DOI:
10.1039/D4RA05596A
(Review Article)
RSC Adv., 2024,
14, 28585-28595
Facile construction of 2-pyrones under carbene catalysis
Received
1st August 2024
, Accepted 2nd September 2024
First published on 6th September 2024
Abstract
2-Pyrones are valuable structural motifs in organic chemistry, found in numerous natural products and pharmaceuticals. The synthesis of these heterocycles has been significantly advanced by the application of N-Heterocyclic Carbene (NHC) catalysis. This review examines the recent advancements in NHC-catalyzed synthesis of 2-pyrones, highlighting key methodologies, mechanisms, and synthetic applications. NHC catalysis has revolutionized the synthesis of 2-pyrones, providing efficient, selective, and versatile methods for constructing these valuable heterocycles.
1. Introduction
2-Pyrones are often found as common scaffolds in various natural products and biologically active molecules with a wide range of activities, such as antibiotic, antifungal, cytotoxic, neurotoxic and phytotoxic activities1–9 (Fig. 1). Luteoreticulin10 and salinipyrones A11,12 are representative examples. Functionalized pyrones also show potential applications in organic materials due to their privileged photophysical properties.13,14 Moreover, due to their privileged heterocyclic structure (conjugated dienes), lactones and arenes (30–35% resonance energy of benzene15), 2-pyrones as facile raw materials were widely used in organic synthetic chemistry, polymer, and medicinal chemistry.16–27 Therefore, a lot of organic chemists paid much more attention in development of strategies for the construction of 2-pyrones. And a lot of facile and efficient methods have been developed promoted by transition-metals and organocatalysis.28,29
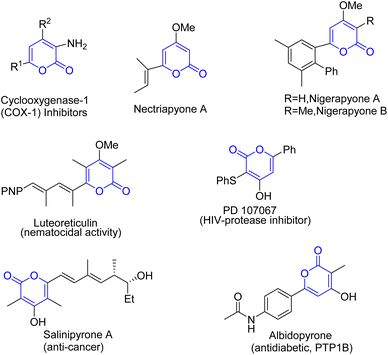 |
| | Fig. 1 Representative biologically important α-pyrones. | |
Notably, organocatalysis meets the development requirements of green chemistry and is widely used in the field of organic synthesis due to their advantages, such as easy catalyst modification, mild reaction conditions, and no residual transition metals. N-heterocyclic carbenes, as one of the most effective organocatalysts, have attracted widespread attention from chemists due to their unique and diverse catalytic activated modes, and have achieved great success in recent years. NHC could activate easily available substrates, such as aldehydes, enals and activated esters, to form several highly reactive and widely used intermediates, such as such as α,β-unsaturated acyl azoliums, alkynyl acyl azoliums, Breslow intermediates, homoenolate and enolate intermediates.30–46 Notably, N-heterocyclic carbene catalysis has also used for the development of a large number of efficient synthetic methods for constructing 2-pyrones. For clarity, this mini-review systematically summarizes the methods for constructing 2-pyranone using N-heterocyclic carbene catalytic strategies, based on the catalytic activation modes of N-heterocyclic carbene (Fig. 2), which are classified into four categories: α,β-unsaturated acyl azoliums, alkynyl acyl azoliums, Breslow intermediates and enolate intermediates. Meanwhile, related substrates are also summarized in Fig. 2c. We evaluate and highlight the progress of this area in this review article, hoping that this short summary will benefit readers in the broad community of chemistry and beyond.
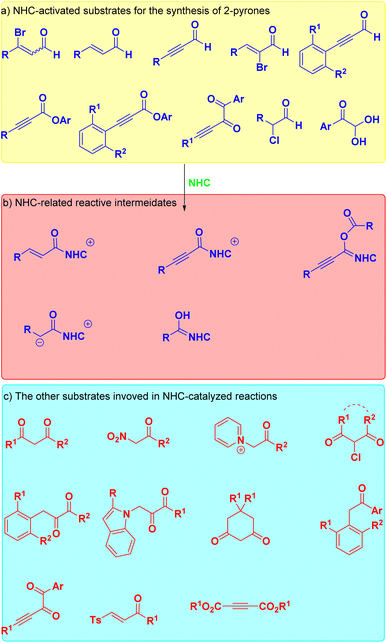 |
| | Fig. 2 Substrates and related intermediates in NHC-catalyzed construction of 2-pyrones: (a) NHC-activated substrates for the synthesis of 2-pyrones; (b) NHC-related reactive intermeidates; (c) the other substrates invoved in NHC-catalyzed reactions. | |
2. Unsaturated acyl intermediates with alpha-leaving group ketones
In 2013, the Ma group reported a highly efficient method for synthesizing multi-substituted 2-pyrones 4 through NHC A catalyzed [3 + 3] cyclization reaction of β-bromo unsaturated aldehydes 1 and 1,3-dicarbonyl compounds 2 by using NaH as a base in THF at 50 °C (Scheme 1).47 Initially, the carbene generated in situ adds to 3-bromoenals, forming the conjugated β-bromo Breslow intermediate Int-1 (Scheme 2), which reacts with an external oxidant to form β-bromo unsaturated acyl azoliums Int-2. The azoliums Int-2 are then attacked by deprotonated 1,3-dicarbonyl compounds 2′, likely forming 1,4-adducts Int-3. The intermediates Int-3 then undergo cyclization and bromide elimination, resulting in the production of 2H-pyran-2-ones 4 and the release of the carbene catalyst. It is worth noting that the oxidant 3 used in this conversion have been introduced for the first time by the Studer group into the field of NHC catalysis, which can efficiently oxidize nucleophilic homoenolate into electrophilic unsaturated intermediate.48
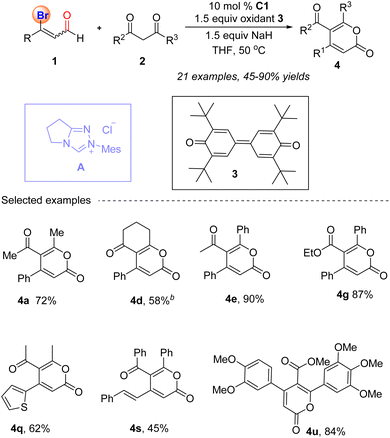 |
| | Scheme 1 NHC-catalyzed synthesis of 2-pyrones from 3-bromoenals and 1,3-dicarbonyl compounds. | |
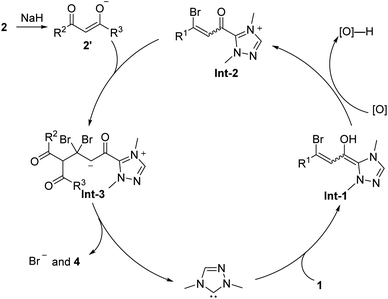 |
| | Scheme 2 Proposed mechanism for Ma's work.47 | |
In 2016, the Studer group achieved the [3 + 3] cyclization reaction of unsaturated aldehydes 5 and aroyl-substituted nitromethane 6, efficiently constructing 4, 6-diaryl-2-pyrones 7 (Scheme 3).49 In this reaction, NHC B was proved as the suitable catalyst with Cs2CO3 as a base in THF. Unsaturated aldehydes undergo a similar process under NHC activity and oxidation of 5 to obtain unsaturated acyl intermediates Int-4 (Scheme 4). It is worth noting that in this reaction, the nitro group in the aroyl-substituted nitromethane is both an activating group and a good leaving group. After HNO2 elimination, it can increase an unsaturation degree, and then undergo lactonization to obtain the final 2-pyrones 7 with moderate to good yields.
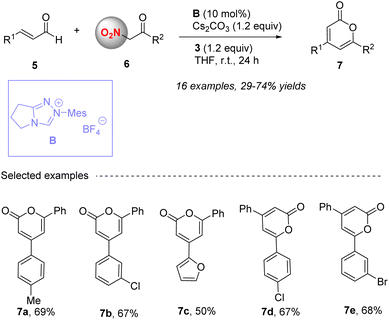 |
| | Scheme 3 The synthesis of 4,6-disubstituted 2-pyrones from aryl-substituted nitromethanes and enals under NHC catalysis. | |
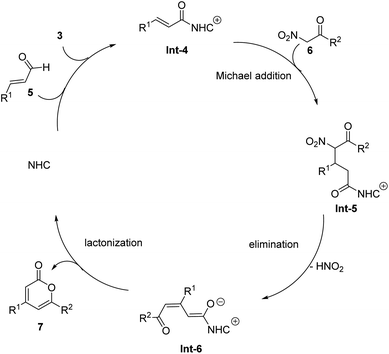 |
| | Scheme 4 Proposed mechanism for Studer's work.49 | |
In 2018, Wang's group reported a cyclization strategy using NHC catalysis of ynals 8 and ylides 9, which directly constructed a series of 4,6-disubstituted 2-pyrones 10 with good to high yields (Scheme 5).50 A series of easily prepared and inexpensive ylides 9, including N-pyridinium, N-quinolinium, and N-imidazolium ylides, were screened, and N-pyridinium ylides have been proven to be more efficient one. NHC activates ynals following by a proton transfer to generate α,β-unsaturated acyl intermediates Int-4, and then undergoes elimination of pyridinium intermediate followed by intramolecular cyclization to give the desired 2-pyrones 10. It is worth noting that N-pyridinium serves as both an activating group and a good leaving group in this conversion. The method is tolerant of various functional groups on both the enal and pyridinium bromide substrates, making it versatile and adaptable to different synthetic needs. Very nearly, Xue, Chi and co-workers51 realized similar work, also using the N-pyridinium ylides 9 to react with α,β-unsaturated acyl intermediate Int-4. The different from Wang group's work, they used enal 5 and oxidant 3 to generate α,β-unsaturated acyl intermediate Int-4, the reaction undergoes similar process to give the desired 2-pyrones 10 with good to excellent yields. Delightingly, for 2′-pyridinium ylides, switching of aryl group to alkyl group also worked well. Notably, both of alkyl ynals and alkyl enals are not compatible with this transformation. For these two works, the optimal conditions, such as catalyst, bases and solvents, are different.
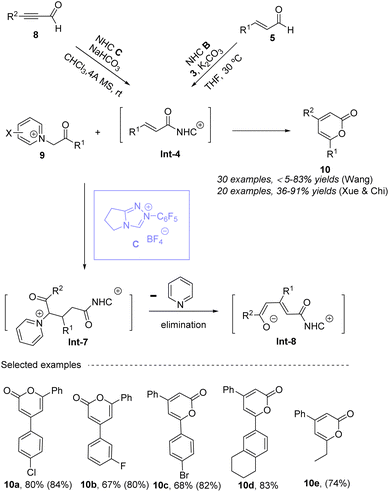 |
| | Scheme 5 The synthesis of 4,6-disubstituted 2-pyrones from ylides and ynals (or enals) under NHC catalysis. | |
In 2022, Xu, Chen and co-workers52 applied inexpensive and easily prepared 2-chloro-1,3-diketones 12 as nucleophiles to construct 4,5,6-trisubstituted α-pyrones 13 efficiently under mild conditions (Scheme 6). Carbene which generated from NHC D deprotonated by Cs2CO3 in THF, activates brominated unsaturated aldehydes 11 to form α,β-unsaturated acyl intermediates Int-4, which are then subjected to Michael addition by 2-chloro-1,3-diketones 12. Subsequently, the departing chlorine increases by one degree of unsaturation, followed by lactonization to give variables 4,5,6-trisubstituted 2-pyrones 13 in generally good to excellent yields. The reactions do not require external oxidants, making the process more environmentally friendly. 2-Pyrones bearing various substituents and substitution patterns are accessible. Besides 6-membered ring scaffold of 2-chloro-1,3-diketones, 6-membered ring or acyclic scaffolds are compatible with this transformation. Notably, alkyl bromoenal also worked well.
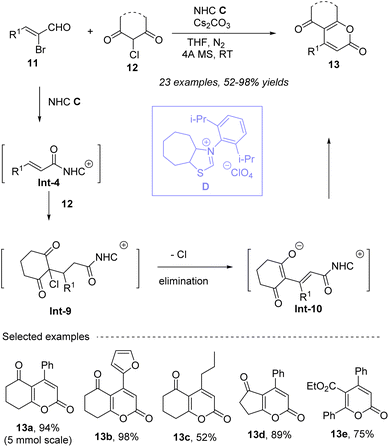 |
| | Scheme 6 Oxidant free synthesis of 2-pyrones from 2-chloro-1,3-diketones and bromoenals under NHC catalysis. | |
In 2022, our group53 realized an NHC-catalyzed atroposelective [3 + 3] annulation reactions from readily available enals 5 and α-aryl pyruvates 14 though Coates-Claisen type rearrangement, after the automatic aromatization, assembling wide-ranging of atropisomerically enriched 5-aryl 2-pyrones 16 in good to excellent enatioselectivities (Scheme 7). Chiral NHC E could give the best outcomes in the term of yields and enationselecitivities by using DBU as a base in THFat −20 °C. Comparison experiments and reported results reveal that the presented strategies involving an atroposelective annulation/heteroaromatization sequence to construct axially chiral aryl-heteroaryl molecules features an inherent advantage in comparison to these rely on alkynylacyl azolium intermediates. The method is compatible with a wide range of substrates, including enals with various electron-withdrawing and donating groups, leading to high yields and enantiomeric ratios. Pleasingly, the resulting axially chiral 2-pyrones were easily converted into widely used biaryl atropisomers in excellent yields without any loss of enantioselectivities via Diels–Alder reaction with benzynes, such as biaryl amino acids and BINAM derivatives, demonstrating the practical utility of the synthesized molecules. Notably, the intermediate could be oxidized to 2-pyrones under reaction conditions (Scheme 8).
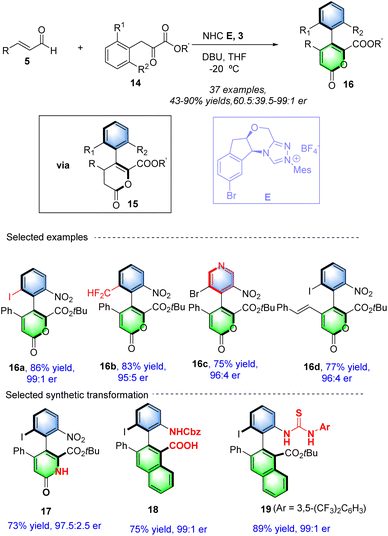 |
| | Scheme 7 NHC-catalyzed synthesis of axially 2-pyrones from enals and α-aryl pyruvates. | |
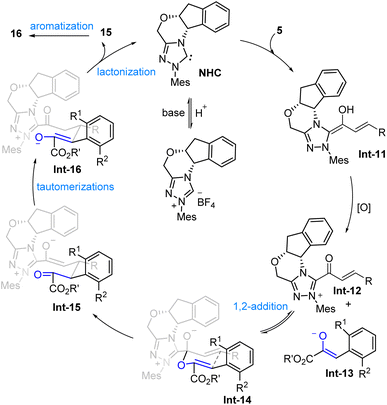 |
| | Scheme 8 Proposed mechanism for Fu's work.53 | |
In 2023, our group54 further developed a carbene-catalyzed oxidative [3 + 3] annulation of indole-1-pyruvate esters 20 and enals 5 to construct a variety of axially enantioenriched 5-indo-1-yl pyran-2-ones 21 with an N–C axis under similar optimal conditions with our previous work (Scheme 9).53 This strategy features direct asymmetric 2-pyrone ring from enals under oxidative NHC catalysis. The method is applicable to a broad range of substrates, including variously substituted indole-1-pyruvate esters and enals. The resulting products exhibit high enantiomeric ratios, making them suitable for further asymmetric synthesis applications. The reaction mechanism is similar with previous work. The resulting products could be converted into important axially chiral N-arylindoles.
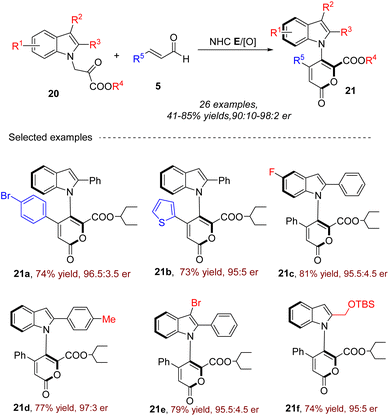 |
| | Scheme 9 The synthesis of 5-indo-1-yl pyran-2-ones with an N–C axis from indole-1-pyruvate esters and enals. | |
3. Alkynyl acyl azoliums with enols
Wang group55 reported a novel method for synthesizing axially chiral α-pyrone-aryls 24 through an N-heterocyclic carbene (NHC)-catalyzed [3 + 3] atroposelective annulation (Scheme 10). This method involves the reaction of cyclic 1,3-diones 23 with ynals 22 in the presence of an NHC precatalyst, base, Lewis acid, and oxidant. The process leads to the formation of axially chiral 2-pyrones in moderate to good yields with high enantioselectivities under NHC F catalysis by using nBu4NOAc as a base in toluene. Notably, Mg(OTf)2 as the additive in this reaction could suppress unexpected intermediates and reaction pathways which results in the formation of byproducts. A wide range of cyclic 1,3-diones and ynals are compatible with this transformation. The use of readily available starting materials and mild reaction conditions makes the method economically attractive and practical for large-scale synthesis. The study also explores the mechanistic pathways and demonstrates the synthetic utility of the products. The addition of the NHC catalyst to ynal followed by oxidation results in the formation of alkynyl acyl azolium intermediate Int-17 (Scheme 11), which subsequently reacts with cyclic 1,3-dione 23 to form intermediate Int-18. Intermediate Int-18 undergoes a Michael addition to the alkynyl azolium moiety, forming an allenolate intermediate Int-19. After a proton transfer from the 1,3-dione to the allenolate, intermediate Int-20 is formed. The subsequent formation of the O–C bond results in intermediate Int-21, releasing the NHC catalyst and ultimately generating product 24. This innovative approach not only expands the toolkit for organic synthesis but also provides a practical and efficient method for producing valuable chemical scaffolds with significant potential in pharmaceuticals and material science.
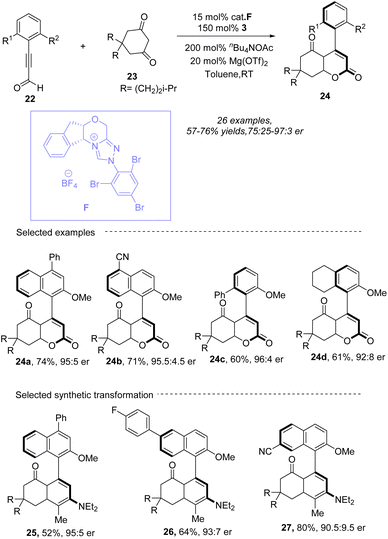 |
| | Scheme 10 NHC-catalyzed synthesis of axially 2-pyrones from ynals and cyclic 1,3-dicarbonyl compounds. | |
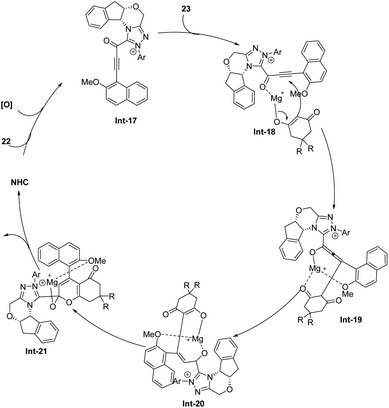 |
| | Scheme 11 Proposed mechanism for Wang's work.55 | |
In 2019, Du and co-workers56 presented a novel method for the efficient construction of pyrano [3,2-b]indol-2-one skeletons 30 in moderate to high yields (up to 95%) under DBU as a base in DCM (Scheme 12), in which the formal [3 + 3] annulation of alkynoic acid esters 28 with indolin-3-ones 29 to construct 2-pyrone ring was facilitated by N-heterocyclic carbene (NHC) B catalysis. The nucleophilic attack of carbene on substrate 28 leads to the formation of alkynyl acylazolium Int-22. Next, the conjugate addition of the C-centered nucleophile derived from 29 with DBU, to intermediate Int-22 results in the formation of intermediate Int-23. This intermediate then undergoes a proton transfer to produce intermediate Int-24. Finally, the lactone formation of Int-24 yields the desired product 30. The resulting pyrano [3,2-b]indol-2-ones 30 can serve as valuable intermediates for the synthesis of other complex molecules with significant biological activities. The use of readily available starting materials and mild reaction conditions makes the method economically attractive and practical for large-scale synthesis.
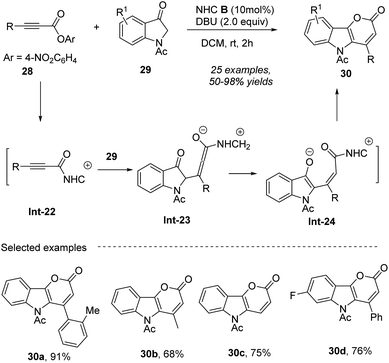 |
| | Scheme 12 Access to functionalized pyrano [3,2-b]indol-2-ones from alkynoic acid esters and indolin-3-one under NHC catalysis. | |
In 2022, Du, Wei and co-workers57 reported a novel method for synthesizing triaryl 2-pyrones 33 with multiple stereogenic axes promoted by NHC G in the presence of Cs2CO3 in DMF (Scheme 13). The research demonstrates a single-step construction of 1,2-diaxially chiral triaryl 2-pyrones under N-heterocyclic carbene organocatalysis. The method involves an asymmetric [3 + 3] annulation of well-designed alkynyl acylazolium precursors with enolizable sterically hindered 2-aryl ketones, resulting in the formation of desired 1,2-diaxially chiral triaryl α-pyrones in excellent diastereoselectivities (most cases >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivities (up to 99.5
1 dr) and enantioselectivities (up to 99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er). The reaction is initiated by nucleophilic addition to 31 by the NHC organocatalyst, which is generated by deprotonation of pre-NHC F, producing intermediate Int-25. The cleavage of the C1–O1 bond occurs, forming intermediate Int-26. Subsequently, Michael addition of 32a′ to intermediate Int-26 generates Int-26, which undergoes proton transfer followed by deprotonation leads to the formation of Int-29. Lactonization of Int-29 results in the formation of the final product 33a and regenerates carbene. Density functional theory (DFT) calculations are performed to rationalize the high diastereoselectivity and enantioselectivity in this [3 + 3] annulation process. TS3 are in four stereoselective pathways. The Gibbs free energy barriers for TS3RS, TS3SR, TS3RR, and TS3SS are 7.7, 11.4, 13.9, and 16.4 kcal mol−1, respectively, indicating that the 4R,5S-configurational pathway is the most energetically favorable. Thus, with the aid of CsHCO3, only the favorable pathway associated with intermediate M3RS is discussed further. Subsequently, intermediate M3RS transforms to intermediate M5RS via a protonated base-assisted1,3-proton shift through stepwise transition states TS4RS (ΔG‡ = 4.9 kcal mol−1) and TS5RS (ΔG‡ = 2.5 kcal mol−1). Following this, the C1–O bond forms through transition state TS6RS (ΔG‡ = 2.2 kcal mol−1), resulting in the ring-closure intermediate M6RS. Finally, the C1–C bond is broken to dissociate the product PRS (3a) with the catalyst through transition state TS7RS (ΔG‡ = 2.3 kcal mol−1). Overall, the pathway associated with the 4R,5S-configurational product is the most energetically favorable, with an energy barrier difference (ΔΔG‡ TS3SR–TS3RS) of 3.7 kcal mol−1, corresponding to an enantiomeric excess (ee) value of 99.4% according to Boltzmann distribution equations (Scheme 14). The resulting 1,2-diaxially chiral triaryl α-pyranones can be further transformed into useful compounds, such as chiral catalysts 34 and ligands 35–37. Notably, Wong, Lu and co-workers also realized similar transformations in which the synthesis of triaryl-2-pyrones with monoaxial or contiguous C–C diaxes the enals has been established via oxidative NHC catalysis (Scheme 15).58
0.5 er). The reaction is initiated by nucleophilic addition to 31 by the NHC organocatalyst, which is generated by deprotonation of pre-NHC F, producing intermediate Int-25. The cleavage of the C1–O1 bond occurs, forming intermediate Int-26. Subsequently, Michael addition of 32a′ to intermediate Int-26 generates Int-26, which undergoes proton transfer followed by deprotonation leads to the formation of Int-29. Lactonization of Int-29 results in the formation of the final product 33a and regenerates carbene. Density functional theory (DFT) calculations are performed to rationalize the high diastereoselectivity and enantioselectivity in this [3 + 3] annulation process. TS3 are in four stereoselective pathways. The Gibbs free energy barriers for TS3RS, TS3SR, TS3RR, and TS3SS are 7.7, 11.4, 13.9, and 16.4 kcal mol−1, respectively, indicating that the 4R,5S-configurational pathway is the most energetically favorable. Thus, with the aid of CsHCO3, only the favorable pathway associated with intermediate M3RS is discussed further. Subsequently, intermediate M3RS transforms to intermediate M5RS via a protonated base-assisted1,3-proton shift through stepwise transition states TS4RS (ΔG‡ = 4.9 kcal mol−1) and TS5RS (ΔG‡ = 2.5 kcal mol−1). Following this, the C1–O bond forms through transition state TS6RS (ΔG‡ = 2.2 kcal mol−1), resulting in the ring-closure intermediate M6RS. Finally, the C1–C bond is broken to dissociate the product PRS (3a) with the catalyst through transition state TS7RS (ΔG‡ = 2.3 kcal mol−1). Overall, the pathway associated with the 4R,5S-configurational product is the most energetically favorable, with an energy barrier difference (ΔΔG‡ TS3SR–TS3RS) of 3.7 kcal mol−1, corresponding to an enantiomeric excess (ee) value of 99.4% according to Boltzmann distribution equations (Scheme 14). The resulting 1,2-diaxially chiral triaryl α-pyranones can be further transformed into useful compounds, such as chiral catalysts 34 and ligands 35–37. Notably, Wong, Lu and co-workers also realized similar transformations in which the synthesis of triaryl-2-pyrones with monoaxial or contiguous C–C diaxes the enals has been established via oxidative NHC catalysis (Scheme 15).58
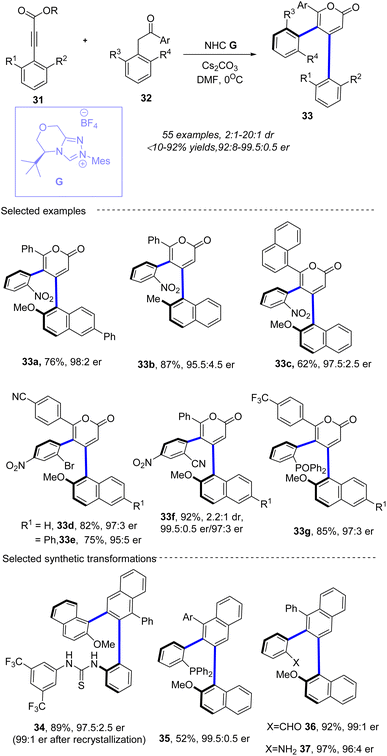 |
| | Scheme 13 NHC-catalyzed synthesis of 1,2-diaxially chiral triaryl 2-pyrones. | |
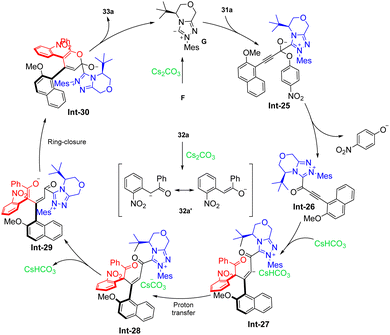 |
| | Scheme 14 Proposed mechanism for Du's work.57 | |
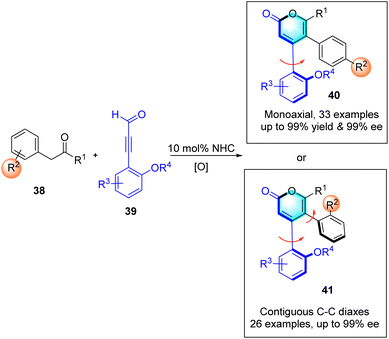 |
| | Scheme 15 NHC-catalyzed synthesis of 1,2-diaxially chiral triaryl 2-pyrones. | |
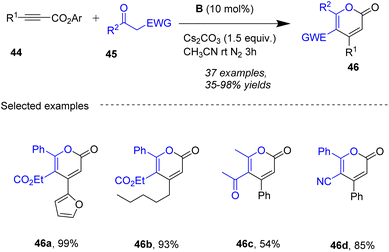
Zhao group59 (Scheme 16) and Du group60 (Scheme 17) further developed a novel strategy for the construction of functionalized 2-pyrones by using the similar strategies.
 |
| | Scheme 16 NHC-catalyzed synthesis of 2-pyrones from ynals with β-keto esters. | |
 |
| | Scheme 17 NHC-catalyzed synthesis of 2-pyrones from alkynyl esters with alkynyl esters. | |
4. O-Acylated allenolate intermediates
Fang and co-workers61 presented a novel method for the rapid synthesis of a variety of α-pyrones 48 through the Umpolung (polarity reversal) of alkynyl 1,2-diketones 47 under N-heterocyclic carbene (NHC) I catalysis in the presence of Cs2CO3 in THF (Scheme 18). The study introduces a new Umpolung pattern involving an O-acylated allenolate as the key intermediate and proposes an unprecedented reaction pathway characterized by group migrations and new bond formations. A variety of substrates, including both diaryl and alkyl-substituted alkynyl diketones are tolerated under reaction condition. Specifically, the nucleophilic attack of a carbonyl group of 47 by the carbene catalyst leads to intermediate Int-31 (Scheme 19). An O-acylated allenolate equivalent Int-32 is formed after the first acyl transfer. Subsequently, the nucleophilic addition of Int-32/33 to a second molecule of 47 affords intermediate Int-34, and intermediate Int-35 is generated via the second acyl transfer. An unusual acyloxy migration occurs between intermediates Int-35 and Int-36, and finally, a lactonization process releases the product 48. Notably, a total of four bonds (one C–C bond and three C–O bonds) are formed in this cycle.
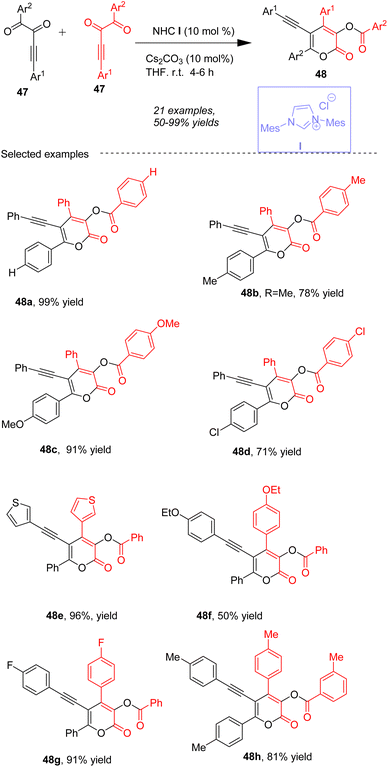 |
| | Scheme 18 NHC-catalyzed synthesis of 2-pyrones from alkynyl 1,2-diketones. | |
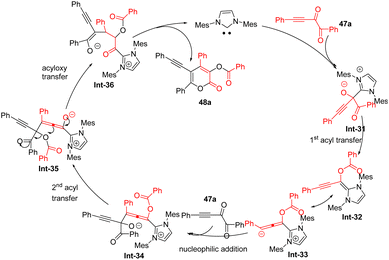 |
| | Scheme 19 Proposed mechanism for Fang's work.61 | |
5. Enolate intermediates
In 2021, Tiwari group62 reported the direct, transition metal-free synthesis of 3,6-disubstituted α-pyrones 51 in moderate to excellent yields (up to 97%) from widely accessible α-chloro aldehydes 49 and β-tosyl enones 50 (Scheme 20). The reactions proceed via a Michael addition/lactonization/elimination cascade, offering a regioselective addition of NHC-bound enolates to enones with bulky functionalities. It should be noted that β-tosyl enones have remained beyond the scope of carbene catalysis. Enolates which are generated from α-chloro aldehydes with carbene via a dechlorination process, undergo Michael addition to enones bearing a leaving group (Ts), lactonization and further detosylation to eventually produce 3,6-disubstituted α-pyrones 51. A wide range of α-chloro aldehydes and β-tosyl enones are compatible with this transformation. The resulting 3,6-disubstituted α-pyrones can be further transformed into valuable compounds such as tetrasubstituted benzenes 54, naphthalenes 53, anthracenes 55, and dihydro-ethenopentacenes 56.
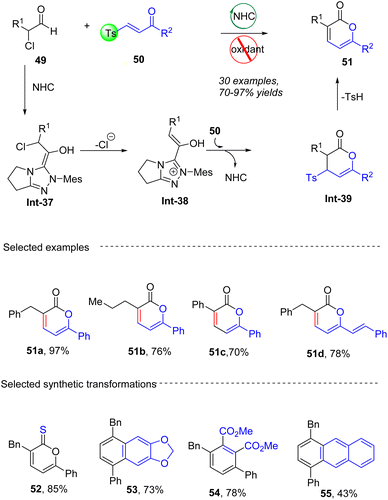 |
| | Scheme 20 NHC-catalyzed synthesis of 2-pyrones from α-chloro aldehydes and β-tosyl enones. | |
6. Breslow intermediates
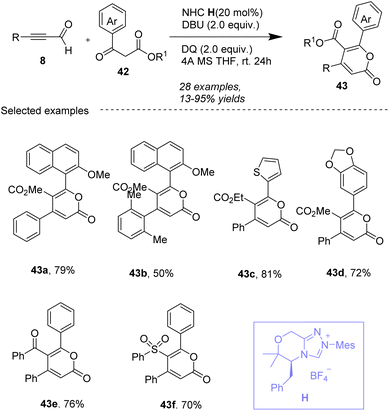
In 2018, Tu, Jiang and co-workers63 developed a new method for synthesizing trisubstituted 2-pyrones 58 using arylglyoxals 57 as a carbonyl source promoted by NHC J in the presence of NaH in CH3CN (Scheme 21). The innovative method involves a thiazolium salt-catalyzed [3 + 2 + 1] cyclization, potentially offering higher yields and better functional group compatibility. The use of arylglyoxals as dual-role reagents enhances the versatility of the reaction, allowing for the incorporation of various functional groups and structural motifs into the final products. This approach is noteworthy for its potential in organic synthesis, offering a novel route to construct 2-pyrones with specific substitution patterns.
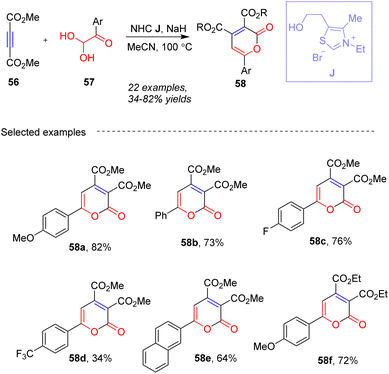 |
| | Scheme 21 NHC-catalyzed synthesis of 2-pyrones from arylglyoxals and substrates 56. | |
For this reaction (Scheme 22), thiazole carbene, generated in situ by the deprotonation of the thiazolium salt I at its most acidic position, reacts with 2-(4-methoxyphenyl)-2-oxoacetaldehyde Int-40, derived from substrate 57, to form zwitterionic intermediate Int-41 (detected by LC-MS, MS = 335.1). This is followed by an Umpolung process to convert Int-41 into intermediate Int-42. Next, the addition of Int-42 to the C–C bond of substrate 56 generates zwitterionic intermediate Int-43 (detected by LC-MS, MS = 477.1), which undergoes an aldol-type reaction with Int-40 to deliver intermediate Int-44 (detected by LC-MS, MS = 641.2). Afterward, proton transfer between Int-44 and H2O gives intermediate Int-45 (detected by LC-MS, MS = 642.2), which is intercepted by the hydroxyl anion to afford Int-46. This step eliminates 4-methoxybenzoic acid (detected by LC-MS, MS = 152.0) to produce intermediate Int-47 (detected by LC-MS, MS = 507.2). Intermediate Int-47 is then converted into the final product 58 through a sequence involving continuous intramolecular nucleophilic addition/elimination of thiazole carbene I and dehydration.
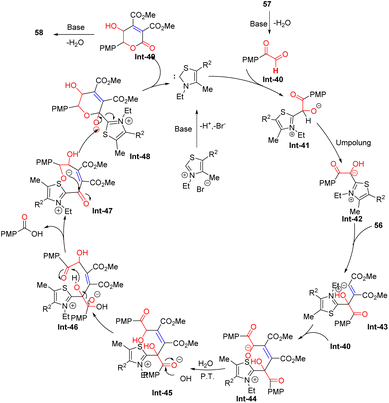 |
| | Scheme 22 Proposed mechanism for Tu, and Jiang's work.63 | |
7. Conclusion and outlook
2-Pyrones have received significant attention in recent years because of their important biological activities and wide applications in synthetic chemistry. The synthesis of these compounds has been significantly enhanced by the application of N-Heterocyclic Carbene (NHC) catalysis. Although significant progress has been made in this field, there are still many issues as follows: (i) most reactions require 10–20% catalyst loading. Further improvements in catalytic efficiency are essential to reduce catalyst loading and enhance overall reaction efficiency; (ii) the range of substrates used in these reactions is relatively limited. Expanding the variety of substrates remains a key challenge, necessitating innovative approaches and the development of new substrates to broaden the applicability of NHC-catalyzed synthesis; (iii) although various 2-pyrone structures have been synthesized, few mimic the structures of natural products or bioactive molecules. Achieving direct synthesis of such compounds through the development of new reaction types and substrate designs is crucial; (iv) despite the synthesis of numerous 2-pyrone molecules, relatively little research has been conducted on their biological activity. Increased focus on biological testing can uncover new bioactive compounds and enhance the relevance of synthesized 2-pyrones in medicinal chemistry. Addressing challenges such as catalytic efficiency, substrate diversity, direct synthesis of bioactive molecules, and biological activity testing will drive the future of 2-pyrone research. Continued exploration in this field promises to expand the synthetic utility and biological relevance of 2-pyrones, with far-reaching implications for organic and medicinal chemistry.
Data availability
Our manuscript entitled “Facile Construction of 2-Pyrones under Carbene Catalysis” should be a review article in RSC Advances. Therefore, there is no need to upload the compound characterisation checklist.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We acknowledge financial support by Natural Science Foundation of Jiangsu Province (BK20221309).
Notes and references
- J. M. Dickinson, Nat. Prod. Rep., 1993, 10, 71–98 RSC.
- G. P. McGlacken and I. J. S. Fairlamb, Nat. Prod. Rep., 2005, 22, 369–385 RSC.
- A. Goel and V. J. Ram, Tetrahedron, 2009, 65, 7865–7913 CrossRef CAS.
- T. F. Schäberle, Beilstein J. Org. Chem., 2016, 12, 571–588 CrossRef PubMed.
- C. P. Sharma, N. M. Gupta, J. Singh, R. A. K. Yadav, D. K. Dubey, K. S. Rawat, A. K. Jha, J.-H. Jou and A. Goel, J. Org. Chem., 2019, 84, 7674–7684 CrossRef CAS PubMed.
- J.-Y. Dong, M.-C. Tang and L. Liu, J. Nat. Prod., 2023, 86, 2496–2501 CrossRef CAS PubMed.
- I. A. Abdelhakim, Y. Futamura, Y. Asami, H. Hanaki, N. Kito, S. Masuda, A. Shibata, A. Muranaka, H. Koshino, K. Shirasu, H. Osada, J. Ishikawa and S. Takahashi, J. Nat. Prod., 2024, 87, 1459–1470 CrossRef PubMed.
- T. Tokizaki, R. Kanehara, H. Maeda, K. Tanaka and M. Hashimoto, J. Nat. Prod., 2024, 87, 1798–1807 CrossRef CAS.
- D. Wang, C. Yuan, Y. Li, S. Bai, J. Feng, Y. Wang, Y. Fang and Z. Zhang, J. Agric. Food Chem., 2024, 72, 3894–3903 CrossRef CAS PubMed.
- M. G. Nair, A. Chandra and D. L. Thorogod, Pestic. Sci., 1995, 43, 361–365 CAS.
- D.-C. Oh, E. A. Gontang, C. A. Kauffman, P. R. Jensen and W. Fenical, J. Nat. Prod., 2008, 71, 570–575 CrossRef CAS.
- P. Ramesh and H. M. Meshram, Tetrahedron, 2012, 68, 9289–9292 CrossRef CAS.
- V. Mahendran, K. Pasumpon, S. Thimmarayaperumal, P. Thilagar and S. Shanmugam, J. Org. Chem., 2016, 81, 3597–3602 CrossRef CAS PubMed.
- C. P. Sharma, N. M. Gupta, J. Singh, R. A. K. Yadav, D. K. Dubey, K. S. Rawat, A. K. Jha, J.-H. Jou and A. Goel, J. Org. Chem., 2019, 84, 7674–7684 CrossRef CAS PubMed.
- R. Pratap and V. J. Ram, Tetrahedron, 2017, 73, 2529–2590 CrossRef CAS.
- R. Pratap and V. J. Ram, Tetrahedron, 2017, 73, 2529–2590 CrossRef CAS.
- Y. Wang, H. Li, Y.-Q. Wang, Y. Liu, B. M. Foxman and L. Deng, J. Am. Chem. Soc., 2007, 129, 6364–6365 CrossRef CAS PubMed.
- C.-L. Sun and A. Fürstner, Angew. Chem., Int. Ed., 2013, 52, 13071–13075 CrossRef CAS PubMed.
- L. Chang, K. Plevová, S. Thorimbert and L. Dechoux, J. Org. Chem., 2017, 82, 5499–5505 CrossRef CAS PubMed.
- Y. Chen, G. Coussanes, C. Souris, P. Aillard, D. Kaldre, K. Runggatscher, S. Kubicek, G. Di Mauro, B. Maryasin and N. Maulide, J. Am. Chem. Soc., 2019, 141, 13772–13777 CrossRef CAS PubMed.
- M. Giardinetti, N. I. Jessen, M. L. Christensen and K. A. Jørgensen, Chem. Commun., 2019, 55, 202–205 RSC.
- T. Ohyoshi, K. Mitsugi, T. Higuma, F. Ichimura, M. Yoshida and H. Kigoshi, RSC Adv., 2019, 9, 7321–7323 RSC.
- C. J. F. Cole, L. Fuentes and S. A. Snyder, Chem. Sci., 2020, 11, 2175–2180 RSC.
- J. Wang, M. A. Márquez-Cadena and R. Tong, Org. Lett., 2020, 22, 5074–5078 CrossRef CAS PubMed.
- M.-M. Xu, X.-Y. You, Y.-Z. Zhang, Y. Lu, K. Tan, L. Yang and Q. Cai, J. Am. Chem. Soc., 2021, 143, 8993–9001 CrossRef CAS PubMed.
- M.-M. Xu, P.-P. Xie, J.-X. He, Y.-Z. Zhang, C. Zheng and Q. Cai, J. Am. Chem. Soc., 2024, 146, 6936–6946 CrossRef CAS PubMed.
- H.-B. Yu, Y.-G. Chen, Y. Tian, M.-S. Xie and H.-M. Guo, ACS Catal., 2024, 14, 8930–8938 CrossRef CAS.
- J. S. Lee, Mar. Drugs, 2015, 13, 1581–1620 CrossRef CAS.
- D. Dobler, M. Leitner, N. Moor and O. Reiser, Eur. J. Org Chem., 2021, 2021, 6180 CrossRef CAS.
- X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC.
- S. De Sarkar, A. Biswas, R. C. Samanta and A. Studer, Chem.–Eur. J., 2013, 19, 4664–4678 CrossRef CAS PubMed.
- S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906–4917 RSC.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS.
- D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307–9387 CrossRef CAS PubMed.
- R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040–5052 RSC.
- X. Y. Chen, Q. Liu, P. Chauhan and D. Enders, Angew. Chem., Int. Ed., 2018, 57, 3862–3873 CrossRef CAS PubMed.
- S. Mondal, S. R. Yetra, S. Mukherjee and A. T. Biju, Acc. Chem. Res., 2019, 52, 425–436 CrossRef CAS PubMed.
- X. Y. Chen, Z. H. Gao and S. Ye, Acc. Chem. Res., 2020, 53, 690–702 CrossRef CAS PubMed.
- H. Ohmiya, ACS Catal., 2020, 10, 6862–6869 CrossRef CAS.
- X. Chen, H. Wang, Z. Jin and Y. R. Chi, Chin. J. Chem., 2020, 38, 1167–1202 CrossRef CAS.
- S. Barika and A. T. Biju, Chem. Commun., 2020, 56, 15484–15495 RSC.
- T. K. Das and A. T. Biju, Chem. Commun., 2020, 56, 8537–8552 RSC.
- A. T. Biju, in N-Heterocyclic Carbenes in Organocatalysis, Wiley-VCH, Weinheim, 2019 Search PubMed.
- T. Li, Z. Jin and Y. R. Chi, Sci. China Chem., 2022, 65, 210–223 CrossRef CAS.
- K.-Q. Chen, H. Sheng, Q. Liu, P.-L. Shao and X.-Y. Chen, Sci. China Chem., 2021, 64, 7–16 CrossRef CAS.
- J. Lv, J. Xu, X. Pan, Z. Jin and Y. R. Chi, Sci. China Chem., 2021, 64, 985–990 CrossRef CAS.
- G. Wang, C. Xia, G.-H. Miao, W.-J. Yao and C. Ma, J. Org. Chem., 2013, 78, 6223–6232 CrossRef CAS.
- S. De Sarkar and A. Studer, Angew. Chem., Int. Ed., 2010, 49, 9266–9269 CrossRef CAS.
- S. Bera and A. Studer, Synthesis, 2017, 49, 121–126 CAS.
- M. Lang, Q. Jia and J. Wang, Chem.–Asian J., 2018, 13, 2427–2430 CrossRef CAS PubMed.
- P. Zheng, C. Li, C. Mou, D. Pan, S. Wu, W. Xue, Z. Jin and Y. R. Chi, Asian J. Org. Chem., 2019, 8, 1067 CrossRef CAS.
- C. Luo, X.-Y. Xu, J.-F. Xu and X.-K. Chen, Org. Biomol. Chem., 2022, 20, 9298–9301 RSC.
- G. Wang, J. Huang, L. Zhang, J. Han, X. Zhang, J. Huang, Z. Fu and W. Huang, Sci. China Chem., 2022, 65, 1953–1961 CrossRef CAS.
- L. Zhang, Q. Wu, M. Ren, H. Zhang, X. Zhang, J. Liu and Z. Fu, Adv. Synth. Catal., 2023, 365, 3467 CrossRef CAS.
- C. Zhao, D. Guo, K. Munkerup, K.-W. Huang, F. Li and J. Wang, Nat. Commun., 2018, 9, 611 CrossRef PubMed.
- K. Sun, S. Jin, S. Fang, R. Ma, X. Zhang, M. Gao, W. Zhang, T. Lua and D. Du, Org. Chem. Front., 2019, 6, 2291 RSC.
- S. Zhang, X. Wang, L.-L. Han, J. Li, Z. Liang, D. Wei and D. Du, Angew. Chem., Int. Ed., 2022, 61, e202212005 CrossRef CAS PubMed.
- S.-C. Zhang, S. Liu, X. Wang, S.-J. Wang, H. Yang, L. Li, B. Yang, M. W. Wong, Y. Zhao and S. Lu, ACS Catal., 2023, 13, 2565–2575 CrossRef CAS.
- C. Jiang, Z. Dong, J. Wang and C. Zhao, Asian J. Org. Chem., 2023, 12, e202300371 CrossRef CAS.
- Z. Liang, J. Li, L. Wang, Z. Wei, J. Huang, X. Wang and D. Du, J. Org. Chem., 2023, 88, 1836–1843 CrossRef CAS.
- X. Kong, G. Zhang, S. Yang, X. Liu and X. Fang, Adv. Synth. Catal., 2017, 359, 2729 CrossRef CAS.
- K. Khatana, V. Singh, M. K. Gupta and B. Tiwari, Adv. Synth. Catal., 2021, 363, 4862 CrossRef.
- L.-C. Xu, P. Zhou, J.-Z. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Org. Chem. Front., 2018, 5, 753–759 RSC.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *b
*b










![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivities (up to 99.5
1 dr) and enantioselectivities (up to 99.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 er). The reaction is initiated by nucleophilic addition to 31 by the NHC organocatalyst, which is generated by deprotonation of pre-NHC F, producing intermediate Int-25. The cleavage of the C1–O1 bond occurs, forming intermediate Int-26. Subsequently, Michael addition of 32a′ to intermediate Int-26 generates Int-26, which undergoes proton transfer followed by deprotonation leads to the formation of Int-29. Lactonization of Int-29 results in the formation of the final product 33a and regenerates carbene. Density functional theory (DFT) calculations are performed to rationalize the high diastereoselectivity and enantioselectivity in this [3 + 3] annulation process. TS3 are in four stereoselective pathways. The Gibbs free energy barriers for TS3RS, TS3SR, TS3RR, and TS3SS are 7.7, 11.4, 13.9, and 16.4 kcal mol−1, respectively, indicating that the 4R,5S-configurational pathway is the most energetically favorable. Thus, with the aid of CsHCO3, only the favorable pathway associated with intermediate M3RS is discussed further. Subsequently, intermediate M3RS transforms to intermediate M5RS via a protonated base-assisted1,3-proton shift through stepwise transition states TS4RS (ΔG‡ = 4.9 kcal mol−1) and TS5RS (ΔG‡ = 2.5 kcal mol−1). Following this, the C1–O bond forms through transition state TS6RS (ΔG‡ = 2.2 kcal mol−1), resulting in the ring-closure intermediate M6RS. Finally, the C1–C bond is broken to dissociate the product PRS (3a) with the catalyst through transition state TS7RS (ΔG‡ = 2.3 kcal mol−1). Overall, the pathway associated with the 4R,5S-configurational product is the most energetically favorable, with an energy barrier difference (ΔΔG‡ TS3SR–TS3RS) of 3.7 kcal mol−1, corresponding to an enantiomeric excess (ee) value of 99.4% according to Boltzmann distribution equations (Scheme 14). The resulting 1,2-diaxially chiral triaryl α-pyranones can be further transformed into useful compounds, such as chiral catalysts 34 and ligands 35–37. Notably, Wong, Lu and co-workers also realized similar transformations in which the synthesis of triaryl-2-pyrones with monoaxial or contiguous C–C diaxes the enals has been established via oxidative NHC catalysis (Scheme 15).58
0.5 er). The reaction is initiated by nucleophilic addition to 31 by the NHC organocatalyst, which is generated by deprotonation of pre-NHC F, producing intermediate Int-25. The cleavage of the C1–O1 bond occurs, forming intermediate Int-26. Subsequently, Michael addition of 32a′ to intermediate Int-26 generates Int-26, which undergoes proton transfer followed by deprotonation leads to the formation of Int-29. Lactonization of Int-29 results in the formation of the final product 33a and regenerates carbene. Density functional theory (DFT) calculations are performed to rationalize the high diastereoselectivity and enantioselectivity in this [3 + 3] annulation process. TS3 are in four stereoselective pathways. The Gibbs free energy barriers for TS3RS, TS3SR, TS3RR, and TS3SS are 7.7, 11.4, 13.9, and 16.4 kcal mol−1, respectively, indicating that the 4R,5S-configurational pathway is the most energetically favorable. Thus, with the aid of CsHCO3, only the favorable pathway associated with intermediate M3RS is discussed further. Subsequently, intermediate M3RS transforms to intermediate M5RS via a protonated base-assisted1,3-proton shift through stepwise transition states TS4RS (ΔG‡ = 4.9 kcal mol−1) and TS5RS (ΔG‡ = 2.5 kcal mol−1). Following this, the C1–O bond forms through transition state TS6RS (ΔG‡ = 2.2 kcal mol−1), resulting in the ring-closure intermediate M6RS. Finally, the C1–C bond is broken to dissociate the product PRS (3a) with the catalyst through transition state TS7RS (ΔG‡ = 2.3 kcal mol−1). Overall, the pathway associated with the 4R,5S-configurational product is the most energetically favorable, with an energy barrier difference (ΔΔG‡ TS3SR–TS3RS) of 3.7 kcal mol−1, corresponding to an enantiomeric excess (ee) value of 99.4% according to Boltzmann distribution equations (Scheme 14). The resulting 1,2-diaxially chiral triaryl α-pyranones can be further transformed into useful compounds, such as chiral catalysts 34 and ligands 35–37. Notably, Wong, Lu and co-workers also realized similar transformations in which the synthesis of triaryl-2-pyrones with monoaxial or contiguous C–C diaxes the enals has been established via oxidative NHC catalysis (Scheme 15).58













