Open Access Article
Open Access ArticleMagnetoresistance effect of nitrogen doped graphdiyne†
Huifang Kang a,
Yi Zhongb,
Peiyan Gaob,
Xiong Lianga,
Huiye Qiu*a and
Yongping Zheng
a,
Yi Zhongb,
Peiyan Gaob,
Xiong Lianga,
Huiye Qiu*a and
Yongping Zheng *b
*b
aSchool of Physics and Mechanical and Electronical Engineering, Longyan University, Longyan 364012, China
bCollege of Physics and Energy, Fujian Normal University, Fujian Provincial Key Laboratory of Quantum Manipulation and New Energy Materials, Fuzhou, 350117, China. E-mail: zyp@fjnu.edu.cn
First published on 25th November 2024
Abstract
This investigation elucidates the influence of nitrogen doping (N-doping) on the magnetoresistance (MR) characteristics of graphdiyne (GDY) by methodically adjusting the levels of N-doping. Through exhaustive experimental analyses, encompassing SEM, XPS, and magneto-transport measurements, we demonstrate that N-doping markedly augments carrier concentration, thereby inducing distinct MR behaviors. Specifically, N30-GDY manifests negative magnetoresistance (NMR) attributable to weak localization phenomena, whereas N60-GDY and N90-GDY exhibit positive magnetoresistance (PMR) characterized by significant resistance enhancements. These results not only clarify the underlying mechanisms governing the MR properties of GDY but also introduce a novel strategy for optimizing MR performance via controlled heteroatom doping, thereby holding considerable implications for the advancement of carbon-based electronic devices.
Introduction
In 2010, the synthesis of a novel two-dimensional (2D) carbon material, graphdiyne (GDY), was successfully achieved through a crosslinking reaction.1 Comprising sp- and sp2-hybridized carbon atoms, GDY has attracted considerable attention owing to its remarkable properties.2–6 As an allotrope of graphene with a direct bandgap structure proximate to the Dirac cone, theoretical predictions estimate GDY's bandgap to range from 0.46 to 1.22 eV,7,8 with carrier mobility comparable to that of graphene.9 Its unique structural configuration and acetylenic bonds render GDY highly conducive to carrier transport, attaining mobilities as high as 104 to 105 cm2 V−1 s−1,10 thereby indicating substantial potential for applications in carbon-based electronic devices.In recent years, GDY has been extensively investigated in domains such as energy,11 catalysis,12 and magnetism,13 however, its magnetoresistance (MR) properties remain largely unexplored. Although several studies have examined the MR characteristics of GDY, the complex factors influencing its MR behavior are still inadequately understood and insufficiently explored. Among the myriad factors affecting GDY's MR properties, carrier concentration emerges as a pivotal parameter. According to the literature, nitrogen doping (N-GDY) has been demonstrated as an effective method for enhancing carrier concentration.14 Nitrogen doping introduces novel electronic states and disrupts the symmetry of the GDY lattice, thereby modulating its electronic, magnetic, and optical properties. Due to the analogous atomic sizes of nitrogen and carbon, nitrogen can be seamlessly integrated into the GDY lattice, resulting in stable nitrogen-doped GDY structures. This incorporation significantly alters the electronic band structure of GDY, facilitating tunable magnetoresistance. Empirical evidence has corroborated that nitrogen doping effectively increases carrier concentration.14 Consequently, nitrogen doping presents a promising strategy for further manipulating the MR properties of GDY.
In this study, we fabricated nitrogen-doped GDY samples with varying nitrogen content, resulting in significant modifications to their MR properties. Specifically, under an applied magnetic field of 9 tesla (T) and at a temperature of 2 kelvin (K), N30-GDY exhibited negative magnetoresistance (NMR) with a maximum MR of 200%, indicative of weak localization effects. In contrast, N60-GDY and N90-GDY demonstrated positive magnetoresistance (PMR) characterized by pronounced resistance enhancements, with N60-GDY exhibiting a particularly robust PMR effect, exceeding a 400% resistance change. At lower levels of nitrogen doping, the increased carrier concentration enhances electron scattering in the magnetic field, thereby augmenting MR. These distinct magnetoresistance behaviors in nitrogen-doped GDY suggest a substantial transformation in its transport properties resulting from nitrogen incorporation.
Experiment
The morphologies of the samples were scrutinized using a scanning electron microscope (SEM, JSM-7500 F, Japan). Raman spectra were obtained with a LABRAM-HR micro-Raman system (Longjumeau, Paris, France). The chemical compositions of the samples were analyzed via X-ray photoelectron spectroscopy (XPS, ESCALAB 250, VG, USA). The thicknesses of the GDY films were measured using atomic force microscopy (Bruker Dimension ICON, Germany).
Four-probe magneto-transport measurements were executed utilizing a physical property measurement system (PPMS, Quantum Design, Dynacool D204, USA) over a temperature range of 2 to 150 K and within magnetic fields up to 9 T. Resistances were gauged by applying a fixed electric current of 0.03 mA, with the magnetic field (B) oriented along the vertical axis, perpendicular to the direction of the electric current. Hall resistivity measurements were conducted under a fixed electric current of 0.038 mA. Electron paramagnetic resonance (EPR) spectra were recorded using an X-band (9.6 GHz) Bruker EMXnano spectrometer at room temperature, with the magnetic field oriented perpendicular to the substrate (approximately 0.335 T).
Result and discuss
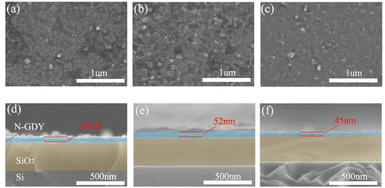
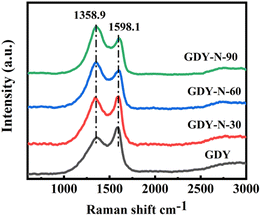
SEM analysis (as illustrated in Fig. 2) was performed to examine the samples with varying nitrogen levels (N30-GDY, N60-GDY, and N90-GDY). The results indicate that all GDY films, irrespective of nitrogen content, exhibit exceptional uniformity and continuity. Notably, the morphology and structural integrity of the films remain virtually unaltered despite variations in N-doping levels. The SEM images display smooth, planar surfaces with minimal disruptions to the GDY films. Furthermore, cross-sectional SEM images reveal consistent features across all samples, including a uniform thickness of approximately 50 nanometers and distinct interlayer boundaries. These structural characteristics establish a robust foundation for subsequent MR investigations.The structural characteristics of GDY and N-GDY films, grown directly on the substrate, were characterized using Raman spectroscopy. As depicted in Fig. 3 and S1,† four primary peaks are discernible: the D peak at 1358.9 cm−1, the G peak at 1598.1 cm−1, and two weaker peaks corresponding to the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– acetylene bonds. The D peak, situated at 1358.9 cm−1, signifies the presence of defects within the sample, with findings suggesting a substantial increase in defect density post N-doping.16 The G peak, at 1598.1 cm−1, is attributed to first-order scattering and the breathing vibrations of the E2g modes within the sp2-hybridized carbon domains of the aromatic ring, characterized by in-phase stretching vibrations.17 The morphology of this peak corroborates the conclusion that the ID/IG ratio, derived from the spectrum, escalates with prolonged annealing durations (30, 60, 90 minutes). This implies a proliferation of defects in GDY correlating with increased NH3 annealing time.
C– acetylene bonds. The D peak, situated at 1358.9 cm−1, signifies the presence of defects within the sample, with findings suggesting a substantial increase in defect density post N-doping.16 The G peak, at 1598.1 cm−1, is attributed to first-order scattering and the breathing vibrations of the E2g modes within the sp2-hybridized carbon domains of the aromatic ring, characterized by in-phase stretching vibrations.17 The morphology of this peak corroborates the conclusion that the ID/IG ratio, derived from the spectrum, escalates with prolonged annealing durations (30, 60, 90 minutes). This implies a proliferation of defects in GDY correlating with increased NH3 annealing time.
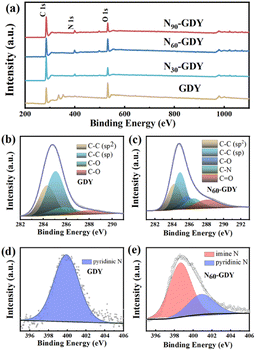
Subsequent XPS analysis was undertaken to further elucidate the chemical structure of N-doped GDY samples. As presented in Fig. 4 and S2,† the comprehensive XPS spectrum confirms that GDY comprises exclusively carbon (C) and oxygen (O). Moreover, with extended NH3 annealing times, the intensity of the nitrogen (N) peak intensifies, unequivocally confirming the successful incorporation of nitrogen into the GDY films. The peak at 284.8 eV corresponds to the C 1s orbital. Due to GDY's exposure to ambient air, an O 1s signal, attributed to adsorbed O2, is detected at 531.6 eV.18,19 Following NH3 treatment at 550 °C, N-GDY was formed, and its N 1s peak was observed at 398.6 eV. The C 1s peak of GDY can be deconvoluted into four components: C–C (sp2) at 284.4 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (sp) at 285.0 eV, C–O at 285.6 eV, and C
C (sp) at 285.0 eV, C–O at 285.6 eV, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 287.7 eV. In contrast, N-GDY exhibits peaks at 284.4 eV, 285.0 eV, 285.6 eV, 286.4 eV, and 287.9 eV, corresponding to C–C (sp2), C
O at 287.7 eV. In contrast, N-GDY exhibits peaks at 284.4 eV, 285.0 eV, 285.6 eV, 286.4 eV, and 287.9 eV, corresponding to C–C (sp2), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (sp), C–O, C
C (sp), C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.16,20,21 The N 1s peak can be further deconvoluted into two distinct peaks at 398.5 eV and 399.9 eV (as depicted in Fig. 4). The peak at 398.5 eV is ascribed to nitrogen derived from NH3, whereas the peak at 399.9 eV corresponds to pyridinic nitrogen.20,22,23
O bonds, respectively.16,20,21 The N 1s peak can be further deconvoluted into two distinct peaks at 398.5 eV and 399.9 eV (as depicted in Fig. 4). The peak at 398.5 eV is ascribed to nitrogen derived from NH3, whereas the peak at 399.9 eV corresponds to pyridinic nitrogen.20,22,23
Electron paramagnetic resonance (EPR) measurements were conducted at ambient temperature to assess the spin concentration and g-factor of the material. As illustrated in Fig. S3,† the spin concentration (NS), directly correlated with the intensity of the EPR peak, was quantified. The spin concentrations for N30-GDY, N60-GDY, and N90-GDY were determined to be 2.34 × 1011 spins per mm3, 7.76 × 1011 spins per mm3, and 16.29 × 1011 spins per mm3, respectively. The experimental g-factors were recorded as 2.0050, 2.0049, and 2.0053 for N30-GDY, N60-GDY, and N90-GDY, respectively. This suggests the presence of nitrogen-related vacancies or defects within the sample, thereby further corroborating the successful doping of nitrogen into the GDY structure.24 Notably, the EPR peak intensity escalates with increasing nitrogen concentrations, indicating that spin–orbit coupling and electron–nuclear interactions remain minimal in typical organic aromatic systems, thereby providing clear evidence for the existence of free radicals.
The transport properties of N30-GDY, N60-GDY, and N90-GDY samples were scrutinized by analyzing their resistivity variations with temperature (ranging from 2 to 120 K) under zero-field conditions. As depicted in Fig. S4,† the resistivity curve exhibits a distinct inflection point, signifying a transition from metallic behavior at low temperatures (2–18 K) to semiconducting characteristics at elevated temperatures (18–100 K). Additionally, Fig. S5† underscores our utilization of density functional theory (DFT) to compute the band gap of N-doped GDY, revealing that N-doping effectively diminishes the band gap, thereby endowing the material with metallic properties.
Further analysis of carrier dynamics in GDY post N-doping was conducted through Hall resistivity measurements. Notably, Fig. S6† illustrates a nonlinear correlation between Hall resistivity and magnetic field, suggesting the involvement of multiple carrier types in the transport mechanism. The coexistence of positive and negative slopes in Hall resistivity across varying temperatures underscores the predominance of electrons as the primary charge carriers. Furthermore, at 1 T, the Hall coefficient (RH) exhibits a pronounced temperature dependence. The carrier concentration (n) and mobility (μ) were quantitatively ascertained using eqn (1) and (2), respectively, based on the Hall resistivity data:14
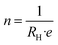 | (1) |
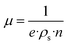 | (2) |
Employing the relationship that links charge carrier density (n) to the electron charge (e), resistivity (ρ), and the Hall coefficient (typically measured in units of m3 C−1), both carrier concentration and mobility were calculated at various temperatures, as delineated in Table S1.† Our findings demonstrate that N-doping in GDY culminates in a substantial enhancement of carrier concentration, approximately by an order of magnitude. This indicates that N-doping significantly elevates the carrier concentration within GDY. Given that nitrogen atoms possess higher electronegativity compared to carbon atoms, N-doping introduces additional electrons into the GDY structure, thereby augmenting carrier concentration. This elevated carrier concentration facilitates enhanced electron transport within the material, ultimately influencing its magnetoresistance (MR) properties.
The MR effect, defined as 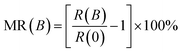 , quantifies the extent of change in electrical resistance in response to external magnetic fields. As depicted in Fig. 5, our measurements conducted at 2 K within a ±9 T magnetic field range reveal distinct behaviors in N-doped GDY samples. Specifically, N30-GDY, characterized by a lower nitrogen concentration, exhibits negative magnetoresistance (NMR) at low temperatures, attaining an MR ratio of approximately 200%. This phenomenon occurs because N atoms, serving as scattering centers, are introduced into GDY, yet their concentration remains insufficient to fully alter the material's transport mechanism. Consequently, the MR characteristics of the N30-GDY sample are predominantly governed by the Weak Localization (WL) effect.25 In contrast, with increasing nitrogen concentration, N60-GDY and N90-GDY display positive magnetoresistance (PMR), with N60-GDY exhibiting a particularly pronounced PMR effect, resulting in a resistance change exceeding 400%. These distinct MR signatures in N-doped GDY indicate a substantial transformation in transport properties due to nitrogen incorporation. The experimental results demonstrate that N-doping effectively modulates the MR characteristics of carbon materials. As N-doping levels escalate, magnetoresistance initially increases and subsequently diminishes. At lower N-doping levels, the enhanced carrier concentration intensifies electron scattering in the magnetic field, leading to elevated magnetoresistance. However, at excessively high N-doping levels, surplus nitrogen atoms may engender defects or impurity centers, thereby diminishing electron transport efficiency and ultimately reducing magnetoresistance.26,27
, quantifies the extent of change in electrical resistance in response to external magnetic fields. As depicted in Fig. 5, our measurements conducted at 2 K within a ±9 T magnetic field range reveal distinct behaviors in N-doped GDY samples. Specifically, N30-GDY, characterized by a lower nitrogen concentration, exhibits negative magnetoresistance (NMR) at low temperatures, attaining an MR ratio of approximately 200%. This phenomenon occurs because N atoms, serving as scattering centers, are introduced into GDY, yet their concentration remains insufficient to fully alter the material's transport mechanism. Consequently, the MR characteristics of the N30-GDY sample are predominantly governed by the Weak Localization (WL) effect.25 In contrast, with increasing nitrogen concentration, N60-GDY and N90-GDY display positive magnetoresistance (PMR), with N60-GDY exhibiting a particularly pronounced PMR effect, resulting in a resistance change exceeding 400%. These distinct MR signatures in N-doped GDY indicate a substantial transformation in transport properties due to nitrogen incorporation. The experimental results demonstrate that N-doping effectively modulates the MR characteristics of carbon materials. As N-doping levels escalate, magnetoresistance initially increases and subsequently diminishes. At lower N-doping levels, the enhanced carrier concentration intensifies electron scattering in the magnetic field, leading to elevated magnetoresistance. However, at excessively high N-doping levels, surplus nitrogen atoms may engender defects or impurity centers, thereby diminishing electron transport efficiency and ultimately reducing magnetoresistance.26,27
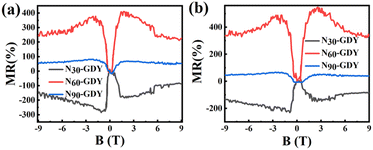 | ||
| Fig. 5 (a) and (b) Depict the magnetoresistance of N30-GDY, N60-GDY, and N90-GDY, measured at 2 K and 10 K, respectively. | ||
Conclusion
We successfully synthesized nitrogen-doped GDY with varying degrees of doping (N30-GDY, N60-GDY, N90-GDY) by meticulously modulating the annealing duration in an NH3 atmosphere. Detailed examinations via SEM and XPS revealed a clear trend: prolonged annealing times augmented the prominence of the nitrogen peak, thereby confirming effective nitrogen doping. Hall resistivity measurements indicated a consistent increase in carrier concentration with enhanced nitrogen doping. Further investigation into the MR properties unveiled intriguing disparities among the doped samples. N30-GDY exhibited NMR, indicative of weak localization effects, whereas N60-GDY and N90-GDY displayed PMR with significant resistance enhancements, signifying a transition in magnetoresponse behavior. We attribute these modifications primarily to the modulation of carrier concentration by nitrogen doping, which substantially impacted the MR properties. Our experiments provide valuable insights into the tuning of electronic and magnetic properties through chemical doping strategies, with significant implications for the development of advanced carbon-based electronic devices.Data availability
The data that support the fundings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Natural Science Foundations of Fujian Province (Grant No. 2024J01470, 2023J01350) and Dr Start Funding from Longyan University (LB2023009).References
- G. X. Li, Y. L. Li, H. B. Liu, Y. B. Guo, Y. J. Li and D. B. Zhu, Chem. Commun., 2010, 46, 3256–3258 RSC
.
- J. Kang, J. B. Li, F. G. Wu, S. S. Li and J. B. Xia, J. Phys. Chem. C, 2011, 115, 20466–20470 CrossRef CAS
.
- S. W. Cranford and M. Buehler, Mechanical properties of graphyne, Carbon, 2011, 49, 4111–4121 CrossRef CAS
.
- Y. Y. Zhang, Q. X. Pei and C. M. Wang, Appl. Phys. Lett., 2012, 101, 081909 CrossRef
.
- S. G. Pari, A. Cuéllar and B. M. Wong, J. Phys. Chem. C, 2016, 120, 18871–18877 CrossRef CAS
.
- R. Pietrzak, Fuel, 2009, 88, 1871–1877 CrossRef CAS
.
- R. H. Baughman, H. Eckhardt and M. Kertesz, J. Chem. Phys., 1987, 87, 6687–6699 CrossRef CAS
.
- N. Narita, S. Nagai, S. G. Suzuki and K. J. Nakao, Phys. Rev. B:Condens. Matter Mater. Phys., 1998, 58, 11009–11014 CrossRef CAS
.
- H. J. Cui, X. L. Sheng, Q. B. Yan, Q. R. Zheng and G. Su, Phys. Chem. Chem. Phys., 2013, 15, 8179–8185 RSC
.
- M. Q. Long, L. Tang, D. Wang, Y. L. Li and Z. G. Shuai, ACS Nano, 2011, 5, 2593–2600 CrossRef CAS PubMed
.
- N. Wang, J. J. He, K. Wang, Y. J. Zhao, T. G. Jiu, C. S. Huang and Y. L. Li, Adv. Mater., 2019, 31, 1803202 CrossRef CAS PubMed
.
- T. Wang and Z. L. Jin, Results Surf. Interfac., 2024, 14, 100188 CrossRef
.
- Y. P. Zheng, Y. H. Chen, L. H. Lin, Y. Y. Sun, H. B. Liu, Y. L. Li, Y. W. Du and N. J. Tang, Appl. Phys. Lett., 2017, 111, 033101 CrossRef
.
- M. Rein, N. Richter, K. Parvez, X. L. Feng, H. Sachdev, M. Kläui and K. Müllen, ACS Nano, 2015, 9, 1360–1366 CrossRef CAS PubMed
.
- H. F. Kang, B. C. Hua, L. Q. Xu, X. L. Zhan, Y. P. Zheng and Z. G. Huang, Carbon, 2021, 184, 526–533 CrossRef CAS
.
- H. Estrade-Szwarckopf, Carbon, 2004, 42, 1713–1721 CrossRef CAS
.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS
.
- H. H. Bao, L. Wang, C. Li and J. Luo, ACS Appl. Mater. Interfaces, 2018, 11, 2717–2729 CrossRef PubMed
.
- J. Li, X. Gao, B. Liu, Q. L. Feng, X. B. Li, M. Y. Huang, Z. F. Liu, J. Zhang, C. H. Tung and L. Z. Wu, J. Am. Chem. Soc., 2016, 138, 3954–3957 CrossRef CAS PubMed
.
- P. Nowicki, R. Pietrzak and H. Wachowska, Energy Fuels, 2010, 24, 1197–1206 CrossRef CAS
.
- K. Ihm, T. H. Kang, D. H. Lee, S. Y. Park, K. J. Kim, B. Kim, J. H. Yang and C. Y. Park, Surf. Sci., 2006, 600, 3729–3733 CrossRef CAS
.
- J. Liu, H. B. Liu, Y. L. Li, Y. P. Yi, X. K. Shang, S. S. Zhang, X. L. Yu, S. J. Zhang, H. B. Cao and G. J. Zhang, Nanoscale, 2014, 6, 11336–11343 RSC
.
- R. Pietrzak, Fuel, 2009, 88, 1871–1877 CrossRef CAS
.
- A. Actis, P. D. Paolo Fornasiero, P. D. Mario Chiesa and P. D. Enrico Salvadori, ChemPhotoChem, 2024, 8, e202300203 CrossRef CAS
.
- I. V. Ovsienko, T. A. Len, L. Yu. Matsuy, Yu. I. Prylutskyy, I. B. Berkutov, V. V. Andrievskii, Yu. F. Komnik, I. G. Mirzoiev, G. E. Grechnev, Yu. A. Kolesnichenko, R. Hayn and P. Scharff, Phys. Status Solidi B, 2015, 252, 1402–1409 CrossRef CAS
.
- L. Lin, J. Y. Li, Q. H. Yuan, Q. H. Li, J. C. Zhang, L. Z. Sun, D. G. Rui, Z. L. Chen, K. C. Jia, M. Z. Wang, Y. F. Zhang, M. H. Rummeli, N. Kang, H. Q. Xu, F. Ding, H. L. Peng and Z. F. Liu, Sci. Adv., 2019, 5, eaaw8337 CrossRef CAS PubMed
.
- F. Joucken, L. Henrard and J. Lagoute, Phys. Rev. Mater., 2019, 3, 110301 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07434c |
| This journal is © The Royal Society of Chemistry 2024 |