Open Access Article
Open Access ArticleEnhanced oxidative stability of deuterated squalene: the impact of labelling extent on chemical resilience†
Johanna Möller‡
 a,
Carl Recsei‡
a,
Carl Recsei‡ *b,
Robert Russell
*b,
Robert Russell b and
Tamim Darwish
b and
Tamim Darwish *bc
*bc
aTechnische Universität München, Arcisstraße 21, 80333 München, Germany
bAustralian Nuclear Science and Technology Organisation, National Deuteration Facility, New Illawarra Rd, Lucas Heights, 2234, New South Wales, Australia. E-mail: tde@ansto.gov.au; recseic@ansto.gov.au
cUniversity of Canberra, Bruce, 2617, Australian Capital Territory, Australia
First published on 19th August 2024
Abstract
The extent to which deuteration affects the oxidative stability of squalene, a nutrient and a commonly-used ingredient in cosmetics and vaccine adjuvants, has been quantified as a function of the extent of labelling. The formation of comedogenic peroxides was inhibited by levels of overall deuteration as low as 19%.
The linear triterpene squalene serves as the universal precursor of sterol molecules in nature as well as a biosynthetic precursor to hormones and vitamins. In its own right, it is produced as a major component of human sebum.1 Its emollient properties have led it to be widely incorporated into cosmetic formulations.2 Squalene is also used as a vaccine adjuvant, for instance against seasonal influenza and H1N1 pandemic flu.3 It may be employed in drug-lipid conjugates to enhance the delivery of drug molecules.4 Other uses include squalene's own medicinal activity5 and in bio-based polymers.6,7
Despite its advantageous properties as a component of cosmetic formulations, squalene tends to form harmful, blemish-causing (i.e. comedogenic) peroxides on skin.8 Thus, even though a protective, antioxidant effect is taking place the product of this reaction is itself damaging. This is not the case for other antioxidants such as ascorbic acid.9 Therefore, techniques for the stabilisation of squalene to allow it to function effectively as a durable emollient in cosmetic formulations or a robust component of drug delivery vehicles are desirable.
While previously sourced almost entirely from shark liver oil, squalene is now also isolated from plant oils, with recent advances permitting microbial biosynthesis to be employed at scale.7,10–12 Microbial biosynthesis favours the creation of isotopically-labelled materials through the use of a suitably labelled carbon source and growth medium.
Since squalene is implicated in breaking lipid peroxidation chains, both in the initial phase and in the propagation phase, the ability to control the tendency of squalene to undergo oxidation reactions could be a useful tool for the study of its endogenous function, particularly its scavenger role in cell stress disease.13 Despite its ubiquity and considerable prior study, there is still considerable uncertainty about the chemical behaviour and biological function of squalene.4 Its role in cardiovascular diseases has been characterised as a ‘double edged sword,’ requiring its function to be better elucidated to facilitate intervention against disease.13 Deuterium labelled squalene is likely to be useful in studying the properties and biological functions of this compound.
Deuterated compounds are also suitable for use as tracers and surrogate standards, for instance in clarifying the fate of squalene-containing formulations in cosmetic and medicinal applications where the endogenously-produced material is also present.14 Stable isotope labelling may also introduce useful chemical characteristics. This is well known for deuterium labelling in medicinal applications where deuteration may confer metabolic stability but can also extend to resistance to other types of chemical degradation.15 These phenomena are consequences of the relative strength of the carbon–deuterium bond with respect to the carbon–protium bond, leading to a uniquely strong hydrogen kinetic isotope effect.
Deuteration of squalene is expected to alter its behaviour as an antioxidant as well as its tendency to form comedogenic peroxides. It is therefore advantageous to be able to produce squalene at a custom level of deuteration and thereby control the level of modulation in chemical behaviour desired. In this work we have explored the tendency of squalene toward the formation of peroxides in air while under UV irradiation, as a function of the degree of deuterium labelling, to mimic the exposure of cosmetic formulations to air and sunlight.
Previous studies have identified characteristic mixtures of hydroperoxides16,17 as well as less common materials such as cyclic peroxides18 generated during free-radical oxidation experiments on squalene. The use of UV-A has proved to be a practical tool to induce oxidation of squalene on human skin in a manner that mimics the conditions under which real formulations operate.19
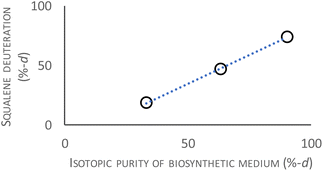
The biosynthesis of deuterated squalene employed Saccharomyces cerevisiae strain Y2805, obtained from Choi and co-workers, because of the high biosynthetic yield of squalene it is known to produce.11 It was used with our previously reported conditions but varying the isotopic purity of the heavy water medium to produce uniformly-deuterated squalene of defined deuteration levels.20 The use of a Lantha D2O analyser allowed for precise determination of the isotopic purity of the medium, permitting the use of heavy water recycled from other biosynthetic work. Fig. 1 shows the relationship between the measured overall deuteration level of the squalene biosynthetic product against the isotopic purity of the heavy water growth medium. The results yield a useful rule of thumb: the isotopic purity of the heavy water growth medium should be set to about 14% higher than the desired deuteration level. Using this rule we produced uniformly-deuterated, i.e. [U-2H]squalene, at overall 19%, 46% and 74%-d, employing the DGet! software platform to analyse mass spectrometric data and determine the overall deuteration level.21
Examination of the methyl, methylene and vinylic integrals of the 2H NMR spectra of [U-2H]squalene at various deuteration levels showed that the level of deuteration is uniformly distributed (a maximum deviation of 5% from the relative integral corresponding to completely uniform deuteration was observed; see: Fig. 2). This means that phenomena subsequently observed in this study are independent of site-specific deuteration effects and can be correlated to overall deuteration.
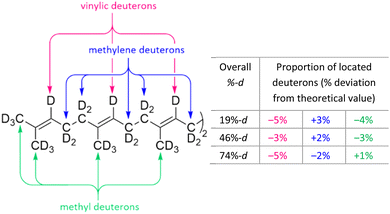 | ||
| Fig. 2 The uniformity of squalene deuteration by chemical environment was assessed by integration of the 2H NMR spectra (see: ESI†). Data is shown as the percentage deviation from the expected integral value if uniform deuteration was observed. | ||
The production of [U-2H]squalene (>85%-d) requires feeding a deuterated carbon source to yeast strain Y2805. For the levels of squalene deuteration used in this study, this is not necessary and only the growth medium was enriched in deuterium. The biosynthetic yield of squalene was not adversely affected by the increasing deuterium content of the medium. At all studied deuteration levels the squalene produced was pure by GC after chromatographic purification, i.e., no other peaks were detected.
We chose to measure the total peroxide content of oxidised samples of squalene via their oxidation of triphenylphosphine, a reaction which has been used extensively as a standard test for degree of lipid peroxidation.22,23 The use of NMR spectroscopy to determine the degree of oxidation has been previously reported, and we considered that using 31P NMR offered the advantage that the spectrum would be unaffected by the presence of overlapping peaks, whereas 1H NMR or GC measurements may be complicated by the presence of reduction products of squalene hydroperoxides.24
The tendency for the differently labelled squalene samples to undergo peroxidation under UV-A irradiation was studied by dispensing equimolar quantities of squalene standard solutions into 1.5 mL glass vials. Following dispensing, a gentle stream of nitrogen was used to blow off the solvent in the dark and the resulting film of squalene around the inside of the vial was subjected to UV-A irradiation in a biocidal cabinet, placing all the (open to the air) vials directly below the fluorescent UV source to permit direct irradiation of the squalene. To ensure that the commercial material (≥98% purity; produced by distillation from shark liver oil) was comparable with the biosynthesised material for the purposes of this study a portion of commercial material was purified by flash column chromatography in analogy to the biosynthesised samples, including filtration and extensive drying in vacuo. The consistent treatment of samples ensured that any observed variability in oxidative stability studies was solely due to the deuteration extent.
The samples for this study were prepared in triplicate, comprising squalene-h50 and purified [U-2H]squalene (19%, 46% and 74%-d). After 1, 3 and 5 days of UV-A irradiation, samples were removed and treated with a triphenylphosphine in CDCl3 standard solution. The samples were agitated several times with a vortex mixer and the ratio of triphenylphosphine and its oxide in the resulting solution measured by quantitative 31P NMR to allow the peroxides formed per squalene molecule to be calculated. The results are shown in Fig. 3. The peroxidation study demonstrated that the tendency toward peroxidation is negatively correlated with deuteration level. This means enhanced resilience of deuterated squalene compared to protiated squalene.
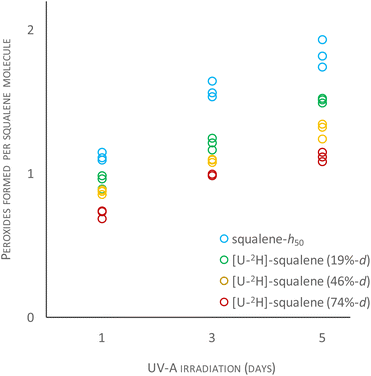 | ||
| Fig. 3 The time-dependent peroxidation studies conducted for different deuteration levels of squalene, displayed as the peroxides formed per squalene molecule as a function of irradiation time. Commercial squalene-h50 (used as received) was also included in the study, behaving similarly to the material subjected to flash column chromatography. For consistency in sample preparation, the data for squalene-h50 (used as received) has been omitted from this figure. See: ESI† (pages 4 and 5) for the data tabulated and graphed. | ||
The peroxidation study shows that the reaction is initially relatively rapid (irradiation time, t ≤ 1 day) then further peroxidation appears slower to occur. NMR analysis showed no formation of aldehydes prior to addition of the triphenylphosphine solution, but we do not rule out other processes that decrease the ability of the oxidised material to oxidise triphenylphosphine through reaction with squalene oxidation products during irradiation. Even if such reactions are occurring, the peroxidation study nevertheless shows that higher deuteration levels result in material with reduced peroxide levels at each studied time point. The measured mean of peroxides formed per squalene as a function of irradiation time is shown in Table 1. An examination of the statistical significance of the results presented in Fig. 3 and Table 1 is found on page 6 of the ESI.†
| Overall %-d | Mean number of peroxides absorbed per squalene molecule (at UV-A irradiation time, t) | ||
|---|---|---|---|
| t = 1 day | 3 days | 5 days | |
| 0%-d | 1.12 | 1.58 | 1.83 |
| 19%-d | 0.95 | 1.21 | 1.51 |
| 46%-d | 0.87 | 1.09 | 1.30 |
| 74%-d | 0.72 | 0.99 | 1.11 |
This peroxidation with increasing deuteration levels is characterised by higher effectiveness per deuterium atom at lower deuteration levels. As an example, taking the measurements in Table 1 after 3 days, the change in the mean extent of peroxidation from squalene-h50 to the lowest level of [U-2H]squalene deuteration studied (a change of +19%-d) is −0.37 peroxides per squalene molecule, while the larger (+27%-d) gap in deuteration level going from [U-2H]squalene (19%-d) to [U-2H]squalene (46%-d) is associated with a smaller change in mean peroxides per squalene of −0.12. A further increase in labelling extent (+28%-d) to [U-2H]squalene (74%-d) shows a yet smaller change in mean peroxides per squalene of −0.10.
Given the uniform deuteration established in Fig. 2, these data suggest a role for deuterium in promoting the termination of chain reactions. If the peroxidation occurring in this study was a series of discrete oxidation events (rate-limited by the breaking or formation of a single C–H or C–D bond) then the peroxides absorbed per molecule ought to be the same for about (100 − x)% of the molecules in [U-2H]squalene (x%-d) as for squalene-h50, discounting secondary isotope effects. In fact, the mean peroxides per squalene-h50 at t = 3 days is 1.58 and therefore one would expect 1.58 × (100 − 19) = 1.28 peroxides per squalene for [U-2H]squalene (19%-d) at t = 3 if C–D bonds did not react at all under the study conditions. Since this anticipated minimum of 1.28 exceeds the total measured peroxides per squalene molecule (1.21) for [U-2H]squalene (19%-d) at t = 3, it is evident that the percentage of deuterium at any specific site in the squalene molecule is an incomplete explanation of the magnitude of the reduction in peroxidation as a function of deuteration level. Since secondary isotope effects are generally marginal and the level of uniform deuteration is the only variable, the effect must therefore be related to the level of deuteration remote from the reaction site. This observation indicates a role for deuteration in suppressing a chain oxidation process rather than process comprised of discrete oxidation events. This putative mechanism for the process of oxidising squalene is in conformance with the known oxidation behaviour of polyunsaturated lipids.25 The suppression of chain oxidation events is classic antioxidant behaviour.
To probe the tendency of deuterated and protiated squalene to endure the conditions of the peroxidation study, rather than measure the degradation products, a second study was performed: using GC to observe unreacted material and thereby avoid deconvoluting NMR spectra (see: Fig. 3). In this kinetic fractionation study equimolar mixtures of [U-2H]squalene (79%-d) and commercial squalene-h50 were irradiated with UV-A under the same conditions as the peroxidation study. This study was restricted to higher levels of overall deuteration to ensure peak separation. The integral ratio differs from unity at t = 0 because deuterated and protiated materials have different response factors when using TIC (EI+) as the ionisation method.§ Upon irradiation the integral ratio of remaining, unoxidized materials was measured. The tendency during the study was for the more rapid destruction of the protiated material and a resulting drift of the integral ratio to favour the deuterated material. The data in Fig. 4 confirm that deuterated squalene has improved durability with respect to protiated squalene. The improved durability of deuterated squalene is linked to the reduced tendency of deuterated squalene to form peroxides (see: Fig. 3).
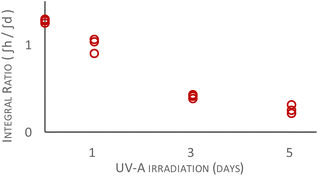 | ||
| Fig. 4 Ratio of integrals (GC) of squalene-h50 (commercial) and [U-2H]squalene (79%-d), as a function of irradiation time. | ||
After one day of irradiation in the kinetic fractionation study there is considerable variability in the integrals (see: ESI,† page 7 for data). This may be due to an induction effect with a variable time for the onset of oxidation, which initially occurs more rapidly and then occurs with a decreasing rate as unoxidized squalene is depleted. The variability at t = 5 days likely reflected the small quantities of unoxidized materials remaining.¶
Deuteration was therefore found both to decrease the tendency of squalene to form undesired, comedogenic peroxides under UV-A irradiation as well as increase the durability of the molecule toward oxidation under UV-A. Our hypothesis that deuteration of squalene would alter its behaviour as an antioxidant as well as its tendency to form comedogenic peroxides in proportion to the extent of labelling was therefore found to be correct, with deuteration enhancing the oxidative stability of squalene.
The ability to reduce the number of comedogenic peroxides formed as well as potentially exert a protective effect on a formulation by terminating chain oxidation events presages a role for moderately deuterated squalene in cosmetic or vaccine formulations. An enhanced ability of deuterated squalene to terminate chain oxidation events also suggests a role in the study and amelioration of cell stress disease.
Data availability
The data supporting the findings of this study are available within the article and its ESI.† This study describes a kinetic fractionation study and a peroxidation study of deuterated squalene. The raw NMR and GC integral data has been provided in tabulated form in the ESI.† In addition, NMR and GC data has been presented to verify the identity and purity of the products. NMR data (provided in graphical form with integrals labelled and the integral curve displayed) has been provided to verify the uniform deuteration of the materials studied.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge Dr Alexander Angeloski for his assistance with instrumentation as well as Dr Eui-Sung Choi for the provision of S. cerevisiae strain Y2805. We acknowledge the Australian Government in supporting ANSTO's National Deuteration Facility, which is partly funded through the National Collaborative Research Infrastructure Strategy (NCRIS).Notes and references
- M. Picardo, M. Ottaviani, E. Camera and A. Mastrofrancesco, Dermatoendocrinol, 2009, 1, 68–71 CrossRef CAS.
- Z. R. Huang, Y. K. Lin and J. Y. Fang, Molecules, 2009, 14, 540–554 CrossRef CAS PubMed.
- P. Nguyen-Contant, M. Y. Sangster and D. J. Topham, Pathogens, 2021, 10, 355 CrossRef CAS PubMed.
- D. Desmaële, R. Gref and P. Couvreur, J. Controlled Release, 2012, 161, 609–618 CrossRef.
- J. M. Lou-Bonafonte, R. Martínez-Beamonte, T. Sanclemente, J. C. Surra, L. V. Herrera-Marcos, J. Sanchez-Marco, C. Arnal and J. Osada, Mol. Nutr. Food Res., 2018, 62, e1800136 CrossRef PubMed.
- S. Grauzeliene, D. Valaityte, G. Motiekaityte and J. Ostrauskaite, Materials, 2021, 14, 2675 CrossRef CAS.
- L. Cheng, T. Ji, M. Zhang and B. Fang, Trends Food Sci. Technol., 2024, 146, 104392 CrossRef CAS.
- D. Saint-Leger, A. Bague, E. Cohen and M. Lchivot, Br. J. Dermatol., 1986, 114, 535–542 CrossRef CAS PubMed.
- M. W. Davey, M. V. Montagu, D. Inzé, M. Sanmartin, A. Kanellis, N. Smirnoff, I. J. J. Benzie, J. J. Strain, D. Favell and J. Fletcher, J. Sci. Food Agric., 2000, 80, 825–860 CrossRef CAS.
- Ç. Yarkent and S. S. Oncel, Biotechnol. Bioprocess Eng., 2022, 27, 295–305 CrossRef.
- J. Y. Han, S. H. Seo, J. M. Song, H. Lee and E.-S. Choi, J. Ind. Microbiol. Biotechnol., 2018, 45, 239–251 CrossRef CAS PubMed.
- K. Paramasivan, P. Kumar Hn and S. Mutturi, Biochem. Eng. J., 2019, 148, 37–45 CrossRef CAS.
- M. Micera, A. Botto, F. Geddo, S. Antoniotti, C. M. Bertea, R. Levi, M. P. Gallo and G. Querio, Antioxidants, 2020, 9, 688 CrossRef CAS.
- F. Beatriz, C. González, M. Lolo and F. J. Sardina, ChemRxiv, 2020, preprint, DOI:10.3390/antiox9080688.
- R. M. C. Di Martino, B. D. Maxwell and T. Pirali, Nat. Rev. Drug Discovery, 2023, 22, 562–584 CrossRef CAS.
- N. Shimizu, J. Ito, S. Kato, Y. Otoki, M. Goto, T. Eitsuka, T. Miyazawa and K. Nakagawa, Sci. Rep., 2018, 8, 9116 CrossRef PubMed.
- D.-M. Pham, B. Boussouira, D. Moyal and Q. L. Nguyen, Int. J. Cosmet. Sci., 2015, 37, 357–365 CrossRef CAS.
- S. Khalifa, M. Enomoto, S. Kato and K. Nakagawa, Antioxidants, 2021, 10, 1760 CrossRef CAS.
- S. E. Mudiyanselage, P. Elsner, J. J. Thiele and M. Hamburger, J. Invest. Dermatol., 2003, 120, 915–922 CrossRef CAS.
- C. Recsei, R. A. Russell, M. Cagnes and T. Darwish, Org. Biomol. Chem., 2023, 21, 6537–6548 RSC.
- T. E. Lockwood and A. Angeloski, J. Cheminf., 2024, 16, 36 Search PubMed.
- T. Nakamura and H. Maeda, Lipids, 1991, 26, 765–768 CrossRef CAS PubMed.
- Z. S. Yeager, PhD thesis, Rutgers University, 2019.
- E. Hafer, U. Holzgrabe, S. Wiedemann, K. M. Adams and B. Diehl, Eur. J. Lipid Sci. Technol., 2020, 122, 1900442 CrossRef CAS.
- H. Yin, L. Xu and N. A. Porter, Chem. Rev., 2011, 111, 5944–5972 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05543h |
| ‡ These two authors are equal contributors to this work and are designated as co-first authors. |
| § The data is uncorrected for the difference in response factor associated with deuteration as the response factor becomes irrelevant when considering the ratio of the integrals as a function of irradiation time; any deviation from the initial ratio means that one compound is changing at a different rate compared to the other, irrespective of the response factor difference. |
| ¶ Evaporation as a substantial contributor to the observed trend was ruled out due to the vial and contents increasing very slightly in weight during the study (this presumably arises from the weight of absorbed oxygen). |
| This journal is © The Royal Society of Chemistry 2024 |