Open Access Article
Open Access ArticleEnhancing the stereoselectivity of Me2GaOR(NHC) species in the ring-opening polymerization of rac-lactide, with the help of the chelation effect†
Paweł Horeglad *,
Anna Rola-Noworyta,
Dawid Tuszyński,
Iga Fabianowska,
Natalia Agnieszka Marek,
Patrycja Gładysz,
Ireneusz Wielgus and
Anna Maria Dąbrowska
*,
Anna Rola-Noworyta,
Dawid Tuszyński,
Iga Fabianowska,
Natalia Agnieszka Marek,
Patrycja Gładysz,
Ireneusz Wielgus and
Anna Maria Dąbrowska
Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, Warsaw, 00-664, Poland. E-mail: Pawel.Horeglad@pw.edu.pl; Tel: +48 22 2345076
First published on 9th September 2024
Abstract
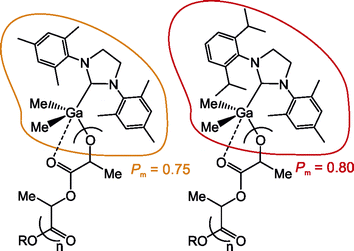
Using dialkylgallium alkoxides with N-hetrocyclic carbenes (Me2GaOR(NHC)) for the ring-opening polymerization of rac-lactide, we have demonstrated the effect of the chelate interaction between the growing PLA chain and gallium on the stereoselectivity of dialkylgallium alkoxide propagating species – Me2Ga(OPLA)(NHC). In order to do so, we have conducted the structure–activity studies of both Me2Ga(OCH2CH2OMe)(NHC) (NHC = SIMes (1) and IMes (2)) and Me2Ga(OCH(Me)CO2Me)(NHC) (NHC = SIMes (3) and IMes (4)), the latter mimicking active species in the ROP of lactide with growing PLA chain. Based on VT NMR and FTIR spectroscopy, the effect of toluene, CH2Cl2 and THF on the structure of 3 and 4 have been demonstrated, especially with regard to the interaction of methyl lactate ligand with gallium. In a combination with the latter, the studies on the activity of 1 and 2 in the ROP of rac-LA, in different solvents, and at temperatures between −40 °C and 40 °C, have shown the extent of the chelation effect on the isoselectivity of Me2Ga(OPLA)(NHC) in the ROP of rac-LA, which varied between Pm of 0.75 and 0.89 depending on the polymerization conditions. Both the latter, and the contribution resulting from the structure of Me2Ga(OPLA)(NHC) (Pm = 0.75) have been decisive for the total isoselectivity observed under specific conditions. Our finding represents the first evidence demonstrating that the chelation effect, resulting from the weak interaction between the growing PLA chain and the metal centre, can be responsible for the enhancement of stereoselectivity in the ROP of rac-LA with metal alkoxide propagating species. It should remain of interest, especially in the case of metal based catalysts, which are able to carry out the stereoselective polymerization of rac-LA at mild conditions, under which the chelation effect can manifest itself.
1. Introduction
For over two decades, scientists have focused on the structure-activity studies of well-defined metal complexes in the ring-opening polymerization (ROP) of lactide, as well as other cyclic esters.1,2 Such an approach has resulted in the extensive understanding of the relationship between the structure of metal alkoxide propagating species and their catalytic properties, which has allowed for the rational design of new catalysts in order to tune the structure and properties of polylactide (PLA) and its copolymers.1–3 Notably, among catalytic properties of metal complexes, the precise control over the stereoselectivity in the ROP of rac-LA,1c,f which strongly affects the stereostructure and properties of the resulting PLA,4 as well as their activity towards different cyclic esters,5 still remain main challenges. On the other hand, with regard to the elements of the structure of metal alkoxide propagating species, which can affect the catalytic properties in the ROP of lactide, the chelation effect resulting from the interaction between the growing polylactide chain and the metal centre (Scheme 1) remains barely described. It was considered based on kinetic studies of the ROP of lactide and ε-caprolactone with aluminium alkoxides, as well as the structure of propagating species.6 With regard to the latter, the studies which showed a considerable role of the chelation effect on the activity of dialkylaluminium alkoxides in the ROP of rac-LA and ε-CL allowed for the X-ray characterization of [Me2Al(μ-(OCH(Me)C(O))2OCH2CH2OMe)]2 resulting from the insertion of lactide into the Al–O bond of [Me2Al(μ-OCH2CH2OMe)]2.7 The only other X-ray structure of species resulting from the insertion of lactide into M–O bonds has been reported by Dagorne et al. for cationic aluminium complexes.8 Additionally, a number of well-defined metal lactate derivatives of Mg,9 Al,10 Ca,11 Sc,12 Zn,9a,13 Ga,14 Y,15 In,14c,16 Sn17 and Bi,18 which are considered to mimic metal alkoxide propagating species in the ROP of lactide, have been reported and used as catalysts in the polymerization studies. In all these cases the intermolecular chelate interaction between the lactate ligand and the metal has been evidenced. Moreover, this interaction has been also considered in the DFT studies on the insertion of lactide into M–OR bond.9b,17b,c,19 However, with regard to the huge number of reported metal based catalysts for the ROP of lactide, the chelation effect in the ROP of lactide, and other cyclic esters, has not been essentially discussed. It is most probably due to the fact that this interaction hasn't necessarily had to manifest its effect on the catalytic properties of metal alkoxide propagating species, which have typically shown a considerable activity in the ROP of lactide mostly at the relatively high temperatures, usually far above room temperatures.1,2 However, there have already been several reports on metal alkoxide catalysts that are able to polymerize lactide at lower temperatures, either in the isoselective20 or heteroselective21 manner. A few years ago, we demonstrated that in the case of Me2GaOR(NHC) complexes, which could polymerize rac-lactide already at −20 °C, in the isoselective fashion,14b,22 the chelation effect between growing PLA chain and Ga had a considerable effect on the activity, average molecular weight (Mn) as well as the dispersity of the obtained PLA (Mw/Mn).14b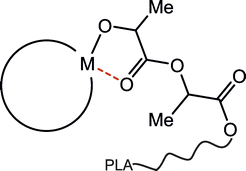 | ||
| Scheme 1 The chelate interaction between the growing polylactide chain and metal for metal alkoxide propagating species in the polymerization of rac-LA. PLA indicates the growing PLA chain. | ||
The revisit of our previous studies on Me2GaOR(NHC) (NHC = 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene (SIMes) and 1,3-bis-(2,4,6-trimethylphenyl)imidazole-2-ylidene (IMes)) complexes, in order to investigate the factors influencing their catalytic properties in the ROP of rac-LA, has demonstrated the considerable role of the chelation effect on their stereoselectivity in the ROP of rac-LA, which is presented and discussed below. As far as we are concerned, the results described herein constitute the first evidence demonstrating that the chelation effect, resulting from the weak interaction between the metal and the growing PLA chain, can be responsible for the enhancement of stereoselectivity in the ROP of rac-LA, and expectedly beyond. They additionally indicate that the chelation effect should be especially important in the case of ROP at low temperatures, at which it can manifest itself, and as a result affect the stereoselectivity of propagating species.
2. Results and discussion
In order to optimize the conditions of the ROP of rac-LA with isoselective Me2GaOR(NHC) (NHC = SIMes, IMes) complexes,14b we initially focused on the effect of the solvent on the chelation effect, resulting from the chelate interaction between gallium and the growing PLA chain, as well as the tendency of investigated complexes for side reactions. Our previous studies on the ROP of rac-LA with Me2GaOR(NHC) at −20 °C in methylene chloride had demonstrated that in the case of propagating species Me2Ga((OCH(Me)C(O))2)nOR(NHC) (Scheme 2), the interaction between the growing PLA chain and gallium, could suppress the activity of the dialkylgallium alkoxide propagating species and lead to the increased dispersity of the resulting PLA, as well as higher Mn in comparison with values expected from LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ratio.14b Therefore, we were interested in whether a change of the solvent, and the use of toluene or THF, which should be expected to compete with a growing PLA chain for the coordination to gallium metal centre,2c,23 could eliminate problems related to the Mn distribution. On the other hand, the side reactions, essentially absent at −20 °C, had been observed at ambient temperature and included both the inter- and intramolecular transesterification reactions. The latter, resulting in the formation of a small fraction of cyclic PLA, was indicative of the insertion of rac-LA into Ga–CNHC bond. In this case we expected to investigate whether the use of toluene or THF could allow for the reduction of side reactions at higher temperatures, i.e. room temperature or 40 °C. Based on our previous studies,14b essentially no chelation effect was expected at these temperatures. In order to address the above questions, we conducted the systematic studies on the effect of toluene, CH2Cl2 and THF on the structure and catalytic properties of Me2Ga(OPLA)(NHC) – propagating species in the ROP of rac-LA – where PLA represents a growing PLA chain, at the temperatures ranging from −40 °C to 40 °C.
Ga ratio.14b Therefore, we were interested in whether a change of the solvent, and the use of toluene or THF, which should be expected to compete with a growing PLA chain for the coordination to gallium metal centre,2c,23 could eliminate problems related to the Mn distribution. On the other hand, the side reactions, essentially absent at −20 °C, had been observed at ambient temperature and included both the inter- and intramolecular transesterification reactions. The latter, resulting in the formation of a small fraction of cyclic PLA, was indicative of the insertion of rac-LA into Ga–CNHC bond. In this case we expected to investigate whether the use of toluene or THF could allow for the reduction of side reactions at higher temperatures, i.e. room temperature or 40 °C. Based on our previous studies,14b essentially no chelation effect was expected at these temperatures. In order to address the above questions, we conducted the systematic studies on the effect of toluene, CH2Cl2 and THF on the structure and catalytic properties of Me2Ga(OPLA)(NHC) – propagating species in the ROP of rac-LA – where PLA represents a growing PLA chain, at the temperatures ranging from −40 °C to 40 °C.
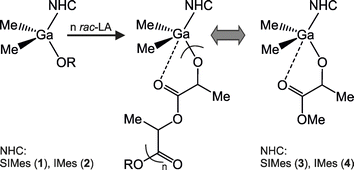 | ||
| Scheme 2 The structure of propagating species in the isoselective polymerization of rac-LA with Me2GaOR(NHC) complexes. | ||
2.1 The effect of the solvent on the structure of Me2Ga(OPLA)(NHC) propagating species
Complexes Me2Ga(OCH2CH2OMe)(NHC) (NHC = SIMes (1) and IMes (2)14b were used as initiators in the ROP of rac-LA in toluene, CH2Cl2 and THF (see below). However, the propagating species – Me2Ga(((OCH(Me)C(O))2)nOCH2CH2OMe)(NHC) (NHC = SIMes, IMes), resulting from the insertion of rac-LA into Ga–OR bond of 1 or 2, also depicted for clarity as Me2Ga(OPLA)(NHC) (OPLA – growing PLA chain), should be considered essentially isostructural with respective methyl lactate (melac) derivatives Me2Ga(OCH(Me)CO2Me)(NHC) (NHC = SIMes (3), IMes (4)) (Scheme 2),14b depicted in the text also as Me2Ga(melac)(NHC). Therefore the latter complexes should be considered to mimic gallium propagating species in the ROP of lactide with 1 and 2, although, it should be noted, that the strength of the chelate interaction between gallium and melac ligand in 3 and 4 was larger in comparison with the analogous interaction of growing PLA chain with gallium for Me2Ga(OPLA)(NHC) propagating species as evidenced by our previous kinetic studies.14b While complexes 1–4 were previously reported in our studies and thoroughly characterized,14b,22a we performed additional analyses of the structure of 3 and 4, using variable temperature 1H NMR spectroscopy (VT 1H NMR) and FTIR spectroscopies in order to get a further insight into the structure of propagating species Me2Ga(OPLA)(NHC) (NHC = SIMes, IMes).For 3 and 4, a presence of the chelate bond resulting from the interaction of the melac ligand with gallium had been previously confirmed in the solution, and in the case of the crystalline complex 4, also in the solid state.14b The molecular structure of 4 revealed two possible coordination modes of the carbonyl group of melac ligand to gallium resulting in the formation of Ga⋯O(Me)C(O) and Ga⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The latter were tentatively ascribed to the FTIR bands at 1747 cm−1 and 1723 cm−1 observed in toluene (1739 cm−1 and 1715 cm−1 in CH2Cl2). Although the FTIR spectroscopy of 3 in CH2Cl2 revealed the presence of one broad band at 1737 cm−1, which initially suggested the presence of free C
C bonds. The latter were tentatively ascribed to the FTIR bands at 1747 cm−1 and 1723 cm−1 observed in toluene (1739 cm−1 and 1715 cm−1 in CH2Cl2). Although the FTIR spectroscopy of 3 in CH2Cl2 revealed the presence of one broad band at 1737 cm−1, which initially suggested the presence of free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,22a the deconvolution revealed the presence of bands at 1737 cm−1 and 1717 cm−1.14b Even though the latter was essentially in agreement with the FTIR of 4, the polymerization studies indicated much weaker chelation effect in the case of both 3 and the respective propagating species.14b In order to focus on the effect of the solvent on the chelate bond between Ga and the melac ligand in the case of 3 and 4, we compared the FTIR spectra of both complexes in toluene, CH2Cl2 and THF (see the ESI, Fig. S27–S42†). In the case of 4 the presence of two strong C
O,22a the deconvolution revealed the presence of bands at 1737 cm−1 and 1717 cm−1.14b Even though the latter was essentially in agreement with the FTIR of 4, the polymerization studies indicated much weaker chelation effect in the case of both 3 and the respective propagating species.14b In order to focus on the effect of the solvent on the chelate bond between Ga and the melac ligand in the case of 3 and 4, we compared the FTIR spectra of both complexes in toluene, CH2Cl2 and THF (see the ESI, Fig. S27–S42†). In the case of 4 the presence of two strong C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands, at 1746 cm−1 and 1724 cm−1 (toluene), 1738 cm−1 and 1715 cm−1 (CH2Cl2), and 1748 cm−1 and 1724 cm−1 (THF) were observed, which is in agreement with previous studies.14b The deconvolution of C
O bands, at 1746 cm−1 and 1724 cm−1 (toluene), 1738 cm−1 and 1715 cm−1 (CH2Cl2), and 1748 cm−1 and 1724 cm−1 (THF) were observed, which is in agreement with previous studies.14b The deconvolution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands of 4 in CH2Cl2 and toluene resulted in two bands with the fitting accuracy of 0.001370 RMS and 0.002934 RMS (RMS − Root Mean Square), respectively (Fig. S42 and S36†). The deconvolution of C
O bands of 4 in CH2Cl2 and toluene resulted in two bands with the fitting accuracy of 0.001370 RMS and 0.002934 RMS (RMS − Root Mean Square), respectively (Fig. S42 and S36†). The deconvolution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands of 4 in THF resulted in three bands at 1748 cm−1, 1733 cm−1 and 1722 cm−1 with the fitting accuracy of 0.001484 RMS (Fig. S40†). The presence of a C
O bands of 4 in THF resulted in three bands at 1748 cm−1, 1733 cm−1 and 1722 cm−1 with the fitting accuracy of 0.001484 RMS (Fig. S40†). The presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band at 1748 cm−1 could be consequently ascribed to the essentially free C
O band at 1748 cm−1 could be consequently ascribed to the essentially free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, which should be considered a result of the stronger coordination of THF to the gallium centre of 4, in comparison with toluene and CH2Cl2. However, in the case of toluene, deconvolution could also lead to the presence of three bands at 1748 cm−1, 1733 cm−1 and 1722 cm−1 with a fitting accuracy of 0.002824 RMS (Fig. S37†). Therefore, the free C
O group, which should be considered a result of the stronger coordination of THF to the gallium centre of 4, in comparison with toluene and CH2Cl2. However, in the case of toluene, deconvolution could also lead to the presence of three bands at 1748 cm−1, 1733 cm−1 and 1722 cm−1 with a fitting accuracy of 0.002824 RMS (Fig. S37†). Therefore, the free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands should be considered present for all the chosen solvents irrespective of their different basicity/donor numbers. In the case of 3, in which the exhibited weaker chelation effect was evidenced in the ROP of rac-LA,14b three C
O bands should be considered present for all the chosen solvents irrespective of their different basicity/donor numbers. In the case of 3, in which the exhibited weaker chelation effect was evidenced in the ROP of rac-LA,14b three C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands at 1749 cm−1, 1733 cm−1 and 1722 cm−1 in THF resulted from the deconvolution (Fig. S32†), and could be distinguished even in the original spectrum (Fig. S30†). For C
O bands at 1749 cm−1, 1733 cm−1 and 1722 cm−1 in THF resulted from the deconvolution (Fig. S32†), and could be distinguished even in the original spectrum (Fig. S30†). For C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands of 3 in CH2Cl2 the deconvolution revealed two bands at 1737 cm−1 and 1715 cm−1 (Fig. S34†), which was in agreement with our previous studies. On the other hand, the deconvolution of C
O bands of 3 in CH2Cl2 the deconvolution revealed two bands at 1737 cm−1 and 1715 cm−1 (Fig. S34†), which was in agreement with our previous studies. On the other hand, the deconvolution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands of 3 in toluene was much better represented by three bands at 1749 cm−1, 1733 cm−1 and 1722 cm−1 (Fig. S29†). Noteworthy, for both 3 and 4 the presence of a free C
O bands of 3 in toluene was much better represented by three bands at 1749 cm−1, 1733 cm−1 and 1722 cm−1 (Fig. S29†). Noteworthy, for both 3 and 4 the presence of a free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in THF and toluene, was observed additionally to the Ga⋯O(Me)C(O) and Ga⋯O
O in THF and toluene, was observed additionally to the Ga⋯O(Me)C(O) and Ga⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Although a similar observation should be expected in methylene chloride, the presence of only two bands, revealed by the deconvolution of the C
C bonds. Although a similar observation should be expected in methylene chloride, the presence of only two bands, revealed by the deconvolution of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands of 3 and 4 in CH2Cl2, could be neither clearly explained nor assigned. Importantly, the presence of equilibrium between Me2Ga(OCH(Me)CO2Me)(NHC) (NHC = SIMes, IMes) with a differently coordinated or pendant melac ligand, which should be considered based on FTIR studies at r.t., was further supported by VT 1H NMR studies.
O bands of 3 and 4 in CH2Cl2, could be neither clearly explained nor assigned. Importantly, the presence of equilibrium between Me2Ga(OCH(Me)CO2Me)(NHC) (NHC = SIMes, IMes) with a differently coordinated or pendant melac ligand, which should be considered based on FTIR studies at r.t., was further supported by VT 1H NMR studies.
The VT 1H NMR spectra of 3 and 4 were performed in toluene-d8, CD2Cl2 and THF-d8, at r.t., 0 °C, –20 °C, and additionally in THF-d8 at –40 °C (Fig. S1–S26†). Irrespectively to the solvent and temperature, the NMR spectra of 3 and 4 revealed the presence of single set of signals, analogous to the NMR spectra registered previously for these complexes at room temperature, both in toluene and CH2Cl2.14b Therefore, they indicated the presence of monomeric species in the whole temperature range. Additionally, singlets corresponding to protons of CH groups in the imidazolium ring of 4, and protons of CH2 groups of the imidazolinium ring of 3, indicated the fast rotation of the NHC ligand along the Ga–CNHC bond at the NMR experiment time scale, even at low temperatures. It was in a full agreement with the rotation's previously reported low energy barriers of 5.5–6.5 kcal mol−1, with solvation correction having only a slight impact on these values.14b The latter excluded the asymmetric arrangement of NHC, which in the case of asymmetric NHC could be expected to affect the stereoselectivity of propagating species.24 Despite the remarkable similarities of the 1H NMR spectra of 3 and 4 at different temperatures, changes in the coordination sphere of gallium were evidenced. For both 3 and 4 in toluene, the lower-field shift of signals corresponding to Ga–Me protons (by around 0.07 ppm (3) and 0.06 ppm (4)) should be considered to indicate weakening coordination of NHC upon decreasing of the temperature from r.t. to −20 °C (Fig. S4 and S17†). It was accompanied by considerable higher-field shifts of signals corresponding to CH protons of imidazolinum (3)/imidazolium (4) ring (by 0.11/0.20 ppm). The simultaneous lower-field shift of protons corresponding to methylene protons of –CH(CH3)– group of melac ligand (by around 0.11 ppm (3) and 0.10 ppm (4)), should be associated with the conformational changes of the melac ligand resulting from the strengthening of the chelate bond between the latter and gallium, in the trans position to the Ga–CNHC bond. Therefore, in the case of toluene solutions of 3 and 4, the equilibrium between Me2Ga(melac)(NHC) with differently coordinated/uncoordinated melac ligand, which was evidenced by FTIR studies at r.t., should be expected to shift towards species I and/or III at lower temperatures (Scheme 3).
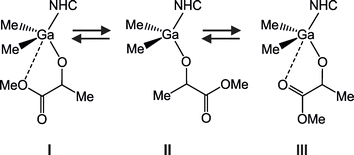 | ||
| Scheme 3 The equilibrium expected in the case of the toluene solution of Me2Ga(melac)(NHC) (NHC = SIMes (3) and IMes (4)) with differently coordinated, or pendant, melac ligands. | ||
In contrast to the NMR spectra in toluene, the decrease of temperature from r.t. to −40 °C in the case of the THF solutions of 3 and 4 resulted, in both cases, in the higher-field shift of signals corresponding to Ga–Me protons (by 0.07 ppm for both 3 and 4) and lower-field shifts of signals corresponding to CH protons of imidazolinum/imidazolium rings of the SIMes/IMes respectively (Fig. S13 and S26†), which was in line with the strengthening of the Ga–CNHC bond. Noteworthy, much smaller shifts of the signals corresponding to the protons of melac ligand were observed, in contrast to NMR spectra in toluene. This should be associated with a shift of the equilibrium, presented on Scheme 4, towards species II′ with essentially no chelate interaction between gallium and the melac ligand, which was due to the interaction of THF with gallium. Additionally, in contrast to the toluene solution, the protons of aromatic rings of SIMes and IMes of 3 and 4 split from singlets to doublets (observed at 0 °C and below), which could indicate an impeded rotation around N–CMes bond upon stabilization of structure II′.
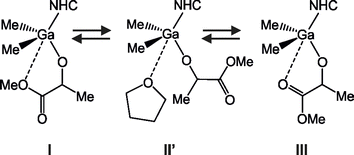 | ||
| Scheme 4 The equilibrium expected in the case of THF solution of Me2Ga(melac)(NHC) (NHC = SIMes (3) and IMes (4)) with differently coordinated or pendant, melac ligands. | ||
In the case of the VT 1H NMR spectra of 3 and 4 in CD2Cl2, only the signals corresponding to Ga–Me protons of 4 were slightly shifted to the higher field with a decrease of temperature (Fig. S8 and S21†). Consequently, essentially no shifts were observed for imidazoilnium/imidazolium protons with the decrease of temperature. For other signals of 4, as well as 3, in dichloromethane only minimal shifts could be observed. These observations could not lead to coherent conclusions and therefore, the combination of both situations observed in the case of toluene and THF solutions of 3 and 4 should be considered instead. Notably, methylene chloride is a polar solvent, which should be therefore expected to weaken the chelate interaction between gallium and the melac ligand in comparison with toluene. On the other hand, the coordination of dichloromethane to the gallium metal centre should not efficiently compete with the formation of the chelate bond, even at low temperatures, due to significantly weaker basicity/donor number in comparison with THF.25 Therefore, the shift of the equilibrium towards I and III species at low temperatures, analogous to those presented on Schemes 3 and 4, should be expected, however to a lower extent in comparison with toluene. Such a reasoning is in line with the polymerization studies presented below.
2.2 Polymerization studies
In order to determine the effect of the solvent on the catalytic properties of propagating species Me2Ga(OPLA)(NHC) (NHC = SIMes, IMes), we extended our previous studies on the catalytic properties of Me2GaOR(NHC) in the ROP of rac-LA, in CH2Cl2, at r.t. and −20 °C, and conducted the ring-opening polymerization of rac-LA with 1 and 2 in toluene, CH2Cl2, and THF at the temperature between −40 °C and 40 °C (Table 1).| No. | Cat. | Solvent | Temp. [°C] | t [min] | Conv. [%] | 10−3 Mna | 10−3 Mnb | 10−3 Mnc | Mw/Mn | Pmd |
|---|---|---|---|---|---|---|---|---|---|---|
| a Determined by gel permeation chromatography (GPC) in methylene chloride.b Determined by1H NMR.c Expected according to rac-LA/Ga ratio and conversion.d Calculated on the basis of 13C NMR;20e,26 see the Experimental section and the ESI for details.e Data from ref. 14b.f Additional distributions for Mn = 1.09 × 106 (4.0%) and Mn = 643 (2.7%). | ||||||||||
| 1 | 1 | Toluene | 40 | 10 | 95 | 7.0 | 5.3 | 6.9 | 1.15 | 0.75 |
| 2 | 1 | DCM | 40 | 10 | 90 | 7.7 | 6.3 | 6.6 | 1.17 | 0.67 |
| 3 | 1 | THF | 40 | 10 | 96 | — | 1.4 | 7.0 | — | — |
| 4 | 1 | Toluene | r.t. | 10 | 89 | 7.4 | 4.8 | 6.5 | 1.10 | 0.75 |
| 5e | 1 | DCM | r.t. | 10 | 70 | 6.4 | 5.9 | 5.1 | 1.32 | 0.66 |
| 6 | 1 | THF | r.t. | 10 | 39 | — | 1.4 | 2.8 | — | — |
| 7 | 1 | Toluene | 0 | 1200 | >99 | 10.8 | 8.5 | 7.3 | 1.26 | 0.82 |
| 8 | 1 | DCM | 0 | 30 | 97 | 13.7 | 9.1 | 7.1 | 1.47 | 0.72 |
| 9 | 1 | Toluene | −20 | 1200 | >99 | 12.3 | 9.3 | 7.3 | 1.12 | 0.85 |
| 10e | 1 | DCM | −20 | 60 | >99 | 32.8 | 17.2 | 7.3 | 1.89 | 0.77 |
| 11 | 1 | THF | −20 | 30 | 66 | 5.7 | 3.8 | 4.8 | 1.30 | 0.73 |
| 12 | 1 | THF | −20 | 1200 | 98 | 7.4 | 8.0 | 7.2 | 1.57 | 0.74 |
| 13 | 1 | Toluene | −40 | 4320 | 67 | — | 4.4 | 4.9 | — | 0.87 |
| 14 | 1 | DCM | −40 | 1200 | >99 | 9.1 | 7.9 | 7.3 | 1.96 | 0.81 |
| 15 | 1 | THF | −40 | 1200 | 97 | 10.2 | 7.1 | 7.1 | 1.62 | 0.75 |
| 16 | 2 | Toluene | 40 | 10 | 95 | 11.0 | 6.5 | 6.9 | 1.27 | 0.75 |
| 17 | 2 | Toluene | r.t | 10 | 78 | 8.8 | 5.6 | 5.7 | 1.34 | 0.76 |
| 18 | 2 | DCM | r.t | 10 | 92 | 8.3 | 6.8 | 6.7 | 1.38 | 0.70 |
| 19 | 2 | THF | r.t | 10 | 76 | 5.1 | 3.8 | 5.5 | 1.48 | 0.68 |
| 20 | 2 | Toluene | 0 | 1200 | >99 | 11.9 | 7.1 | 7.3 | 1.47 | 0.83 |
| 21 | 2 | DCM | 0 | 30 | >99 | 10.7 | 7.9 | 7.3 | 1.62 | 0.74 |
| 22 | 2 | THF | 0 | 30 | 99 | 11.3 | 8.6 | 7.2 | 1.53 | 0.72 |
| 23 | 2 | Toluene | −20 | 1200 | >99 | 11.5 | 10.0 | 7.3 | 1.65 | 0.87 |
| 24 | 2 | DCM | −20 | 30 | >99 | 13.4 | 12.4 | 7.3 | 1.47 | 0.80 |
| 25 | 2 | THF | −20 | 30 | 92 | 12.8f | 13.8 | 6.7 | 1.46 | 0.75 |
| 26 | 2 | Toluene | −40 | 1200 | >99 | 13.9 | 9.4 | 7.3 | 1.69 | 0.89 |
| 27 | 2 | DCM | −40 | 1200 | >99 | 31.0 | 11.7 | 7.3 | 1.36 | 0.81 |
| 28 | 2 | THF | −40 | 1200 | >99 | 30.1 | 16.9 | 7.3 | 1.87 | 0.74 |
| 29 | 5 | DCM | −40 | 1200 | >99 | — | 10.4 | 7.2 | — | 0.84 |
| 30 | 5 | THF | −40 | 1200 | 40 | — | 3.6 | 3.6 | — | 0.80 |
MALDI-TOF of obtained PLA confirmed the insertion of rac-LA into the Ga–O bond, as depicted on Scheme 2, resulting in the formation of PLA with OH and OCH2CH2OMe end groups (Fig. S46–S89†), which was in agreement with the coordination insertion mechanism. In selected cases, it was indicative for the presence of side reactions, mainly transesterification and the insertion of lactide into Ga–CNHC bond, depending on the polymerization conditions. The extent of side reactions is indicated in Fig. 1 and 2, below. In toluene, the ROP of rac-LA with 1 resulted in essentially no side reactions between r.t. and −40 °C (Table 1, entries 4, 7, 9 and 13). Although for the PLA obtained at 40 °C (Table 1, entry 1) the trace distributions referred to the presence of intramolecular transesterifications, as well as the presence of the cyclic PLA, indicating a possibility of the insertion of rac-LA into Ga–CNHC bond. However, the extent of the latter should not considerably affect the structure of the resulting PLA. It was confirmed by both Mn, which was essentially in agreement with values expected from rac-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ratio, and low dispersities of PLA, obtained in a whole temperature range between 40 °C and −40 °C. Similarly, in the case of the ROP of rac-LA with 2 in toluene, the formation of PLA with OH and OCH2CH2OMe end groups was confirmed by the MALDI-TOF at a whole range of temperatures (Table 1, entries 16, 17, 20, 23, 26). Although MALDI-TOF indicated the possibility of the intermolecular transesterification for the polymerizations at 0 °C–40 °C, and the presence of a minor fraction of cyclic PLA obtained at 40 °C, the extent of these side reactions should not considerably affect the structure of resulting PLA. Such reasoning is in line with the Gel Permeation Chromatography (GPC) analyses. Although MALDI-TOF is not a quantitative analytical method, much higher extent of side reactions should be considered in the case of the ROP of rac-LA with 1 and 2 in CH2Cl2, as well as THF. For the ROP in CH2Cl2, MALDI-TOF analysis of PLA obtained with 1 at 40 °C showed, beside the formation of PLA with OH and OCH2CH2OMe end groups, the formation of cyclic PLA, most probably due to the insertion of PLA into Ga–CNHC bond, as well as the extensive intermolecular transesterification. The extent of the latter was similar, or even greater in comparison with the polymerization of rac-LA with Me2GaOR(NHC) complexes at room temperature,14b which confirmed the adverse effect of the increased temperature on the control and stereoselectivity of ROP of rac-LA with these complexes in DCM (Fig. 1 and 2). The ROP of rac-LA with 1 and 2 at 0 °C and below led to essentially only PLA with OH and OCH2CH2OMe end groups based on MALDI-TOF analyses, with a limited intermolecular transesterification in both cases, which was also in line with our previous studies on the ROP of rac-LA with Me2GaOR(NHC).14b,22a In the case of PLA obtained by the polymerizations of rac-LA with 1 and 2 in THF, MALDI-TOF revealed the presence of side reactions even at the temperature of −20 °C and higher, while their extent increased with the temperature. In this case MALDI-TOF revealed the presence of the low molecular weight PLA with the end groups of 138 and 66 Da, the latter resulting from the intermolecular transesterification. However, the end groups could not be reasonably assigned. Upon the decrease of temperature, the PLA with OH and OCH2CH2OMe end groups became dominant.
Ga ratio, and low dispersities of PLA, obtained in a whole temperature range between 40 °C and −40 °C. Similarly, in the case of the ROP of rac-LA with 2 in toluene, the formation of PLA with OH and OCH2CH2OMe end groups was confirmed by the MALDI-TOF at a whole range of temperatures (Table 1, entries 16, 17, 20, 23, 26). Although MALDI-TOF indicated the possibility of the intermolecular transesterification for the polymerizations at 0 °C–40 °C, and the presence of a minor fraction of cyclic PLA obtained at 40 °C, the extent of these side reactions should not considerably affect the structure of resulting PLA. Such reasoning is in line with the Gel Permeation Chromatography (GPC) analyses. Although MALDI-TOF is not a quantitative analytical method, much higher extent of side reactions should be considered in the case of the ROP of rac-LA with 1 and 2 in CH2Cl2, as well as THF. For the ROP in CH2Cl2, MALDI-TOF analysis of PLA obtained with 1 at 40 °C showed, beside the formation of PLA with OH and OCH2CH2OMe end groups, the formation of cyclic PLA, most probably due to the insertion of PLA into Ga–CNHC bond, as well as the extensive intermolecular transesterification. The extent of the latter was similar, or even greater in comparison with the polymerization of rac-LA with Me2GaOR(NHC) complexes at room temperature,14b which confirmed the adverse effect of the increased temperature on the control and stereoselectivity of ROP of rac-LA with these complexes in DCM (Fig. 1 and 2). The ROP of rac-LA with 1 and 2 at 0 °C and below led to essentially only PLA with OH and OCH2CH2OMe end groups based on MALDI-TOF analyses, with a limited intermolecular transesterification in both cases, which was also in line with our previous studies on the ROP of rac-LA with Me2GaOR(NHC).14b,22a In the case of PLA obtained by the polymerizations of rac-LA with 1 and 2 in THF, MALDI-TOF revealed the presence of side reactions even at the temperature of −20 °C and higher, while their extent increased with the temperature. In this case MALDI-TOF revealed the presence of the low molecular weight PLA with the end groups of 138 and 66 Da, the latter resulting from the intermolecular transesterification. However, the end groups could not be reasonably assigned. Upon the decrease of temperature, the PLA with OH and OCH2CH2OMe end groups became dominant.
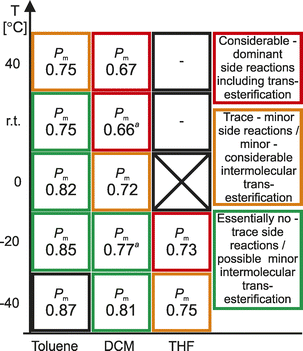 | ||
| Fig. 1 Stereoselectivity of the ROP of rac-LA with 1 depicted as the probability of meso linkages (Pm) in the resulting PLA. | ||
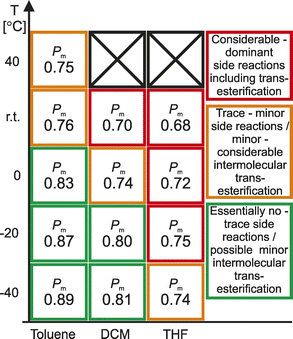 | ||
| Fig. 2 Stereoselectivity of the ROP of rac-LA with 2 depicted as the probability of meso linkages (Pm) in the resulting PLA. | ||
The side reactions for ROP of rac-LA with 1 or 2 in THF were almost fully suppressed only at –40 °C (Fig. S62 and S88† respectively). Even when the side reactions were essentially not present, or could occur to a limited extent, the ROP of rac-LA with 1 led to the PLA of much lower dispersity in comparison to the polymerizations with 2, for which additionally the Mn values were significantly increased in comparison with Mn values expected from LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga ratio. The latter should be associated with a stronger chelate interaction in the case of IMes complexes, which is in line with our previous observations.14b Noteworthy, the chelate interaction between growing PLA chain and gallium, as well as the effect of solvents, limiting to a different extent the interaction of rac-LA with gallium, should be expected in the polymerization studies described above. Most probably, due to the combination of both effects, additionally depending to a different extent on the temperature, no significant correlation between the solvent and the Mn and/or dispersity of the resulting PLA could be observed, regardless of the temperature.
Ga ratio. The latter should be associated with a stronger chelate interaction in the case of IMes complexes, which is in line with our previous observations.14b Noteworthy, the chelate interaction between growing PLA chain and gallium, as well as the effect of solvents, limiting to a different extent the interaction of rac-LA with gallium, should be expected in the polymerization studies described above. Most probably, due to the combination of both effects, additionally depending to a different extent on the temperature, no significant correlation between the solvent and the Mn and/or dispersity of the resulting PLA could be observed, regardless of the temperature.
Remarkably, the activity of 1 and 2 was suppressed in THF in comparison with the ROP of rac-LA in CH2Cl2. In the case of the ROP of rac-LA in toluene, lactide remained largely undissolved at the beginning of the polymerization, and therefore longer times were required, especially at low temperature. The difference in the activity observed for CH2Cl2 and THF should be associated with the lower activity of Me2Ga(OPLA)(NHC)(THF) species, analogous to species II on Scheme 4, in comparison with Me2Ga(OPLA)(NHC) propagating species present in CH2Cl2. The coordination of THF to gallium should be therefore considered to compete with lactide, limiting its access to gallium more efficiently than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Ga chelate interaction in CH2Cl2. Interestingly, based on the structure studies (see above) we initially expected the THF to compete with the growing PLA chain for the coordination to gallium, and as a result to facilitate the monomer insertion into the Ga–O bond. It must be noted that we have previously demonstrated for the ROP of rac-LA in CH2Cl2 at –20 °C that the chelate interaction between the melac ligand and gallium, especially in 4, significantly limits the access of lactide to gallium, and supresses the polymerization, especially at the initiation stage.14b To demonstrate the elimination of the chelation effect in 4 upon the interaction of THF with gallium, we also performed some additional ROP experiments with 4 in CH2Cl2 and THF. Polymerization in THF resulted in a significantly higher conversions, both at –20 °C and –40 °C, than in CH2Cl2 (Scheme 5). It indicated that the competing coordination of THF to gallium resulted in the ease of the intramolecular chelate interaction between gallium and the melac ligand. As a result, the conversions of rac-LA were higher despite the lower activity of Me2Ga(OPLA)(IMes)(THF) propagating species in THF in comparison to Me2Ga(OPLA)(IMes) in CH2Cl2. Significantly, the findings discussed above bring further evidence that THF may significantly weaken the chelate interaction and result in formation of the propagating species Me2Ga(OPLA)(NHC)(THF), analogous to species II′ presented on Scheme 4.
O⋯Ga chelate interaction in CH2Cl2. Interestingly, based on the structure studies (see above) we initially expected the THF to compete with the growing PLA chain for the coordination to gallium, and as a result to facilitate the monomer insertion into the Ga–O bond. It must be noted that we have previously demonstrated for the ROP of rac-LA in CH2Cl2 at –20 °C that the chelate interaction between the melac ligand and gallium, especially in 4, significantly limits the access of lactide to gallium, and supresses the polymerization, especially at the initiation stage.14b To demonstrate the elimination of the chelation effect in 4 upon the interaction of THF with gallium, we also performed some additional ROP experiments with 4 in CH2Cl2 and THF. Polymerization in THF resulted in a significantly higher conversions, both at –20 °C and –40 °C, than in CH2Cl2 (Scheme 5). It indicated that the competing coordination of THF to gallium resulted in the ease of the intramolecular chelate interaction between gallium and the melac ligand. As a result, the conversions of rac-LA were higher despite the lower activity of Me2Ga(OPLA)(IMes)(THF) propagating species in THF in comparison to Me2Ga(OPLA)(IMes) in CH2Cl2. Significantly, the findings discussed above bring further evidence that THF may significantly weaken the chelate interaction and result in formation of the propagating species Me2Ga(OPLA)(NHC)(THF), analogous to species II′ presented on Scheme 4.
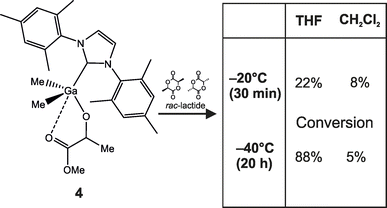 | ||
| Scheme 5 The conversion of rac-LA with 4 in CH2Cl2 and THF – an evidence for the competition between THF and a chelate interaction between the melac ligand and gallium. | ||
The PLA obtained in the ROP of rac-LA with 1 and 2 was isotactically enriched, which was in a full agreement with our previous studies. The considerable effect of solvent on the stereoselectivity was demonstrated by the tacticity of obtained PLA. For the ROP in toluene, the Pm of the resulting PLA increased from 0.75 at 40 °C to 0.87 (for 1) and 0.89 (for 2) at –40 °C, despite essentially no side reactions in the whole temperature range. In the case of the ROP of rac-LA in CH2Cl2, at the temperatures which allowed for essentially absent or limited side reactions, the Pm of the resulting PLA increased from 0.72 (for 1) and 0.74 (for 2) at 0 °C to 0.81 at –40 °C. Noteworthy the PLA obtained in THF at –40 °C, as well as –20 °C, was characterized by Pm of maximum 0.75 while the side reactions observed at higher temperatures resulted in the decreased Pm values. Importantly, the effect of the solvent on the stereoselectivity should be stressed, as it allows for the tuning of the Pm of the resulting PLA. However, regarding the structure–activity studies discussed above, it could indicate the effect of the chelate interaction between the growing PLA chain and gallium on the stereoselectivity, unprecedented in the studies of metal-based catalysts in the ROP of rac-LA.
2.3 The role of the chelate effect on the stereoselectivity of rac-LA polymerization with Me2Ga(OPLA)(NHC)
In our previous studies, we associated the decrease of isoselectivity Me2GaOR(NHC) (NHC = SIMes and IMes) in the ROP of rac-LA at room temperature with the larger tendency for the side reactions, in comparison with the polymerization at –20 °C.14b The polymerization results discussed above do not disprove our earlier observations. However, a strong effect of both the solvent and temperature on the isoselectivity of 1 and 2 in the ROP of rac-LA was observed at conditions under which little to essentially no side reactions were evidenced (Fig. 1 and 2). In these cases, i.e. the ROP of rac-LA with 1 and 2 in toluene at 40 °C–(–40 °C), in CH2Cl2 at 0 °C–(–40 °C) and THF at –40 °C, other factor or factors should be responsible for the isoselectivity of propagating species Me2GaOR(NHC). Notably, the structure of these species, mimicked by model complexes 3 and 4, indicated that the chelate interaction between the growing PLA chain and gallium as essentially only factor which could be affected by the solvent and temperature, and therefore responsible for the observed variations in the stereoselectivity of Me2Ga(OPLA)(NHC), indicated by Pm of the resulting PLA.The above discussed results of the ROP of rac-LA with 1 and 2 in toluene were indicative for the lack of the chelation effect on the polymerization outcome and structure of the resulting PLA already at room temperature. They were supported by the structure of 3 and 4, which showed significant fraction of species with a pending melac ligand, therefore indicating the labile interaction of the growing PLA chain with propagating Me2Ga(OPLA)(NHC) species. Regarding the stereoselectivity, PLA of Pm equal to 0.75 and 0.76 was obtained in the ROP of rac-LA in toluene at r.t., with 1 and 2 respectively. Noteworthy, the increase of temperature, which should be expected to further eliminate the chelation effect in the ROP of rac-LA, had essentially no effect on the Pm of PLA. The stereoselectivity of the polymerization of rac-LA with 1 and 2 in toluene, at both r.t. and 40 °C, was therefore the lowest, unless the presence of side reactions could result in the decrease of Pm of the resulting PLA below 0.75. On the other hand, the decrease of the polymerization temperature, which favoured the interaction of growing PLA chain with gallium, resulted in the substantial increase of the stereoselectivity, at 0 °C, −20 °C and −40 °C, in the latter case resulting in the formation of PLA of the highest Pm equal to 0.87 and 0.89, for 1 and 2, respectively. The slightly increased Pm values in the case of propagating species Me2Ga(OPLA)(IMes), in comparison with their SIMes analogues, should result from stronger chelation effect in the case of IMes.14b The change of solvent in the case of the ROP of rac-LA at –40 °C led to the decrease of Pm of the resulting PLA to 0.81, for both 1 and 2 in CH2Cl2, and to 0.75 and 0.74 for 1 and 2 in THF, respectively. Importantly, essentially the same stereoselectivity in the latter case and in the case of the polymerization of rac-LA in toluene at r.t. and 40 °C was indicative for the lack of the chelation effect in both cases. In other cases, presented on Fig. 1 and 2, the stereoselectivity of the ROP of rac-LA with 1 and 2 in CH2Cl2 and THF were essentially in line with the results discussed above, although could be affected by the side reactions to a different extent (Fig. 1 and 2).
The pattern in which the Pm of PLA obtained with 1 and 2 changed at different temperature, for all three solvents, brought further evidence that it was a chelation effect which influenced the stereoselectivity of the ROP of rac-LA with 1 and 2 (Fig. 1 and 2). The sharp increase in the isoselectivity was observed for the polymerizations in toluene and CH2Cl2 at different temperatures. It excluded the pure temperature effect on the stereoselectivity of propagating species Me2Ga(OPLA)(NHC) and should be associated with the appearance of the chelation effect. Therefore, it should not be surprising that the chelate effect was “switched on” between r.t. (Pm = 0.75 (1) and 0.76 (2)) and 0 °C (Pm = 0.82 (1) and 0.83 (2)) for the ROP of rac-LA in toluene, much higher in comparison with the ROP of rac-LA in CH2Cl2. In the latter case it was observed between 0 °C (Pm = 0.72 (1) and 0.74 (2)) and –20 °C (Pm = 0.77 (1) and 0.80 (2)). Noteworthy, the term ‘switch on’ should be associated with the shift of the equilibrium presented on Schemes 3 and 4 towards species I and III, which due to the interaction of CH2Cl2 with gallium should be expected at a lower temperature. Notably further increase of stereoselectivity, below the ‘switch on’ temperature, could result in a further shift of the equilibrium. Here, we cannot exclude the shift of the equilibrium between species I and III, and I′ and III′, in which the chelate interaction between gallium and the growing PLA chain is strong enough to change their stereoselectivity. On the contrary, the essential elimination of the chelate interaction should not allow for the further decrease in Pm below 0.75 (see above), unless due to the effect of transesterification, which is supported by the PLA of essentially the same Pm, obtained at r.t. and 40 °C with 1 and 2 in toluene. Noteworthy, the stereoselectivity of the polymerizations of rac-LA in THF, did not exceed the Pm of 0.75, even at −40 °C, which was essentially the lowest observed value among the polymerizations with essentially absent or limited side reactions. The latter result was in line with a lack of the chelation effect for the ROP of rac-LA in the whole temperature range.
The coherent results presented on Fig. 1 and 2, supported by structure-activity studies presented above, strongly indicate the chelation effect influenced the stereoselectivity of the ROP of rac-LA with Me2Ga(OPLA)(NHC) propagating species, therefore resulting in the Pm variations in the range between 0.75 and 0.89. It therefore indicated that chelation effect does not decide about the stereoselectivity, but can lead to the stereoselectivity enhancement (Fig. 3).
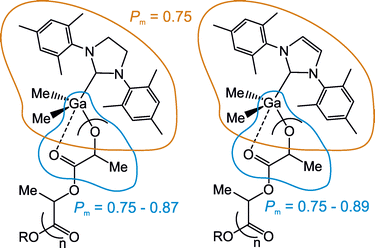 | ||
| Fig. 3 The contribution of elements of the structure of Me2Ga(OPLA)(NHC) to their stereoselectivity in the ROP of rac-LA at −40 °C–40 °C, represented by the Pm of the resulting PLA. | ||
Therefore, it does not question the widely accepted mechanisms for the stereoselectivity control, which include the growing chain or enantiomorphic site control.1 Irrespectively to the mechanism, the structures of Me2Ga(OPLA)(NHC) propagating species, without the chelation effect, were responsible for the Pm of 0.75. In order to confirm our reasoning, we investigated the effect of the solvent on the stereoselectivity of the ROP of rac-LA with Me2GaOMe(SI(Dipp-Mes)) (5),24 for which the presence of an asymmetric SI(Dipp-Mes) was earlier postulated to increase the stereoselectivity in CH2Cl2. Indeed, the ROP of rac-LA with 5 at –40 °C, CH2Cl2, and THF resulted in PLA of Pm equal to 0.84 and 0.80 respectively. The stereoselectivity of the polymerization in THF was substantially larger than in the case of 1 and 2, and therefore especially confirmative for the effect of SI(Dipp-Mes) on the stereoselectivity (Fig. 4). More importantly these results suggest that the enhancement due to the chelation effect can refer to different Pm ranges. This issue is a subject to further studies.
3. Conclusions
We have focused on the effect of solvent on the structure and catalytic properties of propagating species Me2Ga(OPLA)(NHC) in the ring-opening polymerization (ROP) of rac-lactide (rac-LA). The structure–activity studies, using model Me2GaOR(NHC) complexes, showed a substantial effect of solvent, on the chelate interaction between gallium and growing polylactide chain, and subsequently on the activity and the stereoselectivity of Me2Ga(OPLA)(NHC) propagating species. Noteworthy, in contrast to other reports, which have demonstrated the effect of solvent on the stereoselectivity of metal alkoxides due to elimination of transesterification reactions,23b or enhancing the ratio of homochiral to heterochiral dimeric species,14c our finding demonstrates a new way how the solvent can influence the stereoselectivity of metal alkoxides in the ROP of rac-LA, and possibly other chiral cyclic esters. Importantly, with regard to the ongoing studies on the effect of catalysts structures on their properties in the ROP of lactide,2 we have demonstrated for the first time that the interaction between metal and growing PLA chain was responsible for the enhancement of the stereoselectivity of the ROP of rac-LA, in the range of Pm between 0.75–0.89 once the chelation effect is strong enough at the polymerization conditions. The Pm = 0.75 resulted in this case from the structure of Me2Ga(OPLA)(NHC) propagating species, beyond the chelation effect. Therefore, the modification of the chelation effect in the ROP of rac-lactide, with the help of solvent, can be a strategy for the enhancement and in general tuning of the tacticity of PLA. Therefore, it should remain of interests in the case of metal-based catalysts able for the stereoselective polymerization of rac-LA, as well as other chiral heterocyclic monomers, especially at mild conditions, under which the chelation effect between the growing polymer chain and the metal centre can manifest itself.4. Experimental
4.1 Materials and methods
All operations were carried out under dry argon using standard Schlenk techniques. Solvents and reagents were purified and dried prior to use. Solvents were purified using MBRAUN Solvent Purification Systems (MB-SPS-800) and stored over molecular sieves 4 Å. Deuterated solvents were dried over potassium (toluene-d8, THF-d8) or calcium hydride (CD2Cl2) and distilled under argon; subsequently stored over molecular sieves 4 Å. Compounds 1–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14b,22a and 5 (ref. 24) were synthesized according to our previously published procedures. For the latter Me3Ga was purchased from STREM Chemicals, Inc. and used as received. (S)-Methyl lactate (98%, optical purity ee: 97%) and 2-methoxyethanol (anhydrous, 99.8%) were purchased from Sigma-Aldrich and stored over molecular sieves 4 Å. rac-Lactide (99%) was purchased from Aldrich and further purified by crystallization from anhydrous toluene, followed by sublimation under vacuum; subsequently stored under argon. 1H and 13C NMR spectra were recorded on Agilent 400-MR DD2 400 MHz spectrometer with shifts given in ppm according to the deuterated solvent shift. FTIR spectra were recorded on FTIR PerkinElmer System 2000. GPC measurements were recorded on Spectra-Physics chromatograph equipped with two high performance Plgel 5 μm MIXED-C columns and detectors: RI (VE3580 Viscotek) and viscometer (270 Dual Detector Array Viscotek) with conventional calibration according to polystyrene standard. MALDI-TOF spectra were recorded on Bruker model ultrafleXtreme, using DCTB matrix and CF3COOK salt as ion promotor.
14b,22a and 5 (ref. 24) were synthesized according to our previously published procedures. For the latter Me3Ga was purchased from STREM Chemicals, Inc. and used as received. (S)-Methyl lactate (98%, optical purity ee: 97%) and 2-methoxyethanol (anhydrous, 99.8%) were purchased from Sigma-Aldrich and stored over molecular sieves 4 Å. rac-Lactide (99%) was purchased from Aldrich and further purified by crystallization from anhydrous toluene, followed by sublimation under vacuum; subsequently stored under argon. 1H and 13C NMR spectra were recorded on Agilent 400-MR DD2 400 MHz spectrometer with shifts given in ppm according to the deuterated solvent shift. FTIR spectra were recorded on FTIR PerkinElmer System 2000. GPC measurements were recorded on Spectra-Physics chromatograph equipped with two high performance Plgel 5 μm MIXED-C columns and detectors: RI (VE3580 Viscotek) and viscometer (270 Dual Detector Array Viscotek) with conventional calibration according to polystyrene standard. MALDI-TOF spectra were recorded on Bruker model ultrafleXtreme, using DCTB matrix and CF3COOK salt as ion promotor.
Data availability
“Enhancing the stereoselectivity of Me2GaOR(NHC) species in the ring-opening polymerization of rac-lactide, with the help of the chelation effect”. The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization, P. H.; methodology, P. H., A. M. D.; investigation (analyses of the structure of gallium complexes), P. H., A. R.-N., investigation (ROP studies and collecting PLA samples), P. H., D. T., I. F., N. A. M., P. G.; investigation (polymer analyses and interpretation), P. H., I. W.; visualization, P. H., A. R.-N., I. W. Writing – original draft, P. H.; writing – review & editing, P. H., A. M. D.; resources (gallium complexes for ROP), P. H., D. T., N. A. M., P. G., A. M. D.,; supervision, P. H., A. R.-N.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Warsaw University of Technology, POSTDOC-II grant, no. 1820/9/Z01/Z10/2022, within IDUB Programme. The authors thank Matylda Szewczyk-Łagodzińska and Prof. Andrzej Plichta from Warsaw University of Technology for GPC measurements, Dr Grażyna Zofia Żukowska from Warsaw University of Technology for help with FTIR measurements.Notes and references
- For the selected review articles see: (a) B. J. O'Keefe, M. A. Hillmyer and W. B. Tolman, J. Chem. Soc., Dalton Trans., 2001, 2215 RSC; (b) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147 CrossRef CAS; (c) J. Wu, T. L. Yu, C. T. Chen and C. C. Lin, Coord. Chem. Rev., 2006, 250, 602 CrossRef CAS; (d) A. P. Dove, Chem. Commun., 2008, 6446 RSC; (e) C. A. Wheaton, P. G. Hayes and B. Ireland, J. Dalton Trans., 2009, 4832 RSC; (f) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486 RSC; (g) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165 RSC; (h) P. J. Dijkstra, H. Du and J. Feijen, Polym. Chem., 2011, 2, 520 RSC; (i) S. Słomkowski, S. Penczek and A. Duda, Polym. Adv. Technol., 2014, 25, 436 CrossRef.
- For the recent review articles on the topic see: (a) E. Fazekas, P. A. Lowy, M. A. Rahman, A. Lykkeberg, Y. Zhou, R. Chambenahalli and J. A. Garden, Chem. Soc. Rev., 2022, 51, 8793 RSC; (b) X. Dong and J. R. Robinson, New J. Chem., 2022, 46, 444 RSC; (c) W. A. Munzeiwa, B. O. Omondi and V. O. Nyamori, Polym. Bull., 2024, 81, 9419 CrossRef CAS.
- For the recent representative review article see: R. M. Michell, V. Ladelta, E. Da Silva, A. J. Müller and N. Hadjichristidis, Prog. Polym. Sci., 2023, 146, 101742 CrossRef CAS.
- J. M. Becker, R. J. Pounder and A. P. Dove, Macromol. Rapid Commun., 2010, 31, 1923 CrossRef CAS PubMed.
- For the representative reviews see: (a) S. M. Guillaume, E. Kirillov, Y. Sarazin and J.-F. Carpentier, Chem.–Eur. J., 2015, 21, 7988 CrossRef CAS PubMed; (b) A. J. Teator, D. N. Lastovickova and C. W. Bielawski, Chem. Rev., 2016, 116, 1969 CrossRef CAS PubMed.
- (a) A. Duda and S. Penczek, Macromolecules, 1995, 28, 5981 CrossRef CAS; (b) A. Kowalski, A. Duda and S. Penczek, Macromolecules, 1998, 31, 2114 CrossRef CAS.
- J. Lewiński, P. Horeglad, K. Wójcik and I. Justyniak, Organometallics, 2005, 24, 4588 CrossRef.
- S. Dagorne, F. Le Bideau, R. Welter, S. Bellemin-Laponnaz and A. Maisse-François, Chem.–Eur. J., 2007, 13, 3202 CrossRef CAS PubMed.
- (a) V. Balasanthiran, M. H. Chisholm, K. Choojun and C. B. Durr, Dalton Trans., 2014, 43, 2781 RSC; (b) I. E. Nifant’ev, A. V. Shlyakhtin, V. V. Bagrov, M. E. Minyaev, A. V. Churakov, S. G. Karchevsky, K. P. Birin and P. V. Ivchenko, Dalton Trans., 2017, 46, 12132 RSC.
- (a) J. Lewiński, J. Zachara and I. Justyniak, Chem. Commun., 1997, 1519 RSC; (b) Z. Tang, X. Pang, J. Sun, H. Du, X. Chen, X. Wang and X. Jing, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4932 CrossRef CAS; (c) N. Nomura, R. Ishii, Y. Yamamoto and T. Kondo, Chem.–Eur. J., 2007, 13, 4433 CrossRef CAS; (d) C. Kan, J. Ge and H. Ma, Dalton Trans., 2016, 45, 6682 RSC.
- M.-W. Hsiao and C.-C. Lin, Dalton Trans., 2013, 42, 2041 RSC.
- H. Ma, T. P. Spaniol and J. Okuda, Angew. Chem., Int. Ed., 2006, 45, 7818 CrossRef.
- (a) B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229 CrossRef CAS; (b) L. R. Rieth, D. R. Moore, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 15239 CrossRef CAS PubMed; (c) C. A. Wheaton and P. G. Hayes, J. Organomet. Chem., 2012, 704, 65 CrossRef CAS; (d) V. Balasanthiran, M. H. Chisholm, K. Choojun, C. B. Durr and P. M. Wambua, J. Organomet. Chem., 2016, 812, 56 CrossRef CAS.
- (a) P. Horeglad, P. Kruk and J. Pécaut, Organometallics, 2010, 29, 3729 CrossRef CAS; (b) P. Horeglad, M. Cybularczyk, B. Trzaskowski, G. Z. Żukowska, M. Dranka and J. Zachara, Organometallics, 2015, 34, 3480 CrossRef CAS; (c) P. Horeglad, M. Cybularczyk, A. Litwińska, A. M. Dąbrowska, M. Dranka, G. Z. Żukowska, M. Urbańczyk and M. Michalak, Polym. Chem., 2016, 7, 2022 RSC.
- E. Grunova, E. Kirillov, T. Roisnel and J.-F. Carpentier, Organometallics, 2008, 27, 5691 CrossRef CAS.
- N. Yuntawattana, T. M. McGuire, C. B. Durr, A. Buchard and C. K. Williams, Catal. Sci. Technol., 2020, 10, 7226 RSC.
- (a) M. H. Chisholm, E. E. Delbridge and J. C. Gallucci, New J. Chem., 2004, 6, 145 RSC; (b) A. P. Dove, V. C. Gibson, E. L. Marshall, H. S. Rzepa, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2006, 128, 9834 CrossRef CAS; (c) L. Wang, C. E. Kefalidis, S. Sinbandhit, V. Dorcet, J.-F. Carpentier, L. Maron and Y. Sarazin, Chem.–Eur. J., 2013, 19, 13463 CrossRef CAS PubMed.
- Z. R. Turner, J. T. Wilmore, N. H. Rees and J.-C. Buffet, Dalton Trans., 2022, 51, 3060 RSC.
- (a) E. L. Marshall, V. C. Gibson and H. S. Rzepa, J. Am. Chem. Soc., 2005, 127, 6048 CrossRef CAS PubMed; (b) M. Bouyahyi, N. Ajellal, E. Kirillov, C. M. Thomas and J.-F. Carpentier, Chem.–Eur. J., 2011, 17, 1872 CrossRef CAS; (c) J. Fang, I. Yu, P. Mehrkhodavandi and L. Maron, Organometallics, 2013, 32(23), 6950 CrossRef CAS; (d) I. Nifant’ev, A. Shlyakhtin, M. Kosarev, D. Gavrilov, S. Karchevsky and P. Ivchenko, Polymers, 2019, 11, 1641 CrossRef PubMed.
- For the metal complexes, isoselective and highly active at low temperatures see: (a) P. L. Arnold, J.-C. Buffet, R. P. Blaudeck, S. Sujecki, A. J. Blake and C. Wilson, Angew. Chem., Int. Ed., 2008, 47, 6033 CrossRef CAS; (b) I. Yu, A. Acosta-Ramírez and P. Mehrkhodavandi, J. Am. Chem. Soc., 2012, 134, 12758 CrossRef CAS PubMed; (c) M. Normand, V. Dorcet, E. Kirillov and J.-F. Carpentier, Organometallics, 2013, 32, 1694 CrossRef CAS; (d) P. Horeglad, A. Litwińska, G. Z. Żukowska, D. Kubicki, G. Szczepaniak, M. Dranka and J. Zachara, Appl. Organomet. Chem., 2013, 27, 328 CrossRef CAS; (e) S. Abbina and G. Du, ACS Macro Lett., 2014, 3, 689 CrossRef CAS PubMed; (f) C. Bakewell, A. J. P. White, N. J. Long and C. K. Williams, Inorg. Chem., 2015, 54, 2204 CrossRef CAS PubMed; (g) D. C. Aluthge, J. M. Ahn and P. Mehrkhodavandi, Chem. Sci., 2015, 6, 5284 RSC; (h) Z. Dai, Y. Sun, J. Xiong, X. Pan, N. Tan and J. Wu, Catal. Sci. Technol., 2016, 6, 515 RSC; (i) D. Myers, A. J. P. White, C. M. Forsyth, M. Bown and C. K. Williams, Angew. Chem., Int. Ed., 2017, 56, 5277 CrossRef CAS; (j) K. M. Osten and P. Mehrkhodavandi, Acc. Chem. Res., 2017, 50, 2861 CrossRef CAS; (k) D. E. Stasiw, A. M. Luke, T. Rosen, A. B. League, M. Mandal, B. D. Neisen, C. J. Cramer, M. Kol and W. B. Tolman, Inorg. Chem., 2017, 56, 14366 CrossRef CAS; (l) C. Chen, J. Jiang, X. Mao, Y. Cong, Y. Cui, X. Pan and J. Wu, Inorg. Chem., 2018, 57, 3158 CrossRef CAS; (m) X. Li, Z. Jia, X. Pan and J. Wu, Chem.–Asian J., 2019, 14, 662 CrossRef CAS PubMed.
- For the exemplary metal complexes, heteroselective and highly active at low temperatures see: (a) W. Zhao, D. Cui, X. Liu and X. Chen, Macromolecules, 2010, 43, 6678 CrossRef CAS; (b) L. F. Sánchez-Barba, A. Garcés, J. Fernández-Baeza, A. Otero, C. Alonso-Moreno, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2011, 30, 2775 CrossRef; (c) M. Li, S. Behzadi, M. Chen, W. Pang, F. Wang and C. Tan, Organometallics, 2019, 38(2), 461 CrossRef CAS; (d) M. Honrado, A. Otero, J. Fernández-Baeza, L. F. Sánchez-Barba, A. Garcés, A. Lara-Sánchez and A. M. Rodríguez, Organometallics, 2014, 33(7), 1859 CrossRef CAS.
- (a) P. Horeglad, G. Szczepaniak, M. Dranka and J. Zachara, Chem. Commun., 2012, 48, 1171 RSC; (b) M. Cybularczyk-Cecotka, A. M. Dąbrowska, P. A. Guńka and P. Horeglad, Inorganics, 2018, 6, 28 CrossRef.
- (a) N. Ropson, P. Dubois, R. Jérôme and P. Teyssié, Macromolecules, 1995, 28, 7589 CrossRef CAS; (b) M. H. Chisholm, J. Gallucci and K. Phomphrai, Inorg. Chem., 2002, 41, 2785 CrossRef CAS.
- A. M. Dąbrowska, A. Hurko, K. Durka, M. Dranka and P. Horeglad, Organometallics, 2021, 40, 1221 CrossRef.
- Lewis Basicity and Affinity Scales: Data and Measurement, ed. C. Laurence and J.-F. Gal, John Wiley & Sons Ltd, Chichester, United Kingdom, 2010 Search PubMed.
- J. E. Kasperczyk, Macromolecules, 1995, 28, 3937 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: VT 1H NMR data for complexes 3 and 4; FTIR data for complexes 3 and 4; 13C NMR and MALDI-TOF spectra, as well as GPC eluograms, of PLA. See DOI: https://doi.org/10.1039/d4ra05320f |
| This journal is © The Royal Society of Chemistry 2024 |