Open Access Article
Open Access ArticleOxygen activation and transfer for catalytic wet-air oxidation of wastewater: a short review
Pengfei Maa,
Nuowei Zhang b,
Jianzai Shic,
Hongliang Lua,
Jianqiang Fana,
Maoyi Lia,
Qixin Denga,
Zhengzhong Fanga,
Binghui Chen
b,
Jianzai Shic,
Hongliang Lua,
Jianqiang Fana,
Maoyi Lia,
Qixin Denga,
Zhengzhong Fanga,
Binghui Chen b,
Quanxing Zheng*a and
Songshou Ye*b
b,
Quanxing Zheng*a and
Songshou Ye*b
aTechnology Center, China Tobacco Fujian Industrial Co., Ltd, Xiamen 361021, P. R. China. E-mail: zqx10701@fjtic.cn
bDepartment of Chemical and Biochemical Engineering, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, P. R. China. E-mail: clow4648@163.com
cFujian Jinmin Reconstituted Tobacco Development Co., Ltd., Fuzhou 350600, P. R. China
First published on 25th November 2024
Abstract
With the rapid growth of population and industrial production, wastewater pollution has become a major environmental issue. Wastewater pollution also poses a threat to water resources and human health. Catalytic wet-air oxidation (CWAO) is one of the most economical and environmentally friendly technologies, especially for the treatment of toxic and non-biodegradable pollutants in wastewater. Various heterogeneous catalysts have been reported for use in wastewater treatment; however, most of these catalysts are effective only under high temperatures and high pressures. The increasing demand for the removal of wastewater pollutants necessitates the development of low-temperature, high-efficiency catalysts for CWAO technology. To achieve this, the ability of the catalyst to activate O2 and transfer active oxygen species plays a key role in determining the catalytic performance. In this review, we summarize recent advances in various noble and non-noble metal catalysts, oxide catalysts and carbon catalysts for CWAO reactions, focusing on the positive effect of O2 activation and transfer on catalytic performance. We also propose future directions for developing novel CWAO catalysts by optimizing the catalyst's ability to activate O2 and transfer active oxygen species.
1. Introduction
In the last few decades, huge amounts of wastewater containing hazardous and refractory organic pollutants have been produced by industries. Wastewater from pulp and paper, dyeing, chemical, petrochemical, pharmaceutical, and other industries has caused severe environmental problems.1–4 Therefore, hazardous and refractory organic pollutants must be removed from wastewater to meet stringent water quality regulations before discharge.Various technologies, including biological treatment, incineration, membrane separation, Fenton, catalytic ozonation (O3), and catalytic wet-air oxidation (CWAO), have been developed and applied for wastewater treatment.5 These technologies have their own advantages, application scopes, and limitations. Biological treatment is widely used for domestic wastewater as well as non-toxic and low chemical oxygen demand (COD) industrial wastewater. It is generally cheaper but requires a long residence time and a large number of microorganisms to degrade the pollutants.5 Incineration can completely degrade organic contaminants into carbon dioxide and water. However, it is only suitable for wastewater with COD > 100 g L−1.5 Moreover, a large amount of fuel is required to sustain the process. Membrane separation is an effective technology for water purification, though it suffers from membrane fouling over time. The development of novel membranes is the key to addressing this fouling issue. The sunlight-driven self-cleaning layered membrane can effectively avoid fouling and prevent a sharp decrease in permeation flux, thanks to its visible-light catalytic properties.6 The dual superwettabilities of the novel ‘Yin–Yang’ membrane enable continuous separation with high efficiency (over 99.1%) and a large total flux (over 54.4 × 103 L m−2 h−1) for triphase oil/water mixtures.7 Developing membranes with anticontamination properties is a promising future research direction for water treatment.
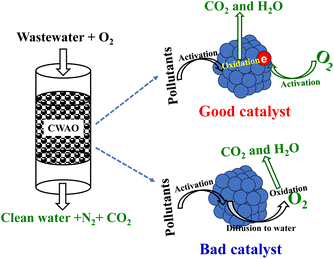
Fenton, catalytic O3, CWAO, etc., are advanced oxidation processes (AOPs), where the produced hydroxyl radicals oxidize various organic compounds.8 The application range of each AOPs depends on the composition of effluent to a great extent. Fenton and catalytic ozonation are preferred at low organic (pollutants) loads with lower treatment capacity. CWAO stands out in AOP technologies. It was considered as the one technology (maybe only) that can effectively deal with the wastewater with high organic loading (10–100 g L−1 of COD). Such wastewater's COD is too high for the Fenton process and catalytic O3. CWAO is also considered as the one of the most economical and technological AOPs for the treatment of wastewater containing toxic or biologically refractory compounds.9 Both homogeneous and heterogeneous catalysts were used in the CWAO process.10 Homogeneous catalysts, dissolved salts, are effective. But an additional separation unit is required for removing or recovering the metal ions from the treated effluent. Heterogeneous catalysts are much more attractive because a separation step is not necessary. Various solid catalysts, including noble and non-noble metal catalysts, oxide catalysts and carbon catalysts, have been widely developed for CWAO technology.11–21 Each type of catalyst has its own advantage and disadvantage. Generally, noble metal catalysts are active and stable, although they are expensive. Oxide catalysts are cheap and active. The limitation of oxide catalysts is their poor stability due to the leaching of the metal ion. Although non-noble metal catalysts and carbon catalysts are cheap, they usually present lower activity. Although many catalysts have been reported for heterogeneous CWAO, most catalysts in the literature only work under high temperatures (150–270 °C) and high pressures (2.5–10 MPa). The reason is that O2 cannot be easily activated to produce hydroxyl radicals at relatively mild reaction conditions due to its chemical inertness. The harsh operating conditions not only increase the operating costs, e.g., gas pumping energy, but also incur more equipment costs and safety risks. Additionally, the reported heterogeneous CWAO catalysts still suffer deactivation due to the formation of by-products during the reaction. The reason is that O2 cannot be effectively activated by the catalyst and there is inadequate active oxygen species to ensure that organic pollutants are completely oxidized to CO2. The partial oxidation of organic pollutants results in the formation of carbonaceous deposit or production of small molecular organic acids.5,9,22,23 There is no doubt that carbonaceous deposits lead to deactivation. Short chain carboxylic acids, including maleic acid, acrylic acid, malonic acid, oxalic acid, and acetic acid, are the main by-productions during the CWAO process.12 The presence of such organic acids will lower the pH, which results in metal leaching and deactivation.23 Small molecular organic acids also have the risk of releasing VOCs, although they are fully biodegraded.5 If a catalyst can provide enough active oxygen species, organic pollutants will be effectively oxidized to CO2 and thus avoid the formation of by-products. The high operating temperature and/or poor stability limit the CWAO industrial applications to a great extent. There is an urgent need to develop low-temperature, high-efficiency and good-stability heterogeneous catalysts for CWAO. Enhancing the catalyst's ability for O2 activation and active oxygen species transfer is essential and perhaps the key parameter.
Some low-temperature catalysts have been reported; however, the catalytic performance needs to be further improved. Table 1 shows the catalytic performance for CWAO of phenol over various catalysts (including noble and non-noble metal catalyst, oxide catalyst and carbon catalyst) at the lowest temperatures that have been reported for the corresponding catalyst. It can be seen that noble metal catalyst and oxide catalyst can obtain higher activity at lower temperature. They are much more active than non-noble metal and carbon catalyst. It should be noted that phenol conversions are always much higher than TOC (total organic carbon) or COD conversions for all the catalysts. This indicates that phenol is not completely oxidized to CO2 and some of it is oxidized to small molecular organic acid. For these low-temperature catalysts, the ability to activate and transfer oxygen needs to be further enhanced to ensure enough active oxygen.
Generally, heterogeneous CWAO proceeds through a Langmuir–Hinshelwood (L–H) or a Eley–Rideal (E–R) mechanism, which strongly depends on the activity of the catalyst, as shown in Fig. 1. The CWAO reaction is initiated by either the activation of oxygen (L–H) or reactant molecules (E–R).28 If the catalyst possesses a high activity, the L–H mechanism will be favoured. For the L–H mechanism, both O2 and organic pollutants are adsorbed and activated by the catalyst. On the other hand, the low activity catalyst prefers to go through an E–R mechanism. In the E–R mechanism, organic pollutant is adsorbed and activated to free radicals by the catalyst while O2 is activated by free radicals in the liquid phase. There is no doubt that a good heterogeneous CWAO catalyst should possess a high oxidation activity and a good ability for facile activation and transfer oxygen. However, there are no reviews in the literature focusing on the relationship among the ability to activate and transfer oxygen, catalyst structure and the CWAO catalytic performance. Therefore, in this paper, the recent advances in various noble and non-noble metal catalysts, oxide catalysts and carbon catalyst are reviewed. The relationships among O2 activation and transfer, catalyst structure and CWAO catalytic performance are discussed.
2. O2 activation for WAO and homogeneous CWAO
The oxidizing agents or oxidants utilized in AOPs process include air, pure oxygen, hydrogen peroxide, and ozone. Among these, molecular O2 is most attractive due to its natural abundance, safety and affordability. However, O2 is kinetically stable, which make CWAO technology only effective at high temperature and high pressure. The stability of O2 is derived from the presence of a spin-triplet and the antibonding nature of the two partially-filled orbitals in its ground state, as shown in Fig. 2. The O2 molecule has two unpaired electrons in the degenerate 2π* orbitals, which renders it susceptible to accepting electrons. The activation of the O2 molecule is realized by the transfer of one or more electrons to an O2 molecule. For WAO and homogeneous CWAO, free radical or M(n−1)+ give electrons to the O2 molecule. In the case of heterogeneous CWAO, the active sites of the solid catalyst provide electrons to O2 molecule. The comparison of WAO, homogeneous CWAO and heterogeneous CWAO is shown in Table 2.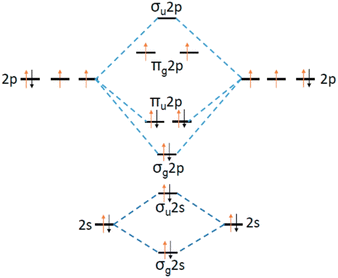 | ||
| Fig. 2 Molecular-orbital energy diagram of an O2 molecule.28 | ||
| Technology | O2 activation |
|---|---|
| WAO | By free radical |
| Homogeneous CWAO | By free radical or M(n−1)+ |
| Heterogeneous CWAO | By active sites of the solid catalyst |
The WAO reaction is generally regarded as a free-radical reaction. The chain reaction is divided into three phases, namely, chain initiation, chain transfer and chain termination.9
Chain initiation:
| RH + O2 → R˙ + HOO˙ | (1) |
| 2RH + O2 → 2R˙ + H2O2 | (2) |
Chain transfer:
| R˙ + O2 → ROO˙ | (3) |
| ROO˙ + RH → ROOH + 2R˙ | (4) |
Chain termination:
| R˙ + R˙ → R–R | (5) |
| ROO˙ + R˙ → ROOR | (6) |
| ROO˙ + ROO˙ → ROOR + O2 | (7) |
During the WAO process, O2 activation is based on the primary dehydrogenation of organic pollution by O2 with an increase in the energy of the system. The mechanism for WAO of carboxylic acids was investigated. It was found that the reaction between O2 and the α, β or γ-CH2 groups of the carboxylic acids led to O2 activation and thus resulted in the formation of free radicals and hydroperoxide-like species (chain initiation). The resultant free radicals reacted immediately with O2 to activate O2 and form peroxy radicals (chain transfer). The peroxy radicals then react with another molecule of carboxylic acid and lead to decarboxylation and formation of CO2 by H abstraction.29
Salts of iron, copper and manganese are added in the WAO process as homogeneous catalysts. The homogeneous catalyst can reduce the severe operating conditions of WAO. The main reason is that Mn+ can catalyze hydrogen abstraction reaction and result in the formation of M(n−1)+. Both R˙ and M(n−1)+ can give electrons and thus activate O2.30,31 However, the dissolved metal salts need be separated from the treated effluent due to their toxicity.
| RH + Mn+ → R˙ + M(n−1)+ + H+ | (8) |
| M(n−1)+ + O2 → O2− + Mn+ | (9) |
3. O2 activation for heterogeneous CWAO reaction
The heterogeneous CWAO system contains three phases, namely, gas phase (O2), liquid phase (wastewater) and solid phase (solid catalyst). For the desirable heterogeneous CWAO catalyst, both O2 and pollution are adsorbed and activated on the surface. Generally, the adsorption of organic pollution is stronger than that of O2. The strong adsorption of organic pollutants results in insufficient reactive oxygen species to oxidized organic pollution, probably leading to the formation of carbonaceous deposit or the production of small molecular organic acid. How to effectively activate O2 and transfer reactive oxygen species to the adsorbed organic pollutants is very essential for the CWAO reaction to proceed. For the heterogeneous catalyst, O2 activation involves adsorption over the active sites, changing the spin-triplet state, weakening the O–O bond and breaking of the O–O bond. O2 is activated by zero-valent metal sites or oxygen vacancies over the solid catalyst, where it can be adsorbed and electrons can be given to O2. The electrons from metal sites or oxygen vacancies can enter into the degenerated 2π* orbitals and then change the spin-triplet state and activate O2.3.1. Noble metal catalysts
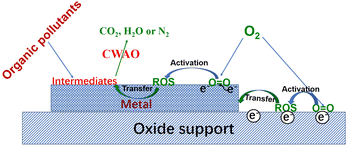
The noble Pt, Ru and Pd are the most promising metals used in the CWAO process for the degradation of pollutants due to their high activity towards oxidation of organic pollutants and good stability under severe reaction conditions. For supported metal catalysts, O2 can be adsorbed and activated on the metal particles and/or the supports (oxygen vacancies of oxide support or edge sites of C support). The mechanism of O2 activation and oxygen transfer over oxide-supported noble metal catalysts is shown in Fig. 3. It can be seen that electrons are transferred from the active sites to the O2 molecule and then weaken or break the O–O bond. Generally, the more the electrons the sites has, the better the ability to activate the O2 molecule the sites have. Apart from activating the O2 molecule, oxide supports also play important roles in transferring active oxygen species due to the presence of oxygen vacancies. The electron transfer between the metal and support can tune the electronic properties and enhance O2 activation. The oxygen transfer between metal and support can accelerate the surface oxidation reaction. The synergistic effect between metal and support determines the catalytic performance to a great extent for CWAO reactions.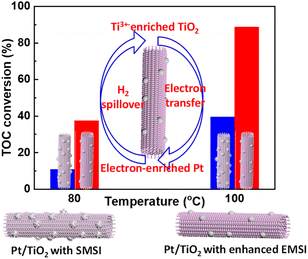 | ||
| Fig. 4 The effect of EMSI between Pt and TiO2 on the CWAO activity.24 | ||
Layered bimetallic oxides (LBMO) support transferred electrons to Pt metal. The electron transfer ensured that Pt/LBMO easily activated O2 at room temperature and possessed excellent catalytic performance for the CWAO of formaldehyde wastewater. Even at 30 °C, Pt/LBMO (H) (LBMO prepared by a hydrothermal method) can completely convert HCHO and 84.1% of HCHO was oxidized to CO2.32 The supports can also effectively activate O2 and promoted the catalytic performance for CWAO reaction. High specific surface area and pore-rich carbon nanoflowers loaded with Pt were highly active and stable for the CWAO of ammonia. The authors thought that Pt was responsible for activating NH3 and increased oxygen transfer, while the positive synergistic effect between the edge carbon and the carboxyl groups promoted the activation of O2 and the generation of reactive oxygen species. Under the synergism of Pt, edge carbon and carboxyl groups, NH3 can be effectively oxidized to N2 and H2O.33 Enhancing the support's oxygen transfer can promote the CWAO catalytic performance. The size and morphologies of the support as well as modification with a reducible promoter promotes the ability for oxygen transfer to a great extent. The size effect of ZrO2 support on the catalytic performance for CWAO of phenol was investigated over Pt/CeO2–ZrO2–Bi2O3/ZrO2. It was found that in the range from 35 to 9 nm, the smaller the ZrO2 particle size, the better the ability to release and store oxygen by the catalyst. Therefore, Pt/CeO2–ZrO2–Bi2O3/ZrO2-9 exhibited the best catalytic performance.34 The effect of MnO2 morphologies on the catalytic performance of Pt/MnO2 in formaldehyde wastewater was investigated. Compared with sheet-like and rod-like MnO2, the cocoon-like MnO2-supported Pt (Pt/C–MnO2) showed much better catalytic performance. Various characterization results indicated that more reactive oxygen species and higher oxidizing ability contribute to the excellent performance of Pt/C–MnO2.35 Novel catalysts of Pt/La1−xBixOF/SBA-16 were prepared for the CWAO of phenol. Lanthanum oxyfluoride (LaOF) was selected as the promoter because of its contribution to the migration of oxygen species in the lattice. The introduction of reducible Bi3+ in the LaOF lattice can further provide oxygen supply ability, which further enhanced the CWAO activity.36 Pt/CexZr1−xO2 catalysts were investigated in the CWAO of formic acid at low-temperature to evaluate the effect of the oxygen mobility on the catalytic performances. It was found that the 0.080 Pt/Ce0.9Zr0.1O2 (wt%) catalyst exhibited the best performances. At low-temperature (T ≤ 313 K), the initial reaction rate was proportional to the dissolved oxygen concentration, indicating that the oxygen transfer to the active site was rate-limiting.37
 | ||
| Fig. 5 The effect of calcination temperature on the performance of Ru/TiO2.39 | ||
Ru–Cu bimetallic catalysts were prepared and investigated for the CWAO of ammonia. Compared with Ru and Cu monometallic catalysts, the Ru–Cu bimetallic catalysts exhibited much higher activity and selectivity to N2 as well as stability. The characterization results indicated that there was strong interaction between Ru and Cu and the co-presence of Ru and Cu adjusted the reactivity and convergence of the oxygen species, which contributed to the good catalytic performance.40 B-site Ru-doped LaFe1−xRuxO3−δ catalyst was developed for the CWAO treatment of chlorine ion wastewater. LaFe1−xRuxO3−δ showed better activity than the supported catalysts RuO2@LaFeO3 and RuO2@TiO2 with the same Ru content. The characterization results confirmed that Fe adsorbed O2 more firmly than Ru and Fe was an active site for the oxidation of acetic acid. Additionally, Fe in LaFe0.85Ru0.15O3−δ adsorbed chlorine ion and protected the Ru site and active oxygen species to exert catalytic activity.41 CeO2 has been widely used as a support or promoter for supported-Ru catalysts in the CWAO reaction due to its excellent redox properties. The effect of supports (CeO2, TiO2, ZrO2) and active metals (Ru, Ir, Pd, Fe, Cu, Ag, Ni, Co, Cr) on the catalytic performance for CWAO of acetic acid wastewater were investigated. The results indicated that Ru catalysts supported on a high-surface area ceria had an excellent activity. The reason was that CeO2 favored the oxygen transfer between the solution and the adsorbed species. Additionally, Ru particles can maintain a close contact with CeO2 clusters when the support had a high-surface area.42 Oliviero et al. compared the catalytic performance of Ru/C and Ru–CeO2/C for the CWAO of phenol and acrylic acid. It was found that Ru–CeO2/C exhibited a better catalytic performance than Ru/C. The authors concluded that the number of contact points between Ru particles and CeO2 crystallites constitutes a key parameter and a high-surface area of ceria was required to ensure O2 activation when the organic molecule was strongly adsorbed.43 The effect of Ce content on the catalytic performance for CWAO over Pt/CexZr1−xO2 and Ru/CexZr1−xO2 was studied. The results suggested that the higher the Ce content, the higher the succinic acid removal rate and the better the mineralization. The reason for such an observation was that higher Ce content promoted the accessibility of oxygen to the active sites.44 The ethanol mediated-CeO2-supported Ru catalyst (Ru/CeO2-A) was prepared and studied for the CWAO of butyric acid. The developed catalyst was much more active than the traditional Ru/CeO2. According to the characterization results, it was concluded that the Ru/CeO2-A catalyst made the active component disperse on the support uniformly and the average particle size of Ru was smaller. Moreover, the catalyst had a high special active surface area and higher content of oxygen vacancies and Ce3+.45 Ru–CeO2–ZrO2 and Ru–Au–CeO2–ZrO2 were prepared by the wet impregnation and deposition–precipitation methods. Ru–Au–CeO2–ZrO2 showed much better catalytic performance for the CWAO of phenol than Ru–CeO2–ZrO2.46 The positive effect of CeO2 was reflected in the selectivity toward CO2, which increased from 88 to 100%. The explanation for the enhanced selectivity was the formation of Ce4+–O2−–M, which favored the oxygen transfer between the catalyst surface and the adsorbed species.47
Although supported-Pd catalysts exhibit good catalytic performance for oxidation reaction, however, there is only little work using them as CWAO catalysts in the literature. To modify the electron and oxygen transfer between the metal and the support, the triple-layer structure Pd/CeO2/C, where Pd is predominantly located on CeO2 and CeO2 on a C support, was designed and prepared for the CWAO of aqueous amide. The triple-layer resulted in the electrons being transferred from carbon to Pd via CeO2, providing excellent leaching resistance, while oxygen was transferred from CeO2 to Pd, providing high oxidation activity and excellent stability.48 The catalytic performance of TiO2-supported Pt, Pd, Ru and Ir catalysts was investigated for the CWAO of ammonia. It was found that among the studied catalysts, Pd/TiO2 was shown to be the most active and the most selective toward molecular nitrogen. The authors thought that the control of the oxygen coverage at the catalyst surface had a key impact on both the activity and selectivity. Increasing the oxygen coverage led to a lower activity (decreasing ammonia adsorption) and a lower selectivity to dinitrogen (increasing nitrites and nitrates formation).49 Nano-hybrid bimetallic Au–Pd catalyst with Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd = 1
Pd = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was highly active and worked efficiently for CWAO of high dye concentrations at reaction temperatures close to even 0 °C. The characterization results indicated that electrons on the surface of Au–Pd could be transferred partially to the adjacent O-vacancy sites, where the absorbed O2 was activated to superoxide radical. The generation of superoxide radical was responsible for the excellent catalytic performance.50
1 was highly active and worked efficiently for CWAO of high dye concentrations at reaction temperatures close to even 0 °C. The characterization results indicated that electrons on the surface of Au–Pd could be transferred partially to the adjacent O-vacancy sites, where the absorbed O2 was activated to superoxide radical. The generation of superoxide radical was responsible for the excellent catalytic performance.50
3.2. Non-noble metal catalysts
Noble metal catalysts possess good catalytic performance for the CWAO reactions; however, they are expensive and suffer from deactivation in the presence of halogens and sulphur group-containing compounds in wastewater. To reduce the cost of catalysts, non-noble metal catalysts have been developed. Among the studied non-noble metal catalysts, supported-Cu catalysts exhibit the best catalytic performance. A series of Cux/CNy@SBA catalysts were prepared by embedding Cu in the framework of g-C3N4 and investigating for the CWAO of phenol. It was found that the strong interaction between the Cu species and the ultrathin g-C3N4 coating reduced the energy gap of Cu+/Cu2+ and facilitated electron transfer, which greatly improved the ability of the catalysts to activate O2 under mild conditions. Both HO2 and ˙OH hydroxyl radicals were responsible for the excellent catalytic performance of the CWAO reaction.51Hou et al. reported that the highly dispersed Cu(II)/Cu(I)–N that embedded in the framework of g-C3N4 (Cux–g-C3N4) was more effective for CWAO than Cu(0) and CuO, and more than 23 toxic containments could be degraded under mild conditions. Fig. 6 shows the O2 activation mechanism for the Cux–g-C3N4 catalyst. It can be seen that the highly dispersed Cu(II)/Cu(I)–N can effectively activate O2 and produce H2O2 in situ, which contributed to the high activity.25
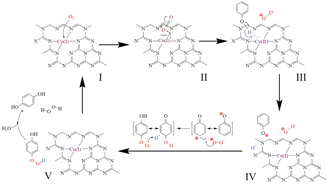 | ||
| Fig. 6 Mechanism of in situ formation of H2O2 over Cux–g-C3N4 in CWAO.25 | ||
A series of M3-Al-500 catalysts (M = Cu, Mn, Fe, Ni, Zn and Co) was prepared and tested for the CWAO of highly concentrated phenol. It was found that Cu3–Al-500 was the most active among these tested catalysts. Characterization results indicated that the good performance of the Cu-based catalyst could be attributed to the redox transitions of Cu2+/Cu+ and/or the formation of H2O2 in the reaction mixture.52 Cu-loaded biomass-derived activated carbon (Cu/AC) catalysts were prepared by precursor impregnation (PI) and activated carbon impregnation (ACI) methods. The characterization demonstrated that Cu/AC-PI have higher specific surface area and Cu content, and Cu species coexisted with Cu2+ and (Cu+ + Cu0). Higher (Cu+ + Cu0) in PI catalysts is more favorable for the generation of ˙O2− and ˙HO and then improved the conversions of phenol and chemical oxygen demand.53 Ce-modified and unmodified Cu–O/Al2O3 catalysts were prepared for the CWAO of printing and dyeing wastewater. Ce modification made the particles of active components become small and uniformly distributed. More importantly, the addition of Ce resulted in the formation of oxygen defects due to its excellent oxygen mobility, which can generate abundant oxygen vacancies into the surface of the mixed oxides, leading to the improvement of catalytic activity and the acceleration of the CWAO reaction. The free-radical experiment indicated that ˙O2−, H2O2, and ˙HO existed in the CWAO reaction system of printing and dyeing wastewater.54 Activated carbon bead (∼0.8 mm)-supported copper (Cu)-dispersed carbon nanofiber (CNF) catalyst was used for the CWAO of aqueous dichlorvos (DDVP) pesticide. The catalyst was shown to be adequately effective in oxidizing the aqueous organics. The authors thought that when the Cu0 was brought in contact with O2 during the CWAO reaction, the initial oxidation converted Cu into Cu+. The monovalent Cu+ further reacted with O2 to generate the unstable CuO2+, which was rapidly converted into the stable Cu2+ and ˙O2−. The ˙O2− radicals were responsible for the formation of H2O2, which in turn produced the reactive ˙OH that directly oxidized DDVP.55 N-doped perovskite La2CuO4 was prepared by a sol–gel rapid calcination method and investigated for the CWAO of high concentration phenol-containing simulated wastewater. The results revealed that N doping favored the formation of more oxygen vacancies and enhanced the charge transfer between Cu and O. More oxygen vacancies improved the ability of the catalyst to adsorb active O2 and the charge transfer between Cu and O facilitated the redox cycle of the catalytically active metal Cu2+/Cu1+. The redox cycle also favored the formation of O2−˙ and HO˙ and thus ensured the more exhaustive degradation of phenol.56
An Fe-supported catalyst was also investigated for CWAO. Activated carbon beads decorated with Fe–CNFs were prepared and used for the CWAO of glyphosate or N-(phosphonomethyl)glycine (PMG) in wastewater. Fe nanoparticles played an efficient role for the degradation of PMG. Active Fe metal sites activated O2 to produce hydroxyl radicals, which oxidized PMG to CO2, H2O, and some inorganic ions through the formation of sarcosine and aminomethyl phosphonic acid intermediates.57
3.3. Oxide catalysts
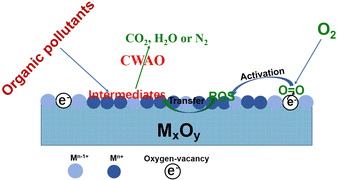
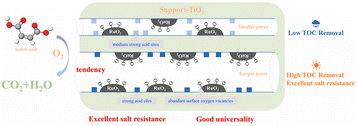
Apart from being good catalyst supports, the oxides with multiple valences (reducible oxides) are also widely used as catalysts for CWAO reactions. Fig. 7 presents the mechanism of O2 activation and oxygen transfer over oxide catalysts. Generally, these metal oxides possess oxygen vacancies or surface defects, produced by the reduction of Mn+ to M(n−1)+. The presence of oxygen vacancies or surface defects not only enhances the adsorption of molecular oxygen but also promotes the transfer of electrons to the O2. Additionally, oxygen vacancies or surface defects can improve the mobility of active oxygen species by Mn+/M(n−1)+ redox cycles. Therefore, the concentrations of oxygen vacancies or surface defects strongly affects the ability of O2 activation and transfer and thus determines the catalytic performance for the CWAO reaction.Manganese oxide shows great application potential in the CWAO process. (100) MnO2 and (001) MnO2 were prepared and studied for the CWAO of phenol. The results indicated that the catalytic degradation phenol rate of (100) MnO2 was three times as much as that of (001) MnO2. EPR and DFT results showed that superoxide (˙O2−) species could exist on (001) MnO2 through one-electron transfer, while the peroxide (O22−) species exist on (100) MnO2 via two-electron transfer. All the results illustrate that birnessite MnO2 with (100) crystal planes possessed better oxygen activation ability, which contributed to the higher activity.58 The δ-MnO2 catalyst with a very high amount of Mn4+ was synthesized and investigated for the CWAO of phenol under very mild conditions. The results indicated that the prepared δ-MnO2 was highly active and stable. The high activity was mainly due to the high concentration of Mn4+ (>90%) and high reactivity of surface oxygen species. Its good stability resulted from the high resistance to Mn leaching and the easy regeneration of Mn4+ species from low valence Mn.59 CeO2 is also an attractive oxide catalyst. A simple but efficient H2O2-ultrasonic treatment was used to modify the surface properties of CeO2 nanorods and enhance its catalytic performance for the CWAO of phenol. It was found that the treatment increased the Ce3+ fraction and created more oxygen vacancies by roughening the surface of CeO2. Higher Ce3+ fraction and surface oxygen vacancies concentration enabled the treated CeO2 catalyst to possess more oxidizing surface and the highest catalytic performance for the CWAO of phenol.60 CeO2 supported on the CNTs-M (treated by HNO3) and CNTs-raw was synthesized and evaluated for the CWAO of DMF. It was found that the acid treatment produced surface functional groups on CNTs, which enhanced the interaction between CNTs and CeO2. Such strong interaction increased the surface oxygen in CeO2/CNTs-M. As a result, CeO2/CNTs-M showed much better catalytic activity and stability than CeO2/CNTs-raw.61 Compared with CeO2-nanorods and CeO2-nanocubes, 3D ball-type CeO2 exhibited better catalytic performance for the CWAO of N,N-dimethylformamide. Detailed characterization results confirmed that 3D ball-type CeO2 contained an abundant fraction of Ce3+ and more active oxygen species.62 Combining MnO2 with CeO2 can effectively enhance the CWAO catalytic performance. The effect of oxygen vacancies on the CWAO of phenol was investigated over CeO2 and MnOx–CeO2 catalysts. It was found that CeO2 and MnOx–CeO2 nanorods were much more active than the corresponding nanocube. The characterization results revealed that the higher concentration of oxygen vacancies in CeO2 and MnOx–CeO2 nanorod increased the oxidizing ability and the catalytic performance. The synergy between Mn and Ce further improved the redox properties, which produce a larger amount of active oxygen species and are the reason why MnOx–CeO2 nanorods were the most active catalysts among the catalysts investigated in the study.63
Synergistic effect between Mn and Ce was investigated for the CWAO of phenol. Four MnCeOx catalysts were active; however, Mn9Ce1Ox possessed much better catalytic performance than Mn15Ce1Ox, Mn5Ce1Ox and Mn1Ce2Ox, as shown in Fig. 8. The characterization results revealed that Mn4+ was favorable for the adsorption and oxidation of phenol, while Mn3+ was favorable for the activation of oxygen. Ce balanced the adsorption and oxidation of phenol and the activation of oxygen by tuning the Mn4+/Mn3+ ratio. Ce was also helpful for retaining the main structure of the catalyst under the reaction conditions and prevented Mn4+ or Mn3+ from being reduced to Mn2+ by facilitating oxygen transfer.26 The mechanistic and kinetic clues for the CWAO of organic pollutants (including phenol, acetic, oxalic and formic acids) over a model MnCeOx catalyst were studied. The results indicated that the CWAO reaction proceeds via an L–H mechanism, involving adsorption as the first reaction step and surface oxidation as the rate-determining step.64
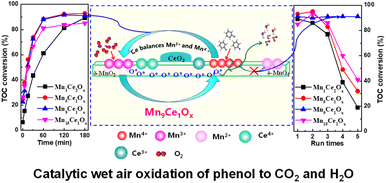 | ||
| Fig. 8 Catalytic wet-air oxidation of phenol over Mn9Ce1Ox.26 | ||
3.4. Carbon catalysts
Carbon materials provide an alternative to replace metal-supported catalysts since they can help to reduce the leaching of the metals during the CWAO process. For carbon catalyst, O2 is absorbed and activated on defect sites, zig-zag and armchair edges, or heteroatoms sites. The unpaired electrons on these sites can reduce O2 to form ˙O2−. The surface carboxylic acid group also contributes to the formation of active oxygen species (such as ˙OH) by providing H atoms.Active carbon was used as a catalyst for the CWAO of high concentration N-methyldiethanolamine (MDEA)-containing wastewater. The conversion of MDEA and COD exceeded 98% and ∼90% at 230 °C and 5.0 MPa for 168 h continuous operation. The surface carboxylic acid groups of AC played a key role in the good catalytic performance. Fig. 9 presents the CWAO process for removing MDEA over AC catalyst. It can be seen that O2 was adsorbed by the carboxyl groups and then dissociated to form ˙O2−. Then, hydroxyl radicals were generated by the electron transfer of ˙O2− or attracting H+ of the carboxyl groups, which could further oxidize organics to small molecules of carboxylic acid or organic compounds. High COD and MDEA conversion were due to the O2 adsorption and dissociation on the carboxylic acid groups of AC to efficiently form ˙OH.65
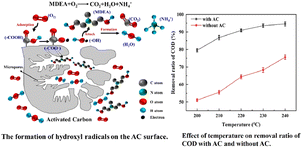 | ||
| Fig. 9 The formation of hydroxyl radicals and the performance of the AC catalyst.65 | ||
Nitrogen-doped carbon nanotube was prepared and used for the CWAO of phenol. It was found that the N-functionalities, including pyridinic and pyrrolic groups and quaternary nitrogen, were related to the higher activity. The reason was that the N-functionalities improved the interaction of oxygen with the carbon surface, enhancing its chemisorption and the activation of the oxygen molecules, which enhanced the activity.27 Herringbone carbon nanotubes (CNTs) that underwent different treatments were used as catalysts for the CWAO of phenol. The results indicated that parallel CNTs (p-CNTs) with cylindrical graphitic walls showed the highest catalytic activity after nitric acid oxidation. The synergistic effect between carboxyl groups and adjacent edge carbons was responsible for the activation of O2 and the improved CWAO activity. The edge carbon atoms contained unpaired electrons constituting the active catalytic sites for the adsorption and activation of O2. The superoxide radical (˙O2−) was formed by electron transport and then abstracted an H atom from the –COOH groups to generate ˙HO2. The formation of ˙HO2 aroused the radical chain reactions of the CWAO of phenol and thus enhanced the activity.66 Aminated activated carbon was used as a catalyst to treat the low-biodegradable coking wastewater. It was found that the aminated AC exhibited better catalytic performance than their parent counterpart. The enhanced activity was explained by the reaction pathway of organic pollutants on the AC surface and activation of oxygen by surface nitrogen groups.67
4. Conclusions
CWAO is one of the most promising technologies for the treatment of various industrial wastewaters containing toxic and non-biodegradable organic pollutants. However, the catalysts reported in the literature only effectively work at high temperature and high pressure. Additionally, the catalyst also suffers from deactivation due to the leaching of active metal or carbonaceous deposition. The poor ability of the CWAO catalyst to activate O2 and transfer oxygen species results in its activity, selectivity and stability not meeting the requirement for commercial application. Further studies are necessary to develop more active and stable catalysts that can effectively work even under mild conditions.The insights into how the O2 molecule is activated to active species on the CWAO catalyst surface will improve the understanding of the chemistry of the low-temperature CWAO. However, the mechanism of O2 activation and transfer during CWAO lacks study. It was reported that various active oxygen species (˙O2−, ˙HO2 and ˙OH) are generated during the CWAO process. However, the effect of the electronic structure on the formation of active oxygen species also lacks study. The relationship between electronic structure, O2 activation and CWAO catalytic performance is needed to be further explored in the future.
The electronic structure and oxidation state of the catalyst strongly affect the oxygen activation and transfer. When metal contacts with reducible oxides, the electrons will be transferred between the metal and oxide due to the difference in the electrochemical potentials. Similar electron transfer occurs between oxide and carbon. Preparing three-component catalysts that consist of metal, oxide and carbon can be one of the most promising directions for developing CWAO technology in the future. Both carbon-modified oxide supports and oxide-modified carbon supports are desirable. For such catalysts, the electron transfer between different materials can effectively enhance the catalytic performance by tuning the electronic structure and oxidation state of the catalyst.
Data availability
No data was used for the research described in the article.Author contributions
PM, NZ and BC the idea, PM, NZ, JS, HL, JF, ML and QD written original manuscript. NZ, ZF, QZ, and SY edited the manuscript. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
All the authors thank the financial support of the National Natural Science Foundation of China (NSFC) (No. 22272137) and Project of Technology Research and Development Program of China Tobacco Fujian Industrial Co., Ltd. (No. FJZYHZJH2023011).Notes and references
- J. Liu and J. Gao, Front. Environ. Sci. Eng., 2023, 17, 26 CrossRef CAS.
- M. Jayanti, K. L. Bibhab, S. Ramesh, M. Nibedita, P. S. Maulin, M. Subhasis, G. Susanta and B. Biswanath, Chemosphere, 2023, 345, 140473 CrossRef.
- L. Lin, J. Wang, Z. Zhao, J. Zhu, A. Zhamaerding, L. Feng, D. Yang, L. Meng, C. He, W. Wang and Y. Zhang, Chem. Eng. J., 2023, 474, 145600 CrossRef CAS.
- Z. Wang, Science, 2012, 338, 604 CrossRef CAS PubMed.
- K. H. Kim and S. K. Ihm, J. Hazard. Mater., 2011, 186, 16–34 CrossRef CAS PubMed.
- Y. Cai, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Adv. Mater., 2020, 32, 2001265 CrossRef CAS PubMed.
- L. Jiao, Y. Huang, Y. Hu, Y. Yang, H. Chen, N. Zhou, Q. Guo, H. Wu, A. Xia, X. Zhao, G. Hu and R. Chen, Sep. Purif. Technol., 2024, 338, 126513 CrossRef CAS.
- V. I. Parvulescu, F. Epron, H. Garcia and P. Granger, Chem. Rev., 2022, 122, 2981–3121 CrossRef CAS.
- S. K. Bhargava, J. Tardio, J. Prasad, K. Fo1ger, D. B. Akolekar and S. C. Grocott, Ind. Eng. Chem. Res., 2006, 45, 1221–1258 CrossRef CAS.
- G. Jing, M. Luan and T. Chen, Arabian J. Chem., 2016, 9, S1208–S1213 CrossRef CAS.
- F. Arena, R. D. Chio, B. Gumina, L. Spadaro and G. Trunfio, Inorg. Chim. Acta, 2015, 431, 101–109 CrossRef CAS.
- M. Bourassi, G. Lafaye and J. Barbier Jr, Catal. Rev., 2024 DOI:10.1080/01614940.2024.2346311.
- M. P. Christophliemk, A. Heponiemi, T. Kangas, T. Hu, H. Prokkola and U. Lassi, Adv. Compos. Hybrid Mater., 2024, 7, 158 CrossRef CAS.
- M. Bourassi, G. Lafaye, B. Gombert, P. Kluson and J. Barbier Jr, J. Cleaner Prod., 2023, 429, 139453 CrossRef CAS.
- H. Shan, R. Oh, J. Fan, X. Zhang, N. Zhang, X. Huang, G. Park, Q. Zheng, H. Lu and B. Chen, J. Environ. Chem. Eng., 2023, 11, 109854 CrossRef CAS.
- G. Li, S. Chai, G. Zhang, J. Liu, Y. Zhang, Y. Lv, Y. Wang and Y. Zhao, J. Environ. Chem. Eng., 2022, 10, 108228 CrossRef CAS.
- M. A. L. Rocha, G. DelÁngel, G. Torres-Torres, A. Cervantes, A. Vázquez, A. Arrieta and J. N. Beltramini, Catal. Today, 2015, 250, 145–154 CrossRef CAS.
- O. S. G. P. Soares, R. P. Rocha, A. G. Goncalves, J. L. Figueiredo, J. J. M. Órfão and M. F. R. Pereira, Appl. Catal., B, 2016, 192, 296–303 CrossRef CAS.
- G. Lafaye, J. J. Barbier and D. Duprez, Catal. Today, 2015, 253, 89–98 CrossRef CAS.
- D. S. Su, G. Wen, S. Wu, F. Peng and R. Schlogl, Angew. Chem., Int. Ed., 2017, 56, 936–964 CrossRef CAS.
- D. F. M. Santos, O. S. G. P. Soares, A. M. T. Silva, J. L. Figueiredo and M. R. Pereira, Appl. Catal., B, 2016, 199, 361–371 CrossRef CAS.
- Sushma, M. Kumari and A. K. Saroha, J. Environ. Manage., 2018, 228, 169–188 CrossRef CAS.
- S. Keav, J. J. Barbier and D. Duprez, Catal. Sci. Technol., 2011, 1, 342–353 RSC.
- J. Fu, X. Zhang, H. Li, B. Chen, S. Ye, N. Zhang, Z. Yu, J. Zheng and B. Chen, J. Hazard. Mater., 2022, 426, 128088 CrossRef CAS PubMed.
- W. Zhang, B. Ye, Z. Zhong, Y. Jiang, R. Zhou, Z. Liu and Z. Hou, J. Hazard. Mater., 2022, 424, 127679 CrossRef CAS PubMed.
- L. Geng, B. Chen, J. Yang, C. Shui, S. Ye, J. Fu, N. Zhang, J. Xie and B. Chen, Appl. Catal., A, 2020, 604, 117774 CrossRef CAS.
- R. P. Rocha, O. S. G. P. Soares, A. G. Gonçalves, J. J. M. Órfão, M. F. R. Pereira and J. L. Figueiredo, Appl. Catal., A, 2017, 548, 62–70 CrossRef CAS.
- J. U. Etim, P. Bai, M. O. Gazit and Z. Zhong, Catal. Rev., 2023, 65(2), 239–425 CrossRef.
- F. Luck, Catal. Today, 1999, 53, 81–91 CrossRef CAS.
- S. Yang, Z. Liu, X. Huang and B. Zhang, J. Hazard. Mater., 2010, 178, 786–791 CrossRef CAS PubMed.
- C. Sergio, L. Adriana and D. Mario, J. Hazard. Mater., 2010, 177, 183–189 CrossRef PubMed.
- H. Chen, N. Liu, N. Zhang and B. Chen, J. Xiamen Univ., Nat. Sci., 2023, 62, 758–765 Search PubMed.
- B. Feng, J. Xue, D. H. Yang, X. M. Song, T. Huang, Q. Zhu and H. Gai, J. Water Process Eng., 2023, 55, 104206 CrossRef.
- A. R. Supandi, N. Nunotani and N. Imanaka, J. Asian Ceram. Soc., 2020, 8, 745–752 CrossRef.
- L. Geng, C. Rong, A. Lin, H. Shi, M. Zhang, N. Zhang and B. Chen, Chin. Sci. Bull., 2021, 66, 2898–2907 CrossRef.
- N. Nunotani, Z. Li and N. Imanaka, Int. J. Appl. Ceram. Technol., 2022, 19, 746–752 CrossRef CAS.
- S. Yang, M. Besson and C. Descorme, Appl. Catal., B, 2010, 100, 28–288 CrossRef.
- J. Qin and K. Aika, Appl. Catal., B, 1998, 16, 261–268 CrossRef CAS.
- W. Zhang, J. Sun, Y. Zhang, D. Yu, W. Piao, H. Wei, X. Liu and C. Sun, Chemosphere, 2023, 312, 137194 CrossRef CAS PubMed.
- J. Fu, K. Yang, C. Ma, N. Zhang, H. Gai, J. Zheng and B. Chen, Appl. Catal., B, 2016, 184, 216–222 CrossRef CAS.
- W. Sun, H. Lv, L. Ma, X. Tan, C. Jin, H. Wu, L. Chen, M. Liu, H. Wei and C. Sun, J. Environ. Sci., 2022, 120, 105–114 CrossRef CAS PubMed.
- J. Barbier Jr, F. Delanoe, F. Jabouille, D. Duprez, G. Blanchard and P. Isnard, J. Catal., 1998, 177, 378–385 CrossRef.
- L. Oliviero, J. Barbier Jr, Da. Duprez, A. Guerrero-Ruiz, B. Bachiller-Baeza and I. Rodriguez-Ramos, Appl. Catal., B, 2000, 25, 267–275 CrossRef CAS.
- S. Yang, M. Besson and C. Descorme, Appl. Catal., B, 2015, 165, 1–9 CrossRef CAS.
- Y. Wang, C. Yu, X. Meng, P. Zhao and L. Chou, RSC Adv., 2017, 7, 39796–39802 RSC.
- A. A. Silahua-Pavon, G. Torres-Torres, J. C. Arevalo-Perez, A. Cervantes-Uribe, Z. Guerra-Que, A. Cordero-Garc, M. A. Espinosa and N. J. Beltramini, RSC Adv., 2019, 9, 11123–11134 RSC.
- G. Torres-Torres, I. A. Cuauhtémoc-López, E. Monteros, D. M. Frías-Márquez, J. C. Arévalo-Pérez and G. Angel, Water Sci. Technol., 2018, 78, 1509–1516 CrossRef CAS PubMed.
- J. Fu, Q. Yue, H. Guo, C. Ma, Y. Wen, H. Zhang, N. Zhang, Y. Zheng, J. Zheng and B. Chen, ACS Catal., 2018, 8, 4980–4985 CrossRef CAS.
- C. Lousteau, M. Besson and C. Descorme, Catal. Today, 2015, 241, 80–85 CrossRef CAS.
- P. Wang, Y. N. Liang, Z. Zhong and X. Hu, Sep. Purif. Technol., 2020, 233, 115960 CrossRef CAS.
- C. Yuan, Y. Zhang, Z. Zong, S. Zhou, H. Cui and H. Tan, Green Chem., 2024, 26, 6601–6615 RSC.
- C. Lai, T. He, X. Li, F. Chen, L. Yue and Z. Hou, Appl. Clay Sci., 2019, 181, 105253 CrossRef CAS.
- H. Wang, G. Li, S. Zhang, Y. Li, Y. Zhao, L. Duan and Y. Zhang, Ind. Eng. Chem. Res., 2020, 59, 2908–2920 CrossRef CAS.
- Y. Zhang, Y. Zhou, C. Peng, J. Shi, Q. Wang, L. He and L. Shi, Appl. Surf. Sci., 2018, 436, 981–988 CrossRef CAS.
- A. Kumara and N. Verma, Chem. Eng. J., 2018, 351, 428–440 CrossRef.
- Z. Ding, C. Liu, B. Yang, C. Ding, S. Mao, M. Shi, X. Hong, F. Wang and M. Xia, Sep. Purif. Technol., 2023, 322, 124310 CrossRef CAS.
- P. Gupta and N. Verma, Chem. Eng. J., 2021, 417, 128029 CrossRef CAS.
- W. Yang, Y. Zhu, F. You, L. Yan, Y. Ma, C. Lu, P. Gao, Q. Hao and W. Li, Appl. Catal., B, 2018, 233, 184–193 CrossRef CAS.
- C. Ma, Y. Wen, C. Rong, N. Zhang, J. Zheng and B. H. Chen, Catal. Sci. Technol., 2017, 7, 3200–3204 RSC.
- C. Ma, J. Fu, J. Chen, Y. Wen, O. P. Fasan, H. Zhang, N. Zhang, J. Zheng and B. Chen, Ind. Eng. Chem. Res., 2017, 56, 9090–9097 CrossRef CAS.
- S. Ali, W. Xiong, Z. Liao, M. J. Aslam, C. Zhou, K. K. Seong, P. Zhang, N. Zhang, J. Zheng, J. Fu and B. Chen, Appl. Catal., A, 2021, 620, 118172 CrossRef CAS.
- S. Ali, Y. Jiang, Z. Lai, P. Zhang, S. Ye, J. Wang, J. Fu, N. Zhang, J. Zheng and B. Chen, J. Rare Earths, 2023, 41, 1179–1188 CrossRef CAS.
- C. Ma, Y. Wen, Q. Yue, A. Li, J. Fu, N. Zhang, H. Gai, J. Zheng and B. H. Chen, RSC Adv., 2017, 7, 27079–27088 RSC.
- F. Arena, C. Italiano, G. D. Ferrante, G. Trunfio and L. Spadaro, Appl. Catal., B, 2014, 144, 292–299 CrossRef CAS.
- J. Zhao, Y. Wei, Z. Liu, L. Zhang, Q. Cui and H. Wang, Chem. Eng. Process., 2022, 171, 108744 CrossRef CAS.
- S. Yang, X. Li, W. Zhu, J. Wang and C. Descorme, Carbon, 2008, 46, 445–452 CrossRef CAS.
- H. Chen, G. Yang, Y. Feng, C. Shi, S. Xu, W. Cao and X. Zhang, Chem. Eng. J., 2012, 198–199, 45–51 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |