenyl) ethanol as substrates under the standard conditions (Table 3, entries 8–10), with some unwanted side reactions having occurred. When this transformation was carried out at 65 °C, the desired product was obtained in a satisfactory yield (Table S2†). This result was similar to that for one-pot synthesis of amides catalyzed by Zn(ClO4)2·6H2O.20 For the substrates substituted with aromatic groups, the yields of the desired amides were more than 90%. The substrates 1-(2-naphthyl) ethanol, 1-(1-naphthyl) ethanol and 1-(4-biphenylyl) ethanol were converted to their corresponding products with high yields of 97.3%, 90.2% and 95.6%, respectively (Table 3, entries 11–13). The improved reactivities for these substrates may be attributed to the electron-donating effect of the phenyl group linked to the aryl ring.
Reactions of the substrates each with a weakly electron-donating group on the phenyl ring also gave the corresponding products in excellent yields (Table 3, entries 14–16). The results of –OCH3– and –CH3– substituted substrates (Table 3, entries 9 and 15) show that the substituted group in the adjacent position might have decreased the reactivity to some extent, owing to the steric hindrance effect.
For the two tested electron-withdrawing-group-containing substrates, markedly decreased yields of the desired products were observed; a lower yield was noted for the more strongly electron-withdrawing group (Table 3, entries 17–18), which is attributed to the reduction of electron density on the reactive site of the substrate.
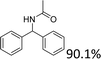
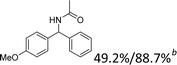
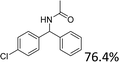
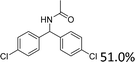
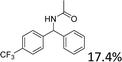
We further tested this protocol with diaryl secondary alcohols, and a similar trend as above was observed: the substrates each with an electron-donating group offered the corresponding amides in good to excellent yields, while the electron-withdrawing group offered low to moderate yields. As shown in Table 3, the diaryl secondary alcohol 1,1-diphenylmethanol could be transformed to the amide in a high yield (90.1%). The product yield for the diaryl secondary alcohol with a methoxyl group on one side of the substrate was also high when using a lower reaction temperature (Table 3, entry 19). Meanwhile, moderate product yields were found when using a substrate with a weakly electron-withdrawing group (–Cl) on one side and when with this group was on both sides (Table 3, entries 21–22). However, for the strongly electron-withdrawing group (–CF3), the according product was offered in a low yield (Table 3, entry 23, 17.4%). Furthermore, we checked whether this approach could be scaled up to a gram scale: a 94.6% isolated yield of corresponding amide was easily prepared in one batch from 0.5 g of 1-phenylethanol when using Fe(NO3)3·9H2O as an additive under standard conditions.
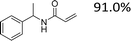
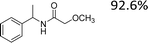
Encouraged by the developed protocol for synthesizing amides from various secondary alcohols with CH3CN, the scope of the reaction was extended to other types of liquid nitriles. The results, summarized in Table 4, showed that this protocol is suitable to other types of liquid nitriles. Specifically, we initiated this aspect of our study by performing the catalytic acetylation of the representative substrate, 1-phenylethanol, using five liquid nitriles as the solvent and reactant. A good range of functional groups was tolerated in this reaction protocol, including a double bond between carbons (Table 4, entry 1), halide (Table 4, entry 4) and ether bond (Table 4, entry 5). Meanwhile, a nitrile substrate containing an N-heterocyclic system, namely pyridine, did not survive our reaction conditions, giving the hydrolysis product to some extent (picolinamide).
| Entry | Nitrile | Product/yield |
|---|---|---|
| a Conditions: substrate (0.5 mmol), Fe(NO3)3·9H2O (0.5 mmol.), nitrile (3 mL), 80 °C, 3 h, isolated yield. | ||
| 1 |  |
 |
| 2 | 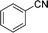 |
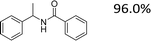 |
| 3 | 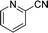 |
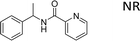 |
| 4 |  |
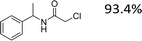 |
| 5 | 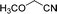 |
 |
Based on our results, a possible albeit speculative mechanism for the formation of the amides was derived and is shown in Scheme 1. According to this mechanism, 1-phenylethanol can be polarized by Fe(NO3)3 to generate the benzylic carbocation B. Then, B would be trapped by MeCN to produce intermediate C, which would further hydrolyse to the amide with H2O.
Conclusions
We developed an efficient, green and convenient protocol for synthesizing amides from secondary alcohols using Fe(NO3)3·9H2O as the mediator, with CH3CN as the N-source and solvent. In this process, various alcohols were successfully reacted with CH3CN to form the corresponding products. Here, substrates with electron-donating group offered the corresponding amides in good to excellent yields, while those with electron-withdrawing groups offered low to moderate yields. Additionally, the reaction proceeded smoothly when other types of liquid nitriles were used as the solvent and reactant. More importantly, this protocol is also applicable to gram-scale synthesis. We are hopeful that this methodology will be very useful in works involving organic synthesis in general and the Ritter reaction specifically.Data availability
The author confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.†Author contributions
Conceptualization, Huanjun Xu and Yiying Li; methodology, Han Li, Xiaodan Wang, and Wenhao Li; formal analysis, Xinmei Wang and Danfeng He; investigation, Han Li, Xiaodan Wang, and Wenhao Li; resources, Yiying Li, Huanjun Xu and Jinhui Wang; writing – original draft preparation, Xiaodan Wang and Ruijing Cheng; writing – review and editing, Huanjun Xu and Jinhui Wang; supervision, Yiying Li and Huanjun Xu; project administration, Huanjun Xu; funding acquisition, Yiying Li, Huanjun Xu, Jinhui Wang and Ruijing Cheng. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (22165009), the Hainan Provincial Natural Science Foundation of China (821QN260, 521MS0786), the Project of Hainan Association for Science and Technology of Youth Science and Talents (QCXM202023), National Science and Technology Major Project of the Ministry of Science and Technology of China (2018ZX09735005), and the Scientific Research Project of Qiongtai Normal University (qtnb202101, qtnb202104).Notes and references
- (a) M. R. Petchey and G. Grogan, Adv. Synth. Catal., 2019, 361, 389 CrossRef; (b) C. L. Feng, G.-B. Yin, B. Yan, J. Q. Chen and M. Ji, Chem. Pap., 2019, 73, 345 CrossRef CAS; (c) A. S. Santos, A. M. S. Silva and M. M. B. Marques, Eur. J. Org Chem., 2020, 17, 2501 CrossRef.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606 RSC.
- (a) T. Ribelin, C. E. Katz, D. G. English, S. Smith, A. K. Manukyan, V. W. Day, B. Neuenswander, J. L. Poutsma and J. Aube, Angew. Chem., Int. Ed., 2008, 47, 6233 CrossRef CAS; (b) S. Lang and J. A. Murphy, Chem. Soc. Rev., 2006, 35, 146 RSC.
- (a) E. Saxo and C. Z. Bertozzi, Science, 2000, 287, 2007 CrossRef PubMed; (b) Y. G. Gololobov and L. F. Kasukhin, Tetrahedron, 1992, 48, 1353 CrossRef CAS.
- (a) R. Wamser, X. Zhang, B. Kuropka, C. Arkona and J. Rademann, Chem. Eur. J., 2024, 30, e202303940 CrossRef CAS PubMed; (b) Q. Feng, J. Zhu, M. Wang and S. Tong, Org. Lett., 2020, 22, 483 CrossRef CAS PubMed; (c) L. Zeng, H. Sajiki and S. Cui, Org. Lett., 2019, 21, 5269 CrossRef CAS.
- D. Jiang, T. He, L. Ma and Z. Y. Wang, RSC Adv., 2014, 4, 64936 RSC.
- (a) K. V. Emelen, T. De Wit, G. J. Hoornaert and F. Compernolle, Org. Lett., 2000, 2, 3083 CrossRef PubMed; (b) F. Ma ertens, A. Van den Bogaert, F. Compernolle and G. J. Hoornaert, Eur. J. Org Chem., 2004, 22, 4648 CrossRef.
- (a) L. R. Jefferies and S. P. Cook, Tetrahedron, 2014, 70, 4204 CrossRef CAS; (b) S. Yaragorla, G. Singh, P. L. Saini and M. K. Reddy, Tetrahedron Lett., 2014, 55, 4657 CrossRef CAS; (c) M. Ueno, R. Kusaka, S. D. Ohmura and N. Miyoshi, Eur. J. Org Chem., 2019, 8, 1796 CrossRef; (d) S. Khaksar, E. Fattahi and E. Fattahi, Tetrahedron Lett., 2011, 52, 5943 CrossRef CAS; (e) P. Salehi and A. R. Motlagh, Synth. Commun., 2000, 30, 671 CrossRef CAS; (f) L. Mamani, A. Heydari and M. Sheykhan, Appl. Catal., A, 2010, 384, 122 CrossRef CAS; (g) R. Sanz, A. Martínez, V. Guilarte, J. M. Álvarez-Gutiérrez and F. Rodríguez, Eur. J. Org Chem., 2007, 28, 4642 CrossRef; (h) S. H. Doan, M. A. Hussein and T. V. Nguyen, Chem. Commun., 2021, 57, 8901 RSC; (i) J. R. Pereira, M. C. Corvo, A. F. Peixoto, A. Aguiar-Ricardo and M. M. B. Marques, ChemCatChem, 2023, 15, e202201318 CrossRef CAS; (j) S. Nandy, A. K. Das and S. Bhar, Synth. Commun., 2020, 50, 3326 CrossRef CAS; (k) S. Jiang, Z. Wang, Z. Jiang, J. Li, S. Zhou and L. Pu, Lett. Org. Chem., 2012, 9, 24 CrossRef CAS; (l) P. Theerthagiri, A. Lalitha and P. N. Arunachalam, Tetrahedron Lett., 2010, 51, 2813 CrossRef CAS; (m) J. S. Yadav, B. V. Subba Reddy, T. Pandurangam, Y. Jayasudan Reddy and M. K. Gupta, Catal. Commun., 2008, 9, 1297 CrossRef CAS.
- S. Rana, J. Biswas, S. Paul, A. a Paik and D. Maiti, Chem. Soc. Rev., 2021, 50, 243 RSC.
- S. Xiang, L. Zhang and N. Jiao, Chem. Commun., 2009, 45, 6487 RSC.
- R. Singh, E. Sathish, A. K. Gupta and S. Goyal, Tetrahedron, 2021, 100, 132474 CrossRef CAS.
- P. Niharika, B. V. Ramulu and G. Satyanarayana, Org. Biomol. Chem., 2014, 12, 4347 RSC.
- B. Anxionnat, A. Guérinot, S. Reymond and J. Cossy, Tetrahedron Lett., 2011, 52, 5943 CrossRef.
- H. Basavaprabhu and V. V. Sureshbabu, Org. Biomol. Chem., 2012, 10, 2528 RSC.
- M. Ji, C. Feng, B. Yan, G. Yin and J. Chen, Synlett, 2018, 29, 2257 CrossRef.
- (a) K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, Org. Lett., 2014, 16, 4630 CrossRef CAS PubMed; (b) S. Xu, J. Wu, P. Huang and C. Lao, Front. Chem., 2020, 8, 151 CrossRef CAS PubMed.
- (a) C. Matt, F. Kölblin and J. Streuff, Org. Lett., 2019, 21, 6909 CrossRef PubMed; (b) Y. Zheng, Q. Hu, Q. Huang and Y. Xie, Org. Lett., 2024, 26, 3316 CrossRef CAS; (c) Y. Gao, Y. Mao, B. Zhang, Y. Zhan and Y. Huo, Org. Biomol. Chem., 2018, 16, 3881 RSC.
- (a) S. Qin, Y. Luo, Y. Sun, L. Tian, S. Jiang, J. Yan and G. Yang, Tetrahedron Lett., 2019, 43, 152267 Search PubMed; (b) Z. Zhang, Y. Ma, S. Dai, L. Li, Y. Zhang and H. Li, Tetrahedron Lett., 2020, 21, 151885 CrossRef.
- C. L. Allen, A. A. Lapkin and J. M. J. Williams, Tetrahedron Lett., 2009, 50, 4262 CrossRef CAS.
- C. Feng, B. Yan, W. Yao, J. Chen and M. Ji, ChemistrySelect, 2018, 3, 11775 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04146a |
| ‡ These authors have contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2024 |