Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnetically nanorized seaweed residue for the adsorption of methylene blue in aqueous solutions†
Xinyi Yang‡
a,
Jingjing Liu‡a,
Xuejin Huanga,
Hemin Cuic,
Ligang Wei *a,
Guolin Shao
*a,
Guolin Shao a,
Xu Fu
a,
Xu Fu *ab,
Na Liua,
Qingda An
*ab,
Na Liua,
Qingda An ab and
Shangru Zhai
ab and
Shangru Zhai ab
ab
aSchool of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian 116034, China. E-mail: weilg@dlpu.edu.cn; fuxumail@163.com; Tel: +86 0411 86323726
bLiaoning Key Lab of Lignocellulose Chemistry and BioMaterials, Liaoning Collaborative Innovation Center for Lignocellulosic Biorefinery, Dalian Polytechnic University, Dalian Polytechnic University, Dalian 116034, China
cDalian Zhonghuida Scientific Instrument Co. Ltd, Dalian 116023, China
First published on 29th July 2024
Abstract
The cost-effective and green separation of dye pollutants from wastewater is of great importance in environmental remediation. Industrial seaweed residue (SR), as a low-cost cellulose source, was used to produce carboxylated nanorized-SR (NSR) via oxalic acid (OA)–water pretreatments followed by ultrasonic disintegration. Fourier transform infrared spectroscopy, X-ray polycrystalline diffraction, nitrogen isotherms, scanning electron microscopy, transmission electron microscopy, vibrating sample magnetometry, X-ray photoelectron spectrometry, particle charge detection, zeta potential and retro titration experiments were utilized to explore the physiochemical properties of samples. The NSRs with carboxyl content of 4.58–6.73 mmol g−1 were prepared using 10–60% OA–water pretreatment. In the case of 20% OA–water pretreatment, the highest NSR yield (73.9%) and nanocellulose content (80.2%) were obtained. Through self-assembly induced by the electrostatic interaction, magnetic NSR composite adsorbents (MNSRs) were prepared with the combination of NSR and Fe3O4 nanoparticles (NPs). The carboxylated NSR with negative charge demonstrated good affinity for Fe3O4 NPs. The Fe3O4 NPs were perfectly microencapsulated with the NSR when the NSR/Fe3O4 mass ratio was higher than 1/1. The adsorption properties of the MNSR for methylene blue (MB) removal from aqueous solution were investigated. The adsorbent with NSR/Fe3O4 mass ratio of 1/1 (MNSR1/1) exhibited optimum performance in terms of the magnetic properties and adsorption capacity. The MNSR1/1 showed high adsorption ability in a pH ≥7 environment. According to the Langmuir fitting, the maximum adsorption capacity of MNSR1/1 for MB reached 184.25 mg g−1. The adsorption of MB complies with the pseudo-second-order kinetic model. MNSR1/1 still maintained good adsorption properties after the fifth cycle of adsorption–desorption. MNSR1/1 could selectively adsorb cationic dye (i.e., MB and methyl violet) from wastewater, with hydrogen bonding and electrostatic interaction as the main force.
1. Introduction
With the rapid development of modern industries, water sources are currently faced with serious environmental problems. A large amount of pollutants, such as organic chemicals and heavy metal ions, exist in industrial wastewater.1 The dyes widely used in textiles, paper-making, leather, coating, printing, and dyeing industries are some of the main organic contaminants that need to be dealt with because they are threatening people's health.2,3 A series of methods have been proposed and employed to treat wastewater, including adsorption,4 membrane separation,5 chemical degradation,6 and electric/photocatalytic degradation.7 The adsorption processes are extensively used because of their advantages of simple operation, high efficiency, low cost, absence of secondary pollution, and zero alterations in the dye's chemical structure.8At present, the use of biomass in water treatment is attracting interest. Cellulose, the most abundant biopolymer on earth, is a remarkable raw biomass material offering wide availability, sustainability, good processability, and the possibility of surface modification.9 Nanostructured adsorbent materials with high specific area offer higher adsorption capacities and better binding affinities than precursors in the macroscale.10 The emergence of nanocellulose (NC) as a novel material with a wide range of potential applications has propelled its application as a new generation of bio-based adsorbents. Cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and bacterial cellulose are the main families of NC, but they differ in their production mode and morphologies.11
He et al.12 prepared CNCs with a high specific area (248 m2 g−1) through the hydrolysis of microcrystalline cellulose in 1 M ammonium persulfate. The adsorption capacity of the prepared CNCs for methylene blue (MB), a kind of cationic dye, reached 101 mg g−1. The Langmuir isotherm model is correlated with the data of MB adsorption on the CNCs, indicating the homogeneous nature of the CNC surface. Chan et al.13 prepared CNFs from a kenaf core via acid–chlorite pretreatment, followed by disintegration using a high-speed blender. The prepared CNFs were used for MB adsorption, and the maximum adsorption capacity was 122.2 mg g−1 under 20 °C and pH 9. Rapid adsorption equilibrium was achieved within a contact time of 1 min. The NC-based adsorbents demonstrated improved adsorption capacities toward dyes. However, the limitations of NC must be addressed before it is considered for real water treatment.14
One of the key challenges for the future growth and integration of NC as a new class of sustainable adsorbent for dye adsorption is adsorbent recovery.15 The adsorbents in powder or particle forms have to be recovered and reused in practical applications. A convenient collection method is required for the adsorbents. Magnetically modified adsorbents can be selectively, rapidly, and easily separated from a specific environment (i.e., wastewater) by using permanent magnets or magnetic separators.16–18 Recently, various magnetic lignocellulose composites have been employed for dye removal from aqueous solutions, such as microcrystalline cellulose, waste flax seeds, tea leaves, corn straw, bagasse, and sawdust.19 Zarei et al.20 prepared Fe3O4–CNC composites through the sol–gel method but investigated the uptake capacities of Hg(II) ions instead of dye adsorption. The abovementioned works allowed us to infer the applicability and availability of magnetic NC composites for dye adsorption, although the relevant information is limited.
Another key challenge for the development and applications of NC-based adsorbents is cost efficiency.15 The preparation of NC from low-cost wastes containing cellulose is an effective way to reduce cost and avoid high energy consumption. In China, a large number of seaweed residues (SRs) rich in cellulose (up to 50%) are produced after alginate extraction.21 SRs are commonly used as animal feeds or fertilizers. The price of SR is lower than that of microcrystalline cellulose or pulp. As the industrial waste of seaweed processing, the supply of SR is more consistent and abundant than that of woody and agricultural biomass. These above issues allow SR to be particularly suitable for the preparation of NC. The preparation of high value-added nanorized SR (NSR) is rarely reported in the literature,21 let alone the application of NSR in dye removal.
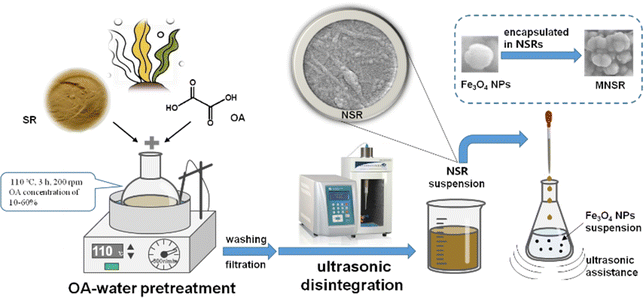
In this study, SR was nanorized through the pretreatment of oxalic acid (OA)–water, followed by ultrasonic disintegration. The obtained NSR could contain NC in the nanoscale and a small amount of undisintegrated SR blocks with size of 1 μm and above. Compared with concentrated mineral acid, OA is environmentally benign and can be easily recovered simply through commercially proven crystalliztaion processes; moreover, the strict equipment requirement for corrosion prevention is not a major concern in OA–water utilization.22 These issues mean that OA–water reagent can be applied as a green reaction for functional NC production. When using OA, the hydrolyzed cellulosic materials can be functionalized with carboxyl groups through Fisher–Speier esterification of one carboxyl group (Scheme S1 in ESI†), which improves the compatibility of materials and the binding with dye or metal ions.22
The effects of the OA content on the adsorption capacity of NSR for cationic dye MB were evaluated. MB is widely used in the textile industry.23 It is frequently studied in the research area as a model adsorbate of organic contaminants in aqueous solutions because of its high affinity for binding to solids.23–25 In this study, a novel magnetic nanocomposite, NSR–Fe3O4 (MNSR), was successfully synthesized by simple electrostatic interaction-induced aggregation with ultrasonic assistance. The resulting magnetic nanocomposite was then utilized for MB adsorption from an aquatic environment. To our knowledge, this is the first time to use MNSR nanocomposite for removing environmental contaminants. The structure, morphology, and properties of MNSR composites were evaluated by a series of measurements. The adsorption characteristics of MNSR for MB dye were investigated in terms of kinetics and thermodynamics, and possible adsorption mechanisms were also proposed.
2. Experimental
2.1 Materials
Magnetic Fe3O4 nanoparticles (NPs; 99.9% metal basis) were provided by Beesley New Materials (Su Zhou) Co., Ltd (China). SR was supplied by Qingdao Hisea Imp. & Exp. Co., Ltd (China). According to the analytical procedures (GB/T 5009.10-2003, GB/T 5009.4-2016, GB/T 14772-2008, GB/T 5750.4-2006, Chinese standard method), the contents of cellulose, ash, lipid, and water-soluble compounds were 65.0 ± 3.2%, 24.7 ± 2.8%, 3.9 ± 0.5%, and 6.6 ± 0.9% (weight percentage; used in subsequent values, unless otherwise stated), respectively, on dry basis. The two kinds of commercial NC samples were purchased from Beijing Weixian Nanomaterials Co. Ltd (NC-1) and Jinan Shengquan Group Share Holding Co., Ltd (NC-2), respectively. MB, methyl orange (MO), methyl violet (MV), and congo red (CR) were of analytical grade and supplied by Shanghai McLean Biochemical Co., Ltd. OA dihydrate (≥99.0%) and anhydrous ethanol (≥99.0%) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. Polydiallyldimethylammonium chloride (PDADMAC) with molecular weight of 100 kDa was supplied by Sigma-Aldrich Co., Ltd. Hydrochloric acid (HCl, 37%) and sulfuric acid (H2SO4, 98%) were purchased from Beijing Tongguang Fine Chemical Co., Ltd. Sodium hydroxide (NaOH, >99.5%) was provided by Tianjin Kemio Chemical Reagent Co., Ltd. Deionized (DI) water used in this study was manufactured by using a DW200 deionized water purifier (Shanghai Hetai Instrument Co., Ltd). All chemicals were used as received without further purification.2.2 Preparation of NSR
About 40 g of SR was added into a beaker containing 1 L of DI water. The mixture was mechanically stirred at room temperature at a speed of 200 rpm for 1 h and left to stand for 1 h. The upper liquid was poured out to remove the water-soluble substance. The above steps were repeated for five times. The obtained sediments were dried at 105 °C until a constant weight was reached. Finally, the washed SR was stored in a sealed bag for future use.The washed SR was pretreated with 10–60% OA aqueous solutions. A typical pretreatment procedure was as follows. Approximately 1.56 g of washed SR was mixed with OA aqueous solution in a three-necked flask, and the solid–liquid mass ratio was 1/80 w/w. Pretreatment was conducted with mechanical stirring (200 rpm) at 110 °C for 3 h. Subsequently, the pretreated SR was washed repetitively with DI water until the washing liquids were detected to be of neutral pH. The pretreated SR was disintegrated to obtain NSR by using an ultrasonic cell crusher (JY96-IIN, Shanghai Huxi Industrial Co., Ltd) under 1 kW and 30 min. The obtained suspension was centrifuged at 3000 rpm for 5 min after ultrasonic disintegration, and the suspension containing NC on the top was collected by removing the precipitate at the bottom. The NSR yield and NC content in NSR were determined using the following equations:
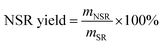 | (1) |
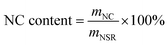 | (2) |
A series of NSRs was prepared with varying OA contents from 10% to 60%. To simplify the discussion, we devised a notation to indicate the OA content in the pretreatment mixture. For example, the NSR obtained with the OA content of 10% in the pretreatment system was named NSR10, and the resulting NSRs were sequentially named NSR20, NSR30, NSR40, NSR50, and NSR60. For comparison, NSR was also prepared by 5% H2SO4 pretreatment followed by ultrasonic disintegration, and the obtained NSR sample was labeled as NSR-SA.
2.3 Fabrication of MNSR materials
The MNSR preparation process is shown in Scheme 1. A series of MNSR samples was prepared by changing the NSR/Fe3O4 mass ratio of 3/1, 2/1, 1/1, 1/2, or 1/3 w/w, hereinafter referred to as MNSR3/1, MNSR2/1, MNSR1/1, MNSR1/2, and MNSR1/3, respectively. Taking the preparation process of MNSR1/1 as an example, Fe3O4 NPs (0.3 g) were added into a 250 mL conical flask with 30 mL of DI water and sonicated at 300 W and 40 kHz for 10 min to disperse Fe3O4 NPs uniformly in the water. About 100 mL of NSR suspension containing 0.3 g of NSR was added dropwise to the Fe3O4 dispersion in an ultrasonic environment. Thereafter, ultrasonic treatment continued for 10 min. The obtained MNSR1/1 composite materials were separated using an external magnetic force and subsequent high-speed centrifugation (8000 rpm and 10 min). Finally, MNSR1/1 was obtained by freeze-drying at −56 °C for 12 h. The composite process of commercial NC (NC-1 and NC-2) and Fe3O4 NPs was also investigated to compare the affinity of different kinds of NC and NSR samples.2.4 Characterization of NSR and MNSR
The method established by Wang et al.26 was used to measure the carboxylic function content per gram of NSR samples for retro titration. All the titrations were carried out in triplicates, and standard deviations were less than ±3.0%. The charge density of the samples was determined at pH 7 with a particle charge detector (PCD 04, BTG Mütek GmbH) using 0.001 M PDADMAC solution as the titrant, as explained elsewhere.27Fourier transform infrared spectrum (FTIR) characterization tests were conducted using the Shimadzu IR Tracer-100 instrument (Japan). The test samples were thoroughly ground, mixed with potassium bromide, and compressed into standard tablets. We selected a spectral range of 500–4000 cm−1 for testing and scanned the area 32 times. X-ray polycrystalline diffraction (XRD) analysis was conducted using a Shimadzu XRD-6100 instrument (Japan). Within the range of 10–80°, the Cu target radiation was 40 kV, the current was set to 40 mA, and the test was conducted at a scanning speed of 5° min−1. The specific surface area of the NSR samples was measured with the Barrett–Emmett–Teller (BET) N2 adsorption method using an Autosorb NOVA2200e volumetric analyzer (Quantachrome, USA).
The morphology and energy-dispersive X-ray spectra (EDS) of the samples were analyzed using an electronic thermal field emission scanning electron microscope (JSM-7800F, Japan), which was equipped with an energy spectrum scanner (X-Mas50, Oxford Instruments). The samples were sprayed with gold before testing. The size distribution of NC in the prepared NSR was determined statistically. A total of 100 samples were randomly selected from the SEM images of each NSR, and their sizes (length and diameter) were measured using Nano Measurer software. Transmission electron microscopy (TEM; Hitachi H-7600) was used to visualize the sample morphology, and it was operated at an accelerating voltage of 100 kV.
During the preparation of magnetic composites, the residual rate of Fe3O4 NPs was calculated to estimate the affinities of NSR and Fe3O4 NPs. The residual rate equals the mass percent of unutilized Fe3O4 NPs. The static magnetic properties of the Fe3O4 NPs and MNSR samples were characterized and tested using a Lake Shore 7404 vibrating sample magnetometer (VSM, USA). About 30–50 mg of the sample was accurately weighed, and the sample quality was recorded. Before testing, the system was calibrated in three steps: Gaussian meter calibration, magnetic moment cancellation, and magnetic moment gain.17 Calibration can eliminate errors caused by sample volume or placement. The surface charge state of Fe3O4 and MNSR was detected using a zeta potentiometer (Nano ZS, Malvern, the UK). The pH values of the solutions were measured by using a PHS-3C pH meter (Shanghai INESA and Scientific Instrument Co. Ltd).
The adsorption mechanism of MNSR was characterized using an ESCALAB Xi+ X-ray photoelectron spectrometer (XPS; Thermo Fisher Scientific, USA). About 15 mg of sample was used for tests under the following conditions: 100 eV full spectrum, 30 eV narrow spectrum, 0.05 eV step size, and residence time of 40–50 ms.
2.5 Adsorption characteristics of NSR and MNSR
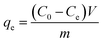 | (3) |
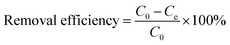 | (4) |
To study the effect of pH, we determined the adsorption of NSR and MNSR in MB aqueous solutions (100 mg L−1) with pH from 3 to 10. The pH was adjusted using the aqueous solutions of HCl or NaOH.
Pseudo first-order model:20
| qt = qe(1 − exp−k1) | (5) |
Pseudo second-order model:20
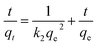 | (6) |
Langmuir model:18
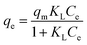 | (7) |
Freundlich model:18
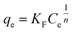 | (8) |
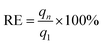 | (9) |
3. Results and discussion
3.1 Yield of NSR
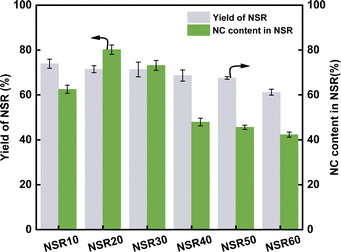
The yield of NSR prepared from SR is indicated in Fig. 1. The yield of NSR monotonously decreased from 73.9% to 61.2% with increasing OA content in pretreatment systems from 10% to 60%. The hydrolysis of cellulose in SR promoted by OA–water systems cleaved part of β-1,4-glycosidic linkages, which resulted in the breakup of cellulose chains.22 The addition of more OA in the pretreatment systems resulted in the release of more H+ ions, which improved the hydrolysis of cellulose. Although hydrolysis was favorable for the dissociation of cellulose fibers in the SR, a part of cellulose could be converted into reducing sugars, which resulted in the decrease in the NSR yields. In addition, SR is rich in ash (about 24.7%). The high OA content in the pretreatment systems could be favorable for the removal of ash from SR, thereby reducing the NSR yield.The NC content in NSR illustrates the nanorization degree of NSR, as shown in Fig. 1. With increasing OA content in pretreatment systems from 10% to 60%, the NC content first increased and then continuously decreased. In particular, at the high OA concentration (40–60%), the NC content in NSR decreased to 47.9–42.3%. The high OA concentration could lead to the excessive hydrolysis of SR cellulose, which contributed to the decrease in NSR–NC production. Moreover, the high OA concentration could favor the cross-linking of cellulose fragments through esterification30. The highest NC content of 80.2% was obtained when the SR was pretreated with the 20% OA–water system. This result suggested that NSR20 had a high nanorization degree.
3.2 Characterization of NSR
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching (1734 cm−1) and carboxyl (C
O) stretching (1734 cm−1) and carboxyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching (1624 cm−1) of the NRS samples showed a slight increase, which could be due to the esterification reaction between cellulose and OA. In particular, this reaction was one of the reasons for the change in the type of –OH groups.
O) stretching (1624 cm−1) of the NRS samples showed a slight increase, which could be due to the esterification reaction between cellulose and OA. In particular, this reaction was one of the reasons for the change in the type of –OH groups.3.3 Adsorption characteristics of NSR
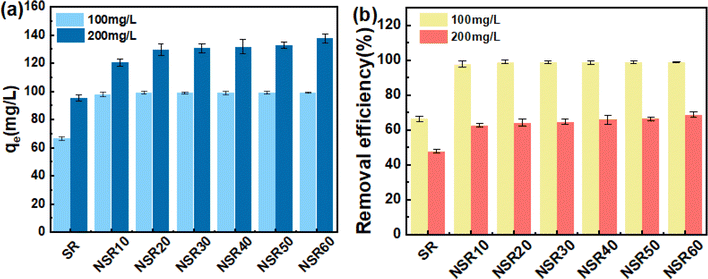
The equilibrium dye uptake of SR and the prepared NSRs under MB dye contents of 100 and 200 mg L−1 is indicated in Fig. 2. qe of the NSR samples was clearly higher than that of SR. For example, at an MB content of 200 mg L−1, qe of NSRs was 125.42–137.57 mg g−1, whereas that of SR was only 95.35 mg g−1 (Fig. 2a). The –OH groups on cellulose materials were the main functional active sites for MB adsorption through the formation of hydrogen bonds and electrostatic interaction.35 More –OH groups on NSR could be exposed after nanorization compared with that on SR. In addition, as shown in Table S1,† the measured SBET values of NSR samples were 57.60 (NSR10), 48.89 (NSR20), 43.54 (NSR30), 62.03 (NSR40), 58.84 (NSR50), and 57.38 m2 g−1 (NSR60), which were commonly higher than that of SR (36.91 m2 g−1). The disassociation of cellulose fiber bundles in SR resulted in a high specific surface area of NSR, which favored MB dye adsorption.At an MB content of 100 mg L−1, we found no clear difference among the prepared NSR samples for qe (around 98 mg g−1), but the removal efficiency exceeded 98% (Fig. 2b). This result indicated that MB in the aqueous solutions had nearly been completely adsorbed by the NSRs. When the MB content increased to 200 mg L−1, the removal efficiency of 62.71–68.79% was obtained for all the NSRs (Fig. 2b). The prepared NSRs were favorable for MB removal at a low dye content (i.e., 100 mg L−1).
Notably, no obvious difference in qe was observed at an MB content of 200 mg L−1. For example, the qe values of NSR10 and NSR20 were 132.76 and 137.57 mg g−1, respectively, whereas those of NSR50 and NSR60 were 128.36 and 131.77 mg g−1, respectively. The adsorption capacity of NSR samples was not correlated with the size distribution of NSR-NC (Fig. S4†) and carboxyl group content. It could be attributed to the complexity of influencing factors of MB adsorption on the NSRs, including active sites, NC content, and microstructure.
The NSR material used in preparing MNSR was selected after evaluating the cost of OA utilization and its adsorption capacity. The adsorption capacity of NSR20 was not lower than that of other NSR samples (Fig. 2). Moreover, the yield of NSR20 was higher than that of other NSR samples (Fig. 1). A low OA content (20%) in the pretreatment system was used in the preparation process, which provided an opportunity to obtain NSR20 materials at low cost. A high NC content in NSR20 was also favorable for the encapulsation of Fe3O4 NPs. Therefore, NSR20 was chosen as the material to prepare MNSRs in subsequent experiments.
3.4 Characterization of MNSR
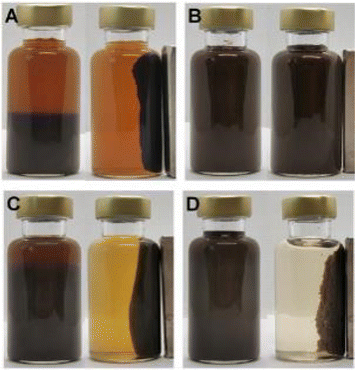 | ||
| Fig. 3 Images of magnetic composite materials prepared from Fe3O4 NPs and NC-1 (A), NC-2(B), NSR-SA5 (C), and NSR20 (D). | ||
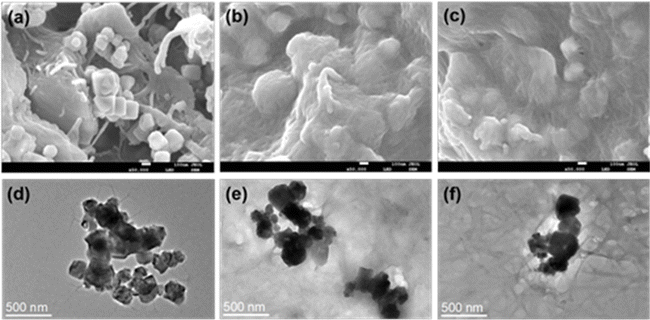
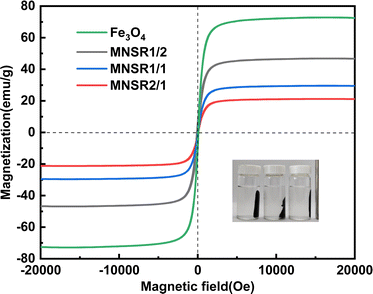
As shown in Fig. 3, NSR20 had the best affinity with Fe3O4 NPs compared with the other materials. For the above used materials, the density of surface charge was in the following order (Table S2†): NC-2 (−13 ± 2 μeq. g−1) < NC-1 (−42 ± 5 μeq. g−1) < NSR-SA (−87 ± 3 μeq. g−1) < NSR-20 (−350 ± 12 μeq. g−1). The carboxylation of NSR20 through the esterification of OA enhanced negative electricity, which was favorable for the electrostatic interactions between NSR20 and Fe3O4 NPs with positive charge. In addition, the affinities of NSR20 and NSR-SA prepared from the SR were better than those of NC-1 and NC-2 derived from other cellulose sources, which could be due to the formation of charged functional groups in SR during seaweed processing. Thus, SR has application potential in the preparation of composite materials.
3.5 Adsorption behavior of MNSRs
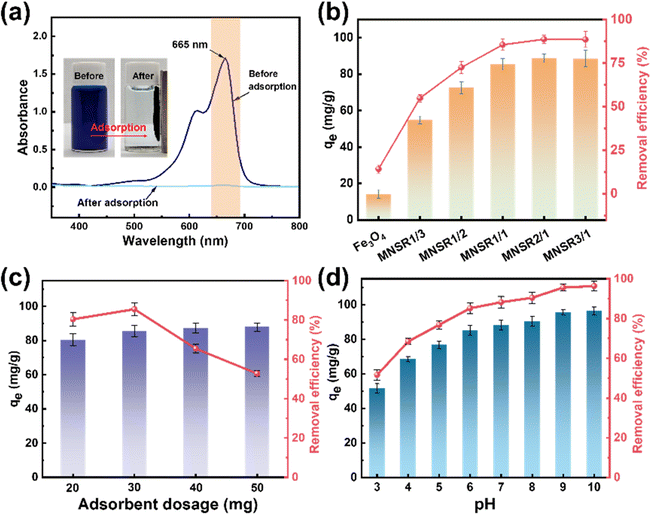 | ||
| Fig. 6 Images of UV-vis spectra before and after adsorption (a), effects of adsorbent type (b), dosage of MNSR1/1 (c), and pH (d) on qe and the removal efficiency for MB adsorption. | ||
When the mass ratio of NSR/Fe3O4 was raised from 1/3 to 1/1, qe increased from 54.89 mg g−1 (MNSR13) to 85.48 mg g−1 (MNSR1/1), as shown in Fig. 6a. However, when the mass ratio of NSR/Fe3O4 continued to increase to 2/1 or 3/1, qe and the removal efficiency of MNSR (MNSR2/1 or MNSR3/1) almost did not increase (∼88 mg g; Fig. 6a). This finding may be due to the complete encapsulation of Fe3O4 NPs by the NSR20 aggregates. The superposition of NSR fibrils limited the number of active sites where adsorption occurred. Moreover, the magnetic property of MNSR1/1 was stronger than that of MNSR2/1 (Fig. 5), which favored the recovery and recycling of MNSR. Thus, MNSR1/1 was selected in the following experiments.
As shown in Fig. 6b, as the dosage of MNSR1/1 increased from 20 mg to 30 mg, qe increased from 80.39 mg g−1 to 85.48 mg g−1, but then it sharply decreased to 65.44 mg g−1 and 52.73 mg g−1 at the adsorbent dosage of 40 or 50 mg. The MB removal efficiency of 85.5–87.8% was achieved at the MNSR1/1 dosage of 30–50 mg, suggesting that MB adsorption reached the saturation state. Unfortunately, under the experimental conditions, the complete removal of MB from the aqueous solutions seemed to be impossible because of the limitations of the adsorption–desorption equilibrium of MNSR1/1.
The pH of aqueous solutions influences the surface charge of the adsorbent and causes variations in the adsorption capacity for the dye.37 Fig. 6c shows the variation tendency of qe and removal efficiency of MB dye (100 mg L−1, initial pH 6.6) with varying pH from 3 to 10. As the pH of the system increased (pH 3–10), qe and the removal efficiency of MB by MNSR1/1 monotonously increased. At pH 10, the maximum removal efficiency of MNSR1/1 (96.3%) was achieved. Under acidic conditions (pH 3–5), the removal efficiency (51.6–76.8%) was relatively low due to protonation and electrostatic repulsion. The active sites on the adsorbent are susceptible to protonation, resulting in their electropositive nature. In this work, pH 6 was deemed close to the initial pH of MB aqueous solution. The decrease in the H+ concentration led to a decrease in the protonation degree of the active sites on the adsorbent, which weakened the electrostatic repulsion effects. This phenomenon was advantageous for capturing MB via mutual electrostatic attraction, thereby improving the adsorption capacity. The significant increase in the removal efficiency of MB dye by MNSR1/1 at pH 7–8 was due to the enhanced electronegativity of surface charges under alkaline conditions, which facilitated the adsorption of cationic dyes.37 At pH 9 or 10, the removal efficiency of MB peaked (95.6% or 96.3%, respectively), possibly due to the saturation of negatively charged active sites on the surface of cellulose. These results indicated that the alkaline conditions were strongly favorable for the adsorption of MB by MNSR1/1.
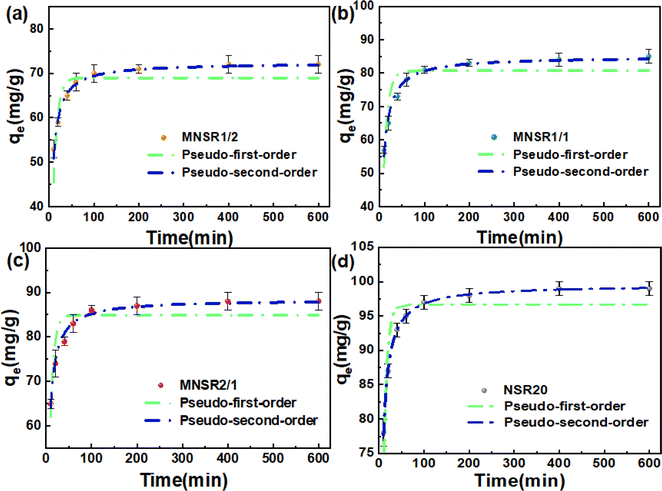
| Adsorbents | Pseudo-first-order model | Pseudo-second-order model | ||||||
|---|---|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | AIC | qe (mg g−1) | K2 (g mg−1 min−1) | R2 | AIC | |
| MNSR1/2 | 68.97 | 0.108 | 0.739 | 74.19 | 72.35 | 0.003 | 0.985 | 24.45 |
| MNSR1/1 | 80.69 | 0.103 | 0.807 | 50.43 | 84.96 | 0.002 | 0.984 | 29.13 |
| MNSR2/1 | 84.89 | 0.131 | 0.811 | 35.70 | 88.42 | 0.003 | 0.990 | 15.51 |
| NSR20 | 96.70 | 0.132 | 0.752 | 60.39 | 99.54 | 0.004 | 0.999 | 13.95 |
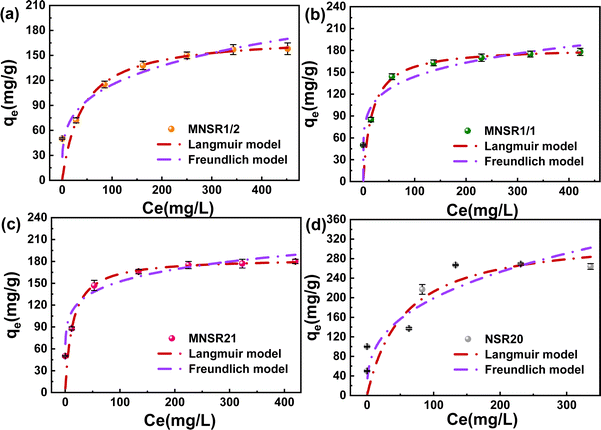
| Materials | Langmuir isotherm | Freundlich isotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (mg L−1) | R2 | AIC | KF (mg g−1) | n | R2 | AIC | |
| MNSR1/2 | 173.67 | 0.024 | 0.998 | 12.63 | 34.59 | 3.840 | 0.921 | 45.69 |
| MNSR1/1 | 184.25 | 0.059 | 0.998 | 12.64 | 62.29 | 5.533 | 0.790 | 66.68 |
| MNSR2/1 | 184.50 | 0.075 | 0.999 | 7.88 | 76.10 | 6.636 | 0.830 | 64.73 |
| NSR20 | 330.49 | 0.018 | 0.648 | 72.60 | 40.36 | 2.890 | 0.953 | 36.37 |
The maximum adsorption capacity (qmax) of MNSR1/1 for MB reached 184.25 mg g−1 qmax of MNSR1/1 for MB adsorption was compared with other adsorbents that have been reported in the literature, which further evaluated the adsorption performance of MNSR1/1 (Table S3†). The research suggested that MNSR1/1 can effectively remove MB dye from the aqueous solution at a initial pH at 25 °C. Thus, MNSR1/1 is a promising adsorbent for wastewater purification.
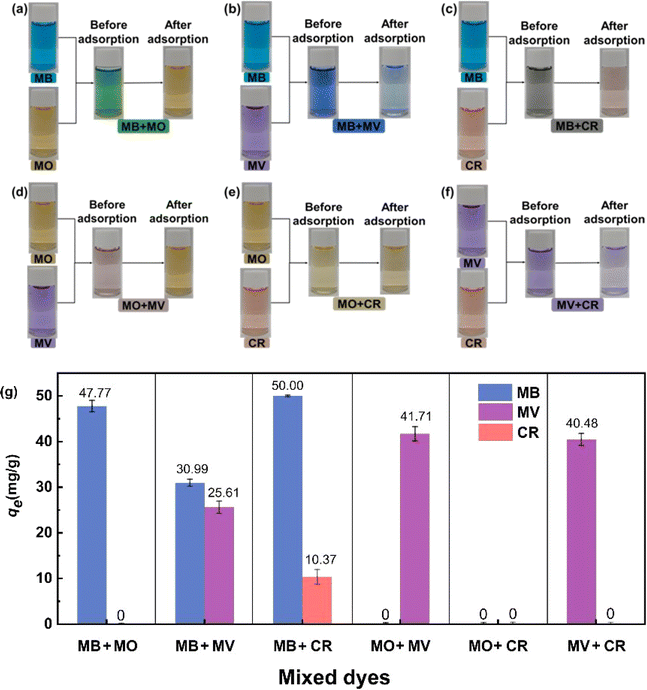
As shown in Fig. 9g, qe for adsorbing cationic dyes (MB and MV) was significantly superior to that for adsorbing anionic dyes (MO and CR) in these coexisting dye mixtures. This finding confirmed that MNSR1/1 exhibited the selective adsorption capacity for cationic dyes (i.e., MB and MV) from the aqueous solutions. This phenomenon could be caused by the negative charge on the surface of MNSR1/1, which was consistent with the results of zeta potential analysis. MNSRs exert electrostatic attraction on positively charged cationic dyes and electrostatic repulsion on negatively charged anionic dyes.43
As shown in Fig. S9,† the type of desorption agents (0.05 M HCl or ethanol) influenced the RE values of MNSR1/1, which may be due to the differences in their desorption role. After the fifth cycle of adsorption–desorption experiment, the RE with 0.05 M HCl as desorption agent was 82.71%, whereas that with ethanol as desorption agent was 77.62%. Thus, the desorption capacity of 0.05 M HCl was better than that of ethanol for MNSR1/1. The recycling experimental results suggested that the stability of MNSR1/1 could be utilized repeatedly under practical situations for the treatment of wastewater.
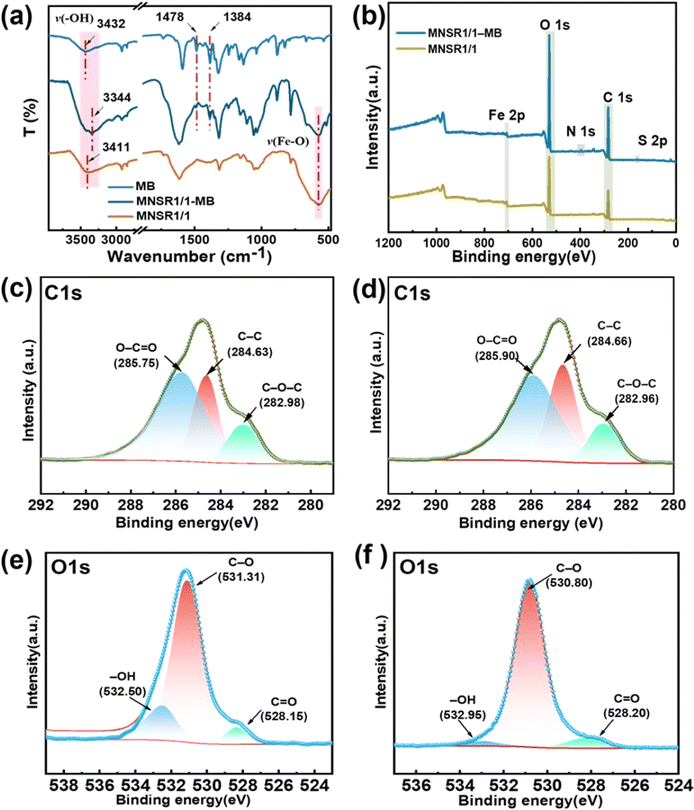
3.6 Possible adsorption mechanism
The MNSR1/1 samples before and after adsorption were characterized by EDS, FTIR, and XPS to explore the possible adsorption mechanism. Fig. S10† displays the detection results for the distribution of elements in MNSR1/1. According to the spectra, the elements C, O, and Fe coexisted in the sample, which indicated that the composites of NSR20 and Fe3O4 NPs were successfully fabricated. Meanwhile, the elemental mapping of S and N could be observed in the recovered MNSR1/1 after adsorption, thereby proving that MB was captured by MNSR1/1.The FTIR spectra of MNSR1/1 before and after adsorption are shown in Fig. 10a. The new absorbance peaks at 1478 and 1384 cm−1 were attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S+ and C
S+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration peaks of MB,43,44 respectively. This finding confirmed that MB molecules were bound with MNSR1/1. The peak of the hydroxyl group (–OH) of cellulose in the spectra of MNSR samples before adsorption shifted from 3423 cm−1 to 3440 cm−1 in the spectra of MNSR1/1 and NSR20 after adsorption (labeled as MNSR1/1-MB and NSR20-MB). This result was possibly due to the hydrogen bonding and electrostatic attraction between MB and –OH groups in MNSR1/1.45
N stretching vibration peaks of MB,43,44 respectively. This finding confirmed that MB molecules were bound with MNSR1/1. The peak of the hydroxyl group (–OH) of cellulose in the spectra of MNSR samples before adsorption shifted from 3423 cm−1 to 3440 cm−1 in the spectra of MNSR1/1 and NSR20 after adsorption (labeled as MNSR1/1-MB and NSR20-MB). This result was possibly due to the hydrogen bonding and electrostatic attraction between MB and –OH groups in MNSR1/1.45
The XPS full spectra (Fig. 10b) confirmed the presence of N1s (396.28 eV) and S2p (160.78 eV) as new elemental peaks after adsorption, indicating successful MB adsorption. For the C 1s spectra, MNSR1/1 (Fig. 10c) had three peaks at 285.75, 284.63, and 282.98 eV corresponding to the bonds of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–C, and C–O–C, respectively.46 The bonding energy of O–C
O, C–C, and C–O–C, respectively.46 The bonding energy of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifted noticeably after adsorption, moving from 285.75 eV to 285.95 eV. This change indicated that an electrostatic interaction occurred between the COO− and MB molecules in the adsorbent.47 The peaks of C–C and C–O–C also shifted from 284.63 eV to 284.70 eV and from 282.98 eV to 282.95 eV, respectively, which were influenced by the interaction between MB and MNSR1/1. In the O1s spectra (Fig. 10e and f), after adsorption, the bonding energy of –OH shifted from 532.50 eV to 532.95 eV, and the bonding energy of C
O shifted noticeably after adsorption, moving from 285.75 eV to 285.95 eV. This change indicated that an electrostatic interaction occurred between the COO− and MB molecules in the adsorbent.47 The peaks of C–C and C–O–C also shifted from 284.63 eV to 284.70 eV and from 282.98 eV to 282.95 eV, respectively, which were influenced by the interaction between MB and MNSR1/1. In the O1s spectra (Fig. 10e and f), after adsorption, the bonding energy of –OH shifted from 532.50 eV to 532.95 eV, and the bonding energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carboxyl groups shifted from 528.15 eV to 528.20 eV. Both peaks were noticeably smaller. This change indicated that oxygen functional groups (–OH and COO−) were the main adsorption functional groups. The shift in these groups was also attributed to the electron transfer between MB molecules and the oxygen functional groups (–OH and COO−) of MNSR1/1, which was consistent with the results shown in Fig. 10c and d.
O in the carboxyl groups shifted from 528.15 eV to 528.20 eV. Both peaks were noticeably smaller. This change indicated that oxygen functional groups (–OH and COO−) were the main adsorption functional groups. The shift in these groups was also attributed to the electron transfer between MB molecules and the oxygen functional groups (–OH and COO−) of MNSR1/1, which was consistent with the results shown in Fig. 10c and d.
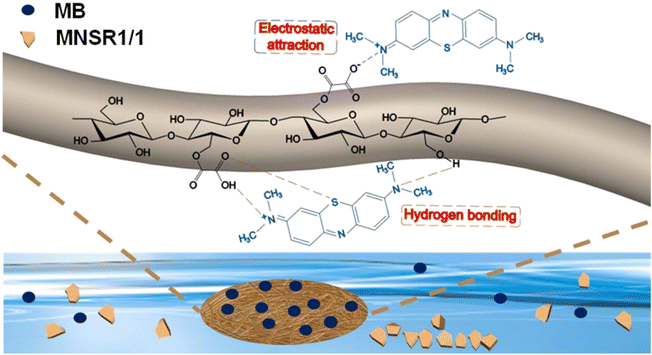
Therefore, Fig. 11 illustrates the adsorption mechanism and interactions. The adsorption mechanism of MNSR1/1 mainly resulted from the combined action of electrostatic attraction and hydrogen bonding. The oxygen functional groups in MNSR1/1 served as the main active sites for MB adsorption. In particular, the negatively charged oxygen atoms in the carboxyl group contributed to hydrogen bonding and electrostatic interactions between the adsorbent and MB.
4. Conclusions
The carboxylated NSR was prepared via OA–water pretreatments followed by ultrasonic disintegration. The yield (73.9%) and nanocellulose content (80.2%) of NSR20 were highest among the obtained NSR samples. With increasing OA content from 10% to 60%, the carboxyl content and surface negative charge of NSR samples monotonously increased, thereby improving their affinity. The MNSR adsorbents were prepared with NSR20 and Fe3O4 NPs via a facile self-assembly method. Comprehensive characterization of the MNSR nanocomposite was performed utilizing various techniques, such as FTIR, XRD, SEM, TEM, and XPS. The MNSRs could selectively adsorb the cationic dye of MB from wastewater via hydrogen bonding and electrostatic interactions. MNSR1/1 is considered a useful biosorbent for environmental remediation. The adsorption capacity of MNSR1/1 without any modification was so low that it was only employed in the removal of ationic dye from a low dye content of wastewater (i.e., 100 mg L−1). In future work, the chemical modification of MNSR will be conducted to improve the adsorption capacity, i.e., amination or carboxylation, because NSR on the surface of MNSR is rich in –OH groups. This work reveals the potential application of MNSR as a dye adsorbent due to its biodegradation, reusability, and low cost.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Xinyi Yang: conceptualization, methodology, software, formal analysis, writing – original draft, investigation; Jingjing Liu: conceptualization, methodology, software, formal analysis; Xuejin Huang: methodology; Hemin Cui: conceptualization; Ligang Wei: writing – review & editing, supervision; Guolin Shao: supervision, investigation; Xu Fu: conceptualization, writing – review & editing, project administration; Na Liu: supervision, investigation; Qingda An: investigation, visualization; Shangru Zhai: investigation, visualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support is gratefully acknowledged from the National Natural Science Foundation of China (22078023); Liaoning Revitalization Talents Program (XLYC1902037); Scientific Research Project of Department of Education of Liaoning Province of China (J2020013, LJKFZ20220212).Notes and references
- N. N. Tran, M. Escribà-Gelonch, M. M. Sarafraz, Q. H. Pho, S. Sagadevan and V. Hessel, Ind. Eng. Chem. Res., 2023, 62, 1195–1214 CrossRef CAS.
- K. Roa, E. Oyarce, A. Boulett, M. Alsamman, D. Oyarzún, G. D. C. Pizarro and J. Sánchez, Sustainable Mater. Technol., 2021, 29, e00320 CrossRef CAS.
- V. Katheresan, J. Kansedo and S. Y. Lau, J. Environ. Chem. Eng., 2018, 6, 4676–4697 CrossRef CAS.
- X. He, C. Ma, Z. Wang and M. Zhang, ChemistrySelect, 2023, 8, e202203701 CrossRef CAS.
- H. J. Tanudjaja, C. A. Hejase, V. V. Tarabara, A. G. Fane and J. W. Chew, Water Res., 2019, 156, 347–365 CrossRef CAS PubMed.
- S. Wang and J. Wang, Chemosphere, 2022, 287, 132365 CrossRef CAS PubMed.
- S.-W. Lv, Y. Cong, X. Chen, W. Wang and L. Che, Chem. Eng. J., 2021, 433, 133605 CrossRef.
- M. A. Ahmed and A. A. Mohamed, Inorg. Chem. Commun., 2022, 148, 110325 CrossRef.
- Z. Jiang, S.-H. Ho, X. Wang, Y. Li and C. Wang, Environ. Pollut., 2021, 290, 118087 CrossRef CAS PubMed.
- M. Adel, M. A. Ahmed, M. A. Elabiad and A. A. Mohamed, Environ. Nanotechnol., Monit. Manage., 2022, 18, 100719 CAS.
- M. N. F. Norrrahim, N. A. M. Kasim, V. F. Knight, M. S. M. Misenan, N. Janudin, N. A. A. Shah, N. Kasim, W. Y. W. Yusoff, S. A. M. Noor, S. H. Jamal, K. K. Ong and W. M. Z. W. Yunus, RSC Adv., 2021, 11, 7347–7368 RSC.
- X. He, K. B. Male, P. N. Nesterenko, D. Brabazon, B. Paull and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2013, 5, 8796–8804 CrossRef CAS PubMed.
- C. H. Chan, C. H. Chia, S. Zakaria, M. S. Sajab and S. X. Chin, RSC Adv., 2015, 5, 18204–18212 RSC.
- A. Qiao, M. Cui, R. Huang, G. Ding, W. Qi, Z. He, J. J. Klemeš and R. Su, Carbohydr. Polym., 2021, 272, 118471 CrossRef CAS PubMed.
- N. Mahfoudhi and S. Boufi, Cellulose, 2017, 24, 1171–1197 CrossRef CAS.
- R. Sivashankar, A. B. Sathya, K. Vasantharaj and V. Sivasubramanian, Environ. Nanotechnol., Monit. Manage., 2014, 1–2, 36–49 Search PubMed.
- I. Hotan Alsohaimi, M. S. Alhumaimess, A. Abdullah Alqadami, G. Tharwi Alshammari, R. Fawzy Al-Olaimi, A. A. Abdeltawab, M. Y. El-Sayed and H. M. Hassan, Inorg. Chem. Commun., 2023, 147, 110261 CrossRef CAS.
- I. H. Alsohaimi, M. S. Alhumaimess, A. A. Alqadami, H. M. A. Hassan, Q. Chen, M. S. Alamri, M. M. J. Alanzi and T. S. Alraddadi, Chem. Eng. Sci., 2023, 280, 119017 CrossRef CAS.
- I. Safarik, E. Baldikova, J. Prochazkova, M. Safarikova and K. Pospiskova, J. Agric. Food Chem., 2018, 66, 2538–2552 CrossRef CAS PubMed.
- S. Zarei, M. Niad and H. Raanaei, J. Hazard. Mater., 2018, 344, 258–273 CrossRef CAS PubMed.
- Z. Liu, X. Li, W. Xie and H. Deng, Carbohydr. Polym., 2017, 173, 353–359 CrossRef CAS PubMed.
- W. Wang, N. Sun, Z.-s. Cai, K. Sun, F. Gu, Y. Jin and H. Xiao, BioResources, 2019, 15, 1014–1025 Search PubMed.
- A. M. Aldawsari, I. H. Alsohaimi, H. M. A. Hassan, M. R. Berber, Z. E. A. Abdalla, I. Hassan, E. A. M. Saleh and B. H. Hameed, J. Saudi Chem. Soc., 2020, 24, 693–703 CrossRef CAS.
- A. M. Aldawsari, I. Alsohaimi, H. M. A. Hassan, Z. E. A. Abdalla, I. Hassan and M. R. Berber, J. Alloys Compd., 2021, 857, 157551 CrossRef CAS.
- H. M. A. Hassan, M. R. El-Aassar, M. A. El-Hashemy, M. A. Betiha, M. Alzaid, A. N. Alqhobisi, L. A. Alzarea and I. H. Alsohaimi, J. Mol. Liq., 2022, 361, 119603 CrossRef CAS.
- S. Wang, W. Linping, W. Kong, J. Ren, C. Liu, K. Wang, R. Sun and D. She, Cellulose, 2013, 20, 2091–2100 CrossRef CAS.
- S. M. Notley and M. Norgren, Biomacromolecules, 2008, 9, 2081–2086 CrossRef CAS PubMed.
- A. Kaushal and S. K. Singh, Appl. Water Sci., 2017, 7, 3191–3196 CrossRef CAS.
- M. Radjai, H. Ferkous, Z. Jebali, H. Majdoub, R. Bourzami, G. Raffin, M. Achour, A. Gil and M. Boutahala, J. Mol. Liq., 2022, 361, 119670 CrossRef CAS.
- S. Song, D. Su, X. Xu, X. Yang, L. Wei, K. Li, G. Shao, Q. An, S. Zhai and N. Liu, Ind. Crops Prod., 2023, 200, 116881 CrossRef CAS.
- D. Li, J. Henschen and M. Ek, Green Chem., 2017, 19, 5564–5567 RSC.
- R. VARMA, A. PRTIHAR, N. PASUMPON and S. VASUDEVAN, Cellul. Chem. Technol., 2022, 56, 949–956 CrossRef CAS.
- C. Du, M. Liu, B. Li, H. Li, Q. Meng and H. Zhan, Bioresearch, 2016, 11, 4017–4024 CAS.
- B. Chen, Y. Cao, H. Zhao, F. Long, X. Feng, J. Li and X. Pan, J. Hazard. Mater., 2020, 392, 122263 CrossRef CAS PubMed.
- J. Hao, M. Zhou, S. Zhou, A. Zhang, X. Pang, J. Sun and X. Qiao, Colloids Surf., A, 2021, 616, 126312 CrossRef CAS.
- J. Wang, L. Wei, Y. Ma, K. Li, M. Li, Y. Yu, L. Wang and H. Qiu, Carbohydr. Polym., 2013, 98, 736–743 CrossRef CAS PubMed.
- M. A. Abdel-Khalek, M. K. A. Rahman and A. A. Francis, J. Environ. Chem. Eng., 2017, 5, 319–327 CrossRef CAS.
- W. Tan, X. Wu, W. Liu, F. Ye and S. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 4352–4363 CrossRef CAS PubMed.
- F. Shafiq, C. Liu, H. Zhou, H. Chen, S. Yu and W. Qiao, Colloids Surf., A, 2023, 672, 131713 CrossRef CAS.
- Y. Zeng, X. Liu, Y. Zhang, Y. Qin, X. Tang, W. Zhang and L. Zhang, New J. Chem., 2023, 47, 6342–6352 RSC.
- M. Garg, N. Bhullar, B. Bajaj and D. Sud, New J. Chem., 2021, 45, 4938–4949 RSC.
- B. Chen, Y. Liu, S. Chen, X. Zhao, W. Yue and X. Pan, Environ. Sci.: Nano, 2016, 3, 670–681 RSC.
- M. I., M. Shahid, H. A. M. Saleh, K. M. A. Qasem and M. Ahmad, CrystEngComm, 2020, 22, 3891–3909 RSC.
- O. V. Ovchinnikov, A. V. Evtukhova, T. S. Kondratenko, M. S. Smirnov, V. Y. Khokhlov and O. V. Erina, Vib. Spectrosc., 2016, 86, 181–189 CrossRef CAS.
- Z. Liu, M. Azharul Islam and J. Huang, J. Mol. Liq., 2022, 366, 120273 CrossRef CAS.
- X. Wang, X. Fan, H. Xie, X. Li and C. Hao, Cellulose, 2022, 29, 483–501 CrossRef CAS.
- W. Chen, H. Ma and B. Xing, Int. J. Biol. Macromol., 2020, 158, 1342–1351 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04416a |
| ‡ These two authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2024 |